Abstract
Background
To determine whether cancer‐associated fibroblasts (CAFs) are associated with microvessel density (MVD) and lymphatic vessel density (LVD) in lung adenocarcinoma (ADC) or are not prognostic.
Methods
Ninety‐three lung adenocarcinoma patients without adjuvant therapy between January 2010 and June 2011 were enrolled. CAFs, MVD, and LVD were identified by α‐smooth muscle actin (α‐SMA), CD34 and D2‐40 staining via immunohistochemistry. Staining intensities were assessed and quantified. For statistics, Pearson's chi‐square test, logistic regression, Kaplan‐Meier, and log‐rank tests were applied. In addition, the Cox proportional hazards model was used for multifactor analysis to predict survival.
Results
CAFs abundance in lung adenocarcinoma is associated with higher MVD and LVD. In addition, a correlation was demonstrated between MVD and LVD (P < 0.05). CAFs, MVD, and LVD are significantly correlating with age, tumor size, differentiation grade, clinical stage, and lymph node metastasis (P < 0.05), but not influenced by gender, tumor location, and smoking history. Three‐year overall survival in the CAFs‐poor group is 64.5%, which is significant higher than that in the CAFs‐rich cohort (41.9%). Further, we found that age, clinical stage, α‐SMA, CD34, D2‐40 positivity, tumor size, differentiation grade, and lymph node metastasis significantly correlate with overall survival of patients with lung adenocarcinoma. However, sex, smoking history, and tumor location have no association with 3‐year survival. The clinical stage is an independent prognostic factor in overall survival (P < 0.05).
Conclusions
The density of CAFs identified by α‐SMA staining is associated with progression and metastasis of lung adenocarcinoma and affects the patient's disease outcome.
Keywords: biomarker, cancer‐associated fibroblasts, lymphatic vessel density, microvessel density, non–small‐cell lung cancer, α‐smooth muscle actin
1. INTRODUCTION
Non–small‐cell lung cancer (NSCLC) is one of the most common malignancies with both high morbidity and mortality rates worldwide, and the fatality rate stands first on the list of cancer‐related deaths.1 Lung adenocarcinoma is one of the most common histological types in NSCLC. Due to the lack of reliable biomarkers for early diagnosis, recurrence, and metastasis, its prognosis is still poor.2 The biological characteristics of the metabolic tumor environment have important influence on proliferation, invasion, migration, adhesion, and neovascularization of the tumor. The tumor stroma thus strongly influences recurrence and formation of metastasis. Most NSCLC are characterized by fibrous connective tissue proliferation,3 and the stromal changes are accompanied by fibroblast differentiation into cancer‐associated fibroblasts (CAFs), which alter the extracellular matrix components in tumor areas and influence neovascularization,4 and consequently contribute to the growth, invasion and metastasis of tumors. Regarding molecular markers, α‐smooth muscle actin (α‐SMA), fibroblast activation protein (FAP), vimentin, etc have been previously used to identify CAFs,5 and the filamentous protein α‐SMA as the most widely accepted one.6, 7
Growth and metastasis of lung cancer depends on the formation of blood vessels.11 Angiogenesis is an important pathophysiological process of occurrence, development, and progression of the tumor. Quantitative indicators include microvascular density (MVD) and levels of vascular endothelial growth factors (VEGF) in the serum. MVD not only reflects the process of angiogenesis, but also predicts tumor growth, metastasis, and recurrence.12 CD34 is a marker of mature endothelial cells and is applied for MVD evaluation. LVD is another critical parameter for predicting disease outcome and it has been used as a major indicator for the treatment regimen. D2‐40, a sialic acid glycoprotein, binds to the mucous membrane glycoprotein of the lymphatic endothelial cells and enables distinction between lymphatic and blood vessels.13
To further understand the NSCLC subgroup and prognostic indicators associated with early onset, development, and metastasis, we analyzed the association between abundance of CAFs in lung adenocarcinoma and MVD and LVD, and correlate these parameters with patient survival. These findings may aid in developing novel therapeutic strategies to tackle this devastating disease.
2. MATERIAL AND METHODS
2.1. Patients and tissue specimens
A total of 125 formalin‐fixed and paraffin‐embedded tumor samples from patients who underwent curative surgical resection from stage I‐III lung adenocarcinoma at the Department of Thoracic Surgery in the Qingdao Municipal Hospital from January 1, 2010, to June 31, 2011, were selected. All patients had definite pathological diagnosis after surgery. Patients who received adjuvant therapy were excluded. Thirty‐two patients were lost during the follow‐up study. Ninety‐three patients were finally enrolled into this study, including 60 male patients and 33 female patients. Among these patients, 11 cases were the central‐type lung adenocarcinoma and 82 cases were peripheral lung adenocarcinoma. Clinical records of the patients, including name, gender, age, smoking history, tumor stage, differentiated degree, and the condition of the follow‐up, are fully documented according to the standard of the Union for International Cancer Control [https://www.uicc.org/resources/tnm]. Written informed consent was obtained from all patients. The study was approved by the Ethical Committee of Qingdao Municipal Hospital.
2.2. Immunohistochemistry
Five micrometer sections were stained with α‐SMA monoclonal antibody (1:100, Maixin, Shanghai) for immunohistochemical evaluation of CAFs. Microvessels were stained with CD34 (1:100, Maixin, Shanghai), and lymphatic vessels were assessed by D2‐40 staining (1:50, Zhongshanjinqiao, Beijing). Immunohistochemical staining was proceeded as follows: After deparaffinization and dehydration, sections were boiled in Tris‐EDTA buffer (10 mmol/L Tris Base, 1 mmol/L EDTA Solution, 0.05% Tween 20, pH 9.0) for antigen unmasking, followed by extensive washing with PBS. Sections were subsequently incubated with 3% H2O2 for 10 minutes, and then rinsed with PBS for three times. Sections were blocked with 2% goat serum albumin in PBS for 20 minutes and incubated with anti‐α‐SMA, anti‐D2‐40 or anti‐CD34 antibodies for 1 hour at 37°C. After three times of washing with PBS, sections were incubated with anti‐mouse or anti‐rabbit antibodies (Envision plus, Dako) for 10 minutes at 37°C. After washing with PBS, peroxidase activity was detected with diaminobenzidine under microscopy. Subsequently, the specimens were counterstained with hematoxylin and dehydrated, hyalinized and embedded. Each section was independently evaluated by two pathological experts who did not understand the clinical status of the patients.
2.3. CAF immunohistochemical scoring
α‐SMA‐positive stained tissues were graded as CAFs‐rich and CAFs‐poor groups according to CAFs staining density as previously described.14, 15 Briefly, the most densely populated staining areas known as hot spot areas were selected and evaluated under microscopy. The α‐SMA positivity was calculated as percentage of α‐SMA‐positive cell numbers/total cell count. A value ≥40% was considered high CAFs group, and <40% was defined as CAFs‐poor group.
2.4. Quantification of MVD
The evaluation method for MVD followed Wang16: Vessel hot spot regions were identified under 40× magnifications and then 400× magnification was applied for counting microvessels in three separate hot spots and the average was calculated and expressed as mean.
2.5. Quantification of LVD
LVD was evaluated as described by Weidner et al17: The lumen in ribbon‐shaped or lacuna‐shaped structures, formed by endothelial cells and positively stained by D2‐40, is identified as lymphatic vessels. Hot spot regions were selected under 40× and 100× magnification. The density of lymph‐vessels was evaluated under 400× magnification and the average numbers from five selected hot spots were calculated and expressed as mean.
2.6. Follow‐up
The follow‐up information was collected until death or disease progression. A routine protocol including detailed inquiry of medical history and radiological examination was preceded over the course of the follow‐up period.
2.7. Statistical analyses
Statistical package SPSS version 20.0 (SPSS Inc. Chicago, IL, USA) was used for data analyses. Pearson's chi‐square test and logistic regression were used for exploring the correlation between clinical data and immunohistochemical results. Kaplan‐Meier method was employed to calculate the survival rate. Difference between groups was done by the log‐rank test. Cox proportional hazards model was used to predict survival rate by multivariate analysis, and P‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. α‐SMA expression and CAFs density in lung adenocarcinoma
α‐SMA‐positive staining was mainly detected in tumor stroma, indicating involvement of CAFs in tumor pathogenesis. Out of the 93 cases, 62 were defined as high CAFs group (Figure 1A) and 31 were low CAFs expressing (Figure 1B).
Figure 1.
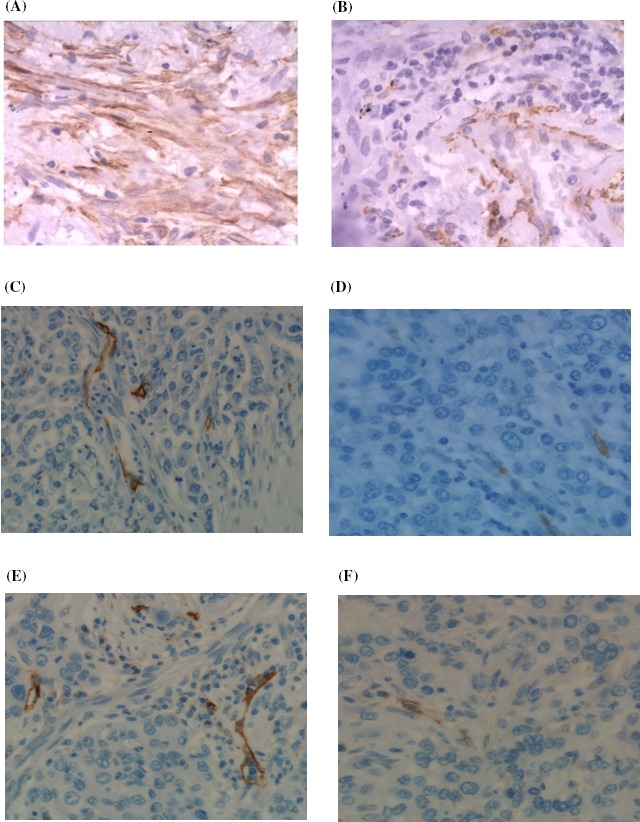
A, α‐SMA staining of lung adenocarcinoma, 400× magnification (CAFs‐rich group); B, α‐SMA staining of lung adenocarcinoma, 400× magnification (CAFs‐poor group); C, CD34 staining of lung adenocarcinoma, 400× magnification (CAFs‐rich group); D, CD34 staining of lung adenocarcinoma, 400× magnification (CAFs‐poor group); E, D2‐40 staining of lung adenocarcinoma, 400× magnification (CAFs‐rich group); F, D2‐40 staining of lung adenocarcinoma, 400× magnification (CAFs‐poor group)
3.2. CAFs density and MVD, LVD
Intratumoral microvessel density was quantified by measuring CD34‐positive staining which was mainly detected in vascular endothelial cell membrane and a lower degree in the cytoplasm. The correlation was evaluated by Pearson's chi‐squared test. MVD was significantly higher in CAFs‐rich group than in the CAFs‐poor group (Figure 1C; P = 0.02, Table 1A), indicating involvement of CAFs in angiogenesis.
Table 1.
(A) Correlation between CAFs density and MVD. (B) Correlation between CAFs density and LVD
| α‐SMA | χ 2 | P | ||
|---|---|---|---|---|
| Negative | Positive | |||
| (A) CD34 | ||||
| Negative | 24 | 33 | 5.099 | 0.020 |
| Positive | 7 | 29 | ||
| (B) D240 | ||||
| Negative | 19 | 12 | 16.355 | 0.000 |
| Positive | 12 | 50 | ||
CAFs, cancer‐associated fibroblasts; LVD, lymphatic vessel density; MVD, microvessel density.
The lymphatic vessel areas with lumen were quantified via D2‐40 staining. LVD area was significantly higher in CAFs‐rich group than in the CAFs‐poor group (Figure 1D; P < 0.001, Table 1B), suggesting a close relationship between CAFs and lymphangiogenesis.
3.3. CAFs density, MVD, LVD and clinicopathological features in lung adenocarcinoma
Correlations between CAFs abundance, MVD, LVD, and other clinicopathological characteristics were determined using Pearson's chi‐squared test and logistic regression. CAFs density, MVD, and LVD are significantly correlating with age, tumor size, degree of differentiated, clinical stages, and lymph node metastasis (P < 0.05), but they were not affected by parameters of gender, tumor location, and smoking history (P > 0.05) (Table 2).
Table 2.
CAFs, MVD, and LVD in 93 patients with lung adenocarcinoma and their correlation with clinical pathological variables
| N | CAFs | P | HR (95%CI) | MVD | P | HR (95%CI) | LVD | P | HR (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Men | 60 | 1.000 | 1.000 (0.406‐2.462) | 0.219 | 1.759 (0.714‐4.331) | 0.170 | 1.864 (0.765‐4.538) | |||
| Women | 33 | |||||||||
| Age | ||||||||||
| ≥60 | 55 | 0.007 | 1.065 (1.017‐1.114) | 0.000 | 1.138 (1.071‐1.209) | 0.013 | 1.059 (1.012‐1.107) | |||
| <60 | 38 | |||||||||
| Location | ||||||||||
| Central | 11 | 0.821 | 1.164 (0.313‐4.323) | 0.626 | 0.729 (0.205‐2.592 ) | 0.821 | 1.164 (0.313‐4.323) | |||
| Peripheral | 82 | |||||||||
| Diameter | ||||||||||
| >3 cm | 65 | 0.000 | 12.273 (4.315‐34.906) | 0.001 | 14.258 (3.124‐65.083) | 0.000 | 16.500 (5.554‐49.021) | |||
| ≤3 cm | 28 | |||||||||
| Differentiation grade | ||||||||||
| High | 18 | 0.007 | 1 | 0.001 | 1 | 0.014 | 1 | |||
| Medium | 53 | 0.048 | 3.056 (1.012‐9.226) | 0.998 | 637687425.543 (0.000‐) | 0.190 | 2.062 (0.698‐6.091) | |||
| Poor | 22 | 0.002 | 15.714 (2.772‐89.098) | 0.998 | 33924971038.902 (0.000‐) | 0.004 | 26.250 (2.877‐239.537) | |||
| TNM staging | ||||||||||
| I | 27 | 0.000 | 1 | 0.000 | 1 | 0.000 | 1 | |||
| II | 35 | 0.000 | 9.643 (3.000‐30.995) | 0.179 | 3.125 (0.593‐16.459) | 0.000 | 10.111 (3.100‐32.980) | |||
| IIIa | 19 | 0.000 | 15.238 (3.387‐68.553) | 0.000 | 46.875 (7.640‐287.595) | 0.000 | 63.00 (6.921‐573.503) | |||
| IIIb | 12 | 0.998 | 4615642473.783 (0.000‐) | 0.998 | 20193435809.473 (0.000‐) | 0.998 | 5654162030.960 (0.000‐) | |||
| Lymphatic metastasis | ||||||||||
| With | 58 | 0.005 | 3.665 (1.482‐9.066) | 0.001 | 14.258 (3.124‐65.083) | 0.001 | 4.552 (1.815‐11.419) | |||
| Without | 35 | |||||||||
| Smoking history | ||||||||||
| With | 47 | 0.558 | 0.772 (0.325‐1.834) | 0.731 | 1.158 (0.502‐2.669) | 0.306 | 0.635 (0.266‐1.516) | |||
| Without | 46 | |||||||||
CAFs, cancer‐associated fibroblasts; LVD, lymphatic vessel density; MVD, microvessel density.
3.4. CAFs density and 3‐year survival and overall survival
Three‐year survival of CAFs‐poor group was 64.5%, which is significantly higher than in CAFs‐rich group (41.9%) (Table 3). Kaplan‐Meier analyses showed that age, clinical stage, CAFs density, MVD, LVD, tumor size, degree of differentiation, and lymph node metastasis all had impact on the overall survival (OS) (P < 0.001; P < 0.001; P < 0.05; P < 0.001; P < 0.05; P < 0.001; P < 0.001; P < 0.001, respectively) (Figure 2), while gender, smoking history, and tumor location had little influence (P = 0.086; 0.096; 0.843, respectively). Cox multifactor analysis identified clinical stage as an independent prognostic factor in lung adenocarcinoma (P < 0.05).
Table 3.
Correlation between OS and CAFs proportion in 93 patients with lung adenocarcinoma
| CAFs | Overall survival | χ 2 | P | |
|---|---|---|---|---|
| Survival | Death | |||
| Poor | 60 | 33 | 9.524 | 0.002 |
| Rich | 39 | 54 | ||
CAFs, cancer‐associated fibroblasts.
Figure 2.
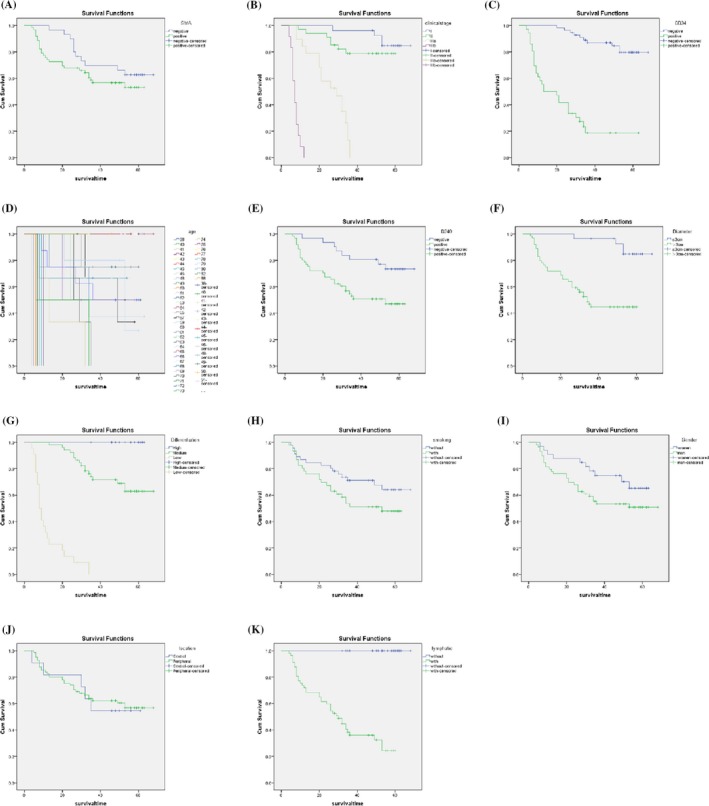
Correlation between OS and each parameter. A, Correlation between OS and α‐SMA; B, Correlation between OS and clinical stage; C, Correlation between OS and MVD; D, Correlation between OS and patient's age (y); E, Correlation between OS and LVD; F, Correlation between OS and tumor diameter; G, Correlation between OS and cancer differentiation; H, Correlation between OS and smoking; I, Correlation between OS and gender; J, Correlation between OS and tumor location; K, Correlation between OS and lymphatic metastasis
4. DISCUSSION
It is well known today that development and progression of malignant tumors rely on the tumor microenvironment. Although the clinical stage is often an independent prognostic factor in OS, the different disease outcome in patients with the same pathological stage significantly depends on the diversity of tumor behaviors. During tumor development, tumor stroma not only supplies nutrition to support tumor cell proliferation, but also offers a platform for tumor invasion and metastasis through angiogenesis and lymphangiogenesis. As a result, components in the tumor stroma may be used as therapeutic targets to reverse the malignant transformation. Specific cells in the stroma such as CAFs may be used for anti‐tumor therapy.18
Previous reports show that CAFs play a crucial role in the progression of lung adenocarcinoma; they are significantly different from normal fibroblasts. These cells express high levels of α‐SMA and show increased proliferative capacity compared to normal fibroblasts.19 These specific characteristics are often closely associated with malignancy of the primary tumor that CAFs support.20, 21 In studies of esophageal, oral cancer, and other malignant diseases, CAFs density show a prognostic value and is strongly associated with increased mortality.22 MVD and LVD are important indicators to reflect tumor metastatic activity and show close relationship with progression of lung adenocarcinoma in the current as well as in previous studies.23, 24 Empirical evidence supports that MVD and LVD are closely related to lymph node metastasis, distant metastasis, differentiation degree, and clinical stage in lung adenocarcinoma and CAF density can be considered as a novel biomarker for disease prognosis in patients with lung squamous cell carcinoma.25
Pathological evaluation showed that in 93 patients with lung adenocarcinoma, 62 cases are CAFs rich (66.7%), and 31 patients (33.3%) are CAFs poor. Pearson's correlation analysis indicated MVD and LVD were significantly higher in the CAFs‐rich patients (P < 0.05), indicating a positive association between CAFs abundance and MVD and LVD. The direct correlation between MVD and LVD (P < 0.05) further demonstrated a close relationship of angiogenesis and lymphangiogenesis. In addition, we showed that CAFs, MVD, and LVD in lung adenocarcinoma significantly correlate with tumor size, degree of differentiation, clinical stages, and lymph node metastasis (P < 0.05). These results further demonstrated that the tumor microenvironment is essential for supporting the progression of lung adenocarcinoma. The 3‐year survival rate of CAFs‐poor (64.5%) was significantly higher than from the CAFs‐rich group (41.9%). LVD is an indicator of favorable survival.26 Additionally, age, clinical stage, MVD, the degree of differentiation, and lymph node metastasis were all found to affect the OS of lung adenocarcinoma patients. These data further emphasize the complexity of the disease. Interestingly, among all analyzed factors, clinical staging was the only parameter that showed an independent prognostic factor for OS in patients with lung adenocarcinoma.
5. CONCLUSIONS
The abundance of CFA, MVD, and LVD is negatively correlated with the prognosis of patients with lung adenocarcinoma. These results demonstrate the important roles of microenvironment in the progression of lung adenocarcinoma and provide useful data to support personalized disease therapy.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Written informed consent was obtained from all patients. The study was approved by the Ethical Committee of Qingdao Municipal Hospital.
Chen L, Qin Y, Zhang T, et al. Clinical significance of cancer‐associated fibroblasts and their correlation with microvessel and lymphatic vessel density in lung adenocarcinoma. J Clin Lab Anal. 2019;33:e22832 10.1002/jcla.22832
Chen and Qin are co‐first authors.
Contributor Information
Zhe Zhang, Email: chengyeguo@outlook.com.
Chengye Guo, Email: zhezhanga@hainan.net.
REFERENCES
- 1. De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE5‐a population‐based study. Lancet Oncol. 2014;15:23‐34. [DOI] [PubMed] [Google Scholar]
- 2. Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non‐small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227‐234. [DOI] [PubMed] [Google Scholar]
- 3. Maeshima AM, Niki T, Maeshima A, Yamada T, Kondo H, Matsuno Y. Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer. 2002;95:2546‐2554. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 5. Jia CC, Wang TT, Liu W, et al. Cancer‐associated fibroblasts from hepatocellular carcinoma promote malignant cell proliferation by HGF secretion. PLoS ONE. 2013;8:e63243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen J, Li H, SundarRaj N, Wang JH. Alpha‐smooth muscle actin expression enhances cell traction force. Cell Motil Cytoskeleton. 2007;64:248‐257. [DOI] [PubMed] [Google Scholar]
- 7. Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension‐dependent recruitment of alpha‐smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawai‐Kowase K, Sato H, Oyama Y, et al. Basic fibroblast growth factor antagonizes transforming growth factor‐beta1‐induced smooth muscle gene expression through extracellular signal‐regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24:1384‐1390. [DOI] [PubMed] [Google Scholar]
- 9. Bogatkevich GS, Tourkina E, Abrams CS, Harley RA, Silver RM, Ludwicka‐Bradley A. Contractile activity and smooth muscle alpha‐actin organization in thrombin‐induced human lung myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2003;285:L334‐L343. [DOI] [PubMed] [Google Scholar]
- 10. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano‐regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349‐363. [DOI] [PubMed] [Google Scholar]
- 11. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182‐1186. [DOI] [PubMed] [Google Scholar]
- 12. Cox G, Walker RA, Andi A, Steward WP, O'Byrne KJ. Prognostic significance of platelet and microvessel counts in operable non‐small cell lung cancer. Lung Cancer. 2000;29:169‐177. [DOI] [PubMed] [Google Scholar]
- 13. Simpson RJ, Dorow DS. Cancer proteomics: from signaling networks to tumor markers. Trends Biotechnol. 2001;19:S40‐48. [DOI] [PubMed] [Google Scholar]
- 14. Vered M, Dobriyan A, Dayan D, et al. Tumor‐host histopathologic variables, stromal myofibroblasts and risk score are significantly associated with recurrent disease in tongue cancer. Cancer Sci. 2010;101:274‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bello IO, Vered M, Dayan D, et al. Cancer‐associated fibroblasts, a parameter of the tumor microenvironment, overcomes carcinoma‐associated parameters in the prognosis of patients with mobile tongue cancer. Oral Oncol. 2011;47:33‐38. [DOI] [PubMed] [Google Scholar]
- 16. Wang GW, Wang ZY, Liu XG. Comparison of CD31 and CD34 in showing microvessel density in non‐small cell lung cancer. Bengbu Yi Xue Yuan Xue Bao. 2009;34:185‐187. [Google Scholar]
- 17. Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early‐stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875‐1887. [DOI] [PubMed] [Google Scholar]
- 18. Polyak K, Haviv I, Campbell IG. Co‐evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30‐38. [DOI] [PubMed] [Google Scholar]
- 19. Sun X, Cheng G, Hao M, et al. CXCL12 / CXCR19 / CXCR19 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma‐associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002‐5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha‐smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730‐2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526‐537. [DOI] [PubMed] [Google Scholar]
- 23. Cheng Y, Wang K, Ma W, et al. Cancer‐associated fibroblasts are associated with poor prognosis in esophageal squamous cell carcinoma after surgery. Int J Clin Exp Med. 2015;8:1896‐1903. [PMC free article] [PubMed] [Google Scholar]
- 24. Mineo TC, Ambrogi V, Baldi A, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB‐IIA non‐small cell lung cancer. J Clin Pathol. 2004;57:591‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen L, Chen M, Han Z, et al. Clinical significance of FAP‐α on microvessel and lymphatic vessel density in lung squamous cell carcinoma. J Clin Pathol. 2018;71(8):721‐728. [DOI] [PubMed] [Google Scholar]
- 26. Niemiec JA, Adamczyk A, Ambicka A, et al. Prognostic role of lymphatic vessel density and lymphovascular invasion in chemotherapy‐naive and chemotherapy‐treated patients with invasive breast cancer. Am J Transl Res. 2017;9(3):1435‐1447. [PMC free article] [PubMed] [Google Scholar]


