Abstract
Background
The purpose of this study is to determine whether Ohio House Bill 341, which mandated the use of Ohio’s Prescription Drug Monitoring Program (PDMP), was an effective regulatory strategy to reduce opioid and benzodiazepine dispensing.
Method
Secondary analysis of Ohio’s PDMP data on prescription opioids and benzodiazepines dispensed from November 2014 to March 2017. An interrupted time series analysis was conducted to determine if there was a significant change in the quantity of opioids and benzodiazepines dispensed.
Results
After HB341 became effective in April 2015, there was a statistically significant decrease in the monthly quantity (number of pills) opioids and benzodiazepines dispensed in Ohio. There was a modest increase in the mean days’ supply of opioids and no change in the mean morphine equivalent dose.
Conclusions
Legislation in Ohio requiring prescribers to check the PDMP was effective in reducing the quantity of opioids and benzodiazepines dispensed.
Keywords: Prescription opioids, Policy, Benzodiazepines, Dispensing
1. Introduction
Drug overdose deaths in the United States (U.S.) have increased every year in the past decade, and in 2015 there were approximately 33,066 overdose deaths involving an opioid (Rudd et al., 2016). Increasing rates of drug overdose deaths have been associated with a parallel increase in prescription opioid sales, as well as treatment admissions for opioid use disorders from 1999 to 2008 (CDC, 2011); however, the national quantity of prescription opioids began to decline in 2012 (Guy et al., 2017). In the absence of the number of patients with legitimate pain that are being appropriately treated with prescription opioids, it’s impossible to know the true excess of prescription opioids dispensed and how that has contributed to the misuse and diversion of these drugs. Annually, approximately 11.5 million people misuse prescription opioids (Han et al., 2017). More than 80% of people that initiate heroin use report that they first used prescription opioids (Jones, 2013) and 80% of people who abuse prescription opioids initiated use from legal prescriptions (Shei et al., 2015). Prescription Drug Monitoring Programs (PDMPs) are state-level electronic registries of prescription drugs dispensed, with the majority of the data being reported by community-based pharmacies for scheduled medications (Bao et al., 2016). Forty-nine states have implemented PDMPs (Manasco et al., 2016; Finley et al., 2017). Early PDMPs (the 1990s) were used to monitor and detect illicit distribution of schedule II medications, and while there is some variation across states regarding the purpose of the PDMP (Katz et al., 2008); many today are being used as a tool to monitor over-prescribing at the provider level and doctor-shopping (obtaining a similar prescription from multiple prescribers) at the patient level (Clark et al., 2012).
Research has evaluated the effectiveness of PDMPs as a regulatory tool to reduce the quantity of opioid prescriptions, the quantity of opioids dispensed, the mean days’ supply of opioids, and the mean opioid morphine milligram equivalence (MME) (Bao et al., 2016; Rasubala et al., 2015; Finley et al., 2017; Brown et al., 2017; Brady et al., 2014; Rutkow et al., 2015). There is mixed evidence on the effectiveness of PDMPs (Finley et al., 2017; Griggs et al., 2015); which in part may reflect different levels of PDMP implementation and/or utilization, variation in the opioid drugs and/or drug schedules included in the analysis, variation in the outcomes measured (i.e., prescriptions written versus dispensed), variations in the prescribing setting (i.e., emergency department, dentist, etc.) and different state-level PDMP characteristics (Pew Charitable Trusts, 2016). For example, a 2001–2010 multistate comparison of prescriptions written for ambulatory pain patients found that PDMPs were associated with a 30% reduction in schedule II opioids; however, there was no impact on the overall number of prescriptions written for opioids (Bao et al., 2016). Florida’s PDMP was associated with a reduction in the volume of opioids dispensed based on a claims dataset; however, this reduction was only statistically significant for high volume patients/prescribers and during this period Florida also passed pill mill legislation (Rutkow et al., 2015).
PDMPs, once implemented, take time to become fully operational. Their utility as a tool to detect doctor shopping relies on the timeliness of dispensing data, and they are unlikely to have a population-level impact until the majority of prescribers are using the PDMP. For example, Ohio passed PDMP legislation in 2006; in the early years, only approximately 25% of prescribers were using it (Burke, 2016). States also need staff and funding to identify over-prescribing, as well as to ensure that the appropriate steps are followed to investigate such cases. Prescriber utilization has increased with the integration of PDMPs within electronic medical records, and many states now require daily reporting of data. It is therefore not surprising that early PDMP studies reported mixed findings (Rutkow et al., 2015; Islam and McRae, 2014; Ringwalt et al., 2015; Chang et al., 2016); given the varying levels of PDMP implementation and utilization by prescribers.
Given the significant between-state variations in PDMPs (Manasco et al., 2016), there is an increasing amount of research investigating whether specific PDMP characteristics are associated with positive outcomes. Seventeen states mandate PDMP enrollment and only eight states require prescribers to review a patient’s PDMP report before prescribing controlled substances (Manasco et al., 2016). Mandatory use of the PDMP is associated with decreases in the quantity of opioids dispensed, the number of opioids prescriptions and multiple provider episodes (MPEs) (Rasubala et al., 2015; Freeman et al., 2015; PDMP Center of Excellence, 2014). There is however mixed evidence regarding whether PDMPs reduce MME or days’ supply, both of which are associated with increased risk of non-medical use and/or overdose (Guy et al., 2017; Paulozzi, 2012). Only one study has used PDMP data to report on benzodiazepine dispensing patterns; however, this study did not investigate the effect of PDMP implementation or regulations on benzodiazepine dispensing patterns (Paulozzi et al., 2015).
Ohio has one of the highest rates of overdose fatalities in the country and some of the highest rates of prescription drug trafficking (Rudd et al., 2016; Winstanley et al., 2012). The Ohio Board of Pharmacy reported that 8.2 million doses of prescription opioids were dispensed in just Scioto County alone in 2011, which was approximately 103.6 doses for every county resident including children (Ohio Department of Health, 2012). Legislation creating Ohio’s PDMP, Ohio’s Automated Rx Reporting System (OARRS), was passed in May 2005 and it became law in January 2006. OARRS is managed by the Ohio Board of Pharmacy, and it incorporates dispensing information on Schedule II–IV drugs and one non-controlled drug, gabapentin. There are approximately 2433 pharmacies, and 48,741 prescribers in Ohio registered to use OARRS. Ohio House Bill 341 (HB341) was first introduced on November 7, 2013, and it was passed on June 3, 2014; with an effective date of April 1, 2015. Rules, recommendations or guidelines previously existed to encourage prescribers to register to use OARRS or to check OARRS prior to prescribing; HB341 was the first legislative mandate that could be enforced. Ohio HB341 requires prescribers to check OARRS prior to initiating a prescription for opioids or benzodiazepines and subsequently re-checking OARRS every 90 days for patients who are continued to be prescribed these medications. HB341 incorporated exemptions to checking OARRS when prescribing or personally furnishing opioids and benzodiazepines when these drugs were for less than a seven-day supply, for hospice patients, for patients with a terminal illness or with cancer, and for patients prescribed opioids post-surgical procedures. Opioids and benzodiazepines administered in a hospital, nursing home or residential care facility were also exempt. Given that regulations may not have an optimal impact unless enforced, the Ohio Board of Pharmacy identified prescribers that were not in compliance with HB341. In August 2016, the Board mailed letters to prescribers that failed to check OARRS before prescribing an opioid or benzodiazepine, informing them they could be fined up to $20,000.
The goal of this project was to evaluate whether the effective date of House Bill 341 was associated with a reduction in the overall quantity of opioids and benzodiazepines dispensed in Ohio. The secondary goals were to evaluate whether HB341 was associated with a reduction in the days’ supply of opioids or benzodiazepines, the mean MME per opioid prescription, and the number of multiple-provider episodes (MPE). Additionally, we investigated whether the HB341 enforcement letters further reduced the quantity of opioids and benzodiazepines dispensed. This study is unique from previous research as it includes all scheduled opioids indicated for pain and it accounts for opioid schedule changes in modeling the impact of PDMP regulations. Further, this is the first study to assess the impact of a PDMP on benzodiazepine prescribing practices.
2. Method
2.1. Data
A reduced dataset was provided by the Ohio Board of Pharmacy, including all records of reported dispensed medications from 2007 through the first quarter of 2017 (March 31, 2017). The dataset included information on the date filled, prescription number, prescription refill number, quantity dispensed, days’ supply, national drug code, drug name, number of authorized refills, payment type, pharmacy business activity code, three-digit pharmacy zip code, patient age, patient sex, patient county, three-digit patient zip code, three-digit prescriber zip code, and prescriber specialty. The dataset included a de-identified unique code for patients, prescribers, and pharmacies.
2.2. Study population and sample
We restricted the data based on whom the bill targeted and was anticipated to benefit. Given that HB341 is only applicable to prescribers licensed in Ohio, we excluded records with an out-of-state prescriber, and only included patients who were Ohio residents. For this study, the dataset was restricted to medications dispensed between November 1, 2014, and March 31, 2017 (n = 52, 603,348). November 1, 2014, to March 31, 2015, was defined as the pre-intervention period, and April 1, 2015, to March 31, 2017, was the post-intervention period. The pre-intervention period was restricted to records after November 1, 2014, because of prior DEA opioid re-scheduling, which is known to have influenced opioid dispensing patterns. In August 2014, tramadol was reclassified from an unscheduled to a schedule IV drug, and in October 2014, hydrocodone was reclassified from a schedule III to schedule II drugs. Between 2007 to March 2016, hydrocodone represented 41.4% and tramadol represented 17.0% of all opioids dispensed in Ohio. Therefore, including opioid dispensing data prior to November 2014, would violate the assumptions of interrupted time series analysis. We developed code to classify opioids as either for the treatment of pain or addiction. The buprenorphine transdermal patch (Butrans), solution for injection, and film (Belbuca) were not categorized as medication-assisted treatment (MAT) because these formulations are indicated in the treatment of pain. Methadone solution was classified as MAT per Ohio regulations. Finally, methadone tablets were not categorized as MAT as they are indicated in the treatment of pain.
Ohio’s PDMP data is entered by pharmacists as part of routine practice and hence data entry errors may occur. We developed a strategy to address data entry errors and had the strategy reviewed by the Ohio Board of Pharmacy to ensure accuracy. The first step was to remove values that were non-permissible and the second step was to remove values that were determined to be data entry errors. A statistical cut point of the top 0.01% was used to identify values that were highly unlikely to be observed; a similar approach has been used else-where (Weiner et al., 2018). For example, all ages greater than 116 years old (0.01%) were coded as missing. This process was used for all applicable variables in the dataset.
2.3. Measures
The primary outcome variable was the quantity of opioids and benzodiazepines dispensed per month and this included only solid doses. Solid doses included tablets, capsules, film, suppositories, and lozenges. All other forms were categorized as non-solid. Quantity was restricted to solid doses because in these cases “days’ supply” represents a quantifiable unit and frequently non-solid formulations cannot be transformed into a similar unit. For opioids, quantity only included drugs with an indication for pain. The secondary outcomes were days’ supply of opioids and benzodiazepines, mean MME per opioid prescription, and the number of multiple provider episodes (MPE). The MME per prescription was calculated per prescription for only solid dose opioids and was determined from the following calculation: (Quantity/Day Supply) × Strength × Conversion Factor. The conversion factors utilized were from the Center Opioid Morphine Equivalent Conversion Factor chart. The Prescription Behavior Surveillance System measure of MPE was used; which defines MPE as a patient that fills prescriptions from five or more prescribers at five or more pharmacies within a six-month period (Paulozzi et al., 2015). We calculated the MPE for benzodiazepines alone, opioids alone, and for benzodiazepines or opioids for six months’ intervals in 2015–2016. Given that this definition of MPE is based on six-month intervals, rather than monthly, we were not able to assess the specific impact of the passage of HB341 in April 2015. Rather, we report the MPE trend during this period.
2.4. Analysis
Stata SE 14.2 (StataCorp, 2013) was the statistical software package used to conduct the analysis. Descriptive statistics were used to summarize the quantity, days’ supply and mean MME. An interrupted time series analysis (ITSA) was used to determine whether there were statistically significant changes in the primary and secondary outcomes between November 2014 to March 2017. A Cumby-Huizinga test (actest) suggested that there was not autocorrelation, hence a Newey-West model was used. For the primary outcomes quantity of opioids and benzodiazepines dispensed, we considered two models and the first included only the effective date of HB341 (April 2015) (Model 1). The second model included the HB341 effective date and the enforcement letter date (August 2016) (Model 2). This research was reviewed by the West Virginia University Institutional Review Board (IRB) and determined not to be human subjects research.
3. Results
Between November 2014 and March 2017, there were 50.6 million prescriptions for 5.5 million unique individuals from 54,272 unique prescribers. The mean age of patients was 46.5 years old and 55.4% were female. Approximately 80% of the records were for new prescriptions and the mean days’ supply was 23.0 (SD = 16.0). The majority (66%) of the prescriptions were paid for with commercial insurance, 24.8% were paid for by Medicaid or Medicare and 7.2% were paid for with cash. Over half (51.6%) of the prescriptions were for opioids, 21.9% were for benzodiazepines and 13.3% for stimulants. Opioids included: Hydrocodone (31.5%), Oxycodone (29.6%), Tramadol (16.8%), buprenorphine (7.1%), Codeine (6.6%), Morphine (3.1%) and other (8.4%).
3.1. Quantity of opioids and benzodiazepines
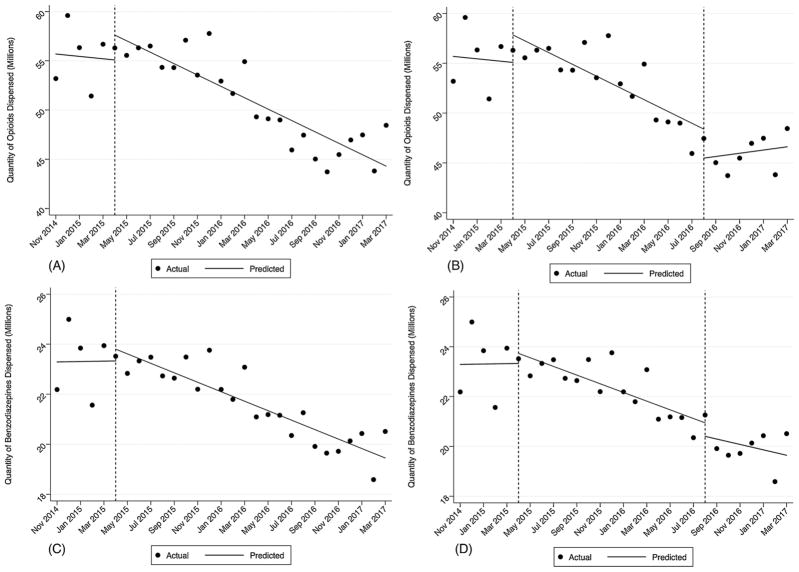
Between November 2014 and March 2017, there were 4.7 million fewer opioids dispensed and 1.6 million fewer benzodiazepines dispensed. The absolute quantity of opioids dispensed decreased by 8.9% and benzodiazepines decreased by 7.5%. In model 1, there was a statistically significant reduction (coef. = −579,000) in the quantity of opioids dispensed after HB341 went into effect (p < 0.000) (see Fig. 1, Panel A). In model 2, there was not an additional reduction in the quantity of opioids dispensed attributable to the enforcement letter. However, in the period following the enforcement letter there was a statistically significant increase in quantity of opioids dispensed (coef. = 753,061; p = 0.04) (see Fig. 1, Panel B). Similarly, there was a statistically significant decrease (coef. = 189,000; p < 0.000) in benzodiazepine dispensing after the passage of HB341 (see Fig. 1, Panel C) and no change after the enforcement letters were sent (see Fig. 1, Panel D).
Fig. 1.
Panel A: Monthly Quantity of Opioids Dispensed and HB341 Effective Date. Panel B: Monthly Quantity of Opioids Dispensed, HB341 Effective Date and Enforcement Letter Date. Panel C: Monthly Quantity of Benzodiazepines Dispensed and HB341 Effective Date. Panel D: Monthly Quantity of Benzodiazepines Dispensed, HB341 Effective Date and Enforcement Letter Date.
3.2. Days’ supply and MME
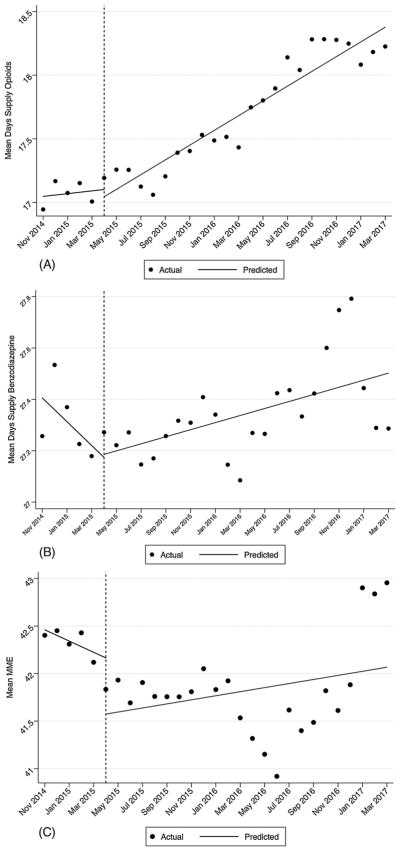
The overall mean days’ supply of solid opioids for pain was 17.6 (SD = 13.5) and 27.5 (SD = 16.2) for benzodiazepines. There was a slight, but statistically significant, increase (coef. = 0.058; p < 0.000) in the mean days’ supply of opioids after the passage of HB341 (see Fig. 2, Panel A) and a similar pattern was observed for benzodiazepines (coef. = 0.014; p < 0.01) (see Fig. 2, Panel A). There was no change in the mean MME (p = 0.200) (see Fig. 2, Panel C).
Fig. 2.
Panel A: Mean Day’s Supply of Opioids and HB341 Effective Date. Panel B: Mean Day’s Supply of Benzodiazepines and HB341 Effective Date. Panel C: Mean MME per opioid prescription and HB341 Effective Date.
3.3. MPE
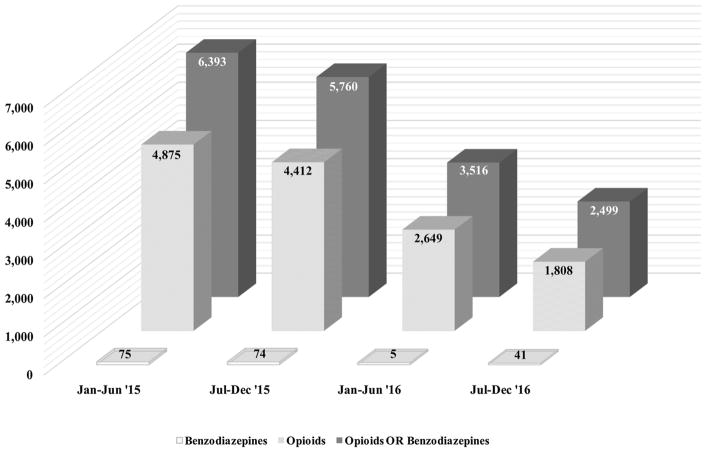
There was a statistically significant decline in MPE episodes between 2015 to 2016 for benzodiazepines (45%), opioids (63%) and opioids or benzodiazepines (61%) (see Fig. 3). The absolute decrease in MPE episodes was 3,067 for opioids and 8,892 for opioids or benzodiazepines.
Fig. 3.
Multiple Provider Episodes from 2015 to 2016 for Opioids and Benzodiazepines.
4. Discussion
After HB341 became effective, there were 4.49 million fewer opioids and 1.68 million fewer benzodiazepines dispensed per month. The mean quantity of opioid dispensed decreased by 8.1%. This finding is consistent with the national study of Dowell et al. (2016) which estimated that mandated PDMP review, combined with pain clinic legislation, was associated with an 8% decrease in the quantity of opioids dispensed (Dowell et al., 2016). Ohio passed pill mill legislation (House Bill 93) in 2011, four years prior to mandating physicians to check a patient’s PDMP report before prescribing an opioid or benzodiazepine. Bao et al. (2016) reported that PDMPs were associated with a statistically significant decline in schedule II opioid prescriptions, but not for all opioid prescriptions (Bao et al., 2016). However, the previous study focused on opioid prescriptions in the ambulatory setting rather than all opioids dispensed. Both of these studies assessed periods prior to 2013 and may not have accounted for the level of PDMP utilization or implementation. As PDMPs have matured and likely improved since their initial implementation, researchers will be able to better determine whether the initial mixed findings reflected sub-optimal prescriber utilization or specific PDMP characteristics.
HB341 was also effective in reducing the quantity of benzodiazepines dispensed in Ohio. Substance use disorders and overdose deaths rarely involve just opioids. Drug users frequently engage in poly-substance abuse, and the combination of opioids with benzodiazepines significantly increases the risk of an overdose fatality (Sun et al., 2017). Our study is the first study to report on how a PDMP may influence benzodiazepine prescribing, and it is interesting to note that there were parallel reductions in benzodiazepines and opioids dispensed. Additional research is needed to determine whether mandatory PDMP legislation should specify checking for individual drug classes or whether it should include all drugs tracked in the state PDMP. It seems possible that as checking a patient’s PDMP report becomes part of routine prescribing practice for controlled medications, that reductions in quantity or MPEs would decrease for all medications reported to a PDMP.
The greatest reduction in MPE was for opioids alone and while there were very few MPEs for benzodiazepines, there was still a statistically significant decline in episodes during the time period. Given the definition of MPE that we used, it is unknown whether the reduction in MPEs is attributable specifically to HB341. There was a slight statistically increase in the days’ supply for both opioids and benzodiazepines after the passage of HB341. During the study period, the mean days’ supply of opioids ranged from 16.9 (November 2014) to 18.3 (October 2016), and benzodiazepines ranged from 27.1 (July 2015) to 27.8 (December 2016). While this increase may not be clinically significant, it may warrant longer-term monitoring. Previous research has not found an association between PDMPs and reductions in MME (Brady et al., 2014); nevertheless, it is important to monitor increasing MME as it could circumvent reduction in the number of opioids dispensed. It is also important to keep in mind that this MME calculation was per prescription and therefore does not reflect an individual’s daily MME, which would account for overlapping prescriptions. While OARRS will report daily MME in the screen view for prescribers, this information is not stored and hence difficult to reconstruct. There are several other PDMP characteristics that likely influence their effectiveness, and full realization of these benefits may require prescribers to check routinely the PDMP before prescribing. However, professional associations such as the American College of Emergency Physicians have released policy statements that PDMPs should be voluntary (American College of Emergency Physicians, 2017). Further, it is unknown to what extent a PDMP’s annual budget and funding may determine its effectiveness. PDMP’s annual costs are estimated to range between $125,000 to 1 million (Finklea et al., 2014). In 2016, Ohio spent $4,232,963 on their PDMP to support the operation of the system; as well as efforts to integrate OARRS directly into electronic health records and pharmacy dispensing systems. These enhancements provide instant access to healthcare providers, and they were implemented to encourage checking the system regularly. It is unknown whether this level of financial investment is needed to observe population-level improvement.
4.1. Study limitations
Over the past decade, Ohio has implemented several strategies to address the opioid epidemic including opioid prescribing guidelines for emergency departments and recommendations to limit opioid prescriptions greater than 80 MME. By limiting the pre-intervention period to after November 2014, we attempted to control for opioid schedule changes and these other earlier state policies that may have influenced opioid prescribing practices. In fact, reports from the Ohio Board of Pharmacy suggest that there have been consistent declines in opioid dispensed since OARRS was implemented in 2006. HB341 required checking a patients’ PDMP report before writing a new prescription for an opioid or benzodiazepine, and it is unknown whether a greater effect would have been found for patients newly prescribed such medications. It would seem reasonable that prescribers would be less concerned about checking the PDMP for patients with whom they already have a regular relationship. Finally, our analyses included only one state and its unknown the extent to which the results would generalize to other states.
5. Conclusion
In conclusion, regulations mandating checking of the PDMP were effective in reducing the quantity of opioids and benzodiazepines dispensed in Ohio. Enforcement of such regulations may be important; however, it does not appear to reduce further the quantity dispensed. The United States President’s Commission on Combating Drug Addiction and the Opioid Crisis’ final draft report was released in November 2017, and it recommends that federal agencies mandate PDMP checking; however, it did not specify when PDMPs should be checked or by whom. This research contributes to the empirical evidence that mandatory PDMP checking for opioids and benzodiazepines is an effective regulatory strategy and additional research is needed to determine whether these reductions, in turn, are associated with less harm including non-medical use of prescription opioids and addiction.
Acknowledgments
Role of funding sources
This publication was supported by grants from the Centers for Disease Control and Prevention (6 NU17CE002738) and from the National Institute of General Medical Sciences of the National Institutes of Health (2U54GM104942-02).
Footnotes
Conflict of interest
No conflict declared.
Contributors
ELW designed the study and obtained the initial funding to support this work. ELW and YZ managed the preparation of the data and performed the statistical analyses. RM, JB, SS and JP developed, reviewed and contributed to the code to accurately categorize medications based on schedule, indication and formulation. NJM, JB and JP contributed to the interpretation of the results. CM reviewed the data cleaning strategy, as well as verified details regarding the utilization of OARRS and the implementation of HB341. All authors contributed to the development of the manuscript and reviewed the revisions.
References
- American College of Emergency Physicians. Electronic prescription drug monitoring program. Ann Emerg Med. 2017;70:116–117. doi: 10.1016/j.annemergmed.2017.03.053. [DOI] [PubMed] [Google Scholar]
- Brown R, Riley MR, Ulrich L, Kraly EP, Jenkins P, Krupa NL, Gadomski A. Impact of New York prescription drug monitoring program, I-STOP, on state-wide overdose morbidity. Drug Alcohol Depend. 2017;178:348–354. doi: 10.1016/j.drugalcdep.2017.05.023. [DOI] [PubMed] [Google Scholar]
- Bao Y, Pan Y, Taylor A, Radhakrishnan S, Luo F, Pincus HA, Schackman BR. Prescription drug monitoring programs are associated with sustained reductions in opioid prescribing by physicians. Health Aff. 2016;35:1045–1051. doi: 10.1377/hlthaff.2015.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 2014;129:139–147. doi: 10.1177/003335491412900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. The value of prescription monitoring programs. [Accessed 22 February 2018];Pharmacy Times. 2016 Available from http://www.pharmacytimes.com/publications/issue/2016/september2016/the-value-of-prescription-monitoring-programs.
- Centers for Disease Control and Prevention (CDC) Vital signs: overdoses of prescription opioid pain relievers – United States, 1999 to 2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- Chang HY, Lyapustina T, Rutkow L, Daubresse M, Richey M, Faul M, Stuart EA, Alexander GC. Impact of prescription drug monitoring programs and pill mill laws on high-risk opioid prescribers: a comparative interrupted time series analysis. Drug Alcohol Depend. 2016;165:1–8. doi: 10.1016/j.drugalcdep.2016.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark T, Eadie J, Kreiner P, Strickler G. [Accessed 22 February 2018];Prescription Drug Monitoring Programs: An Assessment of the Evidence for Best Practices. 2012 Available at http://www.pewtrusts.org/%7E/media/assets/0001/pdmp_update_1312013.pdf.
- Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory provider review and pain clinic laws reduce the amount of opioids prescribed and overdose death rates. Health Aff. 2016;35:1876–1883. doi: 10.1377/hlthaff.2016.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finklea K, Sacco LN, Bagalman E. Prescription drug monitoring programs. J Drug Addict Educ Erad. 2014;10:481–505. [Google Scholar]
- Finley EP, Garcia A, Rosen K, McGeary D, Pugh MJ, Potter JS. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC Health Serv Res. 2017;17:420. doi: 10.1186/s12913-017-2354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman PR, Troske S, Goodin AJ, Blumenschein K, Talbert J. Impact of Kentucky House bill 1 on concurrent prescribing of opioid, alprazolam, and carisoprodol. Value Health. 2015;18:A128. [Google Scholar]
- Griggs CA, Weiner SG, Feldman JA. Prescription drug monitoring programs: examining limitations and future approaches. West J Emerg Med. 2015;16:67–70. doi: 10.5811/westjem.2014.10.24197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP, Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66:697–704. doi: 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167:293–301. doi: 10.7326/M17-0865. [DOI] [PubMed] [Google Scholar]
- Islam MM, McRae IS. An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: pros, cons and tensions. BMC Pharmacol Toxicol. 2014;15:46. doi: 10.1186/2050-6511-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers – United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. 2013;132:95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Katz N, Houle B, Fernandez KC, Kreiner P, Thomas CP, Kim M, Carrow GM, Audet A, Brushwood D. Update on prescription monitoring in clinical practice: a survey study of prescription monitoring program administrators. Pain Med. 2008;9:587–594. doi: 10.1111/j.1526-4637.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- Manasco AT, Griggs C, Leeds R, Langlois BK, Breaud AH, Mitchell PM, Weiner SG. Characteristics of state prescription drug monitoring programs: a state-by-state survey. Pharmacoepidemiol Drug Saf. 2016;25:847–851. doi: 10.1002/pds.4003. [DOI] [PubMed] [Google Scholar]
- Ohio.gov. [Accessed 9 November 2017];Ohio’s Opiate Epidemic. 2012 Available from: http://mha.ohio.gov/Portals/0/assets/Learning/Fact%20Sheets/Opiate%20Fact%20Sheet.pdf.
- Prescription Drug Monitoring Program Center of Excellence at Brandeis. [Accessed 9 November 2017];Briefing on PDMP Effectiveness. 2014 Available from: http://www.pdmpassist.org/pdf/Resources/Briefing%20on%20PDMP%20Effectiveness%203rd%20revision.pdf.
- Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled substance prescribing patterns – prescription behavior surveillance system, eight states, 2013. MMWR Surveill Summ. 2015;64:1–14. doi: 10.15585/mmwr.ss6409a1. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ. Prescription drug overdoses: a review. J Saf Res. 2012;43:283–289. doi: 10.1016/j.jsr.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Pew Charitable Trusts. [Accessed 22 February 2018];Prescription Drug Monitoring Programs: Evidence-Based Practices to Optimize Prescriber Use. 2016 Available from http://www.pewtrusts.org/?/media/assets/2016/12/prescription_drug_monitoring_programs.pdf.
- Rasubala L, Pernapati L, Velasquez X, Burk J, Ren YF. Impact of a mandatory prescription drug monitoring program on prescription of opioid analgesics by dentists. PLoS One. 2015;10:e0135957. doi: 10.1371/journal.pone.0135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwalt C, Garrettson M, Alexandridis A. The effects of North Carolina’s prescription drug monitoring program on the prescribing behaviors of the state’s providers. J Prim Prev. 2015;36:131–137. doi: 10.1007/s10935-014-0381-0. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States: 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. doi: 10.15585/mmwr.mm655051e1. [DOI] [PubMed] [Google Scholar]
- Rutkow L, Chang HY, Daubresse M, Webster DW, Stuart EA, Alexander GC. Effect of Florida’s prescription drug monitoring program and pill mill laws on opioid prescribing and use. JAMA Intern Med. 2015;175:1642–1649. doi: 10.1001/jamainternmed.2015.3931. [DOI] [PubMed] [Google Scholar]
- Shei A, Rice JB, Kirson NY, Bodnar K, Birnbaum HG, Holly P. Sources of prescription opioids among diagnosed opioid abusers. Curr Med Res Opin. 2015;4:779–784. doi: 10.1185/03007995.2015.1016607. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 13. StataCorp LP; College Station, TX: 2013. [Google Scholar]
- Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760. doi: 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner SG, Baker O, Rodgers AF, Garner C, Nelson LS, Kreiner PW, Schuur JD. Opioid prescriptions by specialty in Ohio, 2010–2014. Pain Med. 2018;19:978–989. doi: 10.1093/pm/pnx027. [DOI] [PubMed] [Google Scholar]
- Winstanley EL, Gay J, Roberts L, Moseley J, Hall O, Beeghly BC, Winnhusen T, Somoza E. Prescription drug abuse as a public health problem in Ohio: a case report. Public Health Nurs. 2012;29:553–562. doi: 10.1111/j.1525-1446.2012.01043.x. [DOI] [PubMed] [Google Scholar]