Abstract
One-carbon (C1) compounds are attractive microbial feedstocks as they can be efficiently produced from widely available resources. Formate, in particular, represents a promising growth substrate, as it can be generated from electrochemical reduction of CO2 and fed to microorganisms in a soluble form. We previously identified the synthetic reductive glycine pathway as the most efficient route for aerobic growth on formate. We further demonstrated pathway activity in Escherichia coli after expression of both native and foreign genes. Here, we explore whether the reductive glycine pathway could be established in a model microorganism using only native enzymes. We used the yeast Saccharomyces cerevisiae as host and show that overexpression of only endogenous enzymes enables glycine biosynthesis from formate and CO2 in a strain that is otherwise auxotrophic for glycine. We find the pathway to be highly active in this host, where 0.125 mM formate is sufficient to support growth. Notably, the formate-dependent growth rate of the engineered S. cerevisiae strain remained roughly constant over a very wide range of formate concentrations, 1–500 mM, indicating both high affinity for formate use and high tolerance toward elevated concentration of this C1 feedstock. Our results, as well the availability of endogenous NAD-dependent formate dehydrogenase, indicate that yeast might be an especially suitable host for engineering growth on formate.
Keywords: metabolic engineering, synthetic biology, one-carbon metabolism, carbon labeling, tetrahydrofolate, glycine cleavage system
Reduced one-carbon (C1) compounds are abundant in natural habitats (e.g., methanol in phyllosphere, the aerial parts of plants1) and prevalent as byproducts of industrial processes (e.g., carbon monoxide in the flue gas of the steel industry2). As C1 compounds can also be produced abiotically in an efficient and cost-effective manner—for example, formate from electrochemical reduction of CO23,4—they could potentially serve as ideal feedstocks for sustainable microbial growth and bioproduction,5−7 alleviating the problems associated with sugar feedstocks, the use of which erodes food security and biodiversity.8
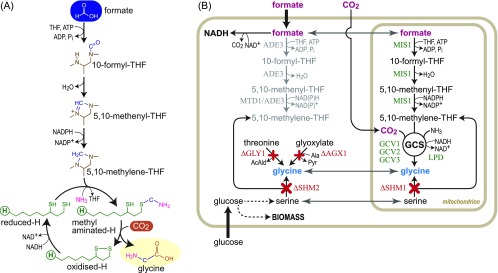
Yet, biological assimilation of C1 compounds is limited to a small number of metabolic pathways and specialized microbial lineages.9−11 Synthetic biology can prove useful by offering tailor-made solutions that can surpass natural alternatives.12 In previous studies, we put forward the reductive glycine pathway as the most efficient route for aerobic growth on formate.7,11,13 In this pathway, formate is first attached to tetrahydrofolate (THF)—the universal C1 carrier—and then reduced to methylene-THF. The glycine cleavage/synthase system (GCS) then condenses the C1-moiety of methylene-THF with CO2 and ammonia to give glycine. Glycine can be further metabolized to biomass and chemical products, e.g., by further condensation with the C1-moiety of methylene-THF to give serine that is deaminated to pyruvate.11
Only a small group of anaerobic purine- and amino-acid-degrading microbes are thought to produce glycine from one carbon units.14,15 In a recently published paper, we demonstrated that the reductive activities of the THF enzymes and GCS can support the net biosynthesis of C2 and C3 compounds from formate and CO2 in E. coli.16 Yet, as E. coli does not harbor an NAD-dependent formate dehydrogenase (FDH)—which is vital for using formate to supply the cell with reducing power and energy—it might not be an ideal host. Furthermore, the activity of the reductive glycine pathway in Escherichia coli was possible only via overexpression of foreign enzymes (from Methylobacterium extorquens). As the enzymatic components of the reductive glycine pathway are prevalent throughout the tree of life, we wondered whether the pathway could be established using only endogenous enzymes of a model host microbe that also naturally harbors NAD-dependent FDH. This would support the premise that C1 assimilation via the reductive glycine pathway could be a “latent” metabolic capability shared by multiple microorganisms, which could be induced by overexpression of naturally occurring components.
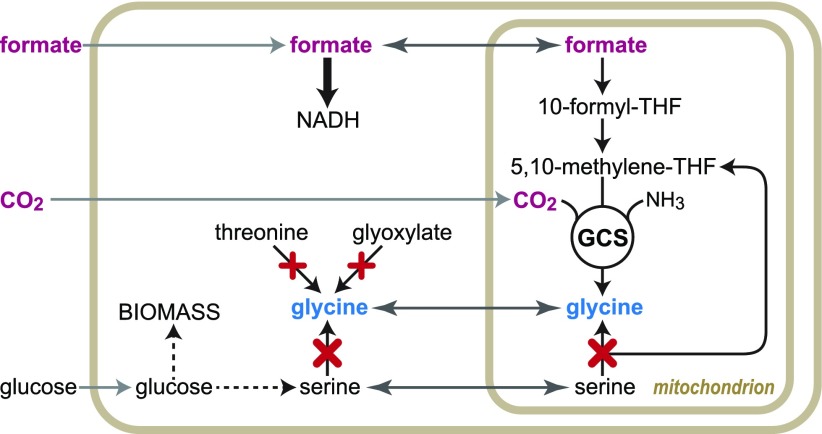
We decided to focus on the model yeast Saccharomyces cerevisiae since it endogenously harbors NAD-dependent FDH as well as all the enzymatic components of the reductive glycine pathway. Furthermore, the GCS of yeast was previously demonstrated to be reversible, such that feeding with 13C-formate resulted in detection of labeled glycine.17,18 However, net production of glycine from formate and CO2 (Figure 1A)—as to indicate the possibility to support growth on C1 compounds—was never demonstrated in any eukaryotic organism. Here, we show the biosynthesis of glycine in a eukaryotic host solelyvia the reductive glycine pathway upon overexpression of native enzymes. We further demonstrate that yeast can sustain a constant growth rate across almost 3 orders of magnitude of formate concentrations, making it an especially promising host to support the assimilation of this key C1 compound.
Figure 1.
Reductive glycine pathway and a selection scheme for its activity in yeast. (A) The “metabolic engine” of the reductive glycine pathway: condensation of C1-moieties into the C2 compound glycine. Substructure of tetrahydrofolate (THF) is shown in brown. Lipoic acid attached to the H-protein of the glycine cleavage/synthase system (GCS) is shown in green. (B) Gene deletions (marked in red) required for the construction of a glycine auxotroph strain, which we used to select for glycine biosynthesis from the activity of the reductive glycine pathway; pathway enzymes are shown in green.
Results
We started with a glycine auxotroph strain—schematically shown in Figure 1B—deleted in the mitochondrial and cytosolic isozymes of serine hydroxymethyltransferase (ΔSHM1 ΔSHM2), as well as in threonine aldolase (ΔGLY1) and alanine:glyoxylate aminotransferase (ΔAGX1).19 This metabolic background was used to select for the biosynthesis of glycine from formate and CO2. We cultivated the strain under high concentrations of formate (100 mM), CO2 (10%), and ammonia (100 mM), in order to kinetically and thermodynamically push the mitochondrial MIS1 enzyme (trifunctional formyl-THF synthetase, methenyl-THF cyclohydrolase, and methylene-THF dehydrogenase20) and the GCS in the reductive direction. Still, we were unable to establish growth without adding glycine to the medium. This indicated that the endogenous activities of MIS1, the GCS, or both are too low to support the required flux.
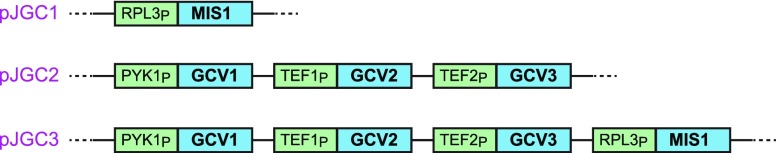
Next, we used the recently developed AssemblX method21 to construct plasmids overexpressing the native MIS1 gene (pJGC1), the genes of the GCS (pJGC2), or both (pJGC3). As shown in Figure 2, each gene was regulated by a (different) strong constitutive yeast promoter to ensure high expression levels. These plasmids were transformed into the glycine auxotroph strain. The transformed strains were then cultivated in the presence of formate and high CO2. Growth of the strains harboring pJGC1 or pJGC2 was not observed without glycine supplement, regardless of the concentrations of formate and CO2. However, the strain harboring pJGC3—expressing both MIS1 and the genes of the GCS—was able to grow with formate substituting for glycine in the medium. This growth was dependent on elevated CO2 concentration (10% CO2) that is needed both thermodynamically, pushing the reversible GCS in the reductive direction, and kinetically, due to the relatively low affinity toward inorganic carbon.22,23
Figure 2.
Three plasmids harboring genes encoding for different subsets of the enzymes of the reductive glycine pathway. pJGC1 harbors only the gene that encodes for MIS1, a trifunctional enzyme that converts formate to methylene-THF. pJGC2 harbors the genes encoding for the subunits of the GCS (the gene encoding for dihydrolipoamide dehydrogenase, LPD1, was not overexpressed since we reasoned its native expression would suffice as it participates in other complexes in the mitochondria, i.e., pyruvate dehydrogenase and 2-ketoglutarate dehydrogenase). pJGC3 harbors the genes encoding for MIS1 and the enzymes of the GCS. Each gene was regulated by a different strong, constitutive promoter as shown in the figure. Each plasmid was based on the pL1A-lc vector backbone as explained in the Methods section.
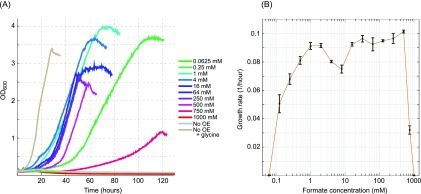
As shown in Figure 3A and B, formate concentrations below 1 mM sufficed to support growth of the glycine auxotroph strain. Maximal growth rate (or close to it) was observed with 1 mM formate and remained nearly constant up to 500 mM formate. At 750 mM formate, growth was severally inhibited, and at 1000 mM formate, no growth was observed. As formate is added as sodium salt, inhibition at concentrations above 500 mM might be attributed to the accumulation of sodium ions. However, while we did observe growth inhibition with NaCl concentrations above 500 mM, the growth inhibition associated with >500 mM sodium formate was considerably more severe. This indicates that at these high concentrations, formate becomes toxic to yeast.
Figure 3.
Formate-dependent growth. (A) Growth of the glycine auxotroph strain harboring the pJGC3 plasmid using different concentrations of formate, 2% glucose and 10% CO2. “No OE” refers to the negative control, i.e., a glycine auxotroph strain without a plasmid, while “No OE + glycine” refers to the positive control, i.e., a glycine auxotroph strain without a plasmid where glycine was added to the medium. Each curve represents the average of three replicates, which were not different by more than 10%. Growth curves were cut after reaching stationary phase. (B) Calculated growth rate as a function of formate concentration. Growth rate increases with increasing formate concentration up to 1 mM, remains rather stable up to 500 mM, and then sharply decreases with higher concentrations. .
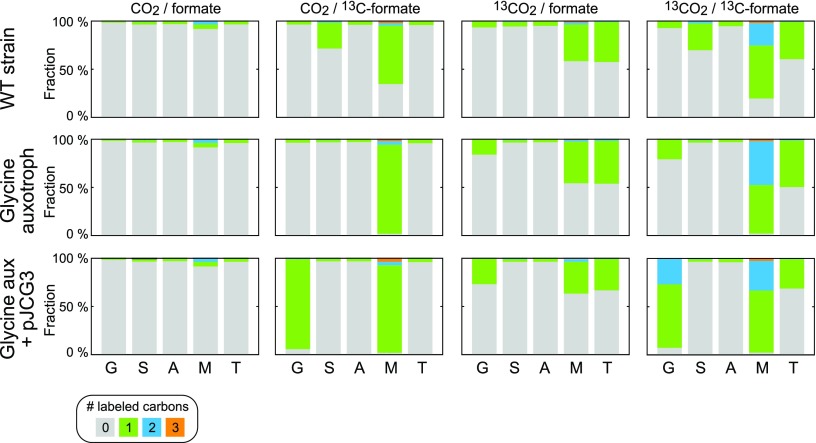
To confirm that glycine is indeed produced solely via the reductive activity of MIS1 and the GCS, we conducted several carbon labeling experiments providing (i) 13C-formate and unlabeled CO2, (ii) 13C-CO2 and unlabeled formate, or (iii) 13C-formate and 13C-CO2. As shown in Figure 4, the results match the expected labeling:
Figure 4.
13C-labeling experiments confirm glycine production from formate. Fraction of labeling of different amino acids in different strains and labeled feedstocks is shown. “G” corresponds to glycine, “S” to serine, “A” to alanine, “M” to methionine, and “T” to threonine. Complete labeling of glycine in the glycine auxotroph strain harboring pJGC3 upon feeding with 13C-formate confirms that glycine biosynthesis occurs only via the reductive glycine pathway. Partial labeling of glycine with 13C-CO2 is attributed to the high production rate of unlabeled CO2 in the mitochondria. See main text for a detailed discussion on the labeling pattern of these amino acids.
Threonine was partially labeled when 13C-CO2 was used, as it is derived from carbon-fixing anaplerosis. The structure of methionine corresponds to that of threonine with the addition of a carbon that originates from methyl-THF. The difference between the labeling of methionine and threonine thus represents the labeling of cytoplasmatic C1 units carried by THF. As shown by the labeling pattern observed upon feeding a WT strain with 13C-formate, this C1 moiety is only partially derived from formate, where the rest originates from serine cleavage. On the other hand, in the glycine auxotroph strain, in which serine hydroxymethyltransferase (SHM1, SHM2) is deleted, all cytoplasmic C1 units originate from formate.
Upon feeding with 13C-formate, alomst all glycine was singly labeled in the glycine auxotroph strain expressing MIS1 and genes of the GCS. This confirms the activity of the reductive glycine pathway where glycine is derived from formate. When feeding with 13C-CO2, glycine was only partially labeled, which can be attributed to the high production rate of unlabeled CO2 by mitochondrial pyruvate oxidation as well as acetyl-CoA oxidation via the TCA cycle. Serine was partially labeled in the WT strain upon feeding with 13C-formate, indicating substantial reductive flux of formate toward methylene-THF and the beta-carbon of serine. This labeling was obviously absent in the glycine auxotroph strain in which serine hydroxymethyltransferase is deleted. As a control, we confirmed that alanine was always unlabeled.
Discussion
The results presented here confirm that the “metabolic engine” of the reductive glycine pathway—net production of the C2 compound glycine from the C1 moieties formate and CO2—can be established within a model microbe using only native enzymes. While this activity was made possible only via overexpression of the necessary endogenous genes (using strong endogenous promoters), once established, it was able to support formate utilization with high affinity, as indicated by the fact that 0.125 mM formate sufficed to support growth. Interestingly, growth rate showed little change with formate concentration varying between 1 and 500 mM. This suggests that, beyond the high efficiency of the reductive glycine pathway, yeast is highly tolerant to formate, a compound that is known to inhibit the growth of other microorganisms at a much lower concentration.24,25 Specifically, many bacteria show severe growth impairment at formate concentrations higher than 100 mM.26 Yeast high tolerance toward formate is in line with previous reports that formate can serve as an auxiliary substrate enhancing growth by providing further reducing power via the endogenous activity of FDH.27,28
Since yeast, as well as many other microorganisms, harbors all the enzymes of the reductive glycine pathway, it is tempting to ask why it cannot support net glycine biosynthesis from formate without the need for gene overexpression. One possible answer is that formate—while being a metabolic intermediate transferred between organelles in eukaryotic organisms29—is not a common compound found in the native habitat of this microorganism. Hence, cells were not adapted to incorporate it efficiently. Another barrier relates to the high concentration of CO2 required to thermodynamically and kinetically support pathway activity—a condition that might not be frequently met in the relevant natural environment.
Luckily, sustaining high CO2 concentration is quite straightforward within a biotechnological context, as is the case in multiple fermentation processes, for example, autotrophic cultivation of acetogens.9 Moreover, in the ultimate yeast strain growing on formate, the oxidation of this compound to CO2 (to provide the cell with reducing power and energy) is expected to surpass CO2 assimilation. Hence, maintaining high CO2 concentration within the bioreactor would be rather straightforward and at most would require the recycling of CO2 from the bioreactor outflow.
In the current study, the dependence of cellular growth on formate is rather low, where only the biosynthesis of glycine and the cellular C1-units requires this C1 feedstock. Confirming this, we did not observe any significant decrease in the concentration of formate in the medium when cultivating our strain for 30 h and up to an OD ∼ 2 (with a starting concentration of 10 mM, see Methods). We speculate, however, that once formate will become a sole carbon source for growth, its consumption rate will become significant.
To conclude, we demonstrate the net production of glycine in a eukaryotic organism. Our findings suggest that S. cerevisiae can become an ideal host for the reductive glycine pathway as it harbors a highly efficient NAD-dependent FDH, requires overexpression of only endogenous enzymes, and supports a rather constant growth rate across ∼3 orders of magnitude of formate concentration. It remains for future studies to engineer the downstream assimilation of glycine to biomass, presumably also via native enzymes, e.g., serine hydroxymethyltransferase and serine deaminase. Beyond yeast, this study suggests that the activity of the reductive glycine pathway might be a “latent” metabolic trait in many microorganisms that endogenously harbor all pathway components, requiring only change in gene expression to support formate assimilation. A recent study that indicates the endogenous activity of the pathway supports this premise.30
Methods
Reagents
PCR reactions were done with PrimeSTAR GXL polymerase (BD Clontech GmbH, Heidelberg, Germany) or Phusion High-Fidelity polymerase (Thermo Fisher Scientific GmbH, Dreieich, Germany), following the manufacturer’s recommendations. All primers were synthesized by Eurofins Genomics GmbH (Ebersberg, Germany). All media and media supplements were ordered from Sigma-Aldrich Chemie GmbH (Munich, Germany). Glucose was ordered from Carl Roth GmbH + Co. KG (Karlsruhe, Germany).
Yeast Strains, Media, and Cultivation
The following Saccharomyces cerevisiae strains were used: YUW1 (MATa ura3-1 trp-1 ade2-1 his3-11-15 leu2-3-112 can1-100 shm1::HIS3 shm2::LEU2 glyΔ0 AGX1::KanMX4),19 and BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0).31 The BY4741 strain was used for in vivo assembly of Level 0 constructs,21 and the glycine auxotroph strain YUW1 was used for in vivo assembly of the final Level 1 multigene plasmids21 and as genetic background for all growth experiments described in the main text.
We used a “semirich” synthetic complete (SC) medium (2% w/v glucose, 0.67% w/v yeast nitrogen base without amino acids, and 0.14% w/v of the appropriate amino acid drop-out mix) to select yeast strains harboring the multigene construct after transformation. Synthetic minimal (SM) medium (2% glucose, 0.67% yeast nitrogen base without amino acids and without ammonium sulfate, 100 mM ammonium sulfate, and 0–1000 mM sodium formate) supplemented with tryptophan and adenine (each 0.0076% w/v) was used to test the YUW1 strain carrying plasmid pJGC1, pJGC2, or pJGC3, for its ability to synthesize glycine from formate and CO2. Additional glycine and/or uracil (0.0076% w/v each) were added to test growth of the YUW1 parental strain, not harboring any plasmid. YPAD medium (2% w/v peptone, 1% w/v yeast extract, 2% w/v glucose, and 0.004% w/v of adenine hemisulfate) was used for yeast recovery during the transformation procedure and for propagation of yeast strains requiring no selection, e.g., plasmid free YUW1 and BY4741.
Yeast liquid cultures were cultivated under shaking at 220 rpm and 30 °C. Different CO2 concentrations (atmospheric or 10%) were used as indicated along with each experiment. Agar plates were prepared using liquid media supplemented with 2% w/v agar and incubated at 30 °C at the indicated CO2 concentration.
Plasmid and Genomic DNA Extraction from Yeast
For PCR amplification of yeast genes, genomic DNA was extracted using the SDS/lithium acetate method.32 In brief, a small amount of a colony was transferred into an SDS/LiAc solution (1% w/v SDS, 200 mM LiAc), incubated for 15 min at 70 °C, and pelleted by centrifugation (21 000g, 2 min). The pellet was subsequently washed with 70% ethanol, dried and resuspended in 10 μL TE buffer. Plasmids from yeast colonies were extracted with the ChargeSwitch Plasmid Yeast Mini Kit (Thermo Fisher Scientific GmbH).
Growth Conditions and Determination of Growth Rate
Growth experiments were performed using a TECAN SPARK 10 M plate reader (Tecan Deutschland GmbH, Crailsheim, Germany) at 30 °C and different CO2 concentrations (atmospheric or 10%, as indicated in the main text). A cycle with 12 individual 60 s shaking steps was programmed with the steps alternating between linear and orbital shaking (2 mm amplitude). To determine the growth rate of yeast cultures, their optical density (OD) at 600 nm was measured immediately after each shaking cycle throughout the complete growth experiment. Growth rate and doubling time were calculated using a custom MATLAB script. Raw data from the plate reader were calibrated to cuvette values according to ODcuvette = ODplate × 3.3. Growth curves were plotted in MATLAB and represent averages of triplicate measurements; in all cases, variability between triplicate measurements was less than 5%.
Yeast Transformation
For plasmid transformation, yeast cells were transformed using the lithium acetate/single-stranded carrier (LiAc/SS) method as described in ref (33). We used 100 ng of each DNA fragment or plasmid to be transformed. For strain YUW1, the cells were heat-shocked at 42 °C for 30 min, recovered in YPDA medium for 4 h at 30 °C, and then plated on appropriate selective SC medium. BY4741 cells were heat-shocked for 40 min at 42 °C and directly plated on appropriate selective SC media without recovery step.
Plasmid Construction
In order to create the different multigene expression plasmids for the enzymes involved in the reductive glycine pathway, we used the AssemblX cloning toolkit, which offers a modular way to create multigene plasmids using a level-based strategy.21
To generate Level 0 constructs (see Supplementary Table S1), all necessary promoters and terminators were PCR-amplified from the AssemblX promoter library, while all CDS that participate in the pathway (GCV1–3, LPD1, and MIS1) were amplified directly from the BY4741 yeast genome. All primers used were designed with the AssemblX webtool or the J5 software34 and contained additional 5′ sequences allowing for homology-directed assemblies. For the list of primers see Supplementary Table S2. For in vivo assembly in yeast BY4741, purified PCR fragments (100 ng per fragment) were mixed with appropriate Level 0 backbone plasmid (linearized with HindIII) according to the assembly protocol—generated by the webtools mentioned above—and transformed into yeast.
Transformants were selected on solid SC medium without uracil and analyzed by colony PCR. Plasmids from potential positive colonies were extracted from yeast with the ChargeSwitch Plasmid Yeast Mini Kit (Thermo Fisher Scientific GmbH), retransformed into E. coli, isolated, and sent for sequencing.
For construction of the final multigene Level 1 plasmids, the Level 0 modules created above were released from their backbones by restriction digestion, according to the AssemblX protocol. During this process, proprietary homology regions, present in the Level 0 backbones, are released along with the previously assembled Level 0 module. These regions overlap between neighboring Level 0 modules and thus allow ordered assembly by in vivo recombination. Following gel purification all Level 0 modules belonging to one intended multigene construct were mixed together with the linearized Level 1 backbone pL1A-lc and transformed directly into yeast YUW1 to allow in vivo assembly.
Selection for successfully assembled plasmids was done on solid SC medium without uracil. Verification of correctly assembled plasmids was done as described above, whereby only the junctions between individual assembly parts were sequenced.
Carbon Labeling
Cells were grown in 3 mL SM media supplemented with adenine, tryptophane and labeled or unlabeled formate (250 mM) in the presence of 10% labeled or unlabeled CO2. After reaching stationary phase, ∼109 cells were harvested by centrifugation for 1 min at 11 500g. The biomass was hydrolyzed by incubation with 1 mL 6 N hydrochloric acid for 24 h at 95 °C. The acid was then evaporated by continued heating at 95 °C and nitrogen streaming. Hydrolyzed amino acids were separated using ultraperformance liquid chromatography (Acquity UPLC, Waters GmbH, Eschborn, Germany) with a C18-reversed-phase column (Waters GmbH). Mass spectra were acquired using an Exactive mass spectrometer (Thermo Fisher Scientific GmbH). Data analysis was performed using Xcalibur software (Thermo Fisher Scientific GmbH). Prior to analysis, amino-acid standards (Sigma-Aldrich Chemie GmbH) were analyzed under the same conditions to determine typical retention times.
Determination of Formate Concentration in Media
The glycine auxotroph strain carrying the pJGC3 plasmid was inoculated, in duplicates, at an OD600 of 0.03 in synthetic minimal medium with 10 mM formate. A sample of the growth medium was taken from each duplicate every ∼2 h during a 34-h fermentation (reaching on OD600 of ∼2). Each sample was centrifuged twice and diluted 1:1000. 500 μL of each diluted sample run in high-performance anion- and cation-exchange chromatography with conductivity detection facilitated by a Dionex ICS-3000 system (Thermo Fisher Scientific GmbH, Dreieich, Germany) with the columns IonPac AS11 Analytical Column 2 × 250 mm (Dionex) and IonPac AG11 Guard Column 2 × 50 mm (Dionex).
Acknowledgments
The authors thank Charlie Cotton and Hai He for critical reading of the manuscript. This work was funded by the Max Planck Society.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssynbio.8b00464.
Table S1: Genetic constructs used in this study; Table S2: DNA primers used in this study (PDF)
The authors declare the following competing financial interest(s): A.B.E. is co-founder of b.fab, aiming to commercialize C1 assimilation. The company was not involved in any way in the conducting, funding, or influencing the research.
Supplementary Material
References
- Vorholt J. A. (2012) Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840. 10.1038/nrmicro2910. [DOI] [PubMed] [Google Scholar]
- Abubackar H. N.; eiga M. C.; Kennes C. (2011) Biological conversion of carbon monoxide: rich syngas or waste gases to bioethanol. Biofuels, Bioprod. Biorefin. 5, 93–114. 10.1002/bbb.256. [DOI] [Google Scholar]
- Kopljar D.; Inan A.; Vindayer P.; Wagner N.; Klemm E. (2014) Electrochemical reduction of CO2 to formate at high current density using gas diffusion electrodes. J. Appl. Electrochem. 44, 1107–1116. 10.1007/s10800-014-0731-x. [DOI] [Google Scholar]
- Yang H.; Kaczur J. J.; Sajjad S. D.; Masel R. I. (2017) Electrochemical conversion of CO2 to formic acid utilizing Sustainion membranes. Journal of CO2 Utilization 20, 208–217. 10.1016/j.jcou.2017.04.011. [DOI] [Google Scholar]
- Bennett R. K.; Steinberg L. M.; Chen W.; Papoutsakis E. T. (2018) Engineering the bioconversion of methane and methanol to fuels and chemicals in native and synthetic methylotrophs. Curr. Opin. Biotechnol. 50, 81–93. 10.1016/j.copbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Bengelsdorf F. R.; Durre P. (2017) Gas fermentation for commodity chemicals and fuels. Microb. Biotechnol. 10, 1167–1170. 10.1111/1751-7915.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yishai O.; Lindner S. N.; Gonzalez de la Cruz J.; Tenenboim H.; Bar-Even A. (2016) The formate bio-economy. Curr. Opin. Chem. Biol. 35, 1–9. 10.1016/j.cbpa.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Naik S. N.; Goud V. V.; Rout P. K.; Dalai A. K. (2010) Production of first and second generation biofuels: a comprehensive review. Renewable Sustainable Energy Rev. 14, 578–597. 10.1016/j.rser.2009.10.003. [DOI] [Google Scholar]
- Drake H. L., Küsel K., and Matthies C. (2013) Acetogenic prokaryotes, In The Prokaryotes, pp 3–60, Springer, Berlin, Heidelberg. [Google Scholar]
- Anthony C. (1982) The Biochemistry of Methylotrophs, Academic Press, London, New York. [Google Scholar]
- Bar-Even A. (2016) Formate Assimilation: The Metabolic Architecture of Natural and Synthetic Pathways. Biochemistry 55, 3851–3863. 10.1021/acs.biochem.6b00495. [DOI] [PubMed] [Google Scholar]
- Erb T. J.; Jones P. R.; Bar-Even A. (2017) Synthetic metabolism: metabolic engineering meets enzyme design. Curr. Opin. Chem. Biol. 37, 56–62. 10.1016/j.cbpa.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Even A.; Noor E.; Flamholz A.; Milo R. (2013) Design and analysis of metabolic pathways supporting formatotrophic growth for electricity-dependent cultivation of microbes. Biochim. Biophys. Acta, Bioenerg. 1827, 1039–1047. 10.1016/j.bbabio.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Fuchs G. (1986) CO2 fixation in acetogenic bacteria: variations on a theme. FEMS Microbiol. Lett. 39, 181–213. 10.1111/j.1574-6968.1986.tb01859.x. [DOI] [Google Scholar]
- Schneeberger A.; Frings J.; Schink B. (1999) Net synthesis of acetate from CO2 by Eubacterium acidaminophilum through the glycine reductase pathway. FEMS Microbiol. Lett. 177, 1. 10.1111/j.1574-6968.1999.tb13705.x. [DOI] [Google Scholar]
- Yishai O.; Bouzon M.; Doring V.; Bar-Even A. (2018) In Vivo Assimilation of One-Carbon via a Synthetic Reductive Glycine Pathway in Escherichia coli. ACS Synth. Biol. 7, 2023. 10.1021/acssynbio.8b00131. [DOI] [PubMed] [Google Scholar]
- Pasternack L. B.; Laude D. A. Jr.; Appling D. R. (1992) 13C NMR detection of folate-mediated serine and glycine synthesis in vivo in Saccharomyces cerevisiae. Biochemistry 31, 8713–8719. 10.1021/bi00152a005. [DOI] [PubMed] [Google Scholar]
- Maaheimo H.; Fiaux J.; Cakar Z. P.; Bailey J. E.; Sauer U.; Szyperski T. (2001) Central carbon metabolism of Saccharomyces cerevisiae explored by biosynthetic fractional (13)C labeling of common amino acids. Eur. J. Biochem. 268, 2464–2479. 10.1046/j.1432-1327.2001.02126.x. [DOI] [PubMed] [Google Scholar]
- Schlosser T.; Gatgens C.; Weber U.; Stahmann K. P. (2004) Alanine: glyoxylate aminotransferase of Saccharomyces cerevisiae-encoding gene AGX1 and metabolic significance. Yeast 21, 63–73. 10.1002/yea.1058. [DOI] [PubMed] [Google Scholar]
- Shannon K. W.; Rabinowitz J. C. (1988) Isolation and characterization of the Saccharomyces cerevisiae MIS1 gene encoding mitochondrial C1-tetrahydrofolate synthase. J. Biol. Chem. 263, 7717–7725. [PubMed] [Google Scholar]
- Hochrein L.; Machens F.; Gremmels J.; Schulz K.; Messerschmidt K.; Mueller-Roeber B. (2017) AssemblX: a user-friendly toolkit for rapid and reliable multi-gene assemblies. Nucleic Acids Res. gkx034. 10.1093/nar/gkx034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. M.; Sagers R. D. (1966) Glycine metabolism. II. Kinetic and optical studies on the glycine decarboxylase system from Peptococcus glycinophilus. J. Biol. Chem. 241, 206–209. [PubMed] [Google Scholar]
- Hiraga K.; Kikuchi G. (1980) The mitochondrial glycine cleavage system. Functional association of glycine decarboxylase and aminomethyl carrier protein. J. Biol. Chem. 255, 11671–11676. [PubMed] [Google Scholar]
- Warnecke T.; Gill R. T. (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb. Cell Fact. 4, 25. 10.1186/1475-2859-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P. (1975) Formate as an inhibitor of cytochrome c oxidase. Biochem. Biophys. Res. Commun. 67, 610–616. 10.1016/0006-291X(75)90856-6. [DOI] [PubMed] [Google Scholar]
- Zaldivar J.; Ingram L. O. (1999) Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol. Bioeng. 66, 203–210. . [DOI] [PubMed] [Google Scholar]
- Overkamp K. M.; Kotter P.; van der Hoek R.; Schoondermark-Stolk S.; Luttik M. A.; van Dijken J. P.; Pronk J. T. (2002) Functional analysis of structural genes for NAD(+)-dependent formate dehydrogenase in Saccharomyces cerevisiae. Yeast 19, 509–520. 10.1002/yea.856. [DOI] [PubMed] [Google Scholar]
- Babel W. (2009) The Auxiliary Substrate Concept: From simple considerations to heuristically valuable knowledge. Eng. Life Sci. 9, 285–290. 10.1002/elsc.200900027. [DOI] [Google Scholar]
- Christensen K. E.; Mackenzie R. E. (2008) Mitochondrial methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase, and formyltetrahydrofolate synthetases. Vitam. Horm. 79, 393–410. 10.1016/S0083-6729(08)00414-7. [DOI] [PubMed] [Google Scholar]
- Figueroa I. A.; Barnum T. P.; Somasekhar P. Y.; Carlstrom C. I.; Engelbrektson A. L.; Coates J. D. (2018) Metagenomics-guided analysis of microbial chemolithoautotrophic phosphite oxidation yields evidence of a seventh natural CO2 fixation pathway. Proc. Natl. Acad. Sci. U. S. A. 115, E92–E101. 10.1073/pnas.1715549114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann C. B.; Davies A.; Cost G. J.; Caputo E.; Li J.; Hieter P.; Boeke J. D. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132. . [DOI] [PubMed] [Google Scholar]
- Looke M.; Kristjuhan K.; Kristjuhan A. (2011) Extraction of genomic DNA from yeasts for PCR-based applications. BioTechniques 50, 325–328. 10.2144/000113672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D.; Schiestl R. H. (2007) Quick and easy yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 35–37. 10.1038/nprot.2007.14. [DOI] [PubMed] [Google Scholar]
- Hillson N. J.; Rosengarten R. D.; Keasling J. D. (2012) j5 DNA assembly design automation software. ACS Synth. Biol. 1, 14–21. 10.1021/sb2000116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.