Abstract
Purpose:
Astrocytes perform a plethora of important functions in the central nervous system (CNS) and are involved in cocaine-evoked synaptic plasticity. Previously, we showed that while cocaine decreased cyclin A2 expression in primary human neural progenitor cells, it increased cyclin A2 expression in human astrocytes. Since cyclin A2 is an essential regulator of the cell cycle, the aim of the present study is to clarify the effect of cocaine on proliferation of human astrocytes and elucidate the underlying molecular mechanisms.
Methods:
Primary human astrocytes were treated with either 1, 10, or 100 μM cocaine for 48 hr, and cell proliferation was measured using the CyQUANT cell proliferation assay. To elucidate the molecular mechanisms through which cocaine affects the proliferation of astrocytes, we analyzed gene expression profiles in cocaine-treated primary human astrocytes using a human focused cDNA array. Gene ontology/pathway enrichment analysis, STRING protein-protein interaction analysis, RT-qPCR, and western blotting were used to identify signal transduction pathways that are involved in cocaine-induced astrocyte dysfunction.
Results:
Cocaine at 10 and 100 μM significantly increased human astrocyte proliferation. Gene expression profiling revealed the JNK MAP kinase pathway as a driver of cell proliferation affected by cocaine in human astrocytes. Further experiments showed that cocaine-induced JNK activation induced up-regulation of cyclin A2, leading to enhanced proliferation of human astrocytes.
Conclusion:
Cocaine-induced abnormal increases in the number of astrocytes may cause disruption in neuron-glia signaling and contribute to synaptic impairment in the CNS. Understanding the mechanisms of cocaine’s effects on human astrocytes may help to reveal the involvement of glial cells in addictive behaviors.
Keywords: Astrocytes, cocaine, reactive astrogliosis, addiction, JNK, cyclin A
1. Introduction
Astrocytes have significant structural, metabolic and trophic roles in modulating and coordinating neuronal structure and function, both in normal brain function and in disease states (Araque & Navarrete, 2010). Astrocytes mediate immune surveillance and inflammatory responses, guide neural development, regulate neuronal excitability, and modulate communication within neural networks (Ricci et al., 2009; Bernardinelli et al., 2014). Astrocytes are essential for brain homeostasis, while brain insults of various types often trigger a complex change known as reactive astrogliosis (Ricci et al., 2009). Increasing evidence shows that prolonged substance abuse can propagate a cascade of cellular insults that ultimately lead to the activation of neuroinflammatory pathways and perturbs CNS networks (Cadet & Bisagno, 2014). Notably, alterations in astrocyte morphology such as elongation of GFAP-positive processes have been identified in the hippocampus of drug abusers (Weber et al., 2013). Nevertheless, effects of drugs of abuse on astrocytes in shaping neuronal dynamics and astrocyte-neuron ensembles remain unclear.
1.1. The role of astrocytes in CNS development
During development of the central nervous system (CNS), astrocytes play significant roles in guiding neuronal differentiation (Johansson & Stromberg, 2002), migration (Cardenas et al., 2014), synaptogenesis (Allen, 2013; Clarke & Barres, 2013; Ullian et al., 2004, 2001), and activity-dependent synaptic remodeling (Chung et al., 2013; Chung et al., 2015; Tasdemir-Yilmaz & Freeman, 2014). They have also been suggested to play a role in activity-dependent myelination (Ishibashi et al., 2006). Therefore, developmental defects in astrocyte proliferation and differentiation can negatively impact neuronal development and formation of the precise neuronal connectivity required for correct development and function of the CNS (Khakh & Sofroniew, 2015; Pekny et al., 2016; Sofroniew & Vinters, 2010; Stipursky et al., 2012). In support of this, overproduction of astrocytes at the expense of neurons has been seen in the brains of fetuses with Down’s syndrome (Zdaniuk et al., 2011), as well as in mouse models of other genetic neurodevelopmental diseases that manifest in humans with cognitive impairment (Sloan & Barres, 2014). These neurodevelopmental diseases include cardio-facio-cutaneous syndrome (Urosevic et al., 2011), Costello syndrome (Paquin et al., 2009), neurofibromatosis type 1 (Hegedus et al., 2007), and Noonan’s syndrome (Gauthier et al., 2007). Many recent studies have also reported upregulation of astrocytic genes in individuals with autism spectrum disorders (ASDs; reviewed by Sloan & Barres, 2014), suggesting that astrocytic dysfunction may be a neuropathological factor in these disorders.
1.2. The role of astrocytes in addiction
In the adult brain, astrocytes are involved in regulating glutamatergic and GABAergic neurotransmission, supplying substrates for neuronal metabolism, and maintaining ionic balance in the brain (Schousboe, 2004; Stobart & Anderson, 2013). They are also a key component of the blood-brain barrier, regulating transport of materials between the brain and the blood supply (Cheslow & Alvarez, 2016), and can be markedly affected by exposure to substances of abuse. For example, exposure of rats to cocaine or methamphetamine resulted in reactive astrogliosis (characterized by increased astrocytic proliferation and expression of glial fibrillary acidic protein, GFAP) in addiction-associated brain regions that undergo neuronal synaptic plasticity in response to these drugs. These brain regions include the nucleus accumbens, prefrontal cortex, and hippocampus (Fattore et al., 2002; Bowers & Kalivas 2003; O’Callaghan & Miller, 1994; Ferrario et al., 2005; Robinson&Kolb, 2004, 1999; Robinsonetal.,2001). Furthermore, cocaine exposure has been shown to decrease astrocytic expression and activity of the glutamate transporter GLT-1 (Knackstedt et al., 2010), a critical regulator of glutamate concentration in the brain (Rothstein et al., 1994). These effects of psychostimulants on astrocytes suggest that alterations in the morphology and physiology of astrocytes in brain areas critical for the manifestation of addictive behaviors may contribute to a vulnerability to initiate and persist in drug addiction.
1.3. Cyclin A2 and cocaine
Cyclin A2, a cell cycle regulator, has been implicated in the control of entry into S phase (Huet et al., 1996). Previously, we showed that cocaine decreased cyclin A2 expression in primary human neural and oligodendrocyte progenitor cells, but did not alter its expression in neurons or microglia (Lee et al., 2008; 2009). In contrast, cocaine increased the cyclin A2 transcript in human astrocytes (Lee et al., 2008; 2009). Cocaine-induced dysregulation of cyclin A2 expression in neural progenitor cells has been shown to inhibit cell proliferation and induce premature neuronal differentiation, ultimately resulting in decreased numbers of post-mitotic neurons (Lee et al., 2008, 2011; Kindberg et al., 2014; Lee et al., 2016).
The cocaine-induced increase in expression of cyclin A2 in astrocytes has led us to hypothesize that cocaine may enhance the proliferation of astrocytes, which could increase their relative numbers in the brain. To identify the specific pathways by which cocaine affects astrocyte proliferation, we examined gene expression profiles in cocaine-treated human astrocytes. More detailed knowledge of cocaine-induced astrocyte plasticity could enhance our understanding of the mechanisms involved in substance use disorders.
2. Methods
2.1. Primary human astrocyte culture
Primary human astrocytes were purchased from ScienCell Research Laboratories and incubated in poly-L-lysine coated culture flasks. Astrocytes were maintained in DMEM/F12 supplemented with 10% fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin (Invitrogen) at 37°C in a humidified atmosphere of 5% CO2 and 95% air, with the medium replaced every three days.
2.2. Immunocytochemistry
Primaryhumanastrocyteswerefixedwith4%PFA for 10 minutes, washed with PBS, and blocked with 0.2% Triton X-100 in PBS supplemented with 5% BSA and 10% goat serum. Cells were then incubated with primary antibody rabbit anti-GFAP (1:1000; DAKO) in 0.2% Triton X-100 in PBS with 5% BSA and 5% goat serum. Corresponding fluorescent-labeled secondary antibodies were used (Alexa Fluor 555 for red; R&D Systems). Images were captured using a Carl Zeiss Axiovert 200M (Jena, Germany) microscope.
2.3. Drugs
Cocaine hydrochloride was provided by the National Institute on Drug Abuse.
2.4. Cell proliferation
Primary human astrocytes were plated onto 96-well plates at 3.5×103 cells/well. After starvation for 24 hr in starvation medium (DMEM/F12 with 0.5% FBS, 50 U/ml penicillin, and 50 μg/ml streptomycin; Invitrogen), astrocytes were treated with cocaine at concentrations of 1, 10, and 100 μM for 48hr, or were left untreated as a control. Cocaine hydrochloride was dissolved in sterile water at 10mM stock concentration. Cell proliferation was then measured using the CyQUANT cell proliferation assay (Invitrogen), according to the manufacturer’s protocol. Fluorescence was measured at an excitation wavelength of 485nm, and an emission wavelength of 530nm using a TECAN fluorescence microplate reader.
2.5. RT-qPCR
Total RNA was extracted from human astrocytes using RNA STAT-60 (Tel-Test) and subsequently treated with TURBO DNase (Ambion) to remove residual genomic DNA. RT-qPCR was employed to quantify cyclin A2 expression using cDNA synthesized from DNase-treated RNA (Transcriptor First Strand cDNA Synthesis Kit; Roche). RT-qPCR of cyclin A2 was accomplished using the LightCycler® 480 SYBR Green I Master with LightCycler 480 Real-Time PCR System (Roche) as described previously (Lee et al., 2015). The primer sequences and sizes of the PCR products for human cyclin A2 were 5’ GCAAACAGTAAACAGCCTGCG 3’ (sense), 5’ TCAACTAACCAGTCCACGAGG 3’ (antisense), 386 bp; and for human GAPDH were 5’ ACCACAGTCCATGCCATCAC 3’ (sense), 5’ TCCACCACCCTGTTGCTGTA 3’ (antisense), 452 bp. Measurements were performed from two separate runs of six independent biological samples, and all results were normalized to GAPDH, which did not differ between samples.
2.6. Human focused cDNA array
Subconfluent primary human astrocytes were starved for 24 hr and treated with 10 or 100 μM cocaine for 24 hr. Total RNA was extracted from astrocyte cultures using TRIzol (Life Technologies). cDNA microarray analysis was performed using a human focused array, which contained 2737 genes, incorporating 1152 genes previously employed in a specialized Neuroarray (Barrett et al., 2001). cDNA probes were prepared by reverse transcription with [33P]-dCTP from 5 μg total RNA obtained from each sample. cDNA probes were purified by using Biospin P-30 spin columns (Bio-Rad). Array membranes were prehybridized in 4ml microhy-bridization buffer (Research Genetics) in a rotating hybridization oven at 55°C for 2 hr, and then heat-denatured cDNA probes were applied to the microarrays for 18 hr at 55°C with rotation. The microarray membranes were sequentially washed in 2×SSC containing 0.1% SDS for 5min twice at room temperature, and in 2×SSC containing 0.1% SDS for 20min twice at 65◦C. The microarrays were exposed to low energy phosphorimager screens (Molecular Dynamics) for 5 days and scanned with a Phosphorimager Storm 860 system (Molecular Dynamics) at 50 μm resolution. ImageQuant software (Molecular Dynamics) was used for image analysis.
Z score transformation normalization (Cheadle et al., 2003) was employed to compare the array data between different treatments. Z score transformation allows analyzing array data independent of the original pixel intensities and can be used in the calculation of p values for significance estimates. To calculate the gene expression changes after cocaine treatments, Z scores were converted to Z ratios, which represent fold-like changes for each gene. To calculate the significant changes in gene expression, Z tests were calculated. Significance thresholds were a z-test criteria of p<0.05 (two-tailed) and a z-ratio criteria of ≥ |1.5|.
Associations of gene ontology terms with cocaine-responsive transcripts in human astrocytes were obtained with the Fast Assignment and Transference of Information (FatiGO) web tool (http://fatigo.bioinfo.cipf.es/). Enrichr (Bio-Carta) was employed for pathway enrichment analysis (http://amp.pharm.mssm.edu/Enrichr/), and STRING analysis was used to elucidate protein-protein interactions involving JNK (http://string-db.org).
The levels (1–8) in GO analysis (Ashburner et al., 2000) indicate increasing degrees of specificity. Level 1 is the most general, so that at level 1 each category contains a large number of individual genes, but is less informative. As levels increase from 1 to 8, there are more categories but each category contains fewer individual genes. Level 8 is the most specific, and can be thought of as providing information about specific molecular pathways. In FATiGo analysis, level 3 is the default and can be considered to indicate general biological processes.
2.7. Western blotting
Western blotting was performed as previously described (Leeetal., 2015). Lysates containing 30 μg total protein were separated by SDS-PAGE and transferred overnight to PVDF-FL membranes at 4°C. The membranes were immunoblotted with primary antibodies against phospho-JNK (1:1000, Santa Cruz), JNK (1:1000, Santa Cruz), and α-tubulin (1:5000, Sigma-Aldrich). Western blots were developed with infrared dye-labeled IRDye 800CW and IRDye 680CW secondary antibodies (Li-Cor Biosciences) while imaging and intensity of immunoreactive bands were quantified using the Odyssey infrared imaging system.
2.8. Statistical analysis
Statistical analyses were performed with GraphPad InStat. Data are shown as means±SEM. For multiple comparisons, data were analyzed by one-way ANOVA followed by Tukey’s compromise post-hoc test. The criterion for statistical significance was p<0.05.
3. Results
3.1. Cocaine stimulates the proliferation of human astrocytes and upregulates expression of cyclin A2
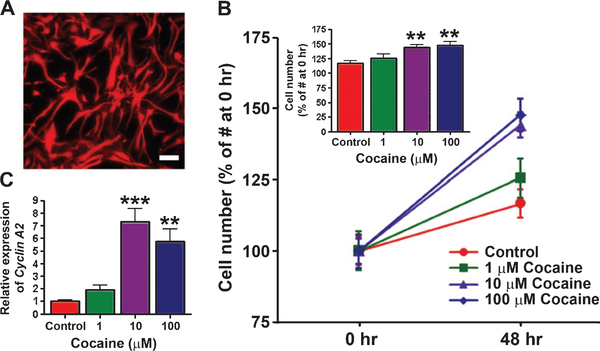
GFAP-positive primary human astrocytes (Fig. 1A) were treated with various concentrations of cocaine (1, 10, and 100 μM) for 48 hr. The number of astrocytes in each condition was then measured and compared to the number at the initiation of the treatment. As shown in Fig. 1B, the numbers of astrocytes in cocaine-treated cultures were significantly increased in a dose-dependent manner as compared to controls. Furthermore, cocaine (10 and 100 μM, 48 hr) significantly increased the expression of cylin A2 in human astrocytes (Fig. 1C).
Fig. 1.
Cocaine induces proliferation and up-regulates expression of cyclin A2 in primary human astrocytes. (A) Immunofluorescence staining for glial fibrillary acidic protein (GFAP) in primary human astrocytes. Scale bar, 50μm. (B) Primary human astrocytes were treated with cocaine at concentrations of 0, 1, 10, and 100 μM for 48 hr. Inset: dose-dependent increase of cell proliferation by 48-hr treatment with cocaine (n=9). (C) Expression of cyclin A2 in primary human astrocytes treated with cocaine. Astrocytes were treated with 0, 1, 10, and 100 μM cocaine for 48 hr, and cyclin A2 was measured by RT-qPCR analysis (n=6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s compromise post-hoc test for B and C; **p<0.01 and ***p<0.001. Data are shown as means±SEM.
3.2. Microarray expression profiling identifies JNK MAP kinase pathway as a proliferation promoter affected by cocaine
For the array data there were 40 and 88 genes differentially regulated by 10 and 100 μM cocaine, respectively, in human astrocytes (28 up-regulated and 12 down-regulated at 10 μM cocaine; 71 upregulated and 17 down-regulated at 100 μM cocaine; Supplementary Table 1).
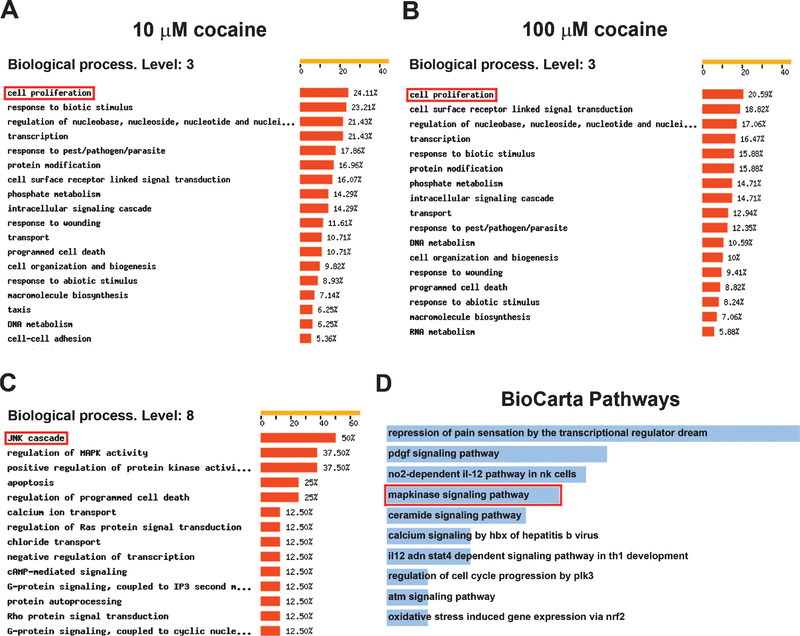
The FatiGO tool, which extracts gene ontology (GO) terms that are significantly represented in clusters of genes (Al-Shahrour et al., 2004), was used to find substantial associations of biologically relevant terms in cocaine-responsive transcripts. The results for biological processes at level 3 indicated that the largest category of genes whose transcription was altered by 10 and 100 μM cocaine treatment included genes associated with cell proliferation (Fig. 2A and B). These findings are coincident with the stimulatory effects of cocaine on human astrocyte proliferation (Fig. 1B). More specific biologically relevant terms could be found by employing the deeper levels of the GO hierarchy (Al-Shahrour et al., 2004). However, there were fewer genes with annotations at deeper GO levels. When analyzing 95 human astrocyte genes affected by either 10 or 100 μM cocaine at level 8, 50% of the genes were associated with the JNK cascade (Fig. 2C). Subsequent pathway enrichment analysis on these 95 cocaine-responsive genes resulted in 94 annotated pathways from the BioCarta database(Supplementary Table 2), the top 10 of which are shown in Fig. 2D. The MAP kinase signaling pathway, which includes the JNK MAP kinases, ranks fourth among the 94 annotated pathways (Fig. 2D and Supplementary Table 2).
Fig. 2.
Functional breakdown of microarray-identified genes which showed significant changes after cocaine treatment. (A-C) FatiGO was employed to extract Gene Ontology (GO) terms from the transcripts changed by cocaine. (A) 40 transcripts changed by 10μM cocaine categorized at biological process level 3. (B) 88 transcripts changed by 100 μM cocaine categorized at biological process level 3. (C) 95 transcripts changed by either 10 or 100 μM cocaine categorized at biological process level 8. Percentages relate to total number of genes changed by cocaine with an ontology at each biological process level. Only categories > 5% of total number of transcripts changed by cocaine are shown. (D) Pathway enrichment analysis on the BioCarta database was employed to identify signal transduction pathways from 95 transcripts changed by either 10 or 100 μM cocaine. The red rectangles indicate the potential gene ontology categories or signal transduction pathways affected by cocaine that were selected for further analysis.
3.3. Protein-protein interactions involving JNK identified by STRING
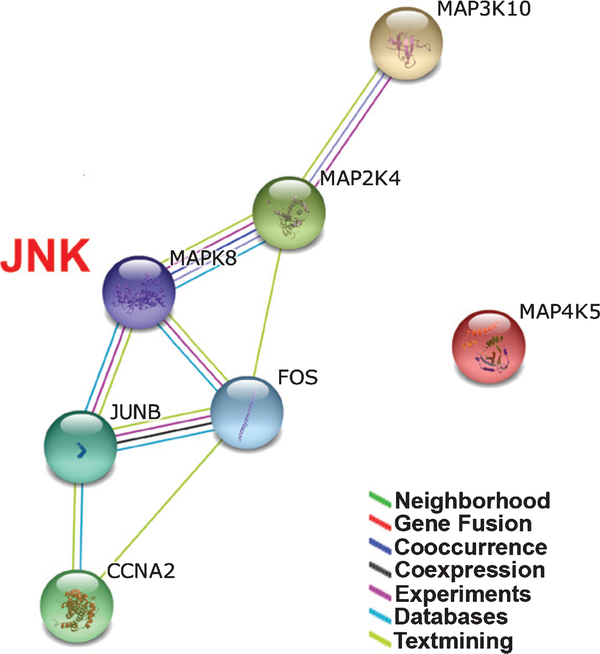
It has been demonstrated that MAP kinase-dependent activation of Fos and Jun family transcription factors is required for cell cycle progression (Zhang & Liu, 2002). Activation of Fos and Jun family transcription factors have been shown to result in the induction of cyclin A2 promoter activity, leading to cell proliferation (Sylvester et al., 1998; Andrecht et al., 2002). We therefore employed the STRING database of known and predicted protein interactions to identify interactions between JNK and other cocaine-responsive genes associated with MAPK cascades, Fos and Jun family transcription factors, and cyclin A2. Figure 3 summarizes the network of predicted protein-protein interactions for JNK (MAPK8) and the protein products of 6 cocaine-regulated genes, including MAP4K5, MAP3K10, MAP2K4, FOS, JUNB, and cyclin A2 (CCNA2). Except for MAP4K5, all of the protein components are linked together (Fig. 3).
Fig. 3.
JNK protein-protein interaction network by STRING analysis. JNK and six cocaine-regulated genes were integrated to the network. Nodes represent proteins, and differently colored lines indicate the various types of evidence for the interaction.
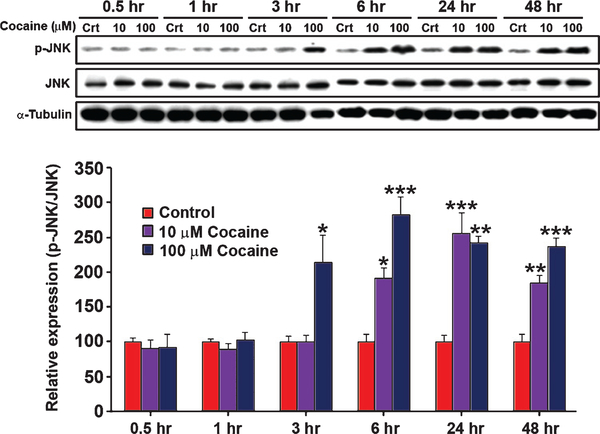
3.4. Cocaine induces JNK activation in primary human astrocytes
To determine whether cocaine activates the JNK pathway in human astrocytes, we examined JNK activation in response to 10 or 100 μM cocaine at various time points. Western blotting showed that 10 μM cocaine significantly increased the phosphorylated form of JNK within 6 hr after treatment (Fig. 4). Cocaine at 100 μM activated JNK even earlier, with a significant increase in phosphorylated JNK within 3 hr after exposure (Fig. 4). Phosphorylation of JNK remained significantly elevated up to 48 hr (Fig. 4).
Fig. 4.
Time course of JNK activation in primary human astrocytes treated with cocaine. Primary human astrocytes were exposed to 10 or 100 μM cocaine for the indicated lengths of time. The phosphorylation status of JNK was determined by normalizing phosphorylated forms to total JNK proteins and expressed as percentage of the control values (n=4). Statistical analysis was performed using one-way ANOVA followed by Tukey’s compromise post-hoc test; *p<0.05, **p<0.01, and ***p<0.001. Data are shown as means±SEM.
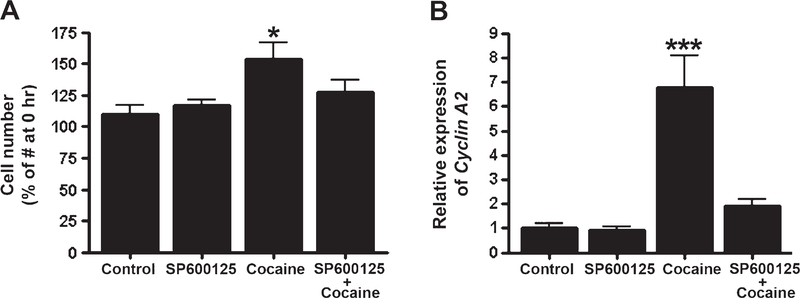
3.5. Reversal of cocaine-induced cell proliferation and increased expression of cyclin A2 in human astrocytes by JNK inhibitor
To determine whether a JNK inhibitor can reverse cocaine-induced proliferation, human astrocytes were pretreated with 10 μM SP600125 for 30min before treatment with 10 μM cocaine. Pretreatment with SP600125 reversed cocaine-induced proliferation of astrocytes (Fig. 5A). Furthermore, SP600125 also inhibited the cocaine-induced increased expression of cyclin A2 in astrocytes (Fig. 5B).
Fig. 5.
Reversal of cocaine-induced changes in cell proliferation and cyclin A2 expression in primary human astrocytes by the JNK inhibitor SP600125. SP600125 (10 μM) was applied to primary human astrocytes 30 min before 10 μM cocaine administration. Cell proliferation and expression of cyclin A2 were measured 48 hr after cocaine treatment. (A) For the cell proliferation assay, data are shown as percentages of control cell numbers at 0 hr (n=6). (B) The expression of cyclin A2 was expressed as fold changes in relationship to the control values (n=6). Statistical analysis was performed using one-way ANOVA followed by Tukey’s compromise post-hoc test; *p<0.05 and ***p<0.001. Data are shown as means±SEM.
4. Discussion
Our study shows that cocaine induces significant increases in both the astrocytic expression of cyclin A2 and the proliferation of primary human astrocytes. Consistent with these findings, gene ontology enrichment analysis of cocaine-responsive transcripts revealed “cell proliferation” as the top enriched GO term on gene sets identified after both 10 and 100 μM cocaine exposure. Subsequent pathway enrichment analysis and STRING protein network analysis suggested the JNK/MAP kinase pathway as a promoter of cocaine-induced proliferation in astrocytes. In the STRING analysis, JNK was modeled as the center of the network and was predicted to have functional interactions with other upstream components MAP3K10 and MAP2K4, as well as downstream components FOS, JUNB, and cyclin A2 (CCNA2).
Involvement of the JNK pathway was then directly tested and verified by inhibition studies using the JNK inhibitor SP600125, which reversed both the cocaine-induced astrocyte proliferation and the upregulation of cyclin A2. These data indicate that cocaine-induced activation of JNK results in an increase in cyclin A2 expression, leading to proliferation of human astrocytes.
The MAP kinase signaling cascade is a major modulator of cell growth, differentiation, and development (Seger & Krebs, 1995). It has been suggested that JNK, a member of the mammalian MAPK family, is activated by cocaine in the rat dorsal striatum and nucleus accumbens (Go et al., 2010; Boudreau et al., 2007). Moreover, cocaine has been shown to activate JNK in primary human and rat neurons (Kovalevich et al., 2015; Yao et al., 2009; Dey & Snow, 2007) as well as to lead to upregulation of transcripts in the JNK signaling pathway in the fetal mouse cerebral wall (Novikova et al., 2005). Despite the link between cocaine and JNK activation, the cellular effects of cocaine-induced JNK activation in the brain are not clear. Although cocaine exposure in vitro has been shown to induce neurotoxicity (Yao et al., 2009; Dey & Snow, 2007), we did not observe any cocaine-induced increase in cell death, as assayed by the Annexin V-FITC Apoptosis Assay (Clontech, data not shown). In our study, cocaine-induced JNK activation in human astrocytes was predicted to be mediated through MLK2 (MAP3K10) and MKK4 (MAP2K4) by STRING analysis (Fig. 3). JNK has been shown to activate FOS and JUNB (Zhang&Liu, 2002), and the expression of both FOS and JUNB was up-regulated by cocaine in human astrocytes (Supplementary Table 1; Fig. 3). Notably, it has been suggested that FOS and JUNB promote cell division through up-regulation of cyclin A2 expression during S-phase. Therefore, it is reasonable to assume that the cocaine-induced activation of JNK signaling through FOS and JUNB amplified cyclin A2 expression, resulting in enhanced proliferation of astrocytes.
Reactive astrogliosis, characterized by increased astrocytic proliferation and GFAP expression, is a common response to CNS injury and disease that leads to pathological dysfunction of astrocytes (Chung et al., 2015). Cocaine exposure has been shown to induce reactive astrogliosis in rodent brain regions responsible for the manifestation of addictive behaviors, such as the prefrontal cortex, nucleus accumbens, and hippocampus (Fattore et al., 2002; Bowers & Kalivas 2003; O’Callaghan & Miller, 1994; Ferrario et al., 2005; Robinson & Kolb, 2004, 1999; Robinson et al., 2001). There is increasing evidence that astrocytes signal with neurons and participate in synaptogenesis and synapse elimination, as well as in synaptic plasticity (Chung et al., 2015; Clarke & Barres, 2013; Ullian et al., 2004; 2001; Chung et al., 2013; Tasdemir-Yilmaz & Freeman, 2014). Drug-evoked synaptic plasticity has been shown to be involved in the development of addiction (Muñoz-Cuevas et al., 2013), yet the mechanisms that underlie these adaptive synaptic changes are not clear. Cocaine-induced reactive astrogliosis might impact astrocyte-dependent synaptic plasticity, ultimately leading to the development of addiction. Additionally, cocaine has been shown to influence synaptic transmission by down-regulation of the astrocyte glutamate transporter, GLT-1 (Knackstedt et al., 2010). Since glutamate signaling is associated with drug-seeking behavior (D’Souza, 2015), decreased uptake of glutamate by astrocytes mayalso contribute to the development of addiction.
Astrogliosis refers to the activation of astrocytes which occurs in response to brain insult or injury of various types. This may involve various changes in astrocyte properties and functions, among which are hypertrophy, process extension, increased expression of markers such as vimentin and GFAP, and increased rates of proliferation (Burda & Sofroniew, 2014; Ekmark-Lewin et al., 2010; Mohn & Koob, 2015; Suzumura et al., 1993). For certain types of brain injury, such as dopaminergic denervation and toluene exposure, astrogliosis is characterized by hypertrophy and increased vimentin and/or GFAP expression without increased astrocyte proliferation (e.g. Gotohda et al., 2000; Morales et al., 2016). Astrocytes are constantly renewed and replenished in the adult brain. Increased astrocyte proliferation is, however, seen in numerous conditions including stroke (Choudhury & Ding, 2015; Buchhold et al., 2007), prion disease (Hafiz & Brown, 2000), chronic ethanol treatment (Scheetz et al., 1988), and, notably, exposure to cocaine (Cai et al., 2016). Although proliferation is only one facet of astrogliosis, the present model may nonetheless be useful in defining the properties of astrocytes which predispose this cell type to proliferate in response to injury.
Cyclin A2 accumulates in the late G1 phase of the cell cycle and regulates S phase entry (Loukil et al., 2014). Cyclin A2 has been shown to be involved in the proliferation of reactive astrocytes (Koguchi et al., 2002). Moreover, TNF-alpha is associated with brain injury-induced reactive gliosis through MAPK signaling pathway (Zhang et al., 2000; Chiang et al., 1994). Our data show that expression of TNFSF12 and TNFRSF1B were significantly increased by 100 μM cocaine(Supplementary Table 1), suggesting a novel role of TNF-alpha signaling in drug-induced astrogliosis.
In summary, our findings demonstrate that cocaine, acting through the JNK/MAP kinase pathway, induces cyclin A2 expression and enhances the proliferation of human astrocytes. Cocaine-induced reactive astrogliosis in the brain might impact astrocyte functions such as synapse formation and regulation of neurotransmission, thereby contributing to subsequent vulnerability to drug addiction. Illuminating the mechanisms by which cocaine induces astrogliosis may lead to the development of new therapies for cocaine-induced synaptic changes caused by astrocyte dysfunction.
It is noteworthy that whereas neural progenitor cells show a decrease in proliferation in response to cocaine (Lee et al., 2008; Kindberg et al., 2014; Lee et al., 2016), astrocytes show the opposite response, an increase in proliferation. In view of the well-established property of astrocytes to proliferate following certain types of brain injury (Burda et al., 2016; Bardehle et al., 2013; Loewen et al., 2016) this differential response of astrocytes to cocaine may reflect a general proliferative tendency of astrocytes in response to various brain injury, damage, or stress. It may be that cocaine provides a model which taps into fundamental properties of astrocytes which differ from those of neurons and neural
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Aging.
Footnotes
Supplementary material
The supplementary table is available in the electronic version of this article: http://dx.doi.org/10.3233/RNN-160676.
References
- Allen NJ (2013). Role of glia in developmental synapse formation. Current Opinion in Neurobiology, 23(6), 1027–1033. [DOI] [PubMed] [Google Scholar]
- Al-Shahrour F, Diaz-Uriate R, & Dopazo J (2004). FatiGO: A web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics, 20, 578–580. [DOI] [PubMed] [Google Scholar]
- Andrecht S, Kolbus A, Hartenstein B, Angel P, & Schorpp-Kistner M (2002). Cell cycle promoting activity of JunB through cyclin A activation. Journal of Biological Chemistry, 277, 35961–35968. [DOI] [PubMed] [Google Scholar]
- Araque A, & Navarrete M (2010). Glial cells in neuronal network function. Philosophical Transactions of The Royal Society B Biological Sciences, 365(1551), 2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM,… & Sherlock G (2000). Gene Ontology: Tool for the unification of biology. Nature Genetics, 25(1), 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardehle S,Krüger M,Buggenthin F,Schwausch J,Ninkovic J, Clevers H,... & Götz M (2013). Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nature Neuroscience, 16(5), 580–586. [DOI] [PubMed] [Google Scholar]
- Barrett T, Cheadle C, Wood WB, Teichberg D, Donovan DM, Freed WJ,... & Vawter MP (2001). Assembly and use of a broadly applicable neural cDNA microarray. Restorative Neurology and Neuroscience, 18(2–3), 127–135. [PubMed] [Google Scholar]
- Bernardinelli Y, Muller D, & Nikonenko I (2014). Astrocyte-synapse structural plasticity. Neural Plasticity, 2014, 232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, & Wolf ME (2007). Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. Journal of Neuroscience, 27(39), 10621–10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, & Kalivas PW (2003). Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. European Journal of Neuroscience, 17(6), 1273–1278. [DOI] [PubMed] [Google Scholar]
- Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Kessler Ch., & A. Popa-Wagner (2007). Environmental enrichment improves functional and neuropathological indices following stroke in young and aged rats. Restorative Neurology and Neuroscience, 25(5–6), 467–484. [PubMed] [Google Scholar]
- Burda JE, & Sofroniew MV (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron, 81(2), 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Bernstein AM, & Sofroniew MV (2016). Astrocyte roles in traumatic brain injury. Experimental Neurology, 275(Pt 3), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, & Bisagno V (2014). Glial-neuronal ensembles: Partners in drug addiction-associated synaptic plasticity. Frontiers in Pharmacology, 5, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yang L, Callen S, & Buch S (2016). Multiple Faceted Roles of Cocaine in Potentiation of HAND. Current HIV Research, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Kong M, Alvarez A, Maldonado H, & Leyton L (2014). Signaling pathways involved in neuron-astrocyte adhesion and migration. Current Molecular Medicine, 14(2), 275–290. [DOI] [PubMed] [Google Scholar]
- Cheadle C, Vawter MP, Freed WJ, & Becker KG (2003). Analysis of microarray data using Z score transformation. Journal of Molecular Diagnostics, 5(2), 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslow L, & Alvarez JI (2016). Glial-endothelial crosstalk regulates blood-brain barrier function. Current Opinion in Pharmacology, 26, 39–46. [DOI] [PubMed] [Google Scholar]
- Chiang CS, Stalder A, Samimi A, & Campbell IL (1994). Reactive gliosis as a consequence of interleukin-6 expression in the brain: Studies in transgenic mice. Developmental Neuroscience, 16(3–4), 212–221. [DOI] [PubMed] [Google Scholar]
- Choudhury GR, & Ding S (2016). Reactive astrocytes and therapeutic potential in focal ischemic stroke. Neurobiology of Disease, 85, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C,...& Barres BA (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature, 504(7480), 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Welsh CA, Barres BA, & Stevens B (2015). Do glia drive synaptic and cognitive impairment in disease? Nature Neuroscience, 18(11), 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, & Barres BA (2013). Emerging roles of astrocytes in neural circuit development. Nature Reviews Neuroscience, 14(5), 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS (2015). Glutamatergic transmission in drug reward: Implications for drug addiction. Frontiers in Neuroscience, 9, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, & Snow DM (2007). Cocaine exposure in vitro induces apoptosis in fetal locus coeruleus neurons through TNF-alpha mediated induction of Bax and phosphorylation of c-Jun NH2-terminal kinase. Journal of Neurochemistry, 103(2), 542–556. [DOI] [PubMed] [Google Scholar]
- Ekmark-Lewén S, Lewén A, Israelsson C, Li GL, Farooque M, Olsson Y,... & Hillered L (2010). Vimentin and GFAP responses in astrocytes after contusion trauma to the murine brain. Restorative Neurology and Neuroscience, 28(3), 311–321. [DOI] [PubMed] [Google Scholar]
- Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, & Spiga S (2002). Astroglial in vivo response to cocaine in mouse dentate gyrus: A quantitative and qualitative analysis by confocal microscopy. Neuroscience, 110(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, & Robinson TE (2005). Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biological Psychiatry, 58(9), 751–759. [DOI] [PubMed] [Google Scholar]
- Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, & Miller FD (2007). Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noon Syndrome. Neuron, 54(2), 245–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go BS, Ahn SM, Shim I, & Choe ES (2010). Activation of c-Jun N-terminal kinase is required for the regulation of endoplasmic reticulum stress response in the rat dorsal striatum following repeated cocaine administration. Neuropharmacology, 59(1–2), 100–106. [DOI] [PubMed] [Google Scholar]
- Gotohda T, Tokunaga I, Kubo S, Morita K, Kitamura O, & Eguchi A (2000). Effect of toluene inhalation on astrocytes and neurotrophic factor in rat brain. Forensic Science International, 113(1–3), 233–238. [DOI] [PubMed] [Google Scholar]
- Hafiz FB, & Brown DR (2000). A model for the mechanism of astrogliosis in prion disease. Molecular and Cellular Neuroscience, 16(3), 221–232. [DOI] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, HartMcMahon EK, Elghazi L,... & Gutmann DH (2007). Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and ras-dependent mechanisms. Cell Stem Cell, 1(4), 443–457. [DOI] [PubMed] [Google Scholar]
- Huet X, Rech J, Plet A, Vié A, & Blanchard JM (1996). Cyclin A expression is under negative transcriptional control during the cell cycle. Molecular and Cellular Biology, 16(7), 3789–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, & Fields RD (2006). Astrocytes promote myelination in response to electrical impulses. Neuron, 49(6), 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, & Strömberg I (2002). Guidance of dopaminergic neuritic growth by immature astrocytes in organotypic cultures of rat fetal ventral mesencephalon. Journal of Comparative Neurology, 443(3), 237–249. [DOI] [PubMed] [Google Scholar]
- Khakh BS, & Sofroniew MV (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nature Neuroscience, 18(7), 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindberg AA, Bendriem RM, Spivak CE, Chen J, Handreck A, Lupica CR,... & Lee CT (2014). An in vitro model of human neocortical development using pluripotent stem cells: Cocaine-induced cytoarchitectural alterations. Disease Models & Mechanisms, 7(12), 1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, & Kalivas PW (2010). Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological Psychiatry, 67, 81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koguchi K, Nakatsuji Y, Nakayama K, & Sakoda S (2002). Modulation of astrocyte proliferation by cyclin-dependent kinase inhibitor p27(Kip1). Glia, 37(2), 93–104. [DOI] [PubMed] [Google Scholar]
- Kovalevich J, Yen W, Ozdemir A, & Langford D (2015). Cocaine induces nuclear export and degradation of neuronal retinoid X receptor-γ via a TNF-α/JNK- mediated mechanism. Journal of Neuroimmune Pharmacology, 10(1), 55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Bendriem RM, Kindberg AA, Worden LT, Williams MP, Drgon T,... & Freed WJ (2015). Functional consequences of 17q21.31/WNT3-WNT9B amplification in hPSCs with respect to neural differentiation. Cell Reports, 10(4), 616–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen J, Hayashi T, Tsai SY, Sanchez JF, Errico SL,... & Freed WJ (2008). A mechanism for the inhibition of neural progenitor cell proliferation by cocaine. PLoS Medicine, 5(6), e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen J, Kindberg AA, Bendriem RM, Spivak CE, Williams MP,... & Freed WJ (2016). CYP3A5 Mediates Effects of Cocaine on Human Neocorticogenesis: Studies using an In Vitro 3D Self-Organized hPSC Model with a Single Cortex-Like Unit. Neuropsychopharmacology, doi: 10.1038/npp.2016.156 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Chen J, Worden LT, & Freed WJ (2011). Cocaine causes deficits in radial migration and alters the distribution of glutamate and GABA neurons in the developing rat cerebral cortex. Synapse, 65(1), 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Lehrmann E, Hayashi T, Amable R, Tsai SY, Chen J,... & Freed WJ (2009). Gene expression profiling reveals distinct cocaine-responsive genes in human fetal CNS cell types. Journal of Addiction Medicine, 3(4), 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewen JL, Barker-Haliski ML, Dahle EJ, White HS, & Wilcox KS (2016). Neuronal injury, gliosis, and glial proliferation in two models of temporal lobe epilepsy. Journal of Neuropathology & Experimental Neurology, 75(4), 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukil A, Zonca M, Rebouissou C, Baldin V, Coux O, Biard-Piechaczyk M,... & Peter M (2014). High-resolution live-cell imaging reveals novel cyclin A2 degradation foci involving autophagy. Journal of Cell Science, 127(Pt 10), 2145–2150. [DOI] [PubMed] [Google Scholar]
- Mohn TC, & Koob AO (2015). Adult Astrogenesis and the Etiology of Cortical Neurodegeneration. Journal of Experimental Neuroscience, 9(Suppl 2), 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales I, Sanchez A, Rodriguez-Sabate C, & Rodriguez M (2016). The astrocytic response to the dopaminergic denervation of the striatum. Journal of Neurochemistry, 139(1), 81–95. [DOI] [PubMed] [Google Scholar]
- Muñoz-Cuevas FJ, Athilingam J, Piscopo D, & Wilbrecht L (2013). Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nature Neuroscience, 16(10), 1367–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikova SI, He F, Bai J, Badan I, Lidow IA, & Lidow MS (2005). Cocaine-induced changes in the expression of apoptosis-related genes in the fetal mouse cerebral wall. Neurotoxicology and Teratology, 27(1), 3–14. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, & Miller DB (1994). Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. Journal of Pharmacology and Experimental Therapeutics, 270, 741–751. [PubMed] [Google Scholar]
- Paquin A, Hordo C, Kaplan DR, & Miller FD (2009). Costello syndrome H-Ras alleles regulate cortical development. Developmental Biology, 330(2), 440–451. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhauser C, Lee J-M, Parpura V,...&Verkhratsky A(2016).Astrocytes:Acentral elementinneurologicaldisease.ActaNeuropathologica,131, 323–345. [DOI] [PubMed] [Google Scholar]
- Ricci G, Volpi L, Pasquali L, Petrozzi L, & Siciliano G (2009). Astrocyte-neuron interactions in neurological disorders. Journal of Biological Physics, 35(4), 317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, & Kolb B (1999). Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. European Journal of Neuroscience, 11(5), 1598–1604. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology, 47(Suppl 1), 33–46. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, & Kolb B (2001). Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse, 39(3), 257–266. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D,... & Kuncl RW (1994). Localization of neuronal and glial glutamate transporters. Neuron, 13(3), 713–725. [DOI] [PubMed] [Google Scholar]
- Scheetz AJ, Markham JA, & Fifková E (1988). Astrocyte proliferation precedes a decrease in basket cells in the dentate fascia following chronic ethanol treatment in mice. Brain Research, 460(2), 246–252. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, & Olsson LM (2004). Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochemistry International, 45(4), 521–527. [DOI] [PubMed] [Google Scholar]
- Seger R, & Krebs EG (1995). The MAPK signaling cascade. FASEB Journal, 9(9), 726–735. [PubMed] [Google Scholar]
- Sloan SA, & Barres BA (2014). Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Current Opinion in Neurobiology, 27, 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, & Vinters HV (2010). Astrocytes: Biology and pathology. Acta Neuropathologica, 119(1), 7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipursky J, Spohr TC, Sousa VO, & Gomes FC (2012). Astroglial interactions in cell-fate commitment and maturation in the central nervous system. Neurochemical Research, 37(11), 2402–2418. [DOI] [PubMed] [Google Scholar]
- Stobart JL, & Anderson CM (2013). Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Frontiers in Cellular Neuroscie, 7, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A, Sawada M, Mokuno K, Kato K, Marunouchi T, & Yamamoto H (1993). Effects of microglia-derived cytokines on astrocyte proliferation. Restorative Neurology and Neuroscience, 5(5), 347–352. [DOI] [PubMed] [Google Scholar]
- Sylvester AM, Chen D, Krasinski K, & Andreś V(1998).Role of c-fos and E2F in the induction of cyclin A transcription and vascular smooth muscle cell proliferation. Journal of Clinical Investigation, 101, 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasdemir-Yilmaz OE, & Freeman MR (2014). Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes & Development, 28(1), 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, & Barres BA (2004). Role for glia in synaptogenesis. Glia, 47(3), 209–216. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, & Barres BA(2001).Controlofsynapsenumberbyglia.Science,291, 657–661. [DOI] [PubMed] [Google Scholar]
- Urosevic J,Sauzeau V,Soto-Montenegro ML,Reig S,Desco M, Wright EMB,... & Barbacid M (2011). Constitutive activation of B-Raf in the mouse germ line provides a model for human cardio-facio-cutaneous syndrome. Proc Natl Acad Sci USA, 108(12), 5015–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Scherf N, Kahl T, Braumann UD, Scheibe P, Kuska JP,... & Franke H (2013). Quantitative analysis of astrogliosis in drug-dependent humans. Brain Research, 1500, 72–87. [DOI] [PubMed] [Google Scholar]
- Yao H,Allen JE,Zhu X,Callen S,&Buch S(2009).Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. Journal of Neuro Virology, 15(2), 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdaniuk G, Wierzba-Bobrowicz T, Szpak GM, & Stepien T (2011). Astroglia disturbances during development of the central nervous system in fetuses with Down’s syndrome. Folia Neuropathologica, 49(2), 109–114. [PubMed] [Google Scholar]
- Zhang L, Zhao W, Li B, Alkon DL, Barker JL, Chang YH,... & Rubinow DR (2000). TNF-alpha induced over-expression of GFAP is associated with MAPKs. Neuroreport, 11(2), 409–412. [DOI] [PubMed] [Google Scholar]
- Zhang W, & Liu HT (2002). MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research, 12(1), 9–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.