Abstract
Introduction
The pro‐ and anti‐inflammatory cytokines play crucial role in the development and functions of placenta. Any changes in these cytokines may be associated with many pregnancy‐related disorders like preeclampsia. Therefore, the present study is aimed to study the expression of pro‐inflammatory (TNF‐α, IL‐6) and anti‐inflammatory (IL‐4, IL‐10) cytokines in placenta and serum of preeclamptic pregnant women.
Material and Methods
For this study, a total of 194 cases of preeclamptic and control cases were enrolled in two Groups as per the gestational age that is, Group I (28‐36 weeks) and II (37 weeks onwards). The number of samples was 55 in Group I and 139 in Group II. The immunohistochemistry (IHC) and enzyme‐linked immunosorbent assay (ELISA) were conducted on placenta and serum of both preeclamptic and normal samples, respectively. IHC results were revalidated by reverse transcriptase PCR (RT‐PCR).
Results
Both Groups (I, II) of preeclampsia showed amended levels of pro‐ and anti‐inflammatory cytokines in placental tissues and serum samples. The levels of TNF‐α and IL‐6 were significantly increased in preeclamptic cases (P = 0.0001, P = 0.0001) while the IL‐4 and IL‐10 were downregulated (P = 0.0001, P = 0.0001) in comparison to control. In addition, a negative correlation was also observed between the two in preeclampsia (P = 0.0001).
Conclusion
The balanced ratio of pro‐ and anti‐inflammatory cytokines is essential to regulate the maternal inflammation system throughout pregnancy. Therefore, the gradual cytokine profiling of the pregnant women may be useful for the management of preeclampsia.
Keywords: cytokines, IL‐10, IL‐4, IL‐6, placenta, preeclampsia, serum, TNF‐α
1. INTRODUCTION
Preeclampsia is considered as life‐threatening pregnancy disorder. It accounts for 11.71% of total pregnancies in India (Federation of Obstetrics and Gynaecological Society of India, FOGSI, 2010 survey).1 Although, a voluminous research had been conducted on preeclampsia, its exact etiology and pathology is still elusive. It is thought that several signaling pathways and immunological modulators like cytokines are responsible for the development as well as progression of this pathological condition. Several evidences suggest that cytokines play a crucial role in ovulation, implantation, placentation, and parturition during pregnancy.2 They are the key mediators of several immunological cell signaling pathways. They are synthesized by both T helper 1 (Th1)‐type and T helper 2 (Th2)‐type immunity cells and exert both positive and negative effects in pregnancy.3 They help in placental invasion, proliferation, and angiogenesis4, 5 and can be classified as pro‐ and anti‐inflammatory cytokines. Both types of cytokines are secreted by immune as well as non‐immune cells like stromal and trophoblastic cells of the placenta, maternal deciduas, endothelial cells of mother, and fetus.6 Their regulatory synthesis is required for various intracellular interactions that are crucial for the normal functioning of maternal and fetal immune system. Any modulation in the amalgation of these may adversely affect the functions of the immune competent cells. In addition, they are also responsible for the changes in the homeostasis of immune system and its functional disruption. The attenuation in the cytokine profile has been observed in many placental disorders like hypertension, preeclampsia, and eclampsia.7, 8 Several researchers had noticed the imbalanced concentration of pro‐ and anti‐inflammatory cytokines in preeclamptic placenta resulting enhanced trophoblastic apoptosis, intrauterine growth retardation or even may lead to preterm delivery.9, 10 Moreover, the exemplified activation of pro‐inflammatory cytokines in preeclamptic pregnant women leads to the development of systemic and local level inflammatory responses.11 Substantial evidences showed the elevated levels of pro‐inflammatory cytokines in the blood of preeclamptic women.12 Despite of these investigations, the data available on these cytokines are controversial and their scrupulous role in the etiology of preeclampsia is not clear. Moreover, it is not clear whether the individual cytokine concentration or their ratio is responsible for the pathophysiology of preeclampsia. Therefore, the aim of the present study was to examine the expression of pro‐inflammatory (IL‐6, TNF‐α) and anti‐inflammatory (IL‐4, IL‐10) cytokines in preeclamptic placental tissues as well as in the serum of preeclamptic pregnant women from 28 weeks of gestation till term in the Indian population. In addition, attempts had been made to find an association among them.
2. MATERIAL AND METHODOLOGY
2.1. Sample
For the present study, placental tissue and serum samples of preeclamptic as well as normal pregnant women were collected from the Department of Obstetrics and Gynaecology, Safdarjung Hospital, New Delhi, India. Ethical clearance from the institute and written informed consent from the patients were taken prior to collection. The inclusion criteria for the preeclamptic patients were hypertension (systolic BP > 140 mm Hg & diastolic BP > 90 mm Hg), proteinuria (>0.3 g/d), and edema after 20th week of gestation. The criteria for inclusion of controls were not having any history of pregnancy‐related complications, diabetes or any other chronic medical illness, vaginal bleeding throughout pregnancy along with no evidence of congenital abnormalities, tuberculosis and not having habits like tobacco, alcohol, and smoking etc A total of 194 cases of preeclampsia and control were included in this study. The samples were then divided into two Groups that is, Group I (28‐36 weeks) and II (37 weeks onwards) according to the gestational age. There were 55 placental tissue and serum samples in Group I of preeclampsia and control. The number of samples of preeclampsia and normal (placenta and serum) in Group II was 139 in total.
2.2. Immunohistochemistry
The placental tissues of both Groups were fixed in 10% buffered formalin and embedded in paraffin. A tissue sections of 5µ thickness was processed for immunohistochemistry of pro‐inflammatory (IL‐6, TNF‐α) and anti‐inflammatory (IL‐4, IL‐10) cytokines. For the antigen retrieval, Tris EDTA (pH‐9.0) buffer was used. The sections were incubated overnight in humid chamber at 4°C with primary antibodies IL‐6 (dilution 1:200, rabbit polyclonal, Abcam Inc, Cambridge, UK), TNF‐α (dilution 1:150, rabbit polyclonal, Abcam Inc), IL‐4 (dilution 1:200, rabbit polyclonal, Abcam Inc), and IL‐10 (dilution 1:150, rabbit polyclonal, Abcam Inc). Polymer based Envision plus Kit (Dako Cytomation, Glostrup, Denmark) was used for secondary antibody and chromogenic visualization reaction. For the negative control, primary antibody was replaced with immunoglobulin G, isotype‐specific.
2.3. RT‐PCR
Total isolation of RNA was done by using Aurum TM‐Total RNA mini kit (Cat. No. 732‐6820, Bio‐Rad Laboratories, Inc, Hercules, CA, USA) as per the instructions of the manufacturer. It was then quantified by absorbance using Nanodrop spectrophotometer, 1000. The cDNA was then amplified by reverse transcription of 2 μg total RNA in the cocktail of MMLV (Moloney Murine Leukemia Virus, MBI Fermentas, Life Sciences) reverse transcriptase by using gene‐specific primers TNFα (414 bp: forward 5′GTGACAAGCCTGTAGCCCA3′; reverse 5′ACTCG GCAAAGT CGAGATAG3′); IL‐6 (277 bp: forward 5′ATGC AATAA CCACCC CT3′; reverse 5′AGTGTCCTAACGCTCATAC3′); IL‐10 (317 bp: forward 5′ AGGCTACGGCGCTGTCATC3′ and reverse 5′GGCATTCTTCAC CTGCTCCA3′); IL‐4 (571 bp: forward 5′CAAGCAGCTGATCCGATTCC3′ and reverse 5′GGAATTCAAGCCCGCCA3′); β‐actin(452 bp: forward 5′CGTACCACTGGCATCGTGAT3′; reverse 5′GTGTTGGCGTACAGGTCTTTG3′) was used as a control. The PCR reaction for each primer set combination consist of 10X Reverse transcriptase buffer (2 μL), 10 mmol/L oligo (dT; 0.6 μL), 7.5 mmol/L forward and reverse primers (1.2 μL) Taq polymerase (0.2 μL), Distilled water (11.8 mL), Reverse‐transcribed cDNA (3 μL).The PCR products were visualized with Chemi Imager IS‐4400 (Alpha Innotech Corp., CA, USA).
2.4. ELISA
The serum samples of both the Group I (N = 55) and Group II (N = 139) were quantitatively analyzed by sandwich enzyme‐linked immunosorbent assay for pro‐inflammatory (IL‐6, TNF‐α) and anti‐inflammatory (IL‐4, IL‐10) cytokines. The protocol of ELISA kits (R&D Systems, Inc) was followed for the quantikine analysis of IL‐6, TNF‐α, IL‐4, IL‐10 (catalog no. D6050, DTA00C, D4050, and D1000B, respectively). The minimum detectable dose for IL‐6, TNF‐α, IL‐4, IL‐10 was typically <0.70 pg/mL, 0.5‐5.5 pg/mL, 10 pg/mL, and 3.9 pg/mL, respectively.
2.5. Scoring of immunohistochemical staining
The immunostained slides of both preeclampsia and control were arbitrarily chosen and blindly investigated by two observers. The slides were scored by following the criteria given by Rath et al13 as H‐score = P(S + 1), where H represents H‐score which is a combination of P (aggregate percentage of stained cells) and S (intensity of cell). The intensity of the stained cells was categorized as “0”: negative; “1”: mild; “2” moderate; and “3”: intense staining.
2.6. Statistical analysis
The SPSS 21.0 software (SPSS Inc, Chicago, IL, USA) was used for the statistical analysis. Student t test (2 tailed) was used to determine the significance of immunohistochemical and ELISA analysis. The data are represented as mean ± standard deviation (mean ± SD). The P‐value <0.05 was considered significant. The Pearson correlation test (2‐tailed) was performed to find the association among the pro‐ and anti‐inflammatory cytokines. The P‐value <0.01 deliberate the significance of results. RT‐PCR results were quantified by using ImageJ software (National Institute of Health, Bethesda, MA, USA).
Further, MedCalc statistical software 15 2.2 (Medcalc softwares., Ostend, Belgium) was used to analyze the ELISA results by receiver operating characteristic (ROC) analysis to find out the significant biomarker among the above cytokines.
3. RESULTS
3.1. Immunohistochemistry
The pro‐inflammatory (IL‐6, TNF‐α) and anti‐inflammatory (IL‐4, IL‐10) cytokines were localized in the cytoplasm of the syntiocytotrophoblast of placental tissues (Figure 1). The intensity of all the proteins was analyzed by calculating H‐Score for each Group (I, II; Table 1).
Figure 1.
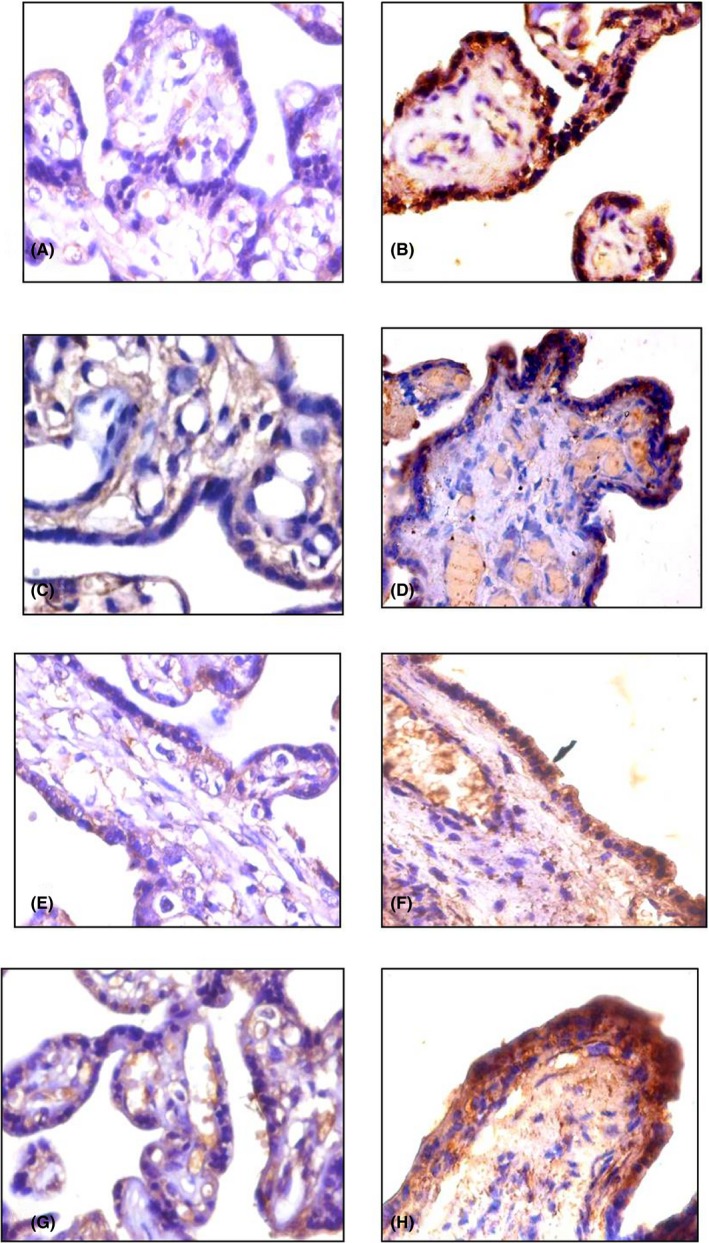
Immunohistochemical expression of pro‐inflammatory cytokines (Magnification: 400×). IL‐6: A, C control placenta (35 wks, 38 wks) showing mild cytoplasmic expression in syncytiotrophoblast (ST); B, D, preeclamptic placenta (32 wks, 38 wks) showing moderate cytoplasmic expression in ST. TNF‐α: E, G control placenta (35 wks, 38 wks) showing mild cytoplasmic expression in ST; F, H preeclamptic placenta (35 wks, 38 wks) showing moderate cytoplasmic expression in ST
Table 1.
H‐Score assessment of IL‐6, TNF‐α, IL‐4, and IL‐10 in placental tissue of preeclampsia and control in Group I and II (mean ± standard deviation)
| Study Group | IL‐6 | TNF‐α | IL‐4 | IL‐10 | ||||
|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group I | Group II | Group I | Group II | Group I | Group II | |
| Control (cytoplasmic) | 199.75 ± 2.65 | 193.88 ± 8.11 | 159.55 ± 6.03 | 189.31 ± 3.13 | 367.13 ± 1.57 | 377.21 ± 7.11 | 299.57 ± 2.31 | 313.17 ± 3.45 |
| Preeclampsia (cytoplasmic) | 321.13 ± 5.78 | 337.17 ± 3.09 | 333.18 ± 4.01 | 309.08 ± 5.43 | 159.45 ± 2.41 | 171.13 ± 4.79 | 169.11 ± 6.21 | 177.87 ± 7.16 |
| P value | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* |
Student t test (2‐ tailed). Data are represented as mean ± standard deviation (SD).
* P < 0.05 is considered significant. No. of patients in Group I: 55, Group II: 139.
The expression of IL‐6 was increased in both Groups I & II of preeclamptic cases (321.13 ± 5.78; 337.17 ± 3.09) as compared to the normal (199.75 ± 2.65; 193.88 ± 8.11; Figure 1A‐D). Similarly, the levels of TNF‐α were upregulated in the preeclamptic Groups I & II (333.18 ± 4.01; 309.08 ± 5.43) while in the control Groups (159.55 ± 6.03; 189.31 ± 7.13), the expression was lower (Figure 1E‐H).
The anti‐inflammatory cytokines IL‐4 and IL‐10 were significantly decreased in both Groups of preeclamptic placenta than that of the control Groups. The cytoplasmic expression of IL‐4 was lower in preeclampsia (159.45 ± 2.41; 171.13 ± 4.79) and it was higher in the control placenta (367.13 ± 1.57; 377.21 ± 7.11; Figure 2A‐D). The levels of IL‐10 were also found to be decreased in the preeclamptic cases (169.11 ± 6.21; 177.87 ± 7.16) than the control (299.57 ± 2.31; 313.17 ± 3.45; Figure 2E‐H). These results suggest the improper expression prevailing in preeclampsia may be responsible for the pathophysiology of preeclampsia.
Figure 2.
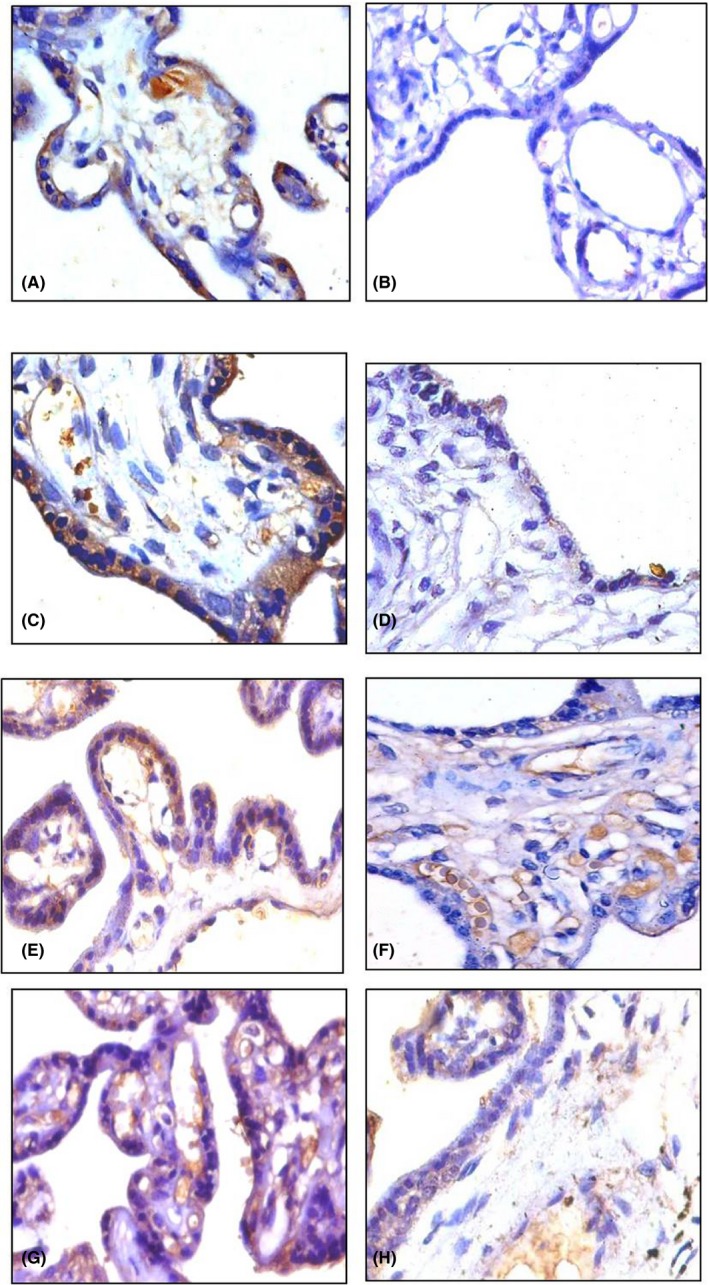
Immunohistochemical expression of anti‐inflammatory cytokines (Magnification: 400×). IL‐4: A, C control placenta (35 wks, 38 wks) showing moderate cytoplasmic expression in ST; B, D, preeclamptic placenta (35 wks, 38 wks) showing mild cytoplasmic expression in ST. IL‐10: E, G, control placenta (35 wks, 38 wks) showing moderate cytoplasmic expression in ST; F, H preeclamptic placenta (32 wks, 38 wks) showing mild cytoplasmic expression in ST
3.2. RT‐PCR
RT‐PCR results were in concordance with IHC findings. The band intensity of IL‐6 and TNF‐α was increased in preeclamptic cases in comparison to control while IL‐4 and IL‐10 were downregulated in preeclampsia (Figure 3). The bands were quantified, and results are mentioned in Table 2.
Figure 3.
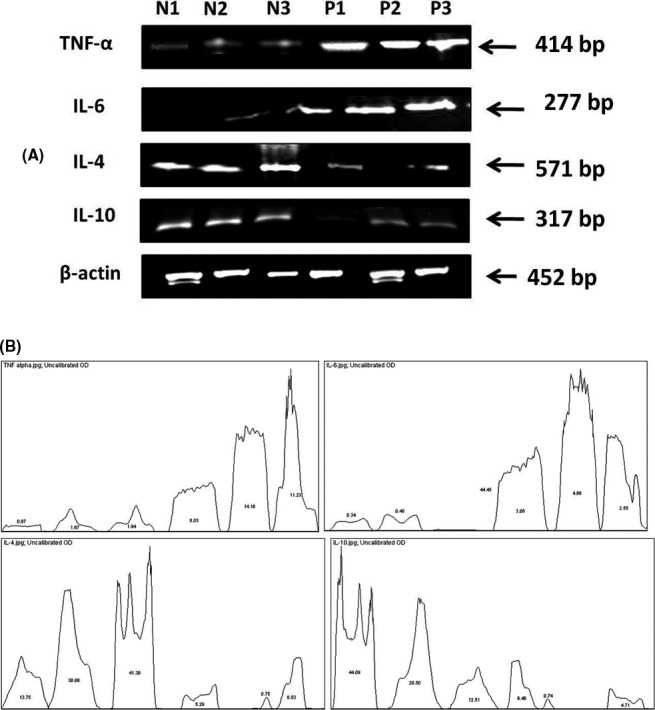
Reverse transcriptase PCR analysis of the preeclamptic (P) and normal (N) placental tissues on representative samples. A, mRNA transcripts of all proteins where N1, P1 represents Group I samples and N2, N3, P2, P3 represents Group II samples. β‐actin was used as control. B, Quantification of the mRNA transcripts of IL‐6, TNF‐α, IL‐4, IL‐10 where the values in the curve represents the percentage of the samples
Table 2.
Quantification of mRNA transcripts of IL‐6, TNF‐α, IL‐4, and IL‐10 in normal and preeclamptic cases in Group I and II
| Samples | TNF‐α | IL‐6 | IL‐4 | IL‐10 | ||||
|---|---|---|---|---|---|---|---|---|
| Area | Percentage | Area | Percentage | Area | Percentage | Area | Percentage | |
| N1 | 834.799 | 0.865 | 1340.991 | 0.337 | 5140.225 | 13.749 | 14292.23 | 44.086 |
| N2 | 1615.255 | 1.675 | 1910.669 | 0.48 | 11245.59 | 30.079 | 9238.296 | 28.496 |
| N3 | 1874.497 | 1.943 | 0001.11 | 0.1 | 15441.93 | 41.303 | 4056.104 | 12.511 |
| P1 | 7743.64 | 8.028 | 12163.76 | 3.054 | 1978.305 | 5.291 | 3067.941 | 9.463 |
| P2 | 13655.23 | 14.157 | 18632.52 | 4.678 | 280.263 | 0.75 | 239.263 | 0.738 |
| P3 | 10835.23 | 11.233 | 10145.52 | 2.547 | 3300.477 | 8.828 | 1525.477 | 4.705 |
N1, P1 represents group I samples and N2, N3, P2, P3 represents group II samples.
3.3. ELISA
The serum concentration of IL‐6 in Group I (mean ± SD = 22.68 pg/mL ± 0.27) and Group II of preeclamptic women (mean ± SD = 26.03 pg/mL ± 0.71) was significantly higher than Group I (mean ± SD = 17.07 pg/mL ± 0.44) and Group II (mean ± SD = 16.12 pg/mL ± 0.33) of control ones (Table 3, P = 0.0001). Hence, the concentration of IL‐6 increases in preeclamptic maternal serum throughout gestation. The serum TNF‐α was significantly upregulated in Group I (mean ± SD = 20.16 pg/mL ± 0.48) and Group II (mean ± SD = 27.62 pg/mL ± 0.64) of preeclampsia as compared with Group I and Group II of control (mean ± SD = 16.04 pg/mL ± 0.69 & 15.30 pg/mL ± 0.61, respectively; Table 3, P = 0.0001). Thus, the serum concentration of TNF‐α kept on increasing until the end of pregnancy during preeclampsia.
Table 3.
The serum concentration (pg/mL) of IL‐6, TNF‐α, IL‐4, and IL‐10 in normal and preeclamptic cases in Group I and II (mean ± standard deviation)
| Study Group | IL‐6 | TNF‐α | IL‐4 | IL‐10 | ||||
|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group I | Group II | Group I | Group II | Group I | Group II | |
| Control | 17.07 ± 0.44 | 16.12 ± 0.33 | 16.04 ± 0.69 | 15.30 ± 0.61 | 25.69 ± 0.11 | 29.25 ± 0.34 | 13.40 ± 0.94 | 19.83 ± 0.64 |
| Preeclampsia | 22.68 ± 0.27 | 26.03 ± 0.71 | 20.16 ± 0.48 | 27.62 ± 0.64 | 18.21 ± 0.05 | 12.77 ± 0.81 | 11.26 ± 0.80 | 7.66 ± 0.74 |
| p value | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* | P < 0.0001* |
Student t test (2‐ tailed). Data are represented as mean ± standard deviation (SD).
* P < 0.05 is considered significant. No. of patients in Group I: 55, Group II: 139.
In preeclamptic Group, the mean serum concentration of IL‐4 was decreased in Group I (mean ± SD = 18.21 pg/mL ± 0.05) and Group II (mean ± SD = 12.77 pg/mL ± 0.81). While in uncomplicated controls, the IL‐4 concentration of Group I (mean ± SD = 25.69 pg/mL ± 0.11) and II (mean ± SD = 29.25 pg/mL ± 0.34) were lesser than the preeclamptic cases (Table 3, P = 0.0001). Therefore, as the pregnancy precedes the IL‐4 concentration decreases in preeclamptic cases. Similarly, the levels of Group I and Group II preeclamptic cases showed a down regulation (mean ± SD = 11.26 pg/mL ± 0.80 & 7.66 pg/mL ± 0.74) in comparison to the control Group I and II (mean ± SD = 13.40 pg/mL ± 0.94 & 19.83 pg/mL ± 0.64; Table 3, P = 0.0001).
Receiver Operating Characteristic curve analysis revealed that IL‐6 in preeclampsia in comparison of control had specificity of 76.6%, 96.25%; sensitivity of 98.5%, 83.7% with area under curve of 0.901, 0.959, respectively, in both the Groups I and II (Table 4, Figure 4,A,B). Both Groups of TNF‐α showed specificity of 66.6%, 68.75%; sensitivity of 92.5%, 87.5% with area under curve of 0.858, 0.925, respectively, in preeclampsia as compared with control (Table 4, Figure 4C,D). While both preeclamptic Group of IL‐4 with respect to control has specificity of 82.5%, 87.5%; sensitivity of 86.25%, 88.61% with area under curve of 0.955, 0.911, respectively (Table 4, Figure 4E,F). The Group I and II of IL‐10 showed specificity of 83.75%, 80.2%; sensitivity of 88.75%, 82.75% with area under curve of 0.925, 0.881, respectively, in preeclampsia than that of control (Table 4, Figure 4G,H).
Table 4.
ROC test performance of serum IL‐6, TNF‐α, IL‐4, and IL‐10 in normal and preeclamptic cases in Group I and II
| ROC parameter | IL‐6 | TNF‐α | IL‐4 | IL‐10 | ||||
|---|---|---|---|---|---|---|---|---|
| Group I | Group II | Group I | Group II | Group I | Group II | Group I | Group II | |
| Sensitivity (%) | 98.5 | 83.7 | 92.5 | 87.5 | 86.25 | 88.61 | 88.75 | 82.75 |
| Specificity (%) | 76.6 | 96.25 | 66.6 | 68.75 | 82.5 | 87.5 | 83.75 | 80.2 |
| Area under curve (AUC) | 0.901 | 0.959 | 0.858 | 0.925 | 0.955 | 0.911 | 0.925 | 0.881 |
| Cut‐off value | 18.27 | 19.32 | 18.25 | 20.32 | 9.17 | 9.25 | 9.99 | 10.45 |
| Mean cut‐off value | 18.79 | 19.28 | 9.02 | 10.22 | ||||
No. of patients in Group I: 55, Group II: 139.
Figure 4.
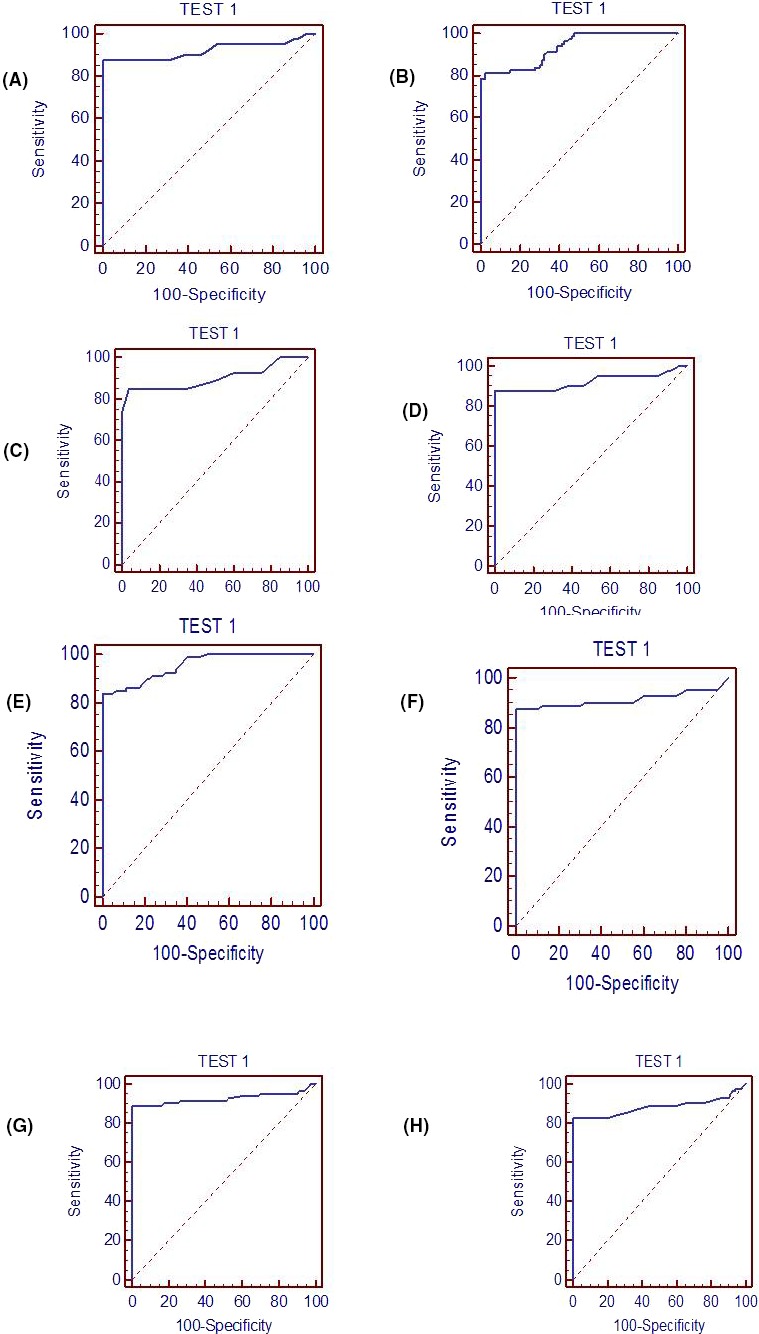
Receiver operating characteristics (ROC) curve showing the expression of serum IL‐6, TNF‐α, IL‐4, IL‐10 to differentiate preeclamptic Group from control. A, B, Group I and Group II of IL‐6; C, D, Group I and Group II of TNF‐α; E, F, Group I and Group II of IL‐4; G, H, Group I and Group II of IL‐10
The mean cut‐off value for IL‐6, TNF‐α, IL‐4, and IL‐10 was 18.79, 19.28, 9.02, and 10.22 pg/mL in (Table 4). On the basis of cut‐off values, it can be predicted that these proteins have the ability to distinguish the normal pregnant women from the preeclamptic ones.
3.4. Inter‐protein correlation
In preeclamptic placental tissues, Group I and II of IL‐6 showed a negative correlation with IL‐4 and IL‐10 (r = −0.793, P = 0.0001, r = −0.532, P = 0.0001 and r = −0.597, P = 0.0001; r = −0.778, P = 0.0001, respectively) and a positive correlation with TNF‐α (r = 0.654, P = 0.0001; r = 0.599, P = 0.0001). In addition, both Groups of TNF‐α depicted a negative association with IL‐4 and IL‐10 (r = −0.543, P = 0.0001, r = −0.687, P = 0.0001 and r = −0.717, P = 0.0001; r = −0.445, P = 0.0001, respectively). However, IL‐4 and IL‐10 were positively correlated with each other in both the Groups (r = 0.846, P = 0.0001; r = 0.527, P = 0.0001; Table S1).
In serum samples of preeclamptic mother, Group I, II of IL‐6 were directly related to TNF‐α (r = 0.711, P = 0.0001; r = 0.562, P = 0.0001) while it was inversely correlated with both Groups of IL‐4 and IL‐10 (r = −0.576, P = 0.0001, r = −0.411, P = 0.0001 and r = −0.669, P = 0.0001; r = −0.537, P = 0.0001, respectively). Similarly, TNF‐α was also negatively related with both Groups I of IL‐4 and IL‐10 (r = −0.519, P = 0.0001, r = −0.649, P = 0.0001 and r = −0.645, P = 0.0001; r = −0.584, P = 0.0001, respectively). While the IL‐4 and IL‐10 were directly related to each other Group I and II (r = 0.724, P = 0.0001; r = 0.865, P = 0.0001; Table S2).
Thus, our findings showed that throughout the pregnancy, these cytokines are intercorrelated with each other in both the placenta and serum of preeclamptic mother.
4. DISCUSSION
The pro‐inflammatory cytokines (IL‐6, TNFα) are important mediators of maternal immune system and are secreted in excess by maternal immune cells in preeclampsia.14 The IL‐6 and TNF‐α cytokine affects the functioning of endothelial cells by increasing the vascular permeability and induces apoptosis of the trophoblastic cells.15, 16 They activate as well as damage the endothelial cells to intricate the maternal inflammatory responses. These are also responsible for the pathophysiological characteristics of preeclampsia.17 Several researchers have proved that IL‐6 and TNF‐α have the capability to induce preeclamptic symptoms in pregnant rats or baboons via activation of endothelium18, 19 by activating the endothelin and renin‐angiotensin system.20, 21 The synthesis of these cytokines is increased in preeclampsia and amends the levels of anti‐inflammatory cytokines.22, 23, 24 In the present study, the protein and mRNA levels of IL‐6 and TNF‐α were found to be significantly increased in the preeclamptic placental tissues than that of the control in Group I and II (P = 0.0001, P = 0.0001). In addition, our results also revealed that the levels of the cytokines increases from Group I to Group II and reaches maximum values. The ELISA analysis on the preeclamptic maternal serum showed that there was also a significant upregulation of these cytokines from Group I to II. Thus, the levels of pro‐inflammatory cytokines kept on increasing from the 28 weeks of gestation till term in both placenta and serum of preeclamptic mother.
The anti‐inflammatory cytokines (IL‐4, IL‐10) are crucial for the functioning of T helper cell 2 (Th2) and regulatory T cells (Treg)25, 26 for the successful progression of pregnancy. Any modulation in their level may affect the functioning of several immunological and apoptotic pathways leading to pregnancy‐associated syndromes like preeclampsia.27 Several experiments on mice had reported that the scarcity of IL‐10 leads to inflammation.28, 29 Hanna et al30 examined that the levels of IL‐10 were increased in the first and second trimester but decreases in the third trimester of normal pregnancy. But, in this study, the intensity of IL‐4 and IL‐10 was found to be decreased in both Groups of preeclamptic placental tissues while upregulated in control ones. In the maternal serum samples, their levels were also found to be decreased in preeclampsia than that of the control. From these observations, it may be presumed that in preeclampsia, IL‐10 and IL‐4 are not able to exert their regulatory effects on pro‐inflammatory cytokines resulting aggravated inflammatory responses.
Several researchers had reported that the abnormal balance of these cytokines may be associated with the disruption of vascular system and preeclampsia.31, 32, 33 In our investigations, the pro‐ and anti‐inflammatory cytokines were strongly negatively correlated with each other in both placenta and serum of preeclamptic women in samples of the enrolled Groups I and II. There was a positive correlation between IL‐4 and IL‐10 as well as in between TNF‐α and IL‐6 in preeclampsia samples. The increased ratio of pro‐ to anti‐inflammatory cytokines in preeclampsia, observed in this study, may suggest that decreased levels of IL‐4 and IL‐10 will accelerate the production of pro‐inflammatory cytokines and exacerbate a Th‐2 cytokine dominant stage resulting excessive inflammation. Therefore, a balance between the two is important to curtail the maternal inflammatory system after 28 weeks of pregnancy.
Thus, the modulation in individual cytokine level along with their amended ratio may be responsible for the progression of pathophysiological characteristics of preeclampsia in later phases of pregnancy. From the present study, it may be concluded that the intensity of these inflammatory cytokines continuously modulates in both placenta and serum of preeclamptic mother immediately after the onset of preeclampsia. Their imbalanced levels cause activation of associated cellular signaling pathways like Toll‐like receptor, NF‐κB.34, 35, 36, 37 The downstream targets of these pathways may further affect the placental immune‐tolerance by impacting a negative effect on the placentation process, secretion of trophoblastic microvesicles, and endothelial cells disruption. This in turn, results in exemplified systemic inflammation leading to several adverse pregnancy outcomes like preeclampsia.
Hence, these studied cytokines can be used as biomarkers for the prediction and better clinical management of preeclampsia in the initial stages. Integrative studies revealing the molecular mechanism responsible for cellular communication between cytokines and signaling pathways are important to determine their exact effect on inflammation process. The new therapeutic strategies targeting the pool of these pro‐ and anti‐inflammatory cytokines may be designed for the treatment of this disorder. Since all the cytokines function in a spatiotemporal manner, a large cohort study including several immune system cytokines may be warranted for better understanding of the immunological etiology and pathophysiology of preeclampsia.
Supporting information
ACKNOWLEDGMENT
We are grateful to Indian Council of medical research (ICMR) for providing the grant. We also thank the staff of Department of Anatomy, VMMC & SJH for their kind support.
Aggarwal R, Jain AK, Mittal P, Kohli M, Jawanjal P, Rath G. Association of pro‐ and anti‐inflammatory cytokines in preeclampsia. J Clin Lab Anal. 2019;33:e22834 10.1002/jcla.22834
Funding information
Senior Research Fellowship from Indian council of medical research (ICMR), Ansari Nagar, New Delhi‐110029, India.
REFERENCES
- 1. Konar H, Chakraborty AB. Maternal mortality: a FOGSI study (based on institutional data). J Obstet Gynae India. 2012;63(2):88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bowen JM, Chamley L, Mitchell MD, Keelan JA. Cytokines of the placenta and extra‐placental membranes: biosynthesis, secretion and roles in establishment of pregnancy in women. Placenta. 2002;23(4):239‐256. [DOI] [PubMed] [Google Scholar]
- 3. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal‐fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14(7):353‐356. [DOI] [PubMed] [Google Scholar]
- 4. Sargent IL, Borzychowski AM, Redman CW. Immunoregulation in normal pregnancy and pre‐eclampsia: an overview. Reprod Biomed Online. 2006;13:680‐686. [DOI] [PubMed] [Google Scholar]
- 5. Keelan JA, Mitchell MD. Placental cytokines and preeclampsia. Front Biosci. 2007;12:2706‐2727. [DOI] [PubMed] [Google Scholar]
- 6. Jones CA, Finlay‐Jones JJ, Hart PH. Type‐1and type‐2 cytokines in human late‐gestation decidual tissue. Biol Reprod. 1997;57(2):303‐311. [DOI] [PubMed] [Google Scholar]
- 7. Steinborn A, Von Gall C, Hildenbrand R, Stutte HJ, Kaufmann M. Identification of placental cytokine‐producing cells in term and preterm labor. Obstet Gynecol. 1998;91:329‐335. [DOI] [PubMed] [Google Scholar]
- 8. Jokhi PP, King A, Loke YW. Cytokine production and cytokine receptor expression by cells of the human first trimester placental uterine interface. Cytokine. 1997;9:126‐137. [DOI] [PubMed] [Google Scholar]
- 9. Raghupathy R, Al‐Azemi M, Azizieh F. Intrauterine growth restriction: cytokine profiles of trophoblast antigen‐stimulated maternal lymphocytes. Clin Dev Immunol. 2012;2012:1‐10. Article ID 734865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raghupathy R. Cytokines as key players in the pathophysiology of preeclampsia. Med Princ Pract. 2013;22(Suppl. 1):8‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Redman CW, Sargent IL. Pre‐eclampsia, the placenta and the maternal systemic inflammatory response–a review. Placenta. 2003;24(Suppl. A):S21‐S27. [DOI] [PubMed] [Google Scholar]
- 12. Mosimann B, Wagner M, Poon LC, Bansal AS, Nicolaides KH. Maternal serum cytokines at 30‐33 weeks in the prediction of preeclampsia. Prenat Diagn. 2013;33(9):823‐830. [DOI] [PubMed] [Google Scholar]
- 13. Rath G, Aggarwal R, Jawanjal P, Tripathi R, Batra A. HIF‐1 alpha and placental growth factor in pregnancies complicated with preeclampsia: a qualitative and quantitative analysis. J Clin Lab Anal. 2014;30(1):75‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leibowitz A, Schiffrin EL. Immune mechanisms in hypertension. Curr Hypertens Rep. 2011;13:465‐472. [DOI] [PubMed] [Google Scholar]
- 15. Chen LM, Liu B, Zhao HB, Stone P, Chen Q, Chamley L. IL‐6, TNF alpha and TGF beta promote nonapoptotic trophoblast deportation and subsequently causes endothelial cell activation. Placenta. 2010;31:75‐80. [DOI] [PubMed] [Google Scholar]
- 16. Kharfi A, Giguere Y, Sapin V, Massé J, Dastugue B, Forest JC. Trophoblastic remodeling in normal and preeclamptic pregnancies: implication of cytokines. Clin Biochem. 2003;36:323‐331. [DOI] [PubMed] [Google Scholar]
- 17. Granger JP. Inflammatory cytokines, vascular function and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286:R989‐R990. [DOI] [PubMed] [Google Scholar]
- 18. Tinsley JH, Chiasson VL, South S, Mahajan A, Mitchell BM. Immunosuppression improves blood pressure and endothelial function in a rat model of pregnancy‐induced hypertension. Am J Hypertens. 2009;22:1107‐1114. [DOI] [PubMed] [Google Scholar]
- 19. Sibai BM. Initiators of severe pre‐eclampsia. Semin Perinatol. 2009;33:196‐205. [DOI] [PubMed] [Google Scholar]
- 20. LaMarca BD, Ryan MJ, Gilbert JS, Ryan MJ, Murphy SR, Granger JP. Inflammatory cytokines in the pathophysiology of hypertension during pre‐eclampsia. Curr Hypertens Rep. 2007;9:480‐485. [DOI] [PubMed] [Google Scholar]
- 21. George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24:964‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Madazli R, Aydin S, Uludag S, Vildan O, Tolun N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82(9):797‐802. [DOI] [PubMed] [Google Scholar]
- 23. Szarka A, Rigo J Jr, Lazar L, Beko G, Molvarec A. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC immunol. 2010;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jonsson Y, Ruber M, Matthiesen L, et al. Cytokine mapping of sera from women with preeclampsia and normal pregnancies. J Reprod Immunol. 2006;70(1‐2):83‐91. [DOI] [PubMed] [Google Scholar]
- 25. Thaxton JE, Sharma S. Interleukin‐10: a multi‐faceted agent of pregnancy. Am J Reprod Immunol. 2010;63(6):482‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin H, Mosmann TR, Guilbert L, Tuntipopipat S, Wegmann TG. Synthesis of T helper 2‐ type cytokines at the maternal‐fetal interface. J Immunol. 1993;151(9):4562‐4573. [PubMed] [Google Scholar]
- 27. Roberts CT, White CA, Wiemer NG, Ramsay A, Robertson SA. Altered placental development in interleukin‐10 null mutant mice. Placenta. 2003;24(Suppl. A):S94‐S99. [DOI] [PubMed] [Google Scholar]
- 28. Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NKcells mediate inflammation‐induced fetal demise in IL‐10 null mice. J Immunol. 2005;175(6):4084‐4090. [DOI] [PubMed] [Google Scholar]
- 29. Jiang PJ, Zhao AM, Bao SM, Xiao SJ, Xiong M. Expression of chemokine recep‐ tors CCR29, CCR29 and CXCR29 on CD4(+) Tcells in CBA/JxDBA/2mousemodel, selectively induced by IL‐4 and IL‐10, regulates the embryo resorption rate. Chin Med J (Engl). 2009;122(16):1917‐1921. [PubMed] [Google Scholar]
- 30. Hanna N, Hanna I, Hleb M, et al. Gestational age‐dependent expression of IL‐10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol. 2000;164(11):5721‐5728. [DOI] [PubMed] [Google Scholar]
- 31. Daneva AM, Hadži‐Lega M, Stefanovic M. Correlation of the system of cytokines in moderate and severe preeclampsia. Clin Exp Obstet Gynecol. 2016;3(2):220‐224. [PubMed] [Google Scholar]
- 32. Nevers T, Kalkunte S, Sharma S. Uterine regulatory T cells, IL‐10 and Hypertension. Am J Reprod Immunol. 2011;66(s1):88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bayraktar M, Peltier M, Vetrano A, et al. IL‐10 modulates placental responses to TLR ligands. Am J Reprod Immunol. 2009;62(6):390‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dabagh‐Gorjani F, Anvari F, Zolghadri J, Kamali‐Sarvestani E, Gharesi‐Fard B. Differences in the expression of TLRs and inflammatory cytokines in pre‐eclamptic compared with healthy pregnant women. Iran J Immunol. 2014;11(4):233‐245. [PubMed] [Google Scholar]
- 35. Taylor BD, Tang G, Ness RB, et al. Mid‐pregnancy circulating immune biomarkers in women with preeclampsia and normotensive controls. Pregnancy Hypertens. 2016;6(1):72‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar A, Begum N, Prasad S, Agarwal S, Sharma S. IL‐10, TNF‐alpha & IFN‐gamma: potential early biomarkers for preeclampsia. Cell Immunol. 2013;283(1‐2):70‐74. [DOI] [PubMed] [Google Scholar]
- 37. Taylor BD, Robert B, Ness RB, et al. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertens. 2016;6(4):388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


