Abstract
Nearly 10 years ago, Frank Gupton and I had a chance encounter where we learned that we both had interest in creating efficient routes to generic medicines. Lucky for me, Frank and I began mentoring each other—Frank teaching me wisdom gained from 30 years in industry and I sharing what I knew about the academic world. As some who know Frank will understand, his lessons in some cases seem devilishly obvious and should be chanted daily like a zen koan while others are subtle and complex, requiring many years of reflection. Frank was asked by OPR&D to offer these lessons in a condensed form so that others might learn what I have. Frank and I decided that a transcript of our many conversations condensed into one document might do the trick.
Introduction
McQuade: Frank, why should academics and industrial chemists/engineers concern ourselves with efficient chemical processes? When you asked me to help you encapsulate our mentor/mentee conversations, I was skeptical that readers would be receptive. I worry that the academics will assume that efficient routes are the province of the industrial chemist and the industrial chemist might feel that we have little to teach them.
Gupton: Tyler, I am glad that you are willing to help, and as usual I am glad that you expressed your reservations. I can always count on you to give it to me straight. Much of what we will discuss below, when taken in isolation, could suffer the criticisms you identify. That being said, the real key here is that our conversation and the approaches we are using/developing in our Medicines for All (M4All) Institute are integrating a holistic approach to creating efficient processes.1 I think that academics can benefit from our message by using our approach to focus their synthetic tools toward active ingredient synthesis in the same way that they have done so with natural products. Creating a more efficient medicine synthesis compared with the state of the practice is hard because the molecules we are often working on are compact. Often entirely new methods and disconnection strategies are required. Herein lies the academic opportunity.
On the other hand, industrial process chemists/engineers might find two sources of benefit from this conversation. The first is affirmation that what modern process chemists/engineers do is increasingly difficult, as cost, safety, and regulation must be balanced while building the process rapidly. The second is that our holistic approach brings themes together that are not always combined. Some of the lessons below will be familiar, but others might not. The benefit then to the industrialist is that we are trying to build a systematic approach that when followed might enable efficient routes to be constructed faster. That both academics and industrialists may embrace our simplicity is a beautiful approach.
McQuade: A process chemist should seek beauty?
Gupton: Tyler, we need to define beauty in this case. In the M4All Institute, we define a beautiful route as one that is low-cost compared with state of practice, easy to transfer, and green. Besides beauty, an efficient process enables many outstanding outcomes. For example, access to affordable medications is a pressing issue in developing countries. For a handful of critical medicines, active pharmaceutical ingredient (API) synthesis represents the most financially important and technically demanding element of pharmaceutical operations.
McQuade: Developing an efficient process might seem simple to the academics and very hard to the industrial readers. How can we reconcile these discordant viewpoints?
Gupton: Creating a streamlined synthesis2 does seem straightforward until you start making lists of possible disconnections and then ranking these possibilities using cost of goods calculations, estimates of implementation, and potential for route consolidation. As we worked through our next-generation synthesis of nevirapine, the first non-nucleoside reverse transcriptase inhibitor used in the treatment of HIV, our core principles became clear to me.3 These principles include:
-
(a)
implementation of innovative chemical methodologies and new manufacturing platforms;
-
(b)
consolidation of high-yielding reactions into a minimal number of unit operations with common solvents and limited intermediate isolations to reduce/eliminate waste;
-
(c)
vertical integration of advanced starting materials prepared from commodity chemicals.
Individually, each principle is easy to understand and implement—the academics are correct. However, when one tries to use each of the principles on the same process, life becomes more difficult—the industrialists are correct. The difficulty is revealed by measuring the cost of goods used and process mass intensity (PMI).4 These measurements are made before a route is attempted and once a route starts to take shape in the laboratory. Measuring often enables one to evaluate successful application of these principles. Sure, one can use a new manufacturing platform and claim success if using the platform is the goal. Success is often much harder to claim if lower cost and PMI are the metrics.
However, there are nuances with both of these metrics that must be clarified and aligned with the objectives of M4All. Because of the significant variability in certain components of manufacturing costs (depreciation, labor, etc.) between suppliers, we have elected to restrict our cost calculations to cost of goods so that we can effectively benchmark against the current state of the art. Likewise, when calculating PMI we place value on solvent recycling because of its financial impact on the cost of goods in addition to environmental considerations. Before we continue this discussion, let’s discuss the framework we use at the M4All Institute to help us develop routes that are optimized with regard to our principles.
The Medicines for All Framework
McQuade: Frank, I think that our core principles and use of measurements to assess success will not wow either side of the audience that we are trying to reach. A process that enables lower cost and more efficient routes to be discovered faster might pique our audience’s interest, however.
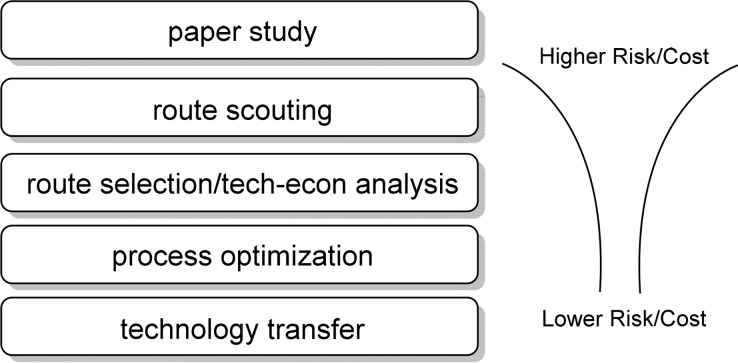
Gupton: Tyler, I totally agree. Our M4All team members use a five-step process that we are continuously refining; this process is shown in Figure 1. These steps are obvious, but when used together, they yield some outstanding results. The first step in the path, “paper study”, involves collecting all known information one can gather regarding a desired target. Gathering literature and patent reports that directly or indirectly relate to the target is critical. The good news is that search engines such as Google Patents, SciFinder, and Web of Science facilitate this knowledge-gathering stage. We have also dipped our toes into the machine learning pool to help identify new synthetic routes but have found the current costs to be too high to proceed.5
Figure 1.
M4All five-step process to guide us toward our objectives.
From this data collection exercise, our team then works together to catalog known routes and to create new possible approaches to our target. Using measurement tools (i.e., cost and PMI), one can rank both known and new possibilities. For the known routes, the most costly or inefficient steps can be targeted for potential improvement, and for the new possibilities, the steps that represent the highest risk can be identified. In addition, key chemical transformations and opportunities (collectively known as key unit operations) to use advanced manufacturing tools can be identified.
The highest-ranking routes and their key unit operations are then assessed in the next step—the route-scouting phase. Here experiments are designed to reduce the unknown to the known. Once the uncertainty is reduced, we redo the cost and PMI measurements—the “route selection and techno-economic” phase. The most efficient routes are then passed to the process optimization phase. Once optimized, the routes exhibiting the most promising cost and PMI are then reproduced, and the collected data are synthesized into technology transfer documents.
McQuade: Frank, you seem to imply that you might create multiple technology transfer documents? Aren’t we shooting for a single optimized process?
Gupton: Creating multiple routes or subroutes might seem wasteful or an unnecessary luxury, but the world is an uncertain place. Creating multiple optimized routes is a risk reduction strategy. Today’s low-cost starting materials might become scarce. One transition partner might be basic in one starting material, and another might be basic in an entirely different starting material. Having multiple high-quality approaches yields the most robust outcome in our networked world. For example, some organizations possess core strengths in photochemistry while others have developed experience in continuous processing. We will discuss this issue in more detail later.
Our Approach to Manufacturing Platforms
McQuade: Frank, I have learned from you that manufacturing platforms are an important consideration when implementing a process on scale. When we started working together, I was a flow chemistry devotee. In our M4All process algorithm shown in Figure 1, we do not explicitly define a stage where manufacturing platforms are considered. Why don’t we have a stage where platforms are explicitly considered?
Gupton: Manufacturing platforms are tools.6 Each has value depending on the context. Whether flow, batch, a combination, or some totally new modality, I want our team to be open to exploring the best combination of tools to achieve the lowest cost, ease of technology transfer, and greenness. We perform chemistry in a wide variety of tools from batch reactors to continuous tank reactors to tubular reactors to wiped film reactors to continuous extractors and so on. We use the optimum tool for the given route. We also try to develop routes that can be implemented using more than one platform. Again, we increase the chances of our process being adopted by partners if we can offer them a fit for whichever platforms they use or have excess capacity.
We also believe that continuous processing methods hold great promise for the pharmaceutical industry, but there is much more work to be done in this area that currently limits its utility.7 Many batch pharma processes are carried out at high concentrations that produce solids and often thick slurries, which is a very good outcome from a process intensification perspective. However, these phases are difficult to translate into flow because of the narrow channels that are used in many flow reactors.8 It is also interesting to note that in general we are more focused on the “chemistry side” of the process, but we also need substantial effort to be placed on the downstream steps like continuous filtration and drying operations.9 This is really the exciting part of our job at the M4All Institute because all of these issues require both chemistry and engineering skills to address these opportunities and we have created a culture in this setting that embraces both.
McQuade: But don’t the tool and the chemistry need to be matched?
Gupton: Yes and no. By leveraging multiple manufacturing platforms, we stress-test the individual unit operations and the overall route. Forcing a route into a continuous platform, we often define a single solvent system that can reduce PMI. That doesn’t preclude the final route being conducted in a batch mode. The ideal output of an M4All effort will be flexible, enabling the route to be implemented in the most cost-effective final manufacturing platform. In our nevirapine effort, we created both batch and continuous routes for both key intermediates and for the registered FDA steps.3a
McQuade: Some who wish to follow our approach may not have the resources to create all possible routes. What do you recommend in this case?
Gupton: Find new chemistry and/or platforms that reduce the solvent volumes and exchanges.10 While we have only published our nevirapine results, we are working through manuscripts on five other medicines, and in all of those cases, solvent volume reductions and removal of exchanges had the single largest impact on cost and efficiency. In every case, we were able to identify “low-hanging fruit” that allowed us to significantly reduce both the cost and the PMI. I’m sure that this may seem obvious to most, but having a high-yielding reaction was the single most important feature that led to the improvements.
Starting Materials, Intermediates, and Active Ingredients Considerations
McQuade: Frank, we have discussed an algorithm to yield processes that optimize each of our guiding principles (Figure 1) and also discussed manufacturing platforms, but the core principles emphasize starting materials, intermediates, and active ingredients. How do raw materials, intermediates, and final products influence how we proceed?
Gupton: Tyler, this is a complex question. For the pharmaceutical industry, the intermediates and the bond-forming steps leading to the target are regulatory defined steps. When creating a new, more cost-effective and efficient route, leaving the existing regulatory steps constant will decrease the cost of implementation. Let’s consider our recently reported nevirapine synthesis. We combine a rotating disk reactor, a continuous stirred tank reactor, and a packed-bed reactor to perform the same bond-forming steps that are already featured in the FDA-approved route to nevirapine. For this endgame, we emphasized our first two guiding principles. Recognizing this regulatory constraint helped us get the process implemented by one of our industrial partners quickly.
McQuade: So this route into nevirapine (Scheme 1) demonstrates the application of the first two core principles, the first being the use of new methods and manufacturing platforms. The new method uses a sodium hydride column to convert the intermediate resulting from the combination of CAPIC and MeCAN into nevirapine, and the continuous process begins with a spinning disk reactor followed by continuous stirred tank and packed-bed reactors, demonstrating our use of new manufacturing platforms. The system also uses a single solvent system from start to finish, all with high yield—our application of the second core principle.
Scheme 1. Final Two Steps Leading from Registered Starting Materials to the Drug Substance Nevirapine; Here We Used Novel Manufacturing Platforms To Achieve Process Intensification.
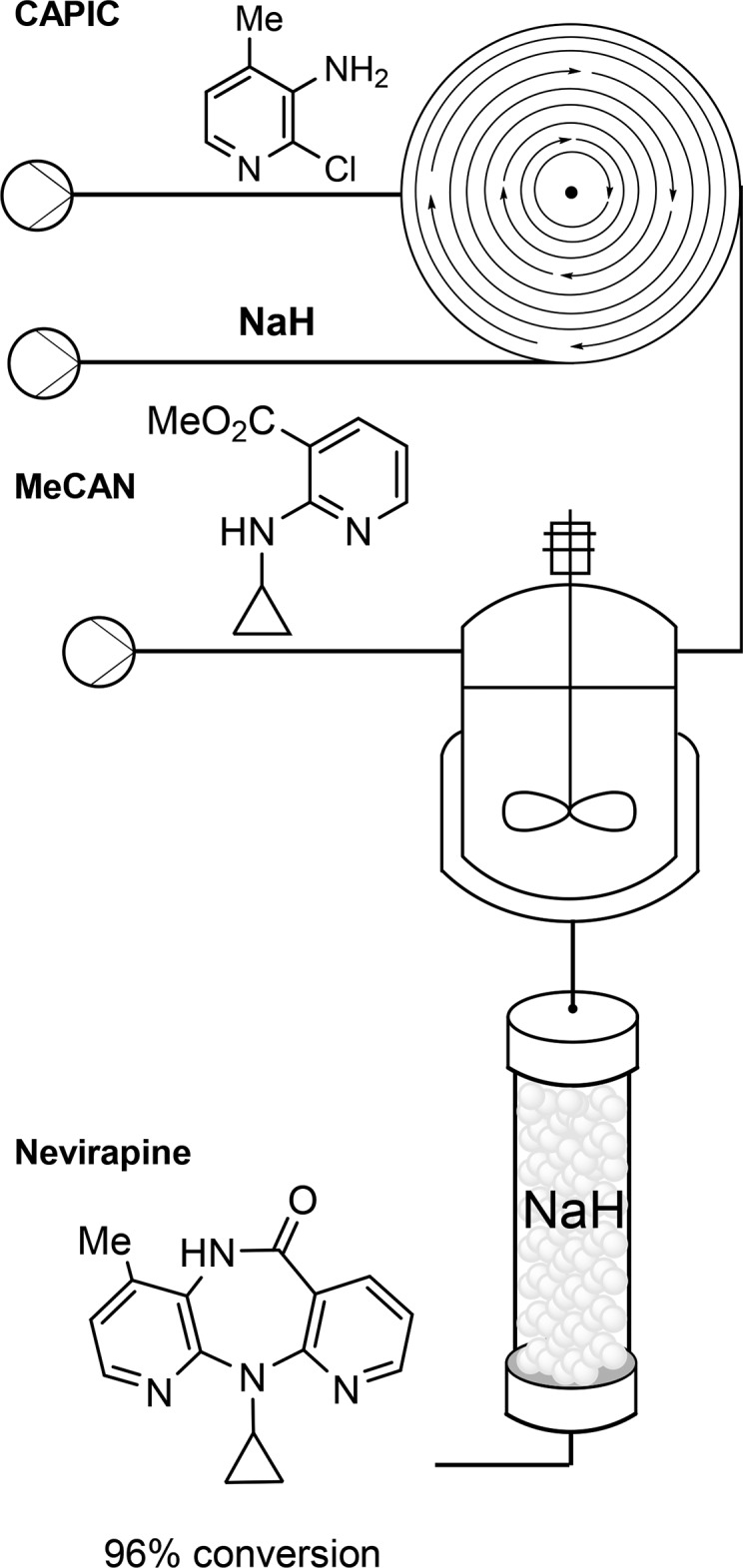
Gupton: Exactly.
McQuade:Scheme 1 clearly illustrates the application of the first two principles, but I could use a refresher on the third core principle. How does vertical integration fit in? If we start from commodity chemicals, won’t we have to submit a new regulatory filing as opposed to an abbreviated new drug application?
Gupton: The third principle does encourage preparation of advanced starting materials from commodity chemicals. The key is to define the advanced starting materials. Routes leading up to and including the registered raw materials can be completely redefined without triggering a new regulatory filing. Scheme 2 shows one of the registered starting materials for nevirapine, denoted as CAPIC. When considering new routes leading to registered starting materials, we look for methods that can transform commodity chemicals such as basic gases (CO, ethylene, acetylene) and high-volume condensed materials such as acetone into our desired intermediates.
Scheme 2. Prior to Our M4All Work on Nevirapine, the State-of-the-Art Starting Materials Were Low-Volume, High-Cost Molecules; We Developed an Efficient Approach That in a Few Steps Realizes CAPIC from Acetone.
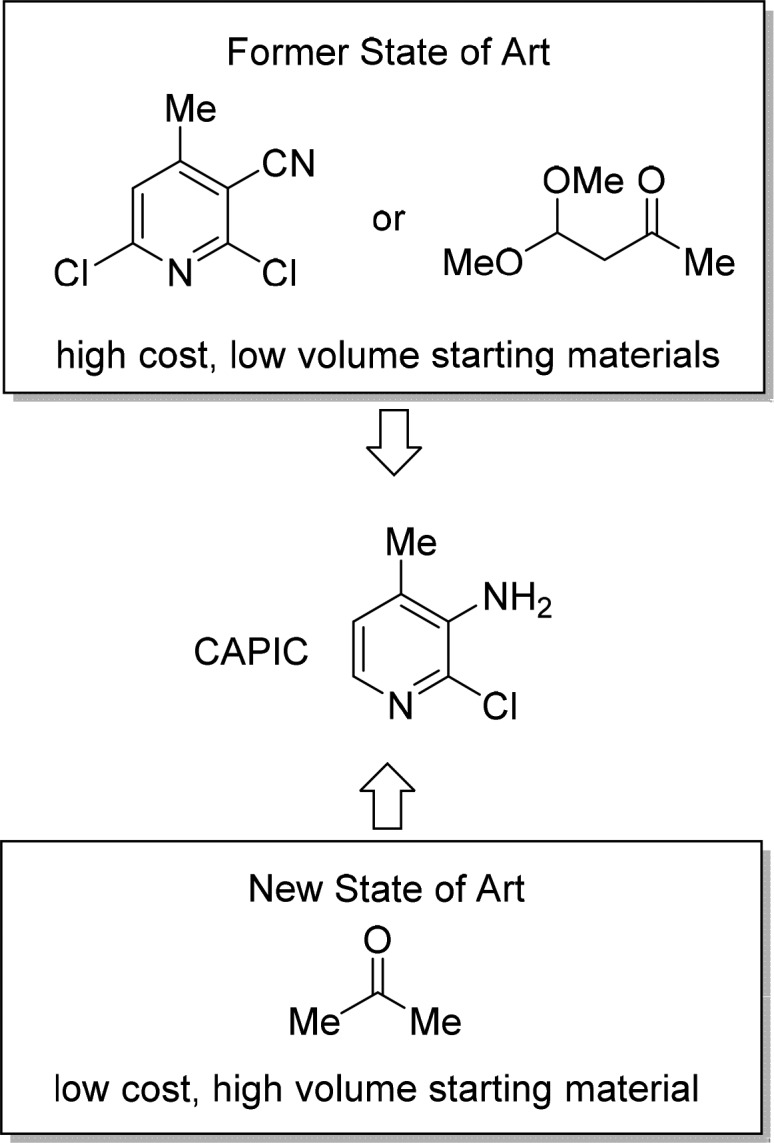
McQuade: Building complexity in the shortest possible route is a concept that both academic and industrial community members should get excited about.11
Gupton: Absolutely—this is where new bond construction strategies and catalysts can really shine. We just need to keep the reagent and ligand costs in check. It is also important to keep in mind that FDA registered starting materials become major cost drivers as APIs mature in commercial operations and move toward generic status.12
McQuade: By emphasizing the registered starting material concept, are you implying that I should discard all routes that do not pass through these intermediates when defining a new route into an active ingredient?
Gupton: New routes that facilitate cost and PMI efficiencies while retaining a path through the registered starting materials will be easier to implement for potential transition partners. That being said, one should never discard alternative routes. One must merely measure them against routes that have less regulatory headwind. An entirely new route might have other advantages such as intellectual property freedom. The bottom line is that we consider use of registered starting materials and intermediates when we rank possible routes to scout/optimize. This can lead to a more holistic strategy that would include both short-term and longer-term benefits.
Cost Models To Guide The Path
McQuade: Our M4All team members constantly use both cost and PMI measurements to help prioritize our efforts. While I think this metrics-driven approach is great, I do worry that there are some hidden issues. While PMI is fairly well defined, arriving at a reasonable cost estimate is challenging. Do you have some thoughts on how we and others can navigate the cost question?
Gupton: Developing a realistic cost model for a route is not as simple as one might expect. A high-quality cost model requires realistic costs for raw materials.13 We rely on phone calls to vendors, web surfing, and subscription service databases to gather our information. Taking the time (and money) to build a strong cost model or models is an essential first step for three reasons: (1) a high-quality cost model helps identify reactions/unit operations in existing manufacturing routes that are inefficient and might benefit from some process intensification; (2) cost models provide a baseline to which novel routes can be compared; and (3) developing approximate cost models for proposed routes is an essential step in prioritizing our route-screening efforts. Our initial cost model for nevirapine identified the registered starting material CAPIC as a major cost driver. We used this information to focus our attention on developing novel routes into CAPIC.
McQuade: Raw material costs fluctuate. How does the M4All team avoid getting buffeted by changing prices?
Gupton: A successful outcome of our approach is not a single route. In our view, a robust outcome is a portfolio of routes leading to the same active ingredient. For example, we often discuss a “make or buy” strategy. Let’s reflect on the nevirapine process again. The key starting materials are CAPIC and MeCAN. A robust process is one where these intermediates can be bought when costs are favorable or produced from multiple commodity starting materials when the intermediates are too expensive. By having multiple paths that lead to the same outcome, we can change paths depending on the emerging economic landscape. We have seen several examples with our current drug targets where disruptions in the supply chain have resulted in dramatic increases in starting material costs. Having the capacity to produce these molecules from simple building blocks really helps to mitigate the risk in this area.
Borrowing Lessons from Our Green Principles
McQuade: How does the M4All team apply green chemistry and engineering principles?14
Gupton: These principles are part of our DNA. We do not need to restate all of the principles here, but they are critical guidelines to help prioritize the routes we move from the paper-study stage to the route-selection stage. A few of the principles are worth underscoring. Our highest-ranked routes avoid protecting group installations and deprotections not because we are adverse to these tools but because routes that contain these steps rarely outcompete those that do not from a cost and PMI perspective. Reducing solvent usage and solvent costs is also very important and flow reactors can help by enabling process intensification. I believe that reduction in solvent usage in our processes is by far the single biggest contributor to lowering the PMI. Finally, we seek catalytic systems where low-cost metals are used or high-cost metals are recycled.
Summary
McQuade: Can you provide a summary of the M4All approach?
Gupton: Synthetic routes are like any technology; they have readiness levels. The discovery chemist will define a route from the most advanced commercial starting materials because the guiding metric is speed to find a lead. The next chemist in the value chain will evolve the discovery chemist’s route so as to enable reliable access to larger quantities, and the chemist tasked with creating a manufacturing route is forced to balance the identification of a low-cost manufacturing route with doing so in a short time. Although industrial chemists are enormously talented, the manufacturing routes they file are compromised by time constraints and quite often have not reached a peak level of readiness.
Achieving a commercial process that is optimized for both cost and environmental efficacy requires that the chemistry be evaluated from many starting positions and that regulatory considerations be evaluated and factored into the process. Cost and PMI are measurement tools that can help rank possible routes. A single ideal route should not be the overarching objective. Developing a portfolio of routes that begin from commodity raw materials and proceed through a set of stable intermediates not only enables the regulatory concerns to be addressed but also opens the possibility to work with nonactive ingredient producers to manufacture preregistered intermediates. Finally, we view success as a route that can be implemented in a number of manufacturing platforms. All of our efforts are oriented toward maximizing the ease of transfer of our technology from the laboratory to the factory, and because the majority of pharma investments are in batch operations, we need to give this some priority. However, by building in the capacity to also carry out these processes in a continuous mode, we have positioned these new synthetic methods for addition cost savings in the future.
Final Thoughts
McQuade: Frank, where do you think all of this is going?
Gupton: My hope is that M4All becomes a center of excellence that forges the next generation of outstanding process chemists.15 Medicine accessibility is correlated with how profitable the medicine is to sell. Keeping manufacturing costs as low as possible will incentivize more participants to enter the marketplace. The presence of more producers will ensure both lower costs and more robust supply. We want our colleagues training with us to be excellent on both the technical side and the cost/PMI measurement side. Elegance to us is a process that is simple, low-cost, and efficient.
Acknowledgments
We thank the Bill and Melinda Gates Foundation for their longstanding support of our collaboration and the jump start that we received from Clinton Health Access Initiative and Corning Inc. We thank Dr. David Snead for his careful reading of this missive.
The authors declare no competing financial interest.
References
- Leng R. B.; Emonds M. V. M.; Hamilton C. T.; Ringer J. W. Holistic Route Selection. Org. Process Res. Dev. 2012, 16, 415–424. 10.1021/op200264t. [DOI] [Google Scholar]
- For an example of a system for selecting synthetic routes, see the following three-part series:; a Parker J. S.; Moseley J. D. Kepner–Tregoe Decision Analysis as a Tool To Aid Route Selection. Part 1. Org. Process Res. Dev. 2008, 12, 1041–1043. 10.1021/op8000349. [DOI] [Google Scholar]; b Moseley J. D.; Brown D.; Firkin C. R.; Jenkin S. L.; Patel B.; Snape E. W. Kepner–Tregoe Decision Analysis as a Tool To Aid Route Selection .Part 2. Application to AZD7545, a PDK Inhibitor. Org. Process Res. Dev. 2008, 12, 1044–1059. 10.1021/op800033c. [DOI] [Google Scholar]; c Parker J. S.; Bower J. F.; Murray P. M.; Patel B.; Talavera P. Kepner–Tregoe Decision Analysis as a Tool To Aid Route Selection. Part 3. Application to a Back-Up Series of Compounds in the PDK Project. Org. Process Res. Dev. 2008, 12, 1060–1077. 10.1021/op8000355. [DOI] [Google Scholar]
- a Verghese J.; Kong C. J.; Rivalti D.; Yu E. C.; Krack R.; Alcázar J.; Manley J. B.; McQuade D. T.; Ahmad S.; Belecki K.; Gupton B. F. Increasing global access to the high-volume HIV drug nevirapine through process intensification. Green Chem. 2017, 19, 2986–2991. 10.1039/C7GC00937B. [DOI] [Google Scholar]; b Longstreet A. R.; Rivalti D.; McQuade D. T. Synthesis and Reactivity Profile of Ylidenemalononitrile Enamines and Their Ester Analogs Towards Electrophiles and Nucleophiles. J. Org. Chem. 2015, 80, 8583–8596. 10.1021/acs.joc.5b01169. [DOI] [PubMed] [Google Scholar]; c Longstreet A. R.; Campbell B. S.; Gupton B. F.; McQuade D. T. Improved Synthesis of Mono- and Disubstituted 2-Halonicotinonitriles from Alkylidene Malononitriles. Org. Lett. 2013, 15, 5298–5301. 10.1021/ol4025265. [DOI] [PubMed] [Google Scholar]
- Green Chemistry: Research Tools. https://www.acs.org/content/acs/en/greenchemistry/research-innovation/tools-for-green-chemistry.html (accessed Dec 14, 2018).
- For example, see:; a Klucznik T.; Mikulak-Klucznik B.; McCormack M. P.; Lima H.; Szymkuc S.; Bhowmick M.; Molga K.; Zhou Y.; Rickershauser L.; Gajewska E. P.; Toutchkine A.; Dittwald P.; Startek M. P.; Kirkovits G. J.; Roszak R.; Adamski A.; Sieredzinska B.; Mrksich M.; Trice S. L. J.; Grzybowski B. A. Efficient Syntheses of Diverse, Medicinally Relevant Targets Planned by Computer and Executed in the Laboratory. Chem. 2018, 4, 522–532. 10.1016/j.chempr.2018.02.002. [DOI] [Google Scholar]; b Kaiser D.; Yang J.; Wuitschik G. Using Data Analysis To Evaluate and Compare Chemical Syntheses. Org. Process Res. Dev. 2018, 22, 1222–1235. 10.1021/acs.oprd.8b00199. [DOI] [Google Scholar]
- Anderson N. G. Using Continuous Processes to Increase Production. Org. Process Res. Dev. 2012, 16, 852–869. 10.1021/op200347k. [DOI] [Google Scholar]
- Porta R.; Benaglia M.; Puglisi A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process Res. Dev. 2016, 20, 2–25. 10.1021/acs.oprd.5b00325. [DOI] [Google Scholar]
- Hartman R. L. Managing Solids in Microreactors for the Upstream Continuous Processing of Fine Chemicals. Org. Process Res. Dev. 2012, 16, 870–887. 10.1021/op200348t. [DOI] [Google Scholar]
- Adamo A.; Beingessner R. L.; Behnam M.; Chen J.; Jamison T. F.; Jensen K. F.; Monbaliu J.-C. M.; Myerson A. S.; Revalor E. M.; Snead D. R.; Stelzer T.; Weeranoppanant N.; Wong S. Y.; Zhang P. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. 10.1126/science.aaf1337. [DOI] [PubMed] [Google Scholar]
- Gupton B. F.; McQuade D. T. The Flow Chemistry Journey—Economics. Chem. Today 2018, 36, 49–64. [Google Scholar]; b Wong S. Y.; Chen J.; Forte L. E.; Myerson A. S. Compact Crystallization, Filtration, and Drying for the Production of Active Pharmaceutical Ingredients. Org. Process Res. Dev. 2013, 17, 684–692. 10.1021/op400011s. [DOI] [Google Scholar]
- Michaudel Q.; Ishihara Y.; Baran P. S. Academia–Industry Symbiosis in Organic Chemistry. Acc. Chem. Res. 2015, 48, 712–721. 10.1021/ar500424a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallu U. R.; Nair A. K.; Sankar J.; Bapatu H. R.; Kumar M. P.; Narla S.; Bhanap T. A.; Thamma N. K.; Raman N. V. V. S. S. Impact of API (Active Pharmaceutical Ingredient) Source Selection on Generic Drug Products. Pharm. Regul. Aff. 2015, 4, 1000136. 10.4172/2167-7689.1000136. [DOI] [Google Scholar]
- Diab S.; McQuade D. T.; Gupton B. F.; Gerogiorgis D. I. Process Design and Optimization for the Continuous Manufacturing of Nevirapine, an Active Pharmaceutical Ingredient for HIV Treatment. Org. Process Res. Dev. 2019, 23, 320–333. 10.1021/acs.oprd.8b00381. [DOI] [Google Scholar]
- a Anastas P. T.Green Chemistry Textbook; Oxford University Press: New York, 2004. [Google Scholar]; b Green Chemical Engineering; Lapkin A., Ed.; Handbook of Green Chemistry, Vol. 12 (Anastas P. T., Series Ed.); Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- We hope to achieve this objective through continued partnerships with other similarly focused organizations. See:; a Brown Ripin D. H.; et al. Process Improvements for the Manufacture of Tenofovir Disoproxil Fumarate at Commercial Scale. Org. Process Res. Dev. 2010, 14, 1194–1201. 10.1021/op1001337. [DOI] [Google Scholar]; b Riley D. L.; Walwyn D. R.; Edlin C. D. An Improved Process for the Preparation of Tenofovir Disoproxil Fumarate. Org. Process Res. Dev. 2016, 20, 742–750. 10.1021/acs.oprd.5b00364. [DOI] [Google Scholar]