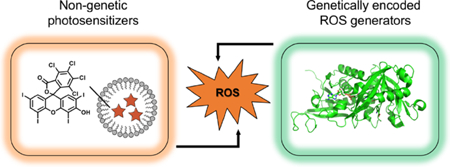
Abstract
Reactive oxygen species (ROS) not only are by-products of aerobic respiration, but also play vital roles in metabolism regulation and signal transductions. It is important to understand the functions of ROS in biological systems. In addition, scientists have made use of ROS to kill bacteria and tumors through a process known as photodynamic therapy (PDT). This paper provides a concise review of current molecular tools that can generate ROS in biological systems via either non-genetic or genetically-encoded way. Challenges and perspectives are further discussed with the hope of broadening the applications of ROS generators in research and clinical settings.
Graphical Abstract
1. Introduction
Reactive oxygen species (ROS), including radicals and non-radical molecules, such as superoxide (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (HO•), and singlet oxygen (1O2), have gained interest for decades as harmful by-products of cellular metabolism.1–4 The production of ROS occurs constantly in chloroplasts, mitochondria, peroxisomes, and the cytosol during photosynthesis and the aerobic respiration process. More importantly, recent work has uncovered ROS as vital cellular signaling molecules in maintaining homeostasis of many physiological processes, such as the cell cycle, apoptosis, autophagy, and immunity.5,6 When the balance between generation and consumption of ROS is dysregulated, diseases may occur.7 Studies have shown that many cancer cells are well adapted to oxidative stress due to their flexible redox regulation systems.8 As such, modulating the redox status of cancer cells has long been recognized as an effective strategy for cancer treatment.8 Photodynamic therapy (PDT), during which a large amount of ROS are generated by exposure of photosensitizers to excitation light, has indeed become a widely used therapeutic approach for superficial cancer.9–11 Moreover, PDT has shown promise as a new approach to combat drug-resistant micro-pathogens, including Gram-positive and Gram-negative bacteria, yeasts and fungi.12 Photosensitizers are typically synthetic molecules or nanoparticles. In addition to these conventional ROS generators, the adventure of using fluorescent proteins and enzymes as genetic encoded ROS generators has created another important research domain. Moreover, synthetic photosensitizers have been integrated with genetically encoded elements to form hybrid ROS generators. This paper, which is not meant to be a thorough review, will convey these basic concepts through a few selected examples.
2. Photodynamic therapy (PDT)
2.1. Brief history of PDT
The history of PDT can be traced back to ~1900.13 Oscar Raab, a German chemist, firstly proposed such conception after observing that micro-organisms could be killed by light when co-cultured with certain dyes.13,14 This phenomenon inspired him and his followers to investigate further and subsequently identify oxygen as an indispensable factor for antibiotic performance. The practice of PDT on skin cancer research was conducted not long after the initial attempt on antibiotic studies.9 However, due to World War II, further development of PDT was largely delayed between 1940s and 1960s. In 1970s, the stagnation was ended by Dr. Thomas Dougherty who introduced “hematoporphyrin derivatives” (HpDs), mixtures of water-soluble porphyrins, into PTD.15 Although HpDs had several practical problems, such as inefficient absorption in the far-red and near-infrared spectral region and low in vivo clearance rate, this seminal work has attracted intensive attention and paved the way for development of modern photosensitizers.13,16 Since then, hundreds of photosensitizers have been chemically synthesized, thereby providing clinicians with a plethora of options for photodynamic agents. In addition to the wide use of PTD in cancer therapy, PDT has recently refocused onto its original purpose as antimicrobial methods because multidrug-resistant ‘super bacteria’ have become one of the greatest, global health challenges.17
2.2. Mechanism of PDT
The detailed photophysics of PDT is beyond the scope of this topical review and interested readers may refer to other publications.18,19 Briefly, the ground-state photosensitizer is excited to a high-energy state after absorbing excitation photons (Figure 1). The excited molecule could then dissipate its energy by either nonradiative pathways (a.k.a. internal conversion or IC), or radiative pathways such as fluorescence emission, or intersystem crossing (ISC). Among all dissipation pathways, ISC is especially important for ROS generation as it converts excited, singlet-state molecules into a triplet state, which is a metastable electronic state and would dissipate its energy via phosphorescence or photochemical reactions. The lifetime of the triplet state is usually within microseconds, which is much longer than the singlet state (nanoseconds), and thus could set stage for efficient, energy-transferring collisions. Two hypothetical types of photochemical processes could take place to form ROS (Figure 1). In the type I process, the excited photosensitizers may directly react with substrates, such as proteins, lipids and nucleic acids, to acquire or lose a single electron to form radical anions or cations. These radicals may further react with molecular oxygen (O2) to generate ROS. Moreover, photosensitizers in the triplet state may directly transfer one electron to nearby oxygen through collisions, resulting in O2•−. O2•− is not very reactive in biological systems and can be disproportionated by superoxide dismutase (SOD) to generate H2O2 and O2. Through a cascade electron acquisition/donation process catalyzed by redox-active transition metal ions such as iron or copper, O2•− and H2O2 could be further converted into highly reactive hydroxyl radical (•OH) through a so-called “Fenton reaction”. The Type II process is mechanistically simpler than the Type I process as energy is directly transferred from the excited triplet molecule to a ground-state triplet oxygen (3O2), resulting in highly reactive singlet oxygen (1O2*). To date, the type II process is considered to be more important for most photosensitizers during PDT.20 It is worthwhile to note that the type I and type II classification refers to the initial electron/proton or direct energy transfer process.21 These highly reactive radicals and singlet oxygen are actually interconvertible. Moreover, for a given photosensitization process, type I and type II reactions may occur simultaneously.19,21
Figure 1. Jablonski diagram and mechanisms for photosensitizer-induced ROS generation.

Photosensitizers in the ground state are excited to a high-energy singlet state, which could subsequently be converted into a triplet state via ISC. This triplet state may further undergo photochemical reactions via either a type I or a type II process to generate ROS. (Abs, absorption; IC, internal conversion; ISC, intersystem crossing; SET, single electron transfer; ET, energy transfer; SOD, superoxide dismutase)
3. Non-genetic ROS generators
3.1. Small-molecule-based photosensitizers
A large number of small-molecule-based photosensitizers have been developed and they are mostly from three categories: tetrapyrrole derivatives, heavy-atom-containing fluorescent dyes, and transition metal complexes. Below we briefly discuss the advantages and disadvantages of photosensitizers in each category. To facilitate the discussion, we provide the information on the absorbance of a few representative, small-molecular-based photosensitizers in Table 1.
Table1.
Representative examples of small-molecule-based photosensitizers.
| Class | Example | Absorbance peak | Reference |
|---|---|---|---|
| porphyrin | Soret band (~400nm) Q band (~630nm) |
22 | |
| Tetrapyrrole derivatives |
chlorin | Soret band (~400nm) Q band (~650nm) |
23 |
| bacteriochlorin | Soret band (~400nm) Q band (~730nm) |
24 | |
| Heavy-atom-containing fluorescent dyes |
DIMPy-BODIPY | 530 nm | 27 |
| Rose-Bengal | 540 nm | 29 | |
| Transition metal complexes |
Ruthenium(Ru) complex |
450 nm | 33 |
| Rhodium(Rh) complex |
450 nm | 35 | |
| Iridium(Ir) complex |
600 nm | 36 | |
3.1.1. Tetrapyrrole derivatives
Tetrapyrrole (Figure 2), which is naturally occurring in heme, chlorophyll, and bacteriochlorophyll, possesses the richest structural diversities among all types of synthetic photosensitizers. Tetrapyrrole derivatives can be further classified into three subgroups (porphyrin, chlorin, and bacteriochlorin), each featuring different numbers of C-C double bonds. As the number of conjugated double-bonds decreases from porphyrin to bacteriochlorin, the Q band absorption is red-shifted (Table 1).22–24 A prominent advantage of tetrapyrrole derivatives is their strong absorption within the near-infrared (NIR) optical window in biological tissue (650–900 nm), enabling an efficient excitation of photosensitizers at a relatively deep depth. However, tetrapyrrole derivatives often have poor solubility, limiting their applications in biological systems. This solubility problem has recently been partially addressed by introducing the sulfonate functional group into tetrapyrrole.20. Photofrin, a mixture of tetrapyrrole derivatives, has been approved by the US FDA to treat cancer since 1995.25 Moreover, several second-generation agents (e.g., benzoporphyrin derivative monoacid) have been recently approved by the US FDA26.
Figure 2.
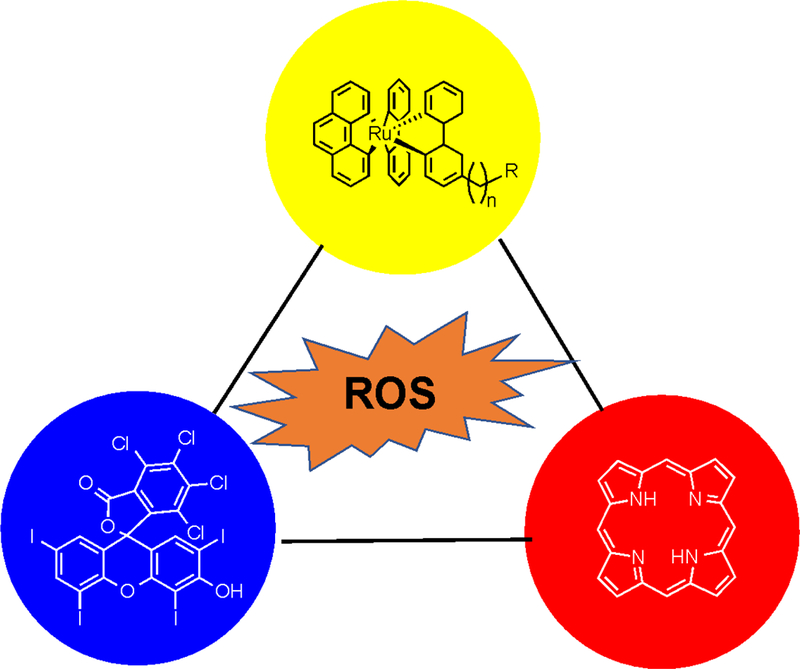
Representative, small-molecule-based photosensitizers from three major categories. Tetrapyrrole (porphyrin) is shown in the red circle. A heavy atom (iodine) containing synthetic dye (Rose Bengal) is shown in the blue circle. A transition metal (ruthenium) complex is shown in the yellow circle.
3.1.2. Heavy-atom-containing fluorescent dyes
Many photosensitizers were developed by structurally modifying classic fluorescent dyes, such as BODIPY,27 fluorescein,28,29 and phenothiazinium salts30 with a number of heavy atoms such as iodine and bromine. Heavy atoms can facilitate ISC and increase the yield of triplet states, thereby increasing ROS production. Some heavy-atom-incorporated fluorescent dyes, such as Rose Bengal (Figure 2), have been explored to treat various cancers or skin conditions.29 A formulated Rose Bengal, known as PV-10, demonstrated good locoregional tumor control of cutaneous melanoma patients.31 PV-10 is currently undergoing clinical trials for melanoma, breast cancer, and liver cancer. Compared to tetrapyrrole derivatives, the blue-shifted absorbance of fluorescein and BODIPY derivatives (Table 1) inevitably decreases their efficiency for ROS generation because of strong tissue scattering and absorption of excitation photons.
3.1.3. Transition metal complexes
Organo-transition metal compounds have demonstrated their versatile roles in catalysis, synthetic chemistry, and development of organic light emitting diodes (OLED).32 Organo-transition metal compounds, such as ruthenium(II) polypyridyl complexes33, have also been examined for light-induced ROS generation. In a recent example, a collection of 17 ruthenium(II) polypyridyl complexes with varying substituent(s), molecular symmetry, electrical charge, and counterions were characterized for absorption coefficient, 1O2 generation quantum yield, and their antibacterial activity in photodynamic assays using Gram-positive and Gram-negative bacteria.34 Interestingly, in addition to 1O2 production efficiency, other parameters, especially those impacting on interaction with bacteria, were identified be key factors for killing of bacteria. In addition, there are a handful of reports on rhodium (Rh)35 and iridium (Ir)36 complexes serving as PDT reagents. The impressively high 1O2 production yields and stability of transitional metal complexes make them a promising category of photosensitizers. However, their poor water-solubility and potentially high cytotoxicity hinder their wide-scale adoption and usage in biomedicine.
3.2. Nanotechnology-enhanced photosensitizers
Nanotechnology has received significant attention in recent years because of its outstanding performance in targeted delivery and controlled release of cargos, which may overcome disadvantages of many synthetic photosensitizers.37,38 The integration of nanotechnology with synthetic photosensitizers has resulted in significant advancement. For example, the low-solubility issue of synthetic photosensitizers has been significantly alleviated by encapsulating photosensitizers within liposomes and micelles.39 Additionally, these modifications are also helpful to enhance tumor accumulation after intravenous administration. In another example, the photosensitizer phthalocyanine was assembled on gold nanoparticles covalently bound with anti-HER2 antibodies to achieve targeted delivery.40 Indeed, the cytotoxicity of phthalocyanine drastically decreased while the drug concentration in breast cancer cells that overexpress the HER2 epidermal growth factor cell surface receptor elevated significantly.
4. Genetically encoded ROS generators
Certain fluorescent proteins have been shown to be effective photosensitizers. In addition, some enzymes can effectively generate ROS via biochemical reactions (Figure 3). These protein-based ROS generators have attracted much attention, because they are excellent research tools for not only understanding the biological functions of ROS,41 but also precisely inactivating specific targets, such as proteins or protein complexes, in live cells and in vivo.42
Fig 3.

Genetically encoded ROS-generating systems, including fluorescent protein-based photosensitizes and enzyme-based ROS generators. Shown in the graph are the structures of KillerRed and RgDAAO based on Protein Data Bank (PDB) entries 3A8S and 1C0I.
4.1. Fluorescent-protein-based photosensitizers
When certain fluorescent proteins are genetically fused to target proteins, excitation of these fluorescent proteins may generate ROS, which can diffuse and inactivate surrounding molecules in living cells.43 By using this chromophore-assisted light inactivation (CALI) technique, one can selectively inactivate proteins within cells. Fluorescent proteins are particularly important for this application, because these genetically encoded photosensitizers can be readily fused to almost any target protein. The absorbance peaks of representative fluorescent-protein-based photosensitizers are given in Table 2.
Table 2.
Representative examples of genetically encoded photosensitizers.
In early studies, green fluorescent protein (GFP) was demonstrated as an effective CALI fluorophore, although its ROS generation capability is worse than synthetic dyes such as fluorescein or malachite green.43–45 From the hydrozoan chromoprotein anm2CP (a GFP homolog), Lukyanov and co-workers developed KillerRed,46 the first fluorescent protein that was specifically engineered for photosensitizing. This red fluorescent, dimeric protein indeed showed 1,000-fold higher phototoxicity than GFP. KillerRed is believed to mainly generate O2•− via the type I reaction.47–49 Recent effort has resulted in its monomeric and/or color-shifted variants, such as SuperNova,50 SuperNova Green,51 KillerOrange,52 and mKillerOrange.53
In addition to GFP analogs, some flavoproteins are also effective photosensitizers. Shu et al. engineered a mini singlet oxygen generator (miniSOG) from the flavin mononucleotide (FMN)-based LOV2 domain of Arabidopsis thaliana phototropin 2. This protein was shown to produce 1O2 upon excitation, suggesting that the photosensitization process occurs via the type II reaction.54 Variants of miniSOG with enhanced 1O2 production, such as SOPP,55 SOPP2, SOPP3,56 and miniSOG2,57 have also been described. In addition, a recent study analyzed eleven LOV-based flavoproteins and all were able to produce 1O2 and/or H2O2, despite that there were remarkable differences in ROS selectivity and yield.58 In particular, two variants, Pp2FbFP and DsFbFP M49I, were demonstrated to be new tools for light-controlled killing of bacteria and studying of specific ROS-induced cell signaling.59
In addition to CALI, genetically encoded photosensitizers have been targeted to mitochondria, chromatin, or plasma membranes to selectively kill cells in cell culture models and in vivo.60,61 Not surprisingly, they have also been explored as phototoxic cancer therapeutic agents.61,62
4.2. Enzymes that produce ROS without need of light
Perhaps one of the most well-known ROS-producing enzymes is nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), which was first characterized in mitochondria of neutrophils and is a major source for endogenous ROS in the form of either O2•− or H2O2.63,64 In addition, xanthine oxidase (XOR) and myeloperoxidase (MPO) are effective ROS generators.65 Furthermore, several components of the mitochondrial respiratory chain can be modulated to generate a large amount of O2•−.66
The most useful ROS-generating tool in this category is D-amino acid oxidase (DAAO), a flavin adenine dinucleotide (FAD)-containing enzyme that catalyzes the conversion of D-amino acids in the presence of molecular oxygen (O2) to alpha-keto acids, leading to enzyme- and D-amino-acid-dependent H2O2 production (Equation 1).67,68
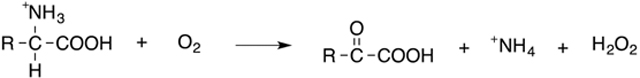 |
(Eq. 1) |
DAAO was first identified by Krebs in 1935.69 In particular, DAAO from the yeast Rhodotorula gracilis (RgDAAO) has been widely used due to its high enzymatic activity toward D-alanine.70 Overexpression of RgDAAO is an effective and innovative way to induce oxidative stress in cell culture models and in vivo. For example, RgDAAO has been used to spatiotemporally control H2O2 concentrations in astrocytes to study the role of H2O2 in astrocyte-dependent neuro-protective mechanisms.71 Recently, RgDAA was expressed in heart of rats fed with D-alanine to induce chronic generation of H2O2.67 The rats developed a dilated cardiomyopathy with significant systolic dysfunction. The study thus showcased a powerful chemogenetic approach to explore redox signaling and physiology in vivo.
5. Hybrid ROS generators
To precisely control the localization of synthetic photosensitizers, hybrid ROS generators have been developed by integrting synthetic photosensitizers with genetically encoded elements. For example, chemical photosensitizers could be conjugated to antibodies and this strategy actually enabled the first CALI experiment.72 Moreover, chemical photosensitizers could be linked to proteins via genetically encoded tags by using FlAsH, ReAsh, or HaloTag technologies.73,74 In another example, a fluorogen-activating single-chain antibody was engineered to bind an iodine-substituted malachite green analog, resulting in ‘on-demand’ activation of the photosensitizer to produces O2•− and fluorescence in the presence of near-infrared (NIR) excitation light.75 This so-called FAP-TAP (fluorogen-activating protein - targeted and activated photosensitizer) technology was validated for light-induced cell ablation in HEK293 cell culture and in live zebrafish. One advantage of these hybrid strategies is the availability of diverse, synthetic photosensitizers, including those excitable with NIR light to facilitate manipulation of proteins and cells in deep tissues of live organisms. Their main disadvantage is the dependence of the uptake of exogenous photosensitizers, whose penetration, localization, and distribution may be problematic for live cell, tissue, and/or in vivo applications.
6. Perspectives
ROS have been recognized as by-products of aerobic respiration and important signaling molecules. Tools that can generate or detect ROS are equally important for understanding biology regulated by ROS signaling and oxidative stress. This topical review discusses tools that have been adapted to control ROS production in biological systems via either photosensitization or enzyme reactions. In particular, a number of genetically encoded photosensitizers are now available for specific generation of O2•− or 1O2 via the type I or type II photosensitization mechanism. However, these current photosensitizers often require blue, green, or orange light for excitation. Further studies are urgently needed to develop red-shifted variants that can be excited in the NIR optical window. In addition, an elegant chemogenetic approach, which uses DAAO and D-amino acids to generate H2O2, may enable a large array of studies. D-amino acids are unfortunately essential in certain tissue types such as the brain.76 Therefore, this DAAO system is not completely bioorthogonal. It remains an open question whether a truly bioorthogonal system can be developed to chemogenetically control ROS in cells and organisms. It is promising that a NanoLuc luciferase was recently fused to MiniSOG to generate 1O2 via energy transfer in a cell culture model.(77) However, it still remains a future task to expand this strategy to in vivo animal models and to the generation of other types of ROS.
Genetically encoded or hybrid photosensitizers are excellent tools for specific deactivation of proteins, a process known as CALI. In addition, photosensitizers may be utilized to selectively destroy organelles, intracellular machineries, or whole cells. When they are applied to therapy, photosensitizers are promising for the treatment of cancer, bacterial infection, and other diseases. A number of synthetic photosensitizers have been approved for uses or clinical trials in cancer patients. Further studies may pursue strategies to achieve selective delivery of photosensitizers and to enhance their tissue penetration, bioavailability, and ROS production efficiency. Moreover, because multidrug resistant pathogens have become a threat to global heath, exploration of ROS generators to combat multidrug resistant pathogens is highly worthwhile.
ACKNOWLEDGMENT
Research reported herein was supported in part by the University of Virginia and the National Institute of General Medical Sciences of the National Institutes of Health under Grants R01GM118675 and R01GM129291.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Zhou Z, Song J, Nie L, and Chen X (2016) Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 45, 6597–6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Tripathy BC, and Oelmüller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signaling & Behavior 7, 1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Ren W, and Ai HW (2013) Genetically encoded fluorescent redox probes. Sensors 13, 15422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Covarrubias L, Hernández-García D, Schnabel D, Salas-Vidal E, and Castro-Obregón S (2008) Function of reactive oxygen species during animal development: passive or active? Dev. Biol. 320, 1–11. [DOI] [PubMed] [Google Scholar]
- (6).Bartosz G (2009) Reactive oxygen species: destroyers or messengers? Biochem. Pharmacol. 77, 1303–15. [DOI] [PubMed] [Google Scholar]
- (7).Casetta I, Govoni V, and Granieri E (2005) Oxidative stress, antioxidants and neurodegenerative diseases. Curr. Pharm. Des. 11, 2033–2052. [DOI] [PubMed] [Google Scholar]
- (8).Takahashi A, Ohtani N, Yamakoshi K, Iida S. i., Tahara H, Nakayama K, Nakayama KI, Ide T, Saya H, and Hara E (2006) Mitogenic signalling and the p16 INK4a–Rb pathway cooperate to enforce irreversible cellular senescence. Nat. Cell Biol. 8, 1291. [DOI] [PubMed] [Google Scholar]
- (9).Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, and Kessel D (2011) Photodynamic therapy of cancer: an update. CA. Cancer J. Clin. 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Liu L, Huang Z, Qiu Z, and Li B (2018) Visualization of Porphyrin-Based Photosensitizer Distribution from Fluorescence Images In Vivo Using an Optimized RGB Camera. J. Appl. Spectros. 84, 1124–1130. [Google Scholar]
- (11).Zhang J, Jiang C, Longo JPF, Azevedo RB, Zhang H, and Muehlmann LA (2018) An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta pharmaceutica sinica B 8, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).F Sperandio F, Huang Y-Y, and R Hamblin M (2013) Antimicrobial photodynamic therapy to kill Gram-negative bacteria. Recent Pat. Anti-Infect. Drug Discovery 8, 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Daniell MD, and Hill JS (1991) A history of photodynamic therapy. Aust. N. Z. J. Surg. 61, 340–8. [DOI] [PubMed] [Google Scholar]
- (14).Raab O (1900) Uber die wirkung Fluorescirender Stoffe auf Infusorien. Z. Biol. 39, 524–546. [Google Scholar]
- (15).Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, and Peng Q (1998) Photodynamic Therapy. JNCI: Journal of the National Cancer Institute 90, 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Huang Z (2005) A review of progress in clinical photodynamic therapy. Technol. Cancer Res. Treat. 4, 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Abrahamse H, and Hamblin MR (2016) New photosensitizers for photodynamic therapy. Biochem. J. 473, 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Foote CS (1968) Mechanisms of photosensitized oxidation. Science 162, 963–970. [DOI] [PubMed] [Google Scholar]
- (19).Castano AP, Demidova TN, and Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 1, 279–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Allison RR, Downie GH, Cuenca R, Hu X-H, Childs CJH, and Sibata CH (2004) Photosensitizers in clinical PDT. Photodiagnosis and photodynamic therapy 1, 27–42. [DOI] [PubMed] [Google Scholar]
- (21).Baptista MS, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente C, Nunez SC, Ribeiro MS, Thomas AH, et al. (2017) Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 93, 912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kessel D (1986) Photosensitization with derivatives of haematoporphyrin. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 49, 901–907. [DOI] [PubMed] [Google Scholar]
- (23).Senge MO, and Brandt JC (2011) Temoporfin (Foscan®, 5,10,15,20-Tetra(m-hydroxyphenyl)chlorin)—A Second-generation Photosensitizer†,‡. Photochem. Photobiol. 87, 1240–1296. [DOI] [PubMed] [Google Scholar]
- (24).Moore CM, Azzouzi A-R, Barret E, Villers A, Muir GH, Barber NJ, Bott S, Trachtenberg J, Arumainayagam N, Gaillac B, et al. (2015) Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU International 116, 888–896. [DOI] [PubMed] [Google Scholar]
- (25).Lightdale CJ, Heier SK, Marcon NE, McCaughan JS Jr., Gerdes H, Overholt BF, Sivak MV Jr., Stiegmann GV, and Nava HR (1995) Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest. Endosc. 42, 507–12. [DOI] [PubMed] [Google Scholar]
- (26).Nyman ES, and Hynninen PH (2004) Research advances in the use of tetrapyrrolic photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B: Biol. 73, 1–28. [DOI] [PubMed] [Google Scholar]
- (27).Carpenter BL, Situ X, Scholle F, Bartelmess J, Weare WW, and Ghiladi RA (2015) Antiviral, Antifungal and Antibacterial Activities of a BODIPY-Based Photosensitizer. Molecules 20, 10604–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Ali MFM (2011) Topical delivery and photodynamic evaluation of a multivesicular liposomal Rose Bengal. Lasers Med. Sci. 26, 267–275. [DOI] [PubMed] [Google Scholar]
- (29).Chang C-C, Yang Y-T, Yang J-C, Wu H-D, and Tsai T (2008) Absorption and emission spectral shifts of rose bengal associated with DMPC liposomes. Dyes and Pigments 79, 170–175. [Google Scholar]
- (30).Jajarm HH, Falaki F, Sanatkhani M, Ahmadzadeh M, Ahrari F, and Shafaee H (2015) A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: a randomized clinical controlled trial. Lasers Med. Sci. 30, 1475–1480. [DOI] [PubMed] [Google Scholar]
- (31).Thompson JF, Hersey P, and Wachter E (2008) Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 18, 405–411. [DOI] [PubMed] [Google Scholar]
- (32).Allardyce CS, Dorcier A, Scolaro C, and Dyson PJ (2005) Development of organometallic (organo‐transition metal) pharmaceuticals. Appl. Organomet. Chem. 19, 1–10. [Google Scholar]
- (33).Lei W, Zhou Q, Jiang G, Zhang B, and Wang X (2011) Photodynamic inactivation of Escherichia coli by Ru(II) complexes. Photochem. Photobiol. Sci. 10, 887–890. [DOI] [PubMed] [Google Scholar]
- (34).Le Gall T, Lemercier G, Chevreux S, Tucking KS, Ravel J, Thetiot F, Jonas U, Schonherr H, and Montier T (2018) Ruthenium(II) Polypyridyl Complexes as Photosensitizers for Antibacterial Photodynamic Therapy: A Structure-Activity Study on Clinical Bacterial Strains. ChemMedChem 13, 2229–2239. [DOI] [PubMed] [Google Scholar]
- (35).Knoll JD, and Turro C (2015) Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 282, 110–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Maggioni D, Galli M, D’Alfonso L, Inverso D, Dozzi MV, Sironi L, Iannacone M, Collini M, Ferruti P, and Ranucci E (2015) A luminescent poly (amidoamine)–iridium complex as a new singlet-oxygen sensitizer for photodynamic therapy. Inorg. Chem. 54, 544–553. [DOI] [PubMed] [Google Scholar]
- (37).Master A, Livingston M, and Gupta AS (2013) Photodynamic nanomedicine in the treatment of solid tumors: perspectives and challenges. J. Controlled Release 168, 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Perni S, Prokopovich P, Pratten J, Parkin IP, and Wilson M (2011) Nanoparticles: their potential use in antibacterial photodynamic therapy. Photochem. Photobiol. Sci. 10, 712–720. [DOI] [PubMed] [Google Scholar]
- (39).Gao Z, Lukyanov AN, Singhal A, and Torchilin VP (2002) Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2, 979–982. [Google Scholar]
- (40).Stuchinskaya T, Moreno M, Cook MJ, Edwards DR, and Russell DA (2011) Targeted photodynamic therapy of breast cancer cells using antibody–phthalocyanine–gold nanoparticle conjugates. Photochem. Photobiol. Sci. 10, 822–831. [DOI] [PubMed] [Google Scholar]
- (41).Toyokuni S. J. P. i. (1999) Reactive oxygen species‐induced molecular damage and its application in pathology. Pathol. Int. 49, 91–102. [DOI] [PubMed] [Google Scholar]
- (42).Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, and Ellisman MHJS (2002) Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507. [DOI] [PubMed] [Google Scholar]
- (43).Rajfur Z, Roy P, Otey C, Romer L, and Jacobson K (2002) Dissecting the link between stress fibres and focal adhesions by CALI with EGFP fusion proteins. Nat. Cell Biol. 4, 286–93. [DOI] [PubMed] [Google Scholar]
- (44).Surrey T, Elowitz MB, Wolf PE, Yang F, Nedelec F, Shokat K, and Leibler S (1998) Chromophore-assisted light inactivation and self-organization of microtubules and motors. Proc. Natl. Acad. Sci. U. S. A. 95, 4293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).McLean MA, Rajfur Z, Chen Z, Humphrey D, Yang B, Sligar SG, and Jacobson K (2009) Mechanism of Chromophore Assisted Laser Inactivation Employing Fluorescent Proteins. Anal. Chem. 81, 1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, and Lukyanov K. A. J. N. b. (2006) A genetically encoded photosensitizer. Nat. Biotechnol. 24, 95. [DOI] [PubMed] [Google Scholar]
- (47).Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, and Tsien RY (2011) A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biology 9, e1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Pletnev S, Gurskaya NG, Pletneva NV, Lukyanov KA, Chudakov DM, Martynov VI, Popov VO, Kovalchuk MV, Wlodawer A, and Dauter Z (2009) Structural basis for phototoxicity of the genetically encoded photosensitizer KillerRed. J. Biol. Chem. 284, 32028–32039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Vegh RB, Solntsev KM, Kuimova MK, Cho S, Liang Y, Loo BLW, Tolbert LM, and Bommarius AS (2011) Reactive oxygen species in photochemistry of the red fluorescent protein “Killer Red”. Chemical Communications 47, 4887–4889. [DOI] [PubMed] [Google Scholar]
- (50).Takemoto K, Matsuda T, Sakai N, Fu D, Noda M, Uchiyama S, Kotera I, Arai Y, Horiuchi M, and Fukui K (2013) SuperNova, a monomeric photosensitizing fluorescent protein for chromophore-assisted light inactivation. Scientific Reports 3, 2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Riani YD, Matsuda T, Takemoto K, and Nagai T (2018) Green monomeric photosensitizing fluorescent protein for photo-inducible protein inactivation and cell ablation. BMC biology 16, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Sarkisyan KS, Zlobovskaya OA, Gorbachev DA, Bozhanova NG, Sharonov GV, Staroverov DB, Egorov ES, Ryabova AV, Solntsev KM, and Mishin AS (2015) KillerOrange, a genetically encoded photosensitizer activated by blue and green light. PLoS One 10, e0145287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Pletneva NV, Pletnev VZ, Sarkisyan KS, Gorbachev DA, Egorov ES, Mishin AS, Lukyanov KA, Dauter Z, and Pletnev S (2015) Crystal Structure of Phototoxic Orange Fluorescent Proteins with a Tryptophan-Based Chromophore. PLoS One 10, e0145740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ruiz-González R. n., Cortajarena AL, Mejias SH, Agut M, Nonell S, and Flors C (2013) Singlet oxygen generation by the genetically encoded tag miniSOG. J. Am. Chem. Soc. 135, 9564–9567. [DOI] [PubMed] [Google Scholar]
- (55).Westberg M, Holmegaard L, Pimenta FM, Etzerodt M, and Ogilby PR (2015) Rational design of an efficient, genetically encodable, protein-encased singlet oxygen photosensitizer. J. Am. Chem. Soc. 137, 1632–42. [DOI] [PubMed] [Google Scholar]
- (56).Westberg M, Bregnhoj M, Etzerodt M, and Ogilby PR (2017) No Photon Wasted: An Efficient and Selective Singlet Oxygen Photosensitizing Protein. J. Phys. Chem. B 121, 9366–9371. [DOI] [PubMed] [Google Scholar]
- (57).Makhijani K, To TL, Ruiz-Gonzalez R, Lafaye C, Royant A, and Shu X (2017) Precision Optogenetic Tool for Selective Single- and Multiple-Cell Ablation in a Live Animal Model System. Cell Chem Biol 24, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Endres S, Wingen M, Torra J, Ruiz-González R, Polen T, Bosio G, Bitzenhofer NL, Hilgers F, Gensch T, and Nonell S (2018) An optogenetic toolbox of LOV-based photosensitizers for light-driven killing of bacteria. Scientific Reports 8, 15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Endres S, Wingen M, Torra J, Ruiz-Gonzalez R, Polen T, Bosio G, Bitzenhofer NL, Hilgers F, Gensch T, Nonell S, et al. (2018) An optogenetic toolbox of LOV-based photosensitizers for light-driven killing of bacteria. Scientific Reports 8, 15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Buckley C, Carvalho MT, Young LK, Rider SA, McFadden C, Berlage C, Verdon RF, Taylor JM, Girkin JM, and Mullins JJ (2017) Precise spatio-temporal control of rapid optogenetic cell ablation with mem-KillerRed in Zebrafish. Scientific Reports 7, 5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Ryumina AP, Serebrovskaya EO, Shirmanova MV, Snopova LB, Kuznetsova MM, Turchin IV, Ignatova NI, Klementieva NV, Fradkov AF, Shakhov BE, et al. (2013) Flavoprotein miniSOG as a genetically encoded photosensitizer for cancer cells. Biochim. Biophys. Acta 1830, 5059–67. [DOI] [PubMed] [Google Scholar]
- (62).Serebrovskaya EO, Edelweiss EF, Stremovskiy OA, Lukyanov KA, Chudakov DM, and Deyev SM (2009) Targeting cancer cells by using an antireceptor antibody-photosensitizer fusion protein. Proc. Natl. Acad. Sci. USA 106, 9221–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Dickinson BC, and Chang CJ (2011) Chemistry and biology of reactive oxygen species in signaling or stress responses. Nature chemical biology 7, 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Aviello G, and Knaus UG (2018) NADPH oxidases and ROS signaling in the gastrointestinal tract. Mucosal Immunol., 1. [DOI] [PubMed] [Google Scholar]
- (65).Kuppusamy P, and Zweier JL (1989) Characterization of free radical generation by xanthine oxidase. Evidence for hydroxyl radical generation. J. Biol. Chem. 264, 9880–4. [PubMed] [Google Scholar]
- (66).Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Steinhorn B, Sorrentino A, Badole S, Bogdanova Y, Belousov V, and Michel T (2018) Chemogenetic generation of hydrogen peroxide in the heart induces severe cardiac dysfunction. Nat. Commun. 9, 4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Pollegioni L, Langkau B, Tischer W, Ghisla S, and Pilone MS (1993) Kinetic mechanism of D-amino acid oxidases from Rhodotorula gracilis and Trigonopsis variabilis. J. Biol. Chem. 268, 13850–13857. [PubMed] [Google Scholar]
- (69).Krebs HA (1935) Metabolism of amino-acids: Deamination of amino-acids. Biochem. J. 29, 1620–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Rosini E, Pollegioni L, Ghisla S, Orru R, and Molla G (2009) Optimization of D-amino acid oxidase for low substrate concentrations--towards a cancer enzyme therapy. FEBS J. 276, 4921–32. [DOI] [PubMed] [Google Scholar]
- (71).Haskew-Layton RE, Payappilly JB, Smirnova NA, Ma TC, Chan KK, Murphy TH, Guo H, Langley B, Sultana R, and Butterfield DA (2010) Controlled enzymatic production of astrocytic hydrogen peroxide protects neurons from oxidative stress via an Nrf2-independent pathway. Proc. Natl. Acad. Sci. USA 107, 17385–17390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Jay DG (1988) Selective destruction of protein function by chromophore-assisted laser inactivation. Proc. Natl. Acad. Sci. USA 85, 5454–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Tour O, Meijer RM, Zacharias DA, Adams SR, and Tsien RY (2003) Genetically targeted chromophore-assisted light inactivation. Nature Biotechnol. 21, 1505. [DOI] [PubMed] [Google Scholar]
- (74).Takemoto K, Matsuda T, McDougall M, Klaubert DH, Hasegawa A, Los GV, Wood KV, Miyawaki A, and Nagai T (2011) Chromophore-assisted light inactivation of HaloTag fusion proteins labeled with eosin in living cells. ACS Chem. Biol. 6, 401–406. [DOI] [PubMed] [Google Scholar]
- (75).He J, Wang Y, Missinato MA, Onuoha E, Perkins LA, Watkins SC, St Croix CM, Tsang M, and Bruchez MP (2016) A genetically targetable near-infrared photosensitizer. Nat. Methods 13, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Wolosker H, Dumin E, Balan L, and Foltyn VN (2008) D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration. FEBS J. 275, 3514–26. [DOI] [PubMed] [Google Scholar]
- (77).Proshkina GM, Shramova EI, Shilova ON, Ryabova AV, and Deyev SM (2018) Phototoxicity of flavoprotein miniSOG induced by bioluminescence resonance energy transfer in genetically encoded system NanoLuc-miniSOG is comparable with its LED-excited phototoxicity. J. Photochem. Photobiol., B 188, 107–115, DOI: 10.1016/j.jphotobiol.2018.09.006 [DOI] [PubMed] [Google Scholar]


