Abstract
Background:
Buprenorphine-naloxone treatment for opioid use disorder has rapidly expanded, yet little is known about treatment outcomes among patients in the general population.
Objective:
To examine predictors of treatment duration, dosage, and continuity in a diverse community setting.
Research Design:
We examined QuintilesIMS RWD data, an all-payer, pharmacy claims database, to conduct an analysis of individuals age ≥18 initiating buprenorphine-naloxone treatment between January 2010 and July 2012 in 11 states. We used logistic regression to assess treatment duration longer than six months. We used accelerated failure time models to assess risk of treatment discontinuation. We used ordinary least squares regression to assess mean daily dosage. For patients with ≥3 fills, we also used logistic regression to assess whether an individual had a medication possession ratio of less than 80% and/or gaps in treatment >14 days. Models adjusted for individual demographics, prescribing physician specialty, state, and county-level variables.
Results:
Overall, 41% of individuals were retained in treatment for at least six months and the mean treatment length was 266 days. Compared to individuals who paid primarily for treatment with cash, adjusted odds of six month retention were significantly lower for individuals with primary payment from Medicaid Fee-for-Service, Medicare Part D, and third-party commercial. There were substantial differences in six-month retention across states with the lowest in Arizona and highest in New York. Low-possession ratios occurred for 30% of individuals and 26% experienced treatment episodes with gaps >14 days. Odds of low-possession and treatment gaps were largely similar across demographic groups and geographic areas.
Conclusion:
Current initiatives to improve access and quality of buprenorphine-naloxone treatment should examine geographic barriers as well as the potential role of insurance benefit design in restricting treatment length.
Keywords: buprenorphine-naloxone, adherence, treatment retention, opioid use disorder
INTRODUCTION
Buprenorphine-naloxone is the most commonly prescribed buprenorphine formulation, a partial opioid agonist used for long-term management of opioid use disorder.1 As part of a comprehensive treatment program, buprenorphine-naloxone is efficacious in reducing symptoms of opioid withdrawal and improving abstinence.1,2 Many patients value the convenience of receiving treatment on a prescription basis as compared to methadone maintenance, which typically is more managed than buprenorphine maintenance.3 Patients generally experience better long-term outcomes when retained in treatment for longer periods of time and with greater continuity.4 Guidelines support buprenorphine-naloxone treatment that is time unlimited,5 but some insurers limit treatment duration and some individuals desire to use buprenorphine-naloxone for relatively short periods of time. While buprenorphine is effective at both lower and higher dosages, studies indicate that patients often experience better abstinence with higher dosages (>16 milligrams/day).6
Research on buprenorphine treatment duration, dosage, and continuity (i.e., adherence) has primarily been derived from clinical trials7,8 and observational studies of select populations,9–13 such as single payers or practices, but less is known about differences in populations representative of individuals receiving care in the community.14–16 Moreover, existing research focuses primarily on the first year; less is known about predictors of treatment lasting longer than 12 months.17 Treatment patterns in community samples are important to study in light of concerns about the quality of care many patients receive and the capacity of office-based providers to effectively manage opioid use disorder.18
Duration, dosage, and continuity in a community sample could be influenced by a variety of factors. Patient demographics such as age and sex may influence these outcomes, but have shown inconsistent relationships in published studies.10,12,13 Treatment outcomes may be further associated with the policies of insurers such as dosage restrictions, prior authorization, and copayments common for buprenorphine-naloxone medication.19 Clinic-level factors, including the specialty of the prescribing physician, could influence treatment outcomes insofar as some physicians may have greater capacity and expertise to manage long-term maintenance treatment.20 Finally, area-level variables, which influence initiation and access to treatment, may also influence treatment outcomes.9,21 For example, patients who travel longer distances to visit their prescribing physician may experience greater barriers maintaining long-term treatment continuity.22
We examined predictors of buprenorphine-naloxone treatment continuity, dosage, and duration, drawing upon a large, diverse database of individuals receiving treatment from prescribers in 11 states. Our sample includes treatment through all potential sources of payment, including cash, providing a more detailed and diverse picture of buprenorphine-naloxone treatment than studies from a single payer. This issue is particularly important since even patients with some public or private insurance may self-pay for some of their prescriptions of buprenorphine-naloxone.23,24 We hypothesized that both duration and continuity of care would be higher for individuals living in areas where travel distance was likely to be less to treatment, and in areas with relatively higher availability of providers and higher socioeconomic status. We also hypothesized that retention and dosage would be highest among individuals who had their care predominantly paid for by private insurance, since insurance may pay for a greater dosage or quantity of treatment than would otherwise be available.
DATA AND METHODS
Prescription Data
We used the QuintilesIMS RWD Anonymized Longitudinal Prescription database, which captures >75% of all prescriptions dispensed in the United States and are automatically reported to QuintilesIMS through weekly feeds from retail, foodstore, independent and mass merchandiser pharmacies (some large networks do not participate in the database). QuintilesIMS then links data using a patented algorithm based on 16 different patient-level characteristics including name, address, date of birth, and gender. These anonymized, individual-level all-payer claims data contain detailed information for each prescription, including the fill date, dosage, days supply, and payment type.
Subjects
Our study was based on a larger cohort derived by identifying any patient filling two or more prescriptions for any opioid during any calendar year between 2006 and 2013 in eleven states (Arizona, California, Florida, Georgia, Louisiana, Maryland, New York, Oregon, Pennsylvania, Texas, Washington). We then extracted the universe of prescriptions for any sampled individual. Our study period ranged from January 1, 2009 through August 31, 2013. We focused on individuals initiating buprenorphine-naloxone between January 1, 2010 and July 31, 2012, thus using a 12-month “washout period” to ensure that incident use was captured while allowing for at least 13 months of follow-up. We limited our analysis to patients filling 100% of their claims at retail pharmacies that consistently reported data to QuintilesIMS during the study period. In our main analyses, we excluded the 27% of individuals exclusively using other buprenorphine formulations (e.g., buprenorphine monotherapy), since this is generally reserved for use in select populations such as pregnant women.25 In sensitivity analysis we found that these individuals were predominantly older and female, and had much shorter treatment episodes, however, including these individuals in our analyses yielded substantively similar results and conclusions. To capture the universe of patients’ prescription fill activity, we limited our sample to patients who filled 100% of their prescriptions from pharmacies that consistently reported data to QuintilesIMS throughout the study period. We also limited our analysis to individuals ≥18 years at the time of their index prescription fill and those who had evidence of prescription activity for any product during both the first and last six months of the study period. Our final sample included 27,273 individuals.
First treatment episode
We defined each patient’s first buprenorphine-naloxone treatment episode as the date of the index fill until the first day of a gap where the patient had no buprenorphine-naloxone on-hand for 90 or more days. In many cases patients had overlapping buprenorphine-naloxone prescriptions as a result of early refills. We chose not to extend the length of the treatment episode for these patients because it was unclear if such overlapping prescription truly represented “stockpiling”. We removed .7% of buprenorphine-naloxone claims with values above the 99th percentile of quantity dispensed (120 pills) or days supply (30 days).
Similar to other investigations12,17, we measured treatment retention by creating a binary measure of treatment episodes 180 days or longer (“six-month retention”). Six months was chosen to facilitate comparison with other studies; findings are comparable when considering alternative cutoffs (3 and 9 months; Appendix). We also quantified total length of the first episode in days. We measured treatment continuity using the medication possession ratio, which reflects percent of days in which the individual had buprenorphine-naloxone available for use. We created a binary indicator reflecting individuals with a possession ratio of less than 80%.26 We measured mean dosage in milligrams/day by calculating the daily dose per day and dividing by the number of days with prescribed medication. Finally, we created a binary indicator for the presence of any treatment interruptions, defined as a gap in the treatment episode of between 14 and 90 days when an individual had no buprenorphine-naloxone available for use and did not fill a prescription; as noted, after 90 days of no fills the episode was defined as terminated. We only calculated low possession ratio and treatment interruptions for individuals with three or more prescriptions, since these measures required multiple fills to calculate.
Patient, Physician, and Area-Level Covariates
We examined the association between each outcome and several patient, physician, and area-level characteristics. We included patient age and sex. We also included an indicator for the patient’s source of payment: third-party payment (a category which includes private insurance plans as well as Medicaid managed care plans), Medicare Part D, Medicaid Fee-for-Service, and self-payment. For the 22% of the sample with multiple sources of payment, we imputed the most common source of payment. We examined the specialty type of physicians prescribing buprenorphine-naloxone: primary care physician (general, internal, or family medicine), psychiatry, or some other specialty, and the county for the physician’s office location. For the 24% of patients visiting multiple physicians, we imputed the most commonly visited physician.
Although we lacked a direct measure of patient’s home address, we used the county of the pharmacy where the first prescription was filled as a proxy. Using the 2015 County Health Rankings, we included the percent of uninsured adults, the median household income, percent of the population that is non-Hispanic white (i.e., non-minority), and the per capita rate of opioid overdose deaths in the year the individual began treatment (additional source information is available in the Appendix). Overdose death data are reported in categories representing rates per 100,000 individuals, which we split into four approximately equal size groups (<10, 10.1–14, 14.1–17.9, ≥18). Other county-level measures were mean standardized to facilitate interpretability. We included a four-level measure of urbanicity of the county using definitions of metropolitan statistical areas (MSAs): large MSA (>1 million people), medium MSA (>250,000–1 million people), small MSA (100,000–250,000 people) and non-MSA (i.e., rural).
Using the Drug Enforcement Administration 2015 directory of physicians with a waiver to prescribe buprenorphine for opioid use disorders, we created a per capita measure by dividing the number of waivered prescribing physicians by the county populations. Finally, comparing the location of the prescribing physician’s county and the location of the pharmacy, we created an indicator for “out-of-county” treatment, reflecting claims where the physician was in a different county than the pharmacy.
Analyses
We examined five outcomes: (1) six-month retention; (2) episode length in days; (3) mean dosage in milligrams/day (4) low possession ratio; and (5) treatment interruptions. We modeled each outcome in a separate regression model, using the complete set of patient-level, physician, and area-level covariates. We fit models to an appropriate functional form for each outcome: logistic regression was used for the binary measures of six-month retention, low possession ratio, and treatment interruption. In addition to deriving odds ratios for these models, we also calculated regression-adjusted means for each covariate, calculating the predicted mean of the outcome at each covariate holding all other variables constant. We calculated ordinary least squares regression models for mean daily dosage.
We used an accelerated failure time model to calculate predictors of treatment length. This parametric model estimates time ratios (constant terms representing the amount by which each covariate accelerates/decelerates time on study). We clustered standard errors at the county-level corresponding to first pharmacy fill.
We tested for the goodness of fit of logistic regression models with Hosmer-Lemeshow statistics. To test for multicollinearity, we calculated variance inflation factors (VIFs) verifying that the VIF for each covariate was <10.
RESULTS
Sample Characteristics
About half the sample (48%) was female and half (50%) were age 18–34 (Table 1). Most had third-party insurance as their main payment source (59.8%), followed by self-payment (25.9%), Fee-for-Service Medicaid (7.9%), and Medicare Part-D (6.4%). Half the sample was in three states: Pennsylvania (18.6%), New York (15.8%), and Florida (15.7%). Almost two-thirds (63.3%) of the sample filled prescriptions in large metropolitan areas, while 9.1% were in rural areas. Almost half (46.5%) of patients visited prescribing physicians across county borders. Half (50.2%) of the physicians most commonly visited by patients were PCPs; psychiatrists (22.7%) and other specialties (28.7%) accounted for the remainder.
Table 1.
Characteristics of the Sample of Individuals Initiating Buprenorphine-Naloxone Treatment
| Variable | Mean |
|---|---|
| Female | 0.48 |
| Age | |
| Age 18–34 | 0.50 |
| Age 35–49 | 0.30 |
| Age >50 | 0.20 |
| Primary source of payment for treatment | |
| Cash payment | 0.26 |
| Medicaid fee-for-service | 0.08 |
| Medicare Part D | 0.06 |
| Third-Party Commercial | 0.60 |
| Primary prescribing physician | |
| Psychiatrist | 0.23 |
| Primary care physician (PCP) | 0.50 |
| Other specialist | 0.28 |
| Annual county opioid overdose death rate | |
| <10 deaths per 100k | 0.27 |
| 10.1–14 deaths per 100k | 0.21 |
| 14.1–17.9 deaths per 100k | 0.25 |
| >18 deaths per 100k | 0.26 |
| Metropolitan status | |
| Large metro area | 0.63 |
| Medium metro area | 0.20 |
| Small metro area | 0.08 |
| Rural area | 0.09 |
| County-level variables | |
| Crossed county lines for treatment | 0.47 |
| County PCP-pop ratio | 76.07 |
| County DEA waivered PCP-pop ratio | 7.38 |
| County median household income | $55,125 |
| County share non-Hispanic white percentage | 0.67 |
| State of first pharmacy fill | |
| Arizona | 0.03 |
| California | 0.05 |
| Florida | 0.16 |
| Georgia | 0.05 |
| Louisiana | 0.04 |
| Maryland | 0.04 |
| New Jersey | 0.01 |
| New York | 0.16 |
| Pennsylvania | 0.19 |
| Texas | 0.08 |
| Washington | 0.03 |
| Other states | 0.17 |
NOTES: Authors’ analysis of individuals initiating first episode of buprenorphine-naloxone treatment in the IMS LifeLink database in 2010–2012. The total sample of individuals=27,273. Data sources for county-level variables are the County Health Rankings (primary care physicians; median income; uninsured rate; non-Hispanic white population); US Department of Agriculture (urbanicity codes); overdose mortality (National Center for Health Statistics); and US Drug Enforcement Administration (overdose death rates).
Retention at Six Months
Overall, 41.4% of individuals were retained at six months (Table 2). After adjusting for all other covariates, individuals with treatment paid for by Medicaid Fee-for-Service and Medicare Part D had very low adjusted means for six-month retention: 22.3% and 21.1%, respectively (Table 3). Individuals with health insurance paying for the majority of their treatment had significantly lower retention than majority cash-paying individuals: Medicaid Fee-for-Service (OR, 0.35; 95% CI, 0.31–0.39), Medicare Part D (OR, 0.33; 95% CI, 0.30–0.37), and third-party commercial (OR, 0.41; 95% CI, 0.39–0.44).
Table 2.
Measures of Treatment Duration and Continuity for Individuals Receiving First Episodes of Buprenorphine-Naloxone Treatment
| Outcome | Value |
|---|---|
| Retained for ≥180 days | 41.4% |
| Mean episode 1 length | 266 days |
| Median episode 1 length | 118 days |
| Mean Daily Dosage | 14.1 mg/day |
| Low possession ratio (<.80) among individuals with ≥3 fills | 30.2% |
| At least 1 interruption of >14 days among individuals with ≥3 fills | 25.5% |
NOTES: Authors’ analysis of individuals initiating first episode of buprenorphine-naloxone treatment in the IMS LifeLink database in 2010–2012. The total sample of individuals=27,273, the sample of individuals with ≥3 fills=18,654.
Table 3.
Predictors of Retention at Six Months and Treatment Length in Days for Individuals Receiving First Episodes of Buprenorphine-Naloxone Treatment
| ≥180 days of treatment | Length of treatment | ||||||
|---|---|---|---|---|---|---|---|
| Adj. Mean |
Odds Ratio |
95% CI | p-value | Time Ratio |
95% CI | p-value | |
| Female | .399 | 0.88 | (0.84–0.93) | <.001 | 0.87 | (0.83–0.9) | <.001 |
| Age (Ref=18–34) | .398 | ||||||
| Age 35–49 | .438 | 1.16 | (1.09–1.23) | <.001 | 1.21 | (1.16–1.26) | <.001 |
| Age >50 | .422 | 1.04 | (0.96–1.13) | 0.282 | 1.13 | (1.06–1.2) | <.001 |
| Majority Payer (Ref=Cash) | .599 | ||||||
| Medicaid Fee-for-service | .223 | 0.35 | (0.31–0.39) | <.001 | 0.47 | (0.43–0.51) | <.001 |
| Medicare Part D | .211 | 0.33 | (0.3–0.37) | <.001 | 0.44 | (0.4–0.48) | <.001 |
| Third-Party Commercial | .336 | 0.41 | (0.39–0.44) | <.001 | 0.49 | (0.47–0.51) | <.001 |
| Majority Prescriber (Ref=PCP) | .429 | ||||||
| Psychiatrist | .415 | 1 | (0.92–1.1) | 0.915 | 1 | (0.93–1.07) | 0.942 |
| Other provider | .364 | 0.73 | (0.68–0.79) | <.001 | 0.82 | (0.77–0.87) | <.001 |
| Metropolitan Status (Ref=nonmetro) | .435 | ||||||
| Large (>1 million people) | .406 | 0.9 | (0.79–1.03) | 0.139 | 0.9 | (0.81–0.99) | 0.027 |
| Medium (>250k-1 million people) | .405 | 0.95 | (0.83–1.09) | 0.472 | 0.92 | (0.84–1.01) | 0.097 |
| Small (100k-250k people) | .434 | 1.1 | (0.95–1.27) | 0.214 | 0.96 | (0.86–1.06) | 0.431 |
| County opioid overdose death rate (Ref=>18.1 per 100,000) | .427 | ||||||
| ≤10 deaths per 100k | .402 | 0.93 | (0.83–1.03) | 0.169 | 0.97 | (0.89–1.06) | 0.462 |
| 10.1–14 deaths per 100k | .402 | 0.93 | (0.83–1.04) | 0.228 | 0.94 | (0.87–1.03) | 0.177 |
| 14.1–17.9 deaths per 100k | .416 | 1.01 | (0.91–1.12) | 0.839 | 1 | (0.93–1.08) | 0.941 |
| Other county covariates | |||||||
| Crossed county lines for treatment | .404 | 0.92 | (0.87–0.97) | 0.004 | 0.93 | (0.88–0.97) | 0.002 |
| PCP to population ratio (standardized) | .420 | 1.03 | (0.98–1.08) | 0.206 | 1.02 | (0.99–1.06) | 0.258 |
| DEA waivered ratio (standardized) | .414 | 1 | (0.96–1.04) | 0.886 | 0.99 | (0.96–1.03) | 0.723 |
| Median income (standardized) | .419 | 1.02 | (0.98–1.07) | 0.377 | 1.03 | (0.99–1.06) | 0.131 |
| Non-minority population (standardized) | .433 | 1.1 | (1.04–1.16) | 0.001 | 1.07 | (1.03–1.11) | 0.001 |
| State of Pharmacy (Ref=Arizona) | .333 | ||||||
| California | .500 | 1.47 | (0.93–2.32) | 0.098 | 1.41 | (1.1–1.81) | 0.007 |
| Florida | .467 | 1.31 | (0.82–2.08) | 0.258 | 1.2 | (0.94–1.55) | 0.147 |
| Georgia | .541 | 1.77 | (1.12–2.79) | 0.014 | 1.6 | (1.26–2.04) | <.001 |
| Louisiana | .563 | 1.94 | (1.23–3.08) | 0.005 | 1.64 | (1.28–2.09) | <.001 |
| Maryland | .616 | 2.48 | (1.57–3.9) | <.001 | 1.99 | (1.57–2.54) | <.001 |
| New Jersey | .612 | 2.37 | (1.44–3.91) | 0.001 | 1.72 | (1.32–2.25) | <.001 |
| New York | .613 | 2.79 | (1.8–4.33) | <.001 | 2.21 | (1.74–2.8) | <.001 |
| Pennsylvania | .563 | 2.2 | (1.41–3.43) | <.001 | 1.84 | (1.46–2.31) | <.001 |
| Texas | .542 | 1.81 | (1.15–2.84) | 0.01 | 1.5 | (1.19–1.9) | 0.001 |
| Washington | .589 | 2.17 | (1.38–3.39) | 0.001 | 1.66 | (1.31–2.09) | <.001 |
| Other states | .535 | 1.86 | (1.19–2.9) | 0.006 | 1.6 | (1.28–2.01) | <.001 |
NOTES: Authors’ analysis of the IMS LifeLink database (N=27,235 individuals initiating first episodes of buprenorphine-naloxone treatment in 2010–2012). Estimates of six-month retention are derived from a logistic regression model and adjusted means represent the predicted value holding all other variables constant at their mean. Standard errors are clustered in both models at the county level. Standardized coefficients represent the marginal difference associated with a 1-standard deviation increase in the predictor variable.
Six-month retention was similar between individuals who primarily visited PCPs versus psychiatrists, but was significantly lower for those visiting other specialists compared to PCPs (OR, 0.73; 95% CI, 0.68–0.79). Differences across county characteristics (e.g., metropolitan status) were small, and generally not statistically significant. There were large differences between Arizona (the state with lowest six-month retention) and every state except California and Florida. Adjusted six-month retention rates exceeded 60% in Maryland, New Jersey, and New York. The largest difference was between Arizona and New York (OR 2.79, 95% CI 1.80–4.33).
Episode Length
The mean treatment length was 266 days and the median was 118 days (Table 2). Time ratios for length of treatment were similar to odds ratios of retention at six months (Table 3). Females had a time ratio of .87 (95% CI, .83-.90). Older individuals had significantly higher time ratios. Compared to individuals with predominantly cash-payment, time ratios were much lower Medicaid fee-for-service (.47; 95% CI, .43-.51), Medicare Part D (.44; 95% CI, .40-.48), and third-party payment (.49; 95% CI, .47-.51). Figure 1 illustrates these dramatic differences in episode discontinuation using plots of the regression-adjusted survival rates by primary payer source from the model displayed in Table 3.
Figure 1.
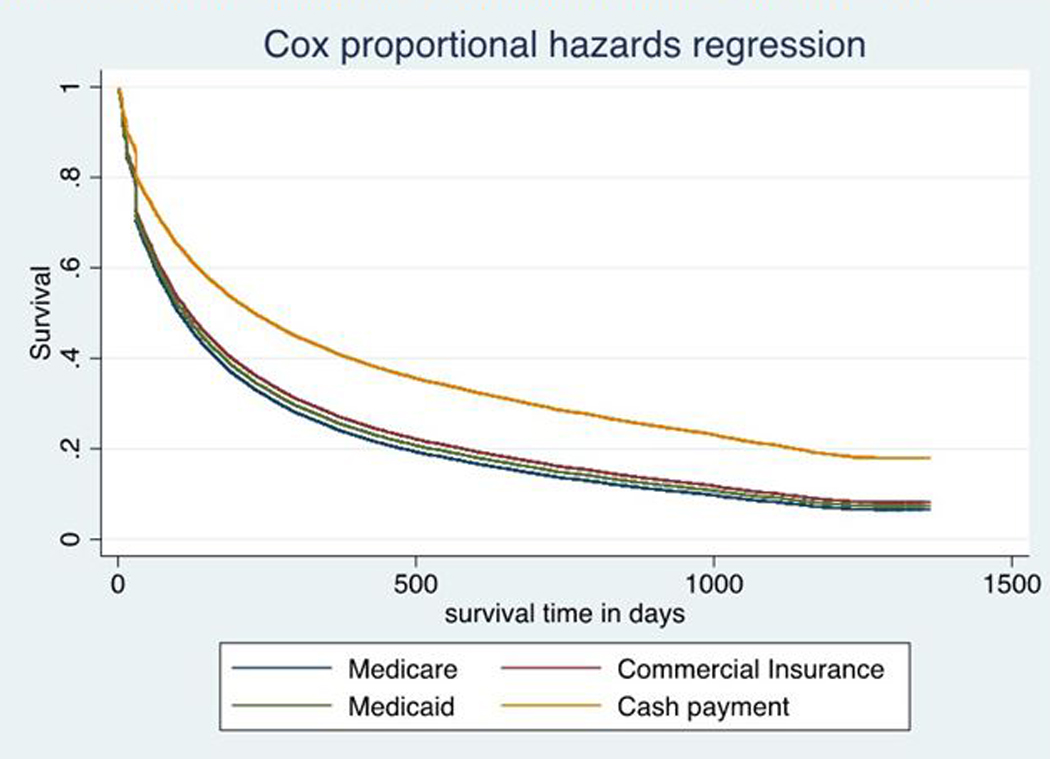
Survival Curves Illustrating Adjusted Differences in Treatment Length for Buprenorphine-Naloxone by Primary Insurance Payment Source
NOTES: Authors’ analysis of the IMS LifeLink database (N=27,235 individuals initiating first episodes of buprenorphine-naloxone treatment in 2010–2012). Each line represents the regression-adjusted survival curve for individuals with Medicare Part D, Medicaid Fee-for-Service, Third-Party Commercial Insurance, or Cash Payment as their primary source of payment. Models adjust for age, sex, prescriber type, county level variables (county urbanicity, an indicator for crossing county lines for treatment, the primary care physician population ratio, the buprenorphine prescriber to population ratio, median income, non-minority population ratio, and county overdose death rate), and state fixed effects.
Compared to individuals visiting PCPs, individuals visiting psychiatrists did not have statistically different time ratios. Those visiting specialists other than psychiatrists had lower ratios (.82; 95% CI, .77-.87). There were substantial differences in time ratios across states that followed similar patterns as six-month retention.
Mean Daily Dosage
The mean daily dosage in the sample was 14.1 mg/day (Table 2). After adjusting for all other covariates, the mean dosage was significantly higher among individuals with any form of insurance than with cash payment, with the mean daily dosage 1.37 mg/day higher among those in Medicaid Fee-for-Service (95% CI: .96–1.78) and 1.14 mg/day higher among Medicare Part D (95% CI: .77–1.51; Table 4). Individuals visiting psychiatrists had mean daily dosage that was −.8 mg/day lower than those visiting PCPs (95% CI:−1.17,−.38). The county-level variables had only a modest association with mean daily dosage. After adjusting for all covariates, there was notable variation across states in mean daily dosage – mean daily dosage was below 13 mg/day in Arizona and California, but was 14.8 mg/day in Pennsylvania and 15.1 mg/day in Louisiana.
Table 4.
Predictors of Mean Daily Dosage (Milligrams/Day) for Buprenorphine-Naloxone Treatment
| Mean Daily Dosage | ||||
|---|---|---|---|---|
| Adj. Mean | Coeff. | 95% CI | p-value | |
| Female | 14.0 | −0.17 | (−0.31--0.02) | 0.023 |
| Age (Ref=18–34) | 13.9 | |||
| Age 35–49 | 14.3 | 0.4 | (0.2–0.59) | <.001 |
| Age >50 | 13.7 | −0.18 | (−0.41–0.06) | 0.14 |
| Majority Payer (Ref=Cash) | 13.1 | |||
| Medicaid Fee-for-service | 14.5 | 1.37 | (0.96–1.78) | <.001 |
| Medicare Part D | 14.2 | 1.14 | (0.77–1.51) | <.001 |
| Third-Party Commercial | 13.5 | 0.41 | (0.18–0.63) | <.001 |
| Majority Prescriber (Ref=PCP) | 14.6 | |||
| Psychiatrist | 13.8 | −0.77 | (−1.17−−0.38) | <.001 |
| Other provider | 14.2 | −0.38 | (−0.74−−0.03) | 0.035 |
| Metropolitan Status (Ref=nonmetro) | 14.2 | |||
| Large (>1 million people) | 14.4 | 0.2 | (−0.31–0.71) | 0.433 |
| Medium (>250k-1 million people) | 14.4 | 0.2 | (−0.33–0.74) | 0.452 |
| Small (100k-250k people) | 14.5 | 0.33 | (−0.31–0.96) | 0.317 |
| County opioid overdose death rate (Ref=>18.1 per 100,000) | 14.2 | |||
| ≤10 deaths per 100k | 14.4 | 0.29 | (−0.42–1) | 0.428 |
| 10.1–14 deaths per 100k | 13.9 | −0.31 | (−0.85–0.23) | 0.263 |
| 14.1–17.9 deaths per 100k | 14.1 | −0.07 | (−0.54–0.4) | 0.78 |
| Other county covariates | ||||
| Crossed county lines for treatment | 14.2 | 0.07 | (−0.2–0.33) | 0.626 |
| PCP to population ratio (standardized) | 14.2 | 0.12 | (−0.05–0.28) | 0.176 |
| DEA waivered ratio (standardized) | 14.1 | −0.04 | (−0.2–0.13) | 0.658 |
| Median income (standardized) | 13.7 | −0.43 | (−0.62−−0.23) | <.001 |
| Non-minority pop. (standardized) | 14.4 | 0.3 | (−0.01–0.61) | 0.058 |
| State of Pharmacy (Ref=Arizona) | 12.4 | |||
| California | 12.6 | 0.17 | (−0.71–1.04) | 0.706 |
| Florida | 13.2 | 0.82 | (−0.02–1.67) | 0.056 |
| Georgia | 14.1 | 1.68 | (0.76–2.61) | <.001 |
| Louisiana | 15.1 | 2.71 | (1.77–3.65) | <.001 |
| Maryland | 14.2 | 1.84 | (0.97–2.7) | <.001 |
| New Jersey | 14.0 | 1.59 | (0.45–2.72) | 0.006 |
| New York | 13.7 | 1.19 | (0.2–2.18) | 0.018 |
| Pennsylvania | 14.8 | 2.4 | (1.36–3.43) | <.001 |
| Texas | 13.0 | 0.58 | (−0.24–1.39) | 0.166 |
| Washington | 13.6 | 1.11 | (0.22–2) | 0.015 |
| Other states | 14.2 | 1.76 | (0.93–2.59) | <.001 |
NOTES: Authors’ analysis of the IMS LifeLink database (N=27,235 individuals initiating first episodes of buprenorphine-naloxone treatment in 2010–2012). Estimates are derived from ordinary least squares regression models and adjusted means represent the predicted value holding all other variables constant at their mean. Standard errors are clustered in both models at the county level. Standardized coefficients represent the marginal difference associated with a 1-standard deviation increase in the predictor variable.
Treatment Continuity
About 30.2% of treatment episodes with ≥3 fills were associated with low possession ratios (Table 2). Odds of low possession ratio were relatively similar across groups by demographics, payment type, prescribing physician, and county. Adjusted rates of low possession ratio ranged from about one-quarter to one-third of all individuals with significantly lower rates among individuals older than 50 (27.4%) and those residing in counties with more non-minority individuals (26.9%). Adjusted low-possession rates were highest in Florida (35.1%) and lowest in New Jersey (22.7%).
Treatment interruptions of 14–90 days were experienced by 25.5% of individuals with ≥3 fills (Table 2). As with low possession, rates were relatively similar across demographic groups (Table 5). Adjusted interruption rates were highest for individuals with Medicare Part D as their primary source of payment (29.4%) and individuals receiving treatment primarily from a psychiatrist (27.6%). No county variables significantly predicted treatment interruptions. Compared to the reference state (Arizona), only New Jersey had a significantly different interruption rate (18.4%; OR, 0.65; 95% CI, 0.48–0.89).
Table 5.
Predictors of Low Possession Ratio and Treatment Interruptions for Buprenorphine-Naloxone Treatment Among Individuals With Three or More Fills
| Low possession ratio | Interruption of more than 14 days | |||||||
|---|---|---|---|---|---|---|---|---|
| Adj. Mean |
Odds Ratio |
95% CI | p-value | Adj. Mean | Odds Ratio |
95% CI | p-value | |
| Female | .311 | 1.08 | (1.02–1.15) | 0.013 | .256 | 1.01 | (0.95–1.07) | 0.806 |
| Age (Ref=18–34) | .320 | .263 | ||||||
| Age 35–49 | .278 | 0.84 | (0.78–0.9) | <.001 | .244 | 0.92 | (0.85–0.99) | 0.031 |
| Age >50 | .274 | 0.84 | (0.76–0.92) | <.001 | .248 | 0.95 | (0.87–1.05) | 0.326 |
| Majority Payer (Ref=Cash) | .307 | .240 | ||||||
| Medicaid Fee-for-service | .296 | 0.97 | (0.85–1.11) | 0.634 | .277 | 1.13 | (0.98–1.3) | 0.082 |
| Medicare Part D | .328 | 1.14 | (0.96–1.34) | 0.13 | .294 | 1.22 | (1.04–1.44) | 0.013 |
| Third-Party Commercial | .313 | 1.13 | (1.05–1.21) | 0.001 | .265 | 1.12 | (1.04–1.21) | 0.005 |
| Majority Prescriber (Ref=PCP) | .314 | .246 | ||||||
| Psychiatrist | .286 | 0.9 | (0.81–0.99) | 0.031 | .276 | 1.15 | (1.06–1.24) | <.001 |
| Other provider | .283 | 0.88 | (0.81–0.96) | 0.003 | .263 | 1.05 | (0.97–1.14) | 0.197 |
| Metropolitan Status (Ref=nonmetro) | .280 | .244 | ||||||
| Large (>1 million people) | .312 | 1.13 | (0.97–1.32) | 0.12 | .260 | 1.07 | (0.93–1.22) | 0.362 |
| Medium (>250k-1 million people) | .325 | 1.14 | (0.98–1.33) | 0.087 | .261 | 1.04 | (0.91–1.19) | 0.596 |
| Small (100k-250k people) | .310 | 1.04 | (0.88–1.24) | 0.654 | .262 | 1.04 | (0.89–1.21) | 0.631 |
| County opioid overdose death rate (Ref=>18.1 per 100,000) | .290 | .25.9 | ||||||
| ≤10 deaths per 100k | .316 | 1.09 | (0.97–1.22) | 0.145 | .252 | 0.98 | (0.89–1.07) | 0.62 |
| 10.1–14 deaths per 100k | .308 | 1.03 | (0.91–1.18) | 0.625 | .248 | 0.95 | (0.85–1.07) | 0.383 |
| 14.1–17.9 deaths per 100k | .309 | 1.04 | (0.94–1.16) | 0.439 | .247 | 0.94 | (0.85–1.04) | 0.215 |
| Other county covariates | ||||||||
| Crossed county lines for treatment | .306 | 1.03 | (0.95–1.11) | 0.463 | .256 | 1.01 | (0.94–1.09) | 0.775 |
| PCP to population ratio (standardized) | .289 | 0.93 | (0.89–0.98) | 0.005 | .253 | 0.99 | (0.95–1.03) | 0.534 |
| DEA waivered ratio (standardized) | .307 | 1.02 | (0.98–1.07) | 0.262 | .257 | 1.01 | (0.98–1.05) | 0.547 |
| Median income (standardized) | .300 | 0.99 | (0.94–1.04) | 0.622 | .252 | 0.98 | (0.94–1.02) | 0.274 |
| Non-minority pop. (standardized) | .269 | 0.83 | (0.79–0.87) | <.001 | .259 | 1.02 | (0.98–1.07) | 0.314 |
| State of Pharmacy (Ref=Arizona) | .301 | .270 | ||||||
| California | .304 | 1.01 | (0.77–1.32) | 0.941 | .242 | 0.93 | (0.79–1.09) | 0.362 |
| Florida | .351 | 1.31 | (1.05–1.64) | 0.017 | .244 | 0.93 | (0.81–1.07) | 0.33 |
| Georgia | .30.1 | 0.99 | (0.75–1.31) | 0.942 | .264 | 1.05 | (0.86–1.28) | 0.631 |
| Louisiana | .296 | 0.97 | (0.76–1.23) | 0.778 | .258 | 1.02 | (0.84–1.23) | 0.864 |
| Maryland | .231 | 0.68 | (0.52–0.88) | 0.004 | .259 | 1.02 | (0.85–1.23) | 0.829 |
| New Jersey | .227 | 0.67 | (0.48–0.93) | 0.015 | .184 | 0.65 | (0.48–0.89) | 0.007 |
| New York | .237 | 0.67 | (0.52–0.85) | 0.001 | .247 | 0.95 | (0.82–1.09) | 0.45 |
| Pennsylvania | .241 | 0.67 | (0.54–0.85) | 0.001 | .252 | 0.98 | (0.86–1.11) | 0.735 |
| Texas | .277 | 0.87 | (0.67–1.13) | 0.298 | .239 | 0.91 | (0.75–1.1) | 0.345 |
| Washington | .276 | 0.87 | (0.66–1.15) | 0.329 | .265 | 1.06 | (0.83–1.34) | 0.659 |
| Other states | .267 | 0.8 | (0.63–1.02) | 0.067 | .250 | 0.97 | (0.84–1.12) | 0.635 |
NOTES: Authors’ analysis of the IMS LifeLink database (N=18,654 individuals initiating first episodes of buprenorphine-naloxone treatment in 2010–2012 with 3 or more fills). Estimates are derived from a logistic regression model and adjusted means represent the predicted value holding all other variables constant at their mean. Length of treatment derived from Cox proportional hazard model. Standard errors are clustered in both models at the county level. Standardized coefficients represent the marginal difference associated with a 1-standard deviation increase in the predictor variable.
DISCUSSION
We evaluated patterns of buprenorphine-naloxone treatment in a multi-state community sample using an all-payer dataset that includes self-paying patients and a longer time window than has previously been considered. Consistent with previously published estimates,12,17 we found that 41% of patients were retained in treatment for six months or longer. The mean length of treatment was 266 days and the median was 118 days. The longer mean length reflects some individuals remaining in treatment for multiple years. Our estimates of six-month retention,12 mean treatment length,27 and adherence27 are fairly consistent with data from studies conducted on more select populations. We also found that mean daily dosage was around 14 mg/day – a dosage generally considered to be in the “medium” range (7–15 mg/day) in clinical studies.6
Individuals who predominantly paid for treatment with cash had longer treatment than those who paid with any form of insurance. This is surprising since cash paying patients are often uninsured and financially burdened to pay for treatment, factors that we hypothesized would lower retention. Several factors may explain this counterintuitive finding. First, many physicians who prescribe buprenorphine-naloxone do not accept health insurance.24 There may be differences in the physicians who treat insured patients, or in the constraints at the practice-level, that could lead to low retention rates. Second, some insurance programs restrict length of treatment, covering buprenorphine-naloxone exclusively for detoxification or imposing a cap on the maximum days covered.28,29 In 2013, several state Medicaid programs imposed lifetime limits on buprenorphine treatment.30 Third, for some patients, cash-payment may be a means of obtaining buprenorphine outside of insurance to divert for resale in the illicit market, as buprenorphine-naloxone has a high street value. Patients “opioid-shopping” for non-buprenorphine opioids are more likely to pay cash,31 but to our knowledge this relationship has not been established for buprenorphine-naloxone.
Other notable differences in retention occurred across states, with northeastern states (New York, New Jersey, and Maryland) having among the highest retention in the sample and Arizona and Florida having the lowest retention. Almost half of all patients crossed county lines for treatment, which may reflect geographic disparities in the location of waivered physicians.32 However, county-level variables such as physician supply, median income, and the opioid overdose death rate had relatively small associations with retention.
Almost one third of patients had episodes with low possession ratios. Age was a marker of higher possession ratio, which may indicate that middle-aged and older individuals have differences in motivation or in socioeconomic barriers that increase their treatment adherence. However, the influence of most characteristics on possession ratio was relatively small, potentially indicating that efforts to improve adherence for buprenorphine-naloxone will need to be broadly targeted.
Our study is subject to limitations. First, our database did not include patient diagnoses; we therefore are unable to adjust for comorbidities. This also means we cannot exclude patients being treated off-label for chronic pain33 (who account for 11% of all buprenorphine prescriptions34). Second, we lacked non-prescription service use, which could provide additional insight into quality of buprenorphine treatment, such as counseling visits with treatment, or outcomes that could be affected by treatment, such as hospitalizations for overdose. We lacked markers of clinical progress, illicit substance use, and relapse, and cannot determine if individuals with shorter treatment duration had worse clinical outcomes. Such information, even if obtained from a select subsample, could provide new insights into issues related to quality and patient health. Third, we could not measure how patients use medications prescribed to them. As mentioned, patients may divert medications prescribed to them for sale to other individuals. Diversion is a problem of growing importance requiring greater oversight in treatment programs.35 Fourth, although we considered county-level characteristics, we could not identify socio-demographic characteristics of the sample such as patient’s insurance status or individual-level income. For example, measuring how much of the cash payment sample is truly uninsured could provide better insights into the motivation and resources of cash paying patients. Among those with third-party payment, we could not separately identify the subgroup with private insurance (a group accounting for 39% of all individuals treated for opioid use disorder).36 Finally, county covariates such as the overdose death rate were measured after the study period, potentially introducing measurement error.
CONCLUSION
Low treatment retention for opioid use disorder is common across a variety of subgroups, and may be addressed through policy intervention. The surprising finding that individuals with insurance have lower retention compared to self-paying patients requires further investigation, and may underscore some issues with current coverage policies in both public and private insurance policies that restrict treatment length. State policies may also be important given the wide variation observed across states not attributable to differences in the county-level measures. These could include state-level differences in health care resources, the severity of the opioid epidemic, or to state funding for treatment programs for opioid use disorder. Identifying potential policies that may narrow these state differences is an important goal for research.
Acknowledgments
The authors have no external funding sources to disclose. Dr. Alexander is Chair of the FDA’s Peripheral and Central Nervous System Advisory Committee; serves as a paid consultant to PainNavigator, a mobile startup to improve patients’ pain management; serves as a paid consultant to IMS Health; and serves on an IMS Health scientific advisory board.
Appendix
APPENDIX TABLE:
Patterns of Buprenorphine-Naloxone Treatment for Opioid Use Disorder in a Multistate Population
| 3 month retention Odds Ratio |
9 month retention Odds Ratio |
|
|---|---|---|
| Female | 0.98*** | 0.96*** |
| Age (Ref=18–34) | ||
| Age 35–49 | 1.01 | 1.05*** |
| Age >50 | 0.98* | 1.02* |
| Majority Payer (Ref=Cash) | ||
| Medicaid Fee-for-service | 0.81*** | 0.77*** |
| Med icare Part D | 0.80*** | 0.76*** |
| Third-Party Commercial | 0.83*** | 0.79*** |
| Majority Prescriber (Ref=PCP) | ||
| Psychiatrist | 1 | 1 |
| Other provider | 0.93*** | 0.93*** |
| Metropolitan Status (Ref=nonmetro) | ||
| Large (>1 million people) | 0.98 | 0.97* |
| Medium (>250k-1 million people) | 1 | 0.98 |
| Small (100k-250k people) | 1.03 | 1.01 |
| County opioid overdose death rate (Ref=>18.1 per 100,000) |
||
| ≤10 deaths per 100k | 1 | 0.98 |
| 10.1–14 deaths per 100k | 0.99 | 0.97* |
| 14.1–17.9 deaths per 100k | 1.01 | 0.99 |
| Other county covariates | ||
| Crossed county lines for treatment | 0.98* | 0.98** |
| PCP to population ratio (standardized) | 1.01 | 1.01 |
| DEA waivered ratio (standardized) | 1 | 1 |
| Median income (standardized) | 1 | 1 |
| Non-minority pop. (standardized) | 1.02*** | 1.02*** |
| State of Pharmacy (Ref=Arizona) | ||
| California | 1.09 | 1.10* |
| Florida | 1.08 | 1.06 |
| Georgia | 1.12* | 1.15** |
| Louisiana | 1.16** | 1.17** |
| Maryland | 1.23*** | 1 24*** |
| New Jersey | 1.22*** | 1.20*** |
| New York | 1 25*** | 1 27*** |
| Pennsylvania | 1.20*** | 1.20*** |
| Texas | 1.13** | 1.14** |
| Washington | 1.20*** | 1.20*** |
| Other states | 1.15** | 1.16** |
P<.05
P<.01
P<.001
Footnotes
Disclosures
This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict of interest policies.
Dr. Saloner and Mr. Daubresse have no conflicts of interest to disclose.
Contributor Information
Brendan Saloner, Department of Health Policy & Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Matthew Daubresse, Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, Maryland, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
G. Caleb Alexander, Center for Drug Safety and Effectiveness, Johns Hopkins University, Baltimore, Maryland; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland; Division of General Internal Medicine, Department of Medicine, Johns Hopkins Medicine, Baltimore, Maryland.
REFERENCES
- 1.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. [DOI] [PubMed] [Google Scholar]
- 2.Mauger S, Fraser R, Gill K. Utilizing buprenorphine-naloxone to treat illicit and prescription-opioid dependence. Neuropsychiatr Dis Treat. 2014;10:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A Comparison of Levomethadyl Acetate, Buprenorphine, and Methadone for Opioid Dependence. N Engl J Med. 2000;343(18):1290–1297. [DOI] [PubMed] [Google Scholar]
- 4.Ward J, Hall W, Mattick RP. Role of maintenance treatment in opioid dependence. The Lancet. 1999;353(9148):221–226. [DOI] [PubMed] [Google Scholar]
- 5.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med. 2015;9(5):358–367. doi: 10.1097/ADM.0000000000000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas CP, Fullerton CA, Kim M, et al. Medication-assisted treatment with buprenorphine: assessing the evidence. Psychiatr Serv Wash DC. 2014;65(2):158–170. doi: 10.1176/appi.ps.201300256. [DOI] [PubMed] [Google Scholar]
- 7.Fudala PJ, Bridge TP, Herbert S, et al. Office-Based Treatment of Opiate Addiction with a Sublingual-Tablet Formulation of Buprenorphine and Naloxone. N Engl J Med. 2003;349(10):949–958. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267(20):2750–2755. [PubMed] [Google Scholar]
- 9.Stein BD, Gordon AJ, Sorbero M, Dick AW, Schuster J, Farmer C. The impact of buprenorphine on treatment of opioid dependence in a Medicaid population: Recent service utilization trends in the use of buprenorphine and methadone. Drug Alcohol Depend. 2012;123(1):72–78. doi: 10.1016/j.drugalcdep.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Stein MD, Patricia Cioe, Friedmann PD. Buprenorphine Retention in Primary Care. J Gen Intern Med. 2005;20(11):1038–1041. doi: 10.1111/j.1525-1497.2005.0228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon AJ, Lo-Ciganic W-H, Cochran G, et al. Patterns and Quality of Buprenorphine Opioid Agonist Treatment in a Large Medicaid Program: J Addict Med. 2015;9(6):470–477. doi: 10.1097/ADM.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 12.Neumann AM, Blondell RD, Azadfard M, Nathan G, Homish GG. Primary care patient characteristics associated with completion of 6-month buprenorphine treatment. Addict Behav. 2013;38(11):2724–2728. doi: 10.1016/j.addbeh.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Soeffing JM, Martin LD, Fingerhood MI, Jasinski DR, Rastegar DA. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat. 2009;37(4):426–430. doi: 10.1016/j.jsat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C. Retention in medication-assisted treatment for opiate dependence: A systematic review. J Addict Dis. 2016;35(1):22–35. doi: 10.1080/10550887.2016.1100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mintzer IL, Eisenberg M, Terra M, MacVane C, Himmelstein DU, Woolhandler S. Treating Opioid Addiction With Buprenorphine-Naloxone in Community-Based Primary Care Settings. Ann Fam Med. 2007;5(2):146–150. doi: 10.1370/afm.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: Five-year experience. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiellin DA, Moore BA, Sullivan LE, et al. Long-Term Treatment with Buprenorphine/Naloxone in Primary Care: Results at 2–5 Years. Am J Addict. 2008;17(2):116–120. doi: 10.1080/10550490701860971. [DOI] [PubMed] [Google Scholar]
- 18.Walley AY, Alperen JK, Cheng DM, et al. Office-Based Management of Opioid Dependence with Buprenorphine: Clinical Practices and Barriers. J Gen Intern Med. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalk M, Alanis-Hirsch K, Woodworth A, Mericle A, Curtis B, McLoyd K. Report of Commercial Health Plan Medication Coverage and Benefits Survey, Treatment Research Institute (TRI), 2013. Bethesda, MD: American Society of Addiction Medicine; 2013. [Google Scholar]
- 20.Thomas CP, Reif S, Haq S, Wallack SS, Hoyt A, Ritter GA. Use of Buprenorphine for Addiction Treatment: Perspectives of Addiction Specialists and General Psychiatrists. Psychiatr Serv. 2008;59(8):909–916. doi: 10.1176/ps.2008.59.8.909. [DOI] [PubMed] [Google Scholar]
- 21.Stein BD, Pacula RL, Gordon AJ, et al. Where Is Buprenorphine Dispensed to Treat Opioid Use Disorders? The Role of Private Offices, Opioid Treatment Programs, and Substance Abuse Treatment Facilities in Urban and Rural Counties. Milbank Q. 2015;93(3):561–583. doi: 10.1111/1468-0009.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenblum A, Cleland CM, Fong C, Kayman DJ, Tempalski B, Parrino M. Distance Traveled and Cross-State Commuting to Opioid Treatment Programs in the United States. J Environ Public Health. 2011;2011:e948789. doi: 10.1155/2011/948789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark HW. Office-Based Practice and Opioid-Use Disorders. N Engl J Med. 2003;349(10):928–930. doi: 10.1056/NEJMp038126. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski AM, Dlugosz MR, Blondell RD. Reimbursement and practice policies among providers of buprenorphine-naloxone treatment. Subst Abuse. 2013;34(2):105–107. doi: 10.1080/08897077.2012.677753. [DOI] [PubMed] [Google Scholar]
- 25.Kraus ML, Alford DP, Kotz MM, et al. Statement of the American Society Of Addiction Medicine Consensus Panel on the use of buprenorphine in office-based treatment of opioid addiction. J Addict Med. 2011;5(4):254–263. doi: 10.1097/ADM.0b013e3182312983. [DOI] [PubMed] [Google Scholar]
- 26.Karve S. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin Suppl. 2009;25(9):2303–2310. doi: 10.1185/03007990903126833. [DOI] [PubMed] [Google Scholar]
- 27.Khemiri A, Kharitonova E, Zah V, Ruby J, Toumi M. Analysis of Buprenorphine/Naloxone Dosing Impact on Treatment Duration, Resource Use and Costs in the Treatment of Opioid-Dependent Adults: A Retrospective Study of US Public and Private Health Care Claims. Postgrad Med. 2014;126(5):113–120. doi: 10.3810/pgm.2014.09.2805. [DOI] [PubMed] [Google Scholar]
- 28.Horgan CM, Stewart MT, Reif S, et al. Behavioral Health Services in the Changing Landscape of Private Health Plans. Psychiatr Serv. 2016;67(6):622–629. doi: 10.1176/appi.ps.201500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merrick EL, Horgan CM, Garnick DW, Reif S, Stewart MT. Accessing Specialty Behavioral Health Treatment in Private Health Plans. J Behav Health Serv Res. 2009;36(4):420–435. doi: 10.1007/s11414-008-9161-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldo S, Rinaldo D. Advancing Access to Addiction Medications: Implications for Opioid Addiction Treatment. Washington, DC: American Society of Addiction Medicine [Google Scholar]
- 31.McDonald DC, Carlson KE. Estimating the Prevalence of Opioid Diversion by “Doctor Shoppers” in the United States. PLOS ONE. 2013;8(7):e69241. doi: 10.1371/journal.pone.0069241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenblatt RA, Andrilla CHA, Catlin M, Larson EH. Geographic and Specialty Distribution of US Physicians Trained to Treat Opioid Use Disorder. Ann Fam Med. 2015;13(1):23–26. doi: 10.1370/afm.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen KY, Chen L, Mao J. Buprenorphine-naloxone therapy in pain management. Anesthesiology. 2014;120(5):1262–1274. doi: 10.1097/ALN.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner L, Kruszewski SP, Alexander GC. Trends in the use of buprenorphine by office-based physicians in the United States, 2003–2013. Am J Addict. November 2014:n/a–n/a. doi: 10.1111/j.1521-0391.2014.12174.x. [DOI] [PubMed] [Google Scholar]
- 35.Monte AA, Mandell T, Wilford BB, Tennyson J, Boyer EW. Diversion of buprenorphine/naloxone coformulated tablets in a region with high prescribing prevalence. J Addict Dis. 2009;28(3):226–231. doi: 10.1080/10550880903014767. [DOI] [PubMed] [Google Scholar]
- 36.Saloner B, Karthikeyan S. Changes in Substance Abuse Treatment Use Among Individuals With Opioid Use Disorders in the United States, 2004–2013. JAMA. 2015;314(14):1515–1517. doi: 10.1001/jama.2015.10345. [DOI] [PubMed] [Google Scholar]


