Abstract
Background
Acute kidney injury (AKI) is a recognized complication of pediatric severe malaria, but its long-term consequences are unknown.
Methods
Ugandan children with cerebral malaria (CM, n = 260) and severe malaria anemia (SMA, n = 219) or community children (CC, n = 173) between 1.5 and 12 years of age were enrolled in a prospective cohort study. Kidney Disease: Improving Global Outcomes (KDIGO) criteria were used to retrospectively define AKI and chronic kidney disease (CKD). Cognitive testing was conducted using the Mullen Scales of Early Learning in children < 5 and Kaufman Assessment Battery for Children (K-ABC) second edition in children ≥ 5 years of age.
Results
The prevalence of AKI was 35.1%, ranging from 25.1% in SMA to 43.5% in CM. In-hospital mortality was 11.9% in AKI compared to 4.2% in children without AKI (p = 0.001), and post-discharge mortality was 4.7% in AKI compared to 1.3% in children without AKI (p = 0.030) corresponding to an all-cause adjusted hazard ratio of 2.30 (95% CI 1.21, 4.35). AKI was a risk factor for short- and long-term neurocognitive impairment. At 1 week post-discharge, the frequency of neurocognitive impairment was 37.3% in AKI compared to 13.5% in children without AKI (adjusted odds ratio (aOR) 2.31 [95% CI 1.32, 4.04]); at 1-year follow-up, it was 13.3% in AKI compared to 3.4% in children without AKI (aOR 2.48 [95% CI 1.01, 6.10]), and at 2-year follow-up, it was 13.0% in AKI compared to 3.4% in children without AKI (aOR 3.03 [95% CI 1.22, 7.58]). AKI was a risk factor for CKD at 1-year follow-up: 7.6% of children with severe malaria-associated AKI had CKD at follow-up compared to 2.8% of children without AKI (p = 0.038) corresponding to an OR of 2.81 (95% CI 1.02, 7.73). The presenting etiology of AKI was consistent with prerenal azotemia, and lactate dehydrogenase as a marker of intravascular hemolysis was an independent risk factor for AKI in CM and SMA (p < 0.0001). In CM, AKI was associated with the presence and severity of retinopathy (p < 0.05) and increased cerebrospinal fluid albumin suggestive of blood-brain barrier disruption.
Conclusions
AKI is a risk factor for long-term neurocognitive impairment and CKD in pediatric severe malaria.
Electronic supplementary material
The online version of this article (10.1186/s12916-019-1332-7) contains supplementary material, which is available to authorized users.
Keywords: Acute kidney injury, Malaria, Child, Cognition, Mortality, Chronic kidney disease
Background
Infection with Plasmodium falciparum is a significant cause of global morbidity and mortality: an estimated 219 million cases of malaria were reported in 2017 with 92% of estimated cases occurring in sub-Saharan Africa [1]. Severe malaria is also a leading cause of acquired neurodisability in African children [2]. Clinical risk factors described to date for neurocognitive impairment in severe malaria are acute neurologic manifestations, e.g., duration of coma and number of seizures [2–6].
Although children with severe malaria may present with signs suggesting a focal insult, multi-organ dysfunction is common [7–9]. Acute kidney injury (AKI) is a common complication of pediatric severe malaria [9, 10] associated with mortality [9–13] and neurologic deficits in survivors [10]. In a meta-analysis of predictors of mortality in African children with severe malaria, AKI was the strongest predictor of death with an odds ratio of 5.96 (95% confidence interval (CI) 2.93 to 12.11) [14]. AKI is an established clinical risk factor for chronic kidney disease (CKD) in adults [15], but information on long-term renal recovery after AKI in pediatric populations is lacking. In particular, there are no data on whether AKI in severe malaria is a risk factor for CKD.
In this prospective cohort study, we evaluated the prevalence of AKI in pediatric severe malaria at admission and investigated the relationship between AKI and clinical and renal recovery, and also with long-term neurocognitive functioning.
Methods
Study participants
The study was performed at Mulago National Referral Hospital in Uganda from 2008 to 2015, enrolling children 18 months to 12 years of age as described [4] (Additional file 1). All children with P. falciparum on blood smear who met the inclusion criteria for cerebral malaria (CM) and severe malarial anemia (SMA) were enrolled. Children with CM had a coma with no other identifiable cause ruling out meningitis, a prolonged postictal state, or hypoglycemia-associated coma reversed by a glucose infusion. Children with SMA had hemoglobin level ≤ 5 g/dL. Children with CM and severe anemia were classified as CM. Age-matched community children (CC) were recruited from the nuclear family, extended family, or household area of children with severe malaria (CM or SMA). Exclusion criteria included prior coma, head trauma, hospitalization for malnutrition, cerebral palsy, or known chronic illness requiring medical care or causing developmental delay.
Children were managed according to the Uganda Clinical Guidelines at the time of the study, including intravenous infusion of 10 mg/kg quinine hydrochloride in 5–10 mL/kg of 5% glucose over a 4-h period for the treatment of severe malaria, repeated every 8 h until the child could take oral medication (quinine or artemether-lumefantrine). Towards the end of the study, the treatment shifted towards the use of parenteral artemisinin-based therapies for the treatment of severe malaria following the 2011 World Health Organization recommendation of injectable artesunate as the first-line treatment for severe malaria. Hypoglycemia was treated with a 1–2-mL/kg 25% dextrose bolus administered intravenously. Fluid resuscitation was managed conservatively according to local guidelines at the time of the study: a fluid bolus of 20 mL/kg of sodium chloride 0.9% intravenously over 1 h was given only for the treatment of shock (systolic blood pressure < 50 mmHg or absent peripheral pulse) with delayed capillary refill (> 2 s). Children without shock but with evidence of dehydration received maintenance intravenous fluids. Furosemide was administered to children with clinical evidence of congestive heart failure or lack of urine output over one or more 8-h shifts, after ruling out dehydration and shock, at a dose of 1 mg/kg up to a maximum of 4 mg/kg daily. Dialysis was not available on site at the time the study was conducted.
All children underwent a medical history and physical examination on enrollment. As a measure of disease severity, we evaluated the number of severe malaria criteria present (Additional file 1: Table S1, Methods). Emotional stimulation was assessed using age-appropriate versions of the Home Observation for the Measurement of the Environment (HOME) [4].
Laboratory assessment
Peripheral blood smears were used to quantify parasite density using Giemsa staining with standard protocols. EDTA anticoagulated plasma was collected at admission and stored at − 80 °C until testing. Plasma PfHRP2 levels were measured to assess parasite biomass (Cellabs, Australia) [16]. Creatinine was tested on cryopreserved enrollment samples using a Beckman Coulter AU680 using the modified Jaffe method (Indiana University, Pathology Laboratory). Samples were sent in batches to the clinical laboratory for creatinine testing between 2012 and 2014, and 1-year follow-up samples were tested in 2016.
Assessment of kidney function
Acute kidney injury (AKI) was defined retrospectively using Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [17] based on a single admission creatinine level, with baseline creatinine estimated using CC as described in Additional file 1. AKI was defined as a 1.5-fold increase in creatinine over baseline and was staged: stage 1, 1.5–1.9× increase in creatinine over baseline; stage 2, 2.0–2.9× increase over baseline; and stage 3, ≥ 3.0× increase over baseline [17]. Chronic kidney disease (CKD) was defined as an eGFR< 90 mL/min/1.73 m2 using the Bedside Schwartz equation and categorized using KDIGO guidelines [18]. The AKI classification was repeated using an epidemiologic method to estimate baseline creatinine [19] (Additional file 1: Table S2). A comparative analysis is presented in Additional file 1.
Neurocognitive assessment
Children were tested at enrollment (CC) or a week after discharge (CM, SMA) and at 1- and 2-year follow-up. Cognition in children less than 5 years of age was assessed by the Mullen Scales of Early Learning. Scores from fine motor, visual reception, receptive language, and expressive language scales were summed to give the early learning composite score, a measure of the overall cognitive ability. Cognitive ability in children 5 years of age and older was assessed by the Kaufman Assessment Battery for Children second edition (KABC-II). The composite score for the mental processing index was used to assess the child’s cognitive ability. Both tests have been validated previously in Ugandan children [2, 4]. Neurocognitive impairment was defined as the presence of a gross deficit on neurologic exam or an age-adjusted cognitive z score more than two standard deviations below the mean.
Statistical analyses
Age-adjusted z scores for cognition were created using the community children [2, 3]. Unadjusted comparisons for continuous and categorical measures used the Wilcoxon rank-sum test or Kruskal-Wallis test and Pearson’s χ2 or Fisher’s exact test, respectively. Logistic regression was used to estimate factors’ association with AKI and to test the association between AKI, retinopathy, and neurocognitive outcomes. Ordinal logistic regression was used to assess the relationship between AKI and the ordinal categories of retinopathy. Cox proportional hazards regression was used to evaluate the association between AKI and death. Logistic regression was used to evaluate the association between AKI and cerebrospinal fluid albumin. Adjusters were included in multivariable analyses if they had p < 0.10 in a bivariate analysis or an a priori hypothesized relationship with the outcome. Holm’s correction was used to adjust for multiple comparisons. Analyses were done using Stata v14.0 (StataCorp. 2015).
Role of the funding source
The funders had no role in the study design, analysis, or decision to publish.
Results
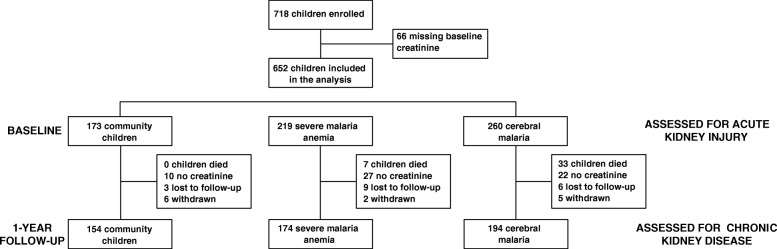
In total, 652 children with enrollment creatinine values were included in the study (Fig. 1). The demographic characteristics of the population are presented in Table 1. The prevalence of AKI was 35.1% overall: 43.5% of children with CM and 25.1% of children with SMA. Children with CM had a more severe AKI than children with SMA (CM: 23.5% stage 1 (n = 61), 12.7% stage 2 (n = 33), 7.3% stage 3 (n = 19); SMA: 16.0% stage 1 (n = 35), 7.3% stage 2 (n = 16), 1.8% stage 3 (n = 4), p < 0.0001). Children with CM had a 2.37-fold (95% CI 1.38, 4.08) increase in the odds of severe AKI (AKI stage 2 or 3) compared to children with SMA (p = 0.010). The prevalence of AKI was similar in children treated with quinine, 34.7% (n = 133), vs. those treated with parenteral artemisinin derivatives, 36.0% (n = 55), p = 0.78.
Fig. 1.
Flow chart of the study population. Children with a creatinine on study enrollment were included in the study and had their kidney function re-assessed at 1-year follow-up
Table 1.
Demographic and laboratory characteristics of study children
| Characteristic | CC (n = 173) | SMA (n = 219) | CM (n = 260) | p value |
|---|---|---|---|---|
| Demographics | ||||
| Age, median (IQR), years | 3.6 (2.6, 4.6) | 2.9 (2.1, 4.5) | 3.5 (2.5, 4.9) | 0.0002 |
| Female sex, (%) no. | 94 (54.3) | 86 (39.3) | 107 (41.2) | 0.006 |
| Weight-for-age z score, median (IQR) | − 1.0 (− 1.5, − 0.3) | − 1.5 (− 2.2, − 0.7) | − 1.2 (− 1.9, − 0.5) | 0.0001 |
| Height-for-age z score, median (IQR) | − 1.1 (− 1.7, − 0.4) | − 1.0 (− 1.9, − 0.3) | − 0.6 (− 1.9, − 0.5) | 0.002 |
| Socioeconomic status score, median (IQR)1 | 9 (8, 12) | 9 (7, 11) | 9 (8, 11) | 0.355 |
| Home environment z score, median (IQR)1 | 0.12 (− 0.71, 0.85) | − 0.05 (− 0.71, 0.67) | − 0.02 (− 0.71, 0.71) | 0.523 |
| Maternal education level, no. (%)1 | ||||
| Primary 6 or lower | 47 (27.2) | 88 (38.6) | 80 (36.7) | 0.348 |
| Primary 7 | 42 (24.3) | 47 (20.6) | 45 (20.6) | |
| Secondary or higher | 74 (42.8) | 80 (35.1) | 83 (38.1) | |
| Not known | 10 (5.8) | 13 (5.7) | 10 (4.6) | |
| Paternal education level, no. (%)1 | ||||
| Primary 6 or lower | 24 (13.9) | 50 (22.9) | 38 (16.7) | 0.076 |
| Primary 7 | 33 (19.1) | 31 (14.2) | 40 (17.5) | |
| Secondary or higher | 90 (52.0) | 98 (45.0) | 98 (43.0) | |
| Not known | 26 (15.0) | 39 (17.9) | 52 (22.8) | |
| Child any education, no. (%)1 | 72 (41.9) | 56 (26.3) | 85 (38.1) | 0.008 |
| Laboratory characteristics2 | SMA vs. CM | |||
| Hemoglobin, g/dL | 11.8 (11.0, 12.6) | 3.9 (3.2, 4.5) | 6.8 (5.1, 8.7) | < 0.0001 |
| Glucose, mmol/L | – | 6.4 (4.7, 8.2) | 6.6 (4.9, 8.9) | 0.162 |
| Lactate, mmol/L | – | 4.8 (3.0, 8.0) | 3.8 (2.2, 6.7) | 0.002 |
| WBC, × 103/μL | 8.6 (7.2, 10.6) | 11.5 (8.2, 16.2) | 9.4 (7.2, 13.9) | 0.0001 |
| Platelet, ×103/μL | 383 (289, 449) | 149 (91, 225) | 60 (34, 111) | < 0.0001 |
| Total bilirubin, mg/dL | 0.2 (0.1, 0.3) | 1.3 (0.7, 2.1) | 1.6 (0.9. 2.7) | 0.004 |
| Lactate dehydrogenase (LDH), U/L | 162 (232, 311) | 762 (621, 990) | 829 (630, 1116) | 0.069 |
| Plasma albumin, g/dL | 3.7 (3.4, 3.9) | 2.6 (2.3, 3.0) | 2.6 (2.4, 2.9) | 0.898 |
| Sodium, mmol/L | 137 (135, 138) | 134 (132, 136) | 132 (128, 136) | < 0.0001 |
| Peripheral parasite density, parasites/uL | 0 (0, 0) | 34,970 (10,085, 134,925) | 48,420 (10,830, 282,400) | 0.0286 |
| Plasma PfHRP2, ng/mL | 5 (5, 118) | 944 (367, 2790) | 2828 (1024, 5546) | < 0.0001 |
| Creatinine, mg/dL | 0.30 (0.24, 0.35) | 0.35 (0.28, 0.46) | 0.42 (0.31, 0.55) | < 0.0001 |
| BUN, mg/dL | 7 (5, 9) | 13 (9, 20) | 17 (12, 25) | < 0.0001 |
Continuous measures presented as median (interquartile range) unless otherwise indicated. Continuous measures compared using the Kruskal-Wallis test. Count measures compared using Pearson’s chi-square or Fisher’s exact, as appropriate
1Assessed on survivors (n = 218 in SMA, n = 228 in CM)
2Data on laboratory characteristics presented for all children, as available, but analyzed using Wilcoxon rank-sum test comparing the differences between SMA and CM
Fluid management, kidney function, and hydration status
The distribution of supportive care and fluid management by severe malaria group and AKI status is shown in Table 2. Blood products were commonly administered with 368 children (76.8%) receiving a transfusion during hospitalization. Details on the type of transfusion were available for 107 children, with 104 (96.3%) receiving packed red blood cells. Seventy-three (15.2%) children enrolled in the study received intravenous fluids (including 0.9% saline, Ringer’s lactate, or albumin) in addition to parenteral antimalarial therapy. No children fulfilled the criteria for an intravenous fluid bolus. Children with SMA and AKI were more likely to receive a dextrose bolus, and children with CM and AKI were more likely to receive intravenous fluids, a blood transfusion, and furosemide (adjusted p < 0.05, following Holm’s correction for multiple comparisons; Table 2).
Table 2.
Supportive care and treatment in children with severe malaria according to AKI status
| Severe malarial anemia (n = 219) | Cerebral malaria (n = 260) | |||||
|---|---|---|---|---|---|---|
| No AKI (n = 164) | AKI (n = 55) | p value | No AKI (n = 147) | AKI (n = 113) | p value | |
| Supportive care | ||||||
| Oxygen | 22 (6.7) | 7 (12.7) | 0.160 | 71 (48.3) | 76 (67.3) | 0.002 |
| Antipyretics | 161 (98.2) | 53 (96.4) | 0.437 | 138 (93.9) | 104 (92.0) | 0.562 |
| Medications | ||||||
| Quinine | 127 (77.4) | 44 (80.0) | 0.691 | 131 (89.1) | 99 (87.6) | 0.707 |
| Artesunate/artemether | 55 (33.5) | 16 (29.1) | 0.619 | 43 (29.3) | 39 (34.5) | 0.420 |
| Anticonvulsants | 1 (0.6) | 2 (3.6) | 0.156 | 109 (74.2) | 96 (85.0) | 0.034 |
| Furosemide | 32 (19.5) | 11 (20.0) | 0.937 | 9 (6.1) | 26 (23.0) | < 0.0001 |
| Bolus dextrose | 28 (17.1) | 28 (50.9) | < 0.0001 | 132 (89.8) | 91 (80.5) | 0.034 |
| Intravenous fluids | 12 (7.3) | 5 (9.1) | 0.671 | 24 (16.3) | 32 (28.3) | 0.020 |
| 0.9% saline | 10 (6.1) | 4 (7.3) | 0.754 | 18 (12.2) | 24 (21.2) | 0.051 |
| Ringer’s lactate | 0 (0.0) | 0 (0.0) | – | 2 (1.4) | 4 (3.5) | 0.246 |
| 10% dextrose | 0 (0.0) | 0 (0.0) | – | 4 (2.7) | 2 (1.8) | 0.700 |
| Bicarbonate | 0 (0.0) | 0 (0.0) | – | 0 (0.0) | 1 (0.9) | 0.435 |
| Darrow’s | 0 (0.0) | 1 (1.8) | 0.251 | 1 (0.7) | 0 (0.0) | 1.000 |
| Albumin | 2 (1.2) | 0 (0.0) | 1.000 | 2 (1.4) | 4 (3.5) | 0.408 |
| Blood transfusion | 164 (100.0) | 55 (100.0) | – | 68 (46.3) | 81 (71.7) | < 0.0001 |
Data presented as n (%) and compared using Pearson’s chi-square or Fisher’s exact, as appropriate
Mortality
Overall, the mortality was 9.2% with 44 deaths during the 2-year study period: 33 (75%) occurring during hospitalization and 11 (25%) occurring during follow-up. With the exception of 1 child with SMA who died of hypoglycemia, all in-hospital study deaths occurred in children with CM and were related to respiratory failure. Four (12.1%) children had clinical signs of cerebral edema identified as a contributing cause of death. Children who died were more likely to have received intravenous fluids during admission (33.3%, n = 11/33) compared to survivors (13.9%, n = 62/446) (p = 0.003) with the effect stronger in children without AKI (OR (95% CI), 3.69 (1.07, 12.68), p = 0.038) compared to children with AKI (OR (95% CI), 2.12 (0.78, 5.77), p = 0.14). Most follow-up deaths (7 of 11) occurred in the first 6 months of follow-up, and all follow-up deaths occurred in children who had severe anemia at the time of study enrollment. Details on the cause of death in follow-up were limited as several deaths occurred out of the hospital, but a history of fever, anemia, and malaria was common.
Association between AKI and clinical recovery
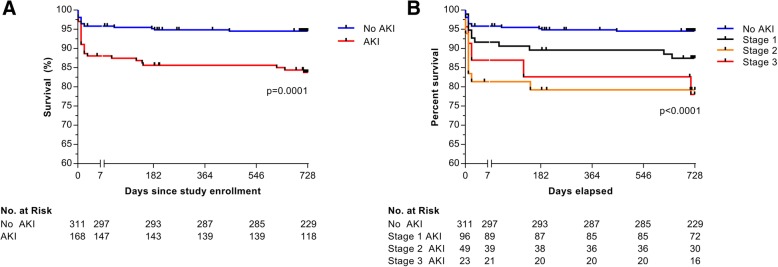
Consistent with previous reports, AKI was associated with increased mortality (Fig. 2). In-hospital mortality was 11.9% (n = 20/168) for children with AKI compared to 4.2% (n = 13/311) in children without AKI (p = 0.001), and post-discharge mortality was 4.7% (n = 7/148) in children with AKI compared to 1.3% (n = 4/298) in children without AKI (p = 0.030) (Additional file 1: Tables S10-S11). Overall, 60.6% of in-hospital deaths and 63.6% of post-discharge deaths occurred in children with AKI. In total, 326 (68.1%) children were treated with intravenous quinine, and 153 (31.9%) were treated with parenteral artemisinin derivatives. In children receiving quinine, 14 (12.4%) children with AKI died compared to 13 (6.1%) children without AKI (p = 0.050). In children receiving artemisinin derivatives, 6 (10.9%) of those with AKI died compared to 0 (0.0%) children without AKI (p = 0.001). From admission to 2-year follow-up, AKI had an adjusted hazards ratio (aHR) for the death of 2.30 (95% CI 1.21, 4.35) following adjustment for age, sex, hemoglobin, disease severity, and parenteral antimalarial treatment (quinine vs. artemisinin derivative) (p = 0.0109).
Fig. 2.
Mortality associated with acute kidney injury and across stages of AKI over 2-year follow-up. Kaplan-Meier plots showing 2-year survival in children with severe malaria based on the presence of KDIGO-defined acute kidney injury (AKI) status at admission (a) or the severity of AKI based on KDIGO-defined AKI stage (b). The break in the horizontal axis separates the first 7 days of follow-up (where the majority of in-hospital deaths occur) from the period of outpatient follow-up. Testing used the log-rank Mantel-Cox test in (a) and log-rank test for trend across stages of AKI (b)
Among the CM survivors, AKI was associated with prolonged fever clearance time (p < 0.0001), coma duration (p < 0.0001), and length of hospitalization (p = 0.0005) (Additional file 1: Table S10-S11). Among the SMA survivors, recovery times were not associated with AKI status.
Association between severe malaria, AKI, and chronic kidney disease 1 year later
In children with a history of severe malaria, AKI was a risk factor for CKD with 9 (7.6%) children with AKI on admission having CKD at 1 year follow-up (7.1% in SMA, 7.8% in CM) compared to 7 (2.8%) children without AKI (odds ratio for CKD 2.81 [95% CI 1.02, 7.73]). In children with a history of CM, severe AKI was associated with a 4.08-fold (95% CI 1.03, 16.09) increase in the odds of CKD. Thirteen children with CKD (81.3%) were GFR category 2 (eGFR, 60–89 mL/min/1.73 m2) and 3 (18.8%) category 3 (eGFR, 30–59). The prevalence of CKD in children treated with quinine was 3.1% (n = 8/257) compared to 7.3% (n = 8/109) in children treated with artemisinin derivatives (p = 0.071). When stratifying by AKI status at enrollment, the prevalence of CKD without AKI was 1.7% (n = 3/173) in children treated with quinine vs. 5.4% (n = 4/74) in children treated with artemisinin derivatives (p = 0.202), and in children with AKI, the prevalence of CKD was 6.0% (n = 5/84) in children receiving quinine vs. 11.4% (n = 4/35) in children treated with artemisinin derivatives (p = 0.446). Children were screened for active illness prior to the blood draw, so the eGFR was unlikely to represent a repeat episode of AKI (detailed in Additional file 1: Methods).
Association between AKI and neurocognitive outcomes
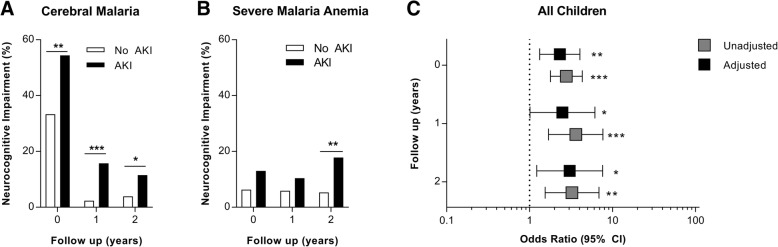
Neurologic deficits were common in CM survivors 1 week post-discharge (36.4%, n = 82) and consisted of speech difficulties (31.7%, n = 26), visual impairment (19.5%, n = 16), motor deficits (54.9%, n = 45), movement disorders (4.9%, n = 4), ataxia (47.6%, n = 39), and hyporeflexia or Babinski sign (32.9%, n = 27). Deficits persisted in only 3.1% of children (n = 7) at 2-year follow-up and were related to speech (71.4%) and movement disorders or motor deficits (85.7%). Overall, 53.3% (n = 48/90) of children with AKI had a neurologic deficit 1 week post-discharge compared to 25.2% (n = 34/135) of children without AKI (p < 0.0001). When stratifying by anti-malarial treatment, the association between AKI and neurologic deficit at discharge remained significant with OR (95% CI) of 3.31 (1.67, 6.56) in children treated with quinine (p = 0.0006) and 5.20 (1.62, 16.74) in children treated with artemisinin derivatives (p = 0.006). The association between AKI and neurologic deficits persisted at 2-year follow-up: 6.7% (n = 6/90) of children with a history of AKI had a neurologic deficit compared to 0.7% (n = 1/136) in children without a history of AKI (p = 0.017).
As children surviving severe malaria are at risk of long-term neurocognitive impairment [5], we tested the association between AKI and neurocognition. AKI was associated with neurocognitive impairment 1 week post-discharge, with aOR (95% CI) of 2.31 (1.32, 4.04), and the effect persisted over time with aORs (95% CI) of 2.48 (1.01, 6.10) and 3.03 (1.22, 7.58) at 1- and 2-year follow-up, respectively (Fig. 3; Additional file 1: Tables S12-S13). The associations remained significant at 2-year follow-up after correction for multiple comparisons. Models were adjusted for demographic factors known to be associated with child development (age, sex, parental education, school attendance, socioeconomic status, enrichment in the home environment, and height- and weight-for-age z scores), a composite measure of disease severity, complications associated with impaired cognition (number of seizures, coma), and parenteral antimalarial treatment (quinine vs. artemisinin derivative).
Fig. 3.
Association between acute kidney injury and neurocognitive recovery in children following severe malaria. Bar graphs showing the frequency of neurocognitive impairment in children with cerebral malaria (a) or severe malarial anemia (b). Neurocognitive impairment was defined as a gross deficit on the neurologic exam or an age-adjusted z score more than two standard deviations below the mean. Data analyzed using Pearson’s chi-square, *p < 0.01, **p < 0.01, ***p < 0.0001. c. Odds ratio of neurocognitive impairment (95% CI) associated with acute kidney injury from logistic regression models, 1 week post-discharge and 1- and 2-year follow-up. Multivariable-adjusted models included child age, sex, height- and weight-for-age z score, parental education, child schooling, an assessment of enrichment in the home environment, socioeconomic status, disease severity on presentation, the presence of coma, and number of seizures during hospitalization and parenteral antimalarial treatment (quinine vs. artemisinin derivative). *p < 0.05, **p < 0.01, ***p < 0.0001 following adjustment for multiple comparisons
Etiology and pathophysiology of AKI in severe malaria
The majority of children with severe malaria had a BUN to creatinine ratio consistent with prerenal azotemia (CM, 96.5%; SMA, 89.6%). There was no association between hydration status (capillary refill time, sunken eyes, decreased skin turgidity, cool peripheries, systolic blood pressure, dry mucous membranes) or comorbidities associated with volume depletion (i.e., gastroenteritis) and prerenal azotemia in children with CM or SMA (p > 0.05 for all). Among children with severe malaria, prerenal azotemia was associated with an increased heart rate (p = 0.004) and lower weight-for-age (p = 0.01) and weight-for-height z scores (p = 0.004). Children with severe malaria and prerenal azotemia had a higher parasite density (p = 0.001) and parasite biomass (plasma HRP-2, p = 0.002) than children without prerenal azotemia. In a multivariable model, a one-unit increase in the natural log of plasma HRP2 was associated with an aOR of prerenal injury of 1.28 (1.15, 1.66) following adjustment for age, sex, heart rate, and weight-for-age and weight-for-height z score.
In bivariate analysis, AKI was associated with disease severity, hemoglobinuria, low glucose concentrations, high concentrations of lactate, total bilirubin, lactate dehydrogenase (LDH), and parasite biomass (plasma HRP-2) (Table 3, Additional file 1: Table S3-S7). However, in multivariable models, LDH was the only measure independently associated with AKI in both CM and SMA following adjustment for multiple comparisons (Additional file 1: Table S4-S5). A one-unit increase in the natural log of LDH was associated with adjusted odds ratios (aOR) of AKI of 5.10 (95% CI 2.22, 11.69) and 8.62 (3.02, 24.60) in CM and SMA, respectively. History of nephrotoxic medication use was not associated with AKI at admission.
Table 3.
Measures associated with acute kidney injury
| Severe malarial anemia (n = 219) | Cerebral malaria (n = 260) | |||||
|---|---|---|---|---|---|---|
| No AKI (n = 164) | AKI (n = 55) | p value | No AKI (n = 147) | AKI (n = 113) | p value | |
| Demographics | ||||||
| Age, years | 2.8 (2.1, 4.2) | 3.2 (2.0, 4.9) | 0.375 | 3.6 (2.7, 5.4) | 3.2 (2.2, 4.5) | 0.014 |
| Sex, F % | 65 (39.6) | 21 (38.2) | 0.849 | 59 (40.1) | 48 (42.3) | 0.705 |
| Weight-for-age z score | − 1.5 (− 2.2, − 0.7) | − 1.8 (− 2.4, − 0.6) | 0.439 | − 1.0 (− 1.8, − 0.4) | − 1.4 (− 1.9, − 0.8) | 0.044 |
| Height-for-age z score | − 1.0 (− 1.8, − 0.3) | − 1.4 (− 2.6, − 0.5) | 0.119 | − 0.7 (− 1.3, 0.3) | − 1.0 (− 1.9, − 0.1) | 0.024 |
| Weight-for-height z score | − 0.9 (− 1.8, − 0.1) | − 0.9 (− 1.6, − 0.1) | 0.697 | − 1.0 (− 1.8, − 0.1) | − 1.0 (− 1.7, − 0.3) | 0.914 |
| HIV-infected, n (%) | 5 (3.1) | 1 (1.9) | 1.000 | 1 (0.7) | 4 (4.0) | 0.165 |
| Sickle cell disease (HbSS), n (%) | 17 (10.4) | 4 (7.3) | 0.605 | 1 (0.7) | 0 (0.0) | 0.380 |
| Admission characteristics | ||||||
| Symptoms | ||||||
| History of fever, days | 4 (3, 5) | 3 (2, 5) | 0.078 | 3 (2, 4) | 3 (2, 4) | 0.537 |
| Tea-colored urine, n (%) | 23 (14.0) | 15 (27.3) | 0.025 | 18 (12.2) | 27 (23.9) | 0.014 |
| Diarrhea, n (%) | 10 (6.1) | 6 (10.9) | 0.235 | 6 (4.1) | 6 (5.3) | 0.640 |
| Vomiting, n (%) | 74 (45.1) | 38 (69.1) | 0.002 | 60 (40.8) | 38 (33.6) | 0.236 |
| Clinical signs | ||||||
| Temperature, °C | 37.7 (37.0, 38.5) | 37.7 (36.7, 38.5) | 0.976 | 38.0 (36.9, 38.5) | 37.6 (37.0, 38.5) | 0.161 |
| Pulse, beats/min | 150 (137, 162) | 154 (138, 168) | 0.158 | 148 (128, 165) | 150 (135, 168) | 0.278 |
| Respiratory rate, breaths/min | 44 (36, 55) | 40 (35, 52) | 0.358 | 44 (34, 54) | 44 (36, 56) | 0.357 |
| Systolic blood pressure, mmHg | 90 (82, 100) | 90 (85, 100) | 0.708 | 96 (90, 105) | 96 (85, 104) | 0.409 |
| Blantyre coma score | 5 (5, 5) | 5 (5, 5) | – | 2 (1, 2) | 2 (1, 2) | 0.684 |
| Glasgow coma score | 15 (15, 15) | 15 (15, 15) | – | 7 (6, 8) | 7 (6, 8) | 0.760 |
| Severe dehydration, n (%)1 | 4 (2.4) | 3 (5.5) | 0.371 | 4 (2.7) | 3 (2.7) | 1.000 |
| Urine hemoglobin positive, n (%) | 10 (7.7) | 8 (19.5) | 0.032 | 14 (10.8) | 28 (33.7) | < 0.0001 |
| Hemoglobinuria, n (%) | 16 (9.8) | 13 (23.6) | 0.009 | 10 (6.9) | 21 (18.6) | 0.004 |
| Laboratory tests | ||||||
| Hemoglobin, g/dL | 4.0 (3.1, 4.6) | 3.8 (3.4, 4.3) | 0.942 | 7.2 (5.7, 9.0) | 6.0 (4.8, 7.9) | 0.0002 |
| Glucose, mmol/L | 6.8 (5.0, 8.2) | 5.3 (4.1, 7.2) | 0.002 | 6.9 (5.4, 9.6) | 6.1 (4.6, 4.6) | 0.032 |
| Lactate, mmol/L | 4.6 (2.8, 7.7) | 5.5 (3.6, 9.5) | 0.052 | 3.3 (2.0, 6.1) | 4.3 (2.8, 8.0) | 0.003 |
| WBC, × 103/μL | 11.2 (8.2, 15.7) | 12.3 (9.2, 22.3) | 0.117 | 8.3 (6.3, 12.3) | 10.8 (7.9, 17.4) | 0.0008 |
| Platelet, × 103/μL | 155 (95, 242) | 133 (82, 197) | 0.179 | 62 (35, 112) | 51 (33, 109) | 0.225 |
| Total bilirubin, mg/dL | 1.1 (0.6, 1.9) | 1.5 (0.9, 2.9) | 0.007 | 1.4 (0.8, 2.0) | 2.0 (1.0, 3.6) | 0.0001 |
| Lactate dehydrogenase (LDH), U/L | 728 (591, 879) | 990 (754, 1510) | < 0.00001 | 712 (537, 906) | 1090 (797, 1513) | < 0.00001 |
| Plasma albumin, g/dL | 2.6 (2.4, 3.0) | 2.6 (2.3, 3.2) | 0.737 | 2.6 (2.3, 3.0) | 2.7 (2.4, 2.9) | 0.467 |
| Sodium, mmol/L | 134 (132, 136) | 135 (132, 137) | 0.383 | 132 (128, 135) | 133 (129, 136) | 0.180 |
| Peripheral parasite density, parasites/uL | 34,680 (10,435, 133,980) | 40,070 (6085, 138,470) | 0.868 | 50,820 (13,910, 287,835) | 42,140 (8000, 249,560) | 0.338 |
| Plasma PfHRP2, ng/mL | 886 (326, 2344) | 1415 (382, 3453) | 0.151 | 2244 (655, 3965) | 4394 (2208, 7469) | < 0.00001 |
| Creatinine, mg/dL | 0.31 (0.26, 0.38) | 0.53 (0.48, 0.67) | < 0.00001 | 0.33 (0.29, 0.39) | 0.58 (0.49, 0.78) | < 0.00001 |
| BUN, mg/dL | 11 (8, 16) | 22 (15, 30) | < 0.00001 | 13 (10, 17) | 26 (18, 41) | < 0.00001 |
| Composite disease severity score | ||||||
| Number of severity criteria2 | 3 (2, 3) | 3 (2, 4) | 0.0029 | 4 (3, 4) | 4 (3, 5) | 0.0004 |
| Nephrotoxic medication history3 | ||||||
| NSAIDs4 | 12 (7.3) | 3 (5.5) | 0.224 | 15 (10.2) | 15 (13.3) | 0.442 |
| Gentamicin | 2 (1.2) | 1 (1.8) | 1.000 | 5 (3.4) | 5 (4.4) | 0.671 |
| Any nephrotoxic medication5 | 14 (8.5) | 4 (7.3) | 1.000 | 19 (12.9) | 19 (16.8) | 0.379 |
| Number of nephrotoxic medications6 | ||||||
| 0 | 148 (91.4) | 50 (92.6) | 0.835 | 127 (87.6) | 98 (82.9) | 0.569 |
| 1 | 13 (8.0) | 4 (7.4) | 17 (11.7) | 18 (16.2) | ||
| 2 | 1 (0.6) | 0 (0.0) | 1 (0.7) | 1 (0.9) | ||
Continuous measures presented as median (interquartile range) unless otherwise indicated. Continuous measures compared using the Wilcoxon rank-sum test. Count measures compared using the Pearson’s chi-square or Fisher’s exact, as appropriate
1Severe dehydration (n = 14) was identified by the presence of sunken eyes (n = 12) or decreased skin turgor (n = 4), hemoglobinuria defined as tea-colored urine on microscopy without red blood cells
2Number of WHO criteria for severe malaria present (description in Additional file 1: Table S1)
3AKI was retrospectively assessed on stored blood samples, and data on AKI was not available on admission
4Non-steroidal anti-inflammatory drug (NSAID), included ibuprofen (n = 5), acetylsalicylic acid (n = 5), and diclophenac (n = 35)
5NSAID or gentamicin
6Sum of ibuprofen, acetylsalicylic acid, diclophenac, and gentamicin
Children in coma underwent an eye exam using indirect ophthalmoscopy. AKI was associated with an increase in the frequency of peripheral and macular whitening, the frequency and severity of retinal hemorrhages, and the number of retinopathy categories positive (Additional file 1: Table S8-S9). Following adjustment for retinopathy risk factors [20] and multiple comparisons, AKI was associated with peripheral and macular whitening and the number of retinopathy categories positive with aOR 2.60 (95% CI 1.20, 5.21), 2.82 (1.49, 5.30), and 2.30 (1.36, 3.89), respectively. AKI was not significantly associated with vessel color changes or papilloedema (p > 0.05). To explore whether AKI was associated with changes in blood-brain barrier integrity, we evaluated albumin concentrations in the CSF of children with CM who had a lumbar puncture to rule out meningitis. A one-unit increase in the natural log of cerebrospinal fluid albumin was associated with an aOR of 2.78 (95% CI 1.56, 4.93; p < 0.001) for having AKI on admission.
Discussion
Acute kidney injury (AKI) is a well-established complication of severe malaria in adults [13], but only recently it has been recognized as a common complication in children with severe malaria [10]. Prior studies established an association between AKI and mortality in severe malaria [9–13]. The present study confirms a strong association of AKI with mortality in children, with over 60% of deaths occurring in children with AKI. This study also demonstrated an association between AKI and persistent neurologic deficits in children with CM, and long-term neurocognitive impairment in children with CM and SMA. Further, this study suggests that AKI in children with severe malaria is associated with CKD. Together, the results show that AKI is a common complication of severe malaria in African children associated with major adverse long-term health outcomes.
The etiology of AKI in pediatric severe malaria is generally attributed to reduced renal perfusion, and this study supports prerenal azotemia as a presenting etiology of AKI. Prerenal azotemia was not associated with clinical measures of dehydration in this cohort apart from an elevated heart rate. However, children with prerenal azotemia had higher parasite density and biomass, consistent with impaired tissue perfusion related to microvascular sequestration of parasitized erythrocytes. Fluid management was conservative with the majority of children receiving intravenous quinine with blood transfusion and maintenance fluids in the first 24 h of hospitalization (Table 2). The relationship between AKI and mortality in the present study does not appear to be related to aggressive fluid replacement therapy. Questions remain regarding the most appropriate and safe approach to fluid resuscitation in the context of severe malaria complicated by severe anemia and impaired tissue perfusion [21, 22], particularly in settings of severe resource constraints where nursing care is limited and mechanical ventilation is unavailable.
AKI was associated with the presence, severity, and extent of retinopathy and the presence of coma and blood-brain barrier impairment in this study. We hypothesize microvascular changes in the retina and brain are associated with generalized systemic microvascular changes in severe malaria. Parasite biomass was related to prerenal injury, consistent with studies in adults with severe malaria-associated AKI [23]. Ultrastructural examination of kidney tissue of adults with fatal malaria reveals parasite sequestration in glomerular and tubulointerstitial vessels and monocyte accumulation in glomerular capillaries [24]. In children, renal sequestration is less frequent, but still occurs, and may potentially contribute to AKI [8].
Histologic findings of malaria pigment in distal convoluted tubules [8] are consistent with the reports of intravascular hemolysis and increased cell-free hemoglobin and heme in malaria-associated AKI [25, 26]. In the present study, LDH was strongly associated with AKI, supporting the hypothesis that hemolysis contributes to oxidative stress and pigment nephropathy. This is further supported by increased bilirubin, another marker of hemolysis, and increased frequency of hemoglobinuria in AKI. A recent clinical trial showed a renoprotective effect of acetaminophen in adults with severe malaria and intravascular hemolysis [27]. Retrospective data from pediatric cardiac surgery suggest early postoperative acetaminophen reduces hemoglobin-mediated oxidative stress and AKI [28]. The present study supports a role for hemolysis and increased cell-free hemoglobin in pediatric AKI and highlights the need for renoprotective adjunctive therapies to reduce the incidence and severity of AKI in pediatric malaria.
In this study, AKI was associated with CKD at 1-year follow-up, consistent with a growing body of evidence that AKI is a risk factor for short-term [29] and long-term CKD and end-stage kidney disease in children [30]. Although AKI can result in persistent and progressive renal dysfunction, even complete recovery is associated with subsequent risk of developing CKD [15]. For example, between 8 and 61% of children who recovered baseline renal function following hemolytic uremic syndrome developed renal complications within 5 to 10 years [31]. The present study is the first to report an association between AKI and CKD in the context of severe malaria. We are currently planning to follow up the study participants to re-evaluate kidney function 5 to 12 years after severe malaria.
Distant organ injury in AKI is well-described, and neurologic complications including central nervous system dysfunction, decreased mental status, and seizures are associated with AKI [32]. The presence of mild-to-moderate CKD is associated with deficits in academic achievement, executive function, and visual and verbal memory [33], but to our knowledge, this is the first report of an association between AKI and neurocognitive impairment. Well-recognized risk factors for neurocognitive impairment in severe malaria relate to neurological complications on admission and inflammatory and neuroactive metabolites in cerebrospinal fluid [2–5, 34–36]. Increased cerebrospinal fluid albumin in children with CM and AKI suggests blood-brain barrier impairment. Many processes known to be important in cognitive impairment, including endothelial dysfunction, oxidative stress, and inflammation, also relate to AKI [32]. The relationship of AKI to neurocognitive impairment could reflect the contribution of these processes to both clinical entities, or reflect an independent role for AKI in neurocognitive impairment, through mechanisms still to be defined.
In the present study, the frequency of CKD was higher in children treated with artemisinin derivatives, though with the small number of children with CKD, these differences did not approach statistical significance. This study highlights the need for additional studies to assess CKD in children treated with artemisinin derivatives. The association between AKI and mortality and neurocognitive impairment was seen in children treated with either drug, so this association occurs independently of severe malaria treatment.
Strengths of the study include its sample size, rigorous clinical definitions, prospective design, and careful neurocognitive follow-up. Including the community controls to estimate premorbid creatinine likely increased our ability to detect AKI. Further, our findings are consistent irrespective of the method used to define premorbid creatinine, suggesting an epidemiologic approach is valid when premorbid creatinine measurements or community controls are unavailable. The study is the first to assess the relationship between AKI and CKD in malaria, and the largest pediatric study to date to assess post-AKI CKD.
Among the study limitations are the retrospective definition of AKI, using a single measurement of creatinine on admission and estimating pre-illness creatinine from community control data. The study definition likely underestimates AKI [37]. Further investigation, including detailed urinalysis and renal biopsy findings, is also needed to understand the pathophysiology of AKI in children with severe malaria. Additional studies are needed to evaluate AKI in low-resource settings to understand whether the findings in this paper are generalizable to all critically ill hospitalized children or specific to severe malaria-associated AKI.
Conclusions
In summary, the present study supports a growing body of evidence that AKI is a risk factor for long-term morbidity and mortality in malaria, providing new evidence that AKI is a risk factor for sustained neurocognitive impairment in survivors. Further, this study suggests that children with severe malaria-associated AKI may be at risk of developing CKD. This has far-reaching implications for the burden of non-communicable disease in Uganda, where the prevalence of CKD is estimated at 18.1% [38] and renal replacement therapy is not available in most areas. To make progress towards the International Society of Nephrology initiative to eliminate preventable deaths from AKI worldwide by 2025 (0by25) [39], a concerted effort to improve diagnosis, and management, of AKI in resource-constrained malaria-endemic settings is urgently needed.
Additional file
I. Methods. Supplementary methods describing the study population and additional details on assessment of disease severity, kidney function, retinopathy, neurocognitive evaluation, and statistical analysis. II. Results. Relationship between AKI and nephrotoxic medication use during hospitalization. III. Supplementary tables. Table S1-S13. (DOCX 89 kb)
Acknowledgements
We thank the children and their parents who participated in this study and the study team for their dedicated effort in treating the children and collecting the data.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke and the Fogarty International Center (grants R01NS055349 and D43 NS078280).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKI
Acute kidney injury
- CC
Community children
- CI
Confidence interval
- CKD
Chronic kidney disease
- CM
Cerebral malaria
- eGFR
Estimated glomerular filtration rate
- HOME
Home Observation for the Measurement of the Environment
- HR
Hazards ratio
- LDH
Lactate dehydrogenase
- OR
Odds ratio
- SMA
Severe malarial anemia
Authors’ contributions
ALC performed the literature search, data analysis and interpretation, and wrote the first draft of the manuscript. ROO, PB, and RI contributed to the study design, data collection, data interpretation and writing of the manuscript. JMS contributed to the data collection, data analysis and interpretation, and writing of the manuscript. JSH contributed to the study design, data analysis and interpretation, and writing of the manuscript. DD contributed to the data collection, data interpretation, and writing of the manuscript. CM contributed to the data analysis, data interpretation, and writing of the manuscript. CCJ designed the primary study and contributed to the data collection, data interpretation, and writing of the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from the parents/guardians of the study participants. Ethical approval was granted by the Institutional Review Boards at Makerere University School of Medicine (SOMREC, reference number: 2008-033; date of first approval: April 7, 2008) and the University of Minnesota (IRB Code Number: 0808 M27022; date of first approval: March 31, 2008). The study was also approved by the Uganda National Council for Science and Technology (UNCST, reference number: HS432; date of first approval: May 16, 2008).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Andrea L. Conroy, Email: conroya@iu.edu
Robert O. Opoka, Email: opokabob@yahoo.com
Paul Bangirana, Email: pbangirana@yahoo.com.
Richard Idro, Email: ridro1@gmail.com.
John M. Ssenkusu, Email: jssenkusu@musph.ac.ug
Dibyadyuti Datta, Email: ddatta@iu.edu.
James S. Hodges, Email: hodge003@umn.edu
Catherine Morgan, Email: cmorgan@ualberta.ca.
Chandy C. John, Email: chjohn@iu.edu
References
- 1.WHO . World malaria report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.John CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics. 2008;122(1):e92–9. [DOI] [PMC free article] [PubMed]
- 3.Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, John CC. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics. 2007;119(2):e360–e366. doi: 10.1542/peds.2006-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangirana P, Opoka RO, Boivin MJ, Idro R, Hodges JS, Romero RA, Shapiro E, John CC. Severe malarial anemia is associated with long-term neurocognitive impairment. Clin Infect Dis. 2014;59(3):336–44. [DOI] [PMC free article] [PubMed]
- 5.Holding PA, Stevenson J, Peshu N, Marsh K. Cognitive sequelae of severe malaria with impaired consciousness. Trans R Soc Trop Med Hyg. 1999;93(5):529–534. doi: 10.1016/S0035-9203(99)90368-1. [DOI] [PubMed] [Google Scholar]
- 6.Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002;23(5):353–364. doi: 10.1097/00004703-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332(21):1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 8.Milner DA, Jr, Whitten RO, Kamiza S, Carr R, Liomba G, Dzamalala C, Seydel KB, Molyneux ME, Taylor TE. The systemic pathology of cerebral malaria in African children. Front Cell Infect Microbiol. 2014;4:104. doi: 10.3389/fcimb.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Seidlein L, Olaosebikan R, Hendriksen IC, Lee SJ, Adedoyin OT, Agbenyega T, Nguah SB, Bojang K, Deen JL, Evans J, et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis. 2012;54(8):1080–1090. doi: 10.1093/cid/cis034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conroy AL, Hawkes M, Elphinstone RE, Morgan C, Hermann L, Barker KR, Namasopo S, Opoka RO, John CC, Liles WC, et al. Acute kidney injury is common in pediatric severe malaria and is associated with increased mortality. Open Forum Infect Dis. 2016;3(2):ofw046. doi: 10.1093/ofid/ofw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imani PD, Odiit A, Hingorani SR, Weiss NS, Eddy AA. Acute kidney injury and its association with in-hospital mortality among children with acute infections. Pediatr Nephrol. 2013;28(11):2199–2206. doi: 10.1007/s00467-013-2544-2. [DOI] [PubMed] [Google Scholar]
- 12.Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, ter Kuile FO, Craddock C, Berry C, Holloway PA, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21(3):577–587. doi: 10.1093/clinids/21.3.577. [DOI] [PubMed] [Google Scholar]
- 13.Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, Than M, Htut Y, Mohanty S, Yunus EB, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis. 2008;47(2):151–157. doi: 10.1086/589287. [DOI] [PubMed] [Google Scholar]
- 14.Sypniewska P, Duda JF, Locatelli I, Althaus CR, Althaus F, Genton B. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med. 2017;15(1):147. doi: 10.1186/s12916-017-0906-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21(6):628–634. doi: 10.1097/MNH.0b013e3283588f24. [DOI] [PubMed] [Google Scholar]
- 16.Park GS, Opoka RO, Shabani E, Wypyszynski A, Hanisch B, John CC. Plasmodium falciparum histidine-rich protein-2 plasma concentrations are higher in retinopathy-negative cerebral malaria than in severe malarial anemia. Open Forum Infect Dis. 2017;4(3):ofx151. doi: 10.1093/ofid/ofx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.KDIGO Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. doi: 10.1038/kisup.2012.1. [DOI] [Google Scholar]
- 18.KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3(1):1–150. [DOI] [PubMed]
- 19.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villaverde C, Namazzi R, Shabani E, Opoka RO, John CC. Clinical comparison of retinopathy-positive and retinopathy-negative cerebral malaria. Am J Trop Med Hyg. 2017;96(5):1176–84. [DOI] [PMC free article] [PubMed]
- 21.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–95. [DOI] [PubMed]
- 22.Hanson J, Anstey NM, Bihari D, White NJ, Day NP, Dondorp AM. The fluid management of adults with severe malaria. Crit Care. 2014;18(6):642. doi: 10.1186/s13054-014-0642-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plewes K, Royakkers AA, Hanson J, Hasan MM, Alam S, Ghose A, Maude RJ, Stassen PM, Charunwatthana P, Lee SJ, et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malar J. 2014;13:91. doi: 10.1186/1475-2875-13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguansangiam S, Day NP, Hien TT, Mai NT, Chaisri U, Riganti M, Dondorp AM, Lee SJ, Phu NH, Turner GD, et al. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Tropical Med Int Health. 2007;12(9):1037–1050. doi: 10.1111/j.1365-3156.2007.01881.x. [DOI] [PubMed] [Google Scholar]
- 25.Elphinstone RE, Conroy AL, Hawkes M, Hermann L, Namasopo S, Warren HS, John CC, Liles WC, Kain KC. Alterations in systemic extracellular heme and hemopexin are associated with adverse clinical outcomes in Ugandan children with severe malaria. J Infect Dis. 2016;214(8):1268–1275. doi: 10.1093/infdis/jiw357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plewes K, Kingston HWF, Ghose A, Maude RJ, Herdman MT, Leopold SJ, Ishioka H, Hasan MMU, Haider MS, Alam S, et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect Dis. 2017;17(1):313. doi: 10.1186/s12879-017-2373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plewes K, Kingston HWF, Ghose A, Wattanakul T, Hassan MMU, Haider MS, Dutta PK, Islam MA, Alam S, Jahangir SM, et al. Acetaminophen as a renoprotective adjunctive treatment in patients with severe and moderately severe falciparum malaria: a randomized, controlled, open-label trial. Clin Infect Dis. 2018:ciy213. [DOI] [PMC free article] [PubMed]
- 28.Van Driest SL, Jooste EH, Shi Y, et al. Association between early postoperative acetaminophen exposure and acute kidney injury in pediatric patients undergoing cardiac surgery. JAMA Pediatr. 2018;172(7):655–663. doi: 10.1001/jamapediatrics.2018.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 30.Calderon-Margalit R, Golan E, Twig G, Leiba A, Tzur D, Afek A, Skorecki K, Vivante A. History of childhood kidney disease and risk of adult end-stage renal disease. N Engl J Med. 2018;378(5):428–438. doi: 10.1056/NEJMoa1700993. [DOI] [PubMed] [Google Scholar]
- 31.Garg AX, Suri RS, Barrowman N, et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA. 2003;290(10):1360–1370. doi: 10.1001/jama.290.10.1360. [DOI] [PubMed] [Google Scholar]
- 32.Nongnuch A, Panorchan K, Davenport A. Brain-kidney crosstalk. Crit Care. 2014;18(3):225. doi: 10.1186/cc13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Didsbury M, van Zwieten A, Howell M, Kim S, Tong A, Howard K, Nassar N, Barton B, Lah S, et al. Neurocognitive and educational outcomes in children and adolescents with CKD: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2018;13(3):387–397. doi: 10.2215/CJN.09650917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangirana P, Musisi S, Boivin MJ, Ehnvall A, John CC, Bergemann TL, Allebeck P. Malaria with neurological involvement in Ugandan children: effect on cognitive ability, academic achievement and behaviour. Malar J. 2011;10:334. doi: 10.1186/1475-2875-10-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holmberg D, Franzén-Röhl E, Idro R, Opoka RO, Bangirana P, Sellgren CM, Wickström R, Färnert A, Schwieler L, Engberg G, et al. Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar J. 2017;16(1):303. doi: 10.1186/s12936-017-1954-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John CC, Panoskaltsis-Mortari A, Opoka RO, Park GS, Orchard PJ, Jurek AM, Idro R, Byarugaba J, Boivin MJ. Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. Am J Trop Med Hyg. 2008;78(2):198–205. doi: 10.4269/ajtmh.2008.78.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, for the AI Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, Patel U. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 39.Mehta RL, Cerdá J, Burdmann EA, Tonelli M, García-García G, Jha V, Susantitaphong P, Rocco M, Vanholder R, Sever MS, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
I. Methods. Supplementary methods describing the study population and additional details on assessment of disease severity, kidney function, retinopathy, neurocognitive evaluation, and statistical analysis. II. Results. Relationship between AKI and nephrotoxic medication use during hospitalization. III. Supplementary tables. Table S1-S13. (DOCX 89 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.