Abstract
Clinical evidence suggests that many cases of serious idiosyncratic drug-induced liver injury (I-DILI) are mediated by the adaptive immune system in response to hepatic drug-protein adducts, also referred to as drug-induced allergic hepatitis (DIAH), but detailed mechanistic proof has remained elusive due to the lack of animal models. We have hypothesized that DIAH is as rare in animals as it is in humans due at least in part to the tolerogenic nature of the liver. Herein we provide evidence that immune tolerance can be overcome in a murine model of halothane-induced liver injury initiated by trifluoroacetylated protein adducts (TFAPA) of halothane formed in the liver. Twenty-four hours after female Balb/cJ mice were initially treated with halothane, perivenous necrosis and an infiltration of CD11b+Gr-1high cells were observed in the liver. Further study revealed a subpopulation of myeloid-derived suppressor cells (MDSCs) within the CD11b+Gr-1high cell fraction, that inhibited the proliferation of both CD4+ and CD8+ T cells. When CD11b+Gr-1highcells were depleted from the liver with Gr-1 antibody treatment, enhanced liver injury was observed at nine days after halothane rechallenge. Toxicity was associated with increased serum levels of IL-4 and immunoglobulins (Ig) IgG1 and IgE directed against hepatic TFAPA, as well as increased hepatic infiltration of eosinophils and CD4+ T cells, all features of an allergic reaction. When hepatic CD4+T cells were depleted 5 days after halothane rechallenge, TFAPA-specific serum Ig and hepatotoxicity were reduced.
Conclusion
Our data provides a rational approach for developing animal models of DIAH mediated by the adaptive immune system and suggests that impaired liver tolerance may predispose patients to this disease.
Keywords: Drug-induced liver injury, Idiosyncratic drug reaction, Eosinophils, Allergic hepatitis, Animal model, Type 2 Immune response, Immunoglobulin E
Idiosyncratic drug-induced liver injury (I-DILI) is a leading cause of acute liver failure and a major reason for abandoning preclinical development, terminating clinical trials, and post-marketing withdrawals of drugs [1]. Unfortunately, it is impossible to accurately predict what new drugs will cause I-DILI and which individuals will be uniquely susceptible.
Clinical evidence suggests I-DILI may be caused by an allergic reaction against the liver initiated in part by an adaptive immune response against liver proteins that have been covalently altered by drugs or drug metabolites [2, 3]. Common clinical features consistent with an allergic hepatitis mechanism of hepatotoxicity include fever, skin rash, eosinophilia, and inflammatory infiltration into the liver by lymphocytes, other mononuclear cells and eosinophils [3]. Additionally, peripheral lymphocytes have been shown to be activated by offending drugs, drug-metabolites, liver proteins and/or protein adducts [4-6] and serum antibodies directed against liver proteins that have been covalently altered and/or the same proteins in their unmodified native form are detected in I-DILI patients [3, 7-10]. Association of I-DILI cases with individuals expressing specific allelic forms of class I and II human leukocyte antigen genes [6] further suggests contribution of the adaptive immune response to the etiology drug-induced allergic hepatitis (DIAH).
While these clinical observations provide correlative support for the role of the adaptive immune system in the pathogenesis of many cases of I-DILI, definitive experimental evidence necessitates the development of animal models that allow for detailed investigation into potential risk factors and immune mechanisms responsible for liver injury and enable preclinical screening of drug candidates for DIAH potential. A major obstacle to establishing such a model is that the pathological process appears to be as idiosyncratic in animals as it is in humans [11].
We [12] and others [11] have hypothesized that the tolerogenic nature of the liver [13] may be a key contributing factor to the low incidence rate of DIAH. One factor contributing to tolerance in the liver is the activity of myeloid-derived suppressor cells (MDSCs) [14]. MDSCs are a heterogenous mixture of immature and mature myeloid cells that expand during cancer, infections and other inflammatory conditions to regulate the immune system by suppressing T cell responses [15]. Depletion of MDSCs significantly improved immune responses against tumors in mice and in cancer patients [16]. Additionally, MDSCs infiltrate the liver and attenuate hepatotoxicity in murine models of immune-mediated liver injury induced by concanavalin A and alpha-galactosamine [17].
Using halothane, a volatile anesthetic with a history of I-DILI resembling prototypical DIAH [2], we have now developed the first model of DIAH by depleting MDSCs from the livers of female Balb/cJ mice prior to halothane treatment. Liver injury appeared to be mediated by an adaptive immune response against the liver initiated by allergenic TFAPA generated in the liver, analogous to allergen-induced inflammatory lung diseases [18].
Materials and Methods
Animals and Treatments
(See Supporting Information for detailed methods)
Sera and Tissue Collection
(See Supporting Information for detailed methods)
Assessment of Hepatotoxicity
Liver injury was assessed as reported previously [19] by measuring serum ALT activity using a microtiter plate adaption of a commercially available kit (Teco Diagnostics, Anaheim, CA) and confirmed with histopathological examination of H&E stained liver sections under light microscopy using Axiovision software (Carl Zeiss Microimaging, Inc.Thornwood, NY) (by D.E.K.).
Isolation of Liver Microsomal Fraction
Livers were removed 10 hours post halothane or vehicle treatment and flash frozen in liquid nitrogen. Sodium deoxycholate (DOC) solubilized extracts of liver microsomal proteins were isolated following an established method [20].
Isolation and Flow Cytometric Analysis of Hepatic Leukocytes
Hepatic leukocytes were isolated using established procedures [21, 22]. Cell surface markers were stained on isolated hepatic leukocytes for identification and quantification of CD4+ T cells, CD8+ T cells, B cells, eosinophils, and dendritic cells (DCs) by flow cytometry analysis. (See Supporting Information for detailed methods).
Purification and Cytological Characterization of CD11b+ Gr-1high Cells
All purification and cytologic characterization of hepatic CD11b+Gr-1high cells were performed as reported previously [21]. (See Supporting Information for detailed methods).
Isolation of Hepatic CD11b+ Gr-1high Cells and T Cell Suppression Assay
(See Supporting Information for detailed methods)
Depletion of CD11b+ Gr-1high Cells
CD11b+Gr-1high cells were depleted by a previously established method in our laboratory [21]. Briefly, 24 hours prior to initial halothane treatment, female Balb/cJ mice were pretreated with 20 μg anti-Gr-1 mAb (clone # RB6-8C5, Bio X cell, West Lebanon, NH) or isotype control (Rat IgG2b, clone # LTF-2, Bio X cell, West Lebanon, NH) administered intraperitoneally in 100 μL of sterile PBS. This concentration depleted the majority of CD11b+Gr-1high cells without significantly affecting the numbers of eosinophils [21] and other immune cell types (Supporting Fig. S2).
Detection of Mouse Serum Antibodies Interacting with Hepatic TFAPA
To detect levels of TFAPA-specific Ig in serum, DOC soluble protein extracts of hepatic microsomes from halothane and vehicle-treated mice and detection antibodies against mouse Ig (H+L), IgG1, IgG2a, IgG2b, IgG3, IgA, IgM, or IgE (SouthernBiotech, Birmingham, AL) were used to develop an ELISA detection system (see Supporting Information for detailed methods).
Measurement of Mouse Cytokines
Serum protein concentrations of IL-4 and IL-10 were quantified using ELISA Ready-Set-Go kits (eBioscience, San Diego, CA) following the manufacturer’s protocols.
Immunohistochemical Detection of Eosinophil Major Basic Protein (MBP)
Detection of MBP in formalin fixed paraffin embedded tissue sections was performed using the established method [23] with rat anti-mouse MBP (MT-14.7, provided by Drs. Nancy and James Lee at Mayo Clinic Arizona, Scottsdale, AZ) or Rat IgG1, κ isotype control (ab18407, Abcam, Cambridge, MA), with some modifications as described previously [21].
T Cell Proliferation in Response to Hepatic TFAPA
(See Supporting Information for detailed methods)
Depletion of CD4+ and CD8+ T Cells
Mice were treated 4 days after halothane rechallenge with either 60 μg of anti-CD4 mAb (GK1.5, Bio X cell) or isotype control Rat IgG2b (LTF-2, Bio X cell) intravenously in 200 μL of sterile PBS to deplete CD4+ T cells, while other mice were treated with 40 μg of anti-CD8 mAb (clone 53-6.72, Bio X cell) or isotype control Rat IgG2b (clone 2A3, Bio X cell) to deplete CD8+ T cells.
Statistical Analysis
All data presented here are reported as the mean ± standard error of the mean (SEM). Statistical significance between two groups was determined by two-tailed Student's t test, while statistical differences between multiple groups were determined by one-way analysis of variance with Newman-Keuls post-test analysis. Relationships between experimental endpoints were assessed by Pearson correlation analysis. Differences were considered significant when P<0.05.
Results
Halothane Treatment of Female Balb/cJ Mice Caused Liver Injury and Induced Hepatic Infiltration of CD11b+ Gr-1high Cells that Suppressed T Cell Proliferation and Produce High Levels of Nitric Oxide
When female Balb/cJ mice were treated with 30 mmol/kg halothane TFAPA were observed in liver and serum at 10 hours and 24 hours post halothane treatment respectively (Supporting Fig. S1A) which correlated with the onset and progression of liver injury as determined by serum ALT activity (Fig. 1A) and histological examination at the peak of injury (Supporting Fig. S1B).
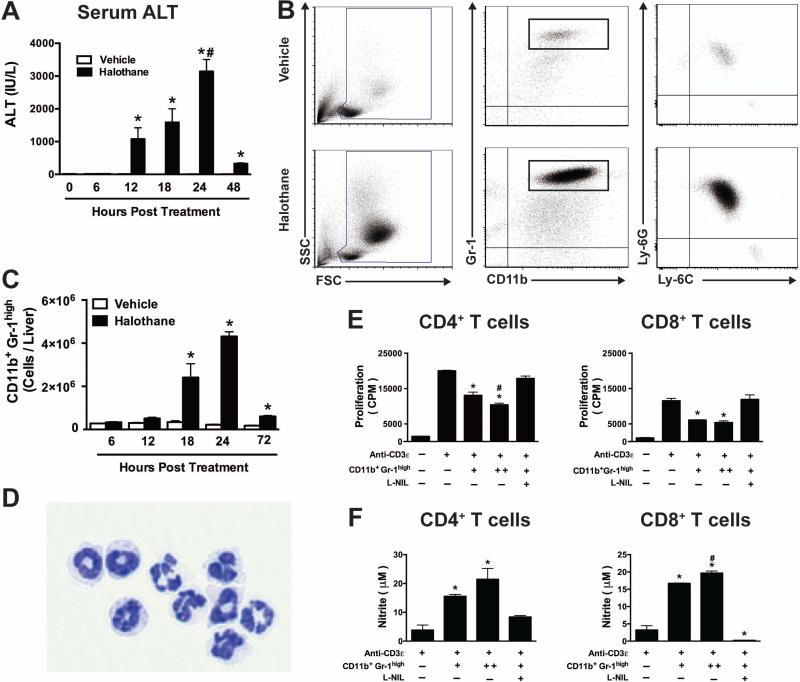
Figure 1. Halothane-induced liver injury in mice is associated with hepatic infiltration of CD11b+Gr-1high cells that suppress T cell proliferation.
(A) Female Balb/cJ mice were injected IP with halothane (30 mmol/kg) or vehicle (olive oil), and serum ALT activities were determined at 6, 12, 18, 24 and 48 hours post-treatment with N=5 mice per group. *P<0.05 versus vehicle-treated control group at the same time point; #P<0.05 versus other time points within the same treatment. (B) Mice were injected with halothane or vehicle and at 24 hours post-treatment hepatic leukocytes were isolated and analyzed by flow cytometry. Representative density dot plots (Left panels) show the SSC- and FSC-area to demonstrate size and granularity of total hepatic leukocytes from individual mice. Middle panels show representative Gr-1 and CD11b staining and CD11b+ Gr-1high cells were then gated and analyzed for Ly-6G and Ly6C staining (Right panels). (C) The total numbers of hepatic CD11b+Gr-1high cells per liver were determined by flow cytometry at 6, 12, 18, 24 and 72 hours post halothane or vehicle treatment with N=4-8 per group. *P<0.05 compared with vehicle-treated group at the same time point. (D) CD11b+ Gr-1high cells were sorted by flow cytometry 24 hours post halothane treatment and visualized by light microscopy on slides following cytospin preparation and DiffQuik staining. (E) CD4+ and CD8+ T cells purified from spleens of naïve mice by cell sorting were incubated with TCR stimulation (anti-CD3ε antibody) along with splenic antigen presenting cells and CD11b+ Gr1high cells isolated from halothane treated mice at equivalent (+) or 2 fold levels (++) with or without L-NIL. Cells were cultured for 96 hours and proliferation was measured by [3H]-thymidine incorporation during the last 24 hours of incubation. (F) NO production was measured in the culture supernatant obtained from the above mentioned experiment where CD4+ T cells or CD8+ T cells were co-cultured with CD11b+, Gr1high cells from halothane treated mice at equivalent (+) or 2-fold levels (++) with or without L-NIL for 72 hours. Data reported is comprised of N=2 experimental replicates per group and is representative of similar results from three independent experiments. *P<0.05 versus anti-CD3ε alone or in the presence of CD11b+ Gr-1high cells and L-NIL; #P<0.05 versus [1:1] MDSC ratio group. All data reported as mean ± SEM.
Consistent with our previous report [21], the number of leukocytes in the liver 24 hours post halothane treatment was increased compared to vehicle-treated mice (Fig. 1B, Left panel). Further examination revealed the majority of these cells were CD11b+Gr-1high (Fig. 1B, Middle panel). As Gr-1 is an indicator of two separate markers; Ly-6C and Ly6G [24], we further characterized these cells and showed that 99 percent of CD11b+Gr-1high cells are Ly6G+Ly6Clow and only 1 percent were Ly6G−Ly6Chigh (Fig. 1B, Right panel). We assessed the kinetics of accumulation of CD11b+Gr-1high cells in the liver and significant infiltration was found 18 hours post halothane treatment, with maximum infiltration at 24 hours (Fig. 1C) – kinetics that match the development of liver injury.
Cytospin preparation of sorted CD11c−CD11b+Gr-1high cells from halothane-treated mice showed morphology consistent with mature neutrophils as well as immature cells with banded nuclei (Fig. 1D). Recent reports have suggested that neutrophils share morphological similarities with granulocytic myeloid-derived suppressor cells (G-MDSCs) [25] and have described MDSCs in mice as cells with CD11b+Gr-1high phenotype [24]. In the absence of exclusive surface markers, CD11b+Gr-1high MDSCs are best defined functionally through their ability to suppress immune responses [15] and previous reports observed the suppressive function of splenic MDSCs identified as “ring cells” (banded nucleus) among the CD11b+Gr-1high cell population [26]. Therefore, we examined the capacity of the CD11b+Gr-1high cells isolated from halothane-treated mice to suppress T cell receptor-mediated T cell proliferation. Hepatic CD11b+Gr-1high MDSCs suppressed both CD4+ and CD8+ T cell proliferation in a dose-dependent manner as measured by [3H]-thymidine incorporation as an index of cell proliferation (Fig. 1E).
Several factors have been implicated in MDSC-mediated immunosuppression, including the expression of arginase and generation of nitric oxide (NO) and reactive oxygen species [24, 27]. The concentration of nitrite increased in the culture supernatant when CD11b+ Gr-1high cells were co-cultured with either CD4+ T cells or CD8+ T cells (Fig. 1F). The addition of L-NIL, a pharmacologic inhibitor of inducible nitric oxide synthase to the culture media completely abolished CD11b+ Gr-1high cell-mediated T cell suppression and nitric oxide production (Fig. 1E and 1F). As the concentration of nitrite was lower in the T cell-only culture supernatant, MDSCs were the main source of nitric oxide.
Depletion of Hepatic MDSCs Prior to Initial Halothane Treatment Resulted in Increased Liver Injury Nine Days Post Halothane Rechallenge
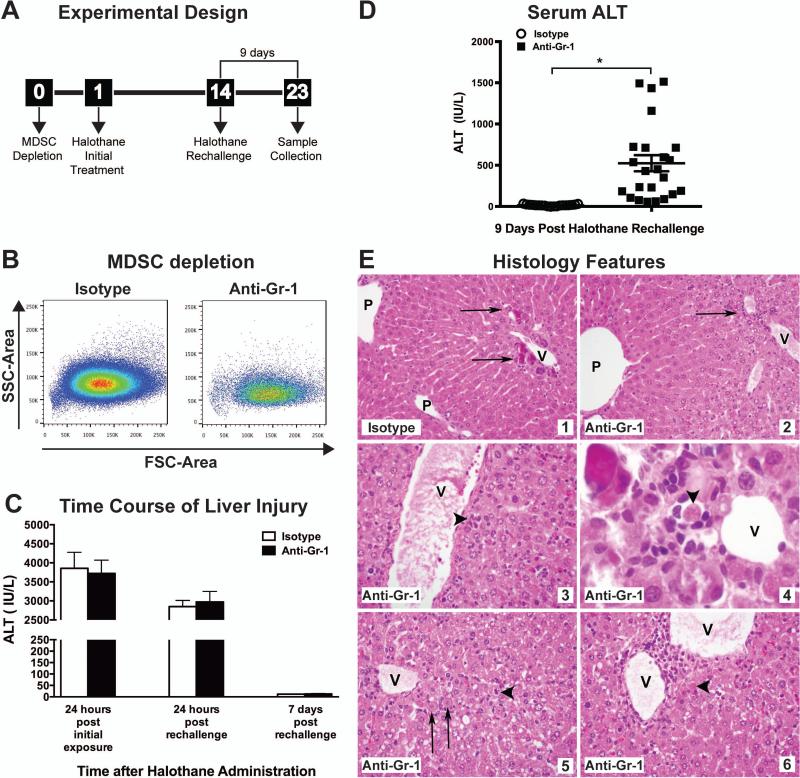
Since the majority of patients with severe halothane hepatitis have been exposed to halothane on two or more occasions [28], we administered halothane at two different times, 14 days apart (Fig. 2A), to model human cases of potential halothane-induced allergic hepatitis and to determine the role of the adaptive immune system in mediating liver injury. Anti-Gr-1 treatment did not alter the acute toxicity observed 24 hours post initial halothane treatment or rechallenge in which ALT elevations and histological evaluations were comparable (Fig. 2C). By 7 days post halothane rechallenge, serum ALT levels had returned to baseline in both treatment groups (Fig. 2C). However, 9 days post halothane rechallenge we observed significantly elevated ALT activity in mice pretreated with Gr-1 antibody compared to isotype-treated control mice (Fig. 2D). As seen in Fig. 2D the extent of liver injury as measured biochemically showed individual variation as did the histological injury. In isotype-treated mice perivenular microcalcifications were observed but no inflammation or evidence of cytopathic injury (Figure 2E, Inset 1), whereas anti-Gr-1 treated mice presented with mild perivenular lymphocytic inflammation in the liver with hepatocytes in zone 3 displaying cytoplasmic vacuolization (Figure 2E, Inset 2). In other anti-Gr-1 treated mice, the perivenular regions showed multiple small foci of inflammation including small clusters of plasma cells (Figure 2E, Inset 3) and some showed hepatocyte dropout and apoptotic hepatocytes were evident with some apoptotic hepatocytes surrounded by small lymphocytes and macrophages (Figure 2E, Inset 4). In Anti-Gr-1 treated mice which developed severe injury, livers also showed inflammation, necrosis and apoptosis in zone 3 with necrotic hepatocytes found mainly near the veins and apoptotic hepatocytes scattered around the edges of the injury (Figure 2E, Inset 3 and 4). As halothane hepatotoxicity is initiated by TFAPA [29, 30], we verified that anti-Gr-1 treatment did not alter halothane metabolism by immunoblotting for hepatic TFAPA to show that similar amounts of TFAPA were present 9 days post halothane rechallenge in anti-Gr-1 or isotype-treated mice (Supporting Fig. S3B).
Figure 2. Procedure for inducing an adaptive immune response against TFAPA released from damaged hepatocytes.
(A) Experimental design: 24 hours prior to the initial halothane treatment, mice were administered either 20 μg/mouse of isotype control antibody or Anti-Gr-1 to deplete MDSCs. Mice were rechallenged with halothane on day 14 and monitored for development of liver injury. (B) Representative dot plots of hepatic MDSCs (SSChigh, CD11b+, Gr-1high) isolated from isotype control or anti-Gr-1 treated mice 24 hours post initial halothane treatment. (C) Mice were injected on Day 0 with Anti-Gr-1 or isotype control antibody. After 24 hours (Day 1), the mice were administered halothane dissolved in olive oil. Serum ALT activity was determined 24 hours post initial halothane exposure. On day 14, mice were rechallenged with halothane and serum ALT activity was determined 24 hours and 7 days following rechallenge. (D) Serum ALT activities were determined at 9 days post halothane rechallenge in isotype or anti-Gr-1 treated mice. Data reported as means ± SEM of 19-23 mice per group from 3 independent experiments. *P<0.05 versus isotype-treated group. Each symbol represents data from an individual mouse. (E) Representative photomicrographs of H&E stained liver sections from mice at 9 days after halothane rechallenge. For all panels, central veins (V) and portal areas (P) are indicated. (Inset 1) Isotype-treated mouse liver showing perivenular microcalcifications (arrows) but no inflammation or evidence of cytopathic injury (H&E, 400x). (Inset 2) Anti-Gr-1 treated mouse liver showing mild perivenular lymphocytic inflammation (arrow) (ALT: 712 IU/L). (H&E, 400x). (Inset 3) Perivenular region showing multiple small foci of inflammation including a small cluster of plasma cells in anti-Gr-1 treated mouse liver (arrowhead) (H&E, 400x). (Inset 4) In an anti-Gr-1 treated mouse liver, hepatocyte dropout and apoptosis was observed and an apoptotic hepatocyte (arrowhead) is surrounded by small lymphocytes and macrophages. (H&E, 600x original magnification). (Inset 5&6) Anti-Gr-1 treated mouse liver shows inflammation, necrosis and apoptosis in zone 3. Necrotic hepatocytes (arrows) have preserved cell size but ghost nuclei and are found mainly near the veins. Apoptotic hepatocytes (arrowheads) were scattered around the edges of the injury (ALT: 1513 IU/L). (H&E, 400x).
Depletion of Hepatic MDSCs Prior to Initial Halothane Treatment Resulted in Increased Antibody Response Against Hepatic TFAPA
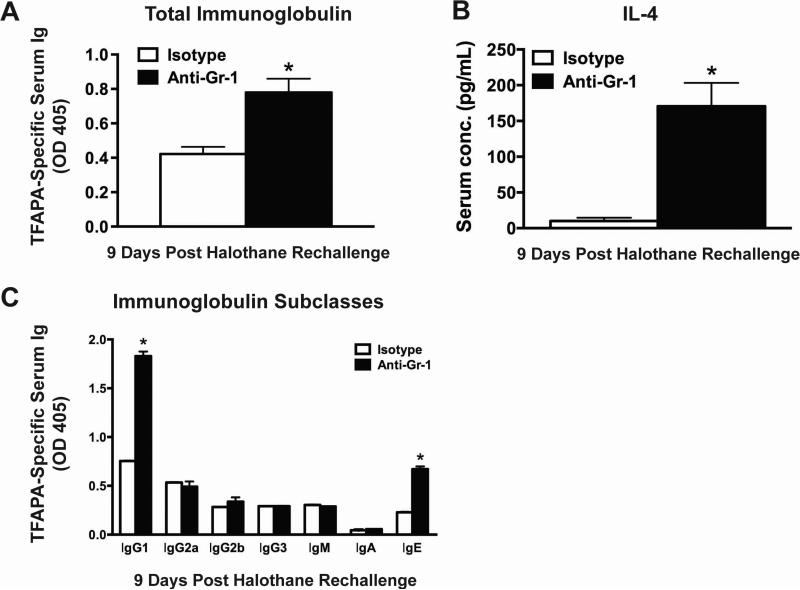
We assessed the generation of immunoglobulins against TFAPA as a measurement of the adaptive immune response following halothane rechallenge. At 9 days post halothane rechallenge, anti-Gr-1 treated mice had increased serum levels of TFAPA-specific immunoglobulin compared to isotype treated mice (Fig. 3A). Moreover, at 9 days post halothane rechallenge anti-Gr-1 treated mice had significantly elevated serum IL-4 compared to isotype-treated mice (Fig. 3B). The majority of TFAPA-specific antibodies were IgG1 and IgE isotypes, which were significantly higher in anti-Gr-1 treated mice compared to isotype-treated mice (Fig. 3C). In anti-Gr-1 treated mice, the severity of liver injury as determined by serum ALT activity was positively correlated with the serum levels of IgG1 and IgE, (r=0.49 and r=0.63 respectively according to Pearson's correlation analysis).
Figure 3. Depletion of hepatic MDSCs prior to initial halothane treatment resulted in increased serum levels of immunoglobulins against hepatic TFAPA.
(A) Serum immunoglobulin against TFA-protein adducts was measured by coating microplates with microsomal extracts from livers of either halothane or oil-treated mice. Antibodies were detected at 1:400 dilution of serum and the results were corrected by subtracting the OD value of wells coated with microsomal proteins from oil-treated mice. Data reported as mean ± SEM of 8-15 mice per group obtained from 3 independent experiments. (B) Serum protein concentrations of IL-4 were measured in isotype or anti-Gr-1 treated mice 9 days after halothane rechallenge with N=5-12 per group from 2 independent experiments. *P<0.05 versus isotype-treated group. (C) Analysis of TFAPA-specific immunoglobulin (Ig) subclasses in serum 9 days post halothane rechallenge. Antibodies were detected at 1:100 dilution of serum and results were corrected by subtracting the OD value of wells coated with microsomal proteins from oil-treated mice. N=4-9 per group from 2 independent experiments. *P<0.05 versus isotype-treated group within the same Ig subclass. All data reported as means ± SEM.
Depletion of Hepatic MDSCs Increased Hepatic Eosinophil Infiltration 9 Days after Halothane Rechallenge
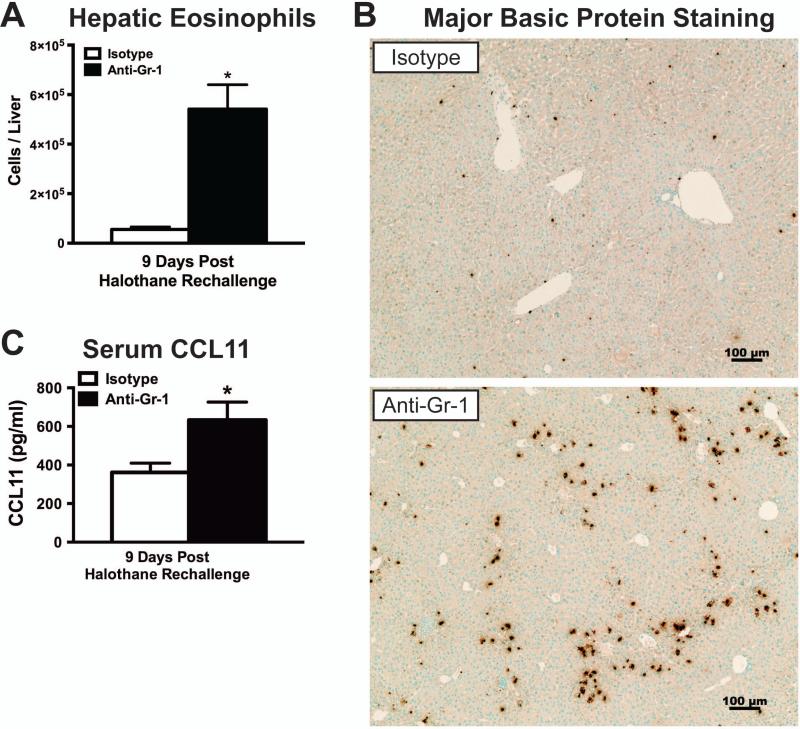
We previously reported the pathogenic role of eosinophils in liver injury after initial acute halothane exposure [21]. In this study we also found increased hepatic eosinophil infiltration in anti-Gr-1 treated mice compared to isotype controls at 9 days post halothane rechallenge, as determined by flow cytometry staining of isolated hepatic leukocytes (Fig. 4A). In addition, increased immunohistochemical staining of major basic protein (MBP) as a measure of eosinophils in the liver was observed in anti-Gr-1 treated mice compared to isotype controls (Fig. 4B). Serum concentration of eotaxin 1 (CCL11), a major chemoattractant of eosinophils to the liver in HILI [21], was increased in anti-Gr-1 treated mice compared to isotype treated mice at 9 days post halothane rechallenge (Fig. 4C).
Figure 4. Depletion of hepatic MDSCs prior to initial halothane treatment resulted in elevated hepatic eosinophil infiltration.
(A) Hepatic eosinophils (SSChigh CD11c− Gr-1low Siglec-Fhigh) were measured by flow cytometry 9 days after halothane rechallenge with N=14 per group from 2 independent experiments. (B) Representative photomicrographs of Major Basic Protein (MBP) staining for the detection of eosinophils in isotype or anti-Gr-1 treated mice 9 days after rechallenge with halothane. (C) Serum protein concentrations of CCL11 were measured in isotype or anti-Gr-1 treated mice 9 days after halothane rechallenge with N=11 per group from 3 independent experiments. All data reported as means ± SEM. *P<0.05 versus isotype-treated group.
Depletion of Hepatic MDSCs Increased Hepatic T Cell Response to TFAPA
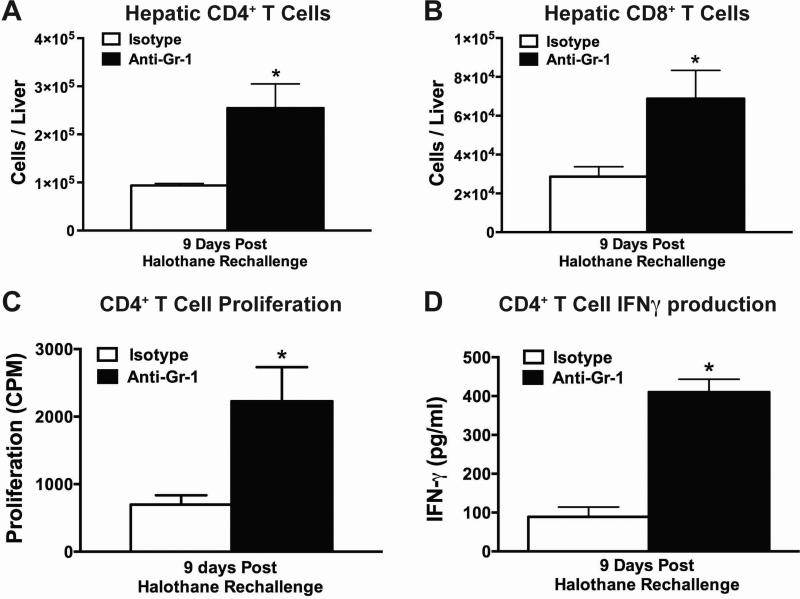
Anti-Gr-1 treated mice had increased hepatic infiltration of CD4+ and CD8+ T cells at 9 days post halothane rechallenge, compared to isotype-treated mice (Fig. 5A and 5B). Furthermore, when CD4+ T cells were isolated 9 days post halothane rechallenge and cultured in the presence of TFA-adducted liver microsomal proteins, cells from anti-Gr-1 treated mice displayed increased proliferation and interferon gamma (IFNγ) production compared to cells from isotype-treated mice (Fig. 5C and 5D). However, comparable results were not observed when the response of CD8+ T cells was evaluated (data not shown).
Figure 5. Depletion of hepatic MDSCs prior to initial halothane treatment resulted in increased number of hepatic CD4+ and CD8+ T cells after halothane rechallenge.
Total number of hepatic CD4+ (A) or CD8+ (B) T cells per liver from isotype or anti-Gr-1 treated mice were quantified at 9 days post halothane rechallenge. (C) Hepatic CD4+ T cells isolated 9 days post halothane rechallenge from either isotype or anti-Gr-1 treated mice were cultured for 96 hours in the presence of microsomal protein extract from halothane or oil-treated mice along with naïve irradiated mouse splenocytes as antigen presenting cells. Proliferation was measured by [3H]-thymidine incorporation during the last 24 hours of incubation. The results were corrected by subtracting the counts per minute (CPM) from the wells incubated with microsomal extracts from oil treated mice. (D) IFNγ protein levels in cell-culture supernatant were assessed in CD4+ T cells isolated, from isotype or anti-Gr-1 treated mice, 9 days post halothane rechallenge. Cells were cultured for 72 hours in the presence of microsomal protein extract from halothane or oil treated mice along with naïve irradiated mouse splenocytes as antigen presenting cells. All data reported as mean ± SEM of N=4-6 mice per group. *P<0.05 versus isotype-treated group.
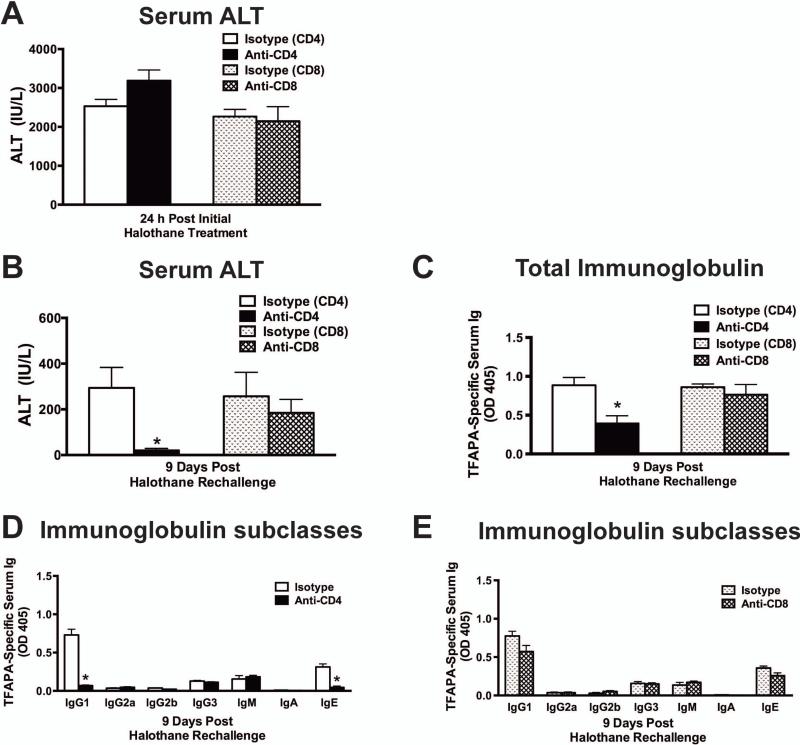
Anti-Gr-1 Treated Mice Were Protected Against Liver Injury by Depletion of CD4+ T Cells
When CD4+ and CD8+ T cells were depleted 24 hours before initial halothane exposure, there was no effect on acute liver toxicity 24 hours after halothane exposure (Fig. 6A). Treatment with anti-CD4 or anti-CD8 antibodies did not decrease the number of other hepatic immune cells measured (Supporting Fig. S4A and S4B: B cells, MDSCs, eosinophils, or DCs). Because T cells remained depleted for at least 4 days following antibody administration (data not shown), we depleted CD4+ or CD8+ T cells 5 days post halothane rechallenge to assess the role of T-cells in the adaptive immune response at 9 days post halothane rechallenge. We found that liver injury was abrogated in CD4+ T-cell depleted mice (Fig. 6B), but CD8+ T cell depleted mice showed no protection against toxicity (Fig. 6B). Additionally, the levels of TFAPA-specific serum immunoglobulins were significantly reduced in CD4+ T cell depleted mice (Fig. 6C) with a diminution of both TFAPA-specific IgG1 and IgE antibodies (Fig. 6D). Mice treated with anti-CD8 or isotype-control antibody had similar levels of TFAPA-specific total immunoglobulin and isotype subclasses (Fig. 6C & 6E).
Figure 6. Depletion of CD4+ T cells attenuated liver toxicity and reduced TFAPA-specific serum immunoglobulin levels after halothane rechallenge in MDSC-depleted mice.
(A) To deplete hepatic CD4+ or CD8+ T cells, Balb/cJ mice were administered either 60 μg/mouse CD4 mAb, 40 μg CD8 mAb or respective isotype controls 24 hours prior to initial halothane treatment and serum ALT activities were determined 24 hours post initial halothane treatment with N=4-9 mice per group from two independent experiments. (B) Hepatic CD4+ or CD8+ T cells were depleted 4 days after halothane rechallenge and serum ALT activities were determined at 9 days post halothane rechallenge with N=8-12 mice per group from 2 independent experiments. *P<0.05 versus respective isotype control-treated groups. (C) TFAPA-specific serum immunoglobulin levels in CD4+ and CD8+ T cells depleted mice were determined 9 days post halothane rechallenge with N=6 mice per group obtained from 2 independent experiments. * P<0.05 versus respective isotype control-treated group. Levels of TFAPA-specific Ig subclasses in serum after CD4+ (D) or CD8+ (E) T cell depletion were assessed 9 days post halothane rechallenge with N=5-6 mice per group obtained from 2 independent experiments. *P<0.05 versus the respective isotype control-treated group within the same Ig subclass. All data reported as mean ± SEM.
Discussion
Although many cases of severe I-DILI are thought to be caused by an allergic reaction against the liver, no concrete evidence for this hypothesis has been provided until now. In this study, we demonstrate a pivotal role for hepatic MDSCs in regulation of the adaptive immune responses in the pathogenesis of DIAH.
Halothane is the prototypical example of a drug thought to cause DIAH and is commonly reported after repeated drug exposure and associated with eosinophilia, which is consistent with an allergic reaction [28]. To mimic these circumstances in a mouse model, we administered an initial dose of halothane on Day 1 and rechallenged mice with halothane on Day 14. Furthermore, we depleted MDSCs prior to initial halothane exposure to disrupt liver tolerance and enhance the adaptive immune response to TFAPAs released from damaged hepatocytes (Supporting Fig. S1A). MDSC-depleted mice (following anti-Gr-1 treatment) developed immune-mediated liver injury 9 days post halothane rechallenge, well after the resolution of the acute effects of halothane in isotype-treated mice (Fig. 2D) that was associated with eosinophilia (Fig. 4A&B). Furthermore, upon histological evaluation of liver sections, we observed apoptotic hepatocytes along with significant infiltration of perivenular lymphocytes, macrophages and plasma cells in anti-Gr-1 treated mice (Fig. 2E). These histological features are seen in patients with immune-mediated liver injury [3] and are indicative of an adaptive immune response in the liver, providing additional evidence for the role of these MDSCs in mediating immune tolerance in this model.
Patients with halothane-induced hepatitis also have serum antibodies against hepatic microsomal proteins that are covalently modified by the trifluoroacetyl chloride reactive metabolite of halothane [10, 31]. In the present study, TFAPA-specific antibody levels were selectively increased in anti-Gr-1 treated mice compared to isotype treatment at 9 days post halothane rechallenge, strongly indicating the involvement of an adaptive immune response and the role of tolerance in regulating this response (Fig. 3A). Moreover, serum concentrations of IL-4 were increased in anti-Gr-1 treated mice at the same time (Fig. 3B). Analysis of TFAPA-specific antibody subclasses revealed increased levels of IgG1 and IgE antibodies in the serum of anti-Gr-1 treated mice compared to isotype-treated mice (Fig. 3C). IL-4 promotes class switching and production of Th2-type antibodies IgG1 and IgE antibodies from plasma cells, which are associated with allergic reactions [32]. IgE can interact with receptors on mast cells, eosinophils and basophils to initiate release of inflammatory cytotoxic mediators in an allergic response [33]. It is possible that halothane hepatitis patients also have IgE antibodies as seen in our studies, although not yet tested, because these individuals do have IgG4 antibodies associated with halothane hepatitis. The synthesis of this antibody isotype, like that of IgE, is dependent on IL-4 [34].
Along with increased liver toxicity and elevated TFAPA-specific IgG1 and IgE levels, anti-Gr-1 treated mice had increased hepatic eosinophil infiltration compared to isotype controls, another indication of an allergic reaction (Fig. 4A). We recently reported the pathogenic role of eosinophils after initial halothane exposure [21] and eosinophilia is often reported as a clinical symptom for patients with DILI [35]. Our findings of hepatic eosinophilia and elevated serum levels of IL-4 after halothane rechallenge strongly supports a type 2 allergic reaction, as seen in inflammatory lung diseases [36].
We now report for the first time in this mouse model of DIAH that CD4+ T-cells are critical effectors of liver injury. In anti-Gr-1 treated mice 9 days post halothane rechallenge, we observed increased numbers of hepatic CD4+ and CD8+ T-cells compared to isotype-treated mice. When hepatic CD4+ T cells were isolated and stimulated ex-vivo with TFAPA, T-cells from anti-Gr-1 treated mice demonstrated an increased proliferative response and elevated production of IFNγ compared to T-cells isolated from isotype-treated mice. However, no response was observed in CD8+ T cells from the same mice (data not shown). CD4+ T cell depletion protected against development of liver injury after halothane rechallenge in anti-Gr-1 treated mice (Fig. 6B). Furthermore, the increase in serum levels of total TFAPA-specific Ig and TFAPA-specific IgG1 and IgE observed 9 days post halothane rechallenge were also abrogated in mice when CD4+ T cells were depleted, suggesting TFAPA-specific antibodies play a pathogenic role in this model, possibly through antibody dependent cellular cytotoxicity, interaction with complement or in the case of IgE through enhancement of eosinophil activity via the high affinity IgE Receptor. The pathogenic role of TFAPA-specific antibodies in this model is further supported by the positive correlation of serum Ig levels with liver injury (as stated in results).
Notably, despite the development of liver injury after halothane rechallenge these mice did not develop fulminant liver failure as in many cases of halothane hepatitis. In this regard, we took blood samples from 5 mice at 14 days post halothane rechallenge in one study and found that ALT values were 80% lower than those of mice 9 days post halothane rechallenge (results not shown). This is likely due to the intrinsic redundancy of tolerogenic factors in the hepatic immune system which include: programmed death-1 (PD-1) signaling, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) expression or the involvement of regulatory T cells (Tregs). These factors may prevent the progression of injury to fulminant liver failure in this model. In this regard, one predominant cytokine with anti-inflammatory and immunosuppressive function is interluekin-10. We found elevated serum levels of IL-10 in anti-Gr-1 treated mice compared to isotype controls at 9 days post halothane rechallenge (Supporting Fig. S5) suggesting that, in the absence of MDSC function, compensatory mechanisms are induced to suppress the immune response.
Other researchers have attempted to develop mouse models of halothane-induced allergic hepatitis by immunizing mice with hepatic proteins that have been chemically trifluoroacetylated. Although this approach to overcome hepatic immune tolerance led to hepatitis, hepatocellular injury was minimal. In a recent report, disruption of both PD-1 and CTLA-4 signaling provided promising results in developing a mouse model of amodiaquine-induce liver injury [37].
Overall, our findings demonstrate a critical role for MDSCs in the regulation of the adaptive immune response to DIAH induced by halothane. Moreover, this report also substantiates DIAH as a real disease with all the features of an allergic reaction where TFAPA serves as an allergen to induce the hepatic cellular and humoral response including antigen specific CD4+ T cells, IgG1 and IgE, as well as production of IL-4 and eosinophilia. As such, this model will allow for further investigation into the pathogenic mechanisms of other cases of DIAH, including those associated with IL-4 (as discussed previously [22], and may be applicable to study the etiologies of other liver diseases or serious drug-induced allergic reactions. Moreover, genetic and environmental factors may exist that can diminish the tolerogenic activity of MDSCs and be important susceptibility factors.
Supplementary Material
Acknowledgments
The authors acknowledge John George for maintaining the mouse colony and the National Heart, Lung and Blood Institute Flow Cytometry core facility for their help with this work. The authors also thank Tami McCoy Graf for her careful review of the manuscript. Financial Support: This work was supported by the Intramural Research Program of the National Institutes of Health and the National Heart, Lung and Blood Institute.
Abbreviations
- ALT
alanine aminotransferase
- BSA
bovine serum albumin
- CCL
chemokine (C-C motif) ligand
- DCs
dendritic cells
- DIAH
drug-induced allergic hepatitis
- DOC
sodium deoxycholate
- ELISA
enzyme-linked immunosorbant assay
- H&E
hematoxylin and eosin
- HILI
halothane-induced liver injury
- I-DILI
idiosyncratic drug-induced liver injury
- IFNγ
interferon gamma
- Ig
immunoglobulin
- IL
interleukin
- L-NIL
LN6-(1-Iminoethyl) lysine dihydrochloride
- MDSC
myeloid-derived suppressor cell
- PBS
phosphate-buffered saline
- SEM
Standard error of the mean
- Th
T helper
- TFAPA
trifluoroacetylated protein-adducts
References
- 1.Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Pohl LR. Drug-induced allergic hepatitis. Semin Liver Dis. 1990;10:305–315. doi: 10.1055/s-2008-1040486. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZX, Kaplowitz N. Immune-mediated drug-induced liver disease. Clin Liver Dis. 2002;6:755–774. doi: 10.1016/s1089-3261(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 4.Warrington RJ, Tse KS, Gorski BA, Schwenk R, Sehon AH. Evaluation of isoniazidassociated hepatitis by immunological tests. Clin Exp Immunol. 1978;32:97–104. [PMC free article] [PubMed] [Google Scholar]
- 5.Tsutsui H, Terano Y, Sakagami C, Hasegawa I, Mizoguchi Y, Morisawa S. Drugspecific T cells derived from patients with drug-induced allergic hepatitis. J Immunol. 1992;149:706–716. [PubMed] [Google Scholar]
- 6.Monshi MM, Faulkner L, Gibson A, Jenkins RE, Farrell J, Earnshaw CJ, et al. Human leukocyte antigen (HLA)-B*57:01-restricted activation of drug-specific T cells provides the immunological basis for flucloxacillin-induced liver injury. Hepatology. 2013;57:727–739. doi: 10.1002/hep.26077. [DOI] [PubMed] [Google Scholar]
- 7.Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39:1430–1440. doi: 10.1002/hep.20205. [DOI] [PubMed] [Google Scholar]
- 8.Bourdi M, Larrey D, Nataf J, Bernuau J, Pessayre D, Iwasaki M, et al. Anti-liver endoplasmic reticulum autoantibodies are directed against human cytochrome P- 450IA2. A specific marker of dihydralazine-induced hepatitis. J Clin Invest. 1990;85:1967–1973. doi: 10.1172/JCI114660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dansette PM, Bonierbale E, Minoletti C, Beaune PH, Pessayre D, Mansuy D. Druginduced immunotoxicity. Eur J Drug Metab Pharmacokinet. 1998;23:443–451. doi: 10.1007/BF03189993. [DOI] [PubMed] [Google Scholar]
- 10.Kenna JG, Satoh H, Christ DD, Pohl LR. Metabolic basis for a drug hypersensitivity: antibodies in sera from patients with halothane hepatitis recognize liver neoantigens that contain the trifluoroacetyl group derived from halothane. J Pharmacol Exp Ther. 1988;245:1103–1109. [PubMed] [Google Scholar]
- 11.Ng W, Lobach AR, Zhu X, Chen X, Liu F, Metushi IG, et al. Animal models of idiosyncratic drug reactions. Adv Pharmacol. 2012;63:81–135. doi: 10.1016/B978-0-12-398339-8.00003-3. [DOI] [PubMed] [Google Scholar]
- 12.Ju C, McCoy JP, Chung CJ, Graf ML, Pohl LR. Tolerogenic role of Kupffer cells in allergic reactions. Chem Res Toxicol. 2003;16:1514–1519. doi: 10.1021/tx0341761. [DOI] [PubMed] [Google Scholar]
- 13.Invernizzi P. Liver auto-immunology: the paradox of autoimmunity in a tolerogenic organ. J Autoimmun. 2013;46:1–6. doi: 10.1016/j.jaut.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol. 2011;11:879–889. doi: 10.1016/j.intimp.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu G, Bi Y, Wang R, Yang H, Zhang Y, Wang X, et al. Targeting S1P1 receptor protects against murine immunological hepatic injury through myeloid-derived suppressor cells. J Immunol. 2014;192:3068–3079. doi: 10.4049/jimmunol.1301193. [DOI] [PubMed] [Google Scholar]
- 18.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 19.Yee SB, Bourdi M, Masson MJ, Pohl LR. Hepatoprotective role of endogenous interleukin-13 in a murine model of acetaminophen-induced liver disease. Chem Res Toxicol. 2007;20:734–744. doi: 10.1021/tx600349f. [DOI] [PubMed] [Google Scholar]
- 20.Butler LE, Thomassen D, Martin JL, Martin BM, Kenna JG, Pohl LR. The calciumbinding protein calreticulin is covalently modified in rat liver by a reactive metabolite of the inhalation anesthetic halothane. Chem Res Toxicol. 1992;5:406–410. doi: 10.1021/tx00027a014. [DOI] [PubMed] [Google Scholar]
- 21.Proctor WR, Chakraborty M, Chea LS, Morrison JC, Berkson JD, Semple K, et al. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2013;57:2026–2036. doi: 10.1002/hep.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proctor WR, Chakraborty M, Fullerton AM, Korrapati MC, Ryan PM, Semple K, et al. Thymic stromal lymphopoietin and interleukin-4 mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology. 2014 doi: 10.1002/hep.27169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denzler KL, Farmer SC, Crosby JR, Borchers M, Cieslewicz G, Larson KA, et al. Eosinophil major basic protein-1 does not contribute to allergen-induced airway pathologies in mouse models of asthma. J Immunol. 2000;165:5509–5517. doi: 10.4049/jimmunol.165.10.5509. [DOI] [PubMed] [Google Scholar]
- 24.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell Mol Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 27.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 28.Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Halothaneinduced hepatitis: A forgotten issue in developing countries:. Hepat Mon. 2011;11:3–6. [PMC free article] [PubMed] [Google Scholar]
- 29.You Q, Cheng L, Reilly TP, Wegmann D, Ju C. Role of neutrophils in a mouse model of halothane-induced liver injury. Hepatology. 2006;44:1421–1431. doi: 10.1002/hep.21425. [DOI] [PubMed] [Google Scholar]
- 30.Bourdi M, Amouzadeh HR, Rushmore TH, Martin JL, Pohl LR. Halothane-induced liver injury in outbred guinea pigs: role of trifluoroacetylated protein adducts in animal susceptibility. Chem Res Toxicol. 2001;14:362–370. doi: 10.1021/tx000244x. [DOI] [PubMed] [Google Scholar]
- 31.Kenna JG, Neuberger J, Williams R. An enzyme-linked immunosorbent assay for detection of antibodies against halothane-altered hepatocyte antigens. J Immunol Methods. 1984;75:3–14. doi: 10.1016/0022-1759(84)90219-9. [DOI] [PubMed] [Google Scholar]
- 32.Snapper CM, Finkelman FD, Stefany D, Conrad DH, Paul WE. IL-4 induces coexpression of intrinsic membrane IgG1 and IgE by murine B cells stimulated with lipopolysaccharide. J Immunol. 1988;141:489–498. [PubMed] [Google Scholar]
- 33.Prussin C, Metcalfe DD. 5. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2006;117:S450–456. doi: 10.1016/j.jaci.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Njoku DB, Mellerson JL, Talor MV, Kerr DR, Faraday NR, Outschoorn I, et al. Role of CYP2E1 immunoglobulin G4 subclass antibodies and complement in pathogenesis of idiosyncratic drug-induced hepatitis. Clin Vaccine Immunol. 2006;13:258–265. doi: 10.1128/CVI.13.2.258-265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, et al. Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology. 2014;59:661–670. doi: 10.1002/hep.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends Immunol. 2014;35:69–78. doi: 10.1016/j.it.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Metushi IG, Hayes MA, Uetrecht J. Treatment of PD-1−/− mice with amodiaquine and anti-CTLA4 leads to liver injury similar to idiosyncratic liver injury in patients. Hepatology. 2014 doi: 10.1002/hep.27549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.