Abstract
Cognitive control (CC)—the ability to regulate attention and memory—plays an important role in a variety of health behaviors, including smoking behavior. In this theoretical review of the literature, we propose a CC and smoking behavior framework that includes (1) the positive influence of CC on the self-regulation of smoking, (2) nicotine-induced improvements in CC that may indirectly reinforce smoking (including withdrawal reversal effects), and (3) the long-term effects of smoking on the brain that may result in reduced CC. Integration of these literatures suggests that CC contributes to both self-regulation (ie, brake pedal) and nicotine-related reinforcement (ie, gas pedal) amid the catastrophic effects of long-term smoking, which may reduce self-regulatory control over smoking while also enhancing indirect reinforcement. Supportive evidence and limitations of this approach will be presented, as well as ideas for future research directions that may fully examine this multifaceted modeling of CC in relation to smoking behavior.
Implications
There is substantial evidence that CC contributes to self-regulation (ie, brake pedal) and reinforcement (ie, gas pedal) of smoking behavior as well as evidence that long-term smoking may cause reduced CC. The proposed model delineates how these opposing influences of CC may mask the unique contribution of self-regulation and reinforcement in maintaining smoking behavior. Targeting CC for treating nicotine dependence will require more nuanced approaches that consider the independent and combined effects of self-regulation and reinforcement to improve smoking cessation success rates.
Cognitive Control
There is growing evidence that cognitive control (CC)—the ability to control attention and memory—is associated with cigarette smoking behavior in multiple respects, including self-regulation,1,2 nicotine-related reinforcement,2 and long-term reduction of CC.3 CC is a general construct that encapsulates multiple cognitive processes that are relevant to the performance of daily activities that involve effort, including working memory, target detection, response inhibition, response preparation, and attentional shifting.4 The “CC” and “executive function”5 labels are often used interchangeably, but the latter is generally viewed more broadly and includes the self-regulation of behavior in addition to CC processes.6,7 With respect to the current model, we utilize the label CC to distinguish between CC processes and the self-regulation of behavior in specific contexts (eg, smoking behavior).
The framing of cognitive processes that comprise CC as a general construct remains challenging, as it is difficult to isolate processes that overlap both empirically and conceptually, especially as these processes operate amid whole brain activity. For example, the majority of CC measures require the capacity to sustain attention, regardless of the more specific CC process (eg, response inhibition, working memory) that are highlighted in the literature. The ability to sustain attention over time might therefore influence multiple CC facets. Similarly, measures of working memory are highly dependent on attentional control.8 Thus, if working memory is the construct of interest then attentional control is unavoidably involved as well. Consistent with this notion, different CC measures activate similar brain regions including the anterior cingulate cortex (ACC) and dorsolateral cortex (DLPFC), suggesting these are core neural substrates recruited by common CC resources.9–11 However, in spite of this apparent overlap among CC processes, measures of CC are often modestly correlated, or even uncorrelated.12 It is important to note that even amid the noted frequent absence of substantive correlations among CC measures, the cognitive processes underlying these measures may additively and/or interactively contribute to the self-regulation of smoking and other CC–smoking links. As described below, there are several reasons to focus on CC as a general construct, as opposed to targeting a single process or subset of processes.
Studies examining the association between CC and smoking involve multiple domains, including response inhibition,13,14 working memory,15–17 multiple aspects of attention,2,3,17 and other processes.18,19 For example, meta-analyses found that nicotine acutely enhances a range of CC processes, including attention, episodic memory, and working memory. Moreover, nicotine may have beneficial effects on indices of response time and accuracy.20 These findings indicate that inclusion of a single CC domain or index of CC functioning may not fully explain the effects of nicotine and tobacco use on CC. While response time as a measure of processing speed may cut across measures, more research is necessary to evaluate whether response time during a working memory task represents the same underlying process as response time during attentional set shifting. There is also compelling evidence that multiple domains of CC are implicated in other CC–smoking links, including the influence of long-term smoking on CC functioning,3,21,22 and the association between CC and the self-regulation of smoking (ie, achieving abstinence).13,15,16
Ultimately, individual studies must rely on a limited set of measures due to time constraints and to avoid participant fatigue, which may ultimately lead to focusing on more specific measures of CC. However, the inclusion of well-validated, standardized measures with normative data (eg, NIH Toolbox) will provide additional rigor and result in reduced variability in CC measurement across studies. Given the above issues relevant to CC and smoking behavior, it is paramount that we examine CC more inclusively, as opposed to focusing more exclusively on a limited range of specific domains. In this theoretical review, we build a model of CC and smoking behavior that involves connecting multiple literatures, some of which are more developed than others. For example, research on the relationship between nicotine and CC is captured in large part by previous reviews and meta-analyses. Thus, our goal is to establish plausible links among self-regulation and both state and trait CC and highlight less developed areas that may inform future research and ultimately bridge gaps in our knowledge.
Self-Regulation and CC
The “CC” and “executive function” labels often seem to be used somewhat interchangeably. In this theoretical review, we discuss CC and self-regulation as distinct components of executive function because it is essential to demonstrate whether a given CC measure is empirically associated with self-regulation (eg, capacity to resist smoking). William James proposed that willpower is dependent on basic attentional processes, noting that “The essential achievement of the will, in short, when it is most ‘voluntary,’ is to attend to a difficult object and hold it fast before the mind.”23 CC may reflect the machinery by which self-regulation is made possible, whereas the execution of self-regulation involves motivations emanating from the “self” in addition to CC processes (eg, the personal desire to resist smoking). The evidence suggests attention (or more generally CC) underlies resources that seem to be used during self-regulation.24
Self-regulation’s dependence on CC has been demonstrated in numerous studies and across behaviors.24–26 Neuroimaging studies reveal that CC (eg, working memory and inhibitory control) and self-regulation may have common neural substrates, including the ACC and DLPFC.11,27,28 Well-validated tasks that measure CC and require sustained, effortful attention acutely reduce self-regulation; similarly, tasks that require self-regulation acutely impair CC.29 The importance of CC in self-regulation extends to various behaviors. For instance, performance on CC measures of working memory (measured by the operation span task) and inhibitory control (measured by the stop signal task) are positively associated with the self-regulation of eating behavior.30 The connection between CC and self-regulation has been well-studied and has served as the foundation for several theories, including Baumeister’s self-control depletion theory,31 theories of behavioral inhibition in attention-deficit hyperactivity disorder (ADHD),32 theories of CC development,26 as well as more general theories of self-regulation and CC.33 Ultimately, the influence of CC processes on self-regulation may impact the ability to resist smoking.34,35 For example, Posner and Rothbart found an association between CC development and the early ability to control distress. Furthermore, performance on CC tasks among children correlated with parent-report of self-regulation amid daily life.36 The converging evidence suggests that CC contributes to goal-directed behaviors, such as maintaining an exercise regimen, focusing on a singular task, or maintaining smoking abstinence.24,25 It also makes intuitive sense (ie, face validity) that various aspects of CC contribute to goal planning and execution, and therefore to smoking abstinence. That is, the ability to shift attention away from smoking-related thoughts and cues while remaining focused on alternative activities is fundamental to maintaining abstinence. We now address research that links CC with the capacity to resist smoking, presumably via improved self-regulation as described earlier.
CC and the Capacity to Resist Smoking
The research linking CC with self-regulation24,25 suggests that CC should be positively associated with the capacity to resist smoking. Indeed, there is evidence showing working memory,15,16 response inhibition,13 and other CC processes37 as predictive factors. In general, these studies reveal that smokers with lower baseline trait CC or who experience greater CC deficits during withdrawal are the most vulnerable to relapse. However, the effect size of CC predicting smoking behavior tends to be small, indicating a need for more sensitive CC indices. Neuroimaging techniques such as functional magnetic resonance imaging (fMRI) have provided insight into the underlying neural mechanisms between CC and smoking behavior. fMRI blood-oxygen-level dependent responses in corticothalamic circuits (eg, right inferior frontal gyrus), which are important for inhibitory control processes, are associated with the ability to resist smoking.38 Specifically, the brain regions activated when exerting CC have been associated with the ability to quit smoking. Similarly, deficits in neural substrates resulting from nicotine withdrawal (ie, reduced working memory–related activity in the left DLPFC and increased activity in the posterior cingulate cortex) during abstinence predicted relapse.16 Several other studies also support a connection between CC and reward-related brain regions with smoking relapse.39,40
While there is some support for the role of CC in smoking outcomes, CC must be considered relative to other factors that influence smoking behavior, such as cue-elicited craving and stress-precipitated smoking. For example, reduced CC may increase susceptibility to cue reactivity, and the exposure to cues may drain CC resources.41 Dual process theories, which involve fluctuations between automatic/implicit processes that encourage drug use (eg, smoking cues) and controlled/explicit processes (ie, CC), may provide insight into how to inhibit automatic processes.42 Tobacco use may be more likely to occur if automatic motivational–emotional processes (eg, craving) override CC-based efforts to resist smoking. While this theoretical review focuses primarily on CC and smoking, the complexities between CC, smoking outcomes, and factors independent of CC all require further analysis. Ultimately, an improved understanding of these interactions will place the field in a better position to develop more targeted treatment strategies.
Nicotine and CC
We have thus far suggested that because CC contributes to generalized self-regulatory capacity, CC may play a prominent role in the regulation of smoking behavior. We will now address the effects of nicotine on CC, and its role in the reinforcement of smoking behavior. The proposed model of CC in relation to smoking behavior is complex due to the opposing roles of self-regulation (ie, both nicotine-related and independent of nicotine) and nicotine-related reinforcement. While CC’s role in self-regulation may actually enable inhibition of smoking behavior, nicotine-induced enhancement of CC may reinforce (ie, maintain) smoking behavior.
In addition to nicotine’s primary reinforcing effects via activating the mesolimbic dopaminergic reward system,43 it has CC-enhancing effects2 which may act as an indirect reinforcer. That is, enhanced CC may positively affect emotional regulation44 and the ability to efficiently complete daily tasks,2 which are likely to be reinforcing. Nicotine’s effects on CC support a self-medication hypothesis, whereby smokers with decrements in CC (due to either withdrawal or lower baseline CC) may find smoking reinforcing due to nicotine’s effects on cognitive functioning.2,17 Beyond nicotine’s enhancing effects on CC, evidence suggests the degree to which nicotine deprivation impairs CC varies as a function of one’s baseline CC (ie, trait CC). Smokers with low trait CC, as measured by electroencephalogram and ERP neural substrates, exhibit greater reductions in CC following nicotine deprivation,19 and nonsmokers demonstrate greater enhancement of CC following nicotine administration.45 Trait and state influences on CC may interact synergistically to produce greater deficits, meaning those with lower baseline CC are potentially doubly challenged and at heightened risk for relapse. More research is needed in this area, including the extent to which this synergy may predict difficulty in maintaining smoking abstinence.
Deleterious Long-Term Effects of Smoking on CC
Research suggests that tobacco use causes declines in CC processes throughout the lifespan. First, chronic smoking via nicotine may disrupt the development of brain neurotransmitter processes that contribute to CC function during adolescence and early adulthood.3 An abundance of animal studies have shown that prenatal exposure to nicotine causes a range of CC deficits, including attention, learning, and memory.46,47 These findings may be explained by interruption of the normal development of prefrontal cortical areas that play important roles in the development of attention and other CC processes that continue into early adulthood.48,49 Evidence also suggests that middle aged smokers show greater decline in CC across a 5-year period compared with nonsmokers.50 Smokers also show age-related declines in brain areas associated with Alzheimer’s disease21, and relative to nonsmokers, smokers perform worse on CC tasks.22 The role of nicotine versus constituents in cigarettes independent of nicotine is often difficult to separate, but the preponderance of evidence nevertheless suggests that long-term smoking causes declines in CC processes across the lifespan.
CC and Smoking Status
The role that CC plays in smoking behavior may be especially relevant among contemporary smokers. The prevalence of smoking among adults in the United States has steadily declined across the past several decades. In 1964 (ie, year of Surgeon General’s seminal report on smoking), the rate of current smokers was 43%.51 As of 2015, the smoking rate had declined to 15%.52 Despite this overall substantial decline in smoking prevalence, the rate of smoking among individuals with psychological disorders53 and lower socioeconomic status (SES)54 has remained high. Individuals diagnosed with psychological disorders are over twice as likely to smoke versus individuals without disorders.55 Individuals diagnosed with ADHD, a disorder characterized by deficits in CC and self-regulation, are more likely to smoke and have more difficulty quitting56 However, evidence is mixed regarding whether individuals with ADHD experience greater withdrawal-related changes in CC, with some studies showing ADHD symptoms may exacerbate CC deficits during withdrawal57 while others do not.58 Among individuals with schizophrenia spectrum disorders, which are also characterized by low CC, the smoking prevalence ranges from 64% to 79%, and existing treatments are less effective in this population.59 Given that more effective treatments are necessary for smokers with comorbid conditions, individuals with these CC-related disorders may garner greater benefit from treatment approaches that target CC. Lower SES (ie, lower income and/or lower educational attainment) is also associated with substantially higher smoking rates.60 Smokers with psychological disorders and/or lower SES also exhibit substantially reduced capacity to successfully quit.61,62 Notably, schizophrenia, mood and anxiety disorders,63 and lower SES60,64 are also associated with compromised CC. The links between psychopathology and SES with elevated smoking rates and lower CC highlight important health disparities that exist among the current population of smokers. To better treat individuals within clinical and low SES populations, it is essential to study the specific factors interacting between CC and smoking behavior more comprehensively.
Proposed Model of CC and Smoking Behavior
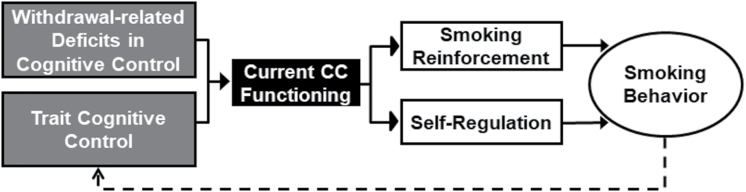
Based on the literature discussed thus far, the proposed model includes three key components. First, the model suggests that CC plays a role in the ability to sustain smoking abstinence due to its effect on self-regulation. That is, all else being equal (eg, motivation to quit, nicotine dependence), the individual with stronger CC would be more likely to successfully maintain smoking abstinence. Current CC functioning may vary according to state factors (eg, disruption during nicotine withdrawal) as well as more chronic, trait-like factors (eg, comorbid psychiatric disorders), which in turn may additively impact self-regulation. For example, evidence that negative affect impairs CC65 may contribute to a further reduction in self-regulation and enhance the reinforcement derived from smoking.2 Indeed, reduced CC has been observed in depression and other negative affect-related disorders,66 which may partially explain high smoking rates among those with psychiatric disorders.67 The second component involves the capacity for nicotine to enhance CC (ie, including nicotine withdrawal reversal). We propose that nicotine’s positive effects on CC provide indirect reinforcement of smoking behavior (ie, self-medication) because CC enhancement may enhance emotion regulation and performance of daily tasks that rely on CC. The model suggests that CC influences smoking in terms of both self-regulation (ie, brakes) and reinforcement (ie, gas pedal). These dual influences may make it difficult to disentangle the role of self-regulation versus the role of reinforcement without carefully designed research that aims to tease these variables apart. The third component involves the potential degrading effects of long-term smoking on CC, which are relevant to understanding CC in the context of the first two components (ie, CC-related self-regulation and indirect nicotine reinforcement). That is, because self-regulation over smoking behavior requires CC, a smoker with fewer CC resources as a consequence of years of chronic smoking may have more difficulty regulating smoking behavior. Additionally, nicotine effects on CC may be reinforcing as difficulty with self-regulation emerges. As shown in Figure 1, the long-term effects of smoking on CC may create a negative feedback loop whereby CC relevant to daily functioning is reduced. As a result, the ability to self-regulate decreases, which in turn reduces the ability to maintain smoking abstinence. Thus, CC-related self-regulation, indirect smoking reinforcement, and consequences of long-term smoking represent three distinct factors that influence the reinforcement and self-regulation of smoking. In tandem with self-regulation, the model suggests that improvements in CC will improve self-regulation and thus the capacity to resist smoking—an assumption we discuss in detail in the next section.
Figure 1.
Model of cognitive control (CC) in relation to smoking behavior. Our proposed model suggests both nicotine withdrawal–related disruption of CC as a state factor and trait CC (gray rectangles) impact current CC functioning, which in turn impacts self-regulation and smoking reinforcement (white rectangles). Depending on whether these factors increase or decrease self-regulation (ie, brake pedal) and/or smoking reinforcement (ie, gas pedal), this will lead to a decision point: smoke or not smoke. This decision influences whether smoking behavior or smoking abstinence is maintained. Finally, long-term smoking may have detrimental effects on CC, thus creating a negative feedback loop (dashed line), which may also impact CC-related indirect reinforcement of smoking behavior.
Additionally, the dual process theory suggests that drug use–related decision making is influenced by competing motivational approach (eg, smoking) and self-regulatory CC systems.68–70 This is consistent with our model which proposes that CC may be an underlying mechanism of self-regulation of smoking behavior. Indeed, dual process theories have been applied to smoking behavior as well.71 An important distinction between our model and the dual process model is the inclusion of CC-related, nicotine-induced indirect reinforcement (ie, gas pedal) as well as the negative feedback loop associated with long-term effects of smoking. The motivational approach activation in our model may be an indirect consequence of the beneficial effects of nicotine on CC. In addition, the model posits the possibility of indirect smoking reinforcement through nicotine’s effects on CC via emotion regulation and task efficiency (including reversal of withdrawal-related deficits). The proposed model and the dual process model also differ in the approach to treatment. The cognitive bias modification approach72 to treatment is focused on the motivational–emotional aspect of traditional dual process modeling. Specifically, the dual process model targets the motivational approach element, whereas interventions described in the next section are intended to enhance self-regulation through improved CC.
CC-Enhancing Therapies as a Strategy to Enhance Smoking Cessation
Interventions that target CC processes may represent novel strategies for smoking cessation73,74 by utilizing behavioral approaches (eg, cognitive training, exercise, mindfulness training), pharmacotherapy (eg, cognitive-enhancing medications), and more recently, noninvasive brain stimulation (NIBS) techniques. It is also important to note that certain subgroups of smokers who may be more vulnerable to CC deficits (eg, people diagnosed with ADHD,75 schizophrenia,76 and other disorders that may involve CC deficits77) may benefit the most from interventions that improve CC.
In the following paragraphs, we provide an overview of studies that have evaluated these strategies as interventions for smoking cessation. Consistent with our broad approach to CC, our examination of interventions intended to improve CC (eg, exercise, mindfulness) encompasses a range of strategies. Overall, these treatment approaches may impact multiple facets of CC. Computerized cognitive training may be an exception in that a single module reflecting a more specific CC process may be targeted (eg, response inhibition; see below).
With respect to behavioral strategies, cognitive training seems like a natural choice in light of evidence showing computerized cognitive training enhances CC in healthy adults.78 Multiple randomized controlled trials (RCTs) show cognitive training’s positive influence on various facets of CC, including information processing speed,79 attention,80 inhibitory control,81 and working memory.82 These effects have been observed across diverse populations such as older adults83 and traumatic brain injury patients.80 Cognitive training has also resulted in beneficial effects on important health outcomes, including depression symptoms,84 schizophrenia symptoms,85 and problem drinking,86 Furthermore, working memory training has been shown to reduce impulsivity (measured via delay discounting),87 suggesting CC enhancements through cognitive training may improve self-regulation and thus enhance the ability to quit smoking. However, it is also important to note that critics have raised valid concerns, including issues related to task validity, inclusion of no-contact control groups, subjective measurements of cognitive changes, lack of consistent compelling evidence of skill transfer, and a paucity of true replications.88 In one of the largest RCTs to date testing this hypothesis, Loughead and colleagues randomized 213 smokers to either computerized cognitive training or a computerized relaxation control group.89 Working memory, attention, and response inhibition training modules were included in the training. Beneficial effects of cognitive training on CC tasks were observed, but these effects did not transfer to higher quit rates among the cognitive training group. While this first set of results does not support cognitive training (ie, null findings) as an efficacious treatment for nicotine dependence, the approach may be more effective for some smokers than others (eg, those with lower baseline CC) and/or combined with additional cognitive-enhancing strategies, such as pharmacotherapy or brain stimulation techniques. Additionally, there were multiple training modules, including working memory, attention, and response inhibition along with other measures not directly related to CC. It is possible that more intensive training in a single domain of CC would yield better training effects (ie, more opportunity for specific training to take hold).
Exercise may also show promise for improving smoking cessation through behavioral CC enhancement. Only one smaller study that we know of has examined exercise and cognitive function in the context of smoking behavior, but this study did not demonstrate that smoking cessation rates were improved by beneficial effects of exercise on CC.90 Additional studies are needed to directly test whether CC mediates the effects of exercise on smoking cessation and whether this varies by type of exercise (eg, aerobic, resistance training, yoga).
The cognitive science literature provides evidence for mindfulness and related meditation practices enhancing neural and behavioral indices of CC functioning91,92 (eg, response inhibition,93 P30094 and error-related negativity95 event-related potential (ERP) neural substrates of CC, and working memory performance96). However, this burgeoning literature is tempered by a lack of precise replications (eg, replication of evidence that mindfulness improves the same outcome measured by a single task). Although more research examining the effects of mindfulness training on smoking cessation is needed, this is nevertheless a potentially promising treatment approach to improving CC processes and consequently improving smoking cessation efforts. No studies have tested whether enhanced CC mediates the effects of mindfulness on smoking cessation. Examining whether individuals with lower baseline CC derive greater benefit from mindfulness practice as a smoking cessation aid may provide insight for developing more targeted treatment strategies.
Despite promising evidence from studies involving potential pharmacotherapy effects on CC and smoking-related behaviors (eg, craving and number of cigarettes smoked), there is little evidence that CC mediates the improvements in smoking cessation. For instance, varenicline, an effective smoking cessation medication, and an α4β2* nicotinic receptor (nAChR) partial agonist and full agonist at α7 nAChRs, has produced positive effects on multiple domains of CC.97,98 However, none of these studies examined whether varenicline’s effects on cognitive performance accounted for its effects on smoking cessation. Acetylcholinesterase inhibitors (AChEIs), which are FDA-approved medications for Alzheimer’s disease, have been proposed as plausible treatment aids. AChEIs increase levels of acetylcholine in the brain by inhibiting the catabolic enzyme responsible for metabolizing acetylcholine.99 AChEIs have been shown to enhance cognition in smokers100 and serve as a substitute for the discriminative stimulus properties of nicotine in humans.101 Specifically, the AChEI galantamine reduces smoking rate, craving, smoking satisfaction, and perceived reward from smoking.101,102 Similar to varenicline, no studies of AChEIs have demonstrated that enhanced CC mediates the effects of pharmacotherapy on smoking cessation success. Additionally, findings for the effects of AChEIs on smoking cessation are mixed.103
Modafinil, which is FDA approved to treat narcolepsy and other sleep conditions,104 is known to enhance dopamine and norepinephrine activity and is another drug that has been found to enhance CC processing.105 A clinical trial that examined modafinil as a smoking cessation treatment was suspended before completion because the drug appeared to be actually increasing smoking behavior in comparison with the placebo condition.106 Similarly, a preclinical experimental study found that modafinil alone or in combination with nicotine replacement did not reduce nicotine withdrawal.107
Transcranial magnetic stimulation (TMS; magnetic fields that evoke electrical current stimulation) and transcranial direct current stimulation (tDCS; soaked surface sponge electrodes on the scalp that deliver electrical current) are well-established forms of NIBS methods that increase/decrease brain activity. Both methods have been shown to increase various facets of CC, including attention, learning, and working memory.108 Recent discoveries present the interesting notion of using NIBS to promote CC as a means to treat substance abuse, including smoking cessation, with the most commonly targeted region being the DLPFC.109 A recent meta-analysis found tDCS targeting the left DLPFC enhanced the working memory.110 Additionally, several reviews of NIBS studies show stimulating the DLPFC may attenuate craving in the context of substance abuse (including nicotine) and highly palatable food,111 making it a promising approach to treat addiction (including smoking cessation) via improved CC.112 Both TMS113 and tDCS114 have been shown to improve cessation rates and enhance the ability to resist smoking in a laboratory paradigm; thus, the NIBS literature supports utilizing these methods as a potentially promising means to promote smoking cessation via improved CC.
We view the above treatment approaches as potential avenues to enhance self-regulation in the context of smoking cessation by enhancing CC. Independent of smoking behavior, interventions to improve CC is a challenging area of research. Only two aforementioned clinical trials have examined therapies intended to improve CC as a means to improve smoking cessation efforts (ie, cognitive training89 and modafinil trials106), and neither of these studies positively impacted smoking cessation. However, we have noted a number of preclinical findings suggestive of CC treatment effects that have translational potential for impacting indices of smoking behavior in humans as well as promising pilot studies indicating a signal for intervention efficacy. While we suggest that this area of research holds promise, the field might be best characterized as being in the earlier stages of development. Future research needs to (1) emphasize basic research that identifies well-validated CC measures as markers of the capacity to quit, and (2) conduct research that shows these CC markers of smoking abstinence can be improved via treatment approaches that improve CC. Ultimately, smoking cessation clinical trials may then be in a better position to examine these CC markers in the context of a therapeutic approach to improve CC.
Finally, it is important to note that individuals who possess fewer CC resources may profit the most from CC-enhancing treatments that are implemented to improve the capacity to remain abstinent.115 Individuals with greater decrements in CC may lack sufficient CC resources to both resist smoking and cope with daily activities and stressors that compete for these limited resources. Thus, research may examine individual differences in CC in an effort to test this hypothesis, which in turn may lead to tailored and/or matching of CC-enhancing treatments based on individual smoker CC characteristics.
Concluding Remarks
We assert that research involving CC via self-regulation on the capacity to resist smoking is still in its formative stage. Empirical work is needed to (1) identify and validate CC measures as predictors of the capacity to resist smoking, (2) evaluate whether treatments that enhance CC improve self-regulation and whether these effects translate into the capacity to remain abstinent, (3) examine whether the effect of CC-enhancing treatments on smoking cessation is mediated by beneficial effects on CC, and (4) identify state and trait factors that may predict for whom and in what manner this approach to smoking cessation might be most appropriate. As shown in Figure 1, we propose a model in which trait CC and nicotine both impact current CC functioning, which in turn impacts self-regulation and indirect reinforcement. Changes in CC and concomitant self-regulation occur amid a variety of states, including nicotine withdrawal, acute stress, and affective changes. The negative impact of long-term smoking on CC is also important to consider, as reduced CC from smoking across time may reduce self-regulatory capacity over smoking, thereby enhancing indirect nicotine-related smoking reinforcement. Because we are connecting diverse literatures to address gaps in the literature, empirical research is needed to test and validate the proposed model. In developing this model, we have attempted to incorporate multiple disciplines that involve varying levels of empirical support ranging from robust (eg, nicotine’s impact on CC processes) to sparse (eg, evidence demonstrating that nicotine’s positive effects on CC actually impact smoking behavior). Investigating interactions between CC, cue reactivity, and stress responses, as well as research to evaluate the efficacy of treatments designed to enhance CC to enhance smoking cessation, will also be important as the field evolves.
Funding
This research was supported by grants from the National Institute on Drug Abuse (R01 DA042682 and K23 DA035295).
Declaration of Interests
None declared.
References
- 1. Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14(1):32–42. [DOI] [PubMed] [Google Scholar]
- 3. Treur JL, Willemsen G, Bartels M, et al. Smoking during adolescence as a risk factor for attention problems. Biol Psychiatry. 2015;78(9):656–663. [DOI] [PubMed] [Google Scholar]
- 4. Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–360. [DOI] [PubMed] [Google Scholar]
- 5. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. [DOI] [PubMed] [Google Scholar]
- 6. Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science. 2011;333(6045):959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welsh MC, Pennington BF. Assessing frontal lobe functioning in children: views from developmental psychology. Dev. Neuropsychol. 1988;4(3):199–230. [Google Scholar]
- 8. Baddeley A. Exploring the central executive. Q J Exp Psychol A. 1996;49(1):5–28. [Google Scholar]
- 9. Barbey AK, Colom R, Grafman J. Dorsolateral prefrontal contributions to human intelligence. Neuropsychologia. 2013;51(7):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. [DOI] [PubMed] [Google Scholar]
- 11. MacDonald AW III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. [DOI] [PubMed] [Google Scholar]
- 12. Evans DE. Individual differences in temperament and attention. Diss Abstr Int. 2004;65(2-B) 1062. [Google Scholar]
- 13. Krishnan-Sarin S, Reynolds B, Duhig AM, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88(1):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luijten M, Kleinjan M, Franken IH. Event-related potentials reflecting smoking cue reactivity and cognitive control as predictors of smoking relapse and resumption. Psychopharmacology (Berl). 2016;233(15–16):2857–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patterson F, Jepson C, Loughead J, et al. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106(1):61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loughead J, Wileyto EP, Ruparel K, et al. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40(6):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: implications for nicotine dependence treatment. Neuropharmacology. 2014;76 (pt B):581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schlienz NJ, Hawk LW Jr, Rosch KS. The effects of acute abstinence from smoking and performance-based rewards on performance monitoring. Psychopharmacology (Berl). 2013;229(4):701–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evans DE, Maxfield ND, Van Rensburg KJ, Oliver JA, Jentink KG, Drobes DJ. Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacology. 2013;38(12):2525–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl). 2010;210(4):453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Durazzo TC, Insel PS, Weiner MW; Alzheimer Disease Neuroimaging Initiative Greater regional brain atrophy rate in healthy elderly subjects with a history of cigarette smoking. Alzheimers Dement. 2012;8(6):513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sabia S, Elbaz A, Dugravot A, et al. Impact of smoking on cognitive decline in early old age: the Whitehall II cohort study. Arch Gen Psychiatry. 2012;69(6):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James W. The Principles of Psychology. Volumes I, II. Reprint. Benton, Chicago. 1950 Dec 1890.
- 24. Kaplan S, Berman MG. Directed attention as a common resource for executive functioning and self-regulation. Perspect Psychol Sci. 2010;5(1):43–57. [DOI] [PubMed] [Google Scholar]
- 25. Hofmann W, Friese M, Schmeichel BJ, Baddeley AD. Working memory and self-regulation. Handbook of Self-Regulation: Research, Theory, and Applications. 2011;2nd ed.:204–225. New York: Guilford Press [Google Scholar]
- 26. Posne MI, Rothbart MK. Developing mechanisms of self-regulation. Dev Psychopathol. 2000;12(3):427–441. [DOI] [PubMed] [Google Scholar]
- 27. Fallgatter AJ, Ehlis AC, Seifert J, et al. Altered response control and anterior cingulate function in attention-deficit/hyperactivity disorder boys. Clin Neurophysiol. 2004;115(4):973–981. [DOI] [PubMed] [Google Scholar]
- 28. Banfield JF, Wyland CL, Macrae CN, Munte TF, Heatherton TF. The cognitive neuroscience of self-regulation. Handbook of Self-Regulation: Research, Theory, and Applications. 2004;62–83. New York: Guilford Press [Google Scholar]
- 29. Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16(3):174–180. [DOI] [PubMed] [Google Scholar]
- 30. Hofmann W, Friese M, Roefs A. Three ways to resist temptation: the independent contributions of executive attention, inhibitory control, and affect regulation to the impulse control of eating behavior. J Exp Soc Psychol. 2009;45(2):431–435. [Google Scholar]
- 31. Baumeister RF, Vohs KD. Self-regulation and the executive function of the self. Handbook of Self and Identity. 2003;1:197–217. New York: Guilford Press [Google Scholar]
- 32. Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. [DOI] [PubMed] [Google Scholar]
- 33. Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. Handbook of Self-Regulation: Research, Theory, and Applications. 2011;2nd ed.:300–320. New York: Guilford Press [Google Scholar]
- 34. Muraven M. Practicing self-control lowers the risk of smoking lapse. Psychol Addict Behav. 2010;24(3):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lesage E, Aronson SE, Sutherland MT, Ross TJ, Salmeron BJ, Stein EA. Neural signatures of cognitive flexibility and reward sensitivity following nicotinic receptor stimulation in dependent smokers: a randomized trial. JAMA Psychiatry. 2017;74(6):632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353(1377):1915–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addict Behav. 2004;29(7):1407–1426. [DOI] [PubMed] [Google Scholar]
- 38. Froeliger B, McConnell PA, Bell S, et al. Association between baseline corticothalamic-mediated inhibitory control and smoking relapse vulnerability. JAMA Psychiatry. 2017;74(4):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sweitzer MM, Geier CF, Addicott MA, et al. Smoking abstinence-induced changes in resting state functional connectivity with ventral striatum predict lapse during a quit attempt. Neuropsychopharmacology. 2016;41(10):2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Janes AC, Pizzagalli DA, Richardt S, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heishman SJ, Boas ZP, Hager MC, Taylor RC, Singleton EG, Moolchan ET. Effect of tobacco craving cues on memory encoding and retrieval in smokers. Addict Behav. 2006;31(7):1116–1121. [DOI] [PubMed] [Google Scholar]
- 42. Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR. Cognitive bias modification and cognitive control training in addiction and related psychopathology mechanisms, clinical perspectives, and ways forward. Clin Psychol Sci. 2013:doi:10.1177/2167702612466547. [Google Scholar]
- 43. Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653(1–2):278–284. [DOI] [PubMed] [Google Scholar]
- 44. Zelazo PD, Cunningham WA. Executive function: mechanisms underlying emotion regulation. Handbook of Emotion Regulation. 2007;135:138 New York: Guilford Press [Google Scholar]
- 45. Sutton SK, Van Rensburg KJ, Jentink KG, Drobes DJ, Evans DE. Nicotine-induced cortical activation among nonsmokers with moderation by trait cognitive control. Psychopharmacology (Berl). 2016;233(12): 2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peters MA, Ngan LL. The effects of totigestational exposure to nicotine on pre- and postnatal development in the rat. Arch Int Pharmacodyn Ther. 1982;257(1):155–167. [PubMed] [Google Scholar]
- 47. Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol Biochem Behav. 1991;40(4):991–993. [DOI] [PubMed] [Google Scholar]
- 48. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nooyens AC, van Gelder BM, Verschuren WM. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am J Public Health. 2008;98(12):2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schroeder SA, Koh HK. Tobacco control 50 years after the 1964 surgeon general’s report. JAMA. 2014;311(2):141–143. [DOI] [PubMed] [Google Scholar]
- 52. Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults—United States, 2005-2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1205–1211. [DOI] [PubMed] [Google Scholar]
- 53. Talati A, Keyes K, Hasin D. Changing relationships between smoking and psychiatric disorders across twentieth century birth cohorts: clinical and research implications. Mol Psychiatry. 2016;21(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Levinson AH. Where the U.S. tobacco epidemic still rages: most remaining smokers have lower socioeconomic status. J Health Care Poor Underserved. 2017;28(1):100–107. [DOI] [PubMed] [Google Scholar]
- 55. Farris SG, Aston ER, Zvolensky MJ, Abrantes AM, Metrik J. Psychopathology and tobacco demand. Drug Alcohol Depend. 2017;177:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62(10):1142–1147. [DOI] [PubMed] [Google Scholar]
- 57. McClernon FJ, Kollins SH, Lutz AM, et al. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology (Berl). 2008;197(1):95–105. [DOI] [PubMed] [Google Scholar]
- 58. Kollins SH, English J, Robinson R, Hallyburton M, Chrisman AK. Reinforcing and subjective effects of methylphenidate in adults with and without attention deficit hyperactivity disorder (ADHD). Psychopharmacology (Berl). 2009;204(1):73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cather C, Pachas GN, Cieslak KM, Evins AE. Achieving smoking cessation in individuals with schizophrenia: special considerations. CNS Drugs. 2017;31(6):471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mani A, Mullainathan S, Shafir E, Zhao J. Poverty impedes cognitive function. Science. 2013;341(6149):976–980. [DOI] [PubMed] [Google Scholar]
- 61. Solberg LI, Asche SE, Boyle R, McCarty MC, Thoele MJ. Smoking and cessation behaviors among young adults of various educational backgrounds. Am J Public Health. 2007;97(8):1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Liu F. Quit attempts and intention to quit cigarette smoking among medicaid recipients in the USA. Public Health. 2010;124(10):553–558. [DOI] [PubMed] [Google Scholar]
- 63. American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 64. Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13(2):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Evans DE, Rothbart MK. A two-factor model of temperament. Pers Individ Dif. 2009;47(6):565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96(4):553–563. [DOI] [PubMed] [Google Scholar]
- 67. Ziedonis D, Hitsman B, Beckham JC, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. 2008;10(12):1691–715. [DOI] [PubMed] [Google Scholar]
- 68. Wiers RW, Stacy AW. Implicit cognition and addiction. Curr Dir Psychol Sci 2006;15(6):292–296. [Google Scholar]
- 69. Wiers RW, Bartholow BD, van den Wildenberg E, et al. Automatic and controlled processes and the development of addictive behaviors in adolescents: a review and a model. Pharmacol Biochem Behav. 2007;86(2):263–283. [DOI] [PubMed] [Google Scholar]
- 70. Fleming KA, Bartholow BD. Alcohol cues, approach bias, and inhibitory control: applying a dual process model of addiction to alcohol sensitivity. Psychol Addict Behav. 2014;28(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mühlig S, Paulick J, Lindenmeyer J, Rinck M, Cina R, Wiers RW. Applying the ‘cognitive bias modification’ concept to smoking cessation—a systematic review. Sucht. 2016;62:333–354. [Google Scholar]
- 72. Elfeddali I, de Vries H, Bolman C, Pronk T, Wiers RW. A randomized controlled trial of web-based attentional bias modification to help smokers quit. Health Psychol. 2016;35(8):870–880. [DOI] [PubMed] [Google Scholar]
- 73. Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ashare RL, Schmidt HD. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin Drug Discov. 2014;9(6):579–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McClernon FJ, Kollins SH. ADHD and smoking: from genes to brain to behavior. Ann N Y Acad Sci. 2008;1141(1):131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2–3):135–157. [DOI] [PubMed] [Google Scholar]
- 77. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284(20):2606–2610. [DOI] [PubMed] [Google Scholar]
- 78. Nouchi R, Taki Y, Takeuchi H, et al. Brain training game boosts executive functions, working memory and processing speed in the young adults: a randomized controlled trial. PLoS One. 2013;8(2):e55518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Edwards JD, Ruva CL, O’Brien JL, Haley CB, Lister JJ. An examination of mediators of the transfer of cognitive speed of processing training to everyday functional performance. Psychol Aging. 2013;28(2): 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. DeGutis JM, Van Vleet T. Tonic and phasic alertness training: a novel behavioral therapy to improve spatial and non-spatial attention in patients with hemispatial neglect. Front Hum Neurosci. 2010;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Spierer L, Chavan CF, Manuel AL. Training-induced behavioral and brain plasticity in inhibitory control. Front Hum Neurosci. 2013;7:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Berry AS, Zanto TP, Clapp WC, et al. The influence of perceptual training on working memory in older adults. PLoS One. 2010;5(7):e11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8(5):e61624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wolinsky FD, Mahncke HW, Weg MW, et al. The ACTIVE cognitive training interventions and the onset of and recovery from suspected clinical depression. J Gerontol B Psychol Sci Soc Sci. 2009;64(5):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Houben K, Nederkoorn C, Wiers RW, Jansen A. Resisting temptation: decreasing alcohol-related affect and drinking behavior by training response inhibition. Drug Alcohol Depend. 2011;116(1–3):132–136. [DOI] [PubMed] [Google Scholar]
- 87. Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69(3):260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shipstead Z, Hicks KL, Engle RW. Cogmed working memory training: does the evidence support the claims?J Appl Res Mem Cogn. 2012;1(3):185–193. [Google Scholar]
- 89. Loughead J, Falcone M, Wileyto EP, et al. Can brain games help smokers quit? Results of a randomized clinical trial. Drug Alcohol Depend. 2016;168:112–118. [DOI] [PubMed] [Google Scholar]
- 90. Janse Van Rensburg K, Taylor AH. The effects of acute exercise on cognitive functioning and cigarette cravings during temporary abstinence from smoking. Hum Psychopharmacol. 2008;23(3):193–199. [DOI] [PubMed] [Google Scholar]
- 91. Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: a systematic review and meta-analysis. Psychiatry Res. 2011;187(3):441–453. [DOI] [PubMed] [Google Scholar]
- 92. Fox KC, Nijeboer S, Dixon ML, et al. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci Biobehav Rev. 2014;43:48–73. [DOI] [PubMed] [Google Scholar]
- 93. Sahdra BK, MacLean KA, Ferrer E, et al. Enhanced response inhibition during intensive meditation training predicts improvements in self-reported adaptive socioemotional functioning. Emotion. 2011;11(2):299–312. [DOI] [PubMed] [Google Scholar]
- 94. Lakey CE, Berry DR, Sellers EW. Manipulating attention via mindfulness induction improves P300-based brain-computer interface performance. J Neural Eng. 2011;8(2):025019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Teper R, Inzlicht M. Meditation, mindfulness and executive control: the importance of emotional acceptance and brain-based performance monitoring. Soc Cogn Affect Neurosci. 2013;8(1):85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P. Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn. 2010;19(2):597–605. [DOI] [PubMed] [Google Scholar]
- 97. Rhodes JD, Hawk LW Jr, Ashare RL, Schlienz NJ, Mahoney MC. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl). 2012;223(2):131–138. [DOI] [PubMed] [Google Scholar]
- 98. Austin AJ, Duka T, Rusted J, Jackson A. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology (Berl). 2014;231(18):3771–3785. [DOI] [PubMed] [Google Scholar]
- 99. Birks JS. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Libr. 2006. doi:10.1002/14651858.CD005593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ashare RL, Ray R, Lerman C, Strasser AA. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug Alcohol Depend. 2012;126(1,2):263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ashare RL, Kimmey BA, Rupprecht LE, Bowers ME, Hayes MR, Schmidt HD. Repeated administration of an acetylcholinesterase inhibitor attenuates nicotine taking in rats and smoking behavior in human smokers. Transl Psychiatry. 2016;6(1):e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sofuoglu M, Herman AI, Li Y, Waters AJ. Galantamine attenuates some of the subjective effects of intravenous nicotine and improves performance on a go no-go task in abstinent cigarette smokers: a preliminary report. Psychopharmacology (Berl). 2012;224(3):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Diehl A, Nakovics H, Mutschler J, Hermann D, Kiefer F. Rivastigmine reduces tobacco craving in alcohol-dependent smokers. Pharmacopsychiatry. 2009;42(3):89–94. [DOI] [PubMed] [Google Scholar]
- 104. Han J, Chen D, Liu D, Zhu Y. Modafinil attenuates inflammation via inhibiting Akt/NF-κB pathway in apoE-deficient mouse model of atherosclerosis. Inflammopharmacology. 2017. doi:10.1007/s10787-017-0387-3 [DOI] [PubMed] [Google Scholar]
- 105. Madras BK, Xie Z, Lin Z, et al. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319(2):561–569. [DOI] [PubMed] [Google Scholar]
- 106. Schnoll RA, Wileyto EP, Pinto A, et al. A placebo-controlled trial of modafinil for nicotine dependence. Drug Alcohol Depend. 2008;98(1,2):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sofuoglu M, Waters AJ, Mooney M. Modafinil and nicotine interactions in abstinent smokers. Hum Psychopharmacol. 2008;23(1):21–30. [DOI] [PubMed] [Google Scholar]
- 108. Coffman BA, Clark VP, Parasuraman R. Battery powered thought: enhancement of attention, learning, and memory in healthy adults using transcranial direct current stimulation. Neuroimage. 2014;85(pt 3):895–908. [DOI] [PubMed] [Google Scholar]
- 109. Gorelick DA, Zangen A, George MS. Transcranial magnetic stimulation in the treatment of substance addiction. Ann N Y Acad Sci. 2014;1327(1):79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mancuso LE, Ilieva IP, Hamilton RH, Farah MJ. Does transcranial direct current stimulation improve healthy working memory? A meta-analytic review. J Cogn Neurosci. 2016;28(8):1063–1089. [DOI] [PubMed] [Google Scholar]
- 111. Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37(10, pt 2):2472–2480. [DOI] [PubMed] [Google Scholar]
- 112. Trojak B, Sauvaget A, Fecteau S, et al. Outcome of non-invasive brain stimulation in substance use disorders: a review of randomized sham-controlled clinical trials. J Neuropsychiatry Clin Neurosci. 2017; doi:10.1176/appi.neuropsych.16080147. [DOI] [PubMed] [Google Scholar]
- 113. Dinur-Klein L, Dannon P, Hadar A, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. 2014;76(9):742–749. [DOI] [PubMed] [Google Scholar]
- 114. Falcone M, Bernardo L, Ashare RL, et al. Transcranial direct current brain stimulation increases ability to resist smoking. Brain Stimul. 2016;9(2):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hall FS, Der-Avakian A, Gould TJ, Markou A, Shoaib M, Young JW. Negative affective states and cognitive impairments in nicotine dependence. Neurosci Biobehav Rev. 2015;58:168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]