Abstract
Background:
Malathion is one of organophosphate pesticides that is widely used in agriculture and crops to control insects. Malathion affects body organs such as the reproductive system by inhibiting acetylcholinesterase activity and induction of oxidative stress. This study is aimed to investigate the effects of malathion on glutathione (GSH) and malondialdehyde (MDA) level in testis of male rats, as well as to study the protective role of Ascorbic Acid.
Methods:
In this study, 30 adult male Wistar rats weighing approximately 200–250 g were divided into 5 groups of 6 rats each. These groups include a control group (no intervention), sham (normal saline 0.9%), experimental Group 1 (malathion 50 mg/kg), experimental Group 2 (Malathion 50 mg/kg + Ascorbic Acid 200 mg/kg), and experimental Group 3 (Ascorbic Acid 200 mg/kg). Malathion, solvents, and ascorbic acid were injected intraperitoneally. After 6 weeks, all groups were anesthetized, and the right testis was used to measure levels of MDA and GSH. MDA as a marker of lipid peroxidation and GSH content was used.
Results:
The results showed that malathion increased MDA level and decreased GSH level compared with the control group (P < 0.001). It was also found that administration of malathion in combination with ascorbic acid reduced MDA level and increased the GSH level.
Conclusions:
Malathion-induced lipid peroxidation and oxidative stress in the testis of rats. In addition, it seems that ascorbic acid, due to its antioxidant capabilities, can improve malathion-induced poisonous changes.
Keywords: Ascorbic acid, glutathione, malathion, malondialdehyde, rats, testis
Introduction
Malathion (diethyl methoxy thio-phosphoryl thio-succinate) is a chemical pesticide organophosphate pesticide family, which is widely used in industry, agriculture to control insects on crops, produce ornamental plants, grasses, fruits, and vegetables as well as in the medical sector for disease vector control in many countries.[1] Despite malathion has the less toxic insecticide than parathion, use of malathion has risen, especially in developing countries as nowadays, this compound has been reported as the third leading cause of poisoning and death in Iran.[2] These pesticides are absorbed through the skin and mucous membranes[3] and affect various organs of the body including liver, kidneys, pancreas, and testis.[4] Malathion in the body, during oxidation-reduction reactions, is converted into a metabolite called malaoxon, the main role of which is to cause toxicity in mammals, insects, and plants so that malaoxon toxicity is 40 times more than that of malathion, the main purpose of which is to disrupt the nervous system of insects.[5] Different mechanisms of organophosphate toxicity have been proposed, including the inhibition of acetylcholinesterase, which lead to the accumulation of acetylcholine and cholinergic activity in muscarinic and nicotine recipients.[6] Oxidative stress is another mechanism affecting the toxicity of organophosphate pesticides, which leads to the production of free radicals such as reactive oxygen species (ROS) and changes in enzyme activity along with antioxidant defense mechanisms in the body,[7] which leads to oxidative damage of biological macromolecules such as nucleic acid, proteins, fats, and carbohydrates.[8,9] Plasma thiol groups are also evaluated as an indicator of oxidative stress. These groups are sensitive to oxidative damage, and their decrease is an important indicator of oxidative stress which is capable of sweeping free radicals.[10] Glutathione (GSH) is one of thiol groups as well as an essential component of the normal immune system and plays an essential role in protecting body cells from damages caused by peroxidase hydrogen and oxygen species such as oxidative stress.[11] The severity of oxidative damage can be measured by estimating aldehyde products of lipid peroxidation such as malondialdehyde (MDA). These aldehydes can covalently bind to proteins through reacting with thiol groups and alter biological macromolecules function.[12] Antioxidants exist in two enzymatic and nonenzymatic forms.[13] Ascorbic acid is a nonenzymatic antioxidant with low molecular weight and soluble in water, which decreases lipid peroxidation and prevents many damaging effects on cells by reducing the production of oxidative stress.[14] With regard to the conditions mentioned above, for the first time, we determined GSH and MDA levels in 50 mg/kg malathion-induced of the testis toxic in adult male Wistar Rats and evaluated the protective effects of Ascorbic Acid.
Methods
In this experimental study, 30 male 2-month-old Wistar rats weighing 200–250 g[15] were randomly divided into 5 groups each containing of 6 rats,[16,17] which include control group (no intervention), sham group receiving daily (50 mg/kg of normal saline 0.9%), experimental Group 1 (malathion 50 mg/kg),[18,19,20] experimental Group 2 (Malathion 50 mg/kg + Ascorbic Acid 200 mg/kg), and experimental Group 3 (Ascorbic Acid 200 mg/kg) [Table 1]. The study lasted for 6 weeks and malathion, solvent (normal saline), and ascorbic acid (product of Sigma CO.) were injected intraperitoneally.[14,21] After 6 weeks,[22] rats were anesthetized with intraperitoneal ketamine/xylazine (60 mg/kg and 6 mg/kg, respectively). Animals were sacrificed, and the right testis were used to measure the levels of MDA and GSH. These rats were kept in the standard conditions (temperature 2 ± 22°C and 12-h light/dark cycle) and had free access to food and water throughout the experiment. All the experimental protocols were approved by the Ethical Committee of Mashhad University of Medical Science (IR. MUMS. REC.1393.136).
Table 1.
Mean±standard deviation of glutathione, malondialdehyde, and compare them with different groups
| Treatments | GSH* | MDA* |
|---|---|---|
| Control | 8868.16±72.35 | 113.06±4.08 |
| Sham (normal saline 50 mg/kg) | 8451.80±151.08 | 113.06±7.10 |
| Experimental Group 1 (malathion 50 mg/kg) | 4473.49±79.05 | 181.57±12.44 |
| Experimental Group 2 (malathion 50 mg/kg + Vitamin C 200 mg/kg) | 5726.82±33.84 | 130.47±7.01 |
| Experimental Group 3 (Vitamin C 200 mg/kg) | 8799.88±112.86 | 112.56±4.92 |
*Values with small different letters in columns shown that are significantly different and similar letters are not significantly different (P<0.05) in various groups. GSH=Glutathione, MDA=Malondialdehyde
Lipid peroxidation testing
Lipid peroxidation content was investigated by measuring the levels of MDA in the testis tissue of Rats. MDA reacts with thiobarbituric acid (TBA) and creates a pink complex with maximum absorbance at 532 nm. To get started, 3 ml phosphoric acid (1%) and 1 ml TBA (6%) were added to 10% tissue homogenates in the potassium chloride and then the mixture was heated in boiling water bath for 45 min. After cooling the mixture, 4 ml of n-butanol was added and was vertexed for 1 min and was centrifuged for 10 min (3000 RPM). The upper layers were later removed for 20–25 min and were transferred to a test tube. The absorbance was read at 532 NM using a spectrometer.[23] The calibration curve is designed using tetrabutylammonium MDA. The MDA level was expressed as nmol/g of tissue (n Mol/g tissue).
Glutathione reduction method
The level of GSH in the testis tissue was investigated using Moron et al.'s method.[24] The task's basis is the formation of yellow color following the addition of 5,5’-Dithiobis-(2-nitrobenzoic acid) (DTNB) to the compound containing sulfhydryl groups. To do this, l300 μ of the homogenated tissue was mixed and vertexed with 300 μl of Trichloroacetic acid 10% (TCA). The upper layers were removed after centrifugation at 2500 RPM for 10 min and were mixed with the reaction mixture containing 2 ml phosphate buffer (pH: 8) and l μ DTNB 500. At the time of 10 min, the absorption at a wavelength of 412 nm was read using a spectrophotometer. At the end, the amount of GSH standard generated curve was determined using commercially available GSH, and GSH level was expressed using nMol/g tissue.
Statistical analysis
In this study, data were analyzed using SPSS software (SPSS 16 Software, Chicago, IL, USA). Results are expressed as mean ± standard deviation. Statistical analysis was performed using one-way ANOVA and Tukey–Kramer test to compare the mean differences between the groups and the difference was considered statistically significant at P < 0.05.
Chemicals
In this study, technical malathion 99% was purchased from Aria shimi Company and the stock solution with a concentration of 50 mg/ml was freshly prepared in normal saline 0.9%. MDA tetrabutylammonium, reduced GSH, DTNB (dithiobis nitrobenzoic acid), and ascorbic acid were purchased from Sigma CO.
Results
The effect of ascorbic acid on lipid peroxidation of rats treated with malathion
As shown in Table 1, a significant increase in MDA levels in the experimental Group 1 (malathion), compared to the control group (P < 0.001). In addition, the level of MDA in the experimental Group 2 (malathion + Ascorbic Acid) was significantly decreased compared with the experimental Group 1 (malathion) (P < 0.001). However, the level of MDA in control, sham, and Ascorbic Acid groups [Figure 1] was not significantly different (P > 0.001).
Figure 1.
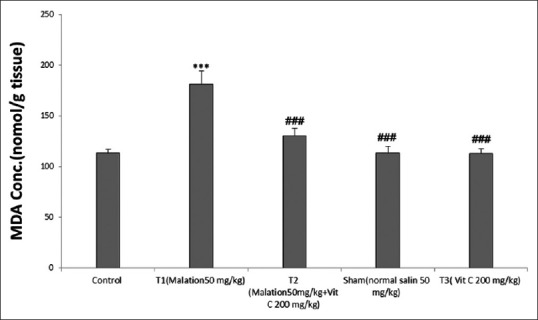
Effect of malathion and Vitamin C on malondialdehyde levels in groups. ***P < 0.001 versus control, ###P < 0.001 versus malation
The effect of ascorbic acid on glutathione levels in the testis tissue of rats after receiving malathion
As shown in Table 1, in the experimental Group 1, rats treated with malathion showed a significant reduction in the levels of GSH compared with the control group (P < 0.001). Furthermore, GSH levels were significantly increased in the experimental Group 2 (vitamins C + malathion) compared with the experimental Group 1 (P < 0.001). Moreover, control, sham and Ascorbic Acid groups showed no significant difference in terms of levels of GSH (P > 0.001) [Figure 2].
Figure 2.
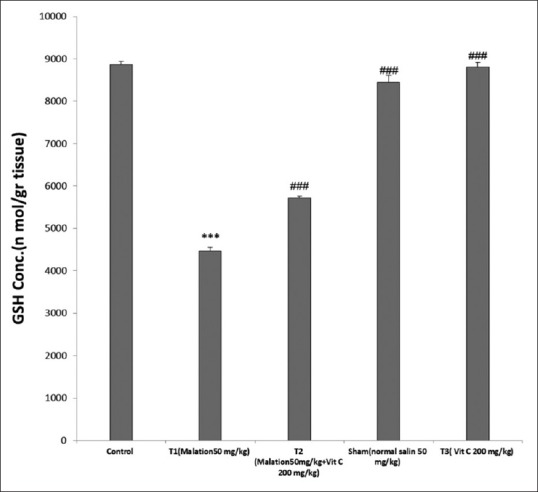
Effect of malathion and Vitamin C on glutathione levels in groups. ***P < 0.001 versus control, ###P < 0.001 versus malation
Discussion
In this study, we investigated MDA and GSH level changes related to the testis tissue of malathion-induced rats and the protective role of ascorbic acid. The results of comparing the average numbers showed that MDA levels in the experimental Group 1 that had only received malathion was significantly more than that compared with the control group (P < 0.001). However, the administration of ascorbic acid along with malathion in the experimental Group 2 reduced the level of MDA compared with the experimental Group 1. Since ascorbic acid, as an effective antioxidant and as a mitigation of the effects of oxidative stress, can contribute to the improvement of blood poisoning, therefore, the results of this study are consistent with the results obtained by Uzun et al. who investigated the effect of malathion on testis of male rats and the protective role of ascorbic acid.[14] MDA as a marker of oxidative stress is the last product of lipid catabolism.[25] Evidence showed that malathion leads to systemic disorders and consequently leads to hormonal changes such as the reproductive system by changing in the cell antioxidant system and increasing membrane lipid peroxidation and by inhibiting acetylcholinesterase enzyme.[26,27]
Furthermore, these pesticides by making some changes in DNA or its binding protein can damage the testis tissues and cause mutations in spermatogonia cells, which ultimately leads to changes in the sperm.[28] According to the similar previous studies, these pesticides cause biochemical changes in reproductive organs, including testis[10] and also, it is proved that any increase in the levels of MDA and reducing the antioxidant immune system.[8,29] Hence, the findings showed that malathion induces toxicity and DNA damage in the cells (human liver carcinoma cells) HepG2 increases levels of MDA through oxidative stress.[30] The results of these studies are consistent with the results of the present study. In this study, the level of MDAandGSH levels also were assessed.[31] In the present study, a comparison between the experimental Group 1 and control group showed that GSH level in the experimental Group 1 was significantly decreased compared to the control group (P < 0.001) that the concomitant use of malathion and ascorbic acid in the experimental Group 2 increase the level of GSH. This difference is statistically significant. The previous studies have also confirmed the reduced MDA levels and increased GSH antioxidant system and lipid peroxidation in rats kidneys and the study are consistent with the results of the present study.[32] In a similar study on one of the organophosphates such as diazinon, it was found that levels of GSH and MDA were significantly decreased and increased in the testis tissue of male rats.[10] Furthermore, the previous investigation showed that GSH levels were significantly decreased in the liver tissue and blood of rats treated with malathion.[33,34] Furthermore, according to the results of recent studies decreased antioxidant activity has been observed in the testis tissue following the use of organophosphate insecticides and ROS production.[10,34] In such circumstances, along with avoiding exposure to organophosphate pesticides, use of an appropriate antioxidant such as ascorbic acid used in this research can reduce adverse effects of exposure to these pesticides. Vitamin C is available in many foods and easily intake an antitoxic effect by daily consuming. The form of ascorbic acid reduction is maintained unchanged thorough reaction with GSH.[24] Ascorbic Acid is a water-soluble vitamin that can decrease the amount of free radicals through its antioxidant properties.[35] Thus, the aim of the present study was to administer ascorbic acid to a group of rats, along with intraperitoneal injection of malathion for 42 days. The results of treatment with ascorbic acid and malathion (experimental Group 2), compared to those who only received malathion showed a significant increase in GSH levels and a significant reduction in the level of MDA (P < 0.002). Thus, according to the results of this study, the key role of ascorbic acid as an antioxidant will improve the oxidative stress.[36] Simultaneous use of vitamins E and C will significantly contribute to the improvement of lipid peroxidation induced by these pesticides in the heart tissue.[37] Another complementary study undertaken by research Sutcu et al. in this regard, revealed that ascorbic acid can, respectively, justify decreased oxidative stress in brain tissue and decreased lipid peroxidation of rats erythrocytes in the organophosphorus pesticide poisoning.[36] Hence, the results of this study are consistent with the results of previous studies that investigated the effect of ascorbic acid on the damage caused by lipid peroxidation.[38]
According to the results obtained in this study, it can be concluded the oxidative stress in rats treated with malathion causes cell toxicity. Furthermore, considering the biochemical effects of malathion in rats’ testis (reducing GSH content and increasing levels of MDA), there is the risk of cytotoxicity in farmers and other people who are in contact with these substances. Thus, according to the results of the present study, there is a dire need to treat and prevent the entry of pesticides into the body, prevent tissue damage under the influence of these pesticides, and prevent other disorders such as dysfunction of the gonads and the risk of infertility. Therefore, the use of ascorbic acid as an antioxidant in this study can protect the testis tissue against this toxicity by after reducing the MDA level and increasing GSH content. This study was limited by the assessment of apoptotic effect, the other antioxidant parameters such as Nrf-2, and sperm quality in expouse to malathion. For this reason, it was necessary to doing superior studies on large sample size to evaluate this factors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The current article is a part of the descriptive science thesis in M. Sc. which is conducted based on the research funding (922,842) in Mashhad University of Medical Sciences.
Therefore, hereby, I would like to appreciate the Vice Chancellor for Research of this university as well as Mrs. Tajadod, a histochemistry laboratory expert in Mashhad University of Medical Sciences for her sincere assistance and technical services as well as the toxicology section of School of the Pharmacy.
References
- 1.Dorri SA, Hosseinzadeh H, Abnous K, Hasani FV, Robati RY, Razavi BM, et al. Involvement of brain-derived neurotrophic factor (BDNF) on malathion induced depressive-like behavior in subacute exposure and protective effects of crocin. Iran J Basic Med Sci. 2015;958:18–66. [PMC free article] [PubMed] [Google Scholar]
- 2.Abdolmaleki M, Ghasemi HH, Hosseinizaijood M, Ranjbar A. Assessing the protective effects of zinc on oxidative injury biomarkers in acute malathion induction in male rats. Sci J Ilam Univ Med Sci. 2014;22:147–52. [Google Scholar]
- 3.Sarabia L, Maurer I, Bustos-Obregón E. Melatonin prevents damage elicited by the organophosphorous pesticide diazinon on the mouse testis. Ecotoxicol Environ Saf. 2009;72:938–42. doi: 10.1016/j.ecoenv.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Shah MD, Iqbal M. Diazinon-induced oxidative stress and renal dysfunction in rats. Food Chem Toxicol. 2010;48:3345–53. doi: 10.1016/j.fct.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Wankhade VW. Effect of malathion on lipid peroxidation and enzymatic activity of liver serum and brain at different exposure periods in mice. Res J Environ Toxicol. 2012;6:142. [Google Scholar]
- 6.Al-Attar AM. Physiological and Histopathological Investigations on the Effects of α-Lipoic Acid in Rats Exposed to Malathion. BioMed Res Int. 2010;2010:8. doi: 10.1155/2010/203503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozkan U, Osun A, Basarslan K, Senol S, Kaplan I, Alp H, et al. Effects of intralipid and caffeic acid phenethyl ester on neurotoxicity, oxidative stress, and acetylcholinesterase activity in acute chlorpyriphos intoxication. Int J Clin Exp Med. 2014;7:837–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Buyukokuroglu ME, Cemek M, Yurumez Y, Yavuz Y, Aslan A. Antioxidative role of melatonin in organophosphate toxicity in rats. Cell Biol Toxicol. 2008;24:151–8. doi: 10.1007/s10565-007-9024-z. [DOI] [PubMed] [Google Scholar]
- 9.Milatovic D, Gupta RC, Aschner M. Anticholinesterase toxicity and oxidative stress. ScientificWorldJournal. 2006;6:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahimi Anbarkeh F, Nikravesh MR, Jalali M, Sadeghnia HR, Sargazi Z, Mohammdzadeh L, et al. Single dose effect of diazinon on biochemical parameters in testis tissue of adult rats and the protective effect of Vitamin E. Iran J Reprod Med. 2014;12:731–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Salehi B, Vakilian K, Ranjbar A. Relationship of schizophrenia with lipid peroxidation, total serum antioxidant capacity and thiol groups. Iran J Psychiatry Clin Psychol. 2008;14:140–5. [Google Scholar]
- 12.Hosseinzadeh Colagar A, Bidmeshkipour A, Gholinezhad Chari M. Total antioxidant capacity and malondialdehyde levels in seminal plasma among the varicocele-suffering men. Sci J Ilam Univ Med Sci. 2009;17:15–23. [Google Scholar]
- 13.Nouri M, Ghasemzadeh A, Farzadi L, Shahnazi V, Ghaffari Novin M. Vitamins C, E and lipid peroxidation levels in sperm and seminal plasma of asthenoteratozoospermic and normozoospermic men. Int J Reprod Biomed. 2008;6:1–5. [Google Scholar]
- 14.Uzun FG, Kalender S, Durak D, Demir F, Kalender Y. Malathion-induced testicular toxicity in male rats and the protective effect of Vitamins C and E. Food Chem Toxicol. 2009;47:1903–8. doi: 10.1016/j.fct.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Kayhan FE. Biochemical evidence of free radical-induced lipid peroxidation for chronic toxicity of endosulfan and malathion in liver, kidney and gonadal tissues of wistar albino rats. Fresenius Environ Bull. 2008;17:1340–3. [Google Scholar]
- 16.Kalender S, Uzun FG, Durak D, Demir F, Kalender Y. Malathion-induced hepatotoxicity in rats: The effects of Vitamins C and E. Food Chem Toxicol. 2010;48:633–8. doi: 10.1016/j.fct.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan K, Balakumar BS, Panneerselvam C. Effects of ascorbic acid and alpha-tocopherol on arsenic-induced oxidative stress. Hum Exp Toxicol. 2002;21:675–80. doi: 10.1191/0960327102ht307oa. [DOI] [PubMed] [Google Scholar]
- 18.Possamai FP, Fortunato JJ, Feier G, Agostinho FR, Quevedo J, Wilhelm Filho D, et al. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Environ Toxicol Pharmacol. 2007;23:198–204. doi: 10.1016/j.etap.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Fortunato JJ, Agostinho FR, Réus GZ, Petronilho FC, Dal-Pizzol F, Quevedo J, et al. Lipid peroxidative damage on malathion exposure in rats. Neurotox Res. 2006;9:23–8. doi: 10.1007/BF03033304. [DOI] [PubMed] [Google Scholar]
- 20.Choudhary N, Goyal R, Joshi SC. Effect of malathion on reproductive system of male rats. J Environ Biol. 2008;29:259–62. [PubMed] [Google Scholar]
- 21.Uzunhisarcikli M, Kalender Y, Dirican K, Kalender S, Ogutcu A, Buyukkomurcu F. Acute, subacute and subchronic administration of methyl parathion-induced testicular damage in male rats and protective role of Vitamins C and E. Pestic Biochem Physiol. 2007;87:115–22. [Google Scholar]
- 22.Ozmen O, Mor F. Apoptosis in adult rabbit testes during subacute endosulfan toxicity. Pestic Biochem Physiol. 2012;102:129–33. [Google Scholar]
- 23.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 24.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione Stransferase activities in rat lung and liver. Biochimica et Biophysica Acta. 1979;582:67–7. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 25.Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf. 2006;64:178–89. doi: 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Sarabia L, Maurer I, Bustos-Obregón E. Melatonin prevents damage elicited by the organophosphorous pesticide diazinon on mouse sperm DNA. Ecotoxicol Environ Saf. 2009;72:663–8. doi: 10.1016/j.ecoenv.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 27.Ranjbar A, Baeeri M. The effect of pentoxifylline on malathion-induced mitochondrial damage in rat liver. J Shahrekord Univ Med Sci. 2013;15:83–92. [Google Scholar]
- 28.Ogutcu A, Uzunhisarcikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of Vitamin E. Pestic Biochem Physiol. 2006;86:93–8. [Google Scholar]
- 29.Jahromi VH, Koushkaki M, Kargar H. The effects of malathion insecticide on ovary in female rats. National park forschung in der schweiz. Switzerland Res Park J. 2012;101:231–5. [Google Scholar]
- 30.Edwards FL, Yedjou CG, Tchounwou PB. Involvement of oxidative stress in methyl parathion and parathion-induced toxicity and genotoxicity to human liver carcinoma (HepG2) cells. Environ Toxicol. 2013;28:342–8. doi: 10.1002/tox.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Shenawy NS, El-Salmy F, Al-Eisa RA, El-Ahmary B. Amelioratory effect of Vitamin E on organophosphorus insecticide diazinon-induced oxidative stress in mice liver. Pestic Biochem Physiol. 2010;96:101–7. [Google Scholar]
- 32.Abbasnejad M, Jafari M, Asgari A, Haji HR, Haji GM, Salehi M, et al. Acute toxicity effect of diazinon on antioxidant system and lipid peroxidation in kidney tissues of rats. Daneshvar Med J. 2009;17:35–42. [Google Scholar]
- 33.Khan SM, Sobti RC, Kataria L. Pesticide-induced alteration in mice hepato-oxidative status and protective effects of black tea extract. Clin Chim Acta. 2005;358:131–8. doi: 10.1016/j.cccn.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 34.El-Demerdash FM. Lipid peroxidation, oxidative stress and acetylcholinesterase in rat brain exposed to organophosphate and pyrethroid insecticides. Food Chem Toxicol. 2011;49:1346–52. doi: 10.1016/j.fct.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Sutcu R, Altuntas I, Yildirim B, Karahan N, Demirin H, Delibas N, et al. The effects of subchronic methidathion toxicity on rat liver: Role of antioxidant Vitamins C and E. Cell Biol Toxicol. 2006;22:221–7. doi: 10.1007/s10565-006-0039-7. [DOI] [PubMed] [Google Scholar]
- 36.Sutcu R, Altuntas I, Buyukvanli B, Akturka O, Ozturka O, Koylu H, et al. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat erythrocytes: Role of Vitamins E and C. Toxicol Ind Health. 2007;23:13–7. doi: 10.1177/0748233707076758. [DOI] [PubMed] [Google Scholar]
- 37.Akturk O, Demirin H, Sutcu R, Yilmaz N, Koylu H, Altuntas I, et al. The effects of diazinon on lipid peroxidation and antioxidant enzymes in rat heart and ameliorating role of Vitamin E and Vitamin C. Cell Biol Toxicol. 2006;22:455–61. doi: 10.1007/s10565-006-0138-5. [DOI] [PubMed] [Google Scholar]
- 38.Uzunhisarcikli M, Kalender Y. Protective effects of Vitamins C and E against hepatotoxicity induced by methyl parathion in rats. Ecotoxicol Environ Saf. 2011;74:2112–8. doi: 10.1016/j.ecoenv.2011.07.001. [DOI] [PubMed] [Google Scholar]


