Abstract
Background
Although interventional methods are the first‐line treatment options in ST‐segment elevation myocardial infarction (STEMI), the incidence of stent restenosis (SR) is frequent. We investigated the relationship between CRP/albumin ratio (CAR), a novel indicator of inflammatory response, and SR in this study.
Method
This study was carried out on the patients who underwent coronary angiography in our clinic between January 2017 and September 2017. Two groups were generated according to restenosis status (group 1: restenosis (−), group 2: restenosis (+)), and clinical biochemical and angiographical features were compared. As baseline demographic and angiographic characteristics are slightly different in two groups, propensity score matching analysis was performed to reduce bias. 45 SR patients were matched on a 1:1 basis were enrolled final cohort.
Results
The mean age of the patients was 55 ± 5.1 and 80% were male; Cox regression model was performed to demonstrate independent predictor of restenosis development; and during the one‐year follow‐up period, CAR (P < 0.001) was found an independent predictor of SR
Conclusion
In this study, we demonstrate that there may be a strong relationship between SR development and CAR. We implied that inflammatory reaction may be an important diagnostic tool for prediction of SR development in STEMI patients.
Keywords: atherosclerosis, coronary artery disease, inflammation
1. INTRODUCTION
Coronary artery disease is the most common cause of cardiovascular deaths in developed countries. Percutaneous coronary interventions (PCI) are widely used in these patients group as a first‐line treatment option.1 Although percutaneous intervention significantly reduces mortality, interventional treatments under emergency conditions may lead to stent restenosis (SR) in ST‐segment elevation myocardial infarction (STEMI) patients during follow‐up period.1, 2, 3, 4
CRP is a sensitive indicator of inflammation and is a positive acute phase reactant. Similarly, albumin shows a decline in inflammatory diseases as a negative acute phase reactant.5 In this context, the CRP/albumin ratio (CAR) was suggested to be a more sensitive indicator of the severity of inflammatory reaction and the progression of the disease. Recently, a few studies have suggested that CAR may be associated with coronary artery disease.5, 6, 7 The aim of this study was to investigate the association between inflammatory parameters and SR in STEMI patients.
2. METHOD
This study was carried out on patients admitted to our hospital with STEMI diagnosis between January 2017 and December 2017, and a total of 1200 patient were enrolled the study. During the follow‐up period of 42 months, preoperative hematologic, biochemical parameters, clinical, and demographic characteristics of patients were recorded. Patients who were diagnosed with non‐ST‐segment elevation myocardial infarction (NONSTEMI) or unstable angina pectoris or patients whose medical records were unavailable due to technical reasons or patients who need blood products were excluded from the study. Furthermore, to eliminate the influences of non‐cancer diseases on inflammation‐based prognostic scores, we excluded patients with rheumatoid diseases and acute infection. Transient ischemia attack or ischemic stroke and peripheral artery diseases and those with baseline level of Hs‐CRP >10 mg/L, which indicated intensively infectious diseases or collagen diseases or potential tumors, were ruled out. Local ethical committee was approved the study.
2.1. Definitions
Patients with new‐onset chest pain >2 mm of ST‐segment elevation in two or more contiguous leads were defined STEMI. Hypertension was defined as the use of chronic medication or blood pressures >140/90 mm Hg in consecutive measurements. Diabetes mellitus was defined according to current ADA guidelines.8 Chronic obstructive pulmonary disease is defined according to the current GOLD guidelines.9 All patients’ medications and medical stories were recorded before the procedure. Peripheral blood samples were taken for hematological and biochemical tests before the procedure.
2.2. Coronary angiography
All patients underwent coronary angiography on the femoral or radial approach with Judkins technique according to operator preference. BMS or DES implantation was performed according to the clinician's choice during angiography. Because of the lesion and patient characteristics, intracoronary G2B3A antagonist was administered in some cases and most of the cases were postdilatated with non‐compliant balloon. All the patients received dual antiaggregant treatment up to one year. In patients who had undergone coronary angiography during a one‐year follow‐up and in‐stent >50%, lesion detection was assessed as SR
2.3. Propensity score
Two groups were generated according to restenosis status (group 1: restenosis (−), group 2: restenosis (+)). As the baseline clinical biochemical and angiographical characteristics of the patients in the group 1 and group 2 were different, the propensity score matching (PSM) analysis (using logistic regression model) was performed to decrease potential bias. After propensity score (PS) calculation of each patient, a 1:1 match analysis was performed using the nearest‐neighbor matching with a caliper distance of 0.001 without replacement. The model fit was examined with the Hosmer‐Lemeshow's goodness‐of‐fit test and the C‐statistic test. The postmatching balance was examined by mean standardized difference, in which less than 10% for a given covariate suggested the adequate balance.
2.4. Statistics
Continuous variables were expressed as mean ± standard deviation or median (interquartile range), and categorical variables were expressed as percentage. The distribution of continuous variables assessed by Kolmogorov‐Smirnov test. Continuous variables were compared using “Student's t test” and the “Mann‐Whitney U test.” Categorical variables were compared using the “chi‐square” test. Two‐tailed P‐values <0.05 were considered to be statistically significant.
To determine independent predictors of SR, Cox regression analysis was performed. In the univariate analysis, variables that found to be significant (P < 0.05) were included to multivariate logistic regression analysis. The receiver operating characteristics (ROC) curve analysis was performed to determine the cutoff value of CAR in prediction of SR A 2‐sided P < 0.05 was considered significant. Data were analyzed using SPSS 23.0 version (IBM, Armonk, NY).
3. RESULTS
The mean age of the patients was 55 ± 5 and 80%. The median follow‐up period was 44 months. After coronary angiography, using PSM method (Figure 1), 45 patients diagnosed with SR were matched with 45 patients who did not diagnosed with SR Baseline demographical, clinical, and angiographical characteristics of the study population are shown in Table 1.
Figure 1.
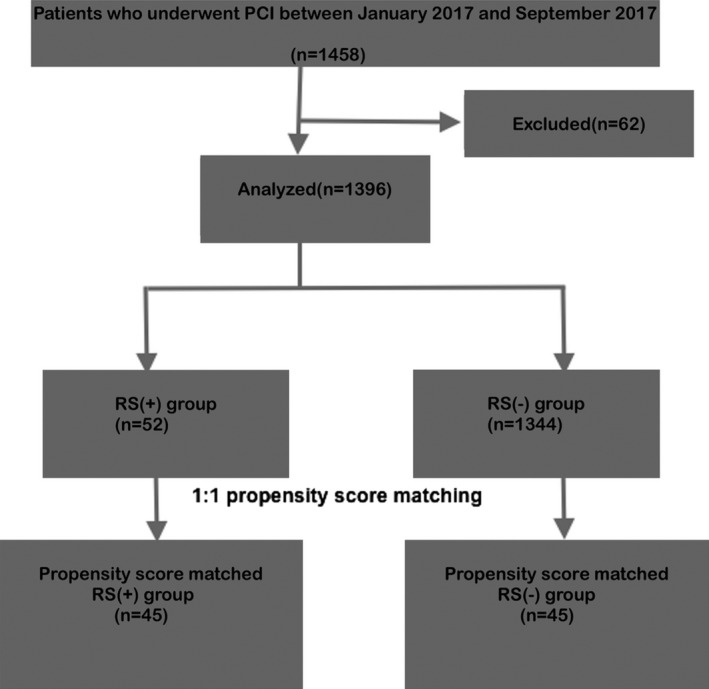
Study flow diagram
Table 1.
Baseline demographic, clinical, and angiographical characteristics of the study populations
| Variables | Group 1 restenosis (−) | Group 2 restenosis (+) | P value |
|---|---|---|---|
| CAR | 1.01 (0.6‐1.38) | 3.8 (1.7‐4.9) | <0.001 |
| SS | 16.5 ± 4.4 | 16.8 ± 4.5 | 0.44 |
| Sex (Male %) | 85.8 | 81.4 | 0.187 |
| Age (y) | 53 ± 11.1 | 56 ± 12 | 0.036 |
| DM (%) | 30.4 | 27.3 | 0.43 |
| HT (%) | 33.6 | 41.9 | 0.05 |
| COPD (%) | 5.5 | 5.5 | 0.95 |
| Smoking (%) | 63.6 | 61.7 | 0.646 |
| Dyslipidemia (%) | 42.7 | 44.7 | 0.654 |
| Aspirin (%) | 1.2 | 4.3 | 0.003 |
| Killip class (%) | |||
| 1 | 87 | 85.5 | 0.67 |
| 2‐4 | 13 | 14.6 | |
| TIMI risk score | 1 (1‐3) | 2 (1‐4) | <0.001 |
| WBC (*103) | 12.1 ± 3.6 | 11.7 (9.6‐14) | 0.94 |
| Neutrophils (*103) | 8.6 (6.7‐11.5) | 8.8 (7‐11.2) | 0.85 |
| Lymphocytes (*103) | 1.7 (1.3‐2.46) | 1.8 (1.2‐2.4) | 0.87 |
| HGB (*103) | 13.9 ± 1.7 | 13.6 ± 1.7 | 0.028 |
| Platelets (*103) | 246 (210‐295) | 249 (217‐294) | 0.55 |
| Cr (mg/dL) | 0.9 ± 0.26 | 0.92 ± 0.32 | 0.77 |
| Glucose (mg/dL) | 125 (105‐185) | 133 (110‐171) | 0.143 |
| Uric acid (mg/dL) | 4.9 ± 0.2 | 5.1 ± 1.4 | 0.156 |
| Albumin (mg/dL) | 3.8 ± 0.49 | 3.7 ± 0.48 | 0.001 |
| CK (U/L) | 276 (146‐447) | 335 (170‐566) | 0.007 |
| CK‐MB (U/L) | 34 (23‐44) | 34 (23‐45) | 0.232 |
| Troponin T (ng/dL) | 1.5 (0.34‐3.7) | 2.2 (0.7‐4.6) | 0.021 |
| LDL (mg/dL) | 113 (87‐145) | 110 (87‐140) | 0.590 |
| Triglyceride (mg/dL) | 116 (79‐168) | 111 (81‐164) | 0.75 |
| CRP (mg/L) | 3.8 (2.5‐5.4) | 11 (6‐18) | <0.001 |
| EF (%) | 48 ± 7.2 | 46 ± 7.6 | 0.02 |
| IRA (%) | |||
| LMCA | 0.4 | 0.4 | 0.213 |
| LAD | 49.8 | 50.2 | |
| CX | 10.7 | 14.2 | |
| RCA | 38.7 | 34.4 | |
| Others | 0.4 | 0.8 | |
| Localization (%) | |||
| Proximal | 49.8 | 55.3 | 0.208 |
| Mid region | 45.3 | 41.1 | |
| Distal region | 4.3 | 3.6 | |
| Stent type (DES, (%) | 36 | 30 | 0.05 |
| No reflow (%) | 20.9 | 30 | 0.169 |
| MBG | 3 (2‐3) | 2 (1‐3) | 0.004 |
| CTFC | 20 (15‐25) | 22 (17‐27) | 0.01 |
CAR, CRP to albumin ratio; CK, creatine kinase; COPD, chronic obstructive pulmonary disease; Cr, creatine; CRP, C‐reactive protein; CTFC, corrected TIMI frame count; EF, ejection fraction; HGB, hemoglobulin; HT, hypertension; IRA, infarct‐related artery; LDL, low‐density lipoprotein; MBG, myocardial blush grade; MD, diabetes mellitus; SS, syntax score; WBC, white blood count.
Bold values indicates statistical significant values.
In univariate analysis, CAR (P < 0.001), age (P = 0.036), HT (P = 0.05), TIMI risk score (P < 0.001), troponin T (P = 0.021), CK (P = 0.007), EF (P = 0.02), stent type (P = 0.05), HGB (P = 0.028), CRP (P < 0.001), albumin (P = 0.001), CTFC (P = 0.01), and MBG (P = 0.004) were associated with the development of SR (Table 1). CAR values according to SR status were demonstrated in Figure 2.
Figure 2.
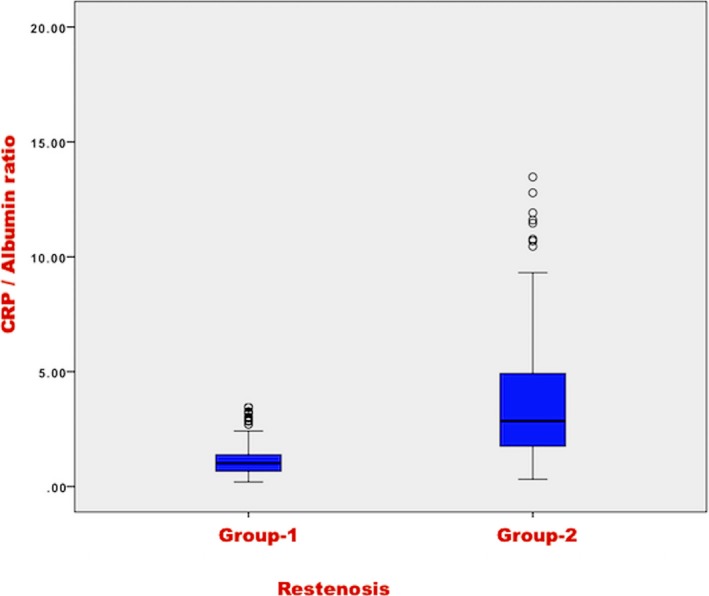
CRP/albumin ratio (CAR) levels according to in‐stent restenosis development status
In Cox regression model, we showed that CAR (OR: 1.2, 95%CI: 1.01‐1.6, P < 0.001), stent type (OR: 2.5, 95% CI: 1.8‐3.6, P < 0.001), and CTFC (0.97, 95%CI: 0.95‐0.98, P = 0.003) are an independent predictor of SR development in STEMI (Table 2).
Table 2.
Independent predictors of SR development in Cox regression model
| Variables | Univariate OR, 95 CI % | P value | Multivariate OR, 95 CI % | P value |
|---|---|---|---|---|
| CAR | 1.17 (1.14‐1.21) | <0.001 | 1.2 (1.01‐1.6) | <0.001 |
| HT | 1.3 (1.06‐1.7) | 0.05 | 1.14 (0.84‐1.55) | 0.37 |
| TIMI risk score | 1.13 (1.06‐1.23) | <0.001 | 1.01 (0.91‐1.12) | 0.78 |
| HGB | 0.95 (0.89‐1.02) | 0.028 | 0.97 (0.91‐1.12) | 0.58 |
| Troponin T | 1.01 (0.97‐1.05) | 0.021 | 1.006 (0.96‐1.04) | 0.74 |
| CK | 1.03 (0.98‐1.06) | 0.007 | 1.02 (0.98‐1.04) | 0.58 |
| EF | 0.97 (0.95‐0.99) | 0.02 | 1.01 (0.98‐1.04) | 0.36 |
| Stent type | 2.7 (2.05‐3.6) | 0.05 | 2.5 (1.8‐3.6) | <0.001 |
| CTFC | 1.003 (0.98‐1.03) | 0.01 | 0.97 (0.95‐0.98) | 0.003 |
| MBG | 0.8 (0.76‐1.01) | 0.004 | 0.98 (0.79‐1.2) | 0.9 |
CAR, CRP to albumin ratio; HT, hypertension; HGB, hemoglobulin; CK, creatine kinase; EF, ejection fraction; CTFC, corrected TIMI frame count; MBG, myocardial blush grade.
Bold values indicates statistical significant values.
In ROC curve analysis, CAR with a cutoff value of 1.25 predicted SR development with 84% sensitivity and 70% specificity (Figure 3).
Figure 3.
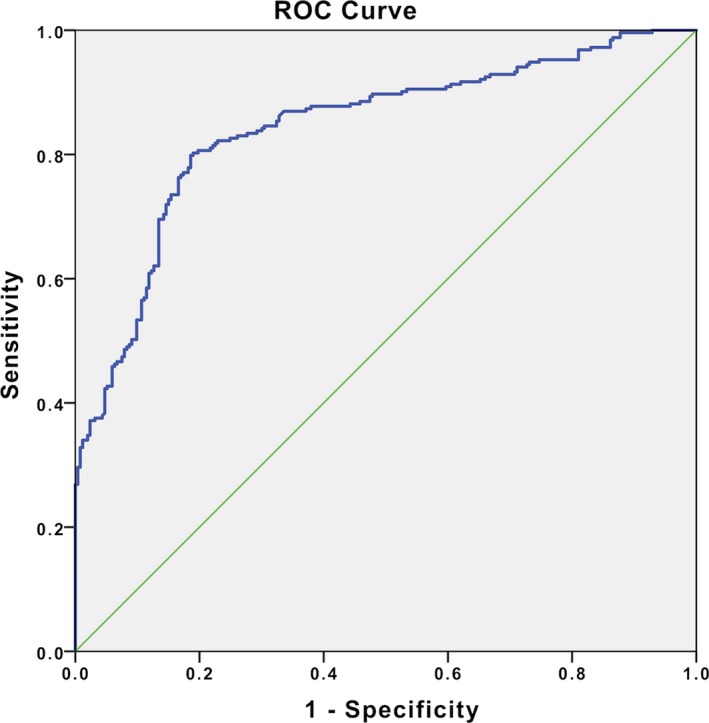
The receiver operating characteristics (ROC) curve analysis for CRP/albumin ratio (CAR) value in prediction of in‐stent restenosis development
4. DISCUSSION
This is the first study to show the relationship between SR and CAR in STEMI patients and we demonstrated that CAR is as effective as the coronary lesion characteristics and stent type in restenosis development and we imply that inflammatory reaction may play an important role in SR development, such as coronary lesion characteristics or traditional risk factors.
Atherosclerotic cardiovascular diseases are the leading cause of death and morbidity in developed countries. The most common cause of death is coronary ischemia and infarction resulting from plaque rupture in the coronary arteries. It has been shown in previous studies that several inflammatory mediators and cytokines were involved in the development of coronary atherosclerotic plaques.10, 11, 12 In the Jupiter study, there was a strong correlation between CRP levels and coronary events. In another study, increased CRP levels were associated with increased mortality and lastly CRP elevation was associated with endothelial dysfunction and a vulnerable coronary plaque.13
Although percutaneous coronary intervention is a successful treatment method, it has some limitations. Because various parameters affect the success of the procedure, such as thrombus burden, lesion characteristics and patient characteristics. Therefore, several risk scores including patient and lesion characteristics were developed for clinical usage, adverse event, and mortality prediction.11 However, endothelial dysfunction and inflammatory response play a major role in coronary events4, 14 and there is no risk classification system including endothelial dysfunction or inflammatory markers in STEMI patients.
Stent restenosis is a major cause of mortality after stent implantation in patients with STEMI. Although rate of RS has decreased significantly with new‐generation drug‐eluting stents, the incidence is relatively frequent and mortality is high.15 Coronary lesion characteristics, stent malposition, and BMS use were shown as a predictors of SR in previous studies.16 As shown in many studies, there is an increased inflammatory reaction in STEMI patients and CRP levels are found high in these patients,17 which is not surprising. Coronary plaque rupture and subsequent inflammatory processes and endothelial dysfunction continue to occur after stent implantation and contribute at least as much as other clinical and angiographic parameters to SR development.4, 14
The most important finding of our study is the relationship between inflammation and SR after PSM analysis, which is consistent with the literature. In light of our study, endothelial function and inflammatory process may play a key role in the SR development as well as coronary lesion characteristics, and the level of inflammatory response may provide information about the prognosis of future adverse events in STEMI and in this context, we implied that the high CAR value at the time of admission could better predict SR development compared to other conventional parameters.
As a result, the inclusion of CAR in risk classification models may provide additional information to predict SR development by providing information about the level of inflammatory reaction.
4.1. Limitations
Our study has several limitations. Small number of patients, instead of a multicenter study, the prognostic value of the CAR should be confirmed in a validation cohort. Meanwhile, this study was a retrospective study, and some criteria for the research methods were not uniform. A multicenter, prospective study should be performed to confirm our findings.
5. CONCLUSION
The inflammatory reaction and endothelial dysfunction play a key role in the development of atherosclerosis. In this context, CAR may be a good diagnostic and prognostic parameter to predict future adverse event in STEMI patients.
Aksu U, Gulcu O, Aksakal E, et al. The association between CRP / Albumin ratio and in‐stent restenosis development in patients with ST‐segment elevation myocardial infarction. J Clin Lab Anal. 2019;33:e22848 10.1002/jcla.22848
REFERENCES
- 1. Palmerini T, Biondi‐Zoccai G, Della Riva D, et al. Stent thrombosis with drug‐eluting and bare‐metal stents: evidence from a comprehensive network meta‐analysis. Lancet. 2012;379(9824):1393‐1402. [DOI] [PubMed] [Google Scholar]
- 2. Kwok CS, Hulme W, Olier I, Holroyd E, Mamas MA. Review of early hospitalisation after percutaneous coronary intervention. Int J Cardiol. 2017;227:370‐377. [DOI] [PubMed] [Google Scholar]
- 3. Nerlekar N, Ha FJ, Verma KP, et al. Percutaneous coronary intervention using drug‐eluting stents versus coronary artery bypass grafting for unprotected left main coronary artery stenosis: a meta‐analysis of randomized trials. Circ Cardiovasc Interv. 2016;9(12):e004729. [DOI] [PubMed] [Google Scholar]
- 4. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2016;389:197–210. [DOI] [PubMed] [Google Scholar]
- 5. Karabag Y, Cagdas M, Rencuzogullari I, et al. Relationship between C‐reactive protein/albumin ratio and coronary artery disease severity in patients with stable angina pectoris. J Clin Lab Anal. 2018;32(7):e22457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geng HH, Wang XW, Fu RL, et al. The relationship between C‐reactive protein level and discharge outcome in patients with acute ischemic stroke. Int J Environ Res Public Health. 2016;13(7):636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asselbergs FW, van den Berg MP, Diercks GF, van Gilst WH, van Veldhuisen DJ. C‐reactive protein and microalbuminuria are associated with atrial fibrillation. Int J Cardiol. 2005;98(1):73‐77. [DOI] [PubMed] [Google Scholar]
- 8. Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, Neuman A. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164(8):542‐552. [DOI] [PubMed] [Google Scholar]
- 9. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557‐582. [DOI] [PubMed] [Google Scholar]
- 10. Head SJ, Farooq V, Serruys PW, Kappetein AP. The SYNTAX score and its clinical implications. Heart. 2014;100(2):169‐177. [DOI] [PubMed] [Google Scholar]
- 11. Thomas MP, Bates ER. Update on primary PCI for patients with STEMI. Trends Cardiovasc Med. 2017;27(2):95–102. [DOI] [PubMed] [Google Scholar]
- 12. Mehilli J, Richardt G, Valgimigli M, et al. Zotarolimus‐ versus everolimus‐eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol. 2013;62(22):2075‐2082. [DOI] [PubMed] [Google Scholar]
- 13. Lee CW, Kang SJ, Ahn JM, et al. Comparison of effects of atorvastatin (20 mg) versus rosuvastatin (10 mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial). Am J Cardiol. 2012;109(12):1700‐1704. [DOI] [PubMed] [Google Scholar]
- 14. Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2016;133(11):1135‐1147. [DOI] [PubMed] [Google Scholar]
- 15. Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 2015;10(9):1024‐1094. [DOI] [PubMed] [Google Scholar]
- 16. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta‐analysis. BMJ. 2013;347:f6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu Y, Cheng Z, Zhao Y, et al. Efficacy and safety of long‐term treatment with statins for coronary heart disease: a Bayesian network meta‐analysis. Atherosclerosis. 2016;254:215‐227. [DOI] [PubMed] [Google Scholar]


