Abstract
Objective
Despite evidence that estrogens and insulin are involved in the development and progression of many cancers, their synergistic role in endometrial carcinoma (EC) has not been analyzed yet.
Methods
Here, we investigated how estrogens act synergistically with insulin to promote EC progression. Cell growth in vitro and in vivo, effects of estradiol and insulin on apoptosis and cell cycle distribution, and expression and activation of estrogen receptor (ER), insulin receptor (InsR), and key proteins in the PI3K and MAPK pathways were examined after combined stimulation with estradiol and insulin.
Results
Compared to EC cells treated with estradiol or insulin alone, those treated with both estradiol and insulin exhibited stronger stimulation. Estradiol significantly induced phosphorylation of InsR-β and IRS-1, whereas insulin significantly induced phosphorylation of ER-α. In addition, treatment with both insulin and estradiol together significantly increased the expression and phosphorylation of Akt, MAPK, and ERK. Notably, InsR-β inhibition had a limited effect on estradiol-dependent proliferation, cell cycle, and apoptosis, whereas ER-α inhibition had a limited insulin-dependent effect, in EC cell lines. Insulin and estradiol individually and synergistically promoted EC xenograft growth in mice.
Conclusions
Estrogen and insulin play synergistic roles in EC carcinogenesis and progression by activating InsR-β and ER-α, promoting a crosstalk between them, and thereby resulting in the activation of downstream PI3K/Akt and MAPK/ERK signaling pathways.
Keywords: Endometrial cancer (EC), estrogen, insulin, InsR-β, ER-α, PI3K/Akt pathway, MAPK/ERK pathway
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in developed countries, with growing incidence worldwide. In 2016, EC was diagnosed in 63,400 patients and accounted for > 21,800 deaths in China 1, exceeding those in the USA2. Nonetheless, EC etiology and pathogenesis remain poorly defined, and molecular mechanisms underlying EC tumorigenesis are not well understood.
Estrogens exert their biological effects primarily by binding and activating estrogen receptors (ERs), a steroid hormone receptor family member primarily acting as a ligand-activated transcription factor3-5. Molecular activities of estrogen are complex and include both ERs-mediated genomic and non-genomic processes, which converge on target genes, and are executed via estrogen-receptor mechanisms, estrogen-non-receptor mechanisms, ERs-ligand mechanisms, and ERs-non-ligand mechanisms6,7. ERs regulate transcription by interacting with estrogen response elements (EREs) located within the promoter regions of target genes, forming a multiprotein complex involved in specific genomic actions of the ERs4. Furthermore, increasing evidence has suggested that biological responses to estrogens can be mediated by membrane-initiated signals, which trigger rapid intracellular transduction pathways, such as the phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways.
Notably, while estrogen can promote cancer progression via both ERs- and ERs-ligand-dependent mechanisms, this hormone can also mediate cancer progression through non-estrogen-receptor dependent mechanisms, including interactions with growth factor receptors, such as insulin-like growth factor receptor (IGFR) and epidermal growth factor receptor. Furthermore, ERs have been shown to interact with insulin-like growth factor (IGF) and promote cancer progression through an ER-non-ligand mechanism8. These studies indicated that crosstalk may occur between estrogen and IGF signaling pathways. With respect to promotion of cancer progression, estrogens have generally been found to stimulate EC cell proliferation; however, the mechanism by which estrogens regulate cancer cell proliferation is also fairly complex.
In our previously study, we showed that estrogen and insulin concentrations in serum were significantly increased in EC patients than those in controls, meanwhile high levels of estrogen and insulin were independent risk factors for EC9. Although estrogens and insulin are synergistically involved in many cancers, the molecular mechanisms underlying their synergistic influence on EC tumorigenesis are not well understood. Our previous studies have shown that insulin acts as a growth factor that promoted EC cell proliferation and inhibited apoptosis via PI3K/Akt and Ras/MAPK pathways10,11. Insulin and IGF-1 belong to the same family, but most studies have focused on IGF-112,13. Notably, many studies have indicated that the ER-α and IGF-1R system share bidirectional crosstalk. In breast cancer, ER-α regulates the IGF-1-dependent pathway and IGF-1 activates ER-α-mediated signaling in a ligand-independent manner14. Similarly, IGF-1 induces phosphorylation of ER-α, which mimics the effects of estrogen-initiated receptor activation, leading to important biological effects in diverse cellular contexts8,15. Given the apparent interplay between ER and IGF-1R and the corresponding network of extensive interactions during EC14,16, a deeper understanding of mechanisms by which ER and the insulin systems cooperate during EC development would be highly relevant in the development of novel pharmacotherapeutic strategies. However, to our knowledge, no studies have been published examining interactions between ER-α signaling and insulin-mediated signaling in EC. In the present study, we established the Ishikawa human endometrial cancer cell line and ECC-1 epithelial adenocarcinoma cell lines with stable knockdown of ER-α and/or insulin receptor-β (InsR-β) to elucidate the mechanisms underlying the effects of estradiol and insulin on EC in vitro and in vivo.
Materials and methods
Regents, kits, drugs, and antibodies
Human recombinant insulin, RIPA cell lysis buffer, protease inhibitors (including phenylmethylsulfonyl fluoride, leupeptin, and aprotinin), and dimethyl sulfoxide solutions were purchased from Sigma-Aldrich (St. Louis, MO, USA). Propidium iodide and RNase A were obtained from Dingguo Biotechnology (Beijing, China). Puromycin (Sigma-Aldrich) was used to identify cells with stable InsR-β and ER-α knockdown. The in situ cell death detection kit used for TUNEL assays was purchased from Roche (Basel, Switzerland); the number of apoptotic cells in EC xenograft tissues was assessed according to the manufacturer's instructions. The BCA Protein Assay Kit was used to quantify total protein and the Enhanced Chemiluminescence Detection Kit was obtained from Pierce Biotechnology (Waltham, MA, USA), and the Annexin V-FITC/PI Apoptosis Detection Kit was purchased from BD Biosciences (San Jose, CA, USA). Matrigel, fibronectin, estradiol, InsR-β shRNA lentiviral particles, ER-α shRNA lentiviral particles, non-targeting shRNA control particles, and polybrene pellets were purchased from Sigma-Aldrich. InsR-β-, ER-α-, cyclin D1-, Ki-67-, and β-actin-specific antibodies and peroxidase-conjugated goat anti-rabbit IgG secondary antibodies were obtained from Santa Cruz Biotechnology (Dallas, TX, USA); antibodies against ERK, phosphorylated (p)-ERK (Thr202/Tyr204), Akt, p-MAPK (Ser217/221), and p-Akt (Thr473) were purchased from Cell Signaling Technology (Danvers, MA, USA).
Cell lines and animals
Ishikawa and ECC-1 human EC cell lines were obtained from the MD Anderson Cancer Center (TX, USA). The immunodeficient female BALB/c-nu mice (5–6 weeks old, 12–13 g each) were used for in vivo experiments [production permit no. SCXK (JING) 2014-0004] and were purchased from Beijing HFK Bioscience Co. (Beijing, China). All animal experiments were performed under standard guidelines approved by the State Key Laboratory of Experimental Hematology [license no. SYXK (JIN) 2009-0002], and all procedures were conducted with the approval of the ethics committee on animal care of the Tianjin Medical University General Hospital. Eighteen mouse groups were utilized for this study with five mice (n = 5) per group.
Cell culture
Cells were grown in Dulbecco’s modified Eagle’s medium without phenol red (Gibco, Grand Island, NY, USA) and supplemented with 10% (v/v) fetal bovine serum (Gibco). Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2. Assays were performed as described previously10,11. Cells were cultured under serum deprivation condition when adding insulin and/or estradiol in some experiments.
Lentivirus-mediated silencing of ER-α and InsR-β
Small hairpin (sh) RNA viral vectors containing a sequence targeting either InsR-β or ER-α and puromycin resistance sequence, for selection of stable clones, were used to infect Ishikawa cells and ECC-1 cells. Sequences used to target InsR-β and ER-α were as follows: InsR (TRCN0000000379), 5'-CCG GGC TCT GTT ACT TGG CCA CTA TCT CGA GAT AGT GGC CAA GTA ACA GAG CTT TTT-3'; and ER (TRCN0000003300), 5'-CCG GCT ACA GGC CAA ATT CAG ATA ACT CGA GTT ATC TGA ATT TGG CCT GTA GTT TTT-3'. Viral infection was performed by incubating the cells in culture medium containing lentiviral particles for 24 h in the presence of 5 μg/mL polybrene17,18. Cells resistant to puromycin (2 μg/mL) were harvested and stored for further analysis.
BrdU cell proliferation enzyme-linked immunosorbent assay (ELISA)
Effects of insulin and estradiol on cell growth and proliferation were examined using a BrdU Cell Proliferation ELISA, as described previously11. Briefly, InsR-β (+/−) and ER-α (+/−) Ishikawa cells and ECC-1 cells were treated with a combination of insulin and estradiol, both at concentrations of 10–8 M, for 24, 48, or 72 hours prior to measurement11.
Cell cycle analysis
Cell cycle distribution of InsR-β (+/−) and ER-α (+/−) Ishikawa cells and InsR-β (+/−) and ER-α (+/−) ECC-1 cells was evaluated via flow cytometry. 5 × 106 cells were treated with insulin and/or estradiol for 24, 48, or 72 hours, after which they were collected, washed with cold phosphate buffered saline (PBS), and fixed in ice-cold 70% ethanol. The samples were then stored at –20 °C for 24 to 48 hours. Before analysis, the cells were washed with cold PBS, and then, suspended in PBS solution containing 0.1% Triton-X and 30 mg/mL DNase-free RNase A (Sigma-Aldrich) for 30 minutes at room temperature. Propidium iodide was added at a final concentration of 10 μg/mL. The samples were then analyzed using a FACS Calibur instrument (BD Bioscience). Data were processed using BD Bioscience software. Data for at least 10,000 cells were collected for each sample. All experiments were repeated three times and the results are reported as mean ± standard error of the mean (SEM).
Annexin V-FITC/PI assay
Apoptosis levels were measured using an Annexin V-FITC/PI Apoptosis Detection Kit, according to the manufacturer’s instructions and as described previously10,11. Briefly, InsR-β (+/−) and ER-α (+/−) Ishikawa cells and InsR-β (+/−) and ER-α (+/−) ECC-1 cells were treated with a combination of insulin and estradiol, both at concentrations of 10–8 M, for 24, 48, or 72 hours. The percentage of apoptotic cells was determined from the number of Annexin V (+)/PI (−) cells relative to the number of Annexin V (−)/PI (−) cells.
Hematoxylin and eosin (H&E) staining
Tumor tissues were fixed in 10% formaldehyde and embedded in paraffin. The tissues were then sectioned (4 μm thickness) and stained with H&E according to standard procedures19.
Immunohistochemistry (IHC) and Western blot analysis
IHC and Western blot analyses were performed as described elsewhere10,20. For IHC, after primary antibody binding, peroxidase-conjugated goat anti-rabbit antibody IgG was bound as a secondary antibody. Tissue sections were then stained with diaminobenzidine (ZSGB-BIO, ZLI-9031), counterstained with hematoxylin (ZSGB-BIO, ZLI-9643), and examined under a light microscope (CKX41; Olympus Corp., Tokyo, Japan). Western blot band intensities were determined by densitometry analysis using the Image J software (National Institutes of Health, Bethesda, MD, USA), and the results were expressed either as the ratio of target protein to β-actin or as the ratio of the phosphorylated form of a protein to the total amount of that protein.
Establishment of mouse models of estradiol and insulin deficiency
The estradiol deficient mice group was generated by bilateral ovariectomy, per standard surgical procedures21, at least 1 week prior to EC cell inoculation. Meanwhile, insulin deficiency was induced via intraperitoneal injection of streptozotocin (STZ) dissolved in citrate buffer (adjusted with citric acid to pH 4.5). The total dose of STZ was 200 mg/kg of body weight. Body weights were recorded for all mice prior to and following STZ injection, and random blood glucose levels were measured using a glucometer (Roche). Diabetes was defined in the insulin deficiency group as having a random blood glucose reading of > 16.7 mM for three continuous days at 72 hours after injection of STZ. Similarly, the insulin and estradiol deficiency group was generated according to the methods described above. Mice were first rendered estradiol-deficient, and then, administered intraperitoneal injections of STZ.
Tumor growth assay
Approximately 5 × 106 Ishikawa cells, including normal [InsR-β (+) and ER-α (+)], InsR-β knockdown [InsR-β (−) and ER-α (+)], ER-α knockdown [InsR-β (+) and ER-α (−)], and double knockdown [InsR-β (−) and ER-α (−)] lines, were injected into the dorsal scapular regions of mice in each group to generate EC tumor xenografts. Resultant tumors were measured with a caliper each week (7 days), and tumor volume was calculated using the following formula: volume = [length × (width)2]/2. After 56 days of observation, mice were sacrificed, and tumors were dissected and weighed. Other detailed characteristics were detected by HE staining, IHC, and Western blot analyses. Apoptosis in xenografts was detected by TUNEL assay.
Statistical analysis
Experimental results were expressed as the means ± SEM of results from at least three independent experiments. Multiple comparisons were analyzed using Wilcoxon’s rank test or one-way analysis of variance (ANOVA). Subsequently, the least significant difference test was adopted if variances were equal, and Tamhane's T2 test was used if the variances were unequal. Statistical significance was assumed at P < 0.05. All calculations were performed using SPSS 13.0 software (SPSS Statistics, Inc., Chicago, IL, USA).
Ethical approval
The study protocol was approved by the Ethics Committee of Tianjin Medical University General Hospital. All animal experiments were performed under standard guidelines approved by the State Key Laboratory of Experimental Hematology [License No. SYXK (JIN) 2009-0002], and all procedures were performed in accordance with the approval of the ethics committee on animal care of Tianjin Medical University General Hospital.
Results
Estradiol and insulin synergistically activate the phosphorylation of Akt and ERK1/2
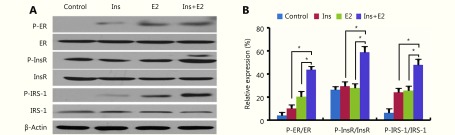
Previous studies have demonstrated that the PI3K and MAPK pathways are involved in EC cell proliferation, cell cycle, and apoptosis10,11. Accordingly, we first investigated whether estradiol exerts its effects on EC via these two pathways. Following estradiol stimulation of EC cells over 15, 30, 60, and 120 minutes, we assayed for phosphorylation of key proteins in these pathways. Our data showed that phosphorylation of AKT and ERK reached their peaks in both Ishikawa and ECC-1 cells at 60 minutes post-stimulation (Figure 1A and 1B).
1.
Estradiol and insulin synergistically activate the phosphorylation of Akt and ERK1/2.
As insulin has also been shown to promote cell proliferation via the Akt and ERK pathways10,11, we next examined whether insulin and estradiol could synergistically activate the PI3K and MEK signaling pathways. As shown in Figure 1C and 1D, the p-Akt/Akt ratio was significantly increased in cells treated with a combination of both insulin and estradiol compared to that in untreated cells or cells treated with either insulin or estradiol alone. In addition, estradiol and insulin synergistically induced MEK and ERK phosphorylation in cells treated with both, compared with cells that either underwent treatment with insulin or estradiol alone or no treatment (P < 0.05; Figure 1C and 1D). These results indicated that estradiol and insulin synergistically activate the PI3K and MEK pathways in EC cells.
Estrogen promotes EC cell progression via InsR to activate the PI3K and MAPK pathways
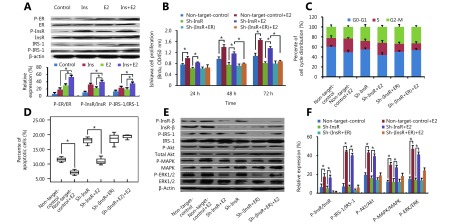
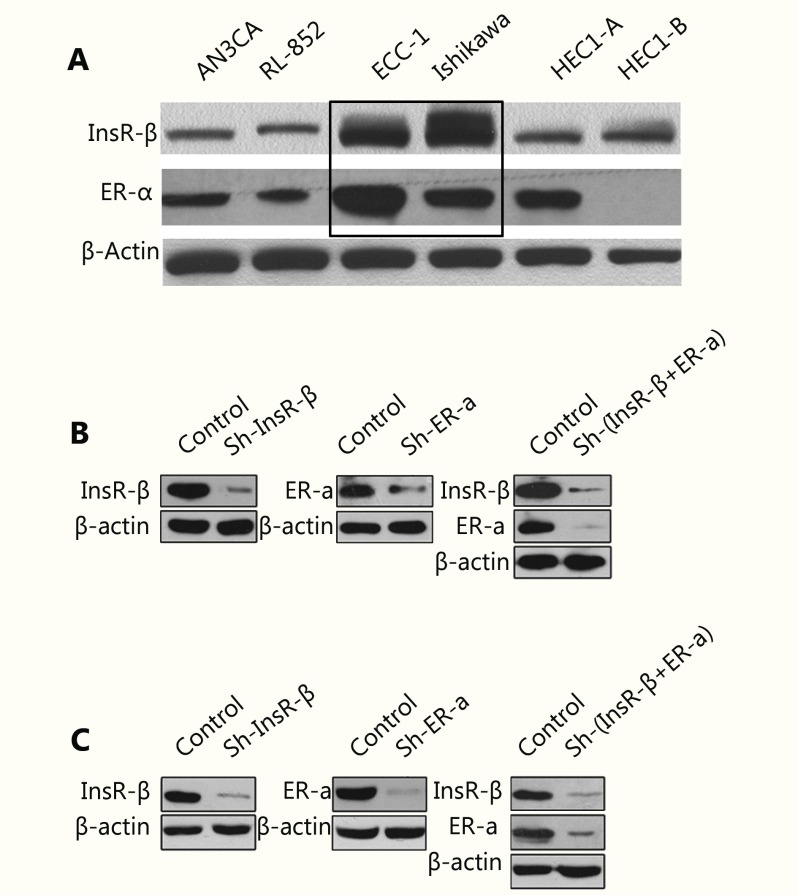
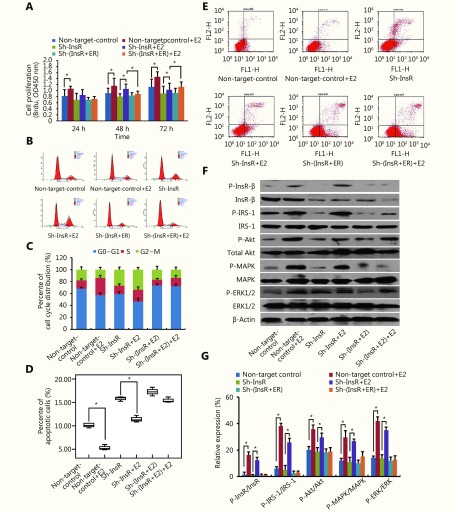
Notably, our results showed that estradiol significantly enhances InsR and InsR substrate (IRS)-1 phosphorylation in Ishikawa cells (P < 0.05; Figure 2A) and ECC-1 cells (P < 0.05; Supplementary Figure S1). We, therefore, speculated that InsR may play an important role in the overall mechanism by which estradiol promotes EC progression. To validate this hypothesis, we knocked down the expression of InsR in EC cells (Figure 3). In our previous study, we found that there were no significant changes in cell proliferation and the percentage of apoptotic cells in control EC cells with non-target shRNA interference compared to the cells without any shRNA10,11. Therefore, in this study, we used EC cells with Non-target shRNA interference as the control group. Notably, the InsR knockdown Ishikawa cells (sh-InsR) exhibited significantly reduced levels of estradiol-induced proliferation, compared to the sh-control-treated cells (P < 0.05; Figure 2B). Moreover, compared to the sh-control cells, there were fewer sh-InsR cells in the S phase (P < 0.05; Figure 2C) but a higher number of apoptotic cells (P < 0.05, Figure 2D), after estradiol treatment. Finally, although estradiol mildly induced Akt, MAPK, and ERK phosphorylation after InsR knockdown, compared with the sh-control, the difference was not statistically significant (P > 0.05, Figure 2E and 2F). Similar results were also obtained for ECC-1 cells (Supplementary Figure S2). Together, these results suggested that estradiol might activate the PI3K and MAPK pathways via interaction with InsR, thereby promoting EC cell progression.
2.
Estrogen promotes EC cell progression via InsR by activating the PI3K and MAPK pathways.
S1.
Analysis of the effects of insulin and estradiol on estrogen receptor (ER), insulin receptor (InsR), and insulin receptor substrate (IRS)-1 in ECC-1 cells. (A, B) Western blot analysis of the effects of insulin and estradiol treatment on the phosphorylation of ER, InsR, and IRS-1 in ECC-1 cells. *P < 0.05.
3.
Analysis of ER-α and InsR-β expression in EC cells.
S2.
Estrogen promotes ECC-1 cell progression via the insulin receptor (InsR) by activating the PI3K and MAPK pathways. (A) BrdU enzyme-linked immunosorbent assay (ELISA) of the effects of estradiol stimulation on ECC-1 cell proliferation after knockdown of estrogen receptor (ER) and/or InsR. (B, C) Flow cytometry analysis of the effects of estradiol stimulation on ECC-1 cell cycle distribution after knockdown of ER and/or InsR. (D, E) The percentage of apoptotic ECC-1 cells (after knockdown of ER and/or InsR) upon treatment with estradiol was determined by flow cytometry using Annexin V-FITC antibodies. (F, G) Western blot analysis of the effects of estradiol treatment on the ratios of phosphorylated (p)-InsR/InsR, p-IRS-1/IRS-1, p-Akt/Akt, p-MAPK/MAPK, and p-ERK/ERK in ECC-1 cells after knockdown of ER and/or InsR. *P < 0.05 for all experiments.
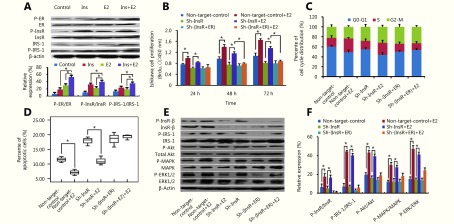
Insulin promotes EC cell progression via ER to activate the PI3K and MAPK pathways
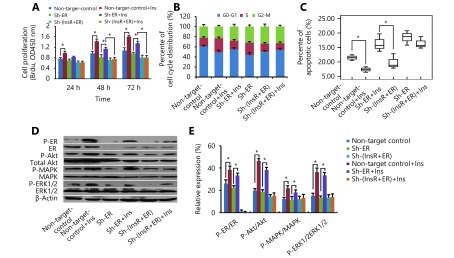
Our previous study demonstrated that insulin promotes EC cell progression via InsR and activates the PI3K and MAPK pathways10,11. Here, we further elucidated these results by investigating whether insulin might also act through ER, resulting in PI3K and MAPK pathway activation. After knockdown of ER (Figure 3), there was reduced insulin-mediated EC cell proliferation (P < 0.05; Figure 4A) and fewer cells in S phase (P < 0.05; Figure 4B), compared with the sh-control group. Conversely, ER-knockdown cells exhibited significantly higher levels of apoptosis after 24 hours of insulin stimulation than did the control cells (P < 0.05; Figure 4C). Finally, while ER-knockdown cells exhibited mild induction of Akt, MAPK, and ERK phosphorylation after insulin stimulation, compared with the sh-control cells, this difference was not statistically significant (P > 0.05; Figure 4D and 4E). Similar results were also obtained for ECC-1 cells (Supplementary Figure S3). These results suggested that insulin might promote EC cell progression via interaction with ER, thereby activating the PI3K and MAPK pathways.
4.
Insulin promotes endometrial cancer (EC) cell progression via estrogen receptor (ER) by activating the PI3K and MAPK pathways.
S3.
Insulin promotes ECC-1 cell progression via the estrogen receptor (ER) by activating the PI3K and MAPK pathways. (A) BrdU enzyme-linked immunosorbent assay (ELISA) of the effects of insulin stimulation on ECC-1 cell proliferation after knockdown of ER and/or insulin receptor (InsR). (B, C) Flow cytometry analysis of the effects of insulin stimulation on ECC-1 cell cycle distribution after knockdown of ER and/or InsR. (D, E) The percentage of apoptotic ECC-1 cells (after knockdown of ER and/or InsR) treated with insulin was determined by flow cytometry analysis using Annexin V-FITC antibodies. (F, G) Western blot analysis of the effects of insulin treatment on the ratios of phosphorylated (p)-ER/ER, p-Akt/Akt, p-MAPK/MAPK, and p-ERK/ERK in ECC-1 cells after knockdown of ER and/or InsR. *P < 0.05 for all experiments.
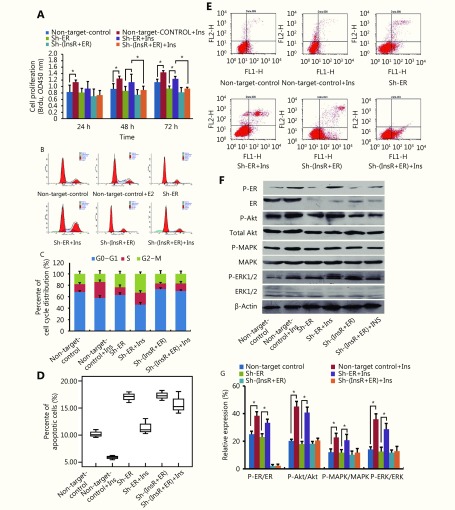
Notably, after dual knockdown of ER and InsR (Figure 3), estradiol and insulin exhibited minor effect on the proliferation of EC cells, compared with that observed in the sh-control group (P > 0.05; Figure 2B and 4A). Treatment with estradiol and insulin also yielded no significant increase in the proportion of sh-ER and sh-InsR-transduced cells in S phase (P > 0.05; Figure 2C and 4B) or in apoptosis after stimulation with estradiol and insulin for 24 hours, compared with control cells (P > 0.05; Figure 2D and 4C). Finally, after knockdown of ER and InsR, estradiol and insulin treatment resulted in no significant induction of Akt, MAPK, or ERK phosphorylation, compared with that in the sh-control cells (P > 0.05; Figure 2E and 2F, Figure 4D and 4E, respectively).
Effects and mechanism of action of insulin and estradiol on EC xenografts in nude mice
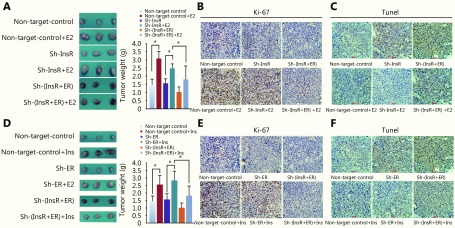
Treatment with either estradiol or insulin promoted the growth of xenografts derived from ER knockdown [InsR-β (+) and ER-α (−)] or InsR knockdown [InsR-β (−) and ER-α (+)] cells (Figure 5A and 5D; P < 0.05). Furthermore, after knockdown of both ER and InsR [InsR-β (−) and ER-α (−)], xenograft growth significantly decreased compared with that observed in either non-target control group or single knockdown groups {[InsR-β (+) and ER-α (−)] or [InsR-β (−) and ER-α (+)]} after treatment with estradiol and insulin ( Figure 5A and 5D; P < 0.05). Lastly, although either estradiol or insulin mildly increased the growth of xenografts derived from dual knockdown cells [InsR-β (−) and ER-α (−)], the increase was not significant ( Figure 5A and 5D, P > 0.05). Our results therefore suggested that estradiol and insulin exerted a synergistic effect on the growth of xenografts, and that both ER and InsR played important roles in xenograft growth.
5.
Tumor growth assay.
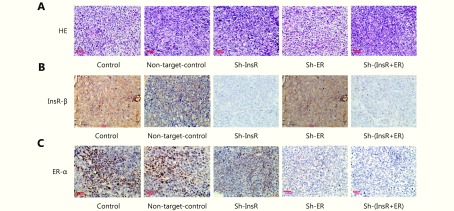
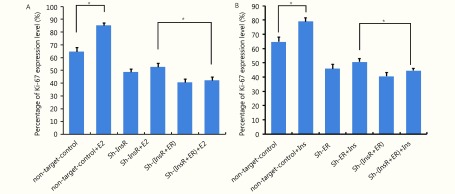
Each of the xenograft tissues was subsequently analyzed by HE staining, IHC, TUNEL assay, and Western blot analyses. HE staining revealed that the xenografts exhibited solid tumor morphology, and their histopathology was interpreted as well-differentiated endometrial adenocarcinoma in all groups (Supplementary Figure S4). InsR expression was almost completely undetectable in the sh-InsR and sh-InsR + ER groups, and ER expression was almost completely undetectable in the sh-ER and sh-InsR + ER groups (Supplementary Figure S4). To understand the impact of insulin and estradiol on EC xenograft growth, we next analyzed the expression of the ubiquitous cell cycle marker, Ki67. Knockdown of either ER-α or InsR-β significantly reduced the number of Ki67+ nuclei (Figure 5B and 5E, Supplementary Figure S6) within xenograft tissues. The addition of estradiol to ER-α knockdown xenografts or insulin to InsR-β knockdown xenografts resulted in mild increases in the number of Ki67+ nuclei; however, these effects were not significantly different from those of the control (Figure 5B and 5E, Supplementary Figure S6). Conversely, ER-α knockdown xenografts stimulated with insulin and InsR-β knockdown xenografts stimulated with estradiol exhibited increased numbers of Ki67+ nuclei (Figure 5D and 5E, Supplementary Figure S6). Apoptosis of the xenografts was analyzed by TUNEL assay. Knockdown of either ER-α or InsR-β significantly increased the number of apoptotic cells (Figure 5C and 5F). Whereas, the addition of estradiol to ER-α knockdown xenografts or insulin to InsR-β knockdown xenografts mildly decreased the number of apoptotic cells; however, these effects did not differ significantly from the control treatment (Figure 5C and 5F). Conversely, ER-α knockdown xenografts stimulated with insulin and InsR-β knockdown xenografts stimulated with estradiol exhibited decreased TUNEL signals (Figure 5C and 5F).
S4.
Evaluation of the morphological characteristics and the expression levels estrogen receptor (ER)-α and insulin receptor (InsR)-β in Ishikawa xenograft tissues. (A) Analysis of xenografts’ morphologic characteristics via hematoxylin and eosin staining (200 ×). (B, C) IHC analysis of (B) InsR-β and (C) ER-α expression in Ishikawa xenograft tissues after knockdown of ER and/or InsR (200 ×).
S6.
Statistical analysis for Ki-67 expression levels of estrogen stimulating insulin receptor (A) and insulin stimulating estrogen receptor (B).
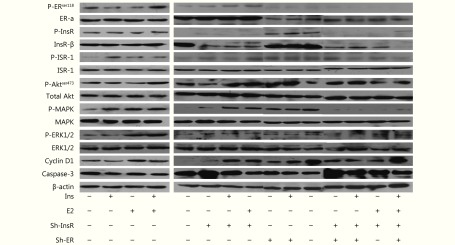
Finally, we analyzed the effects of activation of insulin pathway and estrogen pathway on EC xenografts using Western blot. Insulin significantly induced ER-α phosphorylation, while estradiol significantly induced InsR-β and IRS-1 phosphorylation (P < 0.05; Supplementary Figure S5). Furthermore, co-treatment with insulin and estradiol resulted in significantly upregulated ER-α, InsR-β, IRS-1, Akt, MAPK, ERK, cyclin D1, and downregulated caspase-3 (P < 0.05; Supplementary Figure S5) in xenografts. Conversely, the expression levels of IRS-1, Akt, MAPK, ERK, cyclin D1 upregulated after treatment with insulin and estradiol were restored in InsR and/or ER knockdown xenografts. Notably, after ER and/or InsR knockdown, treatment with estradiol alone upregulated InsR-β, IRS-1, and the key downstream proteins—Akt, MAPK, and ERK—of the PI3K and MAPK signaling pathways (Supplementary Figure S5).
S5.
Evaluation of the expression and activation of major downstream proteins of the PI3K/Akt and MAPK signaling pathways in Ishikawa xenografts.
Discussion
Synergistic combinations of estrogens and IGF-1 have been reported to contribute to breast cancer development and progression22,23. These early reports yielded both epidemiological and cytological evidence of synergy between insulin/IGF and estrogen signaling systems. Briefly, elucidation of the mechanism of this synergistic effect is expected to identify two primary phenomena: i) estrogen signaling could act on insulin receptors and activate downstream pathways and ii) insulin signaling could simultaneously activate estrogen receptors.
Our previous study has shown that insulin promotes EC cell proliferation and inhibits cell apoptosis in a dose- and time-dependent manner10,11. We subsequently demonstrated that estrogen promoted EC cell progression via InsR and, conversely, that insulin promoted EC cell progression via ER, providing preliminary evidence that insulin and estradiol synergistically influence EC growth and progression via these receptors. Moreover, we found that estradiol and insulin synergistically promoted EC cell proliferation, increased the proportion of cells in S-phase, and inhibited EC cell apoptosis, confirming the progression of EC cell development upon estradiol and insulin stimulation. Similar results were also obtained in a tumor xenograft mouse model. Notably, xenografts could not be established after EC cell injection into nude mice that had undergone ovariectomy to deplete estrogen or were deficient in insulin. These observations further indicated that estradiol and insulin have synergistic effects on EC development.
For transduction of insulin signals, insulin binds to the alpha subunit of its receptor resulting in the autophosphorylation of its beta subunit tyrosines. Consequently, InsR is activated, leading to activation of two signaling pathways, PI3K and MAPK. The family of insulin receptors consists of InsR, IGFR, and insulin receptor-related receptor, of which InsR and IGFR share a similar protein structure and biological function. Currently, studies related to the synergistic effects of estrogen signaling and insulin/IGF-1 signaling have been limited to breast cancer. In these studies, estrogens were shown to stimulate the expression of IRS-1, which resulted in an Ins/IGF-1 signal cascade and enhancement of its effect on cell proliferation22,23.
Here, we investigated the mechanisms underlying the observed synergistic effects of estradiol and insulin in EC. The PI3K and MAPK pathways have been reported to regulate cell proliferation and apoptosis in colorectal, non-small cell lung, breast, and endometrial cancers24-26. In addition, we had previously showed that insulin alone exerts its effects in EC via the PI3K and MAPK pathways in dose- and time-dependent manner10,11. Here, we found that estradiol alone promoted cell proliferation, enhanced S phase distribution, and inhibited cell apoptosis in dose- and time- dependent manner via the PI3K and MAPK pathways. Further studies on the molecular crosstalk between the insulin and estrogen signaling pathways revealed a complex network of interactions, some of which involve the ability of IGF-1 to activate ER-α8. In particular, the mechanism by which estrogens induce proliferation of breast cancer cells may involve the ability of ER to regulate the expression of growth factor signaling pathway components. For example, anti-estrogen drugs, such as tamoxifen, have been shown to inhibit IGF-mediated growth, while anti-IGF drugs have been shown to inhibit estrogen-mediated growth, suggesting that IGFs are partially responsible for estrogen-mediated signaling15. Estrogen also increases IGF-1R and IRS-1 expression levels in breast cancer cells, resulting in enhanced IGF signaling and downstream pathway activation27. Together, these studies indicated the existence of a crosstalk between IGF and ER to promote cancer progression.
In the current study, we found that estradiol activated InsR and IRS-1, while insulin promoted the phosphorylation of ER, which was consistent with the earlier findings obtained in breast cancer studies, as insulin and IGF-1 both belong to the insulin/IGF family12,13. Additionally, we found that insulin and estradiol synergistically activated the PI3K and MAPK signaling pathway downstream proteins, Akt, MAPK, and ERK. Furthermore, after knockdown of ER, effects of promoting cell progression promoting effects of estradiol were reduced compared to the control. These results indicated that estrogen might signal via another receptor besides ER. To confirm this possibility, we knocked down InsR or both ER and InsR in EC cells and observed a significant reduction in the effects of estradiol and insulin on cell progression, compared to the control. Notably, after knockdown of both ER and InsR, PI3K and MAPK pathway activation in EC cells treated with estradiol and insulin was significantly reduced compared to the control. Therefore, we inferred that synergistic effects of estrogen and insulin on EC occur via ER and InsR, which in turn activate the PI3K and MAPK pathways. Additional investigations on the underlying molecular mechanisms of the possible interactions between insulin and estrogen and the potential molecules involved in EC progression are ongoing.
Furthermore, in breast cancer MCF-7L xenografts, estrogen has been shown to regulate IRS-1 expression28. Specifically, when tumors were grown in the presence of estrogen, IRS-1 was not only expressed at a high level but was also phosphorylated. These tumors also contained high concentrations of phosphorylated MAPK, indicating an active signaling cascade involving IRS-129. Thus, estrogen-mediated upregulation of both IGF-1R and IRS-1 enhanced IGF-mediated signaling and might explain the observed synergy between the two ligands5. This phenomenon was also investigated in the current study, wherein we found that estradiol upregulated InsR and IRS-1 expression and activation in Ishikawa cell xenografts. The mechanism by which estradiol induces these effects likely involves several components of the complex crosstalk between the InsR and ER pathways. Additionally, estrogen clearly regulates the expression of both the IGF signaling components and the nuclear transcription factors that are necessary for IGF signaling8, and it also modulates the expression of cell cycle regulators, such as cyclin D130. Notably, activation of ERK appears to be required for the induction of cyclin D1 expression in several types of cells31-33. Consistent with this conclusion, in this study, estradiol and insulin treatment upregulated cyclin D1 expression and synergistically promoted the growth of EC xenografts, whereas knockdown of ER-α or InsR-β significantly reduced the effects of estradiol and insulin on the growth of EC xenografts as well as the degree of activation of PI3K/Akt and MAPK signaling pathways.
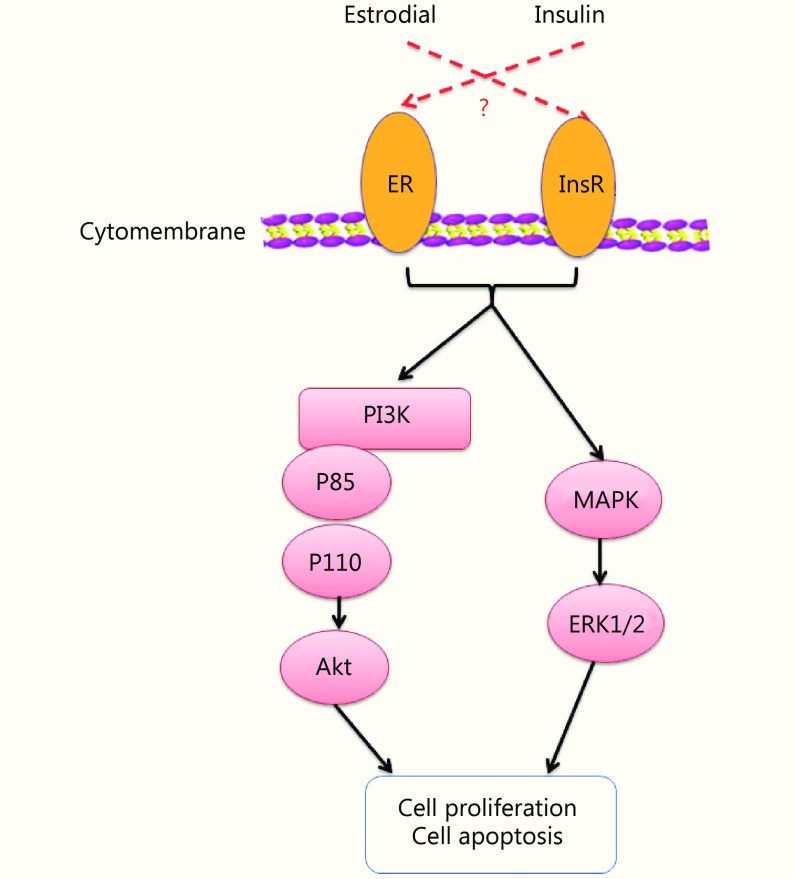
The mechanisms responsible for synergy and crosstalk between estrogen and insulin in EC might involve activation of the PI3K/Akt and MAPK signaling pathways, downstream of both ER and InsR (Figure 6). A better understanding of mechanisms underlying interactions between insulin and ER signaling might have clinical relevance and could reveal novel targets for therapeutic intervention.
6.
Graphical abstract for current work.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 30772316 and 81572568).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Contributor Information
Yingmei Wang, Email: wangyingmei1978@126.com.
Fengxia Xue, Email: fengxiaxue1962@163.com.
References
- 1.Chen WQ, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F, et al Cancer statistics in China, 2015. CA: Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A Cancer statistics, 2016. CA: Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Xu CY, Li JJ, Lu YY, Jiang ZN Estrogen receptor α and hedgehog signal pathway developmental biology of gastric adenocarcinoma. Hepato-Gastroenterology. 2012;59:1319–22. doi: 10.5754/hge11549. [DOI] [PubMed] [Google Scholar]
- 4.Caiazza F, Ryan EJ, Doherty G, Winter DC, Sheahan K Estrogen receptors and their implications in colorectal carcinogenesis. Front Oncol. 2015;5:19. doi: 10.3389/fonc.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen DB, Bird IM, Zheng J, Magness RR Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–25. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- 6.De Marco P, Cirillo F, Vivacqua A, Malaguarnera R, Belfiore A, Maggiolini M Novel aspects concerning the functional cross-talk between the insulin/IGF-I system and estrogen signaling in cancer cells. Front Endocrinol. 2015;6:30. doi: 10.3389/fendo.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrtačnik P, Ostanek B, Mencej-Bedrač S, Marc J The many faces of estrogen signaling. Biochem Med. 2014;24:329–42. doi: 10.11613/issn.1846-7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartella V, De Marco P, Malaguarnera R, Belfiore A, Maggiolini M New advances on the functional cross-talk between insulin-like growth factor-I and estrogen signaling in cancer. Cell Signal. 2012;24:1515–21. doi: 10.1016/j.cellsig.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Tian WY, Teng F, Zhao J, Gao JP, Gao C, Sun DD, et al Estrogen and insulin synergistically promote type 1 endometrial cancer progression. Cancer Biol Ther. 2017;18:1000–10. doi: 10.1080/15384047.2017.1394547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YM, Zhu YX, Zhang LZ, Tian WY, Hua SF, Zhao J, et al Insulin promotes proliferation, survival, and invasion in endometrial carcinoma by activating the MEK/ERK pathway. Cancer Lett. 2012;322:223–31. doi: 10.1016/j.canlet.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Wang YM, Hua SF, Tian WY, Zhang LZ, Zhao J, Zhang HY, et al Mitogenic and anti-apoptotic effects of insulin in endometrial cancer are phosphatidylinositol 3-kinase/Akt dependent. Gynecol Oncol. 2012;125:734–41. doi: 10.1016/j.ygyno.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 13.Samani AA, Yakar S, LeRoith D, Brodt P The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 14.Yu ZH, Gao WM, Jiang EZ, Lu F, Zhang L, Shi ZR, et al Interaction between IGF-IR and ER induced by E2 and IGF-I. PLoS One. 2013;8:e62642. doi: 10.1371/journal.pone.0062642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanzino M, Morelli C, Garofalo C, Panno ML, Mauro L, Ando S, et al Interaction between estrogen receptor alpha and insulin/IGF signaling in breast cancer. Curr Cancer Drug Targets. 2008;8:597–610. doi: 10.2174/156800908786241104. [DOI] [PubMed] [Google Scholar]
- 16.Fox EM, Kuba MG, Miller TW, Davies BR, Arteaga CL Autocrine IGF-I/insulin receptor axis compensates for inhibition of AKT in ER-positive breast cancer cells with resistance to estrogen deprivation. Breast Cancer Res. 2013;15:R55. doi: 10.1186/bcr3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Wang C, Li PC, Zhao Z, Qian C, Wang CX, et al Dual-reporter imaging and its potential application in tracking studies. J Fluoresc. 2016;26:75–80. doi: 10.1007/s10895-015-1673-3. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Zhao Z, Wang C, Wang CX, Li PC, Qian C, et al Bioluminescence imaging of transplanted human endothelial colony-forming cells in an ischemic mouse model. Brain Res. 2016;1642:209–18. doi: 10.1016/j.brainres.2016.03.045. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wang HY, Zhu RR, Liu Q, Fei J, Wang SL Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials. 2015;53:475–83. doi: 10.1016/j.biomaterials.2015.02.116. [DOI] [PubMed] [Google Scholar]
- 20.Tian WY, Zhu YX, Wang YM, Teng F, Zhang HY, Liu GY, et al Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol Oncol. 2013;129:505–12. doi: 10.1016/j.ygyno.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Horsburgh K, Macrae IM, Carswell H Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J Cereb Blood Flow Metab. 2002;22:1189–95. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- 22.Wu MH, Chou YC, Chou WY, Hsu GC, Chu CH, Yu CP, et al Relationships between critical period of estrogen exposure and circulating levels of insulin-like growth factor-I (IGF-I) in breast cancer: evidence from a case-control study. Int J Cancer. 2010;126:508–14. doi: 10.1002/ijc.v126:2. [DOI] [PubMed] [Google Scholar]
- 23.Bradley LM, Gierthy JF, Pentecost BT Role of the insulin-like growth factor system on an estrogen-dependent cancer phenotype in the MCF-7 human breast cancer cell line. J Steroid Biochem Mol Biol. 2008;109:185–96. doi: 10.1016/j.jsbmb.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Chen J, Chen H, Duan Z, Xu QM, Wei MY, et al Leptin regulates proliferation and apoptosis of colorectal carcinoma through PI3K/Akt/Mtor signalling pathway. J Biosci. 2012;37:91–101. doi: 10.1007/s12038-011-9172-4. [DOI] [PubMed] [Google Scholar]
- 25.Yeramian A, Moreno-Bueno G, Dolcet X, Catasus L, Abal M, Colas E, et al Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013;32:403–13. doi: 10.1038/onc.2012.76. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Tang CP, Cao H, Li KF, Pang XL, Zhong L, et al Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther. 2015;16:1220–30. doi: 10.1080/15384047.2015.1056409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaben AM, Sabbah M, Redeuilh G, Bedin M, Mester J Ligand-free estrogen receptor activity complements igf1r to induce the proliferation of the MCF-7 breast cancer cells. BMC Cancer. 2012;12:291. doi: 10.1186/1471-2407-12-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270:1491–4. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 29.Becker MA, Ibrahim YH, Cui XJ, Lee AV, Yee D The IGF pathway regulates ERα through a S6K1-dependent mechanism in breast cancer cells. Mol Endocrinol. 2011;25:516–28. doi: 10.1210/me.2010-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cascio S, Bartella V, Garofalo C, Russo A, Giordano A, Surmacz E Insulin-like growth factor 1 differentially regulates estrogen receptor-dependent transcription at estrogen response element and AP-1 sites in breast cancer cells. J Biol Chem. 2007;282:3498–506. doi: 10.1074/jbc.M606244200. [DOI] [PubMed] [Google Scholar]
- 31.Cubas R, Zhang S, Li M, Chen CY, Yao QZ Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauro L, Pellegrino M, Giordano F, Ricchio E, Rizza P, De Amicis F, et al Estrogen receptor-α drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015;29:2150–60. doi: 10.1096/fj.14-262808. [DOI] [PubMed] [Google Scholar]
- 33.Du MJ, Chen XD, Zhou XL, Wan YJ, Lan B, Zhang CZ, et al Estrogen induces Vav1 expression in human breast cancer cells. PLoS One. 2014;9:e99052. doi: 10.1371/journal.pone.0099052. [DOI] [PMC free article] [PubMed] [Google Scholar]