Abstract
As a newly discovered type of RNA, circular RNAs (circRNAs) are widespread throughout the eukaryotic genome. The expression of circRNAs is regulated by both cis-elements and trans-factors, and the expression pattern of circRNAs is cell type- and disease-specific. Similar to other types of non-coding RNAs, functions of circRNAs are also versatile. CircRNAs have been reported previously to function as microRNA (miRNA) sponges, protein sponges, coding RNAs or scaffolds for protein complexes. Recently, several circRNAs have been reported to play important roles in human malignancies, including glioma. Here, we reviewed several reports related to circRNAs and glioma, as well as the potential diagnostic and therapeutic applications of circRNAs in brain cancer. In general, some circRNAs, such as circSMARCA5 and circCFH, are found to be expressed in a glioma-specific pattern, these circRNAs may be used as tumor biomarkers. In addition, some circRNAs have been found to play oncogenic roles in glioma (e.g., circNFIX and circNT5E), whereas others have been reported to function as tumor suppressors (e.g., circFBXW7 and circSHPRH). Furthermore, circRNA is a good tool for protein expression because of its higher stability compared to linear RNAs. Thus, circRNAs may also be an ideal choice for gene/protein delivery in future brain cancer therapies. There are some challenges in circRNA research in glioma and other diseases. Research related to circRNAs in glioma is comparatively new and many mysteries remain to be solved.
Keywords: Circular RNA, glioma, miRNA sponge, translation, protein scaffold, miRNA target
Introduction
Circular RNA (circRNA) is a newly discovered type of RNA reported to be associated to many human diseases, including glioma. In this review, we sought to summarize the studies related to circRNAs, including their tissue-/disease-specific expression pattern, diverse functions, expression, functional roles, and therapeutic potential in human glioma. Finally, we discussed the current challenges of circRNA research in glioma and some other essential aspects.
Fascinating RNA world
As important intermediate linkers in the central dogma, RNAs have multiple biological functions. RNAs can not only transmit genetic information (such as mRNAs and tRNAs) stored in DNA, but they can also directly store genetic information (such as some RNA viruses)1. In addition, RNAs can also exhibit independent biological activities, including binding to and regulating the function of other biological macromolecules (such as DNA or proteins), and they can also function as enzymes (such as ribozymes)2. There are two categories of RNAs, including coding RNAs and noncoding RNAs (ncRNAs), depending on their protein-coding abilities. The coding RNAs are usually referred to as mRNAs, while non coding RNAs comprise of many subtypes, such as tRNAs, rRNAs, small nucleolar RNAs (snoRNAs), miRNAs, Piwi-interacting RNAs (piRNAs), long noncoding RNAs (lncRNAs), and circRNAs, among others3. The functions of noncoding RNAs are diverse and cover almost all common RNA functions3. As RNA research continues to develop, the boundaries between coding RNA and noncoding RNA are becoming increasingly blurred. Some previously known noncoding RNAs have been found to be able to translate proteins4. For example, toddler is a micropeptide encoded by a noncoding RNA (ENSDARG00000094729 in zebrafish), which plays an important role in zebrafish embryonic signaling5. Some RNAs that were originally considered as coding RNAs have been found to function as noncoding RNAs too, and are thus termed as coding and noncoding RNAs (cncRNAs)6. A typical example of a cncRNA is p53 mRNA, which can not only translate p53 protein in the ribosome but can also directly bind to MDM2 and regulate its function7. Therefore, the functions of RNAs are diverse, and our understanding of RNA functions needs to be improved. It is also necessary to understand the functions of RNAs, especially newly discovered RNA molecules, in many areas.
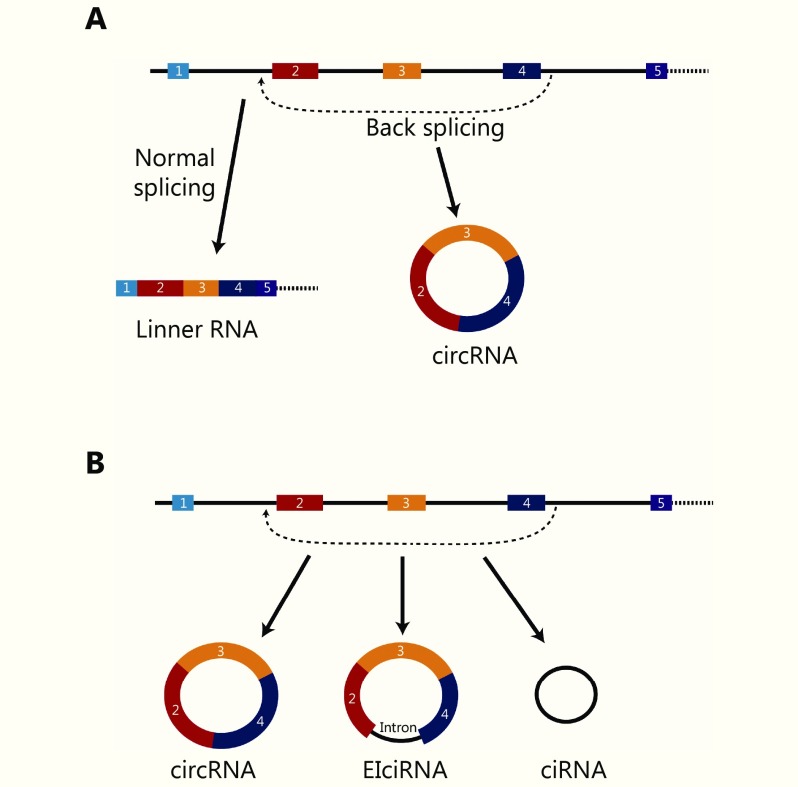
Major types of circRNAs
CircRNAs are generated from back-splicing of primary transcription products. In normal RNA splicing process, exons edited into final RNA products are arranged in the order of host gene. For circRNAs, the downstream exons are connected to the upstream exons, as shown in Figure 1. There are several types of circRNAs. Most circRNAs shared the same exon sequences corresponding to the host gene. Several circRNAs carry some intron sequences of flanking introns, and are thus termed exon-intron circRNAs (EIciRNA)8. Further, some circRNAs could also contain only intron sequences, termed as ciRNA9.
1.
Types of circRNAs. (A) Back-splicing model for circRNAs. (B) Major types of circRNAs.
Brief history of circRNA
CircRNAs are a special type of noncoding RNA that directly and covalently bind their 5’ and 3’ ends together and form a closed single-strand annular structure. The first publication on circRNAs by Sanger et al.10, which appeared in the literature as early as 1976, reported that the plant viroid genome is a single-stranded circRNA. However, for many years, very few reports on circRNAs have been published, generally because most endogenous circRNAs are expressed at a low level and the sequencing technology does not have enough resolution power. Many circRNAs have been identified and characterized after the development of second-generation sequencing technology, and have been found in humans and other species. In 2012, Salzman et al.11 reported hundreds of back-spliced RNA products that were resistant to exonuclease RNase R, which represent circular RNAs. Since then, many circRNAs have been found in a variety of cells and tissue samples from human and other species. According to the circBase database, more than 140,000 circRNAs have been reported in human cells12. Earlier, circRNAs were widely considered as a “byproduct” of the RNA splicing process and were rarely considered as functional molecules. However, with the development of processes for circRNA screening and identification based on second-generation sequencing, many circRNAs have been observed to exhibit tissue- and disease-specific expression patterns, which suggested that it is necessary to further explore the biological functions of circRNAs13. Functional models of circRNAs have also been developed. The fascinating features of circRNAs, including relative low abundance, high sequence diversity, tissue-/disease-specific expression, and novel functions, make these studies more appealing.
CircRNAs are expressed in tissue-/disease-specific patterns
Main mechanisms of circRNAs biogenesis
The biogenesis of circRNAs is regulated at several levels. CircRNAs can be derived from exons, introns, and intergenic sequences and even via chromosome translocation14,15. Most circRNAs are derived from exons of coding RNAs. These exon-derived circRNAs are formed by back-splicing, that is, the joining of downstream exon with upstream exons14. Most exon-derived circRNAs share the same sequences with corresponding sequence of host genes. However, there are many alternative splicing products corresponding to the same junction point16,17. This reminds us that the mechanism of circRNA biogenesis is high complex and still largely unknown. To our knowledge, the expression of circRNAs can be regulated by both cis-elements and trans-factors. The cis-element is mostly based on the reverse complete sequences in the flanking sequence of upstream and downstream introns. In the human genome, the most apparent reverse complete sequence is the Alu element18. Inverted repetitive Alu elements (IRAlus) are distributed in both sides of a reverse spliced exon-intron joint point and can improve the complete reversion of flanking introns that enhances circRNA production. In addition to IRAlus, short reverted sequences also support the generation of circRNAs19. With the exception of the reverse complete sequence elements, RNA binding proteins (RBPs) can also aid in the biogenesis of circRNAs. As of now, many RBPs such as MBL, QKI, DHX9, NF90/NF110, FUS, HNRNPL, ESRP1, RMB20, ADAR1, and some components of the splicing complex have been reported to be involved in circRNA biogenesis14,20. The mechanisms of tissue- and disease-specific circRNA biogenesis still remain largely unclear.
Cell-/tissue-specific expression pattern of circRNAs
In addition to the wide sequence diversity of circRNAs, they are always expressed in low levels, and some circRNAs are expressed in a tissue-/disease-specific manner. Many studies have explored the tissue-specific expression patterns of circRNAs. Salzman et al.21 reported a systematic analysis and identification of circRNAs from online RNA-seq data obtained at the time from several human cell lines as well as Drosophila brain. This study revealed that the expression of circRNAs was cell-type specific. Dang et al.22 analyzed the single cell expression pattern of circRNAs in human pre-implantation embryos, and found that expression pattern of circRNA undergo a very large dynamic process accompanying the progression of embryo. In another single cell circRNA study, Koh et al.23 reported that expression of circRNAs generated from ASXL1 gene exhibited high diversity among cells. Xu et al.24 reported the circRNA expression profiles of several human normal tissues. There were 36 samples from adult tissues in this study, including 15 samples from six adult normal tissues (colon, heart, kidney, liver, lung, and stomach), 12 samples from six fetal normal tissues (colon, stomach, liver, heart, lung and kidney), and nine samples from four normal gland tissues (adrenal gland, mammary gland, pancreas, and thyroid gland). The results showed that the expression patterns of circRNAs in different tissues are highly diverse. In another study, Maass et al.25 also reported similar results for other types of tissues and cells. Among these tissues, the expression pattern of circRNAs in the brain was highly diverse. Rybak-Wolf et al.26 reported that in the mammalian brain, circRNAs are highly abundant, conserved, and dynamically expressed. The authors separated different regions of the brain, primary neurons, and isolated synapses, and found that thousands of circRNAs expressed in these tissues and cells. Some of the circRNAs were conserved between human and mouse, and some were even conserved in Drosophila. The expression of circRNAs in the brain is dynamic, and it was found that ADAR1 negatively regulated the expression of circRNAs26. The expression of circRNAs in different tissues or cells is dynamically regulated by cell-/tissue-specific mechanisms, which are still largely unknown.
CircRNAs in human brain
Compared to other tissues, circRNAs are more diverse in neuronal tissues27. Rybak-Wolf et al.26 reported that circRNAs are dynamically expressed at high level in mammalian brains. In this study, authors combined online data from ENCODE project and RNA-seq in separate brain regions, primary neurons, and isolated synapses samples, and found 65, 731 brain-specific circRNAs26. A wide variety of circRNAs have also been found in mouse and Drosophila28,29. Xia et al.30 collected the tissue-specific circRNAs in human and mouse tissues, and found that there are 89, 137 brain-specific circRNAs in human fetus. Why circRNAs tend to be enriched in brain tissue? One possible reason is abundant alternative splicing factor and RBP in brain tissues30. Many brain-specific circRNAs are gathered in the synaptic neuropil28. However, the cell type specific expression pattern of circRNAs in neuron and glial cells is still unknown. CircRNAs also play some important roles in brain or central nervous system diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and cerebrovascular disease31.
Disease-specific expression pattern of circRNAs
circRNAs are also expressed in disease-specific patterns. The expression of circRNAs is different between diseased and normal tissues, demonstrating a disease-dependent expression. A large number of disease-specific circRNAs has been reported in many types of human diseases, such as cancer, cardiovascular diseases, neurological disorders, diabetes, atherosclerosis, infectious diseases, aging-related diseases, and physiological decline32,33. Guarnerio et al.15 reported that the circRNA that generated from a fusion gene (f-circRNA) can directly function as an oncogene in human leukemia. They found that f-circRNA generated from MLL/AF9 fusion gene (F-circM9) can directly influence cell transformation, growth, and drug resistance, as well as promote tumor formation in vivo15. Shan et al.34 reported that circHIPK3 is significantly upregulated in diabetic retina. In diabetic retinopathy, circHIPK3 inhibited the function of miR-30a and enhanced endothelial proliferation and vascular dysfunction34. Besides, there are large numbers of reports on association between circRNAs and human diseases. This proves the importance of research about circRNAs in human diseases.
General characteristics of circRNAs in human cancers
The expression of circRNAs in many human cancers shows a high degree of diversity. Many studies have aimed to identify cancer-specific circRNA expression, which may play an important role in cancer diagnosis and treatment. Almost all types of human cancers have been studied for circRNA expression, and some important cancer-specific circRNAs have been reported.
A thorough list of human cancer-specific circRNAs has been generated13,35. The functional models of these circRNAs cover the following four main functions: miRNA sponge, protein sponge, protein translation, and scaffold for protein complex13. Among these circRNAs, some act as oncogenes (e.g., circPVT1, CDR1as, and cirMYLK), while others function as tumor suppressors (e.g., circITCH, circFOXO3, and circMTO1)13. Therefore, the cancer-specific expression status and functional mode of circRNAs may be used in cancer diagnosis and treatment in the future.
Diverse functions of circRNAs
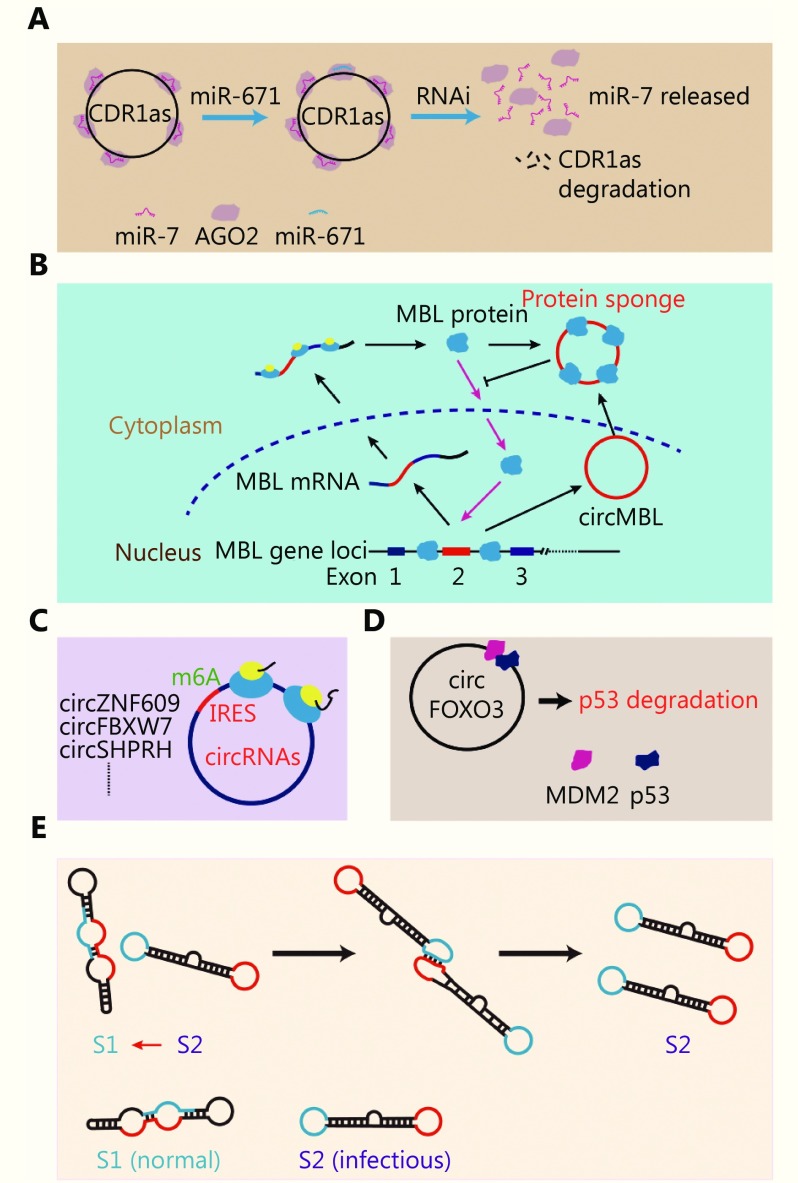
As a newly discovered type of ncRNA, circRNAs share many functions with traditional ncRNAs. At present, five functional models of circRNAs have been reported: (1) circRNAs function as “miRNA sponges”, which competitively bind endogenous miRNAs; (2) circRNAs function as “protein sponges”, which competitively bind endogenous RNA-binding proteins; (3) circRNAs function as “coding RNAs”, which means that they directly encode a micropeptide or protein; (4) circRNAs function as “protein scaffolds”, which means that circRNAs directly bind to proteins and maintain the stability of the protein complex; (5) circRNAs have “prion-like functions”, which indicates that circRNAs exhibit prion-like activity by catalyzing secondary structure changes.
CircRNAs function as miRNA sponges
Competitive endogenous RNAs (ceRNAs) belong to the general functional model family of ncRNAs. In this model, some ceRNAs can regulate target RNAs by competitively binding other RNAs (usually miRNAs), which prevents the binding of miRNAs with their target RNAs and inhibits the function of these miRNAs. Many reports have considered the miRNA sponge function of circRNAs. A typical example of an miRNA sponge is the antisense transcript of the cerebellar degeneration-related protein 1 (CDR1as) gene36,37. Mature CDR1as, which is located in the cytoplasm, has 74 miR-7 binding sites that can specifically absorb miR-7 molecules. As a result, the miR-7 downstream genes are released from binding to miR-7. In this model, the level of molecular miRNA sponging can subtly regulate the binding panel of target miRNAs. In addition to miR-7 binding sites, binding sites for miR-671 have also been studied. When miR-671 binds to CDR1as, pathways that are mediated by AGO2 RNA interference are activated and CDR1as are degraded. Thus, the absorbed miR-7 is released to regulate downstream genes36,37 (Figure 2A).
2.
Function model of circRNAs. (A) MiRNA sponge model. (B) Protein sponge model. (C) Ttranslated into peptides. (D) Complex scaffold. (E) Prion-like activity.
CircRNAs function as protein sponges
Similar to their role as miRNA sponges, circRNAs also act as protein sponges and can bind to certain RNA-binding proteins and influence their location and function. A typical example of a circRNA protein sponge is the muscleblind (MBL/MBNL1) gene38. circMBL and the flanking introns contain several MBL binding sites. This circRNA can competitively bind to MBL protein and influence the function of MBL38. Another example of a protein sponge is circPABPN139. circPABPN1 can bind HuR protein and influence its binding to PABPN1 mRNA39(Figure 2B).
CircRNAs can be directly translated into peptides or proteins
Similar to linear ncRNA, circRNAs also have coding potential. As of now, five reports have confirmed the translation of circRNAs. Yang et al.40 reported that the translation of circRNAs is modified by m6A and a novel cap-independent translation mechanism involving eIF4G2 and m6A reader, YTHDF3. Legnini et al.41 reported that circ-ZNF609 can be translated by a cap-independent translation mechanism. Using ribosome footprint profiling, Pamudurti et al.42 reported that a group of circRNAs is associated with ribosomes, which indicates that these circRNAs can be translated into proteins. Yang. et al.40 reported that circFBXW7 has coding potential and that its translated product could be verified by an antibody targeted to related sequences43. Zhang et al.44 reported that circSHPRH also has coding abilities and that its translated peptide could inhibit the progression of glioblastoma (GBM) (Figure 2C). With further research, we believe that more examples of circRNAs that can be translated into proteins could be found in the near future.
CircRNAs function as protein scaffolds
In contrast to the protein sponge model, as scaffolds, circRNAs provide sites for protein interactions and for the formation of larger functional complexes. Li et al.8 reported that a type of circRNA in which exons are circularized with introns ‘retained’ between exons (EIciRNA) could bind to U1 snRNP, promote the binding of U1 snRNP to RNA polymerase II, and enhance the transcription of host target genes. In this case, EIciRNA acts as a linker of U1 snRNP and the RNA polymerase II complex. Du et al.45 reported that circFOXO3 could bind to both p53 and MDM2 and could enhance ubiquitination and degradation of p53. However, circFOXO3 exhibits low binding affinity to the FOXO3 protein, which results in a reduction in the interaction between FOXO3 and MDM2 and prevention of ubiquitination of FOXO3 by MDM2 (Figure 2D). In this model, circRNAs function as linkers between proteins.
CircRNAs with prion-like activity
Ribozyme is a molecule that can catalyze specific biochemical reactions. Whether circRNAs can act as functional molecules, like ribozymes, remains to be determined. Badelt et al.46 reported a circRNA molecule that could function similarly to a prion (Figure 2E). In this study, the authors designed a circRNA that could trigger the autocatalytic replication of molecules by interaction. The structures of circRNAs are still poorly understood, and thus, further verification is needed to determine if such a molecule actually exists. However, this study provided some clues that circRNAs may also possess properties consistent with those of prions or ribozymes.
CircRNAs in human glioma
Expression pattern of circRNAs in human glioma
According to the Global Cancer Statistics, 2018, new cases of central nervous system (CNS) tumors comprise 1.6% of all new tumor cases and 2.5% of all cancer-related deaths47. Glioma is a common type of CNS tumor and includes two major subgroups: nondiffuse glioma (glioma cells showing a more limited circumscribed growth pattern) and diffuse glioma (glioma cells exhibiting extensive invasive growth into the surrounding CNS)48,49. Diffuse glioma is the most frequent CNS tumor especially in adults48. Diffuse gliomas mainly include IDH-mutant astrocytoma, oligodendroglioma, and glioblastoma (GBM, both IDH-mutant and wild-type)49. Several studies have focused on the expression pattern of circRNAs in glioma.
Song et al.50 conducted a study that included seven oligodendrogliomas, 20 glioblastomas and 19 normal brain specimens to explore the expression level of circRNAs using high-throughput sequencing. The authors developed a computational pipeline termed UROBORUS to analyze the data in this study. They found that the total number of detected circRNAs in GBM was significantly lower than that in normal brain tissue and in oligodendroblastoma samples. However, no difference was observed between normal brain tissue and the oligodendroblastoma samples. In summary, the authors found 572 highly expressed circRNAs (RPM > 0.1) in 46 samples. Among them, 476 circRNAs were differentially expressed between GBM and normal brain tissues. Among the 476 circRNAs, 468 exhibited higher expression in normal brain tissues than in GBM samples, and eight exhibited higher expression in GBM than in normal brain tissues 50. However, even among normal brain samples from different parts of the brain, different circRNA expression was observed50. This study provided strong evidence of tissue- and disease-specific expression patterns of circRNAs.
Wang et al.51 analyzed 33 paired IDH wild-type GBM and para-cancerous tissues by microarray and found 254 upregulated and 361 downregulated circRNAs in GBM compared with para-cancerous tissues (fold-change > 1.5). Wang et al. 52 also reported circRNA expression in GBM by microarray. In this study, they selected three paired GBM and para-cancerous tissues, and subjected them to a microarray analysis (circRNA, lncRNA, mRNA), and found 548 upregulated circRNAs/lncRNAs in GBM (fold-change > 2, P < 0.05) 52. Zhang et al.44 compared the circRNA expression level between ten paired glioma and adjacent normal brain tissues by high-throughput sequencing and found upregulation of 2,709 differentially expressed circRNAs (fold-change > 2). Furthermore, the authors also performed a microarray assay to analyze 12 primary GBMs and five normal brain specimens, and they found 709 differentially expressed circRNAs in GBM. In all, 105 differentially expressed circRNAs were found after cross-matching the above two datasets 44. Xu et al.53 analyzed circRNA expression by downloading RNA-seq data for three paired glioma and normal brain tissues from the Gene Expression Omnibus (GEO) database. Using five different circRNA analysis tools, they found 12 commonly expressed circRNAs. Though many studies have focused on the differential expression of circRNAs in glioma, rare circRNAs have been observed. The expression pattern of circRNAs in glioma may also show a high degree of diversity among individuals. This indicates the necessity of a larger sample size for screening.
In addition to these high-throughput screening studies, several studies have reported that some circRNAs may be potential biomarkers of glioma. Song et al.50 reported that the eight highly expressed GBM-specific circRNAs might be good GBM-specific biomarker candidates. However, the authors did not further verify the expression of these circRNAs in a larger number of samples. Barbagallo et al.54 detected the expression level of circSMARCA5 in 56 formalin-fixed paraffin-embedded (FFPE) GBM biopsy samples and 7 normal controls. The results showed that circSMARCA5 was significantly downregulated in GBM compared with the control, while the levels of linear mRNA of the host gene SMARCA5 were not significantly different54. Bian et al.55 analyzed the expression level of circCFH in 31 glioma tumor samples and paired adjacent normal tissues55, and showed that circCFH was significantly upregulated in both grade I-II and grade III-IV glioma samples55. Xie et al.56 reported the expression of hsa-circ-0012129 in 31 paired glioma tumors and adjacent normal tissues, and the results revealed a significantly higher expression of hsa-circ-0012129 in glioma tissues compared with adjacent tissues.
In summary, circRNAs that may be candidate biomarkers of glioma are listed in Table 1, and the sample number of glioma tissues and normal controls is also shown.
1.
Candidate circRNA biomarkers in glioma
| CircRNAs | Sample information | Expression status in glioma | Screening methods | References |
| (Listed according to the published date) | ||||
| CircCOL1A2, circPTN,
circVCAN, circSMO, circPLOD2, circGLIS3, circEPHB4, circCLIP2 |
7 oligodendroglioma, 20 glioblastoma and 19 normal brain tissues | GBM-specific high expression (P < 0.05, Wilcoxon rank-sum test and Benjamini-Hochberg correction) | RNA-seq | 50 |
| Circ-FBXW7 | 10 paired glioma tumor and adjacent normal tissues for RNA-seq, 100 paired samples for RT-QPCR | Significantly downregulated in glioma samples | RNA-seq and RT-QPCR | 43 |
| Hsa_circ_0046701 | 30 paired glioma tumor and adjacent normal tissues | Significantly upregulated in glioma samples | RT-QPCR | 57 |
| CircSMARCA5 | 56 formalin-fixed paraffin embedded (FFPE) GBM biopsy samples and 7 normal controls | Significantly downregulated in GBM (2.42 fold changed) | RT-QPCR | 54 |
| Circ-SHKBP1 | 5 grade I–II glioma and 5 grade III–IV glioma and 5 normal brain tissues | Significantly upregulated in LGG (2.020 ± 0.2367-fold) and HGG (3.4580 ± 0.2831-fold) | RT-QPCR | 58 |
| Hsa-circ-0012129 | 31 paired glioma tumor and adjacent normal tissues | Significantly upregulated in glioma samples | RT-QPCR | 56 |
| Circ-ITCH | 60 paired glioma tumor and adjacent normal tissues | Significantly downregulated in glioma samples | RT-QPCR | 59 |
| Circ-SHPRH | 60 paired glioma tumor and adjacent normal tissues | Significantly downregulated in glioma samples | RT-QPCR | 44 |
| Hsa_circ_0000177 | 62 paired glioma tumor and adjacent normal tissues | Significantly upregulated in glioma samples | RT-QPCR | 60 |
| Hsa-circ- 0001649 | 64 paired glioma tumor and adjacent normal tissues | Significantly downregulated in glioma samples | RT-QPCR | 61 |
| CircHIPK3 | 48 paired glioma tumor and adjacent normal tissues | Significantly upregulated in glioma samples | RT-QPCR | 62 |
| CircNFIX | 3 paired glioma tumor and adjacent normal tissues | Significantly upregulated in glioma samples | RNA-seq | 53 |
| Circ-CFH | 31 glioma tumor samples and paired adjacent normal tissues | Significantly upregulated both in grade I–II and grade III–IV glioma samples (5.97 fold changed) | RT-QPCR | 55 |
| Hsa_circ_0074362 | 62 paired glioma tumor and adjacent normal tissues | Significantly upregulated in GBM tissues | RT-QPCR | 63 |
| CircMMP9 | 3 paired GBM tumor and adjacent normal tissues | Significantly upregulated in GBM tissues (68.57fold changed) | Microarray | 64 |
Function of circRNAs in human glioma
In addition to the expression analysis of circRNAs in glioma, knowledge of the functions of circRNAs in glioma has also advanced. Here, we summarized the functional models, roles in glioma carcinogenesis, and the values of candidate circRNAs in the treatment of glioma.
Modes of circRNA function in glioma
Along with the development of functional studies of circRNAs, knowledge of the functional models of circRNAs in glioma has also progressed. Several circRNAs function as miRNA sponges that influence many important genes and pathways related to glioma. circFBXW7 and circSHPRH can be directly translated into proteins, and both play a role in the inhibition of GBM progression. circSMARCA5 can bind to SRSF1 and regulate the splicing process. Other functional models of circRNAs have not been reported until now.
Several studies have focused on the miRNA sponge function of circRNAs in glioma. Wang et al.52 reported that circNT5E can bind to miR-422a and inhibit its activity. In this study, they selected miR-422a as a candidate miRNA to screen for circRNAs that directly bind to miR-422a. miR-442a is a brain-enriched miRNA that also plays a role in the progression of head and neck squamous cell carcinoma65, squamous cell lung cancer66, and glioma67. Both bioinformatic prediction analysis and a biotin-labeled miR-442a pull-down assay confirmed the binding of miR-442a with circNT5E, which was significantly upregulated in glioma samples52. In this study, using RNA pull-down assay and fluorescence in situ hybridization (FISH) to verify the interaction between miR-442a and circNT5E, the authors showed that circNT5E could bind to miR-442a and inhibit its function. In this case, the expression levels of miR-442a and circNT5E were opposite of each other. This relationship between circRNA and target miRNA is pervasive in many reports considering the role of circRNA as an miRNA sponge. However, in the earliest miRNA sponge model of CDR1as and miR-7, the expression level of CDR1as was not influenced by miR-7, but rather, was regulated by another miRNA, miR-67136,37. Similarly, Xu et al.53 also found that circNFIX interacted with miR-34a-5p using RNA immunoprecipitation (RIP). In this case, circNFIX could bind to miR-34a-5p, and NOTCH1 was found to be a target of miR-34a-5p. When circNFIX was inhibited by siRNA, the expression level of miR-34a-5p was increased, and NOTCH1 was inhibited53. The relationship of circRNAs and related miRNAs in this model will be discussed in the next part of this review.
The miR-671/CDR1as/miR-7 axis also plays a role in glioma. Barbagallo et al.68 analyzed the expression of miR-671 in 45 GBM samples and five GBM cell lines, and the results showed that the miR-671/CDR1as/miR-7 axis was also present in GBM. Overexpression of miR-671-5p significantly increased migration but had a lower impact on cell proliferation in GBM68. In addition to these cases, many other reports based on the miRNA sponge model in glioma have been published, and these are summarized in Table 2.
2.
CircRNAs function as miRNA sponges in glioma
| CircRNA | MiRNA | Related genes and pathways | References |
| (Listed followed the published date) | |||
| CDR1-AS | MiR-7 | MiR-671-5p/CDR1-AS/CDR1/VSNL1 axis | 68 |
| Circ-TTBK2 | MiR-217 | HNF1β/Derlin-1 pathway | 69 |
| Hsa_circ_0046701 | MiR-142-3p | Sponge of MiR-142-3p that regulates the expression of ITGB8 | 57 |
| Circ-SHKBP1 | MiR-544a | FOXP1 | 58 |
| Circ-SHKBP1 | MiR-379 | FOXP2 | 58 |
| Hsa_circ_0007534 | MiR-761 | ZIC5 | 70 |
| Hsa_circ_0012129 | MiR-661 | -- | 56 |
| Circ-ITCH | MiR-214 | Sponge of MiR-214 that promotes linear ITCH expression | 59 |
| CircNT5E | MiR-422a | Directly binds to MiR-422a and inhibits MiR-422a activity | 52 |
| Hsa_circ_0000177 | MiR-638 | MiR-638-FZD7-Wnt Signaling Cascade | 60 |
| CircHIPK3 | MiR-654 | Interacts with MiR-654 and promotes IGF2BP3 expression | 62 |
| CircNFIX | MiR-34a-5p | Sponge of MiR-34a-5p, an MiRNA that targets NOTCH1 | 53 |
| Circ-CFH | MiR-149 | AKT1 | 55 |
| Hsa_circ_0074362 | MiR-1236-3p | HOXB7 pathway | 63 |
| CircMMP9 | MiR-124 | CDK4 and Aurora A | 64 |
| Hsa_circ_0076248 | MiR-181a | SIRT1 | 71 |
| Hsa_circ_0034642 | MiR-1205 | BATF3 | 72 |
In addition to their ability to act as an miRNA sponge, circRNAs that are translated into peptides or proteins have also been reported. Using ribosome footprinting, Yang et al.43 found that circ-FBXW7 may be translated. circ-FBXW7 contains a spanning junction open reading frame (ORF), which encodes a 185-aa protein, termed as FBXW7-185aa. The translation of circ-FBXW7 is driven by an upstream internal ribosome entry site (IRES). The translation product, FBXW7-185aa, was verified by an antibody generated in-house that targets the related sequences; mass spectrometry fingerprint analysis to determine the related peptide sequences also revealed the translation of circ-FBXW7. Similar to circ-FBXW7, the same group found another circRNA that could be translated. Carrying an IRES and an ORF, the translation product of circ-SHPRH could also be verified by an antibody generated in-house and by mass spectrometry fingerprint analysis44. It is noteworthy that the ORF in circ-SHPRH is an overlapping codon, including the start codon “AUG” and the stop codon “UGA” using the same “A” base44. This might be the first finding of an overlapping codon in mammalian coding genes.
In addition, circRNAs that function as scaffolds for protein complexes have also been reported in glioma. Using enhanced UV crosslinking and immunoprecipitation (eCLIP) to screen for proteins that interact with circSMARCA5, Barbagallo et al.54 found an interaction between circSMARCA5 and SRSF1. Overexpression of circSMARCA5 resulted in the upregulation of SRSF3, which positively regulated glioma cell proliferation54.
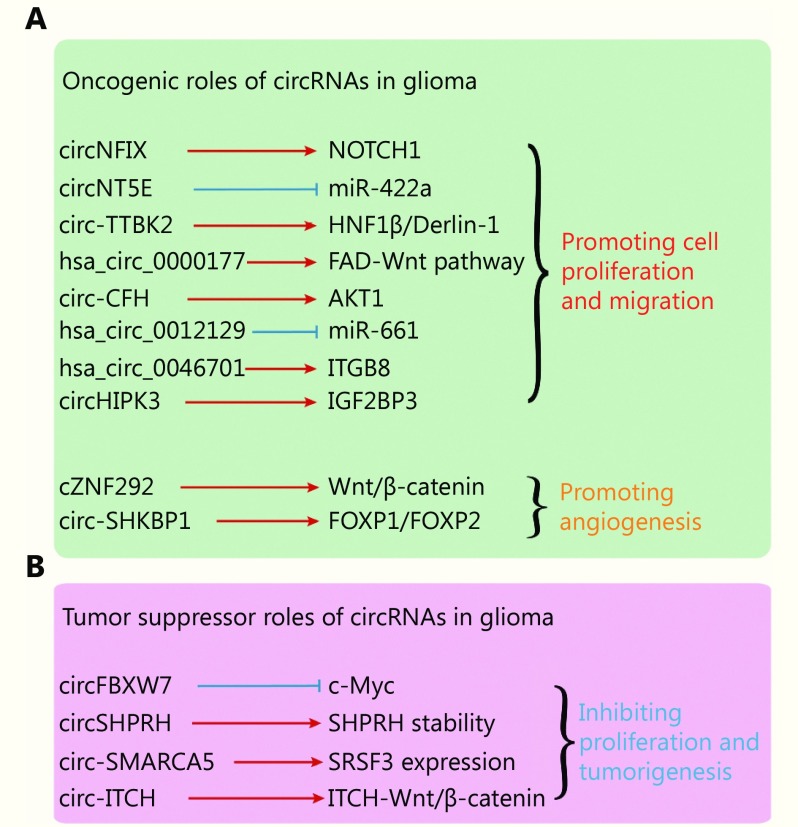
Oncogenic role of circRNAs in glioma
Given the glioma-specific expression pattern of circRNAs, their function in glioma carcinogenesis has also received considerable attention. Functioning in an oncogenic role, a series of circRNAs have been reported to be promoters of the malignant progression of glioma cells (Figure 3A).
3.
Function of circRNAs in glioma. (A) Oncogenic roles of circRNAs in glioma. (B) Tumor suppressor role of circRNAs in glioma.
circNFIX is upregulated in GBM tissues and cell lines53. The inhibition of circNFIX could inhibit cell migration and proliferation by downregulation of the NOTCH1 pathway53, which indicates that circNFIX participates in the improvement of glioma progression. circNT5E can affect the cell proliferation, invasion, and migration abilities of GBM cells by sponging miR-422a52. Zheng et al.69 reported that circ-TTBK2, but not the corresponding TTBK2 linear mRNA, was upregulated in human glioma cells and tumor samples. The overexpression of circ-TTBK2 was found to promote cell proliferation, migration, and invasion in GBM, which further indicates that circ-TTBK2 plays a role in glioma progression69. Chen et al.60 reported that the inhibition of hsa_circ_0000177 by RNAi could significantly inhibit cell proliferation and invasion. Hsa_circ_0000177 participates in glioma progression by sponging miR-638 and influencing the FZD7/Wnt7 pathway60. In addition to the circRNAs mentioned above, circ-CFH55, hsa-circ-001212956, hsa_circ_004670157, and circHIPK362 have also been reported to promote glioma cell proliferation and migration.
In addition to cell proliferation and migration, circRNAs can also promote angiogenesis. He et al.58 found that circ-SHKBP1, but not the corresponding SHKBP1 mRNA, was upregulated in U87 glioma-exposed endothelial cells (GECs) compared with astrocyte-exposed endothelial cells (AECs). The knockdown of circ-SHKBP1 could significantly inhibit cell proliferation, migration, and tube formation of GECs. The circ-SHKBP1 influences GECs via the miR-544a/FOXP1 and miR-379/FOXP2 pathways58. In another study, Yang et al.73 reported that cZNF292 could participate in tube formation in human glioma. cZNF292 circRNA was also found to inhibit tube formation in glioma cells via the Wnt/β-catenin pathway73.
Tumor suppressor role of circRNAs in glioma
On the contrary, many circRNAs also function as tumor suppressors in glioma (Figure 3B). FBXW7-185aa, encoded by circ-FBXW7, can significantly inhibit cell progression, migration, and tumor formation in vivo43. In one study, FBXW7-185aa reduced the half-life of c-Myc by antagonizing USP28-induced c-Myc stabilization43. An in situ GBM mouse model revealed the tumor suppressing effect of FBXW7-185aa but not of circ-FBXW7 circRNA with an IRES mutation43. In addition, circ-SHPRH also could be translated into a peptide of 146aa, termed SHPRH-146aa44. SHPRH-146aa is downregulated in GBM compared with para-cancerous tissues, and in one study, the overexpression of SHPRH-146aa significantly inhibited glioma growth in xenograft mouse models. SHPRH-146aa could also protect full-length SHPRH protein from degradation by the ubiquitin proteasome system44.
In addition to circ-FBXW7 and circ-SHPRH, some other circRNAs have been reported to participate in the inhibition of glioma progression. circSMARCA5 is downregulated in glioma, and the overexpression of circSMARCA5 could increase the expression of SRSF3 and positively regulate the proliferation of glioma cells54. As reported, circ-ITCH59 and hsa_circ_000164961 also function as tumor suppressors in glioma.
CircRNAs as therapeutic targets or strategies in glioma
CircRNAs have various roles in human glioma. In the future, due to their functional characteristics, circRNAs could be used as therapeutic targets or as components of strategies for the treatment of glioma. According to several reports, the knockdown of these circRNAs could significantly inhibit cell proliferation, migration, or angiogenesis. The use of siRNAs specific to these circRNAs might be a good approach for the treatment of glioma in the future. On the contrary, circRNAs that act as tumor suppressors can be used as part of an overexpression strategy.
Besides these traditional ideas, some new progress has been made in engineering circRNAs, which may lead to novel circRNA-based treatments for glioma. Wesselhoeft et al.74 invented a novel method to express proteins by engineering a circRNA vector. Since circRNAs are relatively stable compared with normal linear RNA, protein expression based on circRNAs may improve production efficiency. The results showed that exogenous circRNAs produced exceptionally good protein and exhibited stable production. This suggested that circRNAs might be a good and highly efficient RNA molecule that can be used for protein expression. Meganck et al.75 constructed a tissue-specific circRNA expression vector based on recombinant adeno-associated viral (AAV) vectors. The results showed that this vector efficiently expressed circRNA and translated circRNAs in mouse brain and eyes as well as the heart75. Based on these two reports, we could design circRNAs with specific brain targets based on a protein expression system for the treatment of glioma in the near future.
In addition, the miRNA sponge function of circRNAs can also be used in glioma treatment in the future. Recently, Liu et al.76 reported a circRNA carrying five miR-21 binding sites, termed scRNA21, which can significantly decrease the expression level of miR-21 and inhibit cell proliferation of gastric cancer cell lines. This work is not related to glioma, but the idea of the design and synthesis of a circRNA that targets a certain miRNA is valuable for glioma treatment. miR-21 and many other miRNAs have some important roles in glioma progression77, which has led to the idea that scR21 can also be used to interfere with these miRNAs in glioma. AAV-based circRNA expression vectors are a powerful choice for this idea.
Discussion
The role of RNA in cellular activities is multilayered and multifaceted. CircRNAs, as the most recently discovered RNA molecule, still follow the basic rules of RNA. The current knowledge of circRNAs supports this view. In the human genome, circRNAs are mostly functional molecules in various tissue and cells, and they are expressed in a dynamic manner and function at several levels. However, further detailed important ideas and assumptions still require further exploration.
Challenges of circRNA research in glioma
Although there are a large number of reports about circRNAs in human glioma as well as other diseases, there are many challenges related to circRNA research. Glioma is a highly heterogeneous disease; its pathological manifestation among patients even at different stage of the same individual is ever-changing. In this way, relationship between circRNAs and glioma clinical features is complex and still largely unknown. The mechanism of glioma-specific circRNA expression pattern need to be explored in the future. Furthermore, knock-out model for circRNAs is still a challenge till now. Most circRNAs are generated from coding genes, so accurately knock-out of circRNAs without influencing host gene is still unfeasible for most circRNAs. General low expression level may also hinder the clinical translation for circRNA in glioma and other human diseases.
Nomenclature for circRNAs is still disputed
Not synchronized with the high-speed development of circRNA researches, some important questions have not been solved till now. The nomenclature for circRNAs is a typical question for circRNA. The most used circRNA naming system is based on circBase database ID number12, which consists of a string of numbers and letters. This ID number has no information about the circRNA source and is difficult to remember and orally communicate. The naming rules for circRNAs are urgently needed. A good naming system needs to contain the information of circRNA host gene and should be easy to remember and orally communicate.
Are circRNAs the regulators or targets of miRNAs?
Binding to miRNAs is the most mechanistic model for circRNAs, and experimental evidences have confirmed the binding between circRNAs and miRNAs. The miRNA sponge model is an extension of the ceRNA model, in which RNAs that function as ceRNAs could only bind to target miRNAs, block their free movement, and bind to target molecules. In the case of CDR1as and miR-7, the expression level of CDR1as is not influenced by miR-736,37. MiRNAs function via base-pairing of their seed regions with complementary sequences of their targets78. In the case of mRNAs, miRNAs can target mRNAs via inhibition of protein translation79, regulation of their promoters80, as well as promotion of deadenylation and decay of target mRNAs81,82. In the case of circRNAs, although they can also have translation abilities, few studies have been reported on this topic. The relationship between circRNAs and miRNAs may not simply be due to miRNA sponge activity, which just emphasizes the regulatory role of circRNAs with respect to miRNAs. Conclusive evidence considering circRNAs as the target of miRNAs has already been shown, using miR-671 and CDR1as. Only one site on miR-671 binds to the CDR1as sequence, but this site in miR-671 has near-perfect complementarity and exhibits very little variation across species37. As a result, the expression level of CDR1as is tightly regulated by miR-671 and is based on an RNA interference mechanism. After a review of the relevant reports, in many cases, the expression levels of circRNAs and miRNAs were usually altered in a mutual cause and effect manner. This phenomenon could not be explained solely by the miRNA sponge model.
Are circRNAs the result of alternative splicing?
Alternative splicing is more active in cancer than in normal tissues83. The biogenesis of circRNAs deeply depends on the RNA splicing process14. Many cancer-specific circRNAs are expressed, but the mechanism that underlies their biogenesis has not been fully described. Whether tissue-, cancer-, or disease-specific circRNAs are regulated by alternative splicing remains unknown. The answer to this question may, at least in part, explain the specific expression pattern of circRNAs. Gene fusion by chromosome translocation is also widely found in high grade glioma and secondary GBM (sGBM). Some fusion genes play an oncogenic role in GBM, such as FGFR-TACC, and PTPRZ1-MET84,85. FGFR-TACC fusion gene account for 3% of human glioblastoma cases85; this fusion gene causes a metabolism shift in fusion gene positive cells by activating oxidative phosphorylation and mitochondrial biogenesis pathway, generating sensitivity for inhibitors of oxidative metabolism86. As previously reported, circRNAs derived from fusion gene can also function as oncogenes15. What about circRNAs derived from glioma related fusion gene? Were there any circRNAs that derived from FGFR-TACC, PTPRZ1-MET, and many other glioma-related fusion genes? What are the roles of these circRNAs in GBM? This is an interesting question.
In summary, circRNAs belong to the most recently discovered group of RNA molecules. CircRNAs can function as miRNA sponges, protein sponges, and as scaffolds for protein complexes, and can also be translated into proteins. In some cases, circRNAs may also possess special properties. The expression of circRNAs is cell- or tissue-specific, and many circRNAs are also disease-specific, some of which play important roles in the progression of human cancers. In human glioma, all the major features of circRNAs are exhibited. CircRNAs may also be promising therapeutic targets or components of therapeutic strategies for glioma treatment as well as treatment of other human diseases.
Conflict of interest statement
No potential conflicts of interest are disclosed.
References
- 1.Li GW, Xie XS Central dogma at the single-molecule level in living cells. Nature. 2011;475:308–15. doi: 10.1038/nature10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgs PG, Lehman N The rna world: Molecular cooperation at the origins of life. Nat Rev Genet. 2015;16:7–17. doi: 10.1038/nrg3841. [DOI] [PubMed] [Google Scholar]
- 3.Huang B, Zhang RX Regulatory non-coding RNAs: revolutionizing the RNA world. Mol Biol Rep. 2014;41:3915–23. doi: 10.1007/s11033-014-3259-6. [DOI] [PubMed] [Google Scholar]
- 4.Ransohoff JD, Wei YN, Khavari PA The functions and unique features of long intergenic non-coding RNA. Nat Rev Mol Cell Biol. 2018;19:143–57. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, et al Toddler: an embryonic signal that promotes cell movement via apelin receptors . Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sampath K, Ephrussi A CncRNAs: RNAs with both coding and non-coding roles in development. Development. 2016;143:1234–41. doi: 10.1242/dev.133298. [DOI] [PubMed] [Google Scholar]
- 7.Candeias MM, Malbert-Colas L, Powell DJ, Daskalogianni C, Maslon MM, Naski N, et al p53 mrna controls p53 activity by managing Mdm2 functions . Nat Cell Biol. 2008;10:1098–105. doi: 10.1038/ncb1770. [DOI] [PubMed] [Google Scholar]
- 8.Li ZY, Huang C, Bao C, Chen L, Lin M, Wang XL, et al Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 9.Eger N, Schoppe L, Schuster S, Laufs U, Boeckel JN. Circular RNA splicing. In: Xiao JJ. Circular RNAs: Biogenesis and Functions. Singapore: Springer; 2018; 41-52.
- 10.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 1976;73:3852–6. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glažar P, Papavasileiou P, Rajewsky N CircBase: a database for circular RNAs. RNA. 2014;20:1666–70. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen LS, Hansen TB, Veno MT, Kjems J Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555–65. doi: 10.1038/onc.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Yang L, Chen LL The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71:428–42. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Guarnerio J, Bezzi M, Jeong JC, Paffenholz SV, Berry K, Naldini MM, et al Oncogenic role of fusion-circRNAs derived from cancer-associated chromosomal translocations. Cell. 2016;165:289–302. doi: 10.1016/j.cell.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Wang JF, Zheng Y, Zhang JY, Chen S, Zhao FQ Comprehensive identification of internal structure and alternative splicing events in circular RNAs. Nat Commun. 2016;7:12060. doi: 10.1038/ncomms12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang XO, Dong R, Zhang Y, Zhang JL, Luo Z, Zhang J, et al Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res. 2016;26:1277–87. doi: 10.1101/gr.202895.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–57. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang DM, Wilusz JE Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28:2233–47. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu CY, Li TC, Wu YY, Yeh CH, Chiang W, Chuang CY, et al The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency . Nat Commun. 2017;8:1149. doi: 10.1038/s41467-017-01216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang YJ, Yan LY, Hu BQ, Fan XY, Ren YX, Li R, et al Tracing the expression of circular RNAs in human pre-implantation embryos. Genome Biol. 2016;17:130. doi: 10.1186/s13059-016-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh W, Gonzalez V, Natarajan S, Carter R, Brown PO, Gawad C Dynamic ASXL1 exon skipping and alternative circular splicing in single human cells. PLoS One. 2016;11:e0164085. doi: 10.1371/journal.pone.0164085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu T, Wu J, Han P, Zhao Z, Song X Circular RNA expression profiles and features in human tissues: a study using RNA-seq data. BMC Genomics. 2017;18:680. doi: 10.1186/s12864-017-4029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maass PG, Glažar P, Memczak S, Dittmar G, Hollfinger I, Schreyer L, et al A map of human circular RNAs in clinically relevant tissues. J Mol Med (Berl) 2017;95:1179–89. doi: 10.1007/s00109-017-1582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S, et al Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58:870–85. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Schuman E Circular RNAs in brain and other tissues: a functional enigma. Trends Neurosci. 2016;39:597–604. doi: 10.1016/j.tins.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 28.You XT, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, et al Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18:603–10. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P, et al Genome-wide analysis of Drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation . Cell Rep. 2014;9:1966–80. doi: 10.1016/j.celrep.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia SY, Feng J, Lei LJ, Hu J, Xia LJ, Wang J, et al Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Brief Bioinform. 2017;18:984–92. doi: 10.1093/bib/bbw081. [DOI] [PubMed] [Google Scholar]
- 31.Shao YY, Chen YH Roles of circular RNAs in neurologic disease. Front Mol Neurosci. 2016;9:25. doi: 10.3389/fnmol.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R, et al Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci. 2017;4:38. doi: 10.3389/fmolb.2017.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haque S, Harries LW Circular RNAs (circRNAs) in health and disease. Genes (Basel) 2017;8:353. doi: 10.3390/genes8120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shan K, Liu C, Liu BH, Chen X, Dong R, Liu X, et al Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136:1629–42. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 35.Han B, Chao J, Yao HH Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi: 10.1016/j.pharmthera.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 37.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 38.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, et al CircRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S, et al Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1 . RNA Biol. 2017;14:361–9. doi: 10.1080/15476286.2017.1279788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Fan XJ, Mao MW, Song XW, Wu P, Zhang Y, et al Extensive translation of circular RNAs driven by N6-methyladenosine . Cell Res. 2017;27:626–41. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legnini I, Di Timoteo G, Rossi F, Morlando M, Briganti F, Sthandier O, et al Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, et al Translation of CircRNAs. Mol Cell. 2017;66:9–21. e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang YB, Gao XY, Zhang ML, Yan S, Sun CJ, Xiao FZ, et al Novel role of FBXW7 circular RNA in repressing glioma tumorigenesis. J Natl Cancer Inst. 2018;110:304–15. doi: 10.1093/jnci/djx166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang ML, Huang NN, Yang XS, Luo JY, Yan S, Xiao FZ, et al A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis . Oncogene. 2018;37:1805–14. doi: 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- 45.Du WW, Fang L, Yang WN, Wu N, Awan FM, Yang ZG, et al Induction of tumor apoptosis through a circular RNA enhancing foxo3 activity. Cell Death Differ. 2017;24:357–70. doi: 10.1038/cdd.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badelt S, Flamm C, Hofacker IL Computational design of a circular rna with prionlike behavior. Artif Life. 2016;22:172–84. doi: 10.1162/ARTL_a_00197. [DOI] [PubMed] [Google Scholar]
- 47.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.v68.6. [DOI] [PubMed] [Google Scholar]
- 48.Wesseling P, Capper D Who 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44:139–50. doi: 10.1111/nan.2018.44.issue-2. [DOI] [PubMed] [Google Scholar]
- 49.Masui K, Mischel PS, Reifenberger G Molecular classification of gliomas. Handb Clin Neurol. 2016;134:97–120. doi: 10.1016/B978-0-12-802997-8.00006-2. [DOI] [PubMed] [Google Scholar]
- 50.Song XF, Zhang NB, Han P, Moon BS, Lai RK, Wang K, et al Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res. 2016;44:e87. doi: 10.1093/nar/gkw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang HX, Huang QL, Shen JY, Xu T, Hong F, Gong ZY, et al Expression profile of circular RNAs in idh-wild type glioblastoma tissues. Clin Neurol Neurosurg. 2018;171:168–73. doi: 10.1016/j.clineuro.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Wang RJ, Zhang S, Chen XY, Li N, Li JW, Jia RC, et al CircNT5E acts as a sponge of miR-422a to promote glioblastoma tumorigenesis. Cancer Res. 2018;78:4812–25. doi: 10.1158/0008-5472.CAN-18-0532. [DOI] [PubMed] [Google Scholar]
- 53.Xu HY, Zhang Y, Qi L, Ding LJ, Jiang H, Yu HQ NFIX circular RNA promotes glioma progression by regulating miR-34a-5p via notch signaling pathway. Front Mol Neurosci. 2018;11:225. doi: 10.3389/fnmol.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbagallo D, Caponnetto A, Cirnigliaro M, Brex D, Barbagallo C, D'Angeli F, et al CircSMARCA5 inhibits migration of glioblastoma multiforme cells by regulating a molecular axis involving splicing factors SRSF1/SRSF3/PTB. Int J Mol Sci. 2018;19:480. doi: 10.3390/ijms19020480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bian AM, Wang YP, Liu J, Wang XD, Liu D, Jiang J, et al Circular RNA complement factor H (CFH) promotes glioma progression by sponging mir-149 and regulating AKT1. Med Sci Monit. 2018;24:5704–12. doi: 10.12659/MSM.910180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie G Circular RNA hsa-circ-0012129 promotes cell proliferation and invasion in 30 cases of human glioma and human glioma cell lines U373, A172, and SHG44, by targeting MicroRNA-661(miR-661) Med Sci Monit. 2018;24:2497–507. doi: 10.12659/MSM.909229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li GB, Yang HY, Han K, Zhu DZ, Lun P, Zhao Y A novel circular RNA, hsa_circ_0046701, promotes carcinogenesis by increasing the expression of miR-142-3p target ITGB8 in glioma. Biochem Biophys Res Commun. 2018;498:254–61. doi: 10.1016/j.bbrc.2018.01.076. [DOI] [PubMed] [Google Scholar]
- 58.He QR, Zhao LN, Liu YH, Liu XB, Zheng J, Yu H, et al Circ-SHKBP1 regulates the angiogenesis of U87 glioma-exposed endothelial cells through miR-544a/FOXP1 and miR-379/FOXP2 pathways. Mol Ther Nucleic Acids. 2018;10:331–48. doi: 10.1016/j.omtn.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li F, Ma K, Sun MH, Shi S Identification of the tumor-suppressive function of circular RNA ITCH in glioma cells through sponging miR-214 and promoting linear ITCH expression. Am J Transl Res. 2018;10:1373–86. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Chen ZQ, Duan XB Hsa_circ_0000177-miR-638-FZD7-wnt signaling cascade contributes to the malignant behaviors in glioma. DNA Cell Biol. 2018;37:791–7. doi: 10.1089/dna.2018.4294. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Sui X, Zhao H, Cong L, Li Y, Xin T, et al Decreased circular RNA hsa_circ_0001649 predicts unfavorable prognosis in glioma and exerts oncogenic properties in vitro and in vivo . Gene. 2018;676:117–22. doi: 10.1016/j.gene.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 62.Jin PC, Huang YN, Zhu PL, Zou Y, Shao TT, Wang OY CircRNA circHIPK3 serves as a prognostic marker to promote glioma progression by regulating miR-654/IGF2BP3 signaling. Biochem Biophys Res Commun. 2018;503:1570–4. doi: 10.1016/j.bbrc.2018.07.081. [DOI] [PubMed] [Google Scholar]
- 63.Duan XB, Liu DL, Wang Y, Chen ZQ Circular RNA hsa_circ_0074362 promotes glioma cell proliferation, migration, and invasion by attenuating the inhibition of mir-1236-3p on HOXB7 expression. DNA Cell Biol. 2018;37:917–24. doi: 10.1089/dna.2018.4311. [DOI] [PubMed] [Google Scholar]
- 64.Wang RJ, Zhang S, Chen XY, Li N, Li JW, Jia RC, et al EIF4A3-induced circular RNA MMP9(circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer. 2018;17:166. doi: 10.1186/s12943-018-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonnin N, Armandy E, Carras J, Ferrandon S, Battiston-Montagne P, Aubry M, et al MiR-422a promotes loco-regional recurrence by targeting NT5E/CD73 in head and neck squamous cell carcinoma . Oncotarget. 2016;7:44023–38. doi: 10.18632/oncotarget.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina-Pinelo S, Gutiérrez G, Pastor MD, Hergueta M, Moreno-Bueno G, García-Carbonero R, et al MicroRNA-dependent regulation of transcription in non-small cell lung cancer. PLoS One. 2014;9:e90524. doi: 10.1371/journal.pone.0090524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang HY, Tang CY, Na M, Ma W, Jiang ZF, Gu YF, et al miR-422a inhibits glioma proliferation and invasion by targeting IGF1 and IGF1R. Oncol Res. 2017;25:187–94. doi: 10.3727/096504016X14732772150389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbagallo D, Condorelli A, Ragusa M, Salito L, Sammito M, Banelli B, et al Dysregulated miR-671-5p / CDR1-AS / CDR1/ VSNL1 axis is involved in glioblastoma multiforme. Oncotarget. 2016;7:4746–59. doi: 10.18632/oncotarget.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng J, Liu XB, Xue YX, Gong W, Ma J, Xi Z, et al TTBK2 circular RNA promotes glioma malignancy by regulating miR-217/HNF1β/derlin-1 pathway. J Hematol Oncol. 2017;10:52. doi: 10.1186/s13045-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li GF, Li L, Yao ZQ, Zhuang SJ Hsa_circ_0007534/mir-761/zic5 regulatory loop modulates the proliferation and migration of glioma cells. Biochem Biophys Res Commun. 2018;499:765–71. doi: 10.1016/j.bbrc.2018.03.219. [DOI] [PubMed] [Google Scholar]
- 71.Lei BX, Huang YT, Zhou ZW, Zhao YY, Thapa AJ, Li WP, et al Circular RNA hsa_circ_0076248 promotes oncogenesis of glioma by sponging miR-181a to modulate SIRT1 expression. J Cell Biochem. 2018 doi: 10.1002/jcb.27966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang ML, Li G, Fan LJ, Zhang GF, Xu J, Zhang JW Circular RNA circ_0034642 elevates BATF3 expression and promotes cell proliferation and invasion through miR-1205 in glioma. Biochem Biophys Res Commun. 2019;508:980–5. doi: 10.1016/j.bbrc.2018.12.052. [DOI] [PubMed] [Google Scholar]
- 73.Yang P, Qiu Z, Jiang Y, Dong L, Yang W, Gu C, et al Silencing of cZNF292 circular RNA suppresses human glioma tube formation via the wnt/β-catenin signaling pathway. Oncotarget. 2016;7:63449–55. doi: 10.18632/oncotarget.11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wesselhoeft RA, Kowalski PS, Anderson DG Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meganck RM, Borchardt EK, Castellanos Rivera RM, Scalabrino ML, Wilusz JE, Marzluff WF, et al Tissue-dependent expression and translation of circular RNAs with recombinant AAV vectors in vivo . Mol Ther Nucleic Acids. 2018;13:89–98. doi: 10.1016/j.omtn.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu X, Abraham JM, Cheng YL, Wang ZX, Wang Z, Zhang GJ, et al Synthetic circular RNA functions as a miR-21 sponge to suppress gastric carcinoma cell proliferation. Mol Ther Nucleic Acids. 2018;13:312–21. doi: 10.1016/j.omtn.2018.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Møller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M A systematic review of microRNA in glioblastoma multiforme: Micro-modulators in the mesenchymal mode of migration and invasion. Mol Neurobiol. 2013;47:131–44. doi: 10.1007/s12035-012-8349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartel DP MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA. 2008;105:1608–13. doi: 10.1073/pnas.0707594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E Deadenylation is a widespread effect of miRNA regulation. RNA. 2009;15:21–32. doi: 10.1261/rna.1399509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Djuranovic S, Nahvi A, Green R miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science. 2012;336:237–40. doi: 10.1126/science.1215691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kahles A, Lehmann KV, Toussaint NC, Hüser M, Stark SG, Sachsenberg T, et al Comprehensive analysis of alternative splicing across tumors from 8, 705 patients. Cancer Cell. 2018;34:211–24. doi: 10.1016/j.ccell.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bao ZS, Chen HM, Yang MY, Zhang CB, Yu K, Ye WL, et al RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas . Genome Res. 2014;24:1765–73. doi: 10.1101/gr.165126.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh D, Chan JM, Zoppoli P, Niola F, Sullivan R, Castano A, et al Transforming fusions of FGFR and TACC genes in human glioblastoma . Science. 2012;337:1231–5. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Frattini V, Pagnotta SM, Tala, Fan JJ, Russo MV, Lee SB, et al A metabolic function of FGFR3-TACC3 gene fusions in cancer . Nature. 2018;553:222–7. doi: 10.1038/nature25171. [DOI] [PMC free article] [PubMed] [Google Scholar]