Abstract
Mitochondrial functions are essential for cell viability and rely on protein import into the organelle. Various disease and stress conditions can lead to mitochondrial import defects. Here we found that inhibition of mitochondrial import in budding yeast activated a surveillance mechanism, mitoCPR, that improved mitochondrial import and protected mitochondria during import stress. mitoCPR induced expression of Cis1 which associated with the mitochondrial translocase to reduce the accumulation of mitochondrial precursor proteins at the mitochondrial translocase. Clearance of precursor proteins depended on the Cis1 interacting AAA+ ATPase Msp1 and the proteasome suggesting that Cis1 facilitates degradation of un-imported proteins. mitoCPR was required for maintaining mitochondrial functions when protein import was compromised, demonstrating the importance of mitoCPR in protecting the mitochondrial compartment.
Mitochondrial function is required for cell viability, producing energy and many essential biological molecules such as iron-sulfur clusters and heme (1). Even though mitochondria contain their own genome, the vast majority of their proteins is encoded by the nucleus. Import of nuclear encoded proteins into mitochondria is essential for mitochondrial function and cell viability (1, 2). Defects in mitochondrial protein import are associated with various human diseases, such as Deafness Dystonia Syndrome and Huntington’s disease (3–5). However, even though mitochondrial protein import is essential for all mitochondrial functions, little is known about how cells respond to mitochondrial protein import defects. Recently, two pathways mPOS (mitochondrial precursor over-accumulation stress) and UPRam (unfolded protein response activated by mistargeting of proteins) have been identified in yeast that respond to the accumulation of un-imported mitochondrial proteins in the cytosol (6, 7). UPRam and mPOS reduce global translation and UPRam protects the cytosol from proteotoxic effects of un-imported proteins by accelerating their degradation. In mammals, the Ubiquilin family of proteins has a similar role in mediating the degradation of mitochondrial transmembrane proteins that fail to get imported and remain in the cytosol (8). Whether mechanisms exist that protect mitochondrial functions in the face of mitochondrial import stress is unclear. Here we identified a response to mitochondrial protein import defects that protected mitochondrial functions by reducing the accumulation of precursor proteins at the mitochondrial surface and translocase. This response was brought about by the transcription factor PDR3, previously shown to mediate a multi-drug resistance (MDR) response.
The canonical MDR response is conserved from bacteria to mammals (9). It protects organisms from xenobiotics and can limit the effectiveness of microbial and cancer chemotherapy (9, 10). In budding yeast, the MDR response is activated by a variety of chemical compounds and is primarily mediated by the two related transcription factors Pdr1 and Pdr3 (11–13). They induce the expression of several ABC transporters to mediate efflux of xenobiotics (13). A transcriptional response related to the MDR and specifically mediated by Pdr3 is active in yeast cells with defective mitochondrial DNA (mtDNA) (14). In such cells, Pdr3 induces the expression of genes encoding ABC transporters, sphingolipid biosynthesis enzymes and a number of genes of unknown function (15). We show here that Pdr3 mediates a mitochondrial import defect response.
A system to inhibit mitochondrial protein import acutely
All mitochondrial functions depend on proteins being imported from the cytosol into the organelle. Whether pathways exist that monitor import of proteins into mitochondria and elicit a cellular response under conditions of mitochondrial import stress is unknown. To determine whether cells respond to mitochondrial import stress, we examined the consequences of acutely interfering with mitochondrial protein import. Compounds that uncouple the mitochondrial respiratory chain, such as CCCP (Carbonyl cyanide m-chlorophenyl hydrazine), prevent mitochondrial import, which is dependent on the mitochondrial membrane potential (2). However, these same compounds can also affect potential across other cellular membranes and induce a MDR response, which complicates delineating responses unique to mitochondrial import defects. We hypothesized that acute induction of mitochondrial import stress could be achieved, without drugs, by overloading the mitochondrial import machinery through overexpression of mitochondrial proteins. We overexpressed a number of mitochondrial proteins from the strong galactose inducible GAL1–10 promoter and assessed the mitochondrial import of Cox5a, a nuclear encoded subunit of mitochondrial complex IV. Like most mitochondrial proteins, Cox5a harbors an N-terminal presequence that is cleaved upon import into the mitochondrial matrix (16). In untreated cells, mitochondrial import and precursor cleavage was so efficient that the Cox5a preprotein (henceforth Cox5apre) was not detected (Fig. 1A). Upon disruption of membrane potential and hence protein import with CCCP, Cox5apre accumulated in cells (Fig. 1A).
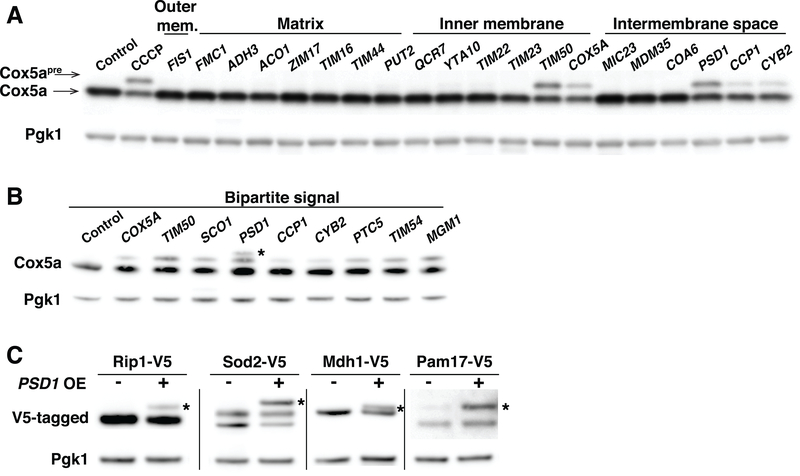
Fig. 1. Overexpression of bipartite signal-containing proteins induces mitochondrial protein import defects.
(A) Immuno-blot of Cox5a-V5 and Cox5apre-V5 (Cox5a preprotein) in control cells, CCCP treated cells (20μM, 1h) or cells overexpressing mitochondrial proteins by the addition of galactose for 4h. Overexpressed proteins are divided by their localization to the outer membrane (outer mem.), matrix, inner membrane and intermembrane space compartments. Pgk1 was used as a loading control. (B) Same as (A). Asterisk represents a non-specific band, result of PSD1 overexpression. (C) Immuno-blot of Rip1-V5, Sod2-V5, Mdh1-V5 and Pam17-V5 (expressed from their endogenous promoter) in control cells or following overexpression of PSD1 for 4h. Asterisks identify the precursor form of the indicated proteins. OE, overexpression. Note that as previously shown (50), Sod2 migrates in SDS PAGE as a doublet under conditions when mitochondria are intact and, as a triplet when its cleavage is inhibited.
Overexpression of the majority of mitochondrial proteins did not affect Cox5a processing, but high levels of Psd1, Ccp1, Cyb2, Cox5a or Tim50 led to Cox5apre accumulation (Fig. 1A). All five proteins use the same mitochondrial import machinery. They contain a bipartite signal that inhibits translocation into the mitochondrial matrix. This results in the lateral release of proteins out of the inner membrane TIM23 translocase into the inner membrane itself (2). A broad survey of mitochondrial proteins known to contain a bipartite signal confirmed this conclusion (Fig. 1B). In contrast, inner membrane proteins that use other import mechanisms (i.e. the TIM22 pathway) or proteins that translocate across the TIM23 translocase, such as matrix proteins, did not affect Cox5a processing (Fig. 1A). Overexpression of bipartite signal-containing proteins affected import of proteins other than Cox5a. High levels of the bipartite signal-containing protein Psd1 interfered with the processing of a number of presequence containing proteins whose import is mediated by the TIM23 complex (Fig. 1C). Thus, saturation of the TIM23 lateral diffusion import pathway leads to the accumulation of mitochondrial preproteins.
The accumulation of mitochondrial preproteins could reflect defects in either translocation into mitochondria or presequence cleavage in the matrix. To test the former possibility we determined the localization of Cox5apre. Both the mature and the preprotein forms of Cox5a were detected in mitochondrial but not cytosolic fractions following overexpression of PSD1 or CCCP treatment (Fig. 2A). Addition of proteinase K to the mitochondrial fractions led to loss of Cox5apre but not mature Cox5a that resides in the inner membrane with its C terminus facing the intermembrane space. Because Cox5a was detected using a C-terminal V5 tag in this analysis, we conclude that at least the C-terminus of Cox5apre resides at the surface of mitochondria facing the cytosol. These results lead to two important conclusions. First, overexpressed bipartite signal containing proteins interfere with mitochondrial protein translocation. Second, Cox5apre’ C-terminus accumulates at the mitochondrial surface when mitochondrial import is impaired.
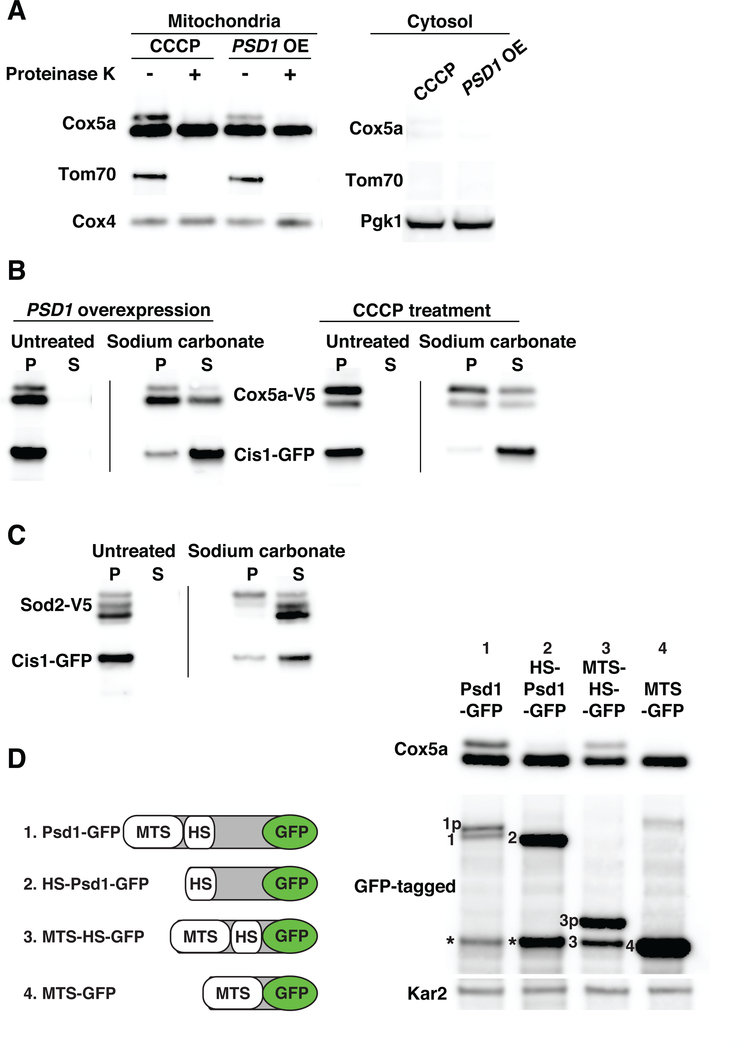
Fig. 2. Mitochondrial precursors accumulate on the surface of the organelle and in the translocase during import stress.
(A) Mitochondria were isolated by differential centrifugation from cells treated with 20μM CCCP for 1h or cells overexpressing PSD1 for 6h. Cox5a-V5, Tom70-mCherry and Cox4 or Pgk1 were detected in mitochondria and cytosol fractions. Mitochondria were treated with 50μg/ml proteinase K. Tom70 served as an outer membrane control protein; Cox4 as a matrix control protein. OE, overexpression. (B) Mitochondria were isolated from cells expressing COX5a-V5 and CIS1-GFP and overexpressing PSD1 for 6h or cells treated with 20μM CCCP for 1h. Sodium carbonate treated or untreated mitochondria were centrifuged to separate insoluble proteins [pellet (P)] from soluble proteins [supernatant (S)]. Samples were analyzed by immuno-blot analysis. Cis1-GFP served as a peripheral outer membrane protein control. (C) Mitochondria were isolated from cells overexpressing PSD1 for 6h. Mitochondria were treated as in (B) to analyze Sod2-V5 by immuno-blot analysis. (D) Left panel: PSD1-GFP constructs used in the analysis. MTS, mitochondrial targeting sequence; HS, hydrophobic segment. Right panel: immuno-blot blot of Cox5a-V5 and Psd1-GFP fusion proteins following overexpression of PSD1-GFP fusion genes for 4h. Kar2 was used as a loading control. Numbering on the immuno-blot indicates the mature form of the GFP-tagged proteins. The letter p following this number identifies the precursor form of proteins. Asterisks identify a proteolytic cleavage product of Psd1 known as the α subunit (51).
Cox5apre could be peripherally associated with the mitochondrial outer membrane by binding to receptors on the mitochondrial surface, be trapped in the translocase, or be incorrectly inserted into the outer membrane via its transmembrane domain. To determine the exact localization of Cox5apre we treated mitochondria preparations with sodium carbonate (pH 11), which extracts peripheral membrane proteins from membranes (17). As expected, the inner membrane localized, mature Cox5a was largely resistant to sodium carbonate extraction, while the peripheral outer membrane protein Cis1 dissociated from mitochondria during this treatment (Fig. 2B). Most of Cox5apre remained associated with mitochondrial membranes during sodium carbonate treatment indicating that a large fraction of Cox5apre was either inappropriately integrated into the outer membrane or stalled in the TOM translocase (Fig. 2B). To distinguish between these possibilities we investigated the localization of Sod2, a mitochondrial matrix protein that lacks any transmembrane domains. Like Cox5apre, Sod2pre accumulated at the mitochondrial outer membrane following overexpression of PSD1; association of the precursor with mitochondrial fractions was sensitive to proteinase K treatment (Fig. S1). Sod2pre was also largely resistant to sodium carbonate extraction (Fig. 2C). In contrast, sodium carbonate treatment solubilized mature matrix localized Sod2. Thus, during import stress mitochondrial preproteins are tightly bound to the mitochondrial outer membrane independently of transmembrane domains. This suggests that at least a fraction of the preproteins is stalled in the mitochondrial translocase during import stress.
How do bipartite signal containing proteins interfere with protein import when overexpressed? To address this question we determined which bipartite signal element interfered with protein import when overexpressed. Bipartite mitochondrial targeting signals are comprised of a mitochondrial targeting sequence, MTS, and a hydrophobic segment, that directs the protein to the inner membrane. Overexpressed Psd1 lacking its MTS did not inhibit mitochondrial protein import demonstrating that Psd1 must be imported into mitochondria to interfere with Cox5a import (Fig. 2D). Consistent with this conclusion, Psd1’s bipartite signal was sufficient to inhibit Cox5a mitochondrial import. Overexpression of GFP fused to Psd1’s bipartite signal inhibited Cox5a import whereas a fusion between only Psd1’s MTS and GFP did not (Fig. 2D). Thus, when present in excess, bipartite signal containing proteins interfere with import only when targeted to the inner membrane. This finding indicates that lateral diffusion out of TIM23 translocase is a rate-limiting step in mitochondrial import that can be saturated by overexpressing proteins imported via this route.
Mitochondrial import defects activate the mitoCPR
Does inhibiting protein import elicit a cellular response? To address this question we examined the transcriptional consequences of overexpressing PSD1. Overexpression of PSD1 upregulated 217 genes and downregulated 11 genes by 2 fold or more (Table S1). Among the upregulated genes was a group of genes previously shown to be induced by the transcription factor Pdr3, but not its close homologs Pdr1, in response to PSD1 overexpression and loss of mtDNA (14, 18). We identified 19 genes whose induction upon mitochondrial import stress depended on PDR3 (Table S1, Fig. 3A, B). This group of genes included MDR response genes such as genes encoding ABC transporters, proteins involved in lipid metabolism and transport, NADPH-dependent enzymes, and a number of proteins of unknown function (Fig. 3A). Other, well characterized mitochondrial stress responses were however not activated by PSD1 overexpression within the time frame of the experiment. PSD1 overexpressing cells did not induce RTG (retrograde) regulated genes, such as CIT2 and PDH1, that are known to be activated in response to defects in Krebs cycle function (Table S1) (19). The finding that overexpression of PSD1 inhibited mitochondrial import suggests that it is mitochondrial import defects that elicit this PDR3-mediated transcriptional response. The finding that cells lacking mtDNA, which exhibit severe mitochondrial import defects (20, 21), also show this transcriptional response is consistent with this idea.
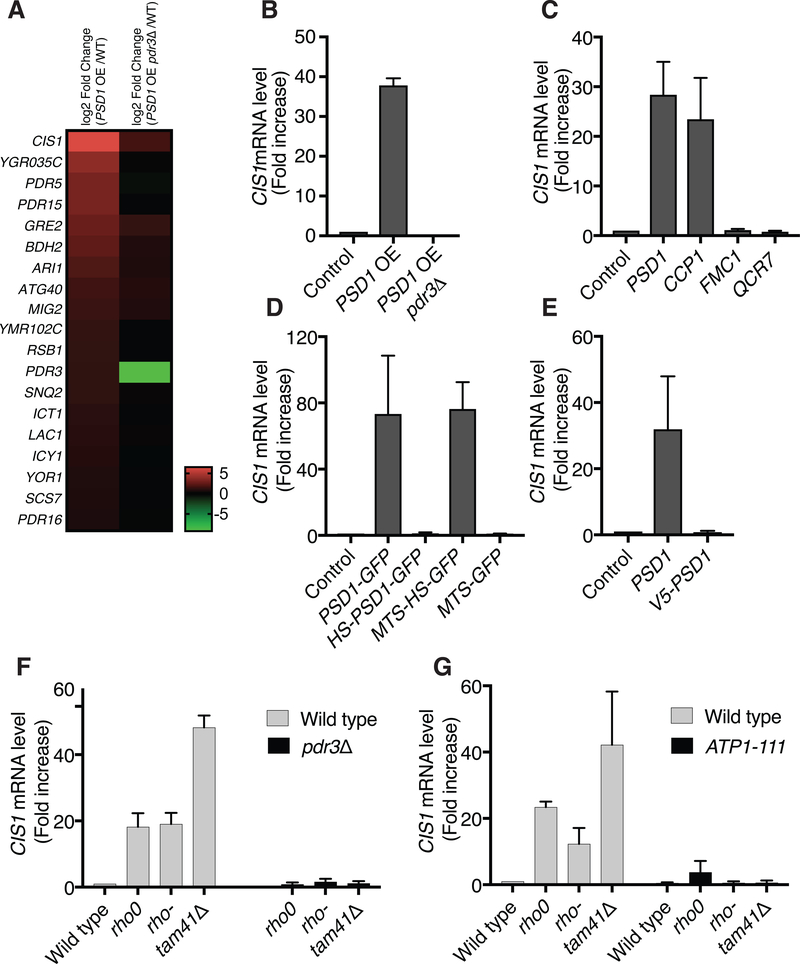
Fig. 3. Inhibition of mitochondrial protein import induces the mitoCPR.
(A) Gene expression analysis of control wild type cells and wild type or pdr3Δ cells that overexpressed PSD1 for 4h by galactose induction. The heat map describes the transcription profiles of cells overexpressing PSD1 and pdr3Δ cells overexpressing PSD1. The 19 genes shown met the following criteria: 1. Genes that exhibited an increase in expression of at least (log2) 0.6 and adjusted p-values that are equal or lower than 0.05 in PSD1 overexpressed cells versus PSD1 overexpressed cells lacking pdr3Δ. 2. Genes that exhibited an increase in expression of at least (log2) 0.6 and adjusted p-values that are equal or lower than 0.05 in PSD1 overexpressed cells versus control cells. WT, wild type. OE, overexpression. (B) CIS1 mRNA levels in wild type cells, cells overexpressing PSD1, or pdr3Δ cells overexpressing PSD1. PSD1 expression was induced by addition of galactose for 4h. OE, overexpression. n=3. Data are mean +/− SD. (C) CIS1 mRNA levels in control cells or cells overexpressing mitochondrial proteins by galactose induction (4h) were analyzed by quantitative RT-PCR. n=3. Data are mean +/− SD. (D) Same as (C) following overexpression of PSD1-GFP fusion genes for 4h. MTS, mitochondrial targeting sequence; HS, hydrophobic segment. n=3. Data are mean +/− SD. (E) Same as (C) following overexpression of PSD1 or V5-PSD1 for 4h. n=3. Data are mean +/− SD. (F) CIS1 mRNA levels of wild type, rho0, rho- and tam41Δ cells in the presence or absence of PDR3. n=3. Data are mean +/− SD. (G) Same as (F) in the presence or absence of the ATP1–111 allele. n=3. Data are mean +/− SD.
To further explore a potential link between the PDR3-mediated transcriptional response and mitochondrial import defects we first asked whether proteins, which when overexpressed inhibited mitochondrial import, also induced the PDR3-mediated transcriptional response. This was the case. All mitochondrial proteins that caused protein import defects when overexpressed induced the PDR3-mediated transcriptional response as judged by upregulation of the PDR3-responsive gene CIS1. Conversely, proteins whose overexpression did not interfere with mitochondrial import did not induce CIS1 (Fig. 3C, Fig. S2A and S2B). The perfect correlation between the ability to inhibit mitochondrial import and induction of a PDR3-mediated transcriptional response was also observed when analyzing cells overexpressing various PSD1 domains. Cells overexpressing Psd1 that lacked its mitochondrial targeting signal or that harbored an N-terminal V5 tag to prevent targeting of the protein to mitochondria failed to induce CIS1 (Fig. 3D, E and Fig. S2C, D) or any other PDR3-mediated transcripts (Table S1). In contrast, GFP fused to the complete Psd1 bipartite signal induced CIS1 when overexpressed whereas GFP fused only to Psd1’s MTS did not (Fig. 3D and Fig. S2C).
The PDR3-mediated transcriptional response was not only induced by acute induction of mitochondrial import defects but was also seen in mutants in which mitochondrial import was constitutively impaired. Cells harboring deletions in mtDNA (rho- cells) or lacking mtDNA (rho0 cells), both of which cause mitochondrial import defects, expressed CIS1 at elevated level (22) (Fig. 3F). Cells lacking TAM41, a gene encoding a cardiolipin biosynthesis enzyme, have severe mitochondrial import defects but intact mtDNA (23, 24). These cells too expressed CIS1 at high levels (Fig. 3F). Not all mitochondrial defects elicited the PDR3-mediated transcriptional response. Deletion of genes encoding subunits of respiration complexes III and IV results in respiration defects (25, 26) but did not cause induction of CIS1 expression (Fig. S2E). In summary, our results reveal a tight correlation between mitochondrial import defects and induction of a PDR3-mediated transcriptional response.
To further test the hypothesis that induction of the PDR3-mediated transcriptional response is caused by mitochondrial import defects we examined the consequences of suppressing mitochondrial import defects on CIS1 expression. The ATP1–111 allele increases membrane potential and improves protein import in rho0 cells by altering the ATP/ADP ratio between the matrix and the intermembrane space (20, 21, 27). Introduction of the ATP1–111 allele into either rho0, rho- or tam41Δ cells caused a large decrease in CIS1 expression (Fig. 3G). Thus, either defects in membrane potential or import defects elicit a PDR3-mediated transcriptional response. The finding that overexpression of PSD1 for 4h, which is sufficient to induce the PDR3-mediated transcriptional response, did not significantly affect mitochondrial membrane potential (Fig. 3B and S2F) suggested that membrane potential defects do not lead to Pdr3 target genes induction. Thus, mitochondrial import defects cause a PDR3-mediated transcriptional response. We termed this response mitoCPR for mitochondrial Compromised Protein Import Response.
The mitoCPR protects mitochondrial functions during import stress
What is the role of the mitoCPR when mitochondrial protein import is impaired? To address this question we first determined the consequences of deleting PDR3 on the fate of Cox5apre under conditions where protein import is impaired. As shown above, overexpression of PSD1 led to the accumulation of Cox5apre (Fig. 1A). Cox5apre had a half-life of approximately 19 minutes in PSD1 overexpressing cells (Fig. 4A, B). The eventual loss of Cox5apre in PSD1 overexpressing cells could be due to import of the preprotein into mitochondria or cytosolic degradation or both. Deletion of PDR3 prolonged the half-life of Cox5apre (Fig. 4A, B). Conversely, overexpression of PDR3 partially suppressed the accumulation of Cox5apre under conditions of mitochondrial import stress (Fig. 4C). Thus, PDR3 and by extension mitoCPR are critical for either maintaining some level of mitochondrial import and/or clearing preproteins from the mitochondrial import machinery during import stress.
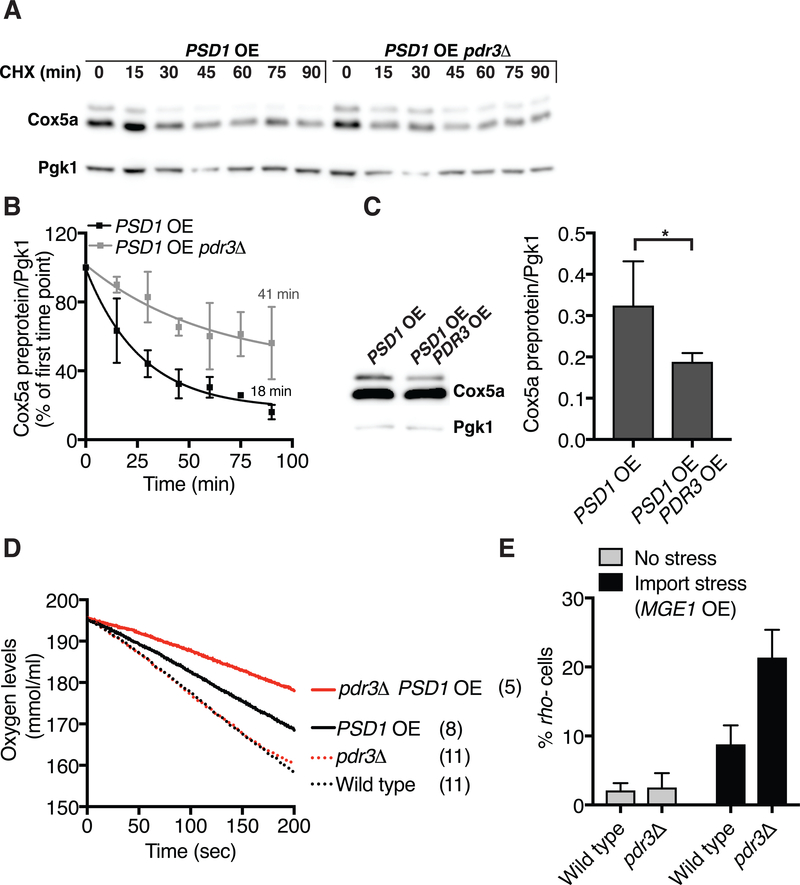
Fig. 4. The mitoCPR protects mitochondrial functions during import stress.
(A) PSD1 was overexpressed for 6h and the half-life of Cox5a preprotein was examined following cycloheximide (0.5 mg/ml) addition in wild type or pdr3Δ cells. CHX, cycloheximide; OE, overexpression. Pgk1 served as a loading control. (B) Quantification of (A)- Cox5a preprotein half-life. n=4. Data are mean +/− SD. (C) Immuno-blot of Cox5a-V5 from GAL-PSD1 cells or GAL-PSD1 cells overexpressing PDR3 (TEF2-PDR3) 6h after galactose induction. OE, overexpression. Quantification of Cox5a preprotein from 3 independent experiments is depicted on the right. Data are mean +/− SD. Statistics were performed using the Student’s t-test. *p ≤ 0.05. (D) Oxygen consumption of wild type and pdr3Δ cells that did or did not overexpress PSD1 for 4h. The oxygen consumption rate (nmole.s−1.ml) of this experiment is shown in brackets. (E) GAL-MGE1 and GAL-MGE1 pdr3Δ cells were grown for 24h in the presence or absence of galactose to induce GAL-MGE1. Mitochondrial DNA loss was analyzed by the appearance of rho- colonies on YEP plates containing 2% ethanol and 0.3% glucose. n=4. Data are mean +/− SD.
Next, we determined whether mitoCPR was important for maintaining mitochondrial functions under conditions of import stress. Upon overexpression of PSD1, oxygen consumption rate decreased (Fig. 4D and Fig. S3A). Deletion of PDR3 further exaggerated this effect (Fig. 4D and Fig. S3A) indicating that PDR3 is critical for maintaining mitochondrial respiration when mitochondrial import is compromised.
PDR1 and PDR3 prevent mtDNA loss resulting from mitochondrial fusion defects (28). We tested whether mitoCPR was important for protecting cells from mtDNA loss during import stress. Respiratory competence is a read out of mtDNA integrity. Assaying respiration, however, requires the analysis of colonies. This prerequisite precluded us from inducing mitochondrial import stress by overexpression of PSD1 because prolonged overexpression of PSD1 is lethal (Fig. S3B). In fact, overexpression of all bipartite signal-containing proteins is lethal (Fig. S3C). The mitochondrial Hsp70 chaperone, Ssc1 and its co- chaperone Mge1 are essential for mitochondrial import (29–31). We hypothesized that overexpression of SSC1 or MGE1 alone would lead to a mitochondrial import defect because the proper ratio of Hsp70 to its co- chaperone is crucial for its chaperone activity in bacteria (32). Overexpression of MGE1, while not lethal (Fig. S3B), caused a mild protein import defect comparable to that of cells lacking mtDNA. Cox5apre did not accumulate in MGE1 overexpressing cells or rho0 cells upon induction of Cox5a expression, from the methionine regulated promoter (MET25) (Fig. S3D). Nevertheless, mature Cox5a levels were reduced in GAL-MGE1 and rho0 cells compared to control cells, while COX5a mRNA expression was comparable in all strains (Fig. S3D and S3E). Thus, less Cox5a is imported into mitochondria in MGE1 overexpressing cells and unimported Cox5apre is rapidly degraded. Consistent with a mild mitochondrial import defect, overexpression of MGE1 induced a mitoCPR as judged by elevated CIS1 levels (as did overexpression of SSC1; Fig. S3F and S3G).
Having established that overexpression of MGE1 causes a mild mitochondrial import defect that is not lethal, we examined its effects on mtDNA stability. Overexpression of MGE1 for 24h led to an increase in rho- cells (Fig. 4E). Inactivation of mitoCPR by deleting PDR3 caused a 3-fold increase in cells harboring defective mtDNA (Fig. 4E). Because maintenance of mtDNA largely depends on nuclear encoded genes (33), we conclude that mitochondrial import stress prevented their import. This caused mtDNA damage and the generation of rho- cells. Furthermore, the mitoCPR protects mtDNA only during import stress. The absence of PDR3 did not affect respiration or mtDNA maintenance under normal growth conditions. Thus, mitoCRP has a protective role specifically during mitochondrial import stress.
Cis1 protects mitochondria during import stress
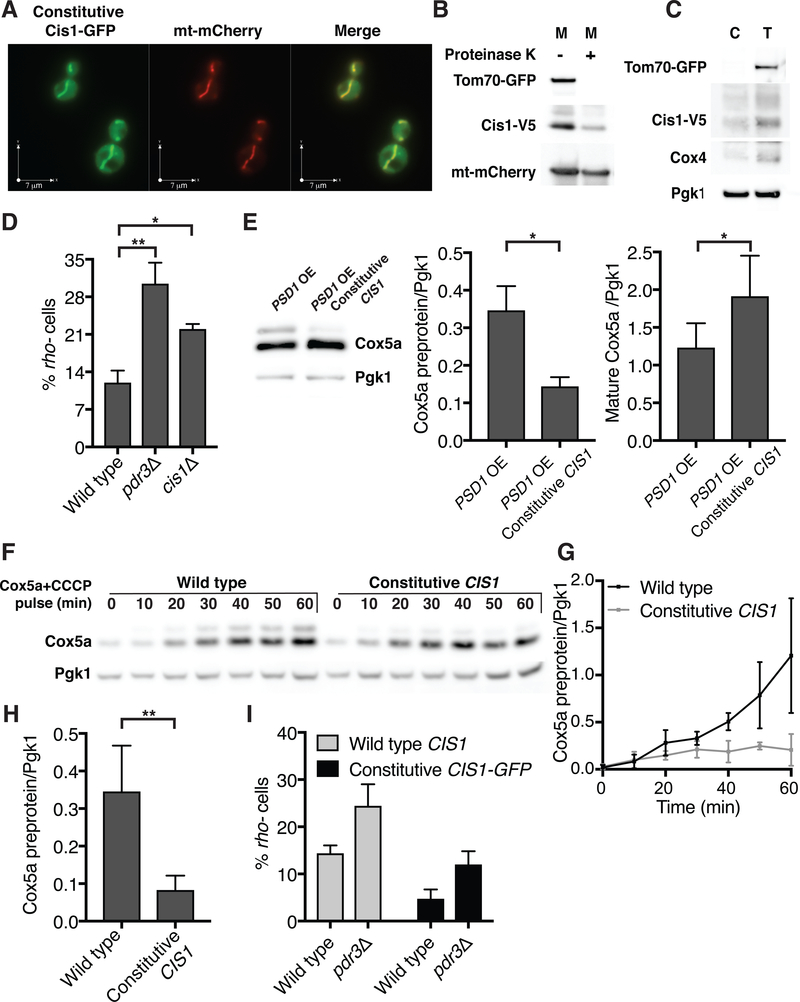
One of the most strongly induced genes following mitochondrial import stress is CIS1 (34) (Fig. 3A). CIS1 overexpression improves cellular fitness in the presence of citrinin, a mycotoxin that reduces mitochondrial membrane potential (35). The protein itself, however, harbors neither domains with known functions nor has homologs in higher eukaryotes. Cis1 protein only accumulated under conditions of mitochondrial import stress and was unstable even when expressed (Fig. S4A and S4B). Cis1 associates with mitochondria in high throughput localization studies (36), which prompted us to investigate whether the protein played a role in protecting mitochondria during import stress. To study Cis1 we placed the gene under the constitutive TEF2 promoter (Fig. S4A). A constitutively expressed Cis1-GFP fusion indeed predominantly localized to the outer membrane of the organelle (Fig. 5A–C). Cis1 is not predicted to have a transmembrane domain. We conclude that Cis1 associates with the outer mitochondrial membrane facing the cytosol.
Fig. 5. Cis1 maintains mitochondrial function during protein import stress.
(A) Live cell fluorescence imaging of cells expressing TEF2-CIS1-GFP and mitochondrial targeted mCherry (mt-mCherry). (B) Mitochondria were isolated from cells expressing TEF2-CIS1-V5 that were grown in 3% glycerol. Mitochondria (M) (+/− proteinase K) are shown. mt-mCherry-matrix control protein, Tom70-GFP-outer membrane control protein. (C) Cytosolic fraction of cells presented in (B). Cytosol (C) fractions as well as total cell lysate (T) are shown. Pgk1 served as a cytosol control protein, Tom70-GFP as an outer membrane control protein and Cox4 as a control matrix protein. (D) Wild type, pdr3Δ and cis1Δ cells were grown for 48h in the presence of galactose to induce GAL-MGE1. Mitochondrial DNA loss was analyzed by appearance of rho- (petite) colonies. n=3. Data are mean +/− SD. Student’s t-test was used. * p ≤ 0.05,** p ≤ 0.005. (E) Immuno-blot analysis of Cox5a from GAL-PSD1 or GAL-PSD1 TEF2-CIS1 cells following PSD1 overexpression (6h). OE, overexpression. Quantifications of Cox5a preprotein (middle) and mature Cox5a (right) are shown. n=3. Data are mean +/− SD. Student’s t-test was used. * p ≤ 0.05. (F) Wild type or TEF2-CIS1 cells were grown in the presence of methionine. MET25-COX5a was then induced by methionine removal in the presence of CCCP. Cox5a-V5 protein levels were analyzed at the indicated times (Pgk1, loading control). (G) Quantification of (F); Cox5a preprotein (n=4). Data are mean +/− SD. (H) Quantification of Cox5a preprotein levels 60 min following induction of MET25-COX5a in the presence of CCCP. n=6. Data are mean +/− SD. Student’s t-test was used. ** p ≤ 0.005. (I) Wild type and pdr3Δ cells (+/− TEF2-CIS1-GFP) were grown for 24h in the presence of galactose to induce GAL-MGE1. Mitochondrial DNA loss was analyzed as in (D). n=4. Data are mean +/− SD.
The expression of Cis1 proved to be important for cells during mitochondrial protein import stress. While deletion of CIS1 did not have a noticeable effect on Cox5apre levels (Fig. S4C) it did cause a defect in mtDNA maintenance during mitochondrial import stress caused by MGE1 overexpression (Fig. 5D). The effects of deleting CIS1 on the mitoCPR were subtle, presumably because proteins acting in parallel could substitute for CIS1 function. The expression of CIS1 from the constitutive TEF2 promoter, however, had a significant protective effect during mitochondrial import stress. It led to a decrease in Cox5apre levels following PSD1 overexpression and an increase in the levels of mature Cox5a (Fig. 5E).
Drugs such as CCCP could not be used to study the role of PDR3 during mitochondrial import stress because the drug caused PDR3 independent expression of mitoCPR genes including CIS1. TEF2 is however not controlled by any MDR response, which allowed us to explore the role of CIS1 expressed from the TEF2 promoter in the mitoCPR using CCCP. We induced expression of COX5a from the MET25 promoter and simultaneously treated cells with CCCP. CCCP treatment partially blocked mitochondrial import causing Cox5apre to accumulate. Constitutive expression of CIS1 prevented this accumulation (Fig. 5F–H). Constitutive Cis1 had the same effect on the matrix proteins Rmd9, Ilv2 and Mss116 (Fig. S4D, E). Thus, high levels of Cis1 affect precursor levels of many, perhaps of all mitochondrial proteins. Constitutive CIS1 (tagged and un-tagged) also protected mtDNA during mitochondrial import stress caused by overexpression of MGE1 and even partially suppressed the detrimental effects of deleting PDR3 on mtDNA maintenance (Fig. 5I and Fig. S4F). Thus, CIS1 was an important effector of the mitoCPR. Cis1 reduces the levels of un-imported proteins and protectes mitochondrial functions during mitochondrial import stress.
Cis1 and Msp1 mediate mitochondrial preprotein clearance during mitochondrial import stress
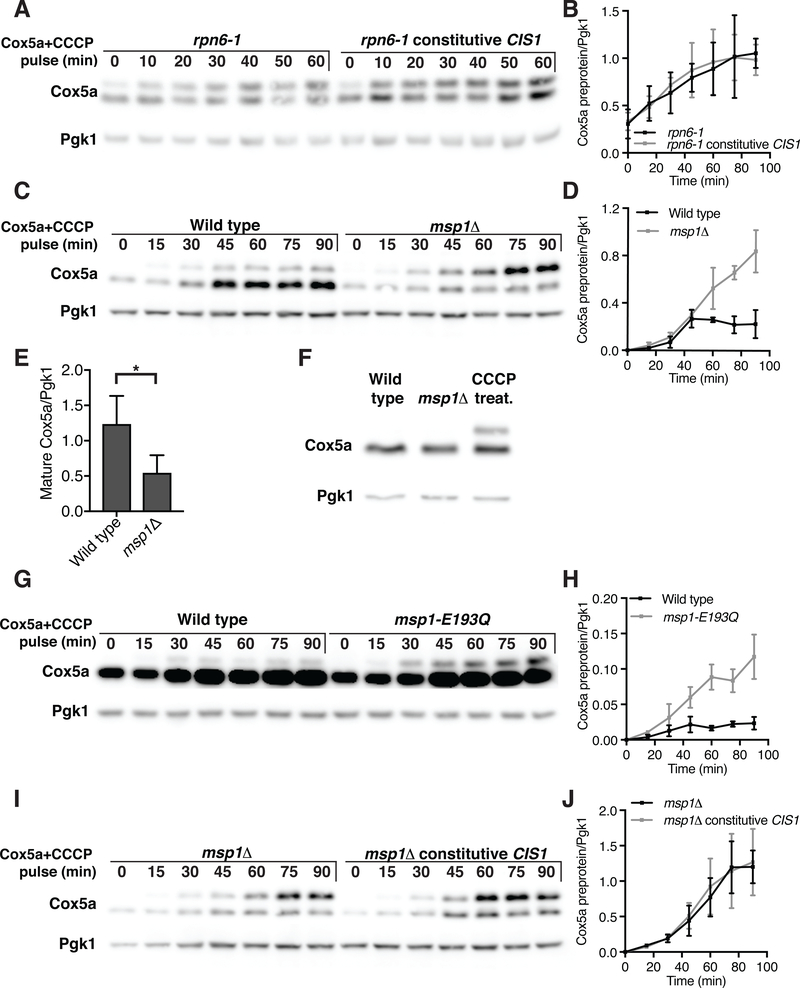
Our results indicate that during mitochondrial import stress Cox5apre accumulated on the surface of mitochondria and appeared to be stalled in the translocase (Fig. 2A and 2B). Cis1 aided in the import of preproteins, or facilitated their degradation at the mitochondrial surface or contributed to both. To test whether Cis1 promoted the degradation of un-imported proteins we asked whether downregulation of Cox5apre brought about by constitutive CIS1 expression depended on the proteasome. Although constitutive CIS1 prevented the accumulation of Cox5apre in wild type cells treated with CCCP (Fig. 5F–H), it failed to do so in cells that carried the temperature sensitive rpn6–1 allele and thus had compromised proteasome function (Fig. 6A, B). Note that MET25-COX5a was likely induced prior to methionine depletion in the rpn6–1 mutant because the transcription factor responsible for activating MET25 is a proteasome substrate (37). Thus, CIS1 promotes proteasomal degradation of un-imported proteins that accumulate at the mitochondrial surface.
Fig. 6. Cis1 and Msp1 are required for preprotein clearance following mitochondrial import stress.
(A) rpn6–1 or rpn6–1 TEF2-CIS1 cells were grown at room temperature in the presence of methionine. Cells were then transferred into medium lacking methionine with 20μM CCCP at 30°C. The accumulation of Cox5a-V5 preprotein (encoded by MET25-COX5a) is shown. (B) Quantification of (A); Cox5a preprotein levels from 4 independent experiments. Data are mean +/− SD. (C) Wild type or msp1Δ cells were grown at 30°C with methionine and treated as in (A). (D) Quantification of (C); Cox5a preprotein levels from 4 independent experiments. Data are mean +/− SD. (E) Quantification of (C); Mature Cox5a levels 60 min following induction. n=4. Data are mean +/− SD. Statistics were performed using the Student’s t-test. *p ≤ 0.05. (F) Immuno-blot analysis of Cox5a-V5 from wild-type cells, wild-type cells treated with 20μM CCCP for 1h, or msp1Δ cells. (G) Wild type cells or cells expressing msp1-E193Q from the inducible GAL1–10 promoter were grown in the presence of galactose for 6h. Cells were then transferred to medium lacking methionine and containing 20μM CCCP and the accumulation of inducible Cox5a-V5 preprotein (encoded by MET25-COX5a) was examined. Note that Cox5a levels were higher in this experiment because MET25-COX5a expression is higher in medium containing raffinose/galactose than glucose (Fig. S5B). (H) Quantification of (G); Cox5a preprotein levels from 3 independent experiments. Data are mean +/− SD. (I) msp1Δ cells or msp1Δ cells expressing TEF2-CIS1 were treated as in (C). Note that the experiment shown in (C) was performed in parallel and results can thus be directly compared. (J) Quantification of (I); Cox5a preprotein levels from 3 independent experiments. Data are mean +/− SD.
How does Cis1 promote the degradation of un-imported proteins? The AAA-ATPase Msp1 is a dislocase that extracts endoplasmic reticulum (ER) and peroxisome membrane proteins mistargeted to the mitochondrial outer membrane for proteasomal degradation (38–41). Our results show that Msp1 has a similar function in reducing preprotein accumulation during mitochondrial import stress. Cells lacking MSP1 accumulated high levels of Cox5apre when Cox5a expression was induced under conditions of mitochondrial import stress (CCCP treatment; Fig. 6C, D). Furthermore, accumulation of mature Cox5a was significantly delayed, suggesting that less Cox5a was imported into mitochondria (Fig. 6C, E). Cells lacking MSP1 neither accumulated Cox5apre nor induced mitoCPR under normal growth conditions (Fig. 6F and Fig. S5A), excluding the possibility that msp1Δ cells were generally defective in importing proteins into mitochondria. An effect on Cox5apre was also observed when the msp1-E193Q allele was overexpressed from the GAL1–10 promoter in cells lacking endogenous MSP1 (Fig. 6G, H, S5B). The E193Q substitution, located in the Walker B motif of the ATPase domain, is predicted to disrupt ATPase activity and stabilizes ER and peroxisome mistargeted proteins in the outer membrane of mitochondria (38–40). Thus, like Cis1, Msp1 limits the accumulation of unimported precursor proteins.
Next we determined the epistatic relationship between MSP1 and CIS1. We asked whether CIS1’s ability to limit the accumulation of Cox5apre required MSP1. Whereas TEF2-CIS1 prevented the accumulation of Cox5apre in wild-type cells (Fig. 5F, G), it failed to do so in cells lacking MSP1 (Fig. 6I, J). Thus, Cis1’s effect on preprotein clearance depended on MSP1.
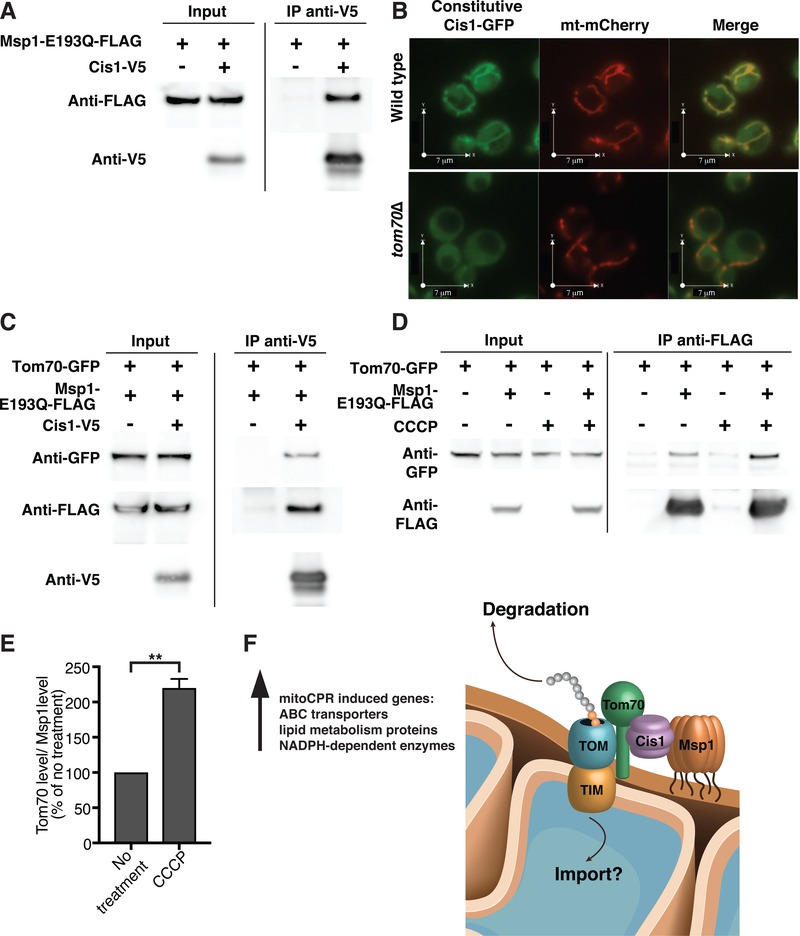
Having established that Cis1 and Msp1 both function in preprotein clearance during mitochondrial import stress, we next asked whether the two proteins act in the same pathway. Cis1 expressed from the TEF2 promoter co-immunoprecipitated with Msp1-E193Q-FLAG and vice versa (Fig. 7A and Fig. S6A). We were not able to detect binding between Cis1 and wild-type Msp1 most likely because this interaction is transient (Fig. S6B). We did however, obtain genetic evidence to indicate that the two proteins interact. In cells lacking GET1, ER membrane proteins accumulate in the mitochondrial outer membrane (38, 39). These conditions did not induce the mitoCPR but caused a growth defect at 37°C (38) (Fig. S5A, C). Overexpression of CIS1, like deletion of MSP1, enhanced this growth defect (Fig. S5C), suggesting that high levels of Cis1 reduce the interaction of Msp1 with ER proteins mis-targeted to the mitochondrial outer membrane.
Fig. 7. Cis1 interacts with Msp1 and with the outer membrane translocase.
(A) Cells expressing msp1-E193Q-FLAG and cells expressing msp1-E193Q-FLAG and TEF2-CIS1-V5 were grown in YPD. Cells were lysed and Cis1-V5 was immunoprecipitated using anti-V5 antibodies. (B) Live cell fluorescence imaging of wild type or tom70Δ cells expressing TEF2-CIS1-GFP and mitochondrial targeted mCherry (mt-mCherry). (C) Cis1-V5 (encoded by TEF2-CIS1-V5) was immunoprecipitated using anti-V5 antibodies from TOM70-GFP and msp1-E193Q-FLAG expressing cells. Cells expressing only TOM70-GFP and msp1-E193Q-FLAG were used as control. (D) Cells expressing TOM70-GFP or msp1-E193Q-FLAG and TOM70-GFP were grown in YPD in the presence or absence of 20μM CCCP for 1h. Msp1-E193Q-FLAG was immunoprecipitated using anti-FLAG antibodies. (E) Quantification of (D); co-immunoprecipitated Tom70 levels (normalized to co-immunoprecipitated Msp1 levels) in non-treated and CCCP treated cells from 3 independent experiments. No treatment was set to 100%. Data are mean +/− SD. Statistics were performed using the Student’s t-test. ** p ≤ 0.005. (F) A model for how Cis1 and Msp1 affect mitochondrial import during import stress. See text for details. IMS, intermembrane space.
The observation that the association of preproteins with mitochondrial membranes was resistant to sodium carbonate treatment suggested that preproteins accumulate at translocases during mitochondrial import stress (Fig. 2B, C). We therefore asked whether Cis1 was also found at translocases. This appeared to be the case. Localization of Cis1 to mitochondria was depended on Tom70, a receptor of the outer membrane translocase (Fig. 7B). Furthermore, Cis1 interacted with Tom70 as assessed by co- immunoprecipitation analysis and also with Msp1 (Fig. 7C).
Because Cis1 is only expressed during mitochondrial import stress (Fig. S4A) we conclude that Cis1 is recruited to mitochondria under import stress, where it interacts with both Tom70 and Msp1. Consistent with this conclusion, the interaction between Tom70 and Msp1 was enhanced during mitochondrial import stress (Fig. 7D and 7E). We propose that upon recruitment to the translocase via Cis1, Msp1 evicts preproteins from the translocase and the mitochondrial surface to target them for proteasomal degradation. It is important to note that our results do not exclude the possibility that Cis1 and Msp1 also improve import efficiency. Indeed, we have some evidence to suggest that this may in fact be the case. Overexpression of CIS1 caused an increase in mature Cox5a levels during prolonged mitochondrial import stress brought about by high levels of Psd1 (Fig 5E). Similarly, msp1Δ cells accumulated less mature Cox5a following CCCP treatment (Fig 6C, E).
Discussion
Here, we describe the discovery of a surveillance mechanism, mitoCPR, that detects mitochondrial import stress and protects mitochondrial functions in response. We propose that the mitoCPR effector Cis1 recruits Msp1 to the outer membrane translocase to clear stalled proteins from the translocase thereby improving mitochondrial import (Fig. 7F). This response is essential to protect mitochondrial functions and to maintain the mitochondrial genome during import stress. Recently, it was discovered that translation by ribosomes at the surface of mitochondria can stall (42). Whether the Msp1-Cis1 complex can clear preproteins from ribosomes during co-translational import or whether the complex only recognizes post-translationally imported proteins remains to be determined. We also do not yet know whether Cis1 and Msp1 improve mitochondrial import solely by clearing un-imported proteins. Our data suggest that they may also aid in the import process itself. Mitochondrial preproteins must be kept unfolded in order to translocate into mitochondria (2). A delay in mitochondrial import could result in premature folding and perhaps even aggregation of preproteins at the organelle’s surface. We speculate that Msp1, whose ATPase domain faces the cytosol, could unfold prematurely folding or aggregated preproteins giving them a second chance to translocate into mitochondria or, when this does not occur, target them for degradation (Fig. 7F).
It is also worth noting that the mitoCPR likely performs additional functions. Mitochondrial import defects lead to wide spread mitochondrial dysfunction. Upregulation of NADPH-dependent enzymes suggests a potential role for mitoCPR in restoring redox potential. Induction of genes involved in lipid metabolism argues for an effort to compensate for lipid biosynthesis disruption. Finally, upregulation of ABC transporter gene expression may be indicative of detoxification efforts aimed at removing toxic metabolic intermediates that could accumulate in the cytosol as a result of mitochondrial dysfunction.
Despite intense efforts we have not been able to identify the signal(s) that activates the mitoCPR. We can thus only speculate as to how the pathway is activated. In the MDR, Pdr1 and Pdr3 are activated by binding to xenobiotics (43). Mitochondrial dysfunction resulting from defects in mitochondrial import could lead to accumulation of metabolic intermediates in the cytoplasm, which in turn bind to and activate Pdr3. It is also possible that specific un-imported proteins activate Pdr3. Such mechanisms have been described for the mitochondrial unfolded protein response and the recognition of damaged mitochondria in mammals (44, 45).
We have studied the mitoCPR in response to overexpression of bipartite signal containing proteins. While this is unlikely to occur under physiological conditions, budding yeast cells are exposed to microorganisms that produce compounds known to interfere with mitochondrial import in the wild (35). Import defects could also result from disease or mitochondrial stress conditions such as high levels of reactive oxygen species. CIS1 and other mitoCPR genes are induced during diauxic shift, a physiological state defined as the switch from glycolysis to respiration that occurs when fermentable carbon sources becomes limiting (46). Switch to respiratory growth requires an expansion of the mitochondria compartment. We propose that this increase in mitochondrial mass, which requires increased mitochondrial import, leads to mitochondrial import stress. Mitochondria of multicellular eukaryotes are less likely to be exposed to mitochondrial poisons in the environment but do undergo increased biogenesis in specific tissues and during development. Whether a mitochondrial import stress response exists in higher eukaryotes remains to be determined.
Materials and Methods
Yeast Strains and Growth Conditions
All strains are derivatives of W303 (AA2587) and are listed in Table S2. Cells were grown overnight in YPD (1% yeast extract, 2% peptone, 2% glucose) at 30°C to saturation, then diluted in fresh YPD (OD600=0.1) and grown until they reached logarithmic phase. To induce the GAL1–10 promoter, cells were grown overnight at 30°C in minimal selective medium containing 2% raffinose or in YPR (1% yeast extract, 2% peptone, 2% raffinose). Cells were then diluted to OD=0.3 or OD=0.1 and recovered for an hour or 3 hours, respectively, following the addition of galactose to a final concentration of 1% for 4h (for measuring mRNA levels) or 6h hours (for protein analysis). To induce MET25-COX5a, cells were grown overnight in YPD supplemented with 8mM methionine. Cells were diluted to OD=0.1, grown for a few hours and then switched to medium lacking methionine [Complete supplement mixture w/o methionine (CSM, MP Biomedicals), yeast nitrogen base w/o amino acids (Difco), 2% glucose, titered to pH 7). CCCP was added to a final concentration of 20 μM.
Wild-type cells were incubated in the presence of 5 μg/ml ethidium bromide in YPD for 72h to obtain rho0 cells. rho0 state was verified by DAPI staining. rho- cells were obtained by deletion of the mitochondrial ribosomal subunit MRPL16 (47). The mrpl16Δ strain was confirmed to be rho- by its inability to grow on medium lacking a fermentable carbon source as a haploid and as a diploid following mating with rho0 cells. The presence of mitochondrial DNA in mrpl16Δ cells was tested by DAPI.
The plasmid pRS426 was used as an empty plasmid control. A plasmid expressing mt-Cherry and integrated into the LEU2 locus was cloned from plasmid pHS12-mCherry (a gift from Benjamin Glick, Addgene plasmid # 25444).
Immuno-blot Analysis
For immuno-blot analyses, ~2 OD600 units of cells were harvested and treated with 5% trichloroacetic acid overnight at 4°C. The acid was washed away with acetone and the cell pellet was subsequently dried. The cell pellet was pulverized with glass beads in 100 μL of lysis buffer (50 mM Tris-HCl at pH 7.5, 1 mM EDTA, 2.75 mM DTT) using a bead-beater. 3×SDS sample buffer was added and the cell homogenates were boiled. Samples were separated by SDS PAGE, blotted onto nitrocellulose membranes, and subsequently incubated with anti-V5 antibodies (1:2000 dilution; Life Technologies), anti-3-PhosphoGlycerate Kinase antibodies (1:5000 dilution; Invitrogen), anti-GFP antibodies (1:1000; Clontech, JL-8), anti-Kar2 (1:200,000 dilution; kindly provided by Mark Rose), anti-Myc antibodies (1:1000 dilution; Sigma, 9E10), anti-Cox4 antibodies (1:1000; Abcam) or anti-FLAG antibodies (1:1000; Sigma). HRP-linked sheep anti-mouse antibodies and HRP-linked donkey anti-rabbit antibodies (GE Healthcare) were used as secondary antibodies. Statistics were performed using the Student’s t-test. The protein half-life in Fig. 4B was analyzed as a one-phase exponential decay chart using Prism software.
Fluorescence Microscopy
Cells were grown overnight in minimal medium at 30°C, diluted to OD=0.1 and grown to logarithmic phase. Images were acquired with a DeltaVision Elite microscope (GE Healthcare Bio-Sciences, Pittsburgh, PA). Images were taken with a 100× plan-Apo objective, an InsightSSI solid-state light source, and a CoolSNAP HQ2 camera.
Real time PCR
Total RNA was isolated using the RNeasy minikit (Qiagen). RNA (750 ng) was used to generate cDNAs using the SuperScript III first strand synthesis system (Life Technologies). Quantitative PCR was performed using a SYBR green mix (Life Technologies) and amplified using a LightCycler 480 II (Roche). Signals were normalized to ACT1 transcript levels and are presented as fold increase of control conditions.
Gene expression analysis
For RNA expression analysis, PSD1 was overexpressed for 4 hours. Total yeast RNA was isolated using the RNeasy mini-kit (Qiagen) and samples were sequenced on a HiSeq 2000. S. cerevisiae RNA-seq reads were aligned to the sacCer3 genome with STAR version 2.5.3a and Ensembl transcripts were quantified using rsem version 1.3.0. Differential expression analysis was performed using deseq2 version 1.16.1 running under R version 3.4.0. Default options were selected for deseq2 runs except cooksCutoff and independent Filtering were both set to false during results preparation and unmoderated fold changes were used. RNA sequencing data can be accessed via the following link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107784
Mitochondrial oxygen consumption
Cells were grown overnight at 30°C in minimal selective medium with 2% raffinose. The cells were then diluted to OD=0.3 and recovered for an hour following the addition of galactose to a final concentration of 1% for 4h. Cells were then transferred to YPG (1% yeast extract, 2% peptone, 3% glycerol) and incubated for 20min. Oxygen consumption rate was measured from 0.75 OD (1ml) cells in YPG using an Oxytherm instrument (Hansatech) for 3 min at 25°C. The slope of the linear range of oxygen depletion was used to measure oxygen consumption rate of 3 experiments. Statistics were performed using the Student’s t-test.
Mitochondrial DNA maintenance assay
The analysis of mtDNA maintenance was described previously (48). Cells were grown overnight at 30°C in minimal selective medium with 2% glucose. Cells were then diluted to OD=0.15 in minimal selective medium with 2% raffinose and were grown for 3h following the addition of galactose to a final concentration of 1% for 24h or 48h. Within these 24h (8h after induction) the cells were diluted 1:20 into the same medium. Yeast cells (~200) were spread on plates containing 1% yeast extract, 2% peptone, 0.3% glucose, 2% ethanol and were grown at 30°C for 3 days until all colonies could be detected. The percentage of small rho- (petite) colonies was determined from 3 different experiments.
Membrane potential measurements
Cells lacking PDR1, PDR3 and PDR5 (to prevent efflux of dyes out of the cells) bearing either an empty plasmid (for control and CCCP treatment) or a GAL-PSD1 containing plasmid and expressing a mitochondria-targeted mCherry (mt-mCherry) were grown overnight at 30°C in minimal selective medium containing 2% raffinose. The cells were diluted to OD=0.3 and recovered for an hour following the addition of galactose to a final concentration of 1% for 4h. CCCP (20 μM) was added for 1h. Cells were then transferred to 1 ml dye buffer (10mM Hepes pH 7.2 and 5% glucose) and incubated with 2.5 μM Rhodamine 123 (Thermo Fisher Scientific) for 15 minutes at room temperature. Cells were washed 5 times in 1.5 ml dye buffer. Mitochondria were identified by mt-mCherry labeling. Membrane potential was analyzed by the following equation: (mitochondrial fluorescence intensity - cytosolic fluorescence intensity)/cytosolic fluorescence intensity cytosol.
Mitochondria isolation
Cells were grown to logarithmic phase, collected by centrifugation and washed once with water. Cells were then resuspended in 0.1 M Tris pH 9.4, 10 mM DTT and incubated for 20 min at 30°C. Cell walls were disturbed by incubation in 1.2M sorbitol, 20mM K2HPO4 pH 7.4, 1% zymolyase for 1 h at 30°C. Dounce homogenization was used to lyse the cells in 0.6M sorbitol, 10mM Tris pH 7.4, 1mM EDTA, fatty acid free 0.2% BSA and 1mM PMSF. Mitochondria were then isolated by differential centrifugation as described previously (49) and resuspended in SEM buffer (0.25M sucrose, 10mM MOPS KOH pH 7.2 and 1mM EDTA). Proteinase K was added to a final concentration of 50 μg/ml for 5min at 37°C and the reaction was stopped by the addition of 4mM PMSF for 15min on ice.
For sodium carbonate extraction, 40 μg of mitochondria were pelleted and resuspended in 500 μl of 100mM sodium carbonate pH 11 or in SEM buffer for the untreated control. The samples were kept on ice for 30 min followed by centrifugation at 90,000 g for 30 min. Supernatants and pellets were incubated with 12.5% TCA overnight at 4°C and separated by SDS PAGE.
Co-immunoprecipitation assays
Cells were grown in YPD to OD=0.9 when not treated or to OD=0.7 following treatment with 20μM CCCP for 1 hour. Approximately 50 OD units of cells were collected, washed once with water and frozen. Cells were lysed with Silica Beads using a FastPrep instrument (speed 6.5, 45 sec, 3 cycles) with 200μ1 IGEPAL buffer (50mM Tris pH 7.5, 150mM NaCl, 1% IGEPAL and Halt Protease Inhibitor Cocktail [Thermo Fisher Scientific]). Lysates were brought up to 1.5 ml with IGEPAL buffer containing 0.2% BSA. Lysates were clarified by centrifugation at 20,000 g for 10 min at 4°C. Twenty microliters of Anti-V5 agarose affinity gel antibody (Sigma) or Anti-FLAG M2 affinity gel (Sigma) were added and lysates were incubated for 2h at 4°C. Beads were then washed 5 times with IGEPAL buffer containing 0.2% BSA. Sample buffer was added to the beads, which were then boiled. Final eluates and two percent of the lysates were separated by SDS-PAGE.
Supplementary Material
Acknowledgements
We are grateful to T. Fox, C. Koehler, P. Perlman, F. Solomon, M. Vander Heiden, J. Rutter and V. Denic for discussions and insights and for critical reading of the manuscript. We thank A. Sandikçi for figure preparation, X. Zhou for help with data analysis and N. Gebert for technical help. We thank D. Gottschling for the ATP1–111 plasmid and P. Walter for the GAL-msp1-E193Q plasmid. We thank C. Whittaker of the Barbara K. Ostrom (1978) Bioinformatics and Computing Facility Koch Institute in the Swanson Biotechnology Center for analyzing the gene expression. We thank J. Falk the members of the Amon lab for critical reading of the manuscript.
Funding: This work was supported by the National Institutes of Health (GM 118066 to A.A.) and by the Koch Institute Support (core) Grant P30-CA 14051 from the National Cancer Institute. A.A is also an investigator of the Howard Hughes Medical Institute and the Glenn Foundation for Biomedical Research. H.W. was supported by the Jane Coffin Childs Memorial Fund, the EMBO Long-Term Fellowship, and the Israel National Postdoctoral Program for Advancing Women in Science.
Footnotes
Competing interests: No competing interests.
Data and materials availability: RNA sequencing data can be accessed via the following link: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE107784
Supplementary Materials
Figs. S1 to S6
Table S1 to S2
References and Notes
- 1.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, Pfanner N, Importing mitochondrial proteins: machineries and mechanisms. Cell. 138, 628–644 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neupert W, Herrmann JM, Translocation of proteins into mitochondria. Annu. Rev. Biochem 76, 723–749 (2007). [DOI] [PubMed] [Google Scholar]
- 3.MacKenzie JA, Payne RM, Mitochondrial protein import and human health and disease. Biochim. Biophys. Acta 1772, 509–523 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C, The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell Metab. 19, 357–372 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Yano H et al. , Inhibition of mitochondrial protein import by mutant huntingtin. Nat. Neurosci 17, 822–831 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrobel L et al. , Mistargeted mitochondrial proteins activate a proteostatic response in the cytosol. Nature. 524, 485–488 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Chen XJ, A cytosolic network suppressing mitochondria-mediated proteostatic stress and cell death. Nature. 524, 481–484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itakura E et al. , Ubiquilins Chaperone and Triage Mitochondrial Membrane Proteins for Degradation. Mol. Cell 63, 21–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkadi B, Homolya L, Szakács G, Varadi A, Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol. Rev 86, 1179–1236 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Kumar S, Mukherjee MM, Varela MF, Modulation of Bacterial Multidrug Resistance Efflux Pumps of the Major Facilitator Superfamily. Int J Bacteriol. 2013, 1–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akache B, MacPherson S, Sylvain M-A, Turcotte B, Complex interplay among regulators of drug resistance genes in Saccharomyces cerevisiae. J. Biol. Chem 279, 27855–27860 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Coorey NVC, Matthews JH, Bellows DS, Atkinson PH, Pleiotropic drug-resistance attenuated genomic library improves elucidation of drug mechanisms. Mol. BioSyst 11, 3129–3136 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Moye-Rowley WS, Transcriptional control of multidrug resistance in the yeast Saccharomyces. Prog. Nucleic Acid Res. Mol. Biol 73, 251–279 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Hallstrom TC, Moye-Rowley WS, Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae. J. Biol. Chem 275, 37347–37356 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Moye-Rowley WS, Retrograde regulation of multidrug resistance in Saccharomyces cerevisiae. Gene. 354, 15–21 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Cumsky MG, Trueblood CE, Ko C, Poyton RO, Structural analysis of two genes encoding divergent forms of yeast cytochrome c oxidase subunit V. Mol. Cell. Biol 7, 3511–3519 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiki Y, Hubbard AL, Fowler S, Lazarow PB, Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell Biol 93, 97–102 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulshan K, Schmidt JA, Shahi P, Moye-Rowley WS, Evidence for the bifunctional nature of mitochondrial phosphatidylserine decarboxylase: role in Pdr3-dependent retrograde regulation of PDR5 expression. Mol. Cell. Biol 28, 5851–5864 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein CB et al. , Genome-wide Responses to Mitochondrial Dysfunction. Mol. Biol. Cell 12, 297–308 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleby RD et al. , Quantitation and origin of the mitochondrial membrane potential in human cells lacking mitochondrial DNA. Eur. J. Biochem 262, 108–116 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Veatch JR, McMurray MA, Nelson ZW, Gottschling DE, Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. 137, 1247–1258 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallstrom TC et al. , Coordinate control of sphingolipid biosynthesis and multidrug resistance in Saccharomyces cerevisiae. J. Biol. Chem 276, 23674–23680 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Gallas MR, Dienhart MK, Stuart RA, Long RM, Characterization of Mmp37p, a Saccharomyces cerevisiae mitochondrial matrix protein with a role in mitochondrial protein import. Mol. Biol. Cell 17, 4051–4062 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura Y et al. , Identification of Tam41 maintaining integrity of the TIM23 protein translocator complex in mitochondria. J. Cell Biol 174, 631–637 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo DK, Phang TL, Trawick JD, Poyton RO, Multiple pathways of mitochondrial-nuclear communication in yeast: intergenomic signaling involves ABF1 and affects a different set of genes than retrograde regulation. Biochim. Biophys. Acta 1789, 135–145 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Golik P, Bonnefoy N, Szczepanek T, Saint-Georges Y, Lazowska J, The Rieske FeS protein encoded and synthesized within mitochondria complements a deficiency in the nuclear gene. Proc. Natl. Acad. Sci. U.S.A 100, 8844–8849 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis BR, White KH, Thorsness PE, Mutations in the Atp1p and Atp3p subunits of yeast ATP synthase differentially affect respiration and fermentation in Saccharomyces cerevisiae. J. Bioenerg. Biomembr 39, 127–144 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Mutlu N, Garipler G, Akdogan E, Dunn CD, Activation of the pleiotropic drug resistance pathway can promote mitochondrial DNA retention by fusion-defective mitochondria in Saccharomyces cerevisiae. G3 (Bethesda). 4, 1247–1258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laloraya S, Gambill BD, Craig EA, A role for a eukaryotic GrpE-related protein, Mge1p, in protein translocation. Proc. Natl. Acad. Sci. U.S.A 91, 6481–6485 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostermann J et al. , Precursor proteins in transit through mitochondrial contact sites interact with hsp70 in the matrix. FEBS Lett. 277, 281–284 (1990). [DOI] [PubMed] [Google Scholar]
- 31.Scherer PE, Krieg UC, Hwang ST, Vestweber D, Schatz G, A precursor protein partly translocated into yeast mitochondria is bound to a 70 kd mitochondrial stress protein. The EMBO Journal. 9, 4315–4322 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugimoto S, Saruwatari K, Higashi C, Sonomoto K, The proper ratio of GrpE to DnaK is important for protein quality control by the DnaK-DnaJ-GrpE chaperone system and for cell division. Microbiology (Reading, Engl.). 154, 1876–1885 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Singh KK, Global genetic determinants of mitochondrial DNA copy number. PLoS ONE. 9, e105242 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devaux F, Carvajal E, Moye-Rowley S, Jacq C, Genome-wide studies on the nuclear PDR3-controlled response to mitochondrial dysfunction in yeast. FEBS Lett. 515, 25–28 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Naranjo S et al. , Dissecting the Genetic Basis of a Complex cis-Regulatory Adaptation. PLOS Genetics. 11, e1005751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh W-K et al. , Global analysis of protein localization in budding yeast. Nature. 425, 686–691 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Rouillon A, Barbey R, Patton EE, Tyers M, Thomas D, Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCFMet30 complex. The EMBO Journal. 19, 282–294 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y-C et al. , Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. The EMBO Journal. 33, 1548–1564 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okreglak V, Walter P, The conserved AAA-ATPase Msp1 confers organelle specificity to tail-anchored proteins. Proc. Natl. Acad. Sci. U.S.A 111, 8019–8024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wohlever ML, Mateja A, McGilvray PT, Day KJ, Keenan RJ, Msp1 Is a Membrane Protein Dislocase for Tail-Anchored Proteins. Mol. Cell 67, 194–202.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir NR, Kamber RA, Martenson JS, Denic V, The AAA protein Msp1 mediates clearance of excess tail-anchored proteins from the peroxisomal membrane. Elife. 6, 13004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izawa T, Park S-H, Zhao L, Hartl FU, Neupert W, Cytosolic Protein Vms1 Links Ribosome Quality Control to Mitochondrial and Cellular Homeostasis. Cell. 171, 890–903.e18 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Thakur JK et al. , A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature. 452, 604–609 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Youle RJ, Narendra DP, Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol 12, 9–14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM, Mitochondrial Import Efficiency of ATFS-1 Regulates Mitochondrial UPR Activation. Science. 337, 587–590 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brauer MJ, Saldanha AJ, Dolinski K, Botstein D, Homeostatic adjustment and metabolic remodeling in glucose-limited yeast cultures. Mol. Biol. Cell 16, 2503–2517 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers AM, Pape LK, Tzagoloff A, Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. The EMBO Journal. 4, 2087–2092 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baruffini E, Ferrero I, Foury F, In vivo analysis of mtDNA replication defects in yeast. Methods. 51, 426–436 (2010). [DOI] [PubMed] [Google Scholar]
- 49.Pfanner N, Meisinger C, Turcotte B, in Yeast Protocol, Xiao W, Ed. (2006), vol. 313, pp. 33–39. [Google Scholar]
- 50.Mossmann D et al. , Amyloid-β peptide induces mitochondrial dysfunction by inhibition of preprotein maturation. Cell Metab. 20, 662–669 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Horvath SE et al. , Processing and topology of the yeast mitochondrial phosphatidylserine decarboxylase 1. J. Biol. Chem 287, 36744–36755 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.