Abstract
Sex differences exist in the regulation of energy homeostasis. Better understanding of the underlying mechanisms for sexual dimorphism in energy balance may facilitate development of gender-specific therapies for human diseases, e.g. obesity. Multiple organs, including the brain, liver, fat and muscle, play important roles in the regulations of feeding behavior, energy expenditure and physical activity, which therefore contribute to the maintenance of energy balance. It has been increasingly appreciated that this multi-organ system is under different regulations in male vs. female animals. Much of effort has been focused on roles of sex hormones (including androgens, estrogens and progesterone) and sex chromosomes in this sex-specific regulation of energy balance. Emerging evidence also indicates that other factors (not sex hormones/receptors and not encoded by the sex chromosomes) exist to regulate energy homeostasis differentially in males vs. females. In this review, we summarize factors and signals that have been shown to regulate energy homeostasis in a sexually dimorphic fashion and propose a framework where these factors and signals may be integrated to mediate sex differences in energy homeostasis.
Introduction
Obesity, resulting from imbalance of energy homeostasis, is now recognized as a serious global health problem, due to its high prevalence and strong association with hypertension, coronary heart disease, stroke, and other metabolic disorders. A clear sexual dimorphism exists in the regulation of feeding behavior and energy homeostasis in rodents (Shi, et al. 2009; Sugiyama and Agellon 2012). For example, total daily energy intake in male rats is higher than that in females, even when corrected by their larger lean body mass and metabolic rate (Woodward and Emery 1989). In addition, high fat-diet (HFD) feeding leads to larger body weight gain in male rats/mice than in female counterparts (Benz, et al. 2012; Dorfman, et al. 2017; Grove, et al. 2010; Morselli, et al. 2016; Stubbins, et al. 2012; Yang, et al. 2014). However, the mechanisms for this sexual dimorphism remain elusive, and better understanding of this fundamental sex difference in energy homeostasis will no doubt benefit our current combat against obesity pandemics.
Males and females are obviously different in the circulating levels of sex hormones and the sex chromosomes their cells carry. Thus, these two categories of factors have been generally thought to be major contributors to the sexual dimorphism in obesity. In this review, we will discuss the functions of sex hormones and sex chromosomes in the context of body weight control. In addition, we will also discuss additional factors, which do not belong to these two categories, as additional contributors to the sex dimorphism of energy homeostasis.
1. Sex hormones
Androgens are described as male sex hormones and estrogens are described as female sex hormones (Chan and O’Malley 1976). The most important biologically relevant forms of estrogens and androgens are 17β-estradiol (E2) and testosterone (T), respectively. Both males and females have these hormones to varying degrees. Androgens are produced in male testis and female ovary. Androgens, mainly testosterone and androstenedione, can be converted to estrogens by aromatase (Jarvie and Hentges 2012). Thus, even in males, despite the low circulating levels of estrogens, estrogens can be produced through local aromatization using circulating testosterone as a substrate. Tissues that express aromatase include not only the gonads, but also the breast, brain, muscle, bone, and adipose tissue (Nelson and Bulun 2001; Simpson 2003; Simpson, et al. 2005). Another major female sex hormone is progesterone, which can also be synthesized by the adrenal glands (Johnston, et al. 2015; Wittmann, et al. 2013) and nervous tissue, especially in the brain (Schumacher, et al. 2004). Classically, androgens, estrogens and progesterone can bind to their nuclear receptors androgen receptors (AR), estrogen receptors (ER) and progesterone receptors (PR), respectively, and these receptors function as transcription factors to regulate gene expression (Yang and Shah 2014). Accumulating evidence also indicates that these sex hormones can bind to membrane receptors and exert rapid signal transduction effects in target cells (Mamounis, et al. 2014). As outlined below, these sex hormones and their receptors contribute to the regulation of energy balance with complex mechanisms.
1.1. Estrogens
1.1.1. Ligand
It is well documented that estrogens play an essential role in preventing body weight gain in females. For example, the withdrawal of endogenous estrogens by ovariectomy (OVX) in female animals leads to body weight gain and hyperadiposity, and these obese phenotypes can be prevented by E2 treatment but not by progesterone (Drewett 1973; Geary, et al. 2001; Roesch 2006; Rogers, et al. 2009; Schwartz and Wade 1981; Wallen, et al. 2001). Since E2 can be produced via conversion from testosterone by the aromatase, aromatase knockout mice represent a good model to examine functions of endogenous E2 not only in female but also in male animals. Female aromatase knockout mice show increased body weight from 3 months of age, while male mutant mice show late onset obesity one year later (Jones, et al. 2000). Both male and female aromatase knockout mice show increased gonadal and infrarenal fat pad compared to control littermates (Jones et al. 2000). This increased adiposity is associated with reduced spontaneous physical activity levels, reduced glucose oxidation, and a decrease in lean body mass (Jones et al. 2000). These findings indicate that endogenous E2 prevent obesity in both sexes.
1.1.2. ERα
Estrogen receptors include estrogen receptor-α (ERα), estrogen receptor-β (ERβ) and G protein-coupled receptor 30 (GPR30). In particular, ERα is perhaps most studied in the context of energy balance. Humans or mice with mutations in the ERα (Esr1) gene are obese (Heine, et al. 2000; Okura, et al. 2003). Further, deletion of ERα in mice blocks the anorexigenic effects of E2 treatment (Geary et al. 2001). Early studies showed that microinjections of E2 into various brain regions change animal’s feeding behavior (Butera and Beikirch 1989; Palmer and Gray 1986), suggesting that ERα expressed in the brain is important for the regulation of food intake. This notion was further supported by observations from various genetic mouse models. For example, we crossed mice carrying loxP-flanked ERα alleles (Esr1fl/fl) (Feng, et al. 2007) to the Nestin-Cre transgenic mice (Bruning, et al. 2000) to produce mice lacking ERα only in the brain (Xu, et al. 2011b). These female mutant mice develop obesity, characteristic of increased body weight and body fat. Obesity in these mice is associated with hyperphagia, decreased energy expenditure and decreased physical activity, which may all contribute to the development of obesity (Xu et al. 2011b). Notably, female mice lacking ERα in the brain display significantly elevated E2 in the circulation (Xu et al. 2011b), presumably due to the impaired negative feedback regulation by estrogens. Given that these mice develop robust obese phenotypes despite the higher E2 in the circulation, these observations argue that compared to ERα expressed in peripheral tissues, brain ERα plays predominant roles in the regulation of energy balance. Notably, male mice lacking ERα in the brain also develop modest obesity, indicating that brain ERα is required to prevent body weight gain in males (Xu et al. 2011b).
ERα is abundantly expressed in multiple brain regions that are implicated in the regulation of feeding behavior. These include the arcuate nucleus (ARH), the ventromedial hypothalamic nucleus (VMH), the nucleus of the solitary tract (NTS), the medial amygdala (MeA), the dorsal Raphe nuclei (DRN), and the medial preoptic area (MPOA) (Merchenthaler, et al. 2004). Interestingly, while many of these brain regions, e.g. ARH, VMH, and MPOA, in female brains express more abundant ERα than in male brains (Cao and Patisaul 2011; Kyi-Tha-Thu, et al. 2015), a few other regions in male brains, including the MeA, show higher ERα expression than in females (Xu, et al. 2015). In particular, about 20–30% pro-opiomelanocortin (POMC) neurons in the ARH co-express ERα (de Souza, et al. 2011; Miller, et al. 1995; Xu, et al. 2011a). E2 can increase excitatory synaptic inputs onto ARH POMC neurons and activate these neurons (Gao, et al. 2007; Malyala, et al. 2008; Saito, et al. 2015). Female mice lacking ERα only in POMC neurons develop hyperphagia and modest body weight gain (Xu et al. 2011b). In addition, E2-induced anorexigenic effects are blunted in these mutant female mice lacking ERα only in POMC neurons (Zhu, et al. 2015). Notably, male mice lacking ERα in POMC neurons do not have any feeding phenotypes (Xu et al. 2011b). Another hypothalamic ERα population with sexually dimorphic functions is that in the VMH. Martinez de Morentin et al. reported that injections of E2 into the VMH promote BAT-mediated thermogenesis in female rats (Martinez de Morentin, et al. 2014). Correa et al. developed a mouse model with loss of 26% of ERα-positive neurons in the VMH; this loss of ERα-positive neurons cause profound obesity only in females but not in males (Correa, et al. 2015). Consistently, genetic deletion of ERα specifically in the VMH leads to female obesity, but does not affect male energy balance in rodents (Musatov, et al. 2007; Xu et al. 2011b). Collectively, these observations indicate that ERα in POMC neurons and VMH neurons contributes to the sex differences in body weight balance.
Other hypothalamic ERα populations may also be involved in body weight control. For example, Santollo et al. reported that microinjections of E2 into the MPOA decreases food intake in female rats (Santollo, et al. 2011). Further, earlier studies showed that E2 implanted in the paraventricular nucleus of the hypothalamus (PVH) decreases food intake and body weight in OVX female rats (Butera and Beikirch 1989). Additionally, the anorexigenic effects of subcutaneous E2 were blunted in female rats with PVH lesions (Butera, et al. 1992). However, subsequent studies failed to reproduce these phenotypes in female rats with PVH implants (Hrupka, et al. 2002). In addition, it needs to be pointed out that the PVH expresses low levels of ERα but high levels of ERβ (Merchenthaler et al. 2004). Thus, the functions of ERα in the MPOA and the PVH warrant further validation with genetic models.
ERα is also present in the hindbrain, including the NTS (Merchenthaler et al. 2004; Osterlund, et al. 1998; Schlenker and Hansen 2006). Treatment of E2 in wild type female mice suppresses food intake and potentiates CCK-induced satiation, which are accompanied by increased activity in NTS neurons (Asarian and Geary 2007; Geary et al. 2001). Interestingly, these responses are all abolished in female mice lacking ERα (Asarian and Geary 2007; Geary et al. 2001). ERα is abundantly expressed in the DRN (Merchenthaler et al. 2004), and the majority of these ERα-positive neurons in the DRN are 5-HT neurons (Cao, et al. 2014). E2 increases neural activities of DRN 5-HT neurons via an ERα-dependent mechanism (Cao et al. 2014; Dalmasso, et al. 2011; Robichaud and Debonnel 2005). Santollo et al. reported that microinjections of E2 into the DRN decreases food intake in female rats (Santollo et al. 2011). Female mice lacking ERα only in 5-HT neurons are resistant to E2’s effects to suppress binge-like eating (Cao et al. 2014), a type of eating behavior that is not driven by hunger but rather than by rewards or hedonic cues. Collectively, ERα in the NTS and DRN mediates the anorexigenic effects of E2 in females, while functions of these ERα populations in male animals remain unknown.
However, it is clear that actions of ERα also prevent obesity in males. For example, ERα gene deficiency results in obesity in male mice (Callewaert, et al. 2009; Heine et al. 2000) and in men (Grumbach and Auchus 1999; Smith, et al. 1994). In addition, administration of E2 or its analogs reduces body weight in male mice (Finan, et al. 2012; Gao et al. 2007). Both male and female aromatase knockout mice develop obesity (Jones et al. 2000). Notably, abundant aromatase is expressed in a few brain regions, including the MeA (Wu, et al. 2009), which makes it possible that ERα in these male brain regions could be exposed to high levels of E2 despite the lack of circulating estrogens. Indeed, loss of ERα in the MeA not only causes obesity in female mice, but also in male mice (Xu et al. 2015). Thus, we speculate that ERα populations in brain regions with aromatase activity may play sexually monomorphic roles in the regulation of energy balance, while ERα in brain regions devoid of aromatase activity contribute to the sex differences in body weight balance.
The role of ERα in the peripheral tissues has been extensively reviewed before (Mauvais-Jarvis, et al. 2013). The deletion of ERα from the liver does not affect body weight fed with chow or HFD in either male or female mice (Matic, et al. 2013). Loss of ERα in the muscle causes obesity in female mice, associated with decreased energy expenditure and physical activity without changes in food intake (Ribas, et al. 2010), while effects of such deletion in male mice were not reported. Deletion of ERα in adipocytes causes obesity in female mice but not in male mice (Davis, et al. 2014). Together, the current literature indicate that ERα in some regions of the brain and in fat contributes to the maintenance of energy homeostasis in a sexually dimorphic fashion, although its effects in males still warrant further investigations. However, ERα in brain regions with enriched aromatase activity (e.g. the MeA) regulates energy balance in both sexes.
1.1.3. Other estrogen receptors
Compared to ERα, ERβ, another classic ER has received less attention at least in the context of body weight balance. An earlier study by Ohlsson et al reported that chow-fed mice (both male and female) with global deficiency in ERβ show normal body weight and fat mass compared to wild type mice (Ohlsson, et al. 2000). Consistent with this, both Santollo et al (Santollo, et al. 2007) and Roesch (Roesch 2006) found that an ERβ agonist, diarylpropionitrile (DPN), has no effect on food intake and body weight in chow-fed OVX female rats, while PPT (the ERα agonist) at similar doses can significantly reduce food intake and body weight. While these earlier studies suggest a minor role of ERβ in body weight control in chow-fed animals, Foryst-Ludwig et al demonstrated that female ERβ knockout mice, when fed on a HFD, developed obesity compared to HFD-fed wild type mice, which is associated with normal food intake, but increased energy expenditure and decreased fat oxidation (Foryst-Ludwig, et al. 2008). However, it has not been reported whether male mice lacking ERβ also show similar obese phenotypes. Certainly, the ERβ-mediated control of energy homeostasis in difference sexes warrants further investigations.
GPR30 (also known as GPER) is a G protein-coupled estrogen receptor, bound to the cell membrane (Thomas, et al. 2005). Body weight phenotypes among several independent GPR30 knockout mouse lines are controversial. For example, both Haas et al (Haas, et al. 2009) and Sharma et al (Sharma, et al. 2013) observed obese phenotypes in male and female GPR30 knockout mice, which were generated by Wang et al (Wang, et al. 2008); however, Liu et al reported no difference in body weight in the same GPR30 knockout strain (Liu, et al. 2009). Otto et al constructed an independent GPR30 knockout line, and found no obese phenotypes in female mutants (Otto, et al. 2009). Interestingly, another GPR30 knockout line generated by Martensson et al show reduced body weight only in females, but not in males (Martensson, et al. 2009). More recently, Davis et al re-characterized Wang’s GPR30 knockout mice and reported that both male and female mutants are significantly heavier than wild type littermates, which appears to depend on reduced energy expenditure independent of physical activity, but not on food intake (Davis et al. 2014). The discrepancy from these studies may be attributed to different strategies to construct the GPR30 knockout alleles, different genetic background that mice were maintained on, and/or different facility environment, etc. Further investigations are warranted to confirm THE roles of GPR30 in estrogenic regulation on body weight homeostasis in both sexes.
Collectively, high levels of circulating estrogens prevent obesity in female animals. Many of these estrogenic actions are mediated by multiple ERα populations, which therefore at least partly account for sex differences in body weight. However, ERα in certain brain regions with enriched aromatase activity, e.g. the MeA, also contributes to the maintenance of male energy balance. ERβ and GPR30 also regulate body weight balance, but their sex-specific functions remain to be further investigated.
1.2. Progesterone
Unlike estrogens, the administration of progesterone alone does not affect food intake, body weight, or adiposity in OVX female mice (Toth, et al. 2001). However, the anorectic effect of estrogens can be attenuated by concurrent administration of progesterone (Schwartz and Wade 1981; Wade 1975). In gonadal intact rodents, exogenous progesterone causes increase in body weight and inguinal white adipose tissue mass in female animals but not in male animals, by regulating the endocrine properties of adipocytes (Komatsu, et al. 2011; Kudo, et al. 2014; Sarton, et al. 2001). However, female mice with the global deletion of progesterone receptor do not show any difference in body weight (Rickard, et al. 2008). Notably, Klump et al reported that in cycling women, the tendencies of binge eating are negatively associated with circulating estrogen level but positively associated with progesterone level (Klump, et al. 2013; Klump, et al. 2014), suggesting that progesterone may promote binge eating. However, administration of progesterone in OVX female rats does not affect binge-like eating behavior (Yu, et al. 2011), although it remains debatable whether the binge-like eating behaviors in rodents really simulates human binge eating. Collectively, some evidence exists to support an orexigenic effect of progesterone, but further investigations are needed to confirm these effects and to explore the underlying mechanisms.
1.3. Androgens
1.3.1. Ligand
Androgens are also implicated to regulate male and female energy balance. Most women diagnosed with polycystic ovary syndrome (PCOS) are hyperandrogenemia, and higher level of serum androgen is correlated with higher BMI (Alexiou, et al. 2017; Dumesic, et al. 2016; Yuan, et al. 2016). In female animals, chronic treatment of dihydrotestosterone (DHT), a non-aromatizable androgen, promotes visceral adiposity with reduced energy expenditure but normal food intake (Nohara, et al. 2014). These effects are associated with reduced POMC neuronal innervations in the hypothalamus (Nohara et al. 2014). Effects of testosterone in males appear to be more complex. Serum testosterone levels are inversely correlated with obesity in men (Jorgensen, et al. 1996; Katznelson, et al. 1998). Man with lower androgen level showed increased fat mass which can be reversed by the testosterone administration (Rolf, et al. 2002). However, in male castrated adult mice, DHT treatment increases adiposity (Moverare-Skrtic, et al. 2006). Consistently, treatment of these male mice with testosterone in the presence of an aromatase inhibitor, but not testosterone alone, induces retroperitoneal fat accumulation (Moverare-Skrtic et al. 2006). Together, these observations demonstrate that in both sexes, DHT promote adiposity, probably via actions on the androgen receptor, while effects of testosterone may be complex due to its direct actions on the androgen receptor combined with indirect effects on estrogen receptors.
1.3.2. Androgen receptor
The role of androgen receptor in body weight control was suggested by an abnormal body weight profile in male mice with global androgen receptor knockout (Fan, et al. 2005; Lin, et al. 2005; Sato, et al. 2003). Interestingly, while young mutant male mice show decreased body weight, aged mutant male mice have increased body weight and adiposity compared to age-matched wild type males (Fan et al. 2005; Lin et al. 2005; Sato et al. 2003). Although selective deletion of androgen receptor in the brain (resulting from crosses between synapsin 1-Cre mice and ARfl/y) does not affect body weight in young male mice, such deletion leads to a late onset obesity (Yu, et al. 2013). These observations indicate that brain androgen receptor is required to prevent aging-associated obesity in males. Further characterization of the brain-specific androgen receptor deletion mouse model revealed increased expression of an anorexigenic neuropeptide, agouti-related peptide, and impaired insulin sensitivity in the hypothalamus, which presumably contribute to development of obesity in these mice (Yu et al. 2013). Interestingly, HFD feeding was found to accelerate obesity development in mice with brain-specific androgen receptor deletion, and this phenomenon is associated with a heightened activity of hypothalamic NF-κB and increased expression of protein-tyrosine phosphatase 1B (PTP1B) (Yu et al. 2013).
Androgen signaling in the liver also contributes to energy homeostasis, as male mice with deletion of androgen receptor from the liver develop obesity when fed with HFD, but female mutants do not (Lin, et al. 2008). Deletion of the androgen receptor in adipocyte (using the AP2-Cre as a putative fat-specific Cre allele) does not affect adiposity in male mice fed with chow diet (Yu, et al. 2008), but this deletion results in increased visceral fat in mice fed with HFD (McInnes, et al. 2012). These results suggest that androgen signaling in the adipose tissue protect against HFD-induced visceral obesity in males. However, the AP2-Cre used for the deletion of androgen receptor may also cause deletion of the receptor in the brain (Mullican, et al. 2013). Thus, the effects of the androgen receptor in the fat need to be further validated. The deletion of the androgen receptor in the muscle decreases the weight of both fat mass, lean mass, and body weight in male mice, despite normal food intake (Ophoff, et al. 2009). These indicate that the androgen signaling in the muscle is essential for the masculinization of energy homeostasis in males. Thus, effects of the androgen receptor in male animals are tissue-specific. While its actions in the brain, liver and adiposity function to prevent male mice from obesity, its actions in the muscle promote growth of both lean mass and adiposity. Notably, except for liver-specific deletion, female phenotypes in mice lacking androgen receptor globally or in specific tissues were not reported in these studies. However, Fagman et al reported that global deletion of androgen receptor in female mice on an apolipoprotein E (apoE)-deficient background are prone to diet-induced obesity compared to apoE-deficient female mice (Fagman, et al. 2015). While these results suggest potential anti-obesity actions of androgen receptor in females, these effects need to be further confirmed in animals with normal apoE functions.
1.4. Developmental Programming
The structure and functions of many organs that regulate energy balance, e.g. the brain, are immature at birth (Markakis 2002). In rodent brains, the majority of neural circuits do not fully develop till about postnatal day 21 (Grove and Smith 2003; Rinaman 2006, 2007). After weaning, the brain still undergoes substantial re-modeling (McNay, et al. 2011). Events (e.g. nutrient and hormone milieu) during the perinatal window have profound effects on the development of neural circuits controlling body weight and program energy balance later in life (Remmers and Delemarre-van de Waal 2011). For example, the placenta undergoes adaptations in response to maternal diet and metabolic status to alter fetal nutrient supply, which contributes to the developmental programming of offspring (Gabory, et al. 2013; Tarrade et al. 2015). This placental programming effect shows sexual dimorphism (Tarrade, et al. 2015). Male placenta decreases size in response to maternal high salt or high fat intake, which is associated with increased inflammation in the placenta and increased fetal exposure to free fatty acids and glucose. In contrast, female placenta is only affected by maternal high fat intake but is resistant to high salt stress (Reynolds, et al. 2015). In addition, it has been reported in mice that maternal high fat feeding during lactation period predisposes the male offspring for obesity and impaired glucose homeostasis, which is associated with an impairment of POMC neuron-originated neural circuitry. Strikingly, these effects are not seen in female offspring (Vogt, et al. 2014). While mechanisms for this sex difference is not clear, sex hormones have been implicated to regulate development of the brain to program energy balance. For example, immediately after birth, male rodents experience a transient testosterone surge in the circulation associated with a concurrent testosterone surge detected in the hypothalamus (Rhoda, et al. 1984). Interestingly, hypothalamic estrogen levels also increase at birth in males (Rhoda et al. 1984), presumably due to aromatization of testosterone. Estrogens are also detectable in female hypothalamus at birth although their levels are lower than those in males during the neonatal period (Amateau, et al. 2004). This neonatal surge of sex hormones may program the long-term regulations of energy balance in both sexes. For example, administration of testosterone in male and female rat pups lead to changes in structures and excitability of hypothalamic neurons (Matsumoto and Arai 1980). Further, neonatal exposure to excess testosterone renders a long-term obese phenotype in female mice, characteristic of increased energy intake and increased visceral adiposity during the adulthood (Nohara, et al. 2013b; Nohara, et al. 2011). These are associated with reduced leptin sensitivity, reduced expression of POMC mRNAs and POMC neuron innervations in the hypothalamus, which may contribute to obesity (Nohara et al. 2011). In males, neonatal exposure to testosterone leads to an early reduction in lean mass, decreases in locomotor activity, energy expenditure and food intake, but a late onset of hyperadiposity (Nohara, et al. 2013a). It is important to note that the reduced leptin sensitivity in females is not recapitulated by neonatal DHT exposure (Nohara et al. 2011), suggesting that this effect of testosterone may be mediated by estrogen receptors. Collectively, the current literature suggests robust effects of sex hormones on programming of energy balance during early development. Notably, most of genetic mouse studies so far use animal models with genes (e.g. ERα, ERβ, and AR) deleted at embryonic stage. Thus data obtained from these models could not fully distinguish effects of the sex hormones during early development vs. adulthood; further investigations using inducible genetic deletion models are warranted to tackle this issue.
2. Sex chromosomes
The sex dimorphism in gonadal hormones is ultimately determined by the sex chromosomes. The Sry gene located in the Y chromosome initiates the differentiation of the testes, which produce androgens and cause the masculinization of male mice (Goodfellow and Lovell-Badge 1993). Without the Sry gene, ovaries will be developed in the female mice and produce ovary hormones including estrogens and progesterone (Goodfellow and Lovell-Badge 1993). To evaluate the pure effect of sex chromosomes, the Sry gene was deleted from the Y chromosome to generate XY mice with ovaries, and translocated to a non-sex chromosome to generate XX mice with testes, together with XX ovary mice and XY testis mice. These ‘‘four core genotypes’’ mouse model were used to distinguish the effects between gonadal sex (testes or ovaries) and sex chromosomes (XX or XY) (De Vries, et al. 2002). Karen Reue’s group found that gonadal males (XX testis and XY testis) are heavier than gonadal females (XX ovary and XY ovary) in the gonad-intact adult mice, indicating that physiologically sex hormones are dominant in the regulation of sex dimorphism of energy homeostasis. However, the body weight difference among the four groups of mice disappeared one month after the gonadectomy, and XX mice (XX ovary and XX testis) gradually become heavier than XY mice (XY ovary and XY testis) 2–10 months after the gonadectomy, associated with increased fat mass, increased daylight time feeding, and decreased lipid oxidation (Chen, et al. 2012; Reue 2017). They also found that XX and XXY mice are much heavier than XO and XY mice, further supporting an important role of the X chromosome dosage in the regulation of energy balance (Chen et al. 2012; Reue 2017).
Thus, the X chromosome dosage is a risk factor for obesity, with one extra X chromosome instead of Y chromosome causing body weight gain (Reue 2017). Notably, XX mice are reported to have lower aromatase expression in the brain than XY mice, independent of gonadal sex (Cisternas, et al. 2015), raising a possibility that the obesity seen in XX mice may be due to decreased brain estrogen bioavailability. In addition, the inactivation of one of the two X chromosomes in XX cells during early development silences most genes on the X chromosome, but some X-linked genes escape this process and cause a higher dose in females than males (Chen et al. 2012; Pessia, et al. 2012; Reue 2017). Some of the X-linked “escape” genes, e.g. Eif2s3x, Kdm6a, Ddx3x, Kdm5c, Usp9x and Uba1, have been validated to be expressed at higher levels in XX fat mass than XY gonadal fat in gonadectomized mice (Chen et al. 2012). However, whether these genes are responsible for the X chromosome’s regulation on energy homeostasis needs to be further studied.
In parallel, a few other X-linked genes have been implicated in the regulation of energy balance. One such example is the gene encoding the androgen receptor, as we have discussed. Another well-studied X-linked gene is O-GlcNAc transferase (OGT), which catalyzes the posttranslational modification of proteins by O-GlcNAc and is regulated by nutrient access. Loss of OGT in orexigenic AgRP neurons inhibits neural activity of these neurons and leads to lean phenotypes in both male and female mice (Ruan, et al. 2014). On the other hand, loss of OGT in glutamatergic neurons causes robust obesity in male mice associated with hyperphagia, and these phenotypes can be rescued by restoration of OGT only in PVH neurons which are largely anorexigenic (Lagerlof, et al. 2016). Female mice carrying the same mutation were not characterized in this study (Lagerlof et al. 2016). Nevertheless, functions of OGT appear to be site-specific, promoting weight gain in AgRP neurons but preventing obesity in PVH neurons, but its sex-specific role needs to be further characterized.
5-HT 2C receptor (5-TH2CR), encoded by an X-linked gene (Ht2cr), also plays essential roles in preventing obesity (Tecott, et al. 1995). Male mice with global deficiency of 5-TH2CR develop a late onset hyperphagic obesity, which can be exacerbated by HFD feeding (Nonogaki, et al. 1998; Tecott et al. 1995). 5-HT and its analogs, e.g. d-fenfluramine, have been shown to suppress food intake in animals, and these effects are largely mediated via the 5-TH2CR expressed by POMC neurons in male animals (Berglund, et al. 2013; Xu, et al. 2008). In addition, the 5-TH2CR expressed by dopamine neurons mediates 5-HT actions to inhibit binge-like eating in male mice (Xu, et al. 2017). Indeed, a selective agonist of the 5-TH2CR, lorcaserin, was approved by FDA as an anti-obesity medicine. Mechanisms for 5-TH2CR’s effects on appetite control involve its actions to activate the transient receptor potential channel 5 (TrpC5) which leads to depolarization of POMC neurons (Gao, et al. 2017). Interestingly, TrpC5 is also encoded by the X chromosome, and it has been shown to mediate actions of multiple hormones on POMC neurons, including estrogens (Qiu, et al. 2018), leptin (Gao et al. 2017; Qiu, et al. 2010) and insulin (Qiu, et al. 2014). Importantly, deletion of TrpC5 in POMC neurons leads to obesity in male mice, which is associated with increased daylight feeding and decreased energy expenditure (Gao et al. 2017). However, effects of both 5-TH2CR and TrpC5 deletion on female energy balance have not been reported.
In summary, the Sry gene on the Y chromosome determines the development of male or female gonads, which influence the circulating sex hormones and therefore energy homeostasis. The X chromosome also influences the energy balance in a dose-dependent manner. A few X-linked genes have been implicated in energy balance, but their contributions to the sex difference in body weight balance remain to be further investigated. In addition, more X-linked genes may be related to the sexual dimorphism in energy balance, which remain to be identified.
3. Other factors
While the majority of the field focus on the functions of sex hormones and sex chromosomal genes, other factors (not sex hormones/receptors and not encoded by the sex chromosomes) may exist to regulate the sex differences in energy homeostasis.
For example, POMC mRNA and POMC neuron projections have been shown to be more abundant in female hypothalamus than in males, corresponding to lower food intake in female mice (Nohara et al. 2011). Further, we recently found that POMC neurons in female mice display higher neural activities compared to male counterparts (Wang, et al. 2018). In addition, enhanced POMC mRNAs and neural activity are both attributed to a transcription factor, namely TAp63. Strikingly, deletion of TAp63 in POMC neurons confers “male-like” diet-induced obesity to female mice, but does not affect body weight in male mice (Wang et al. 2018). Importantly, anorexigenic effects of estrogens are not affected by loss of TAp63 in POMC neurons (Wang et al. 2018), suggesting that TAp63 actions are largely independent of the female sex hormone. Notably, the gene encoding TAp63 is located on an autosome. Thus, these results indicate that TAp63 in POMC neurons is one such example that factors, not related to sex hormones/receptors and not on sex chromosomes, could contribute to the sex differences in energy balance. Interestingly, Sirt1, a nicotinamide adenine dinucleotide (NAD+) dependent deacetylase (Imai, et al. 2000; Michan and Sinclair 2007), is a known transcriptional target of TAp63 (Su, et al. 2012). Deletion of Sirt1 in POMC neurons increases susceptibility to diet-induced obesity specifically in female mice but not in male mice due to reduced energy expenditure, indicating that Sirt1 in POMC neurons plays a sexually dimorphic role in energy homeostasis (Ramadori, et al. 2010). Similarly, loss of signal transducer and activator of transcription 3 (STAT3) in POMC neurons leads to modest obesity in female mice but does not affect male mice (Xu, et al. 2007). Notably, STAT3 is shown to mediate anorexigenic effects of estrogens (Gao et al. 2007). Thus, the sexually dimorphic functions of STAT3 in POMC neurons may result from different levels of circulating estrogens in males vs. females. In contrast, deletion of GABAB receptors in POMC neurons results in obesity in the male mice but not female mice fed a HFD, but it is unclear why GABAB receptors affect male and female differently (Ito, et al. 2013).
In addition to POMC neurons, microglial cells in the hypothalamus also contribute to the sex differences in body weight control. Dorfman et al recently reported that male mice fed a HFD reduce hypothalamic levels of CX3CL1 (a neuron-released chemokine) and its receptor expressed by microglia, CX3CR1, while the CX3CL1-CX3CR1 levels remain normal in the hypothalamus of HFD-fed female mice (Dorfman et al. 2017). Interestingly, female Cx3cr1 knockout mice develop “male-like” hypothalamic microglial accumulation and activation, associated with increased susceptibility to diet-induced obesity (Dorfman et al. 2017). Conversely, increasing brain CX3CL1 levels in the hypothalamus of male mice converts them to a “female-like” metabolic phenotype with reduced microglial activation and reduced body weight gain (Dorfman et al. 2017). These data identify the CX3CL1-CX3CR1 signaling in hypothalamic microglia as an important mediator for sex differences in energy balance.
Kisspeptin (Kiss1) and its receptor, GPR54, are key regulators of reproduction (Murphy 2005). Female mice lacking GPR54 develop massive obesity fed with either chow or HFD, associated with increased fat mass and serum leptin level, and decreased energy expenditure but normal food intake (Tolson, et al. 2016; Tolson, et al. 2014). On the other hand, male mice lacking GPR54 have none to minimal phenotypes in energy balance (Tolson et al. 2016; Tolson et al. 2014). Thus, the Kiss1-GPR54 signaling regulates energy homeostasis in a sexually dimorphic fashion. Notably, since loss of Kiss1-GPR54 signaling substantially reduces the circulating levels of testosterone in males and reduces estrogens in females (d’Anglemont de Tassigny, et al. 2007; Seminara, et al. 2003), the obesity phenotypes observed in GPR54 knockout mice could be at least partially attributed to the altered levels of sex hormones in these mice. Similarly, global deletion of angiotensin II receptor (AT2R) renders female mice more prone to diet-induced obesity with impaired lipid metabolism, but the same mutation does not affect male mice (Samuel, et al. 2013). This female-specific obesity is associated with reduced estrogen level in female mutants, which may account for the sexually dimorphic obesity seen in mice lacking AT2R (Samuel et al. 2013).
While many of the aforementioned factors appear to prevent obesity in females, lecithin cholesterol acyltransferase (LCAT) plays an exactly opposite role. LCAT is an enzyme in the regulation of high density lipoprotein (HDL) metabolism. Deletion of LCAT results in a profound HDL deficiency in both male and female mice (Li, et al. 2011). However, LCAT knockout female mice are protected from high fat high sucrose diet-induced obesity, but male mutant mice are not (Li et al. 2011). The most striking sexually dimorphic pattern was found in mice lacking the cytokine IL-6 in the muscle. On one hand, deletion of IL-6 from the muscle results in decreased body weight in male mice, which is associated with lower core body temperature during the light phase and increased respiratory exchange ratio (Ferrer, et al. 2014; Molinero, et al. 2017). On the other hand, loss of IL-6 in female muscles leads to increased body weight (Ferrer et al. 2014; Molinero et al. 2017). Thus, IL-6 in the muscle regulates energy balance in exactly opposite directions in males vs. females.
Together, these findings indicate that many autosome genes contribute to the sex differences in energy balance through multiple mechanisms. Conceivably, some factors/genes may be transcriptional targets of sex hormones, since estrogens, protesterone and androgens are all known to regulate gene expression via their nuclear receptors. Alternatively, some factors/molecules may mediate body weight-regulatory effects of the sex hormones, e.g. STAT3 being a mediator of anorexigenic effects of estrogens. Further, some factors could regulate levels of sex hormones or receptors, e.g. Kiss1-GPR54 and AT2R. Of course, some genes may be independent of sex hormone actions and function as parallel mechanisms to mediate sex differences in energy balance, e.g. TAp63. Of course, how these factors functionally interact with the sex hormones/chromosomes remain largely unknown and future studies are certainly warranted. It also needs to be pointed out that a lot more factors/molecules in this category may exist but are missed simply because female animals are purposely avoided in most studies to reduce efforts.
Conclusions
The existence of sex difference in biology and physiology, including the energy homeostasis, has been increasingly appreciated (Arnold 2014), and the underlying mechanisms started to emerge (Figure 1). The sex chromosomes are the fundamental difference between males and females and genes in the X or Y chromosome are conceivably major contributors to the sexual dimorphism. The Sry gene in the Y chromosome determines the fate of gonadal development, and therefore influence the levels of sex hormones. The X chromosome dosage adds the risk of obesity in females with mechanisms that are yet to unfold; in parallel, some X-linked genes, including those encoding the androgen receptor, OGT, 5-HT2CR, trpC5, and likely more, are involved in the regulation of energy balance, and some of these molecules are therapeutic targets for obesity treatment. All sex hormones, including estrogens, progesterone and androgens, are involved in the regulation of body weight balance in both sexes, but their actions and mechanisms are largely sex-specific. In addition, increasing number of autosome genes are found to contribute to the sex differences in body weight control. While some of these genes are either downstream targets or upstream factors of sex hormones, some of them appear to provide parallel mechanisms and function independent of the hormones.
Figure 1. A proposed framework for molecular mechanisms underlying sex differences in body weight control.
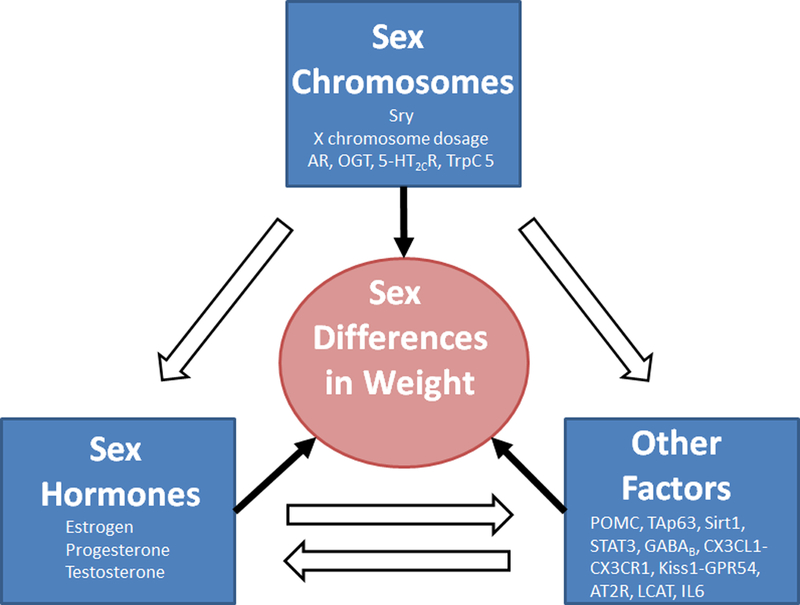
The Sry gene in the Y chromosome determines gonadal development and therefore the levels of sex hormones. The X chromosome dosage adds the risk of obesity in females; some X-linked genes have been implicated in the regulation of energy balance. Sex hormones are involved in the regulation of body weight balance in both sexes. In addition, other factors (autosome-encoded factors that are not sex hormones/receptors) contribute to the sex differences in body weight control. These three categories of factors are integrated through their reciprocal interactions to ultimately determine the sex differences in body weight balance.
Despite the expanding list of factors found to be involved in sex differences in body weight control, and an emerging framework where these factors and signals may be integrated to regulate energy homeostasis in different sexes, a number of areas need further investigations. First, for any tissues or cells that can regulate energy balance, their functions in different sexes would depend on the strength of estrogenic or androgenic tone locally in that region, which is ultimately determined by the availability of the sex hormones, the aromatase activity, and the relevant receptors. Thus, a few important questions need to be systemically addressed. Are these factors (sex hormones, aromatase, and the receptors) present in metabolic organs (including body weight-regulatory brain regions) in each sex? Are their levels dynamic throughout the life period? Most importantly, what are their functions in the context of energy balance? In addition, given the established role of the X chromosome dosage in body weight control, efforts are needed to identify what X-linked genes regulate energy balance and to determine whether these genes regulate body weight only in females, males or both. Further, we predict that a large number of other factors, not directly related to sex hormones/receptors and not encoded by sex chromosomes, also contribute to the sex differences in energy balance. Therefore we call attention to these “other factors” as potential regulators of sex differences in energy balance. Pharmacological and genetic investigations into energy balance in animal models are warranted and should contrast males with females, as required by the funding agencies, e.g. the NIH (Tannenbaum, et al. 2016). After these factors are identified, new studies can be designed to further delineate how they interact with sex hormones as well as sex-linked genes.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (R01DK115761, R01DK101379, R01DK117281 and P01DK113954 to Y. Xu), USDA/CRIS (3092–5-001–059 to Y.Xu), and American Heart Association awards (17GRNT32960003 to Y.Xu; 16POST27260254 to C. Wang).
Footnotes
The authors disclose no interest of conflict.
Reference:
- Alexiou E, Hatziagelaki E, Pergialiotis V, Chrelias C, Kassanos D, Siristatidis C, Kyrkou G, Kreatsa M & Trakakis E 2017. Hyperandrogenemia in women with polycystic ovary syndrome: prevalence, characteristics and association with body mass index. Horm Mol Biol Clin Investig 29 105–111. [DOI] [PubMed] [Google Scholar]
- Amateau SK, Alt JJ, Stamps CL & McCarthy MM 2004. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology 145 2906–2917. [DOI] [PubMed] [Google Scholar]
- Arnold AP 2014. Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Exp Neurol 259 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L & Geary N 2007. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 148 5656–5666. [DOI] [PubMed] [Google Scholar]
- Benz V, Bloch M, Wardat S, Bohm C, Maurer L, Mahmoodzadeh S, Wiedmer P, Spranger J, Foryst-Ludwig A & Kintscher U 2012. Sexual dimorphic regulation of body weight dynamics and adipose tissue lipolysis. PLoS One 7 e37794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y & Elmquist JK 2013. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J Clin Invest 123 5061–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD & Wade GN 1976. Ovarian influences on the meal patterns of female rats. Physiol Behav 17 201–208. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D & Kahn CR 2000. Role of brain insulin receptor in control of body weight and reproduction. Science 289 2122–2125. [DOI] [PubMed] [Google Scholar]
- Butera PC & Beikirch RJ 1989. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res 491 266–273. [DOI] [PubMed] [Google Scholar]
- Butera PC, Willard DM & Raymond SA 1992. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res 576 304–310. [DOI] [PubMed] [Google Scholar]
- Callewaert F, Venken K, Ophoff J, De Gendt K, Torcasio A, van Lenthe GH, Van Oosterwyck H, Boonen S, Bouillon R, Verhoeven G, et al. 2009. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J 23 232–240. [DOI] [PubMed] [Google Scholar]
- Cao J & Patisaul HB 2011. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J Comp Neurol 519 2954–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Xu P, Oyola MG, Xia Y, Yan X, Saito K, Zou F, Wang C, Yang Y, Hinton A, Jr., et al. 2014. Estrogens stimulate serotonin neurons to inhibit binge-like eating in mice. J Clin Invest 124 4351–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L & O’Malley BW 1976. Mechanism of action of the sex steroid hormones (first of three parts). N Engl J Med 294 1322–1328. [DOI] [PubMed] [Google Scholar]
- Chen X, McClusky R, Chen J, Beaven SW, Tontonoz P, Arnold AP & Reue K 2012. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 8 e1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisternas CD, Tome K, Caeiro XE, Dadam FM, Garcia-Segura LM & Cambiasso MJ 2015. Sex chromosome complement determines sex differences in aromatase expression and regulation in the stria terminalis and anterior amygdala of the developing mouse brain. Mol Cell Endocrinol 414 99–110. [DOI] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL & Ingraham HA 2015. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep 10 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, et al. 2007. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A 104 10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso C, Amigone JL & Vivas L 2011. Serotonergic system involvement in the inhibitory action of estrogen on induced sodium appetite in female rats. Physiol Behav 104 398–407. [DOI] [PubMed] [Google Scholar]
- Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM & Clegg DJ 2014. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm Behav 66 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza FS, Nasif S, Lopez-Leal R, Levi DH, Low MJ & Rubinsten M 2011. The estrogen receptor alpha colocalizes with proopiomelanocortin in hypothalamic neurons and binds to a conserved motif present in the neuron-specific enhancer nPE2. Eur J Pharmacol 660 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS & Arnold AP 2002. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22 9005–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman MD, Krull JE, Douglass JD, Fasnacht R, Lara-Lince F, Meek TH, Shi X, Damian V, Nguyen HT, Matsen ME, et al. 2017. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat Commun 8 14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewett RF 1973. Sexual behaviour and sexual motivation in the female rat. Nature 242 476–477. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, Thomas AM, Grogan TR, Haykal R, Schooler TA, et al. 2016. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab 101 4178–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagman JB, Wilhelmson AS, Motta BM, Pirazzi C, Alexanderson C, De Gendt K, Verhoeven G, Holmang A, Anesten F, Jansson JO, et al. 2015. The androgen receptor confers protection against diet-induced atherosclerosis, obesity, and dyslipidemia in female mice. FASEB J 29 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S & Nawata H 2005. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes 54 1000–1008. [DOI] [PubMed] [Google Scholar]
- Feng Y, Manka D, Wagner KU & Khan SA 2007. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci U S A 104 14718–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer B, Navia B, Giralt M, Comes G, Carrasco J, Molinero A, Quintana A, Senaris RM & Hidalgo J 2014. Muscle-specific interleukin-6 deletion influences body weight and body fat in a sex-dependent manner. Brain Behav Immun 40 121–130. [DOI] [PubMed] [Google Scholar]
- Finan B, Yang B, Ottaway N, Stemmer K, Muller TD, Yi CX, Habegger K, Schriever SC, Garcia-Caceres C, Kabra DG, et al. 2012. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med 18 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, et al. 2008. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 4 e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, Moore LG & Junien C 2013. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ 4 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, et al. 2007. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13 89–94. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, Kong X, Yu KJ, Wang RT, Chen H, et al. 2017. TrpC5 Mediates Acute Leptin and Serotonin Effects via Pomc Neurons. Cell Rep 18 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW & Ogawa S 2001. Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-alpha null mice. Endocrinology 142 4751–4757. [DOI] [PubMed] [Google Scholar]
- Goodfellow PN & Lovell-Badge R 1993. SRY and sex determination in mammals. Annu Rev Genet 27 71–92. [DOI] [PubMed] [Google Scholar]
- Grove KL, Fried SK, Greenberg AS, Xiao XQ & Clegg DJ 2010. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 34 989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL & Smith MS 2003. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav 79 47–63. [DOI] [PubMed] [Google Scholar]
- Grumbach MM & Auchus RJ 1999. Estrogen: consequences and implications of human mutations in synthesis and action. J Clin Endocrinol Metab 84 4677–4694. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, et al. 2009. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res 104 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB & Cooke PS 2000. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A 97 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrupka BJ, Smith GP & Geary N 2002. Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol Behav 77 233–241. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M & Guarente L 2000. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403 795–800. [DOI] [PubMed] [Google Scholar]
- Ito Y, Banno R, Shibata M, Adachi K, Hagimoto S, Hagiwara D, Ozawa Y, Goto M, Suga H, Sugimura Y, et al. 2013. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J Neurosci 33 17166–17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvie BC & Hentges ST 2012. Expression of GABAergic and glutamatergic phenotypic markers in hypothalamic proopiomelanocortin neurons. J Comp Neurol 520 3863–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CE, Herschel DJ, Lasek AW, Hammer RP Jr. & Nikulina EM 2015. Knockdown of ventral tegmental area mu-opioid receptors in rats prevents effects of social defeat stress: implications for amphetamine cross-sensitization, social avoidance, weight regulation and expression of brain-derived neurotrophic factor. Neuropharmacology 89 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, et al. 2000. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A 97 12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JO, Vahl N, Hansen TB, Fisker S, Hagen C & Christiansen JS 1996. Influence of growth hormone and androgens on body composition in adults. Horm Res 45 94–98. [DOI] [PubMed] [Google Scholar]
- Katznelson L, Rosenthal DI, Rosol MS, Anderson EJ, Hayden DL, Schoenfeld DA & Klibanski A 1998. Using quantitative CT to assess adipose distribution in adult men with acquired hypogonadism. AJR Am J Roentgenol 170 423–427. [DOI] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Boker S & Hu JY 2013. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. J Abnorm Psychol 122 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL, Boker S & Keel PK 2014. Ovarian Hormone Influences on Dysregulated Eating: A Comparison of Associations in Women with versus without Binge Episodes. Clin Psychol Sci 2 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Ohara A, Sasaki K, Abe H, Hattori H, Hall FS, Uhl GR & Sora I 2011. Decreased response to social defeat stress in mu-opioid-receptor knockout mice. Pharmacol Biochem Behav 99 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Konno K, Uchigashima M, Yanagawa Y, Sora I, Minami M & Watanabe M 2014. GABAergic neurons in the ventral tegmental area receive dual GABA/enkephalin-mediated inhibitory inputs from the bed nucleus of the stria terminalis. Eur J Neurosci 39 1796–1809. [DOI] [PubMed] [Google Scholar]
- Kyi-Tha-Thu C, Okoshi K, Ito H, Matsuda K, Kawata M & Tsukahara S 2015. Sex differences in cells expressing green fluorescent protein under the control of the estrogen receptor-alpha promoter in the hypothalamus of mice. Neurosci Res 101 44–52. [DOI] [PubMed] [Google Scholar]
- Lagerlof O, Slocomb JE, Hong I, Aponte Y, Blackshaw S, Hart GW & Huganir RL 2016. The nutrient sensor OGT in PVN neurons regulates feeding. Science 351 1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hossain MA, Sadat S, Hager L, Liu L, Tam L, Schroer S, Huogen L, Fantus IG, Connelly PW, et al. 2011. Lecithin cholesterol acyltransferase null mice are protected from diet-induced obesity and insulin resistance in a gender-specific manner through multiple pathways. J Biol Chem 286 17809–17820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Xu Q, Yeh S, Wang RS, Sparks JD & Chang C 2005. Insulin and leptin resistance with hyperleptinemia in mice lacking androgen receptor. Diabetes 54 1717–1725. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, Hsu CL, Ma WL, Jokinen J, Sparks JD, et al. 2008. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology 47 1924-1935. [DOI] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS & Mauvais-Jarvis F 2009. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 58 2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malyala A, Zhang C, Bryant DN, Kelly MJ & Ronnekleiv OK 2008. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J Comp Neurol 506 895–911. [DOI] [PubMed] [Google Scholar]
- Mamounis KJ, Yang JA, Yasrebi A & Roepke TA 2014. Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17beta-estradiol’s control of energy homeostasis. Steroids 81 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markakis EA 2002. Development of the neuroendocrine hypothalamus. Front Neuroendocrinol 23 257–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, et al. 2009. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150 687–698. [DOI] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, Lage R, Fernandez-Mallo D, Martinez-Sanchez N, Ruiz-Pino F, Liu J, Morgan DA, Pinilla L, et al. 2014. Estradiol Regulates Brown Adipose Tissue Thermogenesis via Hypothalamic AMPK. Cell Metab 20 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matic M, Bryzgalova G, Gao H, Antonson P, Humire P, Omoto Y, Portwood N, Pramfalk C, Efendic S, Berggren PO, et al. 2013. Estrogen signalling and the metabolic syndrome: targeting the hepatic estrogen receptor alpha action. PLoS One 8 e57458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A & Arai Y 1980. Sexual dimorphism in ‘wiring pattern’ in the hypothalamic arcuate nucleus and its modification by neonatal hormonal environment. Brain Res 190 238–242. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ & Hevener AL 2013. The role of estrogens in control of energy balance and glucose homeostasis. Endocr Rev 34 309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes KJ, Smith LB, Hunger NI, Saunders PT, Andrew R & Walker BR 2012. Deletion of the androgen receptor in adipose tissue in male mice elevates retinol binding protein 4 and reveals independent effects on visceral fat mass and on glucose homeostasis. Diabetes 61 1072–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNay DE, Briancon N, Kokoeva MV, Maratos-Flier E & Flier JS 2011. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J Clin Invest [DOI] [PMC free article] [PubMed]
- Merchenthaler I, Lane MV, Numan S & Dellovade TL 2004. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol 473 270–291. [DOI] [PubMed] [Google Scholar]
- Michan S & Sinclair D 2007. Sirtuins in mammals: insights into their biological function. Biochem J 404 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Tousignant P, Yang U, Pedvis S & Billiar RB 1995. Effects of age and long-term ovariectomy on the estrogen-receptor containing subpopulations of beta-endorphin-immunoreactive neurons in the arcuate nucleus of female C57BL/6J mice. Neuroendocrinology 61 542–551. [DOI] [PubMed] [Google Scholar]
- Molinero A, Fernandez-Perez A, Mogas A, Giralt M, Comes G, Fernandez-Gayol O, Vallejo M & Hidalgo J 2017. Role of muscle IL-6 in gender-specific metabolism in mice. PLoS One 12 e0173675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A & Clegg DJ 2016. A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes (Lond) 40 206–209. [DOI] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Venken K, Andersson N, Lindberg MK, Svensson J, Swanson C, Vanderschueren D, Oscarsson J, Gustafsson JA & Ohlsson C 2006. Dihydrotestosterone treatment results in obesity and altered lipid metabolism in orchidectomized mice. Obesity (Silver Spring) 14 662–672. [DOI] [PubMed] [Google Scholar]
- Mullican SE, Tomaru T, Gaddis CA, Peed LC, Sundaram A & Lazar MA 2013. A novel adipose-specific gene deletion model demonstrates potential pitfalls of existing methods. Mol Endocrinol 27 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KG 2005. Kisspeptins: regulators of metastasis and the hypothalamic-pituitary-gonadal axis. J Neuroendocrinol 17 519–525. [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG & Ogawa S 2007. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A 104 2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LR & Bulun SE 2001. Estrogen production and action. J Am Acad Dermatol 45 S116–124. [DOI] [PubMed] [Google Scholar]
- Nohara K, Laque A, Allard C, Munzberg H & Mauvais-Jarvis F 2014. Central mechanisms of adiposity in adult female mice with androgen excess. Obesity (Silver Spring) 22 1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Liu S, Meyers MS, Waget A, Ferron M, Karsenty G, Burcelin R & Mauvais-Jarvis F 2013a. Developmental androgen excess disrupts reproduction and energy homeostasis in adult male mice. J Endocrinol 219 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, Karsenty G, Burcelin R & Mauvais-Jarvis F 2013b. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab 304 E1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Munzberg H & Mauvais-Jarvis F 2011. Early-life exposure to testosterone programs the hypothalamic melanocortin system. Endocrinology 152 1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF & Tecott LH 1998. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med 4 1152–1156. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, Lindberg MK, Warner M, Angelin B & Gustafsson JA 2000. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochem Biophys Res Commun 278 640–645. [DOI] [PubMed] [Google Scholar]
- Okura T, Koda M, Ando F, Niino N, Ohta S & Shimokata H 2003. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. Int J Obes Relat Metab Disord 27 1020–1027. [DOI] [PubMed] [Google Scholar]
- Ophoff J, Van Proeyen K, Callewaert F, De Gendt K, De Bock K, Vanden Bosch A, Verhoeven G, Hespel P & Vanderschueren D 2009. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology 150 3558–3566. [DOI] [PubMed] [Google Scholar]
- Osterlund M, Kuiper GG, Gustafsson JA & Hurd YL 1998. Differential distribution and regulation of estrogen receptor-alpha and -beta mRNA within the female rat brain. Brain Res Mol Brain Res 54 175–180. [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, et al. 2009. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod 80 34–41. [DOI] [PubMed] [Google Scholar]
- Palmer K & Gray JM 1986. Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav 37 187–189. [DOI] [PubMed] [Google Scholar]
- Pessia E, Makino T, Bailly-Bechet M, McLysaght A & Marais GA 2012. Mammalian X chromosome inactivation evolved as a dosage-compensation mechanism for dosage-sensitive genes on the X chromosome. Proc Natl Acad Sci U S A 109 5346–5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Meza C, Navarro UV, Nestor CC, Wagner EJ, Ronnekleiv OK & Kelly MJ 2018. Estradiol Protects Proopiomelanocortin Neurons Against Insulin Resistance. Endocrinology 159 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Ronnekleiv OK & Kelly MJ 2010. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci 30 1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Ronnekleiv OK & Kelly MJ 2014. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab 19 682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. 2010. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 12 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers F & Delemarre-van de Waal HA 2011. Developmental programming of energy balance and its hypothalamic regulation. Endocr Rev 32 272–311. [DOI] [PubMed] [Google Scholar]
- Reue K. Sex differences in obesity: X chromosome dosage as a risk factor for increased food intake, adiposity and co-morbidities. Physiol Behav. 2017. [DOI] [PMC free article] [PubMed]
- Reynolds CM, Vickers MH, Harrison CJ, Segovia SA & Gray C 2015. Maternal high fat and/or salt consumption induces sex-specific inflammatory and nutrient transport in the rat placenta. Physiol Rep 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoda J, Corbier P & Roffi J 1984. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17 beta-estradiol. Endocrinology 114 1754–1760. [DOI] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ & Hevener AL 2010. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. Am J Physiol Endocrinol Metab 298 E304–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard DJ, Iwaniec UT, Evans G, Hefferan TE, Hunter JC, Waters KM, Lydon JP, O’Malley BW, Khosla S, Spelsberg TC, et al. 2008. Bone growth and turnover in progesterone receptor knockout mice. Endocrinology 149 2383–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L 2006. Ontogeny of hypothalamic-hindbrain feeding control circuits. Dev Psychobiol 48 389–396. [DOI] [PubMed] [Google Scholar]
- Rinaman L 2007. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol 28 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud M & Debonnel G 2005. Oestrogen and testosterone modulate the firing activity of dorsal raphe nucleus serotonergic neurones in both male and female rats. J Neuroendocrinol 17 179–185. [DOI] [PubMed] [Google Scholar]
- Roesch DM 2006. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87 39–44. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Perfield JW 2nd, Strissel KJ, Obin MS & Greenberg AS 2009. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology [DOI] [PMC free article] [PubMed]
- Rolf C, von Eckardstein S, Koken U & Nieschlag E 2002. Testosterone substitution of hypogonadal men prevents the age-dependent increases in body mass index, body fat and leptin seen in healthy ageing men: results of a cross-sectional study. Eur J Endocrinol 146 505–511. [DOI] [PubMed] [Google Scholar]
- Ruan HB, Dietrich MO, Liu ZW, Zimmer MR, Li MD, Singh JP, Zhang K, Yin R, Wu J, Horvath TL, et al. 2014. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Cao X, He Y & Xu Y 2015. Progress in the molecular understanding of central regulation of body weight by estrogens. Obesity (Silver Spring) 23 919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel P, Khan MA, Nag S, Inagami T & Hussain T 2013. Angiotensin AT(2) receptor contributes towards gender bias in weight gain. PLoS One 8 e48425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Torregrossa AM & Eckel LA 2011. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm Behav 60 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Wiley MD & Eckel LA 2007. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293 R2194–2201. [DOI] [PubMed] [Google Scholar]
- Sarton E, Teppema LJ, Olievier C, Nieuwenhuijs D, Matthes HW, Kieffer BL & Dahan A 2001. The involvement of the mu-opioid receptor in ketamine-induced respiratory depression and antinociception. Anesth Analg 93 1495–1500, table of contents. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H & Kato S 2003. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun 300 167–171. [DOI] [PubMed] [Google Scholar]
- Schlenker EH & Hansen SN 2006. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123 89–100. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, et al. 2004. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res 14 Suppl A S18–33. [DOI] [PubMed] [Google Scholar]
- Schwartz SM & Wade GN 1981. Effects of estradiol and progesterone on food intake, body weight, and carcass adiposity in weanling rats. Am J Physiol 240 E499–503. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. 2003. The GPR54 gene as a regulator of puberty. N Engl J Med 349 1614–1627. [DOI] [PubMed] [Google Scholar]
- Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ & Prossnitz ER 2013. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology 154 4136–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Seeley RJ & Clegg DJ 2009. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol 30 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson ER 2003. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86 225–230. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Misso M, Hewitt KN, Hill RA, Boon WC, Jones ME, Kovacic A, Zhou J & Clyne CD 2005. Estrogen--the good, the bad, and the unexpected. Endocr Rev 26 322–330. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB & Korach KS 1994. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med 331 1056–1061. [DOI] [PubMed] [Google Scholar]
- Stubbins RE, Holcomb VB, Hong J & Nunez NP 2012. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51 861–870. [DOI] [PubMed] [Google Scholar]
- Su X, Gi YJ, Chakravarti D, Chan IL, Zhang A, Xia X, Tsai KY & Flores ER 2012. TAp63 is a master transcriptional regulator of lipid and glucose metabolism. Cell Metab 16 511–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama MG & Agellon LB 2012. Sex differences in lipid metabolism and metabolic disease risk. Biochem Cell Biol 90 124–141. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C, Schwarz JM, Clayton JA, de Vries GJ & Sullivan C 2016. Evaluating sex as a biological variable in preclinical research: the devil in the details. Biol Sex Differ 7 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A, Panchenko P, Junien C & Gabory A 2015. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol 218 50–58. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF & Julius D 1995. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374 542–546. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ & Dong J 2005. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146 624–632. [DOI] [PubMed] [Google Scholar]
- Tolson KP, Garcia C, Delgado I, Marooki N & Kauffman AS 2016. Metabolism and Energy Expenditure, But Not Feeding or Glucose Tolerance, Are Impaired in Young Kiss1r KO Female Mice. Endocrinology 157 4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, Smith JT & Kauffman AS 2014. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest 124 3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth MJ, Poehlman ET, Matthews DE, Tchernof A & MacCoss MJ 2001. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab 280 E496–501. [DOI] [PubMed] [Google Scholar]
- Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, et al. 2014. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN 1975. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol 88 183–193. [DOI] [PubMed] [Google Scholar]
- Wallen WJ, Belanger MP & Wittnich C 2001. Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr 131 2351–2357. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, et al. 2008. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, He Y, Xu P, Yang Y, Saito K, Xia Y, Yan X, Hinton A Jr., Yan C, Ding H, et al. 2018. TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nat Commun 9 1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Hrabovszky E & Lechan RM 2013. Distinct glutamatergic and GABAergic subsets of hypothalamic pro-opiomelanocortin neurons revealed by in situ hybridization in male rats and mice. J Comp Neurol 521 3287–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward CJ & Emery PW 1989. Energy balance in rats given chronic hormone treatment. 2. Effects of corticosterone. Br J Nutr 61 445–452. [DOI] [PubMed] [Google Scholar]
- Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S, Harada N & Shah NM 2009. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell 139 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Ste-Marie L, Kaelin CB & Barsh GS 2007. Inactivation of signal transducer and activator of transcription 3 in proopiomelanocortin (Pomc) neurons causes decreased pomc expression, mild obesity, and defects in compensatory refeeding. Endocrinology 148 72–80. [DOI] [PubMed] [Google Scholar]
- Xu P, Cao X, He Y, Zhu L, Yang Y, Saito K, Wang C, Yan X, Hinton AO Jr., Zou F, et al. 2015. Estrogen receptor-alpha in medial amygdala neurons regulates body weight. J Clin Invest 125 2861–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, He Y, Cao X, Valencia-Torres L, Yan X, Saito K, Wang C, Yang Y, Hinton A, Jr., Zhu L, et al. 2017. Activation of Serotonin 2C Receptors in Dopamine Neurons Inhibits Binge-like Eating in Mice. Biol Psychiatry 81 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. 2008. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, et al. 2011a. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab in press. [DOI] [PMC free article] [PubMed]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, et al. 2011b. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab 14 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF & Shah NM 2014. Representing sex in the brain, one module at a time. Neuron 82 261–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Smith DL Jr., Keating KD, Allison DB & Nagy TR 2014. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring) 22 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Lin HY, Liu NC, Sparks JD, Yeh S, Fang LY, Chen L & Chang C 2013. Neuronal androgen receptor regulates insulin sensitivity via suppression of hypothalamic NF-kappaB-mediated PTP1B expression. Diabetes 62 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Lin HY, Liu NC, Wang RS, Sparks JD, Yeh S & Chang C 2008. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology 149 2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Geary N & Corwin RL 2011. Individual effects of estradiol and progesterone on food intake and body weight in ovariectomized binge rats. Physiol Behav 104 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Liu X, Mao Y, Diao F, Cui Y & Liu J 2016. Polycystic ovary syndrome patients with high BMI tend to have functional disorders of androgen excess: a prospective study. J Biomed Res 30 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Xu P, Cao X, Yang Y, Hinton AO, Jr., Xia Y, Saito K, Yan X, Zou F, Ding H, et al. 2015. The ERalpha-PI3K Cascade in Proopiomelanocortin Progenitor Neurons Regulates Feeding and Glucose Balance in Female Mice. Endocrinology 156 4474–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]


