Abstract
Our objective was to determine the effect of vaccination and deworming at arrival (d 0) on bovine respiratory disease (BRD) incidence, mortality, and growth of stocker calves. Calves (n=80) were stratified by d −3 weight and fecal egg count (FEC) into 20 pens of 4 calves. Pens were randomly assigned to treatments in a 2×2 factorial design, testing d 0 vaccination (modified-live respiratory virus and clostridial vaccine, or not) and deworming (oral fenbendazole and levamisole, or not). Body weights were measured on days 0, 14, 28, 42, 56, 70, and 85, and FEC were measured on days −3, 28, 56, and 85. Incidence of BRD was greater for d 0 vaccination (RR=3.2), high fever (≥104°F, ≥40°C) at d 0 (RR=6), and higher d −3 FEC (RR=1.2 per 100 epg). Mortality was greater for d 0 vaccination (OR=8.3) and high fever (OR=41.6). Growth was 10.3 lb (4.7 kg) lower for d 0 vaccination, 24 lb (11 kg) and 16 lb (7.3 kg) lower for moderate (103°F to 103.9°F; 39.4°C to 39.9°C) and high fever, respectively, and 17.6 lb (8 kg) lower for each additional BRD treatment a calf received. Deworming was neither beneficial nor detrimental to any health or performance factors. Health and growth performance of stocker calves may be adversely affected by vaccination at arrival, higher arrival FEC, and fever at arrival.
Keywords: bovine respiratory disease, BRD, vaccination, performance, deworming, stocker cattle
Résumé
Notre objectif était de déterminer l’effet de la vaccination et de la vermifugation à l’arrivée (j0) sur l’incidence du complexe respiratoire bovin (CRB), la mortalité et la croissance des veaux d’élevage. Les veaux (n=80) ont été stratifiés selon le poids et le compte d’œufs fécaux (COF) à j-3 et placés dans 20 enclos avec chacun quatre veaux. Les enclos étaient assignés aléatoirement aux traitements selon un plan factoriel 2×2 avec la vaccination à j0 (avec ou sans vaccin anti-clostridial avec virus respiratoires vivants modifiés) et la vermifugation (avec ou sans injection orale de fenbendazole et de lévamisole) comme facteurs. Le poids corporel a été mesuré aux jours 0, 14, 28, 42, 56, 70 et 85 et le COF a été fait aux jours −3, 28, 56 et 85. L’incidence du CRB était plus élevée suivant la vaccination à j0 (RR=3.2), lorsque la fièvre était élevée à j0 (≥104°F, ≥40°C) (RR=6) et lorsque le COF était plus élevé à j-3 (RR=1.2 par 100 oeufs par gramme). La mortalité était plus élevée suivant la vaccination à j0 (RC=8.3) et lorsque la fièvre était élevée (RC=41.6). Il y a eu une perte de croissance de 10.3 lb (4.6 kg) suivant la vaccination à j0, une perte de 24.1 lb (11 kg) lorsque la fièvre était modérée (103–103.9°F), une perte de 16 lb (7.3 kg) lorsque la fièvre était élevée et une perte de 17.5 lb (8 kg) pour chaque traitement additionnel contre le CRB reçu par un veau. La vermifugation n’a pas eu d’effet bénéfique ou néfaste sur tous les facteurs reliés à la santé ou à la performance. La santé et la croissance des veaux d’élevage peuvent être affectées négativement par la vaccination à l’arrivée, par un COF initialement élevé et par la fièvre à l’arrivée.
Introduction
Bovine respiratory disease (BRD) is the most common illness among post-weaning calves arriving in feedlots or stocker operations.8 Ninety-seven percent of US feedlots report having cattle with BRD, and an estimated 16.2% of cattle placed in feedlots are affected with BRD.8 Cattle moving through market channels are at risk for BRD because they are potentially exposed to multiple pathogens due to commingling cattle from many sources, in addition to environmental stressors such as weaning, transportation, poor nutrient intake, and dehydration. In an attempt to reduce BRD morbidity and mortality, calves are often vaccinated against BRD pathogens at arrival into the stocker or feedlot operation.18 However, vaccinating against viral and bacterial pathogens may be less effective if administered during a period of stress-induced immunosuppression.13 The data supporting vaccination at arrival in North American feedlots are equivocal at best, and some studies of arrival vaccination suggest it does not benefit or may even decrease health performance.6,9,13
Gastrointestinal parasitism is a leading cause of diminished health and productivity of calves arriving into stocker or feedlot operations. Weaned beef calves maintained on pasture are exposed to residual populations of infective larvae leading to new infections on top of existing nematode burdens.2 Parasitic infections may affect feed intake, feed digestibility, and a variety of physiological processes that lead to a decline in herd productivity, and ultimately a decrease in value.10 Anthelmintic treatment of stocker cattle has been recommended to decrease parasitic loads with the goal of improving overall health and growth performance.1,3 However, there is little published research that allows us to quantify the health and performance benefits from deworming stocker cattle at arrival.1,7
Thus, the objective of this study was to determine the effect of on-arrival vaccination and deworming on stocker cattle health and growth performance.
Materials and Methods
Experimental design
The study design was approved by the Mississippi State University Institutional Animal Care and Use Committee (IACUC #15–003). A randomized controlled trial in a 2×2 factorial design was conducted to test 2 levels of vaccination (vaccination or not) and 2 levels of deworming (dewormed or not) on the health and growth performance of stocker calves maintained in grass paddocks.
Sample size calculation
The sample size of 10 pens per main effect with 4 calves per pen was calculated to provide 80% power to detect a difference between 50% and 20% morbidity (relative risk=2.5) at alpha=0.05 and assuming a mild clustering effect of pen. Also, that number of pens was sufficient to provide 80% power to detect a difference between 456 and 484 lb (206.8 and 219.5 kg) of body weight (e.g. at d 56), assuming a standard deviation of 20 lb (9.1 kg) at alpha=0.05.
Treatment allocation
Prior to receiving, all cattle were ear notched to determine bovine viral diarrhea virus persistent infection (BVDV-PI) status, and bulls (n=64) were castrated by the order buyer prior to arrival at the research facility. Eighty multi-source, auction-market derived steers were received into the Leveck Animal Research facility at Mississippi State University on February 27, 2015 (d −3). On d −3 steers were weighed, rectal temperatures were recorded, and fecal samples were collected via manual rectal evacuation. Fecal egg counts (FEC) were quantified using a 1 egg per gram (EPG) sensitivity modified-Wisconsin procedure.
On March 2, 2015 (d 0), cattle were assigned to 20 pens of 4 animals each, stratified by d −3 weight and FEC. Five pens were randomly assigned to each treatment combination in balanced fashion using spreadsheet software.a Different colored ear tags were assigned to cattle in each of the 4 treatment combinations (vaccinated and dewormed, vaccinated and not dewormed, not vaccinated and dewormed, not vaccinated and not dewormed) to help caretakers prevent contact between treatment groups during processing. Pens were arranged such that cattle in different pens did not have nose-to-nose contact. Each pen was a 2.5 acre grass paddock. Grass was sufficiently abundant so that no supplementary sources of protein or energy were provided. A mineral mix with salt and monensin was provided free-choice.
At d 0 calves in the vaccinated treatment pens received a modified-live respiratory virus vaccine labeled to aid in prevention of respiratory disease caused by bovine viral diarrhea virus (BVDV) types 1 and 2, infectious bovine rhinotracheitis (BHV1), and bovine respiratory syncytial virus (BRSV) and to aid in the reduction of respiratory disease caused by parainfluenza 3 (PI3) virus.b Vaccinated calves also received a clostridial bacterin-toxoid labeled to aid in the prevention of disease caused by Clostridium chauvoei, septicum, novyi, sordelli, and perfringens Types C and D.c Also at d 0, calves in the deworming treatment pens received fenbendazoled at 2.3 mg/lb (5 mg/kg PO) and levamisolee at 3.6 mg/lb (8 mg/kg PO). At day 56, cattle in all treatment groups were vaccinated using the same vaccine products.
Cattle were on trial for 85 days, and monitored daily in their paddocks for clinical signs associated with BRD by farm personnel trained to identify the signs, including depression, anorexia, rapid respiratory rate, cough, nasal discharge, and rectal temperature ≥ 104°F (40°C). Individual animal body weights and blood samples from the jugular vein were collected on days 0, 14, 28, 56, 70, and 85, and fecal samples were collected per rectum on days −3, 28, 56, and 85. Serum samples were submitted to the University of Nebraska Veterinary Diagnostic Laboratory, an AAVLD accredited laboratory, for quantification of serum neutralizing (SN) antibodies against BHV1 and BVDV1 using standard methods. Briefly, heat-inactivated serum was diluted in a series of 1:2 in minimum essential medium (MEM) and incubated in the presence of 300 TCID50 of cytopathic stock assay virus (BVDV1 or BHV1) for 1 hour at 98.6°F (37°C) in a 5% CO2 atmosphere. Following incubation, diluted and incubated serum samples were mixed with 4000 washed Bos taurus turbinate cells (BT) per well. Cells were incubated for 4 (BHV-1) or 5 (BVDV1) days at 98.6°F (37°C) in 5% CO2 atmosphere and evaluated for the presence of cytopathic effect by inverted light microscopy. Control serums that have been externally validated were included on each assay run and assay viruses were back titered onto cells to ensure infectivity at appropriate dilutions.
Farm personnel were aware of which pens had received the same treatment because of the ear tag colors, but were blinded to the treatment group associated with ear tag color. Laboratory personnel performing FEC and serology were blinded to treatment group.
Treatment protocol
Cattle with clinical signs of BRD were treated with a 3-drug regimen. Those exhibiting clinical signs for the first time were administered ceftiofur crystalline free acidf at a dosage of 3 mg/lb (6.6 mg/kg) subcutaneously at the base of the ear. Those exhibiting clinical signs 7 days or more after administration of ceftiofur crystalline free acid received retreatment with florfenicolg at a dosage of 18 mg/lb (40 mg/kg) subcutaneously in the neck. Cattle with clinical signs of BRD 3 days or more after treatment with florfenicol were treated with oxytetracycline hydrochlorideh at a dosage of 9 mg/lb (19.8 mg/kg) subcutaneously in the neck. Cattle treated for BRD remained in assigned pens for the duration of the treatment period, and cattle that died underwent a field necropsy performed by a veterinarian.
Fecal egg counts
Five grams of feces were weighed in a small beaker and tap water was added to a total volume of 50 ml. The water and feces were thoroughly mixed before being strained through cheesecloth. The strained mixture was stirred once more and 10 ml were immediately added to a 15 ml tube, topped with water, and centrifuged for 10 minutes at 1500 rpm (250 g). The supernatant was drawn off, being careful not to disturb the fecal pellet, and 10 ml of Sheather’s sugar solution (1000 gm of table sugar in 726 ml of tap water, specific gravity 1.26 ± 0.3) was added and mixed thoroughly with applicator sticks. The mixture was topped off with Sheather’s solution to a positive meniscus and a coverslip was added before it was centrifuged again at 1500 rpm for 10 minutes. The cover slip was carefully removed and a few drops of additional Sheather’s were again added to produce a positive meniscus, and a second cover slip was placed to catch any remaining eggs. The second cover slip was left for 10 minutes, and then both cover slips were examined under 10x objective. All trichostrongyle/strongyle-type eggs on the coverslip were counted to obtain EPG.
Statistical analysis
Rectal body temperature measured on d 0 was categorized as normal (<103°F; <39.4°C), moderate fever (103°F to 103.9°F; 39.4°C to 39.9°C), and high fever (≥104°F; ≥40°C). Antibody titers were log (2) transformed prior to analysis. Fecal egg counts, plus 1 to account for 0 counts, were natural logarithm transformed prior to analysis as an outcome variable. Days at risk for BRD were calculated as the number of days elapsing from the day of vaccination (d 0) until 1) a diagnosis of BRD; 2) removal from the study for other reasons; or 3) completion of the 85-day observation period.
The effects of vaccination and deworming treatments on the incidence density of BRD, BRD mortality, and growth performance were measured using a Poisson, logistic, and linear regression, respectively, using commercial statistical software.i Poisson regression was used to test factors associated with BRD incidence using a generalized linear mixed model (GLMM, PROC GLIMMIX, SAS 9.4) with a Poisson distribution, log link, offset of natural log transformed days at risk, and a random effect of pen. The BRD cases that occurred within the 85-day observation period were included in the analysis. The main treatments of vaccination and deworming were retained in the model. The process of manual forward variable selection was utilized to evaluate several potential effect modifications (vaccination × deworming, vaccination × fever, vaccination × FEC) and the covariates fever, FEC at d −3, and d 0 BW.
Factors associated with mortality were tested in a multilevel logistic regression GLMM model (PROC GLIMMIX) with binary distribution and logit link. Pen was a random effect. Main effects were vaccination and deworming which remained in the model. Covariates of castration status, FEC at d −3, d 0 BW, and fever at d 0 were tested by manual forward selection.
Growth performance was evaluated in a multilevel GLMM linear regression model (PROC MIXED, SAS 9.4) to test for factors associated with the outcome of calf body weight measured every 2 weeks. The model included the random effect of pen and repeated measures of weigh day as fixed effects. Main effects of vaccination and deworming were retained in the model and covariates of castration status, FEC at d −3, BW at d 0, fever at d 0, and a time by treatment interaction were tested using manual forward selection.
Log titers and change in log titers from d 0 to d 28, d 28 to d 56, and d 56 to d 85 were analyzed using multilevel GLMM linear regression model (PROC MIXED, SAS 9.4). Main effects were vaccination and deworming with random effect of pen and covariates of castration status, FEC at d −3, BW at d 0, and fever at d 0 were tested by manual forward selection.
The effect of deworming and vaccination on natural logarithm-transformed FEC was tested using multi-level linear GLMM regression model (PROC MIXED) with random effect of pen and repeated measures of days as a fixed effect. Main effects were vaccination and deworming with random effect of pen. Covariates of castration status, BW at d 0, and fever at d 0 were tested using manual forward selection.
Statistical models were evaluated by model fit (QIC) and score statistic type III P-value. Significance was defined at alpha=0.05.
Results
Eighty market-derived calves weighing between 375 lb (170 kg) and 575 lb (260.8 kg) with an average of 452 lb (205 kg), and confirmed to have a negative BVDV PI status, were enrolled in the study. Sixty-six of the 80 calves (82.5%) completed the 85-day observation period. Thirteen calves (16%) died during the study, and 1 calf was removed from the study due to behavioral issues on d 28.
A greater proportion of non-vaccinated cattle had fever at d 0 than vaccinated, whereas the proportion of fever among dewormed and non-dewormed cattle was more nearly equal. Of the 20 cattle in each treatment combination, moderate or high fever, respectively, were found in: 11 and 4 cattle receiving vaccination and deworming; 14 and 4 cattle receiving vaccination but not dewormed; 16 and 12 cattle receiving no vaccine, but dewormed; and 16 and 10 cattle receiving neither vaccine nor deworming treatment. Therefore, on d 0, 25 of 40 (62.5%) vaccinated and 32 of 40 (80%) non-vaccinated cattle had moderate fevers. Also, 8 of 40 (20%) vaccinated, and 22 of 40 (55%) of non-vaccinated cattle had high fevers.
BRD morbidity
Thirty-seven of the 80 calves (46.25%) were diagnosed with BRD. There were 4,055 total days at risk and overall incidence density was 9.1 cases per 1,000 calf-days. Adjusting for the other variables in the model, calves vaccinated with a modified-live respiratory virus vaccine and a clostridial bacterin-toxoid at d 0 were 3.2 times more likely to be treated for BRD at any point during the 85-day observation period (P=0.02; Figure 1). Calves that presented with a high (≥104°F or 40°C) fever at d 0 were 6 times more likely than calves with no fever to be treated for BRD during the 85-day observation period (P<0.0001; Figure 2). Calves were 1.2 times more likely to be treated for BRD for every additional 100 EPG at d 0 (P<0.0001). Deworming at arrival was not associated with BRD morbidity (P=0.71).
Figure 1.
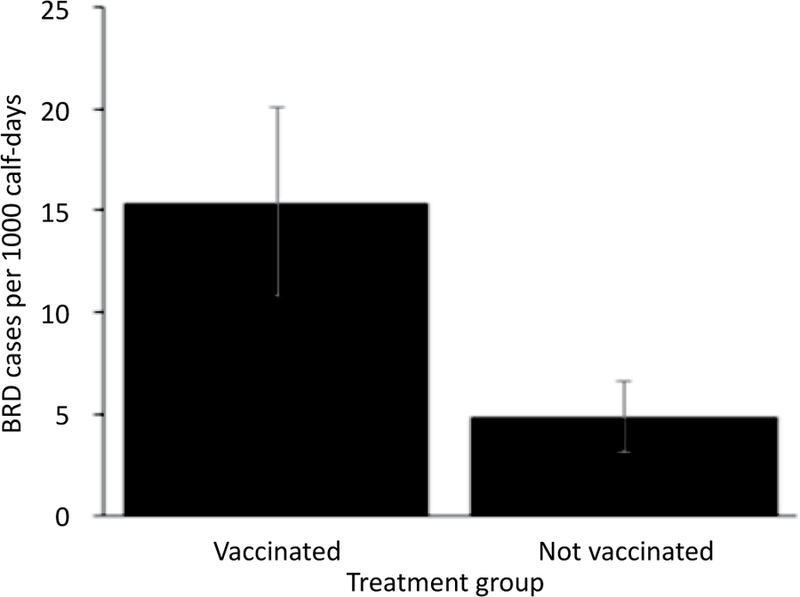
Model-adjusted BRD incidence density for calves vaccinated at arrival (n=40 calves, 10 pens) and calves not vaccinated (n=40 calves, 10 pens) from a Poisson regression model. Calves vaccinated with a modified-live respiratory virus vaccine and a clostridial bacterintoxoid at d 0 were 3.2 times more likely to be treated for BRD at any point during the 85-day observation period. Error bars represent one standard error.
Figure 2.
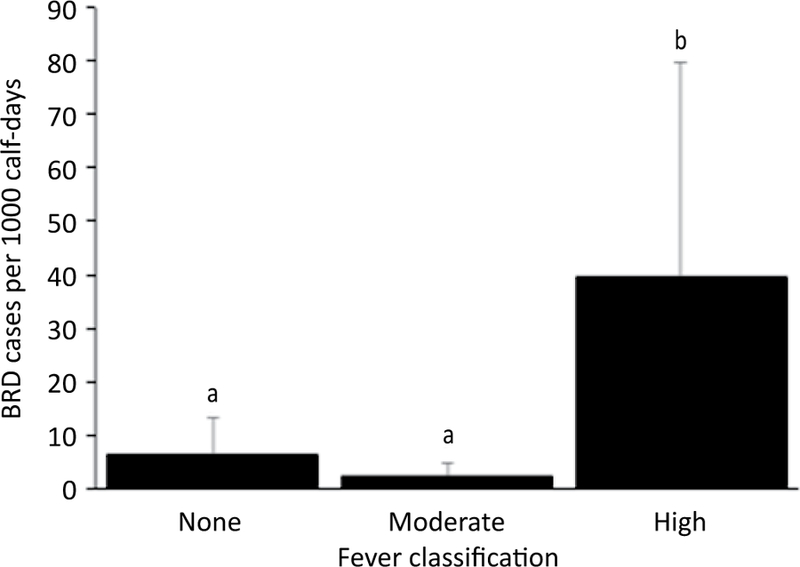
Model-adjusted BRD incidence density according to classification of rectal temperature at arrival (n=80 calves) from a Poisson regression model. Calves that presented with a high (≥104°F or 40°C) fever at d 0 were 6 times more likely than calves with no fever to be treated for BRD during the 85-day observation period. Error bars represent 1 standard error. Different superscripts indicate a statistically significant difference between variables (α=0.05).
BRD mortality
All 13 (16%) deaths were attributed to BRD based on gross postmortem findings of severe bronchopneumonia with cranioventral distribution. The BRD case fatality rate was 35%. Adjusting for other variables in the model, calves vaccinated with a modified-live respiratory virus vaccine and a clostridial bacterin-toxoid at d 0 were at 8.3 times greater odds of death (P=0.03; Figure 3). Calves that presented with a high temperature (≥104°F or 40°C) at d 0 were at 41.6 times greater odds of death compared to calves with no fever (P=0.01; Figure 4). Deworming at arrival was not associated with BRD mortality (P=0.55).
Figure 3.
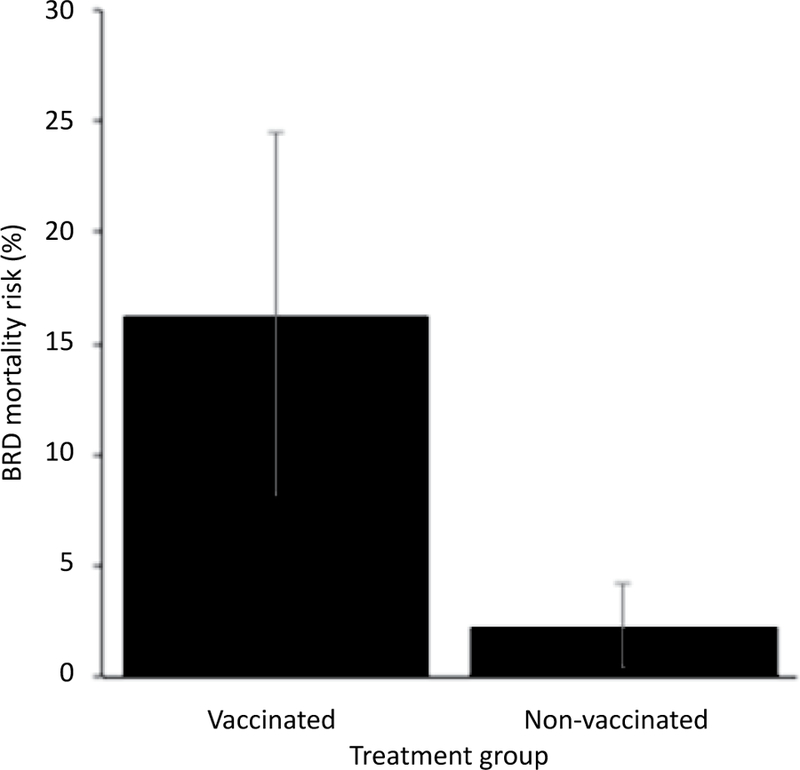
Model-adjusted BRD mortality for calves vaccinated at arrival (n=40 calves, 10 pens) and calves not vaccinated at arrival (n=40 calves, 10 pens) from a logistic regression model. Calves vaccinated with a modified-live respiratory virus vaccine and a clostridial bacterin-toxoid at d 0 had 8.3 times greater odds of death. Error bars represent 1 standard error.
Figure 4.
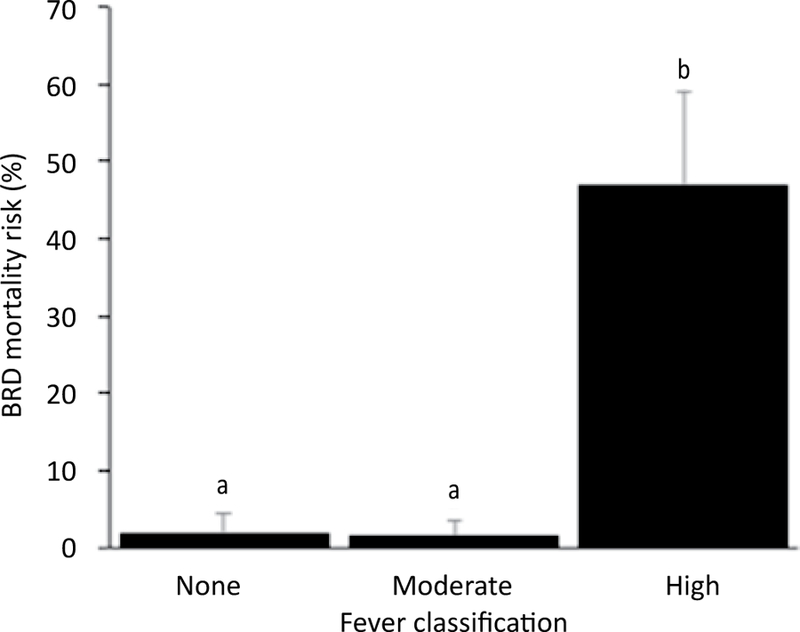
Model-adjusted BRD mortality according to classification of rectal temperature at arrival (n=80 calves, 20 pens) from a logistic regression model. Calves that presented with a high temperature (≥104°F or 40°C) at d 0 had 41.6 times greater odds of death compared to calves with no fever. Error bars represent 1 standard error. Different superscripts indicate a statistically significant difference between variables (α=0.05).
Growth performance
Surviving calves gained a total of 22,484 lb (10,199 kg) over the 85-day study period. Adjusting for other variables in the model, weights on d 28 to d 85 were significantly greater than d 0 weights (P<0.0001). Over the 85-day study period, calves vaccinated at d 0 with a modified-live respiratory virus vaccine and a clostridial bacterin-toxoid averaged 10.3 lb (4.7 kg) less than non-vaccinated calves (P=0.01; Figure 5). Surviving calves that presented with moderate fever (103 to 103.9°F or 39.44 to 39.95°C) and high fever (≥104°F or 40°C) at d 0 averaged 24 lb (10.9 kg) (P<0.0001) and 16 lb (7.3 kg) (P=0.002) less than normal-temperature calves, respectively (Figure 6). Calves lost 17.6 lb (8 kg) for each time they were treated for BRD (P<0.0001). Deworming at arrival was not significantly associated with growth performance (P=0.17).
Figure 5.
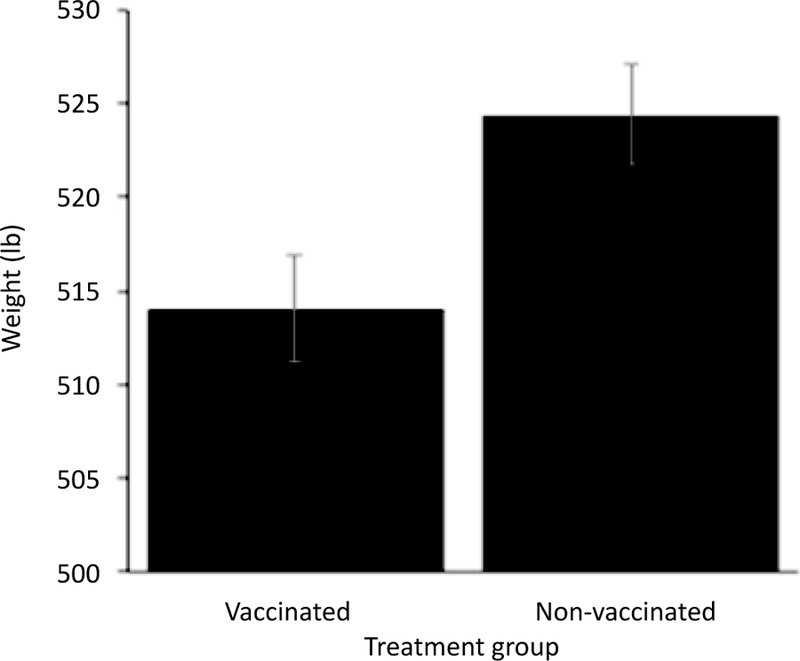
Model-adjusted final weights for calves vaccinated at arrival (n=40 calves, 10 pens) and calves not vaccinated at arrival (n=40 calves, 10 pens) from a linear regression model. Over the 85-day study period, calves vaccinated at d 0 with a modified-live respiratory virus vaccine and a clostridial bacterin-toxoid averaged 10.3 lb (4.7 kg) less than non-vaccinated calves. Error bars represent 1 standard error.
Figure 6.
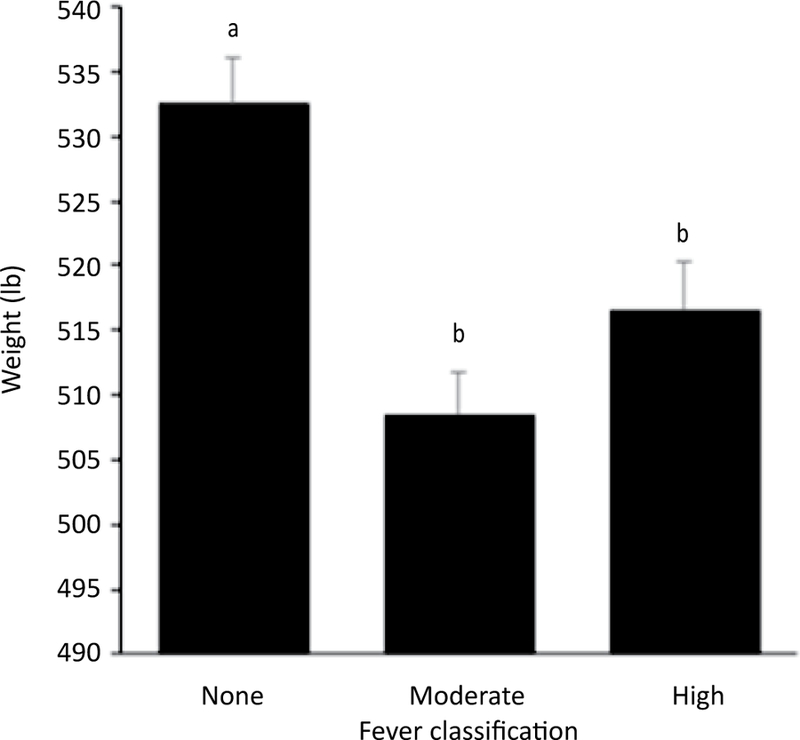
Model-adjusted final weights of calves according to classification of rectal temperature at arrival (n=80 calves, 20 pens) from a linear regression model. Surviving calves that presented with moderately elevated (103 to 103.9°F or 39.44 to 39.95°C) and high (≥104°F or 40°C) temperatures at d 0 averaged 24 lb (10.9 kg) and 16 lb (7.3 kg) less than calves with no fever, respectively. Error bars represent 1 standard error. Different superscripts indicate a statistically significant difference between variables (α=0.05).
Fecal egg counts
The geometric mean FEC at d −3 was 211 EPG, ranging from 25 to 1,703. There was no difference in log-transformed FEC between dewormed and non-dewormed (P=0.73) or vaccinated or non-vaccinated (P=0.15) cattle at arrival. However, calves purchased as bulls, compared to steers (P=0.01), and as lighter weight calves (P=0.05), had higher geometric mean FEC at arrival.
The effect of deworming on log-transformed FEC was modified by days on study. Calves dewormed at arrival had lower FEC compared to untreated controls at d 28 (P<0.0001). However, calves not dewormed also decreased in FEC over the first 56 days, and FECs were not statistically different between dewormed and non-dewormed calves at d 56 (P=0.33) or d 85 (P=0.99; Figure 7). Neither vaccine treatment (P=0.84), castration status (P=0.88), nor d 0 BW (P=0.85) were associated with FEC from d 28 - d 85.
Figure 7.
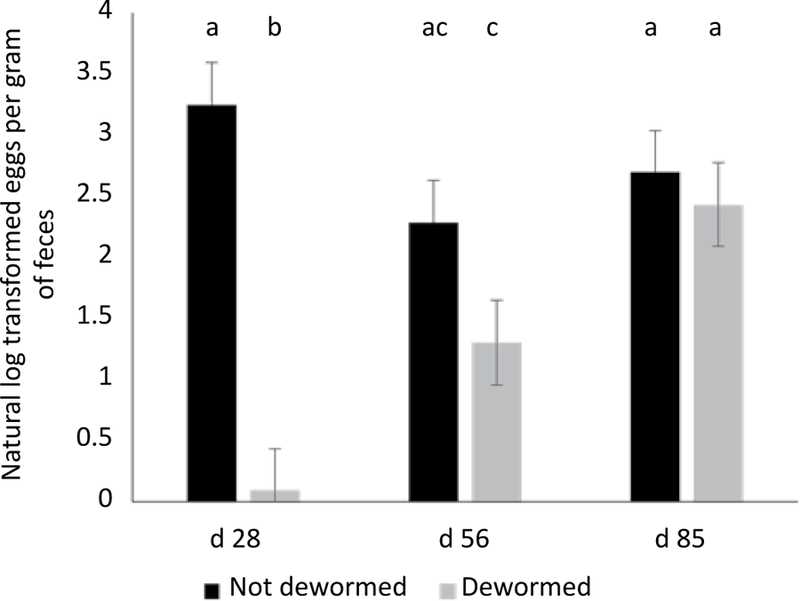
Model-adjusted natural log transformed FEC for calves dewormed (n=40, 10 pens) and not dewormed (n=40, 10 pens) at arrival from a linear regression model. Calves dewormed at arrival had lower FEC compared to untreated controls at d 28. However, calves not dewormed also decreased in FEC over the first 56 days and FECs were not statistically different between dewormed and non-dewormed calves at d 56 and d 85. Error bars represent 1 standard error. Different superscripts indicate a statistically significant difference between variables (α=0.05).
Serology
At d 0 there was no significant difference between treatment groups in SN titers for either BVDV1 (P=0.99) or BHV1 (P=0.99). There were no measured factors associated with arrival BVDV1 or BHV1 SN titers. The BVDV1 SN titers increased for both treatment groups at d 14. Vaccinated calves averaged 4.5 2-fold dilutions higher BVDV1 SN titers at d 28 (P<0.0001) and 5.1 2-fold dilutions higher BVDV1 SN titers at d 56 (P<0.0001) compared to non-vaccinated calves. Calves receiving their first dose of vaccine on d 56 had an increase in BVDV1 titers at d 70 and d 85 to levels comparable to previously vaccinated calves (Figure 8). Vaccinated calves averaged 1.9, 1.8, and 1.9 2-fold dilutions higher BHV1 SN titers at d 14 (P=0.0001), d 28 (P=0.0007), and d 56 (P=0.0004), respectively, compared to non-vaccinated calves. Both treatment groups experienced an increase in BHV1 titers by the same magnitude following vaccination at d 56 (Figure 9).
Figure 8.
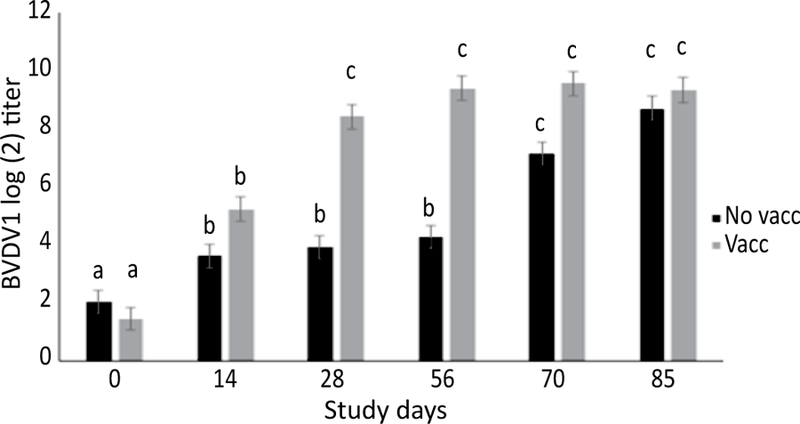
Model adjusted BVDV1 titer concentrations in calves vaccinated (n=40, 10 pens) and not vaccinated (n=40, 10 pens) at arrival (d 0) from a linear regression model. All calves received vaccine on d 56. The BVDV1 SN titers increased for both treatment groups at d 14. Vaccinated calves averaged 4.5 2-fold dilutions higher BVDV1 SN titers at d 28 and 5.1 2-fold dilutions higher BVDV1 SN titers at d 56 compared to non-vaccinated calves. Calves receiving their first dose of vaccine on d 56 had an increase in BVDV1 titers at d 70 and d 85 to levels comparable to previously vaccinated calves. Error bars represent 1 standard error. Different superscripts indicate a statistically significant difference between variables (α=0.05).
Figure 9.
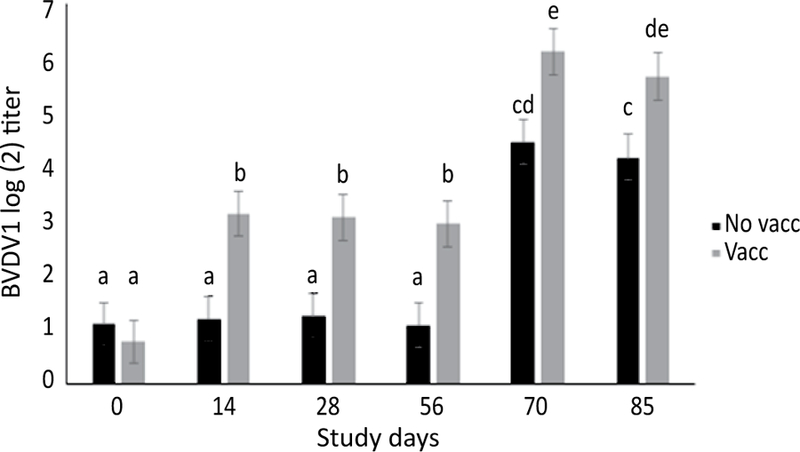
Model adjusted BHV1 titer concentrations in calves vaccinated (n=40, 10 pens) and not vaccinated (n=40, 10 pens) at arrival (d 0) from a linear regression model. All calves received vaccine on d 56. Vaccinated calves averaged 1.9, 1.8, and 1.9 2-fold dilutions higher BHV1 SN titers at d 14, d 28, and d 56, respectively, compared to non-vaccinated calves. Both treatment groups experienced an increase in BHV1 titers by the same magnitude following vaccination at d 56. Error bars represent 1 standard error. Different superscripts indicate a statistically significant difference between variables (α=0.05).
Discussion
The most important findings in this study were that: 1) vaccinating these calves on arrival adversely affected their health and growth performance; 2) deworming these calves did not affect health or growth performance; and 3) certain characteristics of these calves measured at arrival, such as FEC and body temperature, were predictive of future health and growth performance. Calves enrolled in this study typified calves entering the southeastern US market system. Although the pasture stocking rate was similar, the number of calves per paddock in this study was much less than typical grass-based stocker systems. Large populations of cattle may have rates of effective contact or social stressors that affect disease risk differently than in small populations. Therefore, future large pen studies would provide greater external validity for commercial settings.
In this study, vaccinating calves at arrival was associated with increased BRD morbidity, mortality, and lower final weights. These findings support the concept that vaccination of calves that may have been recently exposed to various stressors and are potentially incubating BRD, can increase their risk for illness. This relationship may be due in part to vaccine-induced inflammation. Vaccinating high-risk calves at arrival is a common practice, but there is limited research supporting its efficacy.9,13,16,17 In a recent study, calves vaccinated at arrival had lower average daily gain (ADG) than calves with delayed vaccination at d 14 with no difference in the incidence of respiratory disease.11 A similar study showed no difference in ADG or BRD morbidity between calves vaccinated at arrival and calves with delayed vaccination at d 14.13 Another study performed on high-risk heifer calves found that delaying administration of viral vaccine resulted in a decreased percentage of calves treated twice for BRD.14 In the study presented here, we are unable to determine which component of the vaccine products was responsible for the treatment effects. In a 1982 study, performed to measure factors associated with mortality and treatment costs in feedlot calves, the use of respiratory vaccines within 2 weeks of arrival was associated with increased mortality rates and greater treatment costs.6
The immunosuppressive effects of commingling and environmental stressors such as transportation, poor nutrition, and dehydration on stocker cattle are well documented.4,5,13 However, serology results demonstrated that the calves vaccinated on arrival were able to respond to vaccination. The vaccinated calves had a measurable humoral immune response to 2 of the vaccine components, even though this response did not protect them from BRD morbidity or mortality. Those calves unvaccinated at arrival also exhibited an increase in SN titers following vaccination at d 56.
We chose a relatively simple vaccine regimen to avoid administering an overwhelming amount of antigen or endotoxins, but even so we observed detrimental effects. Calves in this study may have developed BRD in spite of being vaccinated against 5 viral pathogens because the agents responsible for the BRD were not included in the vaccine administered. For example, we did not vaccinate against common bacterial BRD pathogens or their leukotoxins. However, this does not explain why vaccinated calves had an increased incidence of morbidity or mortality compared to non-vaccinates. There was no evidence of an immune response among non-vaccinated calves to BHV1 before they were vaccinated on d 56. There was a small rise in titer of antibodies against BVDV1 among non-vaccinates, but the BVDV1 titer remained lower than that of vaccinated calves. These results suggest that wild-type BHV1 or BVDV1 were not circulating in this group of cattle. We cannot know if these cattle were exposed to other pathogens that may or may not have been components of the vaccine. Regardless, it is possible that the results may have differed under circumstances when the vaccine products offered specific protection against the pathogens responsible for BRD among a given group of cattle. Therefore, we cannot conclude that vaccination at arrival is always detrimental to health and growth performance, although it was in this particular study.
It was interesting to note that uncastrated and lighter-weight calves had higher FEC at arrival. We speculate that this may reflect better parasite management in herds that castrate calves prior to weaning or that wean heavier calves. Regardless, deworming calves at arrival was not associated with any measured beneficial or adverse effect on health or growth performance. Calves dewormed at arrival did have lower fecal egg counts than untreated calves at d 28, but the effect was short-lived. Even though FEC at d 28 were greatly decreased by deworming at arrival, this was not reflected by improved growth performance. It is possible that this study lacked the power to detect small differences in health and growth performance following deworming. However, numerically, the calves not dewormed weighed more than the calves that were dewormed throughout the course of the 85-day study. This finding was unexpected given that many studies2,15,19 have shown that anthelmintic treatment offers a weight gain advantage, at least in the short term. It is unclear why this was the case, but might be due in part to the fact that the EPG of calves not dewormed at arrival also decreased over the first 56 days. We speculate that this natural decrease in EPG was due to improved immunity to helminths as a result of improved nutrition and decreased stress as compared to the time of arrival.
In this study, calves arriving with high FEC were at greater risk for BRD, but deworming failed to mitigate that risk. This supports the concept that gastrointestinal parasitism of stocker calves is an important constraint to animal health and productivity1 that may not be resolved by deworming at arrival. Also, because FEC at arrival was an important predictor of health and growth performance, FEC at arrival may be an important parameter to measure in studies of stocker cattle health and performance.
High fever at arrival was found to be associated with increased BRD morbidity and mortality and lower final weights. Calves arriving with high fevers were likely already incubating respiratory disease, and vaccinating at arrival may have simply been too late to reverse the pathogenesis of BRD; it may even have amplified an established inflammatory response. In this study fever was an important confounder, and had we failed to account for it we might have missed the detrimental effect of vaccination. Therefore, measuring body temperature at arrival may be helpful when performing field studies of BRD.
Calves of this body weight, procured through the auction market system, from multiple auction barns and many different farm sources, are often considered high-risk for BRD and mass medicated with antibiotics as they arrive into the stocker operation. However, we chose not to treat these calves until we observed clinical signs because the primary objective of the study was to test the effect of vaccination or deworming on incidence of BRD. Metaphylactic treatment may have prevented our observation of clinical signs and masked the effect of the treatments on our primary outcomes of BRD morbidity, mortality, and growth performance.
Our choice of vaccine products was based on the use of similar products in other studies.11–13 We did not specifically hypothesize that these products would cause adverse effects. In fact, it seems unlikely these results are a product-specific effect and one would need to evaluate multiple vaccines contemporaneously to test that hypothesis.
Conclusions
Vaccinating some groups of stocker calves at arrival may adversely affect their health and growth performance. Greater FEC and fever at arrival were important predictors of subsequent BRD-related health events and reduced performance. In spite of the importance of FEC on health and performance, deworming calves at arrival did not mitigate losses in health or performance.
Acknowledgements
This is a product of the Beef Cattle Health and Reproduction Program at Mississippi State University. This project was supported by funds from the Department of Pathobiology and Population Medicine at Mississippi State University College of Veterinary Medicine, the Department of Animal and Dairy Sciences at Mississippi State University, Dr. Ray Kaplan’s Parasitology Laboratory at the University of Georgia College of Veterinary Medicine, and the Mikell and Mary Cheek Hall Davis Endowment for Beef Cattle Health and Reproduction.
Footnotes
Endnotes
Microsoft Excel, Microsoft Corporation, Redmond, WA
Express 5, Boehringer-Ingelheim Vetmedica, St. Joseph, MO
Vision 7 with SPUR, Intervet/Merck Animal Health, Madison, NJ
Safeguard, Intervet/Merck Animal Health, Madison, NJ
Prohibit, Agrilabs, St. Joseph, MO
Excede, Zoetis, Inc., Kalamazoo, MI
Nuflor, Intervet/Merck Animal Health, Madison, NJ
Noromycin 300 LA, Norbrook Inc., Lenexa, KS
SAS, Version 9.4, SAS Institute Inc., Cary, NC
The authors declare no conflict of interest.
References
- 1.Ballweber LR. Endoparasite control. Vet Clin North Am Food Anim Pract 2006;22:451–461. [DOI] [PubMed] [Google Scholar]
- 2.Ballweber LR, Smith LL, Stuedemann JA, Yazwinski TA, Skogerboe TL. The effectiveness of a single treatment with doramectin or ivermectin in the control of gastrointestinal nematodes in grazing yearling stocker cattle. Vet Parasitol 1997;72:53–68. [DOI] [PubMed] [Google Scholar]
- 3.Baltzell P, Engelken T, O’Connor AM. A critical review and meta-analysis of the magnitude of the effect of anthelmintic use on stocker calf production parameters in Northern US States. Vet Parasitol 2015;214:2–11. [DOI] [PubMed] [Google Scholar]
- 4.Edwards TA. Control methods for bovine respiratory disease for feedlot cattle. Vet Clin North Am Food Anim Pract 2010;26:273–284. [DOI] [PubMed] [Google Scholar]
- 5.Fulton RW, Cook BJ, Step DL, Confer AW, Saliki JT, Payton ME, Burge LJ, Welsh RD, Blood KS. Evaluation of health status of calves and the impact on feedlot performance: Assessment of a retained ownership program for post-weaning calves. Can J Vet Res 2002;66:173–180. [PMC free article] [PubMed] [Google Scholar]
- 6.Martin SW, Meek AH, Davis DG, Johnson JA, Curtin RA. Factors associated with mortality and treatment costs in feedlot calves: the Bruce County Beef Project, years 1978, 1979, 1980. Can J Comp Med 1982;46:341–349. [PMC free article] [PubMed] [Google Scholar]
- 7.Mertz KJ, Hildreth MB, Epperson WB. Assessment of the effect of gastrointestinal nematode infestation on weight gain in grazing beef cattle. J Am Vet Med Assoc 2005;226:779–783. [DOI] [PubMed] [Google Scholar]
- 8.National Animal Health Monitoring System (NAHMS). Part IV: Health and management on U.S. feedlots with a capacity of 1,000 or more head, 2011.
- 9.Perino LJ, Hunsaker BD. A review of bovine respiratory disease vaccine efficacy Bov Pract 1997;31:59–66. [Google Scholar]
- 10.Perry BD, Randolph TF. Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet Parasitol 1999;84:145–168. [DOI] [PubMed] [Google Scholar]
- 11.Richeson JT, Beck PA, Gadberry MS, Gunter ST, Hess TW, Hubbell DS, Jones C. Effects of on-arrival versus delayed modified-live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly received beef calves. J Anim Sci 2008;86:999–1005. [DOI] [PubMed] [Google Scholar]
- 12.Richeson JT, Beck PA, Poe KD, Gadberry MS, Hess TW, Hubbell DS III. Effects of administration of a modified-live virus respiratory vaccine and timing of vaccination on health and performance of high-risk beef stocker calves. Bov Pract 2015;49:37–42. [Google Scholar]
- 13.Richeson JT, Kegley EB, Gadberry MS, Beck PA, Powell JG, Jones CA. Effects of on-arrival versus delayed clostridial or modified-live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J Anim Sci 2009;87:2409–2418. [DOI] [PubMed] [Google Scholar]
- 14.Rogers KC, Miles DG, Renter DG, Sears JE, Woodruff JL. Effects of delayed respiratory viral vaccine and/or inclusion of an immunostimulant on feedlot health, performance, and carcass merits of auction-market derived feeder heifers. Bov Pract 2016;50:154–162. [Google Scholar]
- 15.Skogerboe TL, Thompson L, Cunningham JM, Brake AC, Karle VK. The effectiveness of a single dose of doramectin pour-on in the control of gastrointestinal nematodes in yearling stocker cattle. Vet Parasitol 2000;87:173–181. [DOI] [PubMed] [Google Scholar]
- 16.Theurer ME, Larson RL, White BJ. Systematic review and meta-analysis of the effectiveness of commercially available vaccines against bovine herpesvirus, bovine viral diarrhea virus, bovine respiratory syncytial virus, and parainfluenza type 3 virus for mitigation of bovine respiratory disease complex in cattle J Am Vet Med Assoc 2015;246:126–142. [DOI] [PubMed] [Google Scholar]
- 17.Tripp HM, Step DL, Krehbiel CR, Moberly HK, Malayer JR. Evaluation of outcomes in beef cattle comparing preventive health protocols utilizing viral respiratory vaccines. Bov Pract 2013;47:54–64. [Google Scholar]
- 18.Wildman BK, Jim GK, Perrett T, Schunicht OC, Hannon SJ, Fenton RK, Abutarbush SM, Booker CW. A comparison of two multivalent viral vaccine programs in feedlot calves at high risk of developing undifferentiated fever/bovine respiratory disease. Bov Pract 2009;43:130–139. [Google Scholar]
- 19.Williams JC, Loyacano AF, DeRosa A, Gurie J, Clymer BC, Guerino F. A comparison of persistent anthelmintic efficacy of topical formulations of doramectin, ivermectin, eprinomectin and moxidectin against naturally acquired nematode infections of beef calves. Vet Parasitol 1999;85:277–288. [DOI] [PubMed] [Google Scholar]


