Abstract
Objective
SHR-1210 is a new and promising anti-PD-1 agent for solid tumors. During the phase I study of SHR-1210, we encountered a novel but prevalent immune-related dermatologic toxicity: reactive capillary hemangiomas (RCHs). Thus we tried to summarize the features of RCHs and estimate their relationship with tumor response.
Methods
This prospective observational study systematically enrolled 98 patients with advanced solid tumors from April 27th, 2016 to June 8th, 2017 in the context of the phase I clinical study of SHR-1210. This report focused on the skin toxicities. Patients underwent entire skin inspection every two weeks while taking medication. The clinical course of RCHs was recorded and their association with tumor response was estimated. The data cut-off date was November 15th, 2017.
Results
After a median follow-up of 242 (range, 29–567) days, RCHs were observed in 85.7% (84/98) of patients on cutaneous/mucosal surfaces; 84.5% (71/84) of the RCHs were evaluated as grade 1 adverse events. No grade 3 or 4 RCHs were observed. The time of onset of RCHs was dose dependent and shortest in the 400 mg-dose cohort (P < 0.001). Spontaneous and complete regression of RCHs was observed both during and after treatment. The objective response rate of tumors for patients with RCHs was 28.9% (24/83). However, no responders were observed among the patients without RCHs.
Conclusions
RCHs were prevalent but manageable during treatment with SHR-1210. It might add to the expanding literature regarding immune-related dermatologic adverse events.
Keywords: Reactive capillary hemangiomas, SHR-1210, skin toxicity, anti-tumor efficacy
Introduction
Antibodies to programmed cell death 1 (PD-1) and its ligand (PD-L1) can restore the immune response by inhibiting the PD-1/PD-L1 pathway. They have remarkably improved the overall survival of many patients with advanced solid tumors. Because of drug-mediated hyperactivation of the immune system, a spectrum of novel immune-related adverse events (irAEs) are put in front of the oncologists. The most common irAEs involve the gastrointestinal tract, liver, endocrine organs, and skin1. Dermatologic adverse events (DAEs) remain significant in 20%–30% of patients undergoing treatment with PD-1 inhibitors (e.g. nivolumab and pembrolizumab), with a broad range of clinical appearances, such as maculopapular, follicular, pruritic, pustular, vesicular, acneiform, and exfoliative lesions2,3. They are different from most chemotherapy and targeted therapy-induced DAEs either in frequency or characteristics4. Curry and his colleagues5 reviewed the published dermatologic toxicities induced by PD-1 antibodies, and categorized the skin lesions into four types: inflammatory, immunobullous, alteration of epidermal keratinocytes, and alterations of epidermal melanocytes or invasive melanoma tumor cells (e.g. vitiligo). However, DAEs of PD-1 antibodies may vary with the histology of cancer or the agents received by patients. For example, vitiligo events are nearly all noted in trials of melanoma2; psoriasiform reactions and vasculopathic changes are seen with pembrolizumab, but not yet with nivolumab5.
SHR-1210 is a kind of humanized, high-affinity IgG4-kappa monoclonal antibody against PD-1, which was under phase I clinical trial at Cancer Hospital Chinese Academy of Medical Sciences recently. At the initiation of the study, we observed multiple emerging skin changes which were suspicious of capillary hemangiomas (CHs) on the extremities of the second participant along with the dosing of SHR-1210. Further biopsy of the lesion led us to the diagnosis of CHs. Since CHs were immune-related and self-limiting, we called them reactive capillary hemangiomas (RCHs). To our knowledge, RCHs had never been reported as immune-related adverse events in trials of other PD-1 inhibitors. SHR-1210 had shown exciting efficacy in many solid tumors, especially for esophageal squamous cell carcinoma and gastric adenocarcinoma6,7. It would be very important to extend our knowledge of this novel toxicity, thus providing appropriate and prompt management in further studies. Hence we focused on the DAEs, trying to make an informative record of RCHs, summarize the features of these lesions, and estimate their correlation with tumor response. Effective managements of RCHs were also investigated.
Materials and methods
Patients
This prospective study systematically enrolled patients with advanced solid tumors in the context of the phase I study (Clinicaltrials.gov identifier NCT02742935) of SHR-1210 conducted at Cancer Hospital, Chinese Academy of Medical Sciences from April 27th, 2016 to June 8th, 2017. This report focused on the skin toxicities. Patients eligibility criteria were as follows: adult patients aged 18–75 years; Eastern Cooperative Oncology Group (ECOG) performance status scored 0 to 2; advanced or metastatic solid tumor identified by surgery, biopsy or dynamic imaging examinations; patients who experienced disease progression or were intolerant of standard therapy or had no effective standard therapy; and patients with adequate organ function. Patients with hemangioma before the initiation of SHR-1210 treatment were also included. All study participants provided written informed consent before enrolling. The data cut-off date was November 15th, 2017.
Study design and treatment
This trial was divided into two parts: dose escalation and dose expansion. Dose escalation was conducted using a traditional 3 + 3 dose-escalation design. SHR-1210 was initially administered at 60 mg by intravenous infusion over 30 minutes on days 1 and 29, and repeated every 14 days thereafter. Then dose escalation proceeded to the next two dose cohorts: 200 mg and 400 mg. The study would continue until intolerable toxicity or disease progression occurred. Expansion for each cohort was allowed following dose escalation. No dose adjustment was allowed throughout the study. The protocol was approved by the institutional review board and independent ethics committee of Cancer Hospital Chinese Academy of Medical Sciences.
Assessment of skin toxicities
The diagnosis of RCHs could be determined by good history and physical examination. Only RCHs that emerged after receiving SHR-1210 were considered as drug-related RCHs. RCHs emerging during treatment were photographed and underwent biopsy for pathologic examination in willing patients. Patients underwent follow-up examinations every two weeks during treatment. Considering that RCHs induced by immunotherapy had never been reported in trials of other PD-1 inhibitors, we defined their severity according to the grading of rash in the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.08, as well as other DAEs. Grade 1 RCHs were defined as skin changes occupying less than 10% of the body surface area (BSA) or without symptoms. Grade 2 RCHs were defined as a more generalized (10%–30% of the BSA) skin change and symptoms not interfering with activities of daily living. Grade 3 or 4 RCHs were defined as ≥ 30% BSA skin reactions or symptoms interfering with daily living or needing hospitalized care. All dermatologic events were characterized based on time to onset, severity, duration, treatment intervention, and evolution.
Statistical analysis
To investigate the association between tumor response and RCHs, imaging information of the tumors was obtained every 8 weeks within the first half year, and extended to every 12 weeks thereafter. Tumor response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by blinded, independent, radiologic review. To ascertain whether this drug could make an influence on internal vascular, enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scanning of the abdomen would run in conjunction with tumor scanning for all the patients. Brain MRI scanning was performed in willing or symptomatic patients. One-way analysis of variance was used to compare measurement data. A P-value < 0.05 was considered statistically significant. All data analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
A total of 98 patients were included in this study, with 12 in the 60 mg-dose cohort, 74 in the 200 mg-dose cohort and 12 in the 400 mg-dose cohort. The median age of the patients was 59 years. Seventy-nine of them were male. The median follow-up time was 242 (range, 29–567) days. The baseline characteristics of the participants are listed in Table 1.
1.
Baseline characteristics of treated patients
| Characteristics | Dose cohort | All patients
(n = 98) |
||
| 60 mg (n = 12) | 200 mg (n = 74) | 400 mg (n = 12) | ||
| ECOG, Eastern Cooperative Oncology Group. | ||||
| Median age, years | 52 (35–66) | 59 (29–75) | 57.5(35–65) | 59 (29–75) |
| Gender, n (%) | ||||
| Male | 8 (66.7) | 61 (82.4) | 10 (83.3) | 79 (80.6) |
| Female | 4 (33.3) | 13 (17.6) | 2 (16.7) | 19 (19.4) |
| ECOG performance status, n (%) | ||||
| 0 | 10(83.3) | 60(81.1) | 10 (83.3) | 81(82.7) |
| 1 | 2(16.7) | 14(18.9) | 2(16.7) | 17(17.3) |
| Tumor types | ||||
| Esophageal squamous cell carcinoma | 3 | 37 | 2 | 42 |
| Small cell carcinoma of esophagus | 0 | 1 | 0 | 1 |
| Triple negative breast cancer | 2 | 4 | 1 | 7 |
| Adenocarcinoma of the esophagogastric junction and stomach | 3 | 25 | 2 | 30 |
| Lung adenocarcinoma | 2 | 0 | 1 | 3 |
| Nasopharyngeal squamous cell carcinoma | 2 | 0 | 1 | 3 |
| Hepatocellular carcinoma | 0 | 3 | 2 | 5 |
| Intrahepatic cholangiocarcinoma | 0 | 1 | 0 | 1 |
| Colorectal adenocarcinoma | 0 | 2 | 2 | 4 |
| Cervical squamous cell carcinoma | 0 | 1 | 0 | 1 |
| Bladder transitional cell carcinoma | 0 | 0 | 1 | 1 |
| Previous systemic therapies n (%) | ||||
| ≤ 1 | 0 (0) | 23 (31.1) | 2 (16.7) | 25 (25.5) |
| 2 | 5 (41.7) | 26 (35.1) | 4 (33.3) | 35 (35.7) |
| 2 | 7 (58.3) | 25 (33.8) | 6 (50.0) | 38 (38.8) |
Emergence of RCHs
By the cut-off time of this study, 12 of the participants were still receiving medication. RCHs were identified in 85.7% (84/98) of the patients, regardless of gender, age and tumor type. The features of RCHs are listed in Table 2.
2.
Clinical features of reactive capillary hemangiomas (RCHs)
| Features | Dose cohort | All patients
(n=98) |
||
| 60 mg (n=12) | 200 mg (n=74) | 400 mg (n=12) | ||
| RCH events n (%) | 8 (66.7) | 64 (86.5) | 12 (100) | 84 (85.7) |
| Time to onset, median (range), days | 53.5 (43–114) | 18.5 (2–144) | 10 (3–32) | 19.5 (2–144) |
| No. of injections before onset, median (range) | 3.5 (3–8) | 1(1–9) | 1 (1) | 1 (1–9) |
| Peak time, median (range), days | 115 (71–169) | 84 (28–171) | 84 (70–98) | 84 (28–71) |
| No. of injections to peak time median (range) | 7 (4–12) | 5.5 (2–11) | 5 (4–6) | 5 (2–12) |
| Severity | ||||
| Grade 1 | 8 | 54 | 9 | 71 |
| Grade 2 | 0 | 10 | 3 | 13 |
| Grade 3–4 | 0 | 0 | 0 | 0 |
| Location | ||||
| Cutaneous only | 8 | 56 | 8 | 72 |
| Mucosal only | 0 | 0 | 0 | 0 |
| Mixed (cutaneous and mucosal) | 0 | 8 | 4 | 12 |
The median time from the initiation of SHR-1210 treatment to onset of RCHs was 20 (range: 2–144) days in the entire population; it was 53.5 days, 18.5 days and 10.0 days in the 60 mg-dose, 200 mg-dose, and 400 mg-dose cohorts, respectively. One-way analysis of variance showed that the median time of onset of RCHs was shortest in the 400 mg-dose cohort (P < 0.001). Grade 1 DAEs comprised 84.5% (71/84) of the RCHs. Grade 2 RCHs were not observed in the 60 mg-dose cohort, but 13.5% (10/74) and 25% (3/12) were found in the 200 mg-dose and 400 mg-dose cohorts, respectively. No grade 3 or 4 RCHs were observed. Nine of the 14 patients without RCHs received only one injection and stopped because of symptomatic disease progression. In such a situation, RCHs would be hardly noticed.
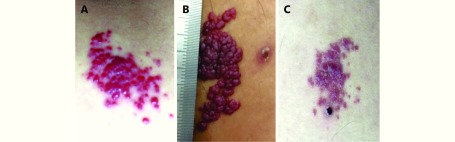
RCHs usually started as red papules or macules with clear boundaries after 1 injection. Some of the lesions were nodule-like, or gathered like mulberry. The most frequent complication was bleeding, without complaints of pain, or pruritus. No ulcerations or infections were found. After repeated hemorrhage, RCHs could be verrucous, and become solid in texture. Nearly all hemangiomas doubled in size after 3 injections, and the growth occurred most rapidly within the first 8 weeks after the initiation of treatment. Maximum size was generally observed at 12 weeks, or after 5 injections. The maximum diameter of RCH in our study was about 40 mm, and located on the inner thigh in one patient (Figure 1B).
1.
Spontaneous regression of RCHs for an esophageal squamous cell carcinoma patient (male, 51 years old) during treatment with SHR-1210. (A) Four weeks after initiation of SHR-1210. (B) Ten weeks after initiation of SHR-1210. (C) Sixteen weeks after initiation of SHR-1210.
Distribution of RCHs
All the RCHs were multiple and disseminated. They developed widespread on body surfaces, and were present most frequently on the head and neck, trunk, and extremities. Lesions of 12 patients were also found on mucosal surfaces: 3 were found on the sclera, with no impact on vision; 3 were observed on the gingiva; others were found in the nasal cavity, on the buccal mucosa, lip or tongue. However, no lower digestive or respiratory tract bleeding occurred. To identify whether the drug could affect the vessels of important internal organs, all the patients underwent abdominal scans regularly, and no new hemangiomas were observed. Twenty-seven willing patients had at least one brain MRI scan randomly after medication (range: day 2 to 376); 16 were conducted within 12 weeks after treatment initiation. No signs of new internal vascular anomalies were found, either. The remaining patients who refused to undergo brain scans were all asymptomatic.
Regression of RCHs
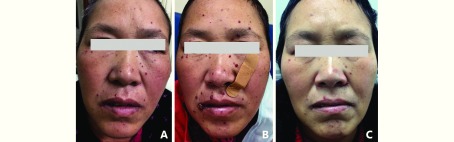
During the two-week interval between injections, new lesions occurred; the pre-existing RCHs became plump and bright red in the first week and then gradually became flat and dull red in the following week. When reaching their full size, old lesions regressed spontaneously. Meanwhile, new lesions still emerged along with medication, but these lesions were milder and more tolerable; the number of new eruptions was much less, and their size was much smaller than the previous ones. At the clinic, apparent involution of RCHs was observed in all participants after peak time. Complete regression of RCHs could be observed both during (Figure 1) and after (Figure 2) treatment. No new lesions occurred after the interruption of the therapy. By the time of analysis, RCHs of 55 patients had disappeared. Fifteen of them were observed during treatment, and the median time gap from the occurrence to the disappearance of RCHs was 221 (range: 14–448) days. Forty were observed after the termination of treatment. The median time from the last dose to the date of complete involution was 53.5 (range: 2–220) days. Seven of the remaining 29 patients without complete regression were still under treatment, 21 died within 3 months after the last dose, and 1 was experiencing gradual and on-going regression after withdrawal of SHR-1210.
2.
Spontaneous regression of RCHs for a triple negative breast cancer patient (female, 50 years old) after termination of SHR-1210. (A) Four weeks after initiation of SHR-1210. (B) Eight weeks after initiation of SHR-1210 (the end of treatment). (C) Eight weeks after the termination of SHR-1210.
Histopathological features of RCHs
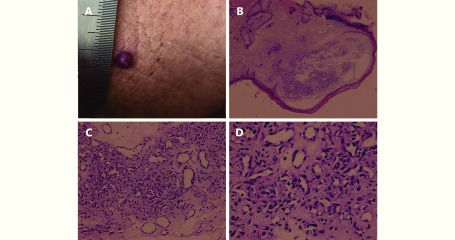
Four willing patients with RCHs underwent skin biopsy. Histopathology showed small capillaries in the papillary dermis or proliferation of endothelial cells. This was reported as capillary hemangioma by the pathologist. Figure 3 shows the histologic features of grade 1 RCHs of an esophageal squamous cell carcinoma patient.
3.
A dome-shaped, red nodule on the chest. (A) Histopathology of the resected specimen showed a well-circumscribed proliferation of small capillaries (H&E staining, B, 4 ×; C, 100 ×; D, 400 ×)
Management of RCHs
Treatment was not required in the majority of cases except for patients with high risk of bleeding (plump lesions or vulnerable ones). The application of imiquimod 5% cream for one grade 2 nodule-like RCHs did not work. RCHs of two patients who took in oral corticosteroids (prednisone 1 mg/kg), could be relieved, but did not completely regress. Topical corticosteroids or timolol hydrogen maleate could also retard the growth of RCHs but was not effective enough. Four patients underwent lesion excision; wound healing was complete and there was no recurrence.
Tumor response and RCHs
The responses of 95 patients were available by cut-off time: 83 of them experienced RCHs, and 12 did not. Tumor response was found in all the tumor types included in the study except for triple negative breast cancer (Supplementary Table S1). For patients with RCHs, the objective response rate was 28.9% (24/83) and 12 patients were still receiving medication by cut-off time, but no responders were observed among the 12 patients without RCHs. There was a significant difference between the RCH and non-RCH groups in objective response (28.9% vs. 0%, P = 0. 031), but 8 patients in the non-RCH group quit the study after only one injection due to rapid disease progression; only one received 4 injections.
S1.
Tumor response in different types of cancer
| Tumor types | No.(n = 98) | Tumor response | ||
| CR+PR | SD | PD | ||
| * Tumor response of the three patients was not available. CR: complete remission; PR: partial remission; SD: stable disease; PD: progression disease. | ||||
| Esophageal squamous cell carcinoma* | 42 | 11 | 11 | 17 |
| Small cell carcinoma of esophagus | 1 | 0 | 0 | 1 |
| Triple negative breast cancer | 7 | 0 | 0 | 7 |
| Adenocarcinoma of the esophagogastric junction and stomach | 30 | 7 | 6 | 17 |
| Lung adenocarcinoma | 3 | 1 | 0 | 2 |
| Nasopharyngeal squamous cell carcinoma | 3 | 1 | 0 | 2 |
| Hepatocellular carcinoma | 5 | 1 | 2 | 2 |
| Intrahepatic cholangiocarcinoma | 1 | 1 | 0 | 0 |
| Colorectal adenocarcinoma | 4 | 1 | 2 | 1 |
| Cervical squamous cell carcinoma | 1 | 0 | 0 | 1 |
| Bladder transitional cell carcinoma | 1 | 1 | 0 | 0 |
Other dermatologic toxicities
Besides RCHs, two other prevalent DAEs were rash (29/98, 29.6%) and pruritus (16/98, 16.3%). Immune-related vitiligo was found on only one patient with gastric adenocarcinoma. No relationship between RCHs and other DAEs was observed (data were not shown). Almost all of these DAEs were grade 1 or 2 events, but two patients had to stop the treatment due to grade 3 pruritus. Pruritus and rash of most patients were self-limiting. Unlike RCHs, pruritus and rash in some patients could be recurrent and deteriorative along with medication. The symptoms could be significantly relieved by antihistamine drugs or discontinuation of treatment.
Discussion
RCHs are different from hemangiomas. Hemangiomas are true neoplasms of endothelial cells, and they are the most common benign vascular tumors in children, affecting 5%–7% of all infants and children9,10. The pathogenic mechanisms of hemangiomas are poorly understood. Perhaps the imbalance between enhancers and inhibitors of angiogenesis may contribute to it11,12. It is very rare for adults to acquire CHs, especially after medication. However, CHs induced by SHR-1210 were very common. Their clinical course resembled that of infantile capillary hemangiomas, characterized by a tripartite growth cycle of proliferation, plateau, and involution13. But different from infantile capillary hemangiomas, all the reactive lesions in this study occurred in adults and emerged after receiving SHR-1210; new lesions still appeared during the “involution phase” and most would completely regress after discontinuation of SHR-1210 if enough observation time was available.
Some types of benign vascular tumors also present as the side-effect of chemotherapy, such as pyogenic granuloma (PG). PG is commonly considered as a reactive inflammatory hyperproliferative vascular response to a variety of stimuli, such as infection, trauma, and female sex hormones14, but drug-induced PG is seldom seen. Only isolated case reports had described them in detail15-18. PG usually presents as a single, lobulated, pedunculated or sessile papule. Case reports provide some clues of disseminated lesions after exfoliative dermatitis or a hypersensitivity drug reaction19,20, but multifocal lesions are extremely rare, too. Unlike drug-induced PG, the incidence of RCHs induced by SHR-1210 was as high as 85.4%, and they were all multifocal lesions, so the management of RCHs could not entirely be the same as that of PGs, either21.
Since the skin is a major organ affected by PD-1 inhibitors, RCHs were supposed to be immune-related DAEs. IrAEs are drug-mediated nonspecific hyperfunctioning of the immune system, notably mediated by the triggering of cytotoxic CD4+/CD8+ T cell activation22. However, the pattern and frequency of DAEs in our study was distinct from that of nivolumab or pembrolizumab1. Instead of rash and pruritus, RCHs were the most prevalent DAEs of SHR-1210. RCHs would become more tolerable as the treatment went on after peak time. Meanwhile, the frequencies of rash and pruritus in this study were still in accordance with previous reports of other PD-1 inhibitors. Vitiligo was relatively rare with an incidence of only 1.04%. Although all DAEs were self-limiting, only RCHs would not be recurrent and deteriorative after peak time. The underlying mechanism still needs further investigation. They dispersed only on cutaneous and mucosal surfaces, not life-threatening or function-impairing, but might contribute to negative self-image evaluation. We also tried to control the rapid enlargement in many ways for some symptomatic patients, but RCHs could only be slightly relieved but not eliminated (e.g. topical or oral corticosteroids) during peak- time. Local treatment (excision) might be a good choice for patients with recurrent hemorrhage. All lesions were expected to disappear gradually after termination of treatment, so we emphasize an approach of careful observation but not active treatment.
Development of rash and melanoma-associated vitiligo after immunotherapy has already been proven to be correlated with improved survival23-25. In our study, patients who developed RCHs seemed to have higher response rate than patients without RCHs, But but we must interpret these findings with caution. Firstly, the number of patients without RCHs was very small (n = 12); secondly, patients in the non-RCH group did not receive the same cumulative dose of SHR-1210 as those who achieved disease control and continued medication. Most patients in the non-RCH group received only one injection, which might be far from the dose required for RCHs to appear.
The limitation of this study is that only four patients underwent skin biopsy for RCHs. Biomarkers of RCHs such as infiltrating lymphocytes or PD-L1 expression was far from known. Besides, the onset times of RCHs for the first four participants were susceptible to recall bias because of potential delayed recognition of RCHs (see the section "Recognition of first RCHs" in Supplementary materials). The major strength of this study is its prospective design, and that the entire clinical course of RCHs was informatively documented. Moreover, it is also the largest study describing DAEs of SHR-1210, which might be instructive in the management of side effects of SHR-1210 in future studies.
There are diverse clinical and histological appearances of DAEs induced by one kind of PD-1 inhibitor, not to mention the DAEs induced by various PD-1 inhibitors. Even some extremely rare DAEs of chemotherapy or targeted therapy, such as psoriasis26, vitiligo1 and Grover’s disease5, are commonly reported in patients receiving PD-1 inhibitors. As a novel anti-PD-1 inhibitor, SHR-1210 had shown a favorable anti-tumor efficacy, but what followed it were new toxicities far from those known. Clinicians are faced with a more challenging situation now. It is imperative for oncologists and dermatologists to be cognizant of novel toxicities of anti-PD-1 treatment so as to reach a more conscious and rational use of such agents. This study of RCHs might add to the expanding literature regarding immune-related and even drug-related adverse events.
Acknowledgements
We would like to acknowledge Jiangsu Hengrui Medicine Co. Ltd for providing the drugs. This work is supported by a grant from CAMS Initiative for Innovative Medicine (Grant No. CAMS-12M-1-010).
Conflict of interest statement
No potential conflicts of interest are disclosed.
Biography
huangjingwg@163.com
References
- 1.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2016;27:1362. doi: 10.1093/annonc/mdw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, et al Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12–25. doi: 10.1016/j.ejca.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccarese C, Alfieri S, Santoni M, Santini D, Brunelli M, Bergamini C, et al New toxicity profile for novel immunotherapy agents: focus on immune-checkpoint inhibitors. Expert Opin Drug Metab Toxicol. 2016;12:57–75. doi: 10.1517/17425255.2016.1120287. [DOI] [PubMed] [Google Scholar]
- 4.Postow MA, Callahan MK, Wolchok JD Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–82. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curry JL, Tetzlaff MT, Nagarajan P, Drucker C, Diab A, Hymes SR, et al Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44:158–76. doi: 10.1111/cup.2017.44.issue-2. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Xu BH, Mo HN, Zhang WL, Chen XL, Wu DW, et al Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 7.Mo HN, Huang J, Xu JC, Chen XL, Wu DW, Qu D, et al Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538–45. doi: 10.1038/s41416-018-0100-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen AP, Setser A, Anadkat MJ, Cotliar J, Olsen EA, Garden BC, et al Grading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0. J Am Acad Dermatol. 2012;67:1025–39. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 9.George A, Mani V, Noufal A Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18:S117–20. doi: 10.4103/0973-029X.141321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luu M, Frieden IJ Haemangioma: clinical course, complications and management. Br J Dermatol. 2013;169:20–30. doi: 10.1111/bjd.12436. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Chen SY, Li K, Li L, Xu C, Xiang B Signaling pathways in the development of infantile hemangioma. J Hematol Oncol. 2014;7:13. doi: 10.1186/1756-8722-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laken PA Infantile Hemangiomas: Pathogenesis and review of propranolol use. Adv Neonatal Care. 2016;16:135–42. doi: 10.1097/ANC.0000000000000254. [DOI] [PubMed] [Google Scholar]
- 13.Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL, Garzon MC, et al Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122:360–7. doi: 10.1542/peds.2007-2767. [DOI] [PubMed] [Google Scholar]
- 14.Harris MN, Desai R, Chuang TY, Hood AF, Mirowski GW Lobular capillary hemangiomas: An epidemiologic report, with emphasis on cutaneous lesions. J Am Acad Dermatol. 2000;42:1012–6. doi: 10.1067/mjd.2000.104520. [DOI] [PubMed] [Google Scholar]
- 15.Fujiwara C, Motegi SI, Sekiguchi A, Amano H, Ishikawa O Pyogenic granuloma possibly associated with capecitabine therapy. J Dermatol. 2017;44:1329–31. doi: 10.1111/jde.2017.44.issue-11. [DOI] [PubMed] [Google Scholar]
- 16.Paul LJ, Cohen PR Paclitaxel-associated subungual pyogenic granuloma: report in a patient with breast cancer receiving paclitaxel and review of drug-induced pyogenic granulomas adjacent to and beneath the nail. J Drugs Dermatol. 2012;11:262–8. [PubMed] [Google Scholar]
- 17.Massa A, Antunes A, Varela P Pyogenic granuloma in a patient on Gefitinib. Acta Med Port. 2016;29:416. doi: 10.20344/amp.6343. [DOI] [PubMed] [Google Scholar]
- 18.Simmons BJ, Chen L, Hu S Pyogenic granuloma association with isotretinoin treatment for acne. Australas J Dermatol. 2016;57:e144–5. doi: 10.1111/ajd.12418. [DOI] [PubMed] [Google Scholar]
- 19.Palmero ML, Pope E Eruptive pyogenic granulomas developing after drug hypersensitivity reaction. J Am Acad Dermatol. 2009;60:855–7. doi: 10.1016/j.jaad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Aliağaoğlu C, Bakan V, Atasoy M, Toker S Pyogenic granuloma with multiple and satellite involvement after a burn in a 5-year-old child. J Dermatol. 2006;33:150–2. doi: 10.1111/jde.2006.33.issue-2. [DOI] [PubMed] [Google Scholar]
- 21.Patil A, Pattanshetti C, Varekar A, Huddar SB Oral capillary haemangioma mimicking pyogenic granuloma: a challenge for diagnosis and management. BMJ Case Rep. 2013;14:2013. doi: 10.1136/bcr-2012-007874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber JS, Yang JC, Atkins MB, Disis ML Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33:2092–9. doi: 10.1200/JCO.2014.60.0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 24.Byrne KT, Turk MJ New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget. 2011;2:684–94. doi: 10.18632/oncotarget.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206–12. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, et al Anti-PD1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol. 2017;31:e254–7. doi: 10.1111/jdv.14011. [DOI] [PubMed] [Google Scholar]