Abstract
Insulin initiation and titration can be challenging for many primary care providers who are involved in the treatment of patients with type 2 diabetes. Despite the introduction of advanced insulin analogs and improvements in insulin delivery devices, many patients with type 2 diabetes continue to experience suboptimal glycemic control. With an increasing number of treatment options available, type 2 diabetes management is moving away from a “one-size-fits-all” approach and toward individualized treatment regimens based on particular patient needs. Given this, nurse practitioners, physician assistants, pharmacists, and certified diabetes educators are becoming increasingly valuable resources in busy primary care practices.
Insulin initiation and titration is a challenge for many primary care providers (PCPs) involved in the treatment of patients with type 2 diabetes (1). Clinical inertia, the failure to initiate or intensify insulin therapy when indicated, is a multifactorial problem resulting from barriers to insulin initiation and intensification, including treatment regimen inconvenience, needle phobia, and fear of hypoglycemia (2–5) and has important consequences for patients, significantly increasing the risk of microvascular complications such as retinopathy, nephropathy, and neuropathy (6).
A desirable basal insulin therapy regimen includes an insulin analog possessing a pharmacokinetic profile that mimics the naturally occurring basal endogenous insulin secretion profile, which can provide adequate and reproducible glucose control. Basal insulin analogs from recombinant DNA technology represent a significant advancement from human NPH insulin, an intermediate-acting insulin that, despite the need for twice-daily dosing and a relatively high incidence of hypoglycemia, remains in use today (7). The first-generation basal insulin analogs, insulin glargine 100 units/mL (Gla-100) and insulin detemir 100 units/mL provided a near–24-hour glucose-lowering effect with low variability in insulin action and a lower incidence of hypoglycemia than NPH insulin (8). The more recently approved second-generation basal insulin analogs, insulin glargine 300 units/mL (Gla-300) and insulin degludec 100 units/mL (IDeg U100) or 200 units/mL (IDeg U200), with their prolonged duration of action and more evenly distributed activity profiles, are associated with reduced incidences of hypoglycemia and similar A1C control compared to earlier basal analogs (9,10). They also provide the advantage of once-daily dosing with reduced intra-individual variability (11).
Despite the introduction of insulin analogs, refinement of insulin regimens, and improvements in injection devices such as pens with fine-gauge needles, advocacy for early insulin initiation, and continuous titration algorithms, many patients with type 2 diabetes continue to experience poor glycemic control (12). This review discusses the initiation and titration of insulin and ways this approach may be optimized by PCPs to help overcome clinical inertia and provide patients with timely glycemic control.
Treatment Delays and Barriers
Patient and clinician factors contribute to delays in adding insulin to treatment regimens or in transitioning from oral antidiabetic agents (OADs) to insulin. Patient barriers are numerous and include the inconvenience of insulin regimens, a need for more frequent self-monitoring of blood glucose (SMBG) (13,14), fear of hypoglycemia, weight gain, and injection pain. Many patients lack confidence in their ability to self-manage their diabetes (15–17), with emotional factors such as an unwarranted sense of failure, guilt, and shame representing some of the most significant barriers to insulin use (13,15,18). In the Diabetes Attitudes, Wishes, and Needs study, 48% of patients initiating insulin believed they had failed to manage their diabetes correctly, 52% were worried about initiating insulin, and only 23% believed insulin would help manage their diabetes (2). Additionally, nonscientific beliefs such as the notion that insulin causes limb loss or kidney failure (18), lack of awareness of improved insulin delivery devices (19), and concerns about treatment costs are important factors that influence insulin adherence (20).
Among PCPs, there is often resonance with patients with regard to fear of hypoglycemia, as well as a lack of confidence in patients’ ability to manage insulin therapy. Factors such as a patient’s presumed unwillingness or inability to inject contribute to the reluctance of PCPs to initiate insulin (16). Importantly, a lack of awareness of patient fears, beliefs, and expectations may affect the quality of PCP-patient relationships and create further barriers to achieving glycemic control (18). Provider or clinician factors, including a lack of integrated care (20), uncertainty regarding insulin type, complexity of titration algorithms, and concerns that the complexity of insulin therapy is too great to be managed in primary care often lead PCPs to delay insulin initiation (19).
Advanced Basal Insulin Analogs and Fixed-Ratio Combinations
Advanced insulin analogs and pre-filled pen delivery devices are helping to overcome some of the barriers to insulin initiation and titration experienced by some patients and PCPs.
Gla-300, a new formulation of insulin glargine, is available in pre-filled pens containing 450 and 900 units and requires only one-third of the injection volume to deliver the same number of units as the Gla-100 pen (21,22). The decreased surface area of the smaller injection depot leads to a slower release rate of insulin, resulting in protracted and stable delivery into the circulation (7,21,23). Gla-300 also provides the advantage of once-daily dosing and reduced intra-individual variability (22), while offering similar glycemic control and a lower risk of hypoglycemia than Gla-100 (10).
It is recommended that unit-to-unit conversions are used when patients are switched from Gla-100 to Gla-300. However, it should be noted that Gla-100 and Gla-300 are not bioequivalent; therefore, when switching from Gla-100 to Gla-300, a higher Gla-300 dose (by ∼10–18%) may be needed to maintain the same level of glycemic control. Conversely, when switching from Gla-300 to Gla-100, the initial dose may need to be similarly reduced to limit the risk of hypoglycemia (24). Overall, it is advised that careful glycemic monitoring and individualized dose adjustments be made when switching from one type of basal insulin to another.
Insulin degludec is a novel, long-acting, once-daily insulin that exists as a stable dihexameric complex that forms long soluble multihexameric chains after injection. Its mechanism of protraction is due to binding to albumin to form bound complexes, from which it dissociates very slowly, thus providing a prolonged and stable delivery of insulin (7,25). Insulin degludec is available in two different concentrations, with IDeg U200 demonstrating similar glycemic control with significantly lower risk of overall and nocturnal hypoglycemia compared to Gla-100 (26). Pre-filled IDeg U100 pens contain 300 units, with a maximum dose per injection of 80 units, whereas pre-filled IDeg U200 pens contain 600 units, with a maximum dose per injection of 160 units. IDeg U200 provides the same dose as 100 units/mL basal insulin, but in half the volume. As with Gla-300, a 1:1 total daily conversion ratio is recommended when transitioning from long- or intermediate-acting basal insulins, followed by individual dosing adjustments (27).
A follow-on Gla-100 insulin available in the United States is a long-acting human insulin analog with similar pharmacological/therapeutic effects and safety profile to Gla-100 (28), which can be administered subcutaneously at any time of the day, providing 24-hour glycemic control (29,30).
Two fixed-ratio combinations of a glucagon-like peptide 1 (GLP-1) receptor agonist and a basal insulin analog delivered in a single injection received approval from the U.S. Food and Drug Administration in 2016 (30,31). GLP-1 receptor agonists provide complementary mechanisms of action to basal insulin by stimulating insulin secretion from pancreatic β-cells, suppressing glucagon secretion from α-cells, and delaying gastric emptying (32,33). Both combinations (iGlarLixi [Gla-100 and lixisenatide] and IDegLira [IDeg U100 and liraglutide] [30,31]) demonstrate improved glycemic control compared to their individual components alone, with no increased risk of hypoglycemia or weight gain (34–36).
The fixed ratio of Gla-100 and lixisenatide in iGlarLixi provides basal insulin doses of 15–60 units as a single daily injection corresponding to lixisenatide doses of 5–20 µg. Starting doses depend on previous therapy; for patients previously treated with lixisenatide or with a basal insulin dose of <30 units daily, the recommended starting dose of iGlarLixi is 15 units (which includes 5 µg lixisenatide), whereas the recommended starting dose for patients previously taking 30–60 units of basal insulin is 30 units (which includes 10 µg lixisenatide). Up or down titration should occur in 2- to 4-unit increments every week, based on individual patient requirements (30).
IDegLira is provided in doses of insulin ranging from 16 to 50 units, corresponding to liraglutide doses of 0.58–1.8 mg. The recommended starting dose is 16 units, which can be titrated up or down in 2-unit increments every 3–4 days. The pens can administer doses as low as 10 units (with 0.36 mg liraglutide), but doses <16 units are only recommended as temporary for down titration; patients persistently requiring <16 units/day should be transitioned to a different treatment (31).
Current Guidelines for Insulin Initiation and Titration
American Diabetes Association Guidelines
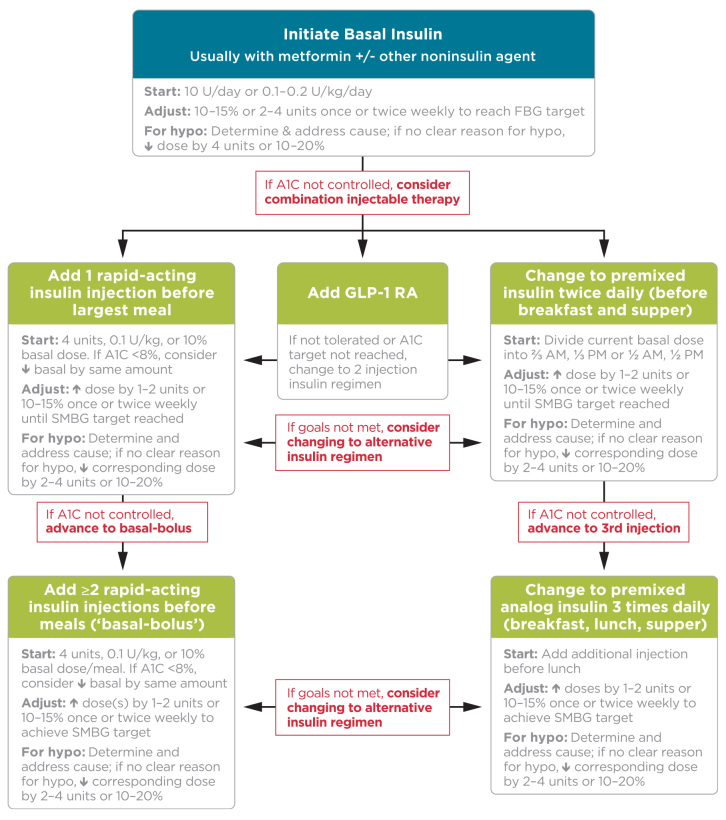
The American Diabetes Association (ADA) recommends initiation of basal insulin at 10 units/day or 0.1–0.2 units/kg/day, adjusted by 10–15% or 2–4 units once or twice weekly to reach a target fasting plasma glucose (FPG) in patients whose A1C remains uncontrolled after >3 months of triple combination therapy, whose A1C is >10%, whose blood glucose is >300 mg/dL, or who are symptomatic of hyperglycemia (37). Figure 1 details treatment intensification recommendations for patients whose A1C remains uncontrolled after basal insulin initiation and titration. Three regimen options should be considered:
Regimen 1: Administer one rapid-acting insulin injection before the meal with the greatest carbohydrate content; if the glycemic target is not met, progress to two or more rapid-acting insulin injections before meals (basal-bolus regimen).
Regimen 2: Add a GLP-1 receptor agonist. If target A1C remains unmet or the regimen is not tolerated, patients may discontinue the GLP-1 receptor agonist and switch to regimen 1 or 3.
Regimen 3: Replace basal insulin with premixed insulin at a 75/25, 70/30, or 50/50 mix twice (usually before breakfast or dinner) or thrice daily (before breakfast, lunch, and dinner). Basal insulin and GLP-1 receptor agonists should be discontinued before initiating premixed insulin.
FIGURE 1.
ADA-recommended approach to initiating and titrating insulin in type 2 diabetes. Reprinted with permission from ref. 37; adapted with permission from ref. 61. ©2015 American Diabetes Association. FBG, fasting blood glucose; GLP-1RA, GLP-1 receptor agonist; hypo, hypoglycemia.
For regimens 1 and 3, insulin doses should be increased by 1–2 units or 10–15% until target A1C and blood glucose values are met. If appropriate, oral agents other than metformin can be discontinued to avoid regimens that are too complex and expensive. If the targets remain unmet, patients may switch to the alternative regimen. If hypoglycemia occurs, the cause should be investigated, and, if no clear cause is found, the insulin dose should be reduced as recommended in Figure 1.
American Association of Clinical Endocrinologists Guidelines
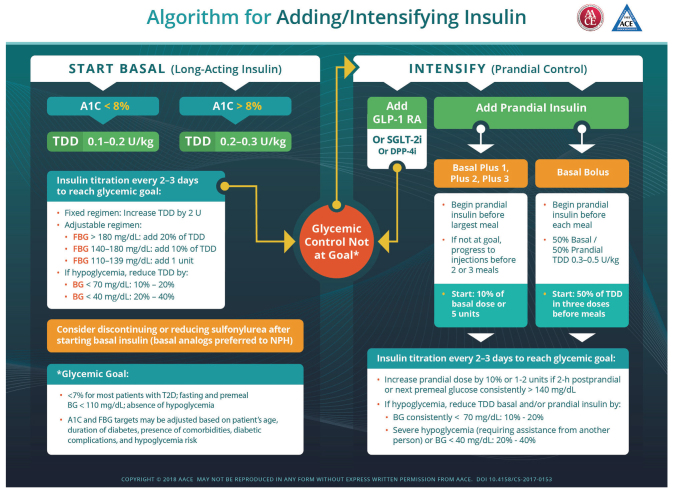
The American Association of Clinical Endocrinologists (AACE) recommends initiating long-acting basal insulin at a total daily dose (TDD) of 0.1–0.2 units/kg for patients with an A1C <8% or 0.2–0.3 units/kg for patients with an A1C >8%, with insulin titration every 2–3 days to reach the glycemic target (38). For those on fixed regimens, the TDD may be increased by 2 units, whereas for those on adjustable regimens, the dose should be adjusted by 1 unit or 10–20% of the TDD according to FPG values, as indicated in Figure 2. For patients taking a sulfonylurea, the dose may have to be reduced or discontinued during titration due to increased risk of hypoglycemia (38). If the A1C target is unmet, a GLP-1 receptor agonist, sodium–glucose cotransporter 2 inhibitor, dipeptidyl peptidase-4 inhibitor, or prandial insulin may be added to the treatment regimen. Two approaches to initiating prandial insulin may be used as follows.
Regimen 1: Begin prandial insulin at 10% of basal dose or 5 units before the largest meal (basal + 1). If A1C target is unmet, progress to injections before meals 2 or 3 (basal + 2 or basal + 3).
Regimen 2: Begin prandial insulin before each meal with a 50% basal/50% prandial ratio to achieve a TDD of 0.3–0.5 units/kg, starting at 50% of the TDD in three divided doses before meals.
FIGURE 2.
AACE-recommended approach to initiating and titrating insulin in type 2 diabetes. Reprinted with permission from ref. 38. ©2018 American Association of Clinical Endocrinologists. BG, blood glucose; DPP-4i, dipeptidyl peptidase-4 inhibitor; GLP-1 RA, GLP-1 receptor agonist; SGLT-2i, sodium–glucose cotransporter 2 inhibitor.
For both regimens, insulin should be titrated every 2–3 days until glycemic targets are met, as recommended in Figure 2.
Insulin Titration Algorithms
A number of titration algorithms have been evaluated that aim to simplify insulin titration and enable patient empowerment through self-titration to effectively participate in the management of their disease (4,39–42), the details of which are summarized in Table 1. Several studies have demonstrated that simple algorithms for titrating basal insulin in small increments at short intervals (e.g., every 3 days based on the mean of three self-monitored FPG values or increasing by 1 unit/day) allow patients to achieve comparable glycemic control as with physician-directed titration (43–45).
TABLE 1.
Titration Algorithms Evaluated in Clinical Trials
| Trial | Study Group | Comparison Group |
|---|---|---|
| Treat-to-target (50) | Algorithm 1: Gla-100 titration | Algorithm 2: NPH titration |
| Increase Gla-100 dose by: | Increase NPH dose by: | |
| • 8 units with FPG ≥180 mg/dL | • 8 units with FPG ≥180 mg/dL | |
| • 6 units with FPG 140–180 mg/dL | • 6 units with FPG 140–180 mg/dL | |
| • 4 units with FPG 120–140 mg/dL | • 4 units with FPG 120–140 mg/dL | |
| • 2 units with FPG ≥100–120 mg/dL | • 2 units with FPG ≥100–120 mg/dL | |
| ATLANTUS study (43) | Algorithm 1: Patient self-titration | Algorithm 2: Physician titration |
| Increase Gla-100 dose by: | Increase Gla-100 dose by: | |
| • 6–8 units with FPG ≥180 mg/dL | • 2 units with FPG ≥180 mg/dL | |
| • 4 units with FPG 140–180 mg/dL | • 2 units with FPG 140–180 mg/dL | |
| • 2 units with FPG 120–140 mg/dL | • 2 units with FPG 120–140 mg/dL | |
| • 0–2 units with FPG ≥100–120 mg/dL | • 0–2 units with FPG ≥100–120 mg/dL | |
| PREDICTIVE study (44) | Patient self-titration | Standard care |
| • Decrease insulin detemir by 3 units with mean FPG <80 mg/dL | ||
| • Keep insulin detemir dose the same with mean FPG 80–100 mg/dL | ||
| • Increase insulin detemir dose by 3 units with FPG >110 mg/dL | ||
| INSIGHT trial (45) | Patient self-titration |
EDITION algorithm |
| • Increase Gla-300 dose by 1 unit/day if FPG >100 mg/dL | • Increase Gla-300 dose by: | |
| 3 units if SMPG value >100 and <140 mg/dL | ||
| 6 units if SMPG value ≥140 mg/dL | ||
| • Decrease by 3 units if SMPG value <79 mg/dL |
SMPG, self-monitoring of plasma glucose.
Simplified self-titration algorithms have also been successfully evaluated for basal-bolus regimens (46,47) and prandial regimens (48). Several studies have also revealed that, in some cases, certain patients can achieve adequate A1C control using more complex titration algorithms combined with regular support from PCPs (49).
When Too Much Insulin Has Little Effect on Glycemic Target
Current use of basal insulin has been shaped by treat-to-target trials that have emphasized systematically titrating the insulin dose without limit until an FPG of 100–130 mg/dL is reached (50). “Overbasalization” is said to occur when FPG is uncontrolled despite uptitration of basal insulin and the A1C target remains unmet (51). Since basal insulin is not designed for postprandial coverage, patients may experience hypoglycemia in the fasting state without seeing any additional reduction in their A1C. Furthermore, patients are prone to experience postprandial hyperglycemia due to a lack of mealtime insulin as a result of β-cell failure. Overbasalization may cause hypoglycemia during the day and evening hours (in the nonfasting state) if insulin dose adjustments are based on fasting blood glucose values. Increased activity levels during the day can lead to hypoglycemia if too much basal insulin is given daily.
Therefore, it is important to ensure that patients monitor their blood glucose values at different times of the day. This practice was derived from the concept of “fix fasting first,” which developed as a consequence of fasting hyperglycemia being shown to be the primary contributor to hyperglycemia at higher A1C levels in patients taking only OADs (52). Riddle et al. (53) expanded this concept by showing how treatment intensification affects the relative contributions of fasting hyperglycemia and postprandial hyperglycemia in patients with an A1C >7% taking only OADs (53). The observed changes depend on the main effect of the treatment used. Treatment intensification with basal insulin results in a marked reduction in fasting hyperglycemia, and this accounts for approximately one-third of total hyperglycemia in patients who are close to their A1C target. These findings highlight the importance of addressing both fasting and postprandial hyperglycemia to normalize glycemic exposure.
A basal insulin dose >0.5 units/kg (37,54), a >50 mg/dL difference between bedtime (Be) and the next morning’s (AM) SMBG value (known as the “BeAM value”), or an absolute morning glucose level <70 mg/dL (54) should be recognized as potential overbasalization. Increasing basal insulin to >0.5 units/kg has been shown to not improve A1C or mean FPG and is associated with weight gain (55). Patients whose FPG is near or within the target range but whose A1C remains elevated after treatment intensification with basal insulin should be considered for therapy that effectively reduces postprandial glycemia (37).
Managing Insulin Regimens in the Primary Care Setting
It is important to gain an understanding of a patient’s background and lifestyle before initiating insulin to ensure that the treatment regimen takes into account the patient’s needs and preferences as well as clinical characteristics (37,56,57). The key to successful initiation and titration of insulin is to communicate effectively the benefits of insulin and develop a shared decision-making process that enables patients to feel confident in and in control of their treatment regimens, facilitating their decision to start and engage with insulin therapy. Factors to consider when initiating insulin regimens include patients’ age, daily schedule, activity level, eating pattern, social situation, cultural factors, diabetes-related complications, comorbidities, preferences for self-management, and life expectancy (14).
Although it has been demonstrated that some patients can successfully manage their insulin regimen (43,44), the titration regimen must be simple and easy to manage and support both patients and PCPs in optimizing insulin therapy. Careful support and education about available treatments are instrumental to intensifying insulin therapy and should be provided to help overcome barriers such as fear of injections, hypoglycemia, and lack of knowledge and to manage patients’ expectations (58).
Patients should be closely monitored during titration, and their therapy should be adjusted accordingly until their A1C target is achieved (56). Some patients may require more frequent contact with their PCPs (59) and diabetes management team during titration to reduce the risk of overbasalization (60). As the target FPG is approached, smaller and less frequent insulin dose adjustments should be used to reduce the risk of hypoglycemia. If hypoglycemia occurs, its cause should be investigated because it may be due to non–insulin-related factors such as a missed meal or increased physical activity. If no cause can be found, the insulin dose should be reduced accordingly (59). It also may be necessary to adjust other noninsulin therapies when insulin is added, especially agents that increase hypoglycemia risk—namely, insulin secretagogues (i.e., sulfonylureas and glinides).
Once a stable insulin dose and adequate A1C control have been achieved, the frequency of patient evaluation and monitoring should be reviewed (59). PCPs should continue to communicate with patients in a timely manner to ensure that they are persistent with treatment, successfully managing their disease, and kept up to date on new guidelines, treatment options, and insulin delivery devices.
Conclusion
Multiple insulin algorithms have been developed to help PCPs with insulin initiation and titration and to enable patient self-management. New insulin formulations such as Gla-300 and IDeg U100/U200 have helped to address barriers such as fear of hypoglycemia, the need for multiple daily injections, and pain associated with large injection volumes, while the introduction of pen devices has helped simplify insulin delivery. Although these advances have helped to simplify treatment regimens, effective communication that takes patients’ beliefs and preferences into account is essential to educating patients about the benefits of insulin therapy. Enhanced support from PCPs, including assistance with insulin titration and timely follow-up, may help to improve adherence to insulin regimens by patients with type 2 diabetes.
Acknowledgments
The authors received writing/editorial support in the preparation of this manuscript, provided by Georgina Bowden, PhD, of Excerpta Medica and funded by Sanofi US.
Duality of Interest
J.C. is an advisory board member for Sanofi and is on a Speakers bureau for AstraZeneca. J.S. is on speakers bureaus for AstraZeneca, Boehringer Ingelheim/Lilly, Janssen, Novo Nordisk, and Sanofi; is an advisory board member for Ascensia Diabetes, Novo Nordisk, and Sanofi; is a consultant for the American Diabetes Association and Novo Nordisk; and has received authorship support from Sanofi. S.U. is on speakers bureaus for AstraZeneca and Novo Nordisk; is an advisory board member for Sanofi; and is a consultant for Novo Nordisk. No other potential conflicts of interest were reported.
Author Contributions
The contents of this article and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication. All authors researched data, reviewed and edited the manuscript, and contributed to the discussion. J.C. is the guarantor of this work and, as such, takes responsibility for the integrity of the information and the accuracy of the data interpretation.
References
- 1.Lovre D, Fonseca V. Benefits of timely basal insulin control in patients with type 2 diabetes. J Diabetes Complications 2015;29:295–301 [DOI] [PubMed] [Google Scholar]
- 2.Peyrot M, Rubin RR, Lauritzen T, et al. , on behalf of the International DAWN Advisory Board. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs study (DAWN). Diabetes Care 2005;28:2673–2679 [DOI] [PubMed] [Google Scholar]
- 3.Rubin RR, Peyrot M, Kruger DF, Travis LB. Barriers to insulin injection therapy: patient and health care provider perspectives. Diabetes Educ 2009;35:1014–1022 [DOI] [PubMed] [Google Scholar]
- 4.Dailey G, Aurand L, Stewart J, Ameer B, Zhou R. Comparison of three algorithms for initiation and titration of insulin glargine in insulin-naive patients with type 2 diabetes mellitus. J Diabetes 2014;6:176–183 [DOI] [PubMed] [Google Scholar]
- 5.Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825–834 [DOI] [PubMed] [Google Scholar]
- 6.Zoungas S, Chalmers J, Ninomiya T, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia 2012;55:636–643 [DOI] [PubMed] [Google Scholar]
- 7.Heise T, Mathieu C. Impact of the mode of protraction of basal insulin therapies on their pharmacokinetic and pharmacodynamic properties and resulting clinical outcomes. Diabetes Obes Metab 2017;19:3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenstock J, Schwartz SL, Clark CM Jr, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care 2001;24:631–636 [DOI] [PubMed] [Google Scholar]
- 9.Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-long acting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet 2012;379:1498–1507 [DOI] [PubMed] [Google Scholar]
- 10.Riddle MC, Bolli GB, Ziemen M, Muehlen-Bartmer I, Bizet F, Home PD. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care 2014;37:2755–2762 [DOI] [PubMed] [Google Scholar]
- 11.Lamos EM, Younk LM, Davis SN. Concentrated insulins: the new basal insulins. Ther Clin Risk Manag 2016;12:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garber AJ. Will the next generation of basal insulins offer clinical advantages? Diabetes Obes Metab 2014;16:483–491 [DOI] [PubMed] [Google Scholar]
- 13.Williams R. Breaking down barriers to insulin management in primary care. Prim Care Diabetes 2010;4(Suppl. 1):vii–viii [DOI] [PubMed] [Google Scholar]
- 14.Ng CJ, Lai PS, Lee YK, Azmi SA, Teo CH. Barriers and facilitators to starting insulin in patients with type 2 diabetes: a systematic review. Int J Clin Pract 2015;69:1050–1070 [DOI] [PubMed] [Google Scholar]
- 15.Barag SH. Insulin therapy for management of type 2 diabetes mellitus: strategies for initiation and long-term patient adherence. J Am Osteopath Assoc 2011;111(Suppl. 5):S13–S16 [PubMed] [Google Scholar]
- 16.Arnolds S, Heise T, Flacke F, Sieber J. Common standards of basal insulin titration in type 2 diabetes. J Diabetes Sci Technol 2013;7:771–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gough SC, Bhargava A, Jain R, Mersebach H, Rasmussen S, Bergenstal RM. Low-volume insulin degludec 200 units/mL once daily improves glycemic control similarly to insulin glargine with a low risk of hypoglycemia in insulin-naïve patients with type 2 diabetes. Diabetes Care 2013;36:2536–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janes R, Titchener J, Pere J, Pere R, Senior J. Understanding barriers to glycaemic control from the patient’s perspective. J Prim Health Care 2013;5:114–122 [PubMed] [Google Scholar]
- 19.Petznick AM. Identifying and addressing barriers to insulin acceptance and adherence in patients with type 2 diabetes mellitus. J Am Osteopath Assoc 2013;113(Suppl. 2):S6–S16 [PubMed] [Google Scholar]
- 20.Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 2016;10:1299–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunton SA, Kruger DF, Funnell MM. Role of emerging insulin technologies in the initiation and intensification of insulin therapy for diabetes in primary care. Clin Diabetes 2016;34:34–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanofi. FDA approves Toujeo® Max SoloStar®. 27 March 2018. Available from www.news.sanofi.us/2018-03-27-FDA-approves-Toujeo-R-Max-SoloStar-R. Accessed 28 September 2018.
- 23.Bailey TS, Pettus J, Roussel R, et al. Morning administration of 0.4U/kg/day insulin glargine 300U/mL provides less fluctuating 24-hour pharmacodynamics and more even pharmacokinetic profiles compared with insulin degludec 100U/mL in type 1 diabetes. Diabetes Metab 2018;44:15–21 [DOI] [PubMed] [Google Scholar]
- 24.U.S. Food and Drug Administration Toujeo 300 units/ml solution for injection in a pre-filled pen, 2017. Available from www.accessdata.fda.gov/drugsatfda_docs/label/2015/206538lbl.pdf. Accessed 14 May 2018 [Google Scholar]
- 25.Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 2012;29:2104–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab 2013;15:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novo Nordisk. Tresiba® [prescribing information]. Basgsvaerd, Denmark, Novo Nordisk, 2015. Available from novo-pi.com/tresiba.pdf. Accessed 10 July 2018.
- 28.Rosenstock J, Hollander P, Bhargava A, et al. Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the ELEMENT 2 study). Diabetes Obes Metab 2015;17:734–741 [DOI] [PubMed] [Google Scholar]
- 29.Eli Lilly and Company, Boehringer Ingelheim BASAGLAR® [prescribing information]. Indianapolis, Ind., Lilly USA, LLC, Ridgefield, CT, Boehringer Ingelheim, 2016. Available from pi.lilly.com/us/basaglar-uspi.pdf. Accessed 14 May 2018 [Google Scholar]
- 30. Sanofi. SOLIQUA™ 100/33 (insulin glargine and lixisenatide injection) [prescribing information]. Bridgewater, NJ, sanofi-aventis U.S. 2017. Available from products. sanofi.us/Soliqua100-33/Soliqua100-33.pdf. Accessed 14 May 2018.
- 31. Novo Nordisk. XULTOPHY® 100/3.6 (Insulin degludec and liraglutide injection) [prescribing information]. Bagsvaerd, Denmark, Novo Nordisk, 2016. Available from www.novo-pi.com/xultophy10036.pdf. Accessed 14 May 2018.
- 32.Baynes KC. The evolving world of GLP-1 agonist therapies for type 2 diabetes. Ther Adv Endocrinol Metab 2010;1:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tasyurek HM, Altunbas HA, Balci MK, Sanlioglu S. Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev 2014;30:354–371 [DOI] [PubMed] [Google Scholar]
- 34.Rosenstock J, Aronson R, Grunberger G, et al.; LixiLan-O Trial Investigators . Benefits of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled with oral agents: the LixiLan-O randomized trial. Diabetes Care 2016;39:2026–2035 [DOI] [PubMed] [Google Scholar]
- 35.Aroda VR, Rosenstock J, Wysham C, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care 2016;39:1972–1980 [DOI] [PubMed] [Google Scholar]
- 36.Gough SCL, Jain R, Woo VC. Insulin degludec/liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Rev Endocrinol Metab 2016;11:7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Diabetes Association 8. Pharmacological approaches to glycemic treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41 (Suppl. 1):S73–S85 [DOI] [PubMed] [Google Scholar]
- 38.Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2018 executive summary. Endocr Pract 2018;24:91–120 [DOI] [PubMed] [Google Scholar]
- 39.Philis-Tsimikas A, Brod M, Niemeyer M, Ocampo Francisco AM, Rothman J. Insulin degludec once-daily in type 2 diabetes: simple or step-wise titration (BEGIN: Once Simple Use). Adv Ther 2013;30:607–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg SK, Admane K, Freemantle N, et al. Patient-led versus physician-led titration of insulin glargine in patients with uncontrolled type 2 diabetes: a randomized multinational ATLAS study. Endocr Pract 2015;21:143–157 [DOI] [PubMed] [Google Scholar]
- 41.Bajaj HS, Venn K, Ye C, Aronson R. Randomized trial of long-acting insulin glargine titration web tool (LTHome) versus enhanced usual therapy of glargine titration (INNOVATE Trial). Diabetes Technol Ther 2016;18:1–6 [DOI] [PubMed] [Google Scholar]
- 42.Pfützner A, Stratmann B, Funke K, et al. Real-world data collection regarding titration algorithms for insulin glargine in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2016;10:1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care 2005;28:1282–1288 [DOI] [PubMed] [Google Scholar]
- 44.Meneghini L, Koenen C, Weng W, Selam J-L. The usage of a simplified self-titration dosing guideline (303 Algorithm) for insulin detemir in patients with type 2 diabetes: results of the randomized, controlled PREDICTIVE™ 303 study. Diabetes Obes Metab 2007;9:902–913 [DOI] [PubMed] [Google Scholar]
- 45.Yale JF, Harris SB, Berard L, Groleau M, Javadi P, Stewart J. A pragmatic self-titration 1 unit/day (INSIGHT) algorithm for insulin glargine 300 U/mL (GLA-300) (Abstract). Diabetes 2016;65 (Suppl. 1A): 93-LB [Google Scholar]
- 46.Harris SB, Yale JF, Berard L, et al. Does a patient-managed insulin intensification strategy with insulin glargine and insulin glulisine provide similar glycemic control as a physician-managed strategy? Results of the START (Self-Titration with Apidra to Reach Target) study: a randomized noninferiority trial. Diabetes Care 2014;37:604–610 [DOI] [PubMed] [Google Scholar]
- 47.Gerety G, Bebakar WM, Chaykin L, et al. Treatment intensification with insulin degludec/insulin aspart twice daily: randomized study to compare simple and step-wise titration algorithms. Endocr Pract 2016;22:546–554 [DOI] [PubMed] [Google Scholar]
- 48.Oyer DS, Shepherd MD, Coulter FC, et al. A1c control in a primary care setting: self-titrating an insulin analog pre-mix (INITIATEplus Trial). Am J Med 2009;122:1043–1049 [DOI] [PubMed] [Google Scholar]
- 49.Kennedy L, Herman WH, Strange P, Harris A. Impact of active versus usual algorithmic titration of basal insulin and point-of-care versus laboratory measurement of HbA1c on glycemic control in patients with type 2 diabetes: the Glycemic Optimization with Algorithms and Labs at Point of Care (GOAL A1C) trial. Diabetes Care 2006;29:1–8 [DOI] [PubMed] [Google Scholar]
- 50.Riddle MC, Rosenstock J, Gerich J. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 51.LaSalle J, Berria R. Insulin therapy in type 2 diabetes mellitus: a practical approach for primary care physicians and other health care professionals. J Am Osteopath Assoc 2013;113:152–162 [PubMed] [Google Scholar]
- 52.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–885 [DOI] [PubMed] [Google Scholar]
- 53.Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011;34:2508–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reid T, Gao L, Gill J, et al. How much is too much? Outcomes in patients using high-dose insulin glargine. Int J Clin Pract 2016;70:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zisman A, Morales F, Stewart J, Stuhr A, Vlajnic A, Zhou R. BeAM value: an indicator of the need to initiate and intensify prandialtherapy in patients with type 2 diabetes mellitus receiving basal insulin. BMJ Open Diabetes Res Care 2016;4:e000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirsch IB, Bergenstal R, Parkin C, Parkin CG, Wright E, Buse JB. A real-world approach to insulin therapy in primary care practice. Clin Diabetes 2005;23:78–86 [Google Scholar]
- 57.Shubrook JH., Jr Insulin therapy for challenging patient cases. J Am Osteopath Assoc 2013;113(Suppl. 2):S17–S28 [PubMed] [Google Scholar]
- 58.Kruger D. Intensifying insulin treatment: options, practical issues, and the role of the nurse practitioner. J Am Acad Nurse Pract 2012;24(Suppl. 1):260–269 [DOI] [PubMed] [Google Scholar]
- 59.Inzucchi S, Bergenstal R, Buse J, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuddihy RM, Philis-Tsimikas A, Nazeri A. Type 2 diabetes care and insulin intensification: is a more multidisciplinary approach needed? Results from the MODIFY survey, Diabetes Educ 2011;37:111–123 [DOI] [PubMed] [Google Scholar]
- 61.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]