Abstract
Introduction
Population-based studies show inconsistent effects of cigarette smoking on olfactory function. We aimed to identify direct and indirect associations between measures of smoking exposure/nicotine dependence and altered olfaction in a nationally representative sample of adults.
Methods
NHANES 2011–2014 (n = 7418) participants (mean age = 57.8 ± 12.2 years) self-reported olfaction and related health and demographic risks. Affirmative answers to three questions defined altered olfaction (olfactory problems in the past years; worse ability since age 25; phantom smells). Smoking (never, former, current) was self-reported by chronicity (pack years, PY) and dependency (time to first cigarette upon waking) and verified by serum cotinine. Associations were tested with logistic regression, reporting odds ratios (ORs) and 95% confidence intervals (CIs), and mediation models.
Results
Estimated prevalence of altered olfaction was 22.3%, with age-related increases. Nearly half of the sample were former/current smokers (47.4%). Controlling for olfactory-related risks, ≥10 PY smokers had significantly greater odds of altered olfaction versus never smokers (OR 1.36, CI: 1.06–1.74). The odds of altered olfaction were heightened among current smokers (≥10 PY) who also had high nicotine dependence (smoked ≤30 min of waking) (OR 1.41, CI: 1.01–1.99). Light smokers (≤10 PY smokers) did not show increased odds versus never smokers. Current smokers who also were heavy drinkers (≥4 drinks/day) had the highest odds for altered olfaction (OR 1.96, CI: 1.20–3.19). Olfactory-related pathologies (sinonasal problems, serious head injury, tonsillectomy, xerostomia) partially mediated the association between smoking and altered olfaction.
Conclusions
Chronic cigarette smoking was associated with increased odds of self-reported olfactory alterations, directly and indirectly via olfactory-related pathologies.
Implications
Analysis of the US nationally representative data revealed significant positive associations between chronic smoking and alterations in the sense of smell. Rates of smell alteration (self-reported problems in the past year, losses with aging, and phantom smells) increased from 23% among adults to 33% for chronic smokers and 38% for chronic smokers who also reported heavy drinking. Chronic smoking showed associations with smell alteration that were direct and indirect through exposure to olfactory-related pathologies (naso-sinus problems, dry mouth, head/facial injury). Smell alteration can impact smokers’ quality of life by challenging the ability to sense warning odors, food flavor, and olfactory-stimulated emotions and memories.
Introduction
Analysis of NHANES 2011–2012 data indicates that olfactory dysfunction is a prevalent problem, affecting nearly 13% of the US adults ≥40 years of age.1 Ranging from partial (hyposmia) to complete (anosmia) loss and phantom sensations, olfactory dysfunction can result from the loss of olfactory receptors, inability of odors to reach and bind to these receptors, interrupted transmission of the odor message to the central olfactory systems, or inability to correctly identify and label odors. Clinical and population-based studies2,3 show that modifiable and nonmodifiable risks of olfactory dysfunction include frequent sinonasal problems,4 head or face trauma,5 exposure to certain chemicals,6 neurodegenerative disorders,7 and advanced age.1,2 Individuals with olfactory dysfunction are more likely to experience hazardous exposures in the environment and food,8 poorer nutritional status,9 and reduced quality of life.10 Healthy People 2020 has goals to increase the proportion of adults who seek diagnosis and treatment for chemosensory disorders,11 compelling greater attention to prevention and treatment of olfactory dysfunction.10
Cigarette smoking may be a modifiable risk factor for olfactory dysfunction. In animal models, chronic exposure to aqueous cigarette smoke decreased functional olfactory receptors.12 Population-based findings are less consistent. From the Epidemiology of Hearing Loss Study, current smokers (relative to former and never smokers) at baseline had greater odds of olfactory dysfunction in odor identification task,2 yet the 5-year follow-up revealed no significant association between baseline smoking status and incidence of olfactory dysfunction.13 Among 1300 Swedish adults, there was no significant association between odor identification ability and cigarette smoking, whether defined as current smoking, heavy smoking, or by pack years.14 A Spanish study (n = 9348) using home-administered odor identification tests, found former or current smoking as mildly protective of olfactory function.3 Conversely, current smoking was a risk factor for measured olfactory dysfunction in a German study (n = 1312), with dose-response relationships between cigarettes/day and frequency of impairment.15 Similarly, dose-response relationships were reported between chronic smoking and olfactory impairment in a community-based study (n = 638).16
Chronic cigarette exposure could indirectly impair olfactory function via known risk factors, including upper respiratory track infections,17 sinonasal problems,18 and xerostomia.19 Analysis of NHANES 2011–2012 data suggested that significant risk factors for self-reported olfactory alteration included persistent cold/flu, persistent xerostomia, frequent nasal congestion, head injury, and heavy alcohol consumption.20 Smokers appear more susceptible to viral respiratory colds,21 acute and chronic rhinitis, nasal inflammation,22 xerostomia;23 have longer recovery after mild traumatic brain injury;24 and report greater alcohol consumption.25 Excessive alcohol consumption has been linked to depressed olfactory function measured by odor identification26 and/or odor discrimination27 testing, and olfactory dysfunction was observed among those with neurological complications of alcohol dependence.28
Presently, we examined the independent and joint effects of smoking status and olfactory-related risks on self-reported olfactory alteration in the NHANES 2011–2014 dataset. Olfactory alteration was operationalized as an index based on three responses, recorded at the time of the NHANES interview, and treated as a binary classification.20 Smoking exposure was defined in terms of chronicity, severity of nicotine dependence, and presence of current smoking. We hypothesized that defining smoking status by chronicity, dependence, and nicotine biomarker would strengthen its association with self-reported olfactory alteration. We also hypothesized a synergistic effect of dependent smoking and heavy alcohol consumption on olfactory alteration. Finally, since self-reported olfactory alterations showed good association with clinically identified risks of olfactory dysfunction,20 we tested the hypothesis that smoking would have an indirect effect on self-reported olfactory alteration via other olfactory-related pathologies. Findings from this study have implications for smoking cessation—olfactory abilities may improve with smoking cessation.16,29,30
Methods
Data for the present study were abstracted from the NHANES. Conducted each year by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention, NHANES involves cluster, multistage sampling to randomly select US households to provide insight into emerging health issues, disease risk, and changes in health problems over time.31 The resulting sample is nationally representative of civilian, noninstitutionalized residents, selected for assessment of health and nutrition via interview questionnaires, laboratory tests, and physical examinations.
For this study, the continuous NHANES 2011–2012 and 2013–2014 cycles were merged, and data from adults, aged 40 years and older (n = 7418), who answered questions on olfactory-related problems, cigarette smoking, and other potential risk factors were analyzed. The NCHS Research Ethics Review Board approved all procedures, and participants provided written, informed consent.
Measures
Self-Rated Olfactory Alteration
The NHANES Chemosensory Questionnaire (CSQ) included items regarding self-reported olfactory ability as well as symptoms, medical treatment, and presence of related risk factors for olfactory dysfunction.31 These questions were content-validated by experts in chemosensation and tested to ensure consistency in participant understanding, processing, and interpretation.1 Since combining questions on current olfactory function and losses with aging improved the sensitivity and specificity of self-reported olfactory ability,32 three CSQ questions were used in an index to classify olfactory alteration: perceived olfactory problems within the past 12 months [yes], or phantom odor sensations [yes], or perceived changes in function since age 25 [worse now].20 A positive response on any of these questions resulted in a positive score for olfactory alteration. The dichotomous measure [‘yes’ or ‘no’] for olfactory alteration was the outcome variable in data analyses. This classification has shown excellent test–retest reliability over 6 months33 and good correspondence with clinically supported risk factors.20 Classification with this index has reasonable specificity (78.1%) and modest sensitivity (54.4%) in identifying anosmia/severe hyposmia via single screening measure,1 a specificity/sensitivity pattern expected of conditions, such as olfactory dysfunction, which are rarely measured.34
Cigarette Smoking and Smoking Status Classification
The NHANES home interview included questions about daily cigarette use, history of use, details about length of time being a smoker, and time to first cigarette upon waking in the morning.35 Serum cotinine was measured in the NHANES mobile examination center. The interview responses and cotinine levels yielded five smoking status classifications (for details see Supplementary Table S1).
Smokers were classified based on an affirmative response to ever smoking 100 cigarettes in their lifetime; never smokers answered ‘no.’ Current smokers answered ‘yes’ to the question ‘do you now smoke cigarettes’ whereas former smokers answered ‘no.’ Former smokers also reported the length of time since quitting cigarettes, which was converted into a continuous measure (years, portion of years). Light smokers were uniquely defined for comparison with each smoking status classification (Supplementary Table S1) and included the 2.7% of smokers who reported smoking ‘some days’ or 1 cigarette/day.
Chronic Smoker
Packs smoked per year (packs/day × years smoked) defined light [<10 pack years (PY), n = 1343] or chronic (≥10 PY, n = 1922) smokers. Chronic smokers were classified further as current (n = 915; reporting cigarette use in the last 30 days) or former (n = 1007; reporting cigarette use around the time when they smoked regularly). Years smoked was calculated for current (interview age − age reported started smoking) and former [interview age − (age reported started smoking − reported number of years since quitting)] smokers.
Current Chronic, High Dependent Smoker
Smoking status was refined by a proxy for nicotine dependence, time to first cigarette of the day (TTFC), which is linked with negative health outcomes.36,37 Current chronic high dependent smokers were defined as ≥10 PY and ≤30 minutes of waking (n = 582). Current light smokers were either <10 PY or >30 minutes TTFC (n = 697).
Chronic Active Smoker
PY was combined with available serum cotinine measures (NHANES 2011–2012 subset). Serum cotinine, the main nicotine metabolite, is regarded as the best biomarker of smoking exposure.38 Typical levels among nonsmokers are <1 ng/mL; those with heavier exposure (eg, secondhand smoke) are 1–10 ng/mL38. We used ≥10 ng/mL cotinine to define a smoker39 and distinguish false self-reports of nonsmoking. Thus, chronic active smokers were: ≥10 PY smokers with cotinine levels ≥10 ng/mL (n = 418); current light smokers <10 PY or <10 ng/mL cotinine (n = 422); and never/former smokers <10 ng/mL cotinine. Because cotinine metabolism varies between race/ethnicity groups,40 multivariate analyses were verified using race/ethnicity-specific cotinine exposure levels.41
High Dependent Active Smoker
Smoking status was defined with both TTFC and cotinine levels to compare to never/former smokers with <10 ng/mL cotinine. High dependent active smokers reported TTFC ≤30 minutes and ≥10 ng/mL cotinine (n = 297); light smokers were TTFC >30 minutes (n = 450) or <10 ng/mL cotinine (n = 450).
Heavy Alcohol Drinking and Defining High Dependent Smoker-Drinkers
The NHANES alcohol use questionnaire probed current and lifetime alcohol use trends. Heavy drinking, defined as answering ‘yes’ to there being a time/times in their life that they drank 4/5 drinks on almost every day (versus ‘no’), was examined independently as a risk factor for olfactory alteration and combined with high dependent smoking (TTFC ≤ 30 minutes). Adults were classified as either never/former smoker and heavy drinker (n = 628), high dependent smoker and nonheavy drinker (n = 391), or high dependent smoker/heavy drinker (n = 214) to compare with neither smokers nor heavy drinkers (n = 4642).
Olfactory-Related Pathologies and Sociodemographic Risk Factors
Potential risk factors of olfactory alterations were assessed as covariates, including sociodemographic variables and olfactory-related pathologies. Education status was dichotomized (<high school education and ≥high school education). Race was classified as Mexican American, Other Hispanic, nonHispanic white, nonHispanic black, nonHispanic Asian, or Other nonHispanic/Multi-Race. Income-to-poverty ratio (family income divided by federal poverty threshold) was dichotomized as below (≤1) or above (>1) the poverty line. Marital status was defined as married or not (widowed, divorced, separated, and never married). Self-rated health status was dichotomized (poor/fair health and excellent/very good/good health). Sinonasal problem during the past 12 months was defined as reporting persistent cold/flu (lasting more than a month) or frequent nasal congestion from allergies. Other examined risks included xerostomia (persistent dry mouth) during the past 12 months, history of serious head or face injury, history of tonsillectomy, and history of frequent ear infections (3+).
Data Analysis
Because of the complex sampling design of NHANES, sample weights were combined across 2-year data collection cycles to adjust for over-sampling of selected population subgroups, survey nonresponse, and poststratification. Statistical analyses were completed using SAS version 9.4 (Cary, NC), and mediation models were computed in SPSS using the PROCESS macro. All tests were two-tailed and p values <.05 were considered statistically significant.
Univariate associations between self-reported olfactory alterations and potential risks were assessed with chi-square tests (categorical variables) and two-tailed t-tests (continuous variables). Only chronic/dependent/active smokers (not light smokers; Supplementary Table S1) were compared to never/former smokers in univariate analyses. Post hoc analyses were completed for chi-square tests, when necessary, using adjusted standardized residuals.
Potential risks for self-reported olfactory alteration, including all smoking status classifications, were examined in unadjusted and adjusted logistic regression models. Odds ratios were considered significant if the confidence interval did not include the value one. Variables that were statistically significant in the unadjusted model were included as covariates in the multivariable (adjusted) models. Age and sex were selected a priori due to their known association with both smoking and olfactory function. Separate multivariable models were tested for each smoking status classification (Supplementary Table S1). Former smokers were grouped with never smokers for the analyses, except for the >10 PY (chronic) measurement; former smokers did not have significantly greater odds of olfactory alteration (versus never smokers) and greater years since quitting smoking was not associated with lower odds of an olfactory alteration when controlling for age and sex (OR: 1.00; 95% CI = 0.99 to 1.01). For the smoking status classifications including cotinine, only the NHANES 2011–2012 cycle was available, creating unequal sample sizes of nonsmokers versus chronic active/high dependent smokers. Accordingly, logistic regression models were tested using age and sex matched nonsmokers and smokers using propensity scores derived from the MatchIt package (www.r-project.org), using the ‘nearest’ option.
Two mediation models were examined in the smoking status classifications that showed greatest odds of self-reported olfactory alteration in multivariable analysis (ie, current chronic high dependent smokers, high dependent smoker-drinkers). The PROCESS macro allowed a logistic regression analytical framework with bootstrapping to estimate indirect and direct effects with a dichotomous outcome variable. The mediator variable in each model was an olfactory risk score—the significant risk factors for olfactory alteration in multivariable analyses (score 0 to 5) based on equally-weighted ‘yes’ responses to: frequent nasal congestion; persistent cold/flu; xerostomia; tonsillectomy; or history of a serious head/face injury. The first model tested whether the olfactory risk score (m) mediated the association between current chronic high dependent smoking (x) and olfactory alteration (y). The second model tested whether the olfactory risk score (m) mediated the association between high dependent smoker-drinkers (x) and olfactory alteration (y). Correlations between all variables were assessed prior to mediation modeling to test for expected bivariate relationships (between x and y, x and m, and m and y), controlling for x. Beta estimates, standard errors, and 95% confidence intervals were used through bootstrapping procedure using 5000 resampling to estimate the mediation relationships. Relationships were considered significant if the confidence intervals did not include the value zero. The ratio of indirect effect to total effect was used to quantify the proportion mediated. Covariates in both models on the a and b paths were age, sex, race, and income-to-poverty ratio. Alternate models, with the direction of causality switched, were tested; these models failed to adequately fit the data.
Results
Nearly half of the total sample (52.3%) were never smokers versus former/current smokers (47.4%). Table 1 provides demographic characteristics of the total sample and by smoking status. Smokers, in all five classifications, were more frequently male, non-Hispanic White, with lower education level, living below the poverty line, and heavy drinkers.
Table 1.
Demographic Characteristics of Participants in the NHANES 2011–2014 Sample and Stratified by Smoking Status Classification
| Entire NHANES sample | Current and former chronic smokersa | Current chronic high dependent smokers b | Chronic active smokers c | High dependent active smokers d | High dependent smoker-drinkers e | |
|---|---|---|---|---|---|---|
| Number of participants | 7418 | 1922 | 582 | 418 | 297 | 214 |
| Gender (%) | ||||||
| Male | 47.2 | 56.6 | 53.7 | 58.4 | 54.5 | 70.6 |
| Female | 52.8 | 43.4 | 46.4 | 41.6 | 45.5 | 29.4 |
| Age (years) | 57.8 ± 12.2 | 59.4 ± 11.6 | 59.8 ± 9.6 | 54.5 ± 10.5 | 54.0 ± 10.2 | 53.2 ± 8.8 |
| Race (%) | ||||||
| Mexican American | 6.2 | 3.1 | 1.1 | 1.9 | 2.4 | 1.8 |
| Other Hispanic | 4.9 | 3.0 | 2.7 | 2.5 | 2.2 | 1.9 |
| NonHispanic white | 71.2 | 79.9 | 80.5 | 79.1 | 76.5 | 81.7 |
| NonHispanic black | 10.7 | 9.0 | 9.5 | 9.6 | 11.3 | 10.3 |
| NonHispanic Asian | 4.8 | 2.0 | 1.1 | 1.0 | 1.1 | <1 |
| Other/multi-race | 2.2 | 3.0 | 5.1 | 5.8 | 6.5 | 4.0 |
| Education (%) | ||||||
| < High school | 17.1 | 21.2 | 27.5 | 24.6 | 26.6 | 32.7 |
| ≥High school | 82.9 | 78.8 | 72.5 | 75.4 | 73.4 | 67.3 |
| Income-to-poverty ratio (% ≤1) | 12.9 | 15.6 | 25.5 | 17.9 | 23.1 | 30.8 |
| Marital status | ||||||
| Married(%) | 63.2 | 56.6 | 49.9 | 51.4 | 46.9 | 48.3 |
| Heavy drinkers (%) | 15.5 | 30.5 | 37.4 | 33.6 | 32.7 | 100.0 |
a≥10 PY (packs smoked per day × years smoked) smokers.
b≥10 PY smokers who report Time to First Cigarette (TTFC) as <30 minutes.
c≥10 PY smokers with serum cotinine ≥ 10 ng/ml.
dSmokers who report TTFC as <30 minutes with serum cotinine ≥ 10 ng/ml.
eSmokers who report TTFC as <30 minutes as well as consuming 4/5 alcoholic drinks on most/every day.
Olfactory alteration was reported by 22.3% (n = 1609) of the total sample. Of those, 32.4% reported loss since age 25, 26.2% reported a problem in the past year and loss since age 25, and 6.5% reported all three (smell problems in past year, loss with aging, phantom smells). Phantom smells were reported by 20.4%. These results are comparable to prevalence estimates in the NHANES 2011–201220.
Olfactory Alteration Risks—Univariate Analysis
Table 2 reports distribution of olfactory alteration by separate potential risks, including smoking status classifications. Adults of age 80 years and more most frequently reported olfactory alteration. By post hoc testing, significantly higher proportion of olfactory alteration was reported by nonHispanic White and other nonHispanic/multirace; lower proportion by nonHispanic Black and Asians. Adults who were unmarried, lived below the poverty line, had self-rated fair/poor health, or were heavy drinkers had significantly greater reported frequency of olfactory alteration. Additionally, greater frequency of olfactory alteration was reported by those with history of serious head/face injury, tonsillectomy, ear infections, persistent cold/flu, dry mouth, and frequent nasal congestion. These risks are consistent with the NHANES 2011–2012 analysis.20
Table 2.
Distribution of Self-Reported Olfactory Alteration by Separate Potential Risk Factors, Including Smoking Status Classification
| Total sample | Olfactory alteration | % of self-reported olfactory alteration | |||
|---|---|---|---|---|---|
| N | N | % Yes | % No | Statistic | |
| Age, years (mean) | 57.8 ± 12.2 | 58.6 ± 12.4 | 57.6 ± 12.2 | T = 2.13* | |
| Age strata | χ2 = 16.10* | ||||
| 40–49 years | 1934 | 378 | 20.4 | 79.6 | |
| 50–59 years | 1852 | 402 | 22.8 | 77.2 | |
| 60–69 years | 1848 | 406 | 22.3 | 77.7 | |
| 70–79 years | 1069 | 216 | 22.1 | 77.9 | |
| 80+ years | 715 | 207 | 28.3 | 71.7 | |
| Sex | χ2 = 0.28 | ||||
| Male | 3556 | 747 | 22.4 | 77.6 | |
| Female | 3862 | 862 | 22.2 | 77.8 | |
| Race/Ethnicity | χ2 = 30.52*** | ||||
| Mexican American | 803 | 172 | 21.3 | 78.7 | |
| Other Hispanic | 737 | 162 | 21.9 | 78.1 | |
| NonHispanic black | 1777 | 351 | 19.4 | 80.6 | |
| NonHispanic white | 3047 | 761 | 23.2 | 76.8 | |
| NonHispanic Asian | 894 | 118 | 13.1 | 86.9 | |
| Other/multi-race | 160 | 45 | 30.9 | 69.1 | |
| Marital Status | χ2 = 10.32*** | ||||
| Married | 4301 | 844 | 21.0 | 79.0 | |
| Not Married | 3108 | 762 | 24.3 | 75.7 | |
| Education | χ2 = 2.58 | ||||
| < High school | 1925 | 433 | 24.0 | 76.0 | |
| ≥High school | 5484 | 1176 | 22.0 | 78.0 | |
| Income-to-poverty ratio | χ2 = 16.71*** | ||||
| IPR ≤ 1 (poverty) | 1426 | 377 | 27.9 | 72.1 | |
| IPR > 1 | 5322 | 1112 | 21.7 | 78.3 | |
| Self-rated health | χ2 = 50.20*** | ||||
| Fair or poor | 1793 | 514 | 30.0 | 70.0 | |
| Excellent, very good, good | 4704 | 924 | 20.8 | 79.2 | |
| Heavy Alcohol Use | χ2 = 55.91*** | ||||
| Yes | 986 | 281 | 31.8 | 68.2 | |
| No | 5464 | 1142 | 21.0 | 79.0 | |
| Smoking Status Classification | |||||
| Chronic Smokers | 1922 | 544 | 29.3 | 70.7 | χ2 = 77.82*** |
| Never smokers | 3942 | 725 | 19.1 | 80.9 | |
| Current Chron High Dependent Smokers | 582 | 181 | 32.8 | 67.2 | χ2 = 44.96*** |
| Never/former smokers | 6058 | 1243 | 21.2 | 78.8 | |
| Chronic Active Smokers | 418 | 134 | 30.6 | 69.4 | χ2 = 15.98* |
| Never/former smoker | 2468 | 542 | 22.0 | 78.0 | |
| High Dependent Active Smokers | 297 | 97 | 31.3 | 68.7 | χ2 = 13.99* |
| Never/former smoker | 2468 | 542 | 22.0 | 78.0 | |
| High Dependent Smoker-drinkers | 214 | 70 | 37.6 | 62.4 | χ2 = 37.37*** |
| Never/former smoker and nondrinker | 4642 | 936 | 20.5 | 79.5 | |
| Olfactory risks, “Yes,” ever had… | |||||
| Serious head/face injury | 1573 | 465 | 28.2 | 71.8 | χ2 = 53.26*** |
| Ear infections, 3+ times | 1397 | 423 | 27.8 | 72.2 | χ2 = 44.67*** |
| Tonsils removed | 1853 | 476 | 26.0 | 74.0 | χ2 = 28.23*** |
| “Yes,” in last 12 months | |||||
| Cold/flu for >1 month | 478 | 182 | 38.8 | 61.2 | χ2 = 76.22*** |
| Persistent dry mouth | 1115 | 424 | 37.5 | 62.5 | χ2 = 149.82*** |
| Frequent nasal congestion | 2055 | 671 | 32.2 | 67.8 | χ2 = 168.09*** |
*p < .05; ***p < .001.
Across smoking status classifications, more smokers reported an olfactory alteration compared to never/former smokers. The highest frequency of olfactory alteration was reported by high dependent smoker-drinkers (37.6%).
The unadjusted odds ratios and 95% CI for these risk factors were examined prior to multivariable analysis and identified the following significant factors for the final adjusted models (Supplementary Table S2): age, being unmarried, income-to-poverty ratio ≤1, sinonasal problems, xerostomia, head/face injury, tonsillectomy, multiple ear infections, self-rated fair/poor health, and smoking (not light smoking) defined by all five smoking status classifications (Supplementary Table S1).
Smoking Status Classification and Olfactory Alteration—Multivariable Analysis
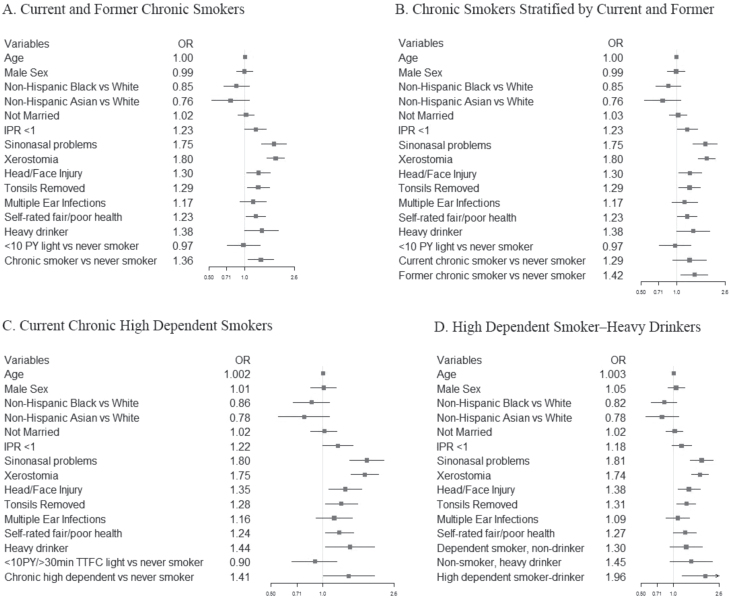
Each five smoking status classifications (Supplementary Table S1) was tested in separate adjusted models (Figure 1; tabular form in Supplementary Table S2). Across fully adjusted models, smoking status classified by chronic (≥10 PY) or chronic high dependent smokers remained significant risk factors. The odds of olfactory alteration were greater among high dependent smoker-drinkers. Sinonasal problems and xerostomia were significant independent risk factors for olfactory alteration in all models. The significance of other risk factors (history of a serious head/face injury, tonsillectomy, poor self-rated health, heavy alcohol use, poverty) varied between the five adjusted models.
Figure 1.
Forest plots of adjusted odds ratios and 95% confidence intervals of risk factors associated with self-reported olfactory alteration in the US adults in models by different smoking status classifications (A–D; results shown in tabular form in Supplementary Table S2).
Chronic Smokers
Chronic smokers (former, current) versus never smokers had significantly greater odds of olfactory alteration (1.36, 95% CI = 1.06 to 1.74; Model A). However, examined separately (Model B), only former chronic smokers remained at significantly greater odds (1.42, 95% CI = 1.09 to 1.84), current chronic smokers did not (1.29, 95% CI = 0.93 to 1.80).
Current Chronic, High Dependent
TTFC < 30 minutes was not a significant risk factor alone (1.30, 95% CI = 0.94 to 1.79), but current chronic, high dependent smokers were at significantly greater odds of an olfactory alteration versus never/former smokers (1.41, 95% CI = 1.01 to 1.99) (Model C). No significant difference was seen between light smokers and never/former smokers in any model.
Chronic Active Smoker
The cotinine biomarker did not add predictive ability. There were nonsignificant greater odds that chronic active smokers [1.22, 95% CI = 0.72 to 2.05] or high dependent active smokers [1.22, 95% CI = 0.77 to 1.93] had olfactory alteration versus never/former smokers.
Chronic Active Smokers and High Dependent Active Smokers
Chronic active smokers and high dependent active smokers compared with age and sex matched never/former smokers also did not have increased odds of olfactory alteration. Furthermore, cotinine alone as a continuous measure of smoking, or by race specific cut-off points, was not significantly related to olfactory alteration.
High Dependent Smoker-Drinkers
High dependent smoker-drinkers had higher odds for olfactory alteration than any other smoker group (1.96, 95% CI = 1.20 to 3.19) (Model D). Heavy drinking alone was not significant or just significant contributor to olfactory alteration (Supplementary Table S2). Being a dependent smoker and nonheavy drinker or nonsmoker and heavy drinker were not significant risk factors. All smoking variables were tested with heavy alcohol use; TTFC with heavy alcohol produced the highest odds ratios with olfactory alteration.
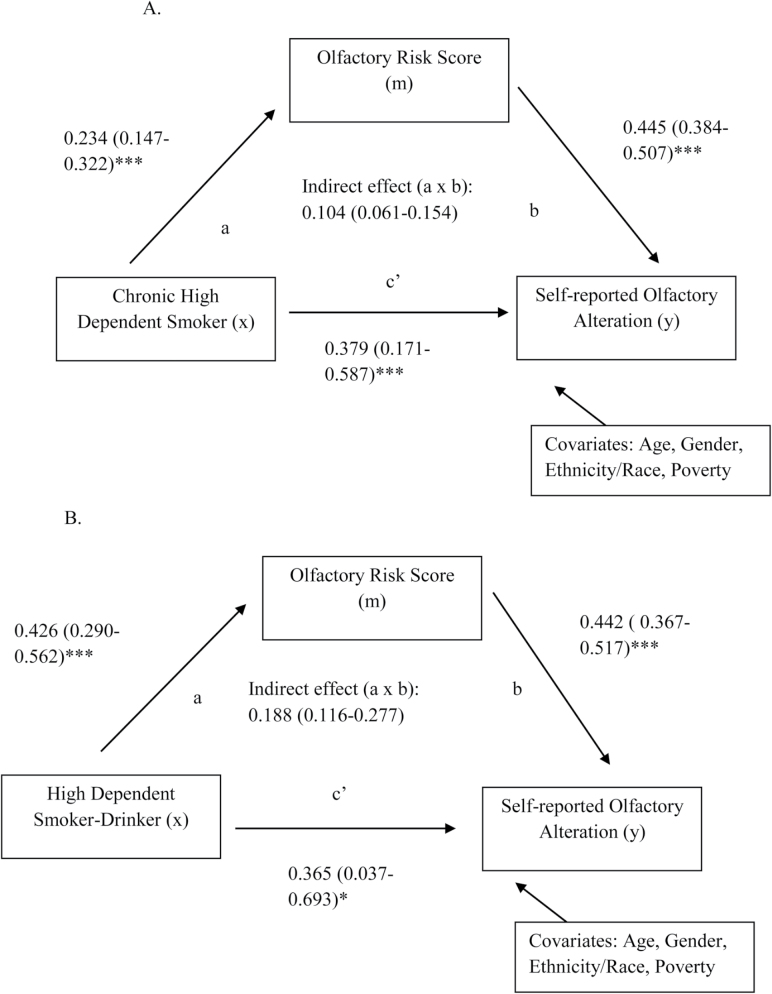
Examination of the Indirect Relationship With Olfactory Alteration Through Mediation Modeling
Figure 2 displays the two mediation models, regression coefficient estimates (beta estimates) with 95% confidence intervals of pathways, and indirect and direct effects, when controlling for age, gender, race/ethnicity, and poverty. The first model displays the indirect relationship between current chronic high dependent smokers with olfactory alteration through olfactory risk score. All beta estimates (path a and b, Figure 2) were positive—there were greater number of olfactory risk factors with moving from never/former smokers to chronic high dependent smokers, which associated with greater odds of olfactory alteration. Both direct and indirect effects were significant (0.3786, 95% CI = 0.1707 to 0.5865; 0.1043, 95% CI = 0.0611 to 0.1538), indicating partial mediation. Of the association between smoking and olfactory alteration, 21.6% was mediated via olfactory risk factor score.
Figure 2.
Models of the association between (A) chronic high dependent smoking or (B) high dependent smoking-drinking and self-reported olfactory alterations mediated by olfactory risk score in data from the National Health and Nutrition Examination Survey 2011–2014. The a paths represent the relationship between x and m, and the b paths represent the relationship m and y. The product of path a and b represents the indirect effect of chronic dependent smoking or dependent smoking-drinking on olfactory alteration; the c’ path represents the direct effect on olfactory alteration. Results displayed for each path include regression coefficient estimates (95% confidence intervals). *p ≤ .05, **p ≤ .01, ***p ≤ .001.
The second model showed similar, but more significant results. Moving from never/former smoker and nondrinker to high dependent smoker-drinker was associated with greater olfactory risk factor score, and then with greater odds of olfactory alteration. The direct (0.3651, 95% CI = 0.0373 to 0.6929) and indirect (0.1884, 95% CI = 0.1155 to 0.2767) effects were both significant. Of the association between smoking-drinking and olfactory alterations, 34.0% was mediated via olfactory risk factor score.
Discussion
Analysis of data from NHANES 2011–2012 and 2013–2014 cycles revealed an estimated prevalence of altered olfaction at 22.3% across adults ages ≥40 years, including self-reported problems in the past year, perceived losses of olfaction since age 25, and/or phantom olfactory sensations. This prevalence is nearly equivalent to the 2011–2012 NHANES estimates.20 A significantly greater frequency of smokers reported olfactory alterations (range 29.3–32.9%) depending on how smoking was characterized. Chronic smokers, who reported high dependency and heavy drinking, had the highest prevalence of olfactory alteration (37.6%). Some of the association between chronic smoking was mediated by an index of exposure to pathologies associated with olfactory dysfunction (frequent nasal congestion, persistent cold/flu, xerostomia, tonsillectomy, serious head/face injury). These findings suggest more negative effects may be added to the list of smoking’s harms. Olfactory alterations can impair ability to detect warning odors (eg, smell of smoking), perceive food flavor and enjoy eating, as well as experience odors related to emotion, memory and behaviors.10
The prevalence of self-reported olfactory alteration was almost double that of 12.4% dysfunction in the 2012 NHANES data,1 measured by brief odor identification task. A single odor identification task cannot detect perception of smell loss with aging or phantosmia, both of which were captured in the self-reported classification used here. Clinicians and public health professionals42 accept self-report as an efficient means of assessing individual and population characteristics, risk factors and diseases. Importantly, self-report assesses perception of illness, which may differ from clinical assessment of disease.42 Individuals may perceive an illness that does not fall within the diagnostic criteria of a disease. Importantly, olfactory perception is complex and the brief odor identification task, measured in NHANES,1 only captures part of this complexity. Self-reported olfactory alteration also associated significantly with identified risks for olfactory dysfunction20—sinonasal problems,13 xerostomia,23 history of serious head/face injury,5 tonsillectomy,43 and poverty.44
Interestingly, in recent analyses of the NHANES 2012 data, cigarette smoking appeared to be a protective factor for measured olfactory function1 with nonsignificant effects on self-reported function.20 However, in the current analyses, cigarette smoking was associated positively with self-reported olfactory alteration, when characterized by chronicity (≥10 PY), dependency (time to first cigarette <30 minutes), and combined with heavy drinking. The chronicity of smoking (≥10 PY) significantly increased odds of olfactory alteration in former and current smokers when examined together, but not in current smokers alone. Only current smokers, who were chronic and dependent, had elevated odds for olfactory alteration.
Four other studies used pack years to characterize the smoking–olfaction association. One found no association when examining pack years in former and current smokers, or heavy use (>20 cigarettes/day) among current smokers.14 The other three studies reported dose-related responses in current smokers (defined by pack year) by decreased olfactory sensitivity45 and poorer odor discrimination or identification.16,46 These previous studies did not control for other risk factors for olfactory dysfunction (eg, sinonasal issues, head trauma, xerostomia) that may also afflict smokers and partially mediate relationships between smoking and olfactory alteration as shown in the present analysis. None of the other studies examined the relationship between olfaction and smoking dependency. Time to first cigarette, a fast and inexpensive screening of nicotine dependence, has associated with quitting success,47 pulmonary impairment,37 and corresponds with smoking biomarkers (eg, nicotine, cotinine, hydroxycotinine concentrations).48 The present study results suggest value in characterizing smoking thoroughly to accurately assess its association with olfactory alteration.
We failed to detect an association with years since quitting smoking and improvement in olfaction in former smokers, as observed in other studies previously.16,29 One potential reason for the lack of association could be the older age of our sample (mean age 57.8 versus 42.9 years in a previous study16). Advanced age associates with a greater risk of olfactory dysfunction.1,2 Increase in years since quitting also corresponds with increasing age, which may attenuate positive effects on olfactory function. The present study controlled for many demographic and pathology-related risks for olfactory dysfunction, which may explain a lack of association between years quitting and olfactory alteration.
Although time to first cigarette has been shown to associate with cotinine levels, we failed to find that serum cotinine strengthened the association with olfactory alteration. There is no universally accepted cotinine level to distinguish smoking status or heaviness of smoking. A systematic review of self-reported smoking status and serum cotinine levels found cut-off points that ranged from 8 to 100 ng/mL48. Race, gene expression and medications competing with binding substrates,49 and sex41 affect cotinine metabolism and inter-individual variation. Although cotinine has a longer half-life than nicotine, it remains in the system for about 16 hours,38 which may miss chronic, dependent smokers most at risk for olfactory alteration. The cotinine measure is expensive, and, according to this analysis, appears less predictive of altered olfaction than self-reported smoking behaviors.
Chronic smoking and heavy drinking showed synergistic effects on altered olfaction. Although smokers more often tend to be heavy drinkers,25 their joint effect had not been examined previously as a risk factor for olfactory alteration. Instead, studies use smoking as a covariate when examining the association with alcohol use.28,50 Presently, the odds of altered olfaction were greatest among chronic, dependent smokers who also reported heavy alcohol drinking. These addictions may increase risk of olfactory alteration through pathologies associated with olfactory dysfunction. An olfactory risk score comprised of five pathologies (frequent nasal congestion, persistent cold/flu, presence of xerostomia, tonsillectomy, or history of a serious head/face injury) explained 22–34% of the association between smoking alone or with heavy drinking. Although the relationship between smoking, olfactory alterations, and these other olfactory pathologies have been examined independently, no study to our knowledge has examined this complex relationship simultaneously. Mediation modeling allowed us to test all variables and their relationships together, displaying their complex associations.
Although this study utilized a nationally representative sample of adults, there are limitations to acknowledge, including the cross-sectional design. Self-report of olfactory function, related risk factors, smoking, and alcohol behavior are potentially biased by factors such as social desirability. From NHANES 2012 data, the index of self-report of olfactory function had relatively reasonable sensitivity in the ability to match the diagnosis of ansomia/severe hyposmia from a single odor identification task. Thus, the self-report index likely misses chronic smokers who have milder olfactory dysfunction (hyposmia), have not been tested for olfactory functioning previously, or had progression of olfactory loss that was gradual and unnoticed. The relationship between self-report and measured olfactory function likely also varies by sociodemographic characteristic or cause of the olfactory problem. In addition, unexamined risks for olfactory alteration probably exist. The analysis strategy may not have detected complex interactions between demographic factors (eg, education, income, marital status) on the associations between chronic smoking and olfactory function. The analyses with cotinine levels were on a subset (NHANES 2011–2012 cycle) and did not consider other types of tobacco products, which could influence olfactory function. Finally, other complex mediation relationships and combined lifestyle factors should be examined to fully understand the relationship of cigarette smoking on olfactory function.
Conclusion
In analysis of NHANES among adults of age 40 years and more, chronic dependent cigarette smoking alone or with heavy alcohol consumption was associated with increased odds of self-reported olfactory alteration, a classification that captured problems during the past year, losses noticed with age, and experiencing phantom smells. Some of this association was direct and some was explained by an increased frequency of pathologies associated with olfactory dysfunction (frequent nasal congestion, persistent cold/flu, presence of xerostomia, tonsillectomy, and history of a serious head/face injury). The associations between smoking and altered olfaction were uncovered by characterizing smoking by chronicity and level of dependence. The simple olfactory alteration questions used in this study could be used by clinicians with their chronic smoking patients to detect smell alterations. Realization that altered sense of smell may recover with smoking cessation, may give smokers one more reason to stop.
Funding
Grant 1 R01 DA036492 from the National Institute on Drug Abuse as well as an Interagency Agreement (Y1-DC-0013) between the National Institute on Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH) and the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention (CDC).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
Author contributions—SGG: conception of analysis design, data analysis and interpretation of data, initial drafting of manuscript; THM: statistical assistance and interpretation of data, drafting and revision of manuscript; SR: interpretation of data, drafting and revision of manuscript; HJH: interpretation of data, drafting and revision of manuscript; MDL: interpretation of data, drafting and revision of manuscript; VBD: conception of study, interpretation of data, drafting and revision of manuscript. The authors thank the study participants and the dedicated staff who conducted the chemosensory (taste and smell) interview and examination components in the National Health and Nutrition Examination Survey (NHANES).
References
- 1. Hoffman HJ, Rawal S, Li CM, Duffy VB. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): first-year results for measured olfactory dysfunction. Rev Endocr Metab Disord. 2016;17(2):221–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288(18):2307–2312. [DOI] [PubMed] [Google Scholar]
- 3. Mullol J, Alobid I, Marino-Sanchez F, et al. . Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ Open. 2012;2(6):pii:e001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirsch AG, Stewart WF, Sundaresan AS, et al. . Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017;72(2):274–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coelho DH, Costanzo RM. Posttraumatic olfactory dysfunction. Auris Nasus Larynx. 2016;43(2):137–143. [DOI] [PubMed] [Google Scholar]
- 6. Gobba F. Occupational exposure to chemicals and sensory organs: a neglected research field. Neurotoxicology. 2003;24(4–5):675–691. [DOI] [PubMed] [Google Scholar]
- 7. Doty RL. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol. 2012;8(6):329–339. [DOI] [PubMed] [Google Scholar]
- 8. Pence TS, Reiter ER, DiNardo LJ, Costanzo RM. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol Head Neck Surg. 2014;140(10):951–955. [DOI] [PubMed] [Google Scholar]
- 9. Aschenbrenner K, Hummel C, Teszmer K, et al. . The influence of olfactory loss on dietary behaviors. Laryngoscope. 2008;118(1):135–144. [DOI] [PubMed] [Google Scholar]
- 10. Boesveldt S, Postma EM, Boak D, et al. . Anosmia-a clinical review. Chem Senses. 2017;42(7):513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. Dept of Health and Human Services. Healthy People 2020- Hearing and Other Sensory or Communication Disorders 2016. https://www.healthypeople.gov/2020/topics-objectives/topic/hearing-and-other-sensory-or-communication-disorders/objectives. Accessed June 29, 2017.
- 12. Ueha R, Ueha S, Kondo K, et al. . Damage to olfactory progenitor cells is involved in cigarette smoke-induced olfactory dysfunction in mice. Am J Pathol. 2016;186(3):579–586. [DOI] [PubMed] [Google Scholar]
- 13. Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. 2011;121(4):873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brämerson A, Johansson L, Ek L, Nordin S, Bende M. Prevalence of olfactory dysfunction: the skövde population-based study. Laryngoscope. 2004;114(4):733–737. [DOI] [PubMed] [Google Scholar]
- 15. Vennemann MM, Hummel T, Berger K. The association between smoking and smell and taste impairment in the general population. J Neurol. 2008;255(8):1121–1126. [DOI] [PubMed] [Google Scholar]
- 16. Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA. 1990;263(9):1233–1236. [PubMed] [Google Scholar]
- 17. Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicola ML, Carvalho HB, Yoshida CT, et al. . Young “healthy” smokers have functional and inflammatory changes in the nasal and the lower airways. Chest. 2014;145(5):998–1005. [DOI] [PubMed] [Google Scholar]
- 19. Rad M, Kakoie S, Niliye Brojeni F, Pourdamghan N. Effect of long-term smoking on whole-mouth salivary flow rate and oral health. J Dent Res Dent Clin Dent Prospects. 2010;4(4):110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011-2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 2016;41(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen S, Tyrrell DA, Russell MA, Jarvis MJ, Smith AP. Smoking, alcohol consumption, and susceptibility to the common cold. Am J Public Health. 1993;83(9):1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benninger MS. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999;13(6):435–438. [DOI] [PubMed] [Google Scholar]
- 23. Dyasanoor S, Saddu SC. Association of xerostomia and assessment of salivary flow using modified schirmer test among smokers and healthy individuals: a preliminutesary study. J Clin Diagn Res. 2014;8(1):211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durazzo TC, Abadjian L, Kincaid A, Bilovsky-Muniz T, Boreta L, Gauger GE. The influence of chronic cigarette smoking on neurocognitive recovery after mild traumatic brain injury. J Neurotrauma. 2013;30(11):1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zizza CA, Sebastian RS, Wilkinson Enns C, ISIK Z, Goldman JD, Moshfegh AJ. The contribution of beverages to intakes of energy and myplate components by current, former, and never smokers in the United States. J Acad Nutr Diet. 2015;115(12):1939–1949. [DOI] [PubMed] [Google Scholar]
- 26. Kesslak JP, Profitt BF, Criswell P. Olfactory function in chronic alcoholics. Percept Mot Skills. 1991;73(2):551–554. [DOI] [PubMed] [Google Scholar]
- 27. Maurage P, Callot C, Chang B, Philippot P, Rombaux P, de Timary P. Olfactory impairment is correlated with confabulation in alcoholism: towards a multimodal testing of orbitofrontal cortex. PLoS One. 2011;6(8):e23190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brion M, de Timary P, Vander Stappen C, et al. . Chemosensory dysfunction in alcohol-related disorders: a joint exploration of olfaction and taste. Chem Senses. 2015;40(9):605–608. [DOI] [PubMed] [Google Scholar]
- 29. Ajmani GS, Suh HH, Wroblewski KE, Pinto JM. Smoking and olfactory dysfunction: a systematic literature review and meta-analysis. Laryngoscope. 2017;127(8):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. CDC. Know Your Reasons for Quitting - Why Do You Want to Quit? Tips from Former Smokers 2017; https://www.cdc.gov/tobacco/campaign/tips/quit-smoking/guide/reasons-to-quit.html. Accessed June 29, 2017.
- 31. CDC. National Health and Nutrition Examination Survey (NHANES). 2011 - 2012 Data Documentation, Codebook, and Frequencies: Taste and Smell Disorders https://wwwn.cdc.gov/nchs/nhanes/2011-2012/CSQ_G.htm. Accessed June 29, 2017.
- 32. Rawal S, Hoffman HJ, Chapo AK, Duffy VB. Sensitivity and Specificity of Self-Reported Olfactory Function in a Home-Based Study of Independent-Living, Healthy Older Women. Chemosens Percept. 2014;7(3–4):108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rawal S, Hoffman HJ, Honda M, Huedo-Medin TB, Duffy VB. The taste and smell protocol in the 2011-2014 US National Health and Nutrition Examination Survey (NHANES): test-retest reliability and validity testing. Chemosens Percept. 2015;8(3):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oksanen T, Kivimäki M, Pentti J, Virtanen M, Klaukka T, Vahtera J. Self-report as an indicator of incident disease. Ann Epidemiol. 2010;20(7):547–554. [DOI] [PubMed] [Google Scholar]
- 35. CDC. National Health and Nutrition Examination Survey (NHANES) 2011 - 2012 Data Documentation, Codebook, and Frequencies: Smoking - Cigarette Use https://wwwn.cdc.gov/nchs/nhanes/2011-2012/SMQ_G.htm. Accessed on June 28, 2017.
- 36. Fagerström K. Time to first cigarette; the best single indicator of tobacco dependence?Monaldi Arch Chest Dis. 2003;59(1):91–94. [PubMed] [Google Scholar]
- 37. Selya AS, Oancea SC, Thapa S. Time to First Cigarette, a Proxy of Nicotine Dependence, Increases the Risk of Pulmonary Impairment, Independently of Current and Lifetime Smoking Behavior. Nicotine Tob Res. 2016;18(6):1431–1439. [DOI] [PubMed] [Google Scholar]
- 38. CDC. Biomonitoring Summary- Cotinine 2013; http://www.cdc.gov/biomonitoring/Cotinine_BiomonitoringSummary.html. Accessed on June 28, 2017.
- 39. Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 40. Jain RB. Serum cotinine and urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanonol levels among nonHispanic Asian American smokers and nonsmokers as compared to other race/ethnicities: data from NHANES 2011-2012. Chemosphere. 2015;120: 584–591. [DOI] [PubMed] [Google Scholar]
- 41. Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248. [DOI] [PubMed] [Google Scholar]
- 42. Lenderink AF, Zoer I, van der Molen HF, Spreeuwers D, Frings-Dresen MH, van Dijk FJ. Review on the validity of self-report to assess work-related diseases. Int Arch Occup Environ Health. 2012;85(3):229–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Snyder DJ, Bartoshuk LM. Oral sensory nerve damage: Causes and consequences. Rev Endocr Metab Disord. 2016;17(2):149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schubert CR, Cruickshanks KJ, Fischer ME, et al. . Olfactory impairment in an adult population: the Beaver Dam Offspring Study. Chem Senses. 2012;37(4):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayes JE, Jinks AL. Evaluation of smoking on olfactory thresholds of phenyl ethyl alcohol and n-butanol. Physiol Behav. 2012;107(2):177–180. [DOI] [PubMed] [Google Scholar]
- 46. Katotomichelakis M, Balatsouras D, Tripsianis G, et al. . The effect of smoking on the olfactory function. Rhinology. 2007;45(4):273–280. [PubMed] [Google Scholar]
- 47. Baker TB, Piper ME, McCarthy DE, et al. . Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007;9(suppl 4):S555–S570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res. 2009;11(1):12–24. [DOI] [PubMed] [Google Scholar]
- 49. Bramer SL, Kallungal BA. Clinical considerations in study designs that use cotinine as a biomarker. Biomarkers. 2003;8(3–4):187–203. [DOI] [PubMed] [Google Scholar]
- 50. Rupp CI, Kurz M, Kemmler G, et al. . Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcohol Clin Exp Res. 2003;27(3):432–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.