Abstract
Background:
Gestational diabetes mellitus (GDM) is defined by World Health Organization as glucose intolerance diagnosed for the first time during pregnancy; GDM affects 7% of pregnancies. Women with earlier GDM have higher risk to develop type 2 diabetes (T2D). The aim of our study was to evaluate the outcomes of GDM and to assess the impact of recalling patients in the postpartum stage by phone, the target was to assess T2D or impaired glucose tolerance in women with a history of GDM.
Methods:
This prospective study included 200 patients with GDM that have received education sessions regarding the major interest of screen T2D using 75 g of oral glucose tolerance testing in the 3rd month after birth.
Results:
Only 22.5% (n = 45) women spontaneously complied to assess T2D. About 15% have had developed T2D and 28% prediabetes. Risk factors of T2D onset were younger gestational age at the occurrence GD, higher fasting blood glucose, and frequent use of insulin.
Conclusions:
Women with GD history demonstrated high risk of developing T2D. Simple changes of lifestyle were shown to be an efficient prevention protocol. Despite therapeutical education, few women spontaneously complied with T2D screening. The telephone reminders could improve the screening observance therefore patient's outcome.
Keywords: Diabetes mellitus, follow-up, gestational diabetes
Introduction
According to World Health Organization (WHO), gestational diabetes (GD) is a disorder of glucose tolerance that is diagnosed for the first time during the pregnancy.[1] It is a public health problem since diagnosed in 7% of pregnancies with shorter and longer term impact on maternal and fetal health.[1,2] Former pregnant women with GD have higher risk to developing a diabetes mellitus, especially type 2 diabetes (T2D), metabolic syndrome, and obesity.[3,4,5] Appropriate care has significantly reduced the maternal and fetal morbidity.[5] The compliance to screening was demonstrated to be low; fewer patients adjust their lifestyle to prevent later occurrence of T2D.[3]
The mail reminders have allowed increasing T2D screening compliance in women with earlier GD; however, the impact of telephone reminder was never assessed.[6]
The goal of our study was to assess the prospect of GD, to highlight the predictive factors of T2D occurrence, and to evaluate the impact of phone reminder on the compliance to T2D screening in postpartum in women with earlier GD.
Methods
This prospective was carried along 5 years between January of 2010 and 2015. The study included 200 patients followed for GD in the Department of Endocrinology and Diabetology of University hospital of Fez, Morocco.
According to diagnosis criteria of “Collège national des gynécologues et obstétriciens français” established in 2010; any FBG higher than 0.92 g/L or oral glucose tolerance test (OGTT) achieved between 24th and 28th week of amenorrhea would allow establishing the diagnosis of GD.
In our study, the diagnosis of GD was based on any of both assessing fasting blood glucose (FBG) in the first trimester of pregnancy. All patients have had benefited of therapeutical education, this emphasized the importance of follow-up of diabetes using OGTT with 75 g of carbohydrates after the thirds month of the postpartum. The statistical analysis was realized by SPSS Inc. Released 2009. PASW Statistics for Windows, Version 18.0. Chicago, USA.
Results
The average age of patients was 33.5 ± 5.5 years old, and their middleweight before pregnancy was 70 ± 9 kg. The average gestational age of GD assessment was 19.36 weeks of amenorrhea. GD screening in our study was focused on risk factors in 80% of cases and systematic in 20% of cases. These risk factors consisted of being older than 30 years-old found in 71% of patients, presence of maternal heredity of T2D, history of polymicrocyst in ovaries, and history of high arterial blood pressure, in utero fetal death, former GD as well as of macrosomia [Figure 1].
Figure 1.
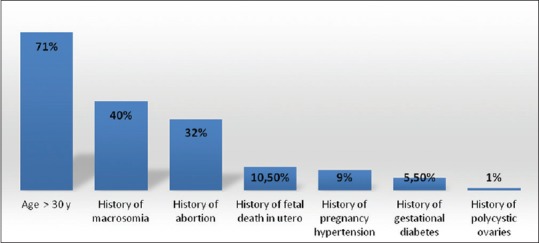
Risk factors of developing a diabetes mellitus
The postpartum consultation was spontaneously achieved in 22.5% (n = 45) of patients. About 77.5% of patients were followed up by phone reminder and 51.5% of called responded positively; while 26% (n = 52 patients) were not reachable despite multiple recall [Table 1].
Table 1.
Postpartum follow-up
| Patient’s followed up spontaneously | Patient’s followed up phone call | Patients lost at the follow-up | |
|---|---|---|---|
| n (%) | 45 (22.5) | 103 (51.5) | 52 (26) |
| Patient’s motivations | Self-motivation Heredity of diabetes Education impact | Busy with the baby Forgotten Carelessness Fear of discovering T2D | False phone number Changed phone number Unreachable patient |
T2D=Type 2 diabetes
The “self-dismiss” of follow-up was mainly due to being busy with baby care, negligence, and fear of discovering diabetes, despite the follow-up of patients lasted 6 months.
Screening protocol consisted of achieving OGTT in 67.6% of patients, followed by glycosylated hemoglobin test (HbA1C) achieved in 25.7% of cases and FBG realized in 6.7% of cases. The upcoming of our GD patients is summarized in Figure 2.
Figure 2.
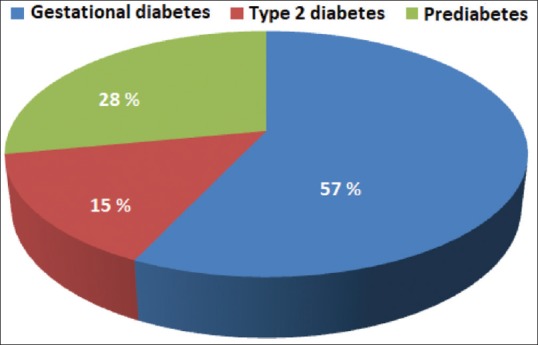
Reclassification of becoming of former gestational diabetes after birth child
The risk factors to develop T2D after delivery were marked by the lower gestational age at the GD diagnosis associated with higher FBG, requiring insulin treatment in 86% of cases. The obesity was shown in 9% of cases.
Neonates were females in 58% of cases and benefited of an exclusive breastfeeding in 83% of cases along an average duration of 6 months.
The postpartum contraception was established in 52.5% of cases with an intrauterine contraceptive device in 13.5%, estrogen-progesterone microdose in 27%, estrogen-progesterone minidose in 2%, and mechanical contraception in 10%.
In postpartum, patients have had benefited of care mainly based on rigorous healthy lifestyle with particular rejection of rapid sugars, a balanced diet, and a regular physical activity.
Regarding cardiovascular risk factors, patients have established an education taking into account regular control follow-up of glycemia and glycosuria, proteinuria, and the blood pressure.
T2D was treated by insulin during the feeding stage, which was substituted by oral antidiabetics. Metformin was indicated for patients with prediabetes when associating obesity when they are not successful in modifying their lifestyle.
Discussion
“Prediabetes” is an intermediate state between a normal homeostasis of glucose and confirmed T2D and includes two clinical entities: (i) moderate hyperglycemia of fasting individual and (ii) glucose intolerance (GI). According to WHO, the fasting hyperglycemia (FG) is defined by FBG ranging between 1.10 and 1.26 g/L. GI is defined by a glycemia measured 2 h after an oral load of 75 g of glucose with values ranging between 1.40 and 2 g/L.
Despite the therapeutical education, only 22.5% (n = 45) of cases spontaneously complied to T2D screening. Most patients required phone reminder. Among 148 patients that were included in our study, 15% have developed T2D and 28% prediabetes. The risk factors of occurrence of T2D include a younger gestational age at the assessment of GD, higher FBG, and a more frequent use of insulin.
The GD is defined by WHO as a disorder of sugar tolerance, diagnosed for the first time during the pregnancy.[1] GD includes preexisting diabetes, as well as glycoregulation disorders initiated by the pregnancy, and might evolve and/or vanish after delivery. GD screening is based on risk factors, rather than a universal screening.[5] Indeed, the assessment of FBG in the first trimester of pregnancy in higher risk patients would allow diagnosing of any unrevealed T2D.[5]
The benefits of diabetological care were demonstrated with a significant reduction of the maternal and fetal morbidity.[5] Although the sugar tolerance quickly normalizes after the delivery in most women with history of GD, the risk of developing later diabetes or an intolerance to glucose is 43% in our study [Table 2].[7,8] A history of GD increases the risk of T2D by 7 times, metabolic syndrome by 2–5 times, and cardiovascular diseases by 1–7 times.[9] The recurrence of GD is estimated between 30% and 84% and even more frequent in GD cases with insulinotherapy.[5] The outcome of gestational diabetic patients after delivery is summarized in Table 2.[10,11,12,13,14]
Table 2.
Outcome of gestational diabetic patients after delivery
| Outcome of glycemia after delivery | T2D (%) | Prediabetes (%) | GD (%) | Lost at follow-up (%) |
|---|---|---|---|---|
| Notre série (200 cases) | 15 | 28 | 57 | 26 |
| Cundy et al.[10] (1110 cases) | 41 | 16 | ||
| Schaefer-Graf et al.[11] (4041 cases) | 14 | - | - | 40 |
| Russell et al.[12] (344 cases) | 8 | - | - | 55 |
| Vambergue et al.[13] (466 cases) | 18 | 22 | 60 | |
| Kjos et al.[14] (671 cases) | 22 |
T2D=Type 2 diabetes, GD=Gestational diabetes
The American College of Obstetricians and Gynecologists[15] and the American Diabetes Association (ADA)[16] recommend systematic screening within 6th and 12th week after delivery. The feeding and contraception do not justify delaying diabetes assessment. Indeed, the screening could be achieved using FBG or OGTT.[17] The OGTT with 75 g of glucose has the highest sensitivity of 100%.[17] The FBG has lower sensitivity compared to OGTT but HbA1C could supplant these assessments.[3] Indeed, FBG allows assessing moderate hyperglycemia and diabetes in fasting patients, but it is not sensitive to GI. According to ADA recommendations,[16] a HbA1C indicates increased risk of diabetes requiring a greater supervision, but it does not allow a positive diagnosis in the view of insufficient predictive value.[18] The collaborative work of ATLANTIC DIP group has used a threshold value of HbA1C that was fixed at 5.9%.[18] In our series, OGTT of 75 g glucose were prescribed to patients at the end of pregnancy, which has increased the achievement rate [Table 3].[12,19,20]
Table 3.
Outcome of glycemia assessment according to used diagnostic approach
| Diagnostic method of T2D (postpartum stage) | FBG | OGTT (75 g) | HbA1C |
|---|---|---|---|
| Our series (148 cases) | 6.7 | 67.6 | 25.7 |
| Morrison et al.[19] (1372 cases) | 72.7 | 27.3 | - |
| El Feleh et al.[20] (73 cases) | - | 82.2 | - |
| Russell et al.[12] (344 cases) | 8 | - | - |
HbA1C=Glycosylated hemoglobin, FBG=Fasting blood glucose, OGTT=Oral glucose tolerance testing
ADA recommends performing a T2D assessment every 3 years in each GD patient with a first negative screening in postpartum and yearly assessments in cases presenting CHI, a moderate fasting hyperglycemia or both abnormalities.[21] However, literature reported half of GD patients achieved a screening after delivery.[11,12,22] In our series, we recorded higher rate of 74%, indeed this can be elucidated by the active reminding of former GD by phone in postpartum stage. Van Ryswyk et al. reported a study comparing results of a reminding system by messages sent to 276 women after GD episodes and delivery; randomized patients were split into two groups: the first group included 140 patients that were reminded by 6th week, 3rd and 6th month; while the second group enclosed 136 patients reminded only in the 6th month. The screening compliance and follow-up results were similar in both groups; hence, they suggested a single reminder by the 6th after delivery since sufficient to motivate the follow-up.[23]
The predictive factors of T2D development after a GD reported in our series agreed with earlier literature; these predictive factors includes obesity, the use of insulin during the pregnancy, higher FBG and HbA1C at the diagnosis, GD occurrence before 24th week of amenorrhea.[19,20,24,25,26]
Schaefer-Graf et al. calculated the prevalence and risk of diabetes attributed to each factor.[11] Hence, raised FBG during OGTT at the time of the diagnosis was the most discriminating since glycemia ≤0.95 g/l reflects minor risk of 0.5%. The risk rises to 36.7% with a glycemia over 1.21 g/L.[27] The gestational age at the diagnosis seems to be significant because the risk is 21.4% for GD diagnosed at 19th week, while dropping to 8.7% after the 31st week. The risk is 29% with existing former history of GD.[27]
The results of O’Sullivan tests are also a predictive factor; the risk of T2D is 2.6% when glycemia is ≤1.55 g/L and increases to 11.8% for glycemia ranging between 1.75 and 2.02 g/L and raises to 27.3% when glycemia is >2.02 g/L. Our series did not reveal that raised body mass index as a predictive factor, while other studies did.[10] The female gender of fetus seems to present higher risk factor of developing T2D; this was noticed in our study and also reported in earlier study involving 23363 patients.[27]
All these factors are easily detectable during the pregnancy and allow outlining females with higher risks of diabetes; therefore allows warning on the high requirement of a regular metabolic follow-up targeting a preventive or curative therapeutic care. Studies considering adjustment of lifestyle including regular physical activity and balanced diet were achieved in this population with higher risks of cardiovascular disorders and T2D; it showed the prevention efficiency.[23,28] The study Diabetes Prevention Program showed the efficiency of using metformin in the prevention of T2D.[29] After GD, women presenting of minor glycoregulation disorders and excess of weight have shown to half the risk of T2D if they do consider change in the lifestyle with metformin intake.[3]
Conclusions
This study established the GD prospective and defined risk factors of evolvement of GD toward T2D; it has also revealed motivations behind neglecting the follow-up. The women with GD have higher risk to become T2D. Indeed, the pregnancy offers an important opportunity to instigate very early therapeutical education on the interest of regular follow-up. Simple preventive strategies showed their efficiency to prevent T2D, therefore any associated complications. Reminding by phone besides to therapeutical education might improve the involvement of patients in T2D screening during the postpartum stage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21(Suppl 2):B161–7. [PubMed] [Google Scholar]
- 3.Vérier-Mine O. Outcomes in women with history of gestational diabetes mellitus. Screening and prevention of type 2 diabetes mellitus. Literature review. J Gynecol Obstet Biol Reprod (Paris) 2010;39:S299–321. doi: 10.1016/S0368-2315(10)70056-9. [DOI] [PubMed] [Google Scholar]
- 4.Burguet A. Long-term outcome in children of mothers with gestational diabetes. Diabetes Metab. 2010;36:682–94. doi: 10.1016/j.diabet.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Vambergue A. Gestational diabetes: Diagnosis, short and long term management. Presse Med. 2013;42:893–9. doi: 10.1016/j.lpm.2013.02.316. [DOI] [PubMed] [Google Scholar]
- 6.Middleton P, Crowther CA. Reminder systems for women with previous gestational diabetes mellitus to increase uptake of testing for type 2 diabetes or impaired glucose tolerance. Cochrane Database Syst Rev. 2014:CD009578. doi: 10.1002/14651858.CD009578.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiéronimus S, Le Meaux JP. Relevance of gestational diabetes mellitus screening and comparison of selective with universal strategies. J Gynecol Obstet Biol Reprod (Paris) 2010;39:S200–13. doi: 10.1016/S0368-2315(10)70047-8. [DOI] [PubMed] [Google Scholar]
- 8.Vambergue A, Valat AS, Dufour P, Cazaubiel M, Fontaine P, Puech F. Pathophysiology of gestational diabetes. J Gynecol Obstet Biol Reprod (Paris) 2002;31(6 Suppl):4S3–4S10. [PubMed] [Google Scholar]
- 9.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 10.Cundy T, Gamble G, Townend K, Henley PG, MacPherson P, Roberts AB, et al. Perinatal mortality in type 2 diabetes mellitus. Diabet Med. 2000;17:33–9. doi: 10.1046/j.1464-5491.2000.00215.x. [DOI] [PubMed] [Google Scholar]
- 11.Schaefer-Graf UM, Buchanan TA, Xiang AH, Peters RK, Kjos SL. Clinical predictors for a high risk for the development of diabetes mellitus in the early puerperium in women with recent gestational diabetes mellitus. Am J Obstet Gynecol. 2002;186:751–6. doi: 10.1067/mob.2002.121895. [DOI] [PubMed] [Google Scholar]
- 12.Russell MA, Phipps MG, Olson CL, Welch HG, Carpenter MW. Rates of postpartum glucose testing after gestational diabetes mellitus. Obstet Gynecol. 2006;108:1456–62. doi: 10.1097/01.AOG.0000245446.85868.73. [DOI] [PubMed] [Google Scholar]
- 13.Vambergue A, Schaller S, Lenne X, Lemaire C, Dognin C, Fontaine P. How become the mother eleven years after gestational diabetes (GD) or moderate hyperglycemia (MHG) in Nord-Pas de Calais region. Diabetes & Metabolism. 2009;35 Supplement 1:A1. [Google Scholar]
- 14.Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA, et al. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes. 1995;44:586–91. doi: 10.2337/diab.44.5.586. [DOI] [PubMed] [Google Scholar]
- 15.Vambergu A. Gestational diabetes mellitus: Expert-consensus from CNGOF and SFD – 2010 Avant-propos. Medecine des Maladies Metaboliques. 2010;4:713–7. [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2010;33(Suppl 1):S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verier-Mine O. Outcomes in women with a history of gestational diabetes. Screening and prevention of type 2 diabetes. Literature review. Diabetes Metab. 2010;36:595–616. doi: 10.1016/j.diabet.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Noctor E, Crowe C, Carmody LA, Avalos GM, Kirwan B, Infanti JJ, et al. ATLANTIC DIP: Simplifying the follow-up of women with previous gestational diabetes. Eur J Endocrinol. 2013;169:681–7. doi: 10.1530/EJE-13-0491. [DOI] [PubMed] [Google Scholar]
- 19.Morrison MK, Collins CE, Lowe JM. Postnatal testing for diabetes in Australian women following gestational diabetes mellitus. Aust N Z J Obstet Gynaecol. 2009;49:494–8. doi: 10.1111/j.1479-828X.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 20.El Feleh E, Mahjoub F, Sebai I, Mhalla H, Ksira I, Berriche O, et al. The gestational diabetes in postpartum stage. Ann Endocrinol (Paris) 2015;76:531. [Google Scholar]
- 21.Janin C, Fontanie M, Sallée X, Ducloux R, Altman J. Postpartum screening for diabetes after gestational diabetes: An example of improved practices. Médecine des Maladies Métaboliques. 2014;8:169–75. [Google Scholar]
- 22.Kim C, Tabaei BP, Burke R, McEwen LN, Lash RW, Johnson SL, et al. Missed opportunities for type 2 diabetes screening among women with a history of GDM. Am J Public Health. 2006;96:1643–8. doi: 10.2105/AJPH.2005.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Ryswyk EM, Middleton PF, Hague WM, Crowther CA. Postpartum SMS reminders to women who have experienced gestational diabetes to test for type 2 diabetes: The DIAMIND randomized trial. Diabet Med. 2015;32:1368–76. doi: 10.1111/dme.12769. [DOI] [PubMed] [Google Scholar]
- 24.Olesen CR, Nielsen JH, Mortensen RN, Bøggild H, Torp-Pedersen C, Overgaard C, et al. Associations between follow-up screening after gestational diabetes and early detection of diabetes – A register based study. BMC Public Health. 2014;14:841. doi: 10.1186/1471-2458-14-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metzger BE, Cho NH, Roston SM, Radvany R. Prepregnancy weight and antepartum insulin secretion predict glucose tolerance five years after gestational diabetes mellitus. Diabetes Care. 1993;16:1598–605. doi: 10.2337/diacare.16.12.1598. [DOI] [PubMed] [Google Scholar]
- 26.Dornhorst A, Bailey PC, Anyaoku V, Elkeles RS, Johnston DG, Beard RW, et al. Abnormalities of glucose tolerance following gestational diabetes. Q J Med. 1990;77:1219–28. doi: 10.1093/qjmed/77.3.1219. [DOI] [PubMed] [Google Scholar]
- 27.Retnakaran R, Shah BR. Sex of the baby and future maternal risk of type 2 diabetes in women who had gestational diabetes. Diabet Med. 2016;33:956–60. doi: 10.1111/dme.12989. [DOI] [PubMed] [Google Scholar]
- 28.Rautio N, Jokelainen J, Korpi-Hyövälti E, Oksa H, Saaristo T, Peltonen M, et al. Lifestyle intervention in prevention of type 2 diabetes in women with a history of gestational diabetes mellitus: One-year results of the FIN-D2D project. J Womens Health (Larchmt) 2014;23:506–12. doi: 10.1089/jwh.2013.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubin RR, Fujimoto WY, Marrero DG, Brenneman T, Charleston JB, Edelstein SL, et al. The Diabetes Prevention Program: Recruitment methods and results. Control Clin Trials. 2002;23:157–71. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]


