Abstract
Objective:
Arterial ageing is characterized by increasing arterial stiffness as measured by pulse wave velocity (PWV). This process is enhanced in participants with early vascular ageing (EVA), but slowed in participants with healthy vascular ageing (HVA). We aimed to describe characteristics of EVA and HVA in a transcontinental study including 11 cohorts.
Methods:
In all, 18 490 participants from the global MARE Consortium, free of cardiovascular disease, participated with data on PWV and cardiometabolic risk factors. We defined HVA as the lowest 10% and EVA as the highest 10% of the standardized PWV distribution, adjusted for age intervals. HVA individuals were compared with the 90% of non-HVA individuals with ANCOVA, adjusted for age, sex and hypertension.
Results:
The 1723 HVA participants were at the same age as the rest of the population, more likely women (59.4 vs 57.0%), and with significantly lower levels of established cardiovascular risk factors (blood pressure, lipids, glucose). Similarly, the prevalence rate of obesity, diabetes mellitus, hypertension and the metabolic syndrome was lower in the HVA participants. In the presence of similar levels of cardiovascular risk factors, HVA participants in the 50–64 years of age group presented lower PWV 5.8 (SD 0.5) vs. 7.4 (1.4) m/s (P < 0.0001) than control individuals in the 35–49 years of age group, corresponding to an estimated difference in chronological age of 14 years.
Conclusion:
Participants with healthy vascular ageing (HVA), belonging to the lowest end of the PWV distribution, are in general characterized by an up to 14 years estimated younger biological (vascular) age than those with higher PWV values, and have lower levels of risk factors.
Keywords: age, arterial, blood pressure, cohort, epidemiology, health, metabolic syndrome, risk factor
INTRODUCTION
Large arteries, as the aorta and its main branches, have a conduit function by delivering the blood from the center to the periphery, as well as a cushioning function, transforming the pulsatile flow at the heart level into a steady flow at the tissue level. The cushioning function depends on the viscoelastic properties of arterial walls and on the arterial geometry [1,2]. With advancing age, there is decreased elastin and increased collagen content in the arterial wall, as well as increased cross-linking of collagen fibres. This stiffening process is enhanced by the mechano-transduction of shear stress and elevated blood pressure on the smooth muscle cell differentiation of the arterial wall [3]. Increased arterial stiffness, indexed as pulse wave velocity (PWV), is an established hall-mark of arterial ageing and can effectively be used to calculate individual risk of cardiovascular events and related disability [4–5]. Notably, at older ages, the stiffening of large artery becomes progressively independent of blood pressure levels [6].
Arterial ageing also reflects a genetic predisposition [7,8] and exposure to multiple risk factors often occurring together [9,10]. Arterial stiffening begins early in life [11], as indicated and described by the early vascular ageing (EVA) concept [12,13] with recent data showing high prevalence of the syndrome in individuals below 50 years of age [14]. Therefore, a life-course approach aimed at the early identification of individuals with accelerated arterial ageing has been proposed as a mean to implement effective prevention of cardiovascular events and improved hypertension control [15].
Not all individuals, however, undergo this vascular ageing process in a uniform way. In fact, some individuals have lower than expected PWV for a certain chronological age, the opposite of EVA, and this has recently been called Healthy Vascular Ageing (HVA) [16]. In the Framingham Heart Study, it was reported that participants without hypertension and with PWV below a low threshold (determined from European reference values [17]), as markers of HVA, had less cardiovascular risk factors in general and less changes to the arterial system, associated with a lower prospective risk of cardiovascular events [16]. Another recent American study focused on coronary artery calcium (CAC) nondevelopment as hallmarks of HVA in the MESA Study [18], and concluded that participants with persistent CAC = 0 were individuals with lower long-term cardiovascular risk than expected from individual cardiovascular risk factors. As an alternative to the two previous definitions, hypothesizing that ageing cannot be avoided but active and healthy ageing is an achievable goal, HVA will account the lowest end of the PWV distribution of a given population: for example, the lowest 10% according to age category, without consideration of hypertension or treatment status.
We used data from the international MARE cohort collaboration [19], including PWV measurements in approximately 20 000 individuals free of clinical overt cardiovascular disease to apply this simplified definition. The primary aim of the present analyses was, therefore, to describe the risk factor profile of HVA participants (using an alternative definition of HVA – 10% lowest of PWV distribution) from MARE for a better representativeness of a global population and based on data from a wide age range of both sex. A secondary aim was to investigate whether the prevalence and the risk factor profile of HVA changed with advancing age.
PARTICIPANTS AND METHODS
The Metabolic syndrome and Artery REsearch Consortium
The original MARE (Metabolic syndrome and Artery REsearch) Consortium was established as a collaboration among European, American, and Asian Centers studying population-based cohorts to identify any cross-cultural differences in clustering of metabolic syndrome, its altered components and associations with arterial ageing; to disentangle the specific role of genes and lifestyle factors (and their interactions) on the clinical presentation of MetS and on the cardiovascular risk attributable to MetS; and to develop new strategies to prevent cardiovascular events through identification of lifestyle changes. The MARE Consortium and measured variables has been previously described, for details see [19].
Each study had been approved by local Institutional Review Board or Ethical Committee and each participant gave informed consent. All studies participating in the MARE Consortium adhere to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects, Revised 13 November 2001, effective 13 December 2001. The cohorts participating to the present analysis are briefly described in Supplemental Material, http://links.lww.com/HJH/A961.
Pulse wave velocity measurements
PWV was measured noninvasively and calculated from foot-to-foot delay between carotid and femoral artery waveforms and body surface measurement of the distance between carotid and femoral pulse recording sites. In accordance with recent recommendations [17], PWV measured in each cohort was ‘normalized’ as follows:
For devices entering the direct distance (common carotid artery-common femoral artery):
PWV ‘normalized’ = PWV measured × 0.8.
For devices entering the subtracted distance (common carotid artery–common femoral artery):
PWV ‘normalized’ = PWV measured.
Definition of healthy vascular ageing and early vascular ageing
For this analysis, we excluded all participants with coronary and cerebrovascular disease according to self-reported medical history and medical records. Thus, 18 490 participants from the MARE Consortium free of cardiovascular disease in whom PWV had been assessed represented our study population.
There is at present no clear definition of HVA. Therefore, we adopted two definitions of HVA: the first one was undertaken based on the idea that ageing is not an avoidable phenomenon, but healthy ageing is possible and represents a life-long condition. The second definition referred to arterial ageing as an avoidable phenomenon.
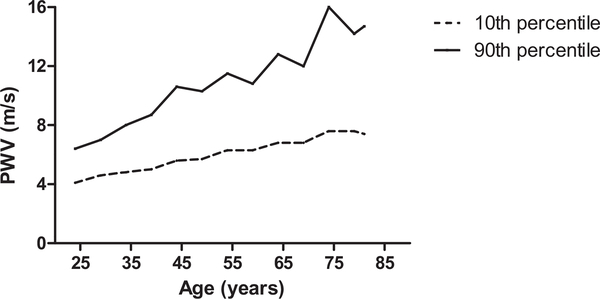
Therefore, in the first definition, HVA was defined as a PWV value below the age-quintile specific 10th percentile - given that PWV is a highly age-associated trait. For the second definition, we adopted an approach similar (but not identical) to the one proposed by a previous study [15], that is, we identified the lower 10th percentile of PWV distribution in younger participants (age <35 years) with normal blood pressure levels as reference. The first definition was adopted for the primary analysis of the current study. Correspondingly, EVA was defined as a PWV value above the age-quintile specific 90th percentile. Figure 1 illustrates the trend in the age-specific threshold values of PWV adopted to define HVA and EVA.
FIGURE 1.
Age-specific lower (10th) and upper (90th) percentile for PWV in participants without prevalent cardiovascular disease (myocardial infarction and/or stroke).
Statistical methods
All analyses were performed using the SAS package for Windows (9.1 Version Cary, North Carolina, USA). Data are presented as mean ± SD or proportions, unless otherwise specified. Student’s t-test was adopted to compare means in participants without and with HVA. ANOVA analysis was adopted to compare variables across age groups. ANCOVA analysis was adopted to compare means across groups after controlling for age, sex, and hypertension.
The definition of HVA based upon age-specific PWV lower percentiles was adopted for further statistical modelling. To have some insight as to whether factors protecting from arterial ageing are the same that, when elevated, accelerates arterial ageing, we compared cardiometabolic risk profile in participants with HVA or with EVA, and with all those without HVA. Multivariable logistic regression analysis was, therefore, adopted to identify determinants of HVA and EVA. A two-sided P value less than 0.05 indicated statistical significance.
RESULTS
Primary analysis was conducted adopting age-specific (quintile) lower 10th percentile to define HVA. The 1723 HVA participants were at same age as the rest of the population, more likely women (59.4 vs. 57.0%), and with significantly lower levels of established cardiovascular risk factors (blood pressure, lipids, glucose) - as illustrated in Table 1, left columns. Similarly, the prevalence rate of obesity (but not overweight), diabetes mellitus, hypertension, low HDL cholesterol, and the metabolic syndrome was much lower in the HVA participants. By construction, PWV was significantly lower in the HVA group, 5.5 (SD: 0.9) vs. 8.8 (2.9) m/s (P < 0.0001).
Table 1.
Characteristics of participants with healthy vascular ageing and the general study population (controls), based on the two definitions (see Methods)
| HVA defined as PWV value below the age-quintile specific 10th percentile |
HVA defined as PWV lower than 4.3 m/s, that is, 10th percentile in the younger (<35 years) normotensive population |
|||||
|---|---|---|---|---|---|---|
| Control (n = 16803) | HVA (n = 1723) | P value | Control (n = 18238) | HVA (n = 288) | P value | |
| Age (years) | 52.5 ± 16.5 | 52.4 ± 17.1 | 0.76 | 53.0 ± 16.2 | 22.3 ± 10.6 | 0.0001 |
| Women (%) | 57.0 | 59.4 | .05 | 57.1 | 64.2 | 0.01 |
| BMI (kg/m2) | 27.1 ± 5.2 | 25.2 ± 4.2 | .0001 | 27.0 ± 5.1 | 21.1 ± 2.9 | 0.0001 |
| Waist circumference (cm) | 91.0 ± 14.0 | 85.1 ± 12.8 | 0.0001 | 90.7 ± 13.9 | 73.3 ± 7.8 | 0.0001 |
| Fasting glucose (mg/dl) | 96.1 ± 24.6 | 88.9 ± 18.2 | 0.0001 | 95.7 ± 24.3 | 79.4 ± 9.3 | 0.0001 |
| Total cholesterol (mg/dl) | 218.4 ± 50.2 | 204.3 ± 41.8 | 0.0001 | 217.8 ± 49.5 | 171.7 ± 30.7 | 0.0001 |
| HDL cholesterol (mg/dl) | 58.4 ± 18.7 | 63.4 ± 17.5 | 0.0001 | 58.8 ± 18.8 | 61.8 ± 13.7 | 0.0001 |
| Non-HDL cholesterol (mg/dl) | 160.1 ± 51.9 | 140.9 ± 40.7 | 0.0001 | 159.1 ± 51.2 | 109.9 ± 27.8 | 0.0001 |
| Triglycerides (mg/dl) | 117.4 ± 123.9 | 87.1 ± 59.2 | 0.0001 | 115.5 ± 120.3 | 57.9 ± 31.7 | 0.0001 |
| SBP (mmHg) | 131.5 ± 19.5 | 120.5 ± 16.7 | 0.0001 | 130.7 ± 19.4 | 111.6 ± 10.7 | 0.0001 |
| DBP (mmHg) | 79.7 ± 11.5 | 73.3 ± 10.2 | 0.0001 | 79.3 ± 11.6 | 68.5 ± 7.0 | 0.0001 |
| MAP (mmHg) | 96.8 ± 13.0 | 89.0 ± 11.3 | 0.0001 | 96.3 ± 13.0 | 82.9 ± 7.3 | 0.0001 |
| PP (mmHg) | 52.0 ± 14.1 | 47.4 ± 12.4 | 0.0001 | 51.7 ± 14.1 | 43.4 ± 8.4 | 0.0001 |
| HR (bpm) | 67.7 ± 11.1 | 64.6 ± 10.2 | 0.0001 | 67.4 ± 11.0 | 67.3 ± 11.2 | 0.89 |
| Central SBP (mmHg)a (n) | 125.7 ± 19.8 (8354) | 117.3 ± 18.5 (367) | 0.0001 | 125.4 ± 19.8 (8710) | 103.4± 13.7 (11) | 0.0001 |
| Central PP (mmHg)a | 43.7 ± 14.4 | 41.4 ± 14.5 | 0.01 | 43.6 ± 14.4 | 31.3 ± 7.4 | 0.01 |
| PP amplification (%)a | 131.7 ± 30.7 | 128.2 ± 21.9 | 0.05 | 131.5 ± 30.4 | 160.1 ± 27.6 | 0.01 |
| Serum creatinine (mg/ml)a (n) | 0.85 ± 0.26 (13632) | 0.84 ± 0.19 (1685) | 0.01 | 0.85 ± 0.26 (15029) | 0.76 ± 0.15 (288) | 0.0001 |
| PWV (m/s) | 8.8 ± 2.9 | 5.5 ± 0.9 | 0.0001 | 8.6 ± 2.9 | 4.0 ± 0.2 | 0.0001 |
| PWV/MBP | 9.2 ± 2.8 | 6.3 ± 1.2 | 0.0001 | 9.0 ± 2.8 | 4.9 ± 0.5 | 0.0001 |
| CCA IMT (mm)a (n) | 0.70 ± 0.25 (9916) | 0.58 ± 0.13 (1349) | 0.0001 | 0.69 ± 0.24 (10992) | 0.48 ± 0.06 (273) | 0.0001 |
| Hypertension (%) | 41.8 | 21.0 | 0.0001 | 40.5 | 27.8 | 0.0001 |
| Diabetes mellitus (%) | 10.1 | 4.7 | 0.0001 | 9.7 | 3.5 | 0.0001 |
| Overweight (%) | 14.2 | 17.0 | 0.01 | 14.6 | 5.2 | 0.0001 |
| Obesity (%) | 23.4 | 12.5 | 0.0001 | 22.7 | 1.7 | 0.0001 |
| Metabolic syndrome | 23.0 | 8.4 | 0.0001 | 22.0 | 0 | 0.0001 |
| N of altered MetS components | 1.5 ± 1.3 | 0.8 ± 1.1 | 0.0001 | 1.5 ± 1.3 | 0.2 ± 0.4 | 0.0001 |
| Low HDL cholesterol | 24.1 | 13.1 | 0.0001 | 23.2 | 11.1 | 0.0001 |
| Elevated waist circumference | 39.9 | 27.0 | 0.0001 | 39.3 | 2.4 | 0.0001 |
| Elevated triglycerides | 21.6 | 9.6 | 0.0001 | 20.8 | 1.4 | 0.0001 |
| Elevated blood pressure | 49.2 | 25.6 | 0.0001 | 47.7 | 4.5 | 0.0001 |
| Elevated fasting glucose | 18.2 | 7.9 | 0.0001 | 17.6 | 0.3 | 0.0001 |
Means (SD) and proportions (%). Definitions of the metabolic syndrome [17] needed for elevated variables: low HDL cholesterol less than 40 mg/dl for men or less than 50 mg/dl for women. Elevated waist circumference greater than 102 cm for men or greater than 88 cm for women. Elevated triglycerides at least 150 mg/dl. Elevated blood pressure at least 130 mmHg/at least 85 mmHg or use of antihypertensive medications. Elevated fasting glucose at least 10 mg/dl or use of antidiabetic medications. HDL, high-density lipoprotein; HR, heart rate; HVA, healthy vascular ageing; PP, pulse pressure; PWV, pulse wave velocity.
Measurement not available for each participant.
A multivariable logistic regression model was constructed to identify determinants of the HVA condition as illustrated in Table 2, left columns. As expected, controlled cardiometabolic risk factors were all associated with a lower likelihood of presenting HVA. Advancing chronological age was associated with a significantly decreased likelihood of having HVA. Per each 5 years increase in age, there was approximately a 7% lower likelihood of having HVA characteristics.
Table 2.
Determinants of healthy vascular ageing by multivariable logistic regression analysis, by use of the two definitions (see Methods)
| HVA defined as PWV value below the age-quintile specific 10th percentile |
HVA defined as PWV lower than 4.3 m/s, that is, 10th percentile in the younger (<35 years) normotensive population |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (per year) | 0.985 | 0.982–0.988 | 0.0001 | 1.17 | 1.15 – 1.19 | 0.0001 |
| Female sex | 1.06 | 0.95 – 1.18 | 0.32 | 1.44 | 1.10–1.89 | 0.01 |
| Diabetes mellitus | 1.30 | 0.92 – 1.86 | 0.14 | N /A | N /A | |
| Low HDL cholesterol | 0.60 | 0.52 – 0.70 | 0.0001 | 0.86 | 0.57 – 1.28 | 0.45 |
| Elevated waist circumference | 0.76 | 0.66 – 0.86 | 0.0001 | 0.22 | 0.10 – 0.47 | 0.0001 |
| Elevated triglycerides | 0.57 | 0.48 – 0.68 | 0.0001 | 0.40 | 0.15 – 1.11 | 0.07 |
| Elevated blood pressure | 0.36 | 0.32 – 0.40 | 0.0001 | 0.37 | 0.21 – 0.66 | 0.001 |
| Elevated fasting glucose | 0.43 | 0.32 – 0.57 | 0.0001 | 0.35 | 0.08 – 2.61 | 0.30 |
95% CI, 95% confidence intervals; HDL, high-density lipoprotein; HVA, healthy vascular ageing; OR, odds ratios; PWV, pulse wave velocity.
Secondary analysis was conducted adopting the younger and healthier (normal BP) segment of population as reference. Notably the overall prevalence of HVA in the study population was much lower with this definition (288 vs.1723 participants with the other definition). This was attributable to the lack of HVA in participants older than age 40 years. Even with such definition, participants with HVA had significantly lower levels of established cardiovascular risk factors (blood pressure, lipids, glucose) - as illustrated in Table 1, right columns and elevated BP and abdominal obesity were associated with lower likelihood of having HVA (Table 2, left columns).
The definition of HVA based upon age-specific PWV lower percentile was adopted for further statistical modelling. To have some insight as to whether factors protecting from arterial ageing are the same that accelerates arterial ageing, we compared cardiometabolic risk profile in participants with HVA, with EVA, and with controls. PWV remained significantly lower in the HVA group after adjustment for age, sex, and hypertension (P < 0.0001), Table 3.
Table 3.
Characteristics and differences between participants with healthy vascular ageing (first definition), controls, and early vascular ageing in the Metabolic syndrome and Artery Research Consortium
| HVA (n = 1723) | Control (n = 14866) | EVA (n = 1937) | ANCOVA (adjusted for age, sex, hypertension) P for trend | |
|---|---|---|---|---|
| Age (years) | 52.4 ± 17.1 | 52.5 ± 16.5 | 52.2 ± 16.2 | — |
| Women (%) | 59.4 | 58.4 | 45.7 | — |
| BMI (kg/m2) | 25.2 ± 4.2 | 27.1 ± 5.1 | 27.8 ± 5.3 | 0.0001 |
| Waist circumference (cm) | 85.1 ± 12.8 | 90.7 ± 14.0 | 93.3 ± 13.8 | 0.0001 |
| Fasting glucose (mg/dl) | 88.9 ± 18.2 | 95.7 ± 23.4 | 99.2 ± 30.1 | 0.0001 |
| Total cholesterol (mg/dl) | 204.3 ± 41.8 | 217.8 ± 50.2 | 223.2 ± 50.1 | 0.0001 |
| HDL 0cholesterol (mg/dl) | 63.4 ± 17.5 | 59.0 ± 18.8 | 53.8 ± 17.6 | 0.0001 |
| Non-HDL cholesterol (mg/dl) | 140.9 ± 40.7 | 158.9 ± 51.9 | 169.5 ± 51.1 | 0.0001 |
| Triglycerides (mg/dl) | 88.8 ± 56.0 | 114.6 ± 120.0 | 138.7 ± 148.4 | 0.0001 |
| SBP (mmHg) | 120.5 ± 16.7 | 130.3 ± 18.9 | 140.2 ± 21.6 | 0.0001 |
| DBP (mmHg) | 73.3 ± 10.2 | 79.0 ± 11.2 | 84.8 ± 12.8 | 0.0001 |
| MAP (mmHg) | 89.0 ± 11.3 | 96.0 ± 12.6 | 103.1 ± 14.4 | 0.0001 |
| PP (mmHg) | 47.4 ± 12.4 | 51.5 ± 13.8 | 46.0 ± 16.9 | 0.001 |
| HR (bpm) | 64.6 ± 10.2 | 67.3 ± 11.0 | 70.9 ± 11.6 | 0.0001 |
| Central SBP (mmHg)a | 117.3 ± 18.5 (367) | 125.1 ± 19.1 (7290) | 130.1 ±23.5 (1064) | 0.0001 |
| Central PP (mmHg)a | 41.4 ± 14.5 | 43.4 ± 13.9 | 46.0 ± 16.9 | 0.0001 |
| Serum creatinine (mg/ml)a | 0.84 ± 0.19 | 0.85 ± 0.25 | 0.89 ± 0.37 | 0.0001 |
| PWV (m/s) | 5.5 ± 0.9 | 8.2 ± 2.2 | 13.1 ± 3.8 | 0.0001 |
| PWV/MBP | 6.3 ± 1.2 | 8.7 ± 2.2 | 12.8 ± 3.8 | 0.0001 |
| CCA IMT (mm)a | 0.58 ± 0.13 (1349) | 0.69 ± 0.24 (9148) | 0.85 ± 0.31 (768) | 0.0001 |
| PP amplification (%)a (n) | 128.2 ± 21.9 (367) | 131.6 ± 30.4 (7290) | 132.4 ±33.0 (1064) | 0.01 |
| Hypertension (%) | 26.3 | 44.1 | 48.3 | — |
| Diabetes mellitus (%) | 4.6 | 8.7 | 11.0 | 0.0001 |
| Overweight (%) | 17.0 | 14.3 | 13.5 | 0.01 |
| Obesity (%) | 12.4 | 23.0 | 26.0 | 0.0001 |
| Metabolic syndrome | 8.4 | 24.4 | 31.9 | 0.0001 |
| N of altered MetS components | 0.8 ± 1.1 | 1.5 ± 1.3 | 1.9 ± 1.3 | 0.0001 |
| Low HDL cholesterol | 13.1 | 21.8 | 31.5 | 0.0001 |
| Elevated waist circumference | 27.0 | 39.8 | 40.9 | 0.0001 |
| Elevated triglycerides | 9.6 | 20.6 | 29.6 | 0.0001 |
| Elevated blood pressure | 25.6 | 46.9 | 67.0 | 0.0001 |
| Elevated fasting glucose | 7.9 | 17.8 | 21.8 | 0.0001 |
Means (SD) and proportions (%). Definitions of the metabolic syndrome [17] needed for elevated variables: Low HDL cholesterol less than 40 mg/dl for men or less than 50 mg/dl for women. Elevated waist circumference greater than 102 cm for men or greater than 88 cm for women. Elevated triglycerides at least 150 mg/dl. Elevated blood pressure at least 130 mmHg/at least 85 mmHg or use of antihypertensive medications. Elevated fasting glucose at least 110 mg/dl or use of antidiabetic medications. CCA IMT, common carotid artery intima-media thickness; EVA, early vascular ageing; HDL, high-density lipoprotein; HR, heart rate; HVA, healthy vascular ageing; MetS, metabolic syndrome; PP, pulse pressure; PWV, pulse wave velocity.
Measurement not available for each participant.
Age and age-associated changes in profile of healthy vascular ageing
As PWV significantly increases with advancing age and that cardiometabolic risk factors impacting on arterial ageing vary with advancing age, we compared the characteristic of HVA participants in four different age groups: less than 35 years, 35–40 years, 50–64 years, 65 years and older, in Table 4.
Table 4.
Cardiometabolic risk profile in participants with healthy vascular ageing or early vascular ageing as compared with controls when stratified by age groups
| < 34 years | ||||
| HVA (n = 281) | Control (n = 2404) | EVA (n = 320) | ANCOVA P for trend | |
| Age (years) | 24.2 ± 6.7 | 25.8 ± 5.7 | 26.3 ± 5.0 | 0.0001a |
| Women (%) | 69.0 | 57.1 | 46.9 | 0.0001a |
| BMI (kg/m2) | 21.5 ± 2.8 | 23.1 ± 3.7 | 24.7 ± 5.0 | 0.0001 |
| Waist circumference (cm) | 74.4 ± 9.0 | 79.7 ± 10.9 | 85.9 ± 13.6 | 0.0001 |
| Fasting glucose (mg/dl) | 79.1 ± 8.5 | 80.7 ± 16.6 | 79.8 ± 16.3 | 0.0001 |
| Total cholesterol (mg/dl) | 176.8 ± 32.8 | 186.0 ± 36.3 | 186.5 ± 39.1 | 0.0001 |
| HDL cholesterol (mg/dl) | 62.3 ± 13.6 | 60.5 ± 15.3 | 54.6 ± 14.8 | 0.0001 |
| Not HDL cholesterol (mg/dl) | 114.5 ± 29.2 | 125.4 ± 33.7 | 131.8 ± 39.6 | 0.0001 |
| Triglycerides (mg/dl) | 62.0 ± 32.8 | 76.1 ± 45.4 | 91.4 ± 72.5 | 0.0001 |
| SBP (mmHg) | 110.8 ± 10.2 | 117.0 ± 12.2 | 123.1 ± 15.4 | 0.0001 |
| DBP (mmHg) | 69.1 ± 7.5 | 71.6 ± 7.9 | 75.6 ± 10.5 | 0.0001 |
| MBP (mmHg) | 83.1 ± 7.4 | 86.7 ± 8.3 | 91.3 ± 11.3 | 0.0001 |
| PP (mmHg) | 42.2 ± 8.3 | 45.7 ± 9.7 | 47.6 ± 10.3 | 0.0001 |
| HR (bpm) | 66.7 ± 10.5 | 67.7 ± 11.7 | 71.2 ± 11.8 | 0.0001 |
| Serum creatinine (mg/ml)b (n) | 0.76 ± 0.14 (281) | 0.79 ± 0.16 (2394) | 0.82 ± 0.17 (316) | 0.0001 |
| PWV (m/s) | 4.1 ± 0.4 | 5.4 ± 0.8 | 8.5 ± 2.3 | 0.0001 |
| PWV/MAP | 5.0 ± 0.5 | 6.3 ± 1.0 | 9.3 ± 2.8 | 0.0001 |
| 35–49 years | ||||
| HVA (n = 376) | Control (n = 3345) | EVA (n = 438) | ANCOVA P for trend | |
| Age (years) | 42.3 ± 4.0 | 42.2 ± 3.9 | 42.2 ± 4.0 | 0.88a |
| Women (%) | 64.1 | 48.4 | 34.0 | 0.0001a |
| BMI (kg/m2) | 24.3 ± 3.6 | 26.5 ± 4.7 | 28.2 ± 5.4 | 0.0001 |
| Waist circumference (cm) | 81.0 ± 10.9 | 88.6 ± 13.8 | 93.1 ± 14.1 | 0.0001 |
| Fasting glucose (mg/dl) | 84.0 ± 11.5 | 91.0 ± 20.2 | 95.5 ± 20.9 | 0.0001 |
| Total cholesterol (mg/dl) | 202.9 ± 35.2 | 215. 6 ±46.0 | 220.6 ± 45.1 | 0.01 |
| HDL cholesterol (mg/dl) | 64.0 ± 14.3 | 57.6 ± 17.0 | 50.6 ± 16.0 | 0.0001 |
| Non-HDL cholesterol (mg/dl) | 138.8 ± 34.8 | 158.0 ± 48.5 | 170.3 ± 47.6 | 0.0001 |
| Triglycerides (mg/dl) | 77.1 ± 45.0 | 126.8 ± 64.8 | 170.6 ± 182.8 | 0.0001 |
| SBP (mmHg) | 116.1 ± 12.4 | 124.9 ± 17.0 | 134.5 ± 21.1 | 0.0001 |
| DBP (mmHg) | 74.0 ± 9.0 | 80.3 ± 11.4 | 87.5 ± 13.1 | 0.0001 |
| MAP (mmHg) | 88.1 ± 9.5 | 95.0 ± 12.5 | 103.0 ± 14.9 | 0.0001 |
| PP (mmHg) | 42.5 ± 7.9 | 44.7 ± 10.4 | 47.1 ± 13.2 | 0.05 |
| HR (bpm) | 64.1 ± 10.6 | 67.9 ± 11.2 | 70.3 ± 11.5 | 0.0001 |
| Serum creatinine (mg/ml)b (n) | 0.80 ± 0.15 (373) | 0.84 ± 0.25 (2679) | 0.92 ± 0.50 (295) | 0.01 |
| PWV (m/s) | 5.0 ± 0.4 | 7.4 ± 1.4 | 11.6 ± 2.0 | |
| PWV/MAP | 5.8 ± 0.7 | 7.9 ± 1.6 | 8.9 ± 2.3 | |
| 50–64 years | ||||
| HVA (n = 613) | Control (n = 5268) | EVA (n = 692) | ANCOVA P for trend | |
| Age (years) | 56.6 ± 4.6 | 56.7 ± 4.5 | 56.3 ± 4.5 | 0.05a |
| Women (%) | 54.7 | 48.0 | 50.6 | 0.0001a |
| BMI (kg/m2) | 26.7 ± 4.2 | 29.3 ± 5.2 | 29.2 ± 5.4 | 0.0001 |
| Waist circumference (cm) | 88.9 ± 12.5 | 95.9 ± 13.6 | 96.5 ± 13.6 | 0.0001 |
| Fasting glucose (mg/dl) | 92.8 ± 21.9 | 100.9 ± 25.1 | 104.8 ± 34.3 | 0.0001 |
| Total cholesterol (mg/dl) | 218.9 ± 44.2 | 240.2 ± 53.1 | 243.4 ± 48.6 | 0.0001 |
| HDL cholesterol (mg/dl) | 64.3 ± 19.2 | 57.8 ± 19.5 | 53.7 ± 17.5 | 0.0001 |
| Non-HDL cholesterol (mg/dl) | 154.7 ± 44.5 | 182.4 ± 56.3 | 189.7 ± 50.1 | 0.0001 |
| Triglycerides (mg/dl) | 103.1 ± 75.7 | 146.0 ± 134.7 | 166.6 ± 178.7 | 0.0001 |
| SBP (mmHg) | 123.0 ± 15.9 | 134.4 ± 18.0 | 145.1 ± 20.2 | 0.0001 |
| DBP (mmHg) | 76.3 ± 10.5 | 83.1 ± 10.5 | 88.7 ± 11.9 | 0.0001 |
| MAP (mmHg) | 91.8± 11.5 | 100.0 ± 12.1 | 107.3 ± 13.5 | 0.0001 |
| PP (mmHg) | 46.7 ± 10.2 | 51.3 ± 12.6 | 56.4 ± 14.5 | 0.0001 |
| HR (bpm) | 64.8 ± 9.9 | 67.1 ± 10.6 | 70.2 ± 11.7 | 0.0001 |
| Serum creatinine (mg/ml)b (n) | 0.83 ± 0.17 (596) | 0.83 ± 0.22 (3935) | 0.86 ± 0.22 (324) | 0.01 |
| PWV (m/s) | 5.8 ± 0.5 | 8.8 ± 1.4 | 13.6 ± 2.5 | 0.0001 |
| PWV/MAP | 6.4 ± 1.0 | 8.9 ± 1.7 | 13.0 ± 2.9 | 0.0001 |
| 65+ years | ||||
| HVA (n = 452) | Control (n = 3849) | EVA (n = 487) | ANCOVA P for trend | |
| Age (years) | 72.5 ± 5.7 | 72.5 ± 5.5 | 72.3 ± 5.1 | 0.74a |
| Women (%) | 56.0 | 60.4 | 48.9 | 0.0001a |
| BMI (kg/m2) | 26.1 ± 3.9 | 27.2 ± 4.3 | 27.6 ± 4.5 | 0.0001 |
| Waist circumference (cm) | 90.0 ± 11.7 | 92.5 ± 12.1 | 93.9 ± 12.0 | 0.001 |
| Fasting glucose (mg/dl) | 94.0 ± 18.1 | 102.5 ± 23.4 | 107.6 ± 31.5 | 0.0001 |
| Total cholesterol (mg/dl) | 202.9 ± 39.5 | 209.8 ± 43.7 | 221.4 ± 48.4 | 0.0001 |
| HDL cholesterol (mg/dl) | 62.4 ± 19.7 | 60.6 ± 21.0 | 56.1 ± 20.3 | 0.001 |
| Not HDL cholesterol (mg/dl) | 140.4 ± 37.5 | 149.2 ± 42.2 | 165.3 ± 47.1 | 0.0001 |
| Triglycerides (mg/dl) | 89.4 ± 49.2 | 86.6 ± 55.3 | 102.3 ± 66.6 | 0.0001 |
| SBP (mmHg) | 126.6 ± 20.1 | 138.2 ± 19.1 | 149.5 ± 19.3 | 0.0001 |
| DBP (mmHg) | 71.2 ± 10.9 | 77.1 ± 10.9 | 83.0 ± 11.8 | 0.0001 |
| MAP (mmHg) | 89.5 ± 12.6 | 97.3 ± 12.4 | 105.0 ± 12.7 | 0.0001 |
| PP (mmHg) | 45.9 ± 17.7 | 61.0 ± 14.6 | 56.1 ± 16.6 | 0.0001 |
| HR (bpm) | 63.4 ± 9.7 | 66.8 ± 10.8 | 72.2 ± 11.3 | 0.0001 |
| Serum creatinine (mg/ml)b (n) | 0.94 ± 0.23 (435) | 0.93 ± 0.31 (3378) | 0.98 ±0.46 (311) | 0.07 |
| PWV (m/s) | 6.4 ± 0.7 | 10.1 ± 1.9 | 16.6 ± 3.8 | 0.0001 |
| PWV/MAP | 7.3 ± 1.3 | 10.5 ± 2.1 | 16.0 ± 3.8 | 0.0001 |
ANCOVA, analysis of covariance; EVA, early vascular ageing; HDL, high-density lipoprotein; HR, heart rate; HVA, healthy vascular ageing; MAP, mean arterial pressure; MetS, metabolic syndrome; PP, pulse pressure; PWV, pulse wave velocity.
ANOVA (analysis of variance).
Measurement not available for each participant.
No significant difference in the age-distribution or sex-distribution between HVA participants and controls were observed in any age group. Furthermore, age-group belonging did not impact on relative differences in cardiometabolic risk factors between HVA participants and the rest.
Notably, in the presence of similar levels of cardiometabolic risk factors, those without HVA in the age group 35–49 years (mean age 42.5 ± 4.0 years) had significantly stiffer (older) arteries (PWV: 7.4± 1.4 vs. 5.8±0.5 m/s, respectively) as compared with HVA participants in the age group 50–64 years (mean age 56.5 ± 4.5 years), even in the presence of similar average levels in blood pressure, adiposity, blood lipids, and glucose. Similar comparisons were made for the metabolic syndrome and its components in participants with HVA and EVA as compared with the rest of the population when stratified by age groups, Table 5.
Table 5.
Metabolic syndrome and its components in participants with healthy vascular ageing and early vascular ageing, as compared with controls, when stratified by age groups
| < 34 years | ||||
| HVA (n = 281) | Control (n = 2404) | EVA (n = 320) | ANCOVA P for trend | |
| Hypertension (%) | 4.6 | 8.7 | 21.6 | - |
| Diabetes mellitus (%) | - | 0.5 | 1.9 | 0.21 |
| Overweight (%) | 6.4 | 10.6 | 13.4 | 0.0001 |
| Obesity (%) | 1.4 | 4.9 | 10.9 | 0.20 |
| Metabolic syndrome | - | 2.0 | 7.8 | 0.0001 |
| N of altered MetS components | 0.2 ± 0.4 | 0.5 ± 0.7 | 0.9 ± 1.0 | 0.0001 |
| Low HDL cholesterol | 8.9 | 13.6 | 22.5 | 0.0001 |
| Elevated waist circumference | 4.6 | 11.5 | 22.2 | 0.0001 |
| Elevated triglycerides | 0.7 | 5.4 | 11.9 | 0.001 |
| Elevated blood pressure | 3.2 | 13.8 | 28.8 | 0.0001 |
| Elevated fasting glucose | - | 1.0 | 3.1 | 0.01 |
| 35–49 years | ||||
| HVA (n = 376) | Control (n = 3345) | EVA (n = 438) | ANCOVA P for trend | |
| Hypertension (%) | 10.6 | 28.2 | 48.9 | - |
| Diabetes mellitus (%) | 0.3 | 4.9 | 8.0 | 0.05 |
| Overweight (%) | 18.6 | 14.4 | 13.9 | 0.10 |
| Obesity (%) | 6.1 | 18.5 | 23.5 | 0.01 |
| Metabolic syndrome | 2.1 | 15.8 | 29.2 | 0.0001 |
| N of altered MetS components | 0.4 ± 0.7 | 1.2 ± 1.2 | 1.8 ± 1.3 | 0.0001 |
| Low HDL cholesterol | 6.9 | 20.8 | 36.1 | 0.0001 |
| Elevated waist circumference | 12.5 | 27.4 | 32.6 | 0.0001 |
| Elevated triglycerides | 5.9 | 22.8 | 38.6 | 0.0001 |
| Elevated blood pressure | 15.2 | 38.1 | 60.5 | 0.0001 |
| Elevated fasting glucose | 0.8 | 10.3 | 16.2 | 0.0001 |
| 50–64 years | ||||
| HVA (n = 613) | Control (n = 5268) | EVA (n = 692) | ANCOVA P for trend | |
| Hypertension (%) | 22.6 | 44.3 | 66.0 | - |
| Diabetes mellitus (%) | 6.0 | 13.4 | 16.6 | 0.05 |
| Overweight (%) | 20.0 | 13.4 | 11.1 | 0.001 |
| Obesity (%) | 19.9 | 35.7 | 34.2 | 0.001 |
| Metabolic syndrome | 12.5 | 34.3 | 44.1 | 0.0001 |
| N of altered MetS component | 1.1 ± 1.2 | 2.0 ± 1.3 | 2.3 ± 1.3 | 0.0001 |
| Low HDL cholesterol | 14.7 | 29.1 | 34.1 | 0.0001 |
| Elevated waist circumference | 34.7 | 56.6 | 52.6 | 0.0001 |
| Elevated triglycerides | 15.0 | 33.2 | 41.6 | 0.0001 |
| Elevated blood Pressure | 33.4 | 55.6 | 77.0 | 0.0001 |
| Elevated fasting glucose | 10.6 | 23.8 | 25.0 | 0.0001 |
| 65+ years | ||||
| HVA (n = 452) | Control (n = 3849) | EVA (n = 487) | ANCOVA P for trend | |
| Hypertension (%) | 37.6 | 62.4 | 82.3 | - |
| Diabetes mellitus (%) | 9.5 | 14.2 | 22.0 | 0.001 |
| Overweight (%) | 18.1 | 17.6 | 16.4 | 0.64 |
| Obesity (%) | 14.6 | 20.9 | 26.5 | 0.001 |
| Metabolic syndrome | 13.3 | 22.3 | 32.6 | 0.0001 |
| N of altered MetS components | 1.2 ± 1.1 | 1.7 ± 1.1 | 2.1 ± 1.1 | 0.0001 |
| Low HDL cholesterol | 18.6 | 22.8 | 29.6 | 0.0001 |
| Elevated waist circumference | 42.7 | 45.2 | 43.7 | 0.86 |
| Elevated triglycerides | 10.8 | 10.8 | 16.2 | 0.01 |
| Elevated blood pressure | 37.6 | 63.3 | 83.8 | 0.0001 |
| Elevated fasting glucose | 15.0 | 26.6 | 34.5 | 0.0001 |
ANCOVA, analysis of covariance; EVA, early vascular ageing; HDL, high-density lipoprotein; HVA, healthy vascular ageing; MetS, metabolic syndrome.
Determinants of healthy vascular ageing and early vascular ageing in age groups by multiple regression analysis
We investigated determinants of HVA and EVA, as compared with their counterparts and stratified by age groups. As illustrated in Table 6, low HDL cholesterol, elevated triglycerides, and elevated BP were associated with lower likelihood of HVA in any age group. Elevated glucose was associated with lower likelihood of having HVA in younger and older participants, whereas abdominal adiposity reduced the likelihood of having HVA in participants less than 35 years of age. Elevated glucose and BP were consistently associated with greater likelihood of having EVA regardless of age. Abdominal adiposity and elevated triglycerides conferred greater likelihood of having EVA up to the age of 65, whereas low HDL cholesterol was associated with greater likelihood of EVA in all but the youngest age group.
Table 6.
Determinants of healthy vascular ageing and early vascular ageing as compared with controls, stratified by age groups (multinomial logistic regression analysis)
| < 35 years |
35–49 years |
50–64 years |
65+ years |
|||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| HVA | ||||||||
| Age | 1.00 | 0.98–1.02 | 0.97 | 0.94–0.99 | 0.84 | 0.82–0.85 | 0.99 | 0.98–1.01 |
| Female sex | 0.72 | 0.55–0.93 | 0.75 | 0.59–0.94 | 0.64 | 0.54–0.77 | 0.64 | 0.52–0.79 |
| Elevated fasting glucose | 0.30 | 0.17–0.80 | 0.85 | 0.62–1.16 | 1.04 | 0.85–1.26 | 0.75 | 0.61–0.92 |
| Low HDL cholesterol | 0.64 | 0.47–0.87 | 0.54 | 0.43–0.68 | 0.78 | 0.65–0.92 | 0.66 | 0.53–0.82 |
| Elevated triglycerides | 0.66 | 0.44–1.00 | 0.69 | 0.54–0.87 | 0.78 | 0.65–0.93 | 0.75 | 0.57–0.98 |
| Elevated blood pressure | 0.51 | 0.38–0.68 | 0.45 | 0.37–0.56 | 0.38 | 0.31–0.46 | 0.34 | 0.26–0.44 |
| Elevated waist circumference | 0.50 | 0.36–0.70 | 1.15 | 0.90–1.46 | 1.22 | 1.02–1.46 | 1.10 | 0.89–1.35 |
| EVA | ||||||||
| Age | 0.96 | 0.94–0.99 | 1.07 | 1.04–1.10 | 1.01 | 0.99–1.03 | 1.00 | 0.99–1.02 |
| Female sex | 1.56 | 1.18–2.06 | 1.32 | 1.04–1.67 | 0.81 | 0.67–0.97 | 0.80 | 0.64–0.99 |
| Elevated fasting glucose | 7.54 | 2.37–24.0 | 1.75 | 1.33–2.31 | 1.97 | 1.50–2.58 | ||
| Low HDL cholesterol | 1.50 | 0.97–2.31 | 2.38 | 1.57–3.62 | 1.57 | 1.23–2.00 | 1.33 | 1.03–1.73 |
| Elevated triglycerides | 5.38 | 1.32–22.0 | 2.27 | 1.43–3.60 | 1.98 | 1.55–2.52 | 0.85 | 0.62–1.18 |
| Elevated blood pressure | 3.46 | 1.74–6.86 | 2.70 | 1.99–3.66 | 2.21 | 1.84–2.64 | 2.90 | 2.37–3.56 |
| Elevated waist circumference | 2.46 | 1.37–4.39 | 1.66 | 1.18–2.31 | 1.57 | 1.28 −1.91 | 0.88 | 0.71–1.10 |
Definitions of the metabolic syndrome [17] needed for elevated variables: low HDL cholesterol less than 40 mg/dl for men or less than 50 mg/dl for women. Elevated waist circumference greater than 102 cm for men or greater than 88 cm for women. Elevated triglycerides at least 150 mg/dl. Elevated blood pressure at least 130 mmHg/at least 85 mmHg or use of antihypertensive medications. Elevated fasting glucose at least 110 mg/dl or use of antidiabetic medications. 95% CI, 95% confidence intervals; EVA, early vascular ageing HDL, high-density lipoprotein; HVA, healthy vascular ageing; OR, odds ratios.
DISCUSSION
Overall we found that participants characterized by HVA, that is, with PWV values in the lowest 10% range of the age-specific and quintile-specific distribution, had a more favourable cardiovascular risk profile, and that both the HVA and EVA phenotypes were determined by the same cardiovascular risk factors. However, for the same levels of cardiovascular risk factors, PWV was lower in HVA participants, even 14 years older in chronological age than younger control participants.
The recently published data from the Framingham Heart Study (FHS) on HVA used another definition, based on the absence of hypertension in combination with PWV values below a specified low threshold [16] that even could be regarded as arbitrary. The mean PWV of the HVA participants in the Framingham study was 6.8 (SD: 0.5) m/s, as compared with 5.5 (0.9) m/s in our MARE study. In Cox regression models adjusted for traditional CVD risk factors, including blood pressure, HVA in Framingham was associated with a hazard ratio of 0.45 (95% confidence interval 0.26–0.77) for CVD relative to absence of HVA [16]. The authors concluded that HVA was rare beyond 70 years of age [16]. The advantage of the FHS is that outcomes could be calculated during follow-up, but, on the other hand, the MARE consortium offers a larger total study data-base and age group-based sub-analyses for description of HVA on a global scale. Their PWV lower values (<7.6 m/s) correspond to the HVA participants (lowest 10th percentile) of age-group 70–74 years, or to EVA participants (upper 90th percentile) of age-group 30–34 years in the present study.
It is also worth noting that if we were to use the FHS definition of HVA [16], we would not find a single participant fitting this definition in our population above the age of 40 years. This could be partially explained by a selection bias in our global population, but we would also like to discuss this as a possible hint that the definition of HVA based on the concept of ‘no aging after 30 years of age’ is probably inadequate to characterize HVA after the age of 40. We consider that the ageing process has been, up until now, shown to be an inevitable process that can, at its best, be slowed down, but not reversed, by controlling cardiovascular risk factors and pursuing healthy lifestyle.
In the MARE collaboration, we preferred to use a simplified approach, only focusing on participants at the lower 10% end of the PWV. However, we also adjusted for the use of cardiovascular drugs, when hypertension status was a proxy for such drug usage. However, at present, we do not have access to follow-up prospective data in the MARE cohort collaboration making prospective analyses for risk of incident cardiovascular events impossible. Furthermore, the residual risk associated with genetic, cultural, lifestyle, and ethnic diversity will most likely influence the long-term health consequences associated with HVA, why direct comparisons with Framingham will be difficult.
We adopted our approach (definition) because we did not want to recommend to avoid ageing, as indicated in the Framingham paper [16], but rather to age well. To have some insight as to whether factors protecting from arterial ageing are the same that accelerates arterial ageing, we compared the cardiometabolic risk profile in participants with HVA, with EVA, and with controls.
Although cardiovascular morbidity and mortality remains very high even at older ages, it has been reported that traditional cardiovascular risk factors, such as obesity and serum cholesterol, are associated with lower, rather than higher, risk of cardiovascular events in older individuals [10,20]. This is the so called ‘reverse epidemiology’ or ‘risk factor paradox.’ Regardless of whether this paradox is attributable to healthy selection survival bias or, more simply, to a reduced burden of factors risky at younger ages, we compared cardiometabolic risk factor distribution across participants belonging to HVA, EVA, or controls in four age groups: less than 35 years, 35–49 years, 50–64 years, 65+ years. When stratified for age groups (Tables 4–6) the trend in cardiovascular risk factor levels were roughly similar across age groups.
The major limitation of the present study is represented by its cross-sectional design and use of different methods for estimation of reported cardiovascular risk factors and PWV, as previously described [19]. Selection bias could vary between cohorts with shifting representativeness of the background population in each country. On the other hand, a major strength is represented by the adoption of the lowest 10% of the PWV distribution within age groups of each studied population (cohort) to define HVA as compared with previous report adopting ‘external reference value’ [17].
The global nature of the MARE consortium makes our data of interest to understand the HVA phenotype in a broader sense, reflecting the global perspective. As the cardiovascular risk in general undergoes time-dependent changes over time, with decreasing age-adjusted trends in many western countries, but increasing trends in countries with populations in transition, this could mean that the importance of HVA is also time-dependent and cohort-dependent, reflecting cohort characteristics at a certain historical time window. Increased knowledge about HVA and its characteristics could benefit the design of preventive projects for vascular protection by lifestyle improvement or drug intervention in EVA participants [12,13,21], the inverse of HVA. The increased use of drugs believed to limit or reverse PWV increasing with age, and the complex nature of drug treatments in each individual makes this confounding factor difficult, if not impossible to control in the present study.
Future studies, including genetic mapping of HVA and related protective mechanisms could eventually reveal possible new drug targets, to be tested in controlled studies.
From the wider perspective of Active and Healthy Aging (AHA), it is thus of importance to better understand determinants of HVA (genetics, environment, lifestyle, and drugs) to support precision care [22]. The observation that both HVA and EVA was determined by the same cardiovascular risk factors indicates that treatment of these cardiovascular risk factors might shift the vascular ageing away from EVA towards HVA.
The future perspective may thus change from the treatment of cardiovascular risk factors only, to a more integrative approach associating protective factors to preserve functional reserve and independency in the daily activity [23]. It is necessary that these aspects are studied in different ethnic populations, and a recent report from Shanghai concluded that target organ damage was associated with elevated PWV, but at the lower end of the PWV distribution, corresponding to HVA in Chinese individuals, far less TOD was noticed [24].
In conclusion, we report characteristics of HVA on a global scale and that these participants, belonging to the lowest end of the PWV distribution, are in general characterized by an up to 14 years younger biological age than controls, selected from the rest of the distribution. We have also shown that different factors contribute to HVA in different age groups, paving the way for differentiated public health policies according to age and sex. These insights could guide preventive work and the communication with participants at elevated risk to improve their own vascular age as reflected by elevated PWV.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all participants in the cohorts for contributing data to the MARE consortium as well as the funding agencies supporting these cohorts.
Financial support: The Asklepios Study is supported by the Fund for Scientific Research - Flanders (FWO research grants G042703 and G083810N).
The Rotterdam Study is supported by the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research; the Netherlands Organization for Health Research and Development (ZonMw); the Research Institute for Diseases in the Elderly (RIDE); the Netherlands Heart Foundation; the Ministry of Education, Culture and Science; the Ministry of Health Welfare and Sports; the European Commission; and the Municipality of Rotterdam.
The SardiNIA team was supported by Contract NO1–AG– 1–2109 from the NIA. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging (USA).
The Baltimore Longitudinal Study of Aging (BLSA) is supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
The Kingmen Aging Study was supported, in part, by a grant from the National Science Council (NSC 99–2314–B–010–034–MY3), an intramural grant from the Taipei Veterans General Hospital (grant V102C–119), Research and Development contract NO1–AG–1–2118, and the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
The Malmoe Diet Cancer - Cardiovascular Study was supported by grants from the Swedish Research Council (K2008–65X–20752–01–3, K2011–65X–20752–04–6), the Lund-ströms Foundation, the Swedish Heart-Lung Foundation (2010–0244; 2013–0249) and ALF government grants (Dnr: 2012/1789).
The Guimarães Study was funded by the Life and Health Research Institute, Medical School, Minho University, Portugal
Abbreviations:
- AHA
active and healthy ageing
- EVA
early vascular ageing
- FHS
Framingham Heart Study
- HVA
healthy vascular ageing
- MAP
mean arterial pressure
- MARE Consortium
Metabolic syndrome and Artery Research Consortium
- PWV
pulse wave velocity
Footnotes
Conflicts of interest
There are no conflicts of interest.
Full list of authors in the Supplemental Material.
REFERENCES
- 1.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 2005; 46:200–204. [DOI] [PubMed] [Google Scholar]
- 2.Scuteri A, Chen CH, Yin FCP, Yin FC, Chih-Tai T, Spurgeon HA, Lakatta EG. Functional correlates of central arterial geometric phenotypes. Hypertension 2001; 38:1471–1475. [DOI] [PubMed] [Google Scholar]
- 3.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol Rev 2017; 97:1555–1617. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scuteri A, Tesauro M, Guglini L, Lauro D, Fini M, Di Daniele N. Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol 2013; 169:371–377. [DOI] [PubMed] [Google Scholar]
- 6.Scuteri A, Morrell CH, Orrù M, Strait JB, Tarasov KV, Ferreli LA, et al. Longitudinal perspective on the conundrum of central arterial stiffness, blood pressure, and aging. Hypertension 2014; 64:1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarasov KV, Sanna S, Scuteri A, Strait JB, Orrù M, Parsa A, et al. COL4A1 is associated with arterial stiffness by genome-wide association scan. Circ Cardiovasc Genet 2009; 2:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GF, Verwoert GC, Tarasov KV, Isaacs A, Smith AV, Yasmin. et al. Common genetic variation in the 3’-BCL11B gene desert is associated with carotid-femoral pulse wave velocity and excess cardiovascular disease risk: the Aorta Gen Consortium. Circ Cardiovasc Genet 2012; 5:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scuteri A, Najjar SS, Orru’ M, Usala G, Piras MG, Ferrucci L, et al. The central arterial burden of the metabolic syndrome is similar in men and women: the SardiNIA Study. Eur Heart J 2010; 31:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scuteri A, Orru’ M, Morrell CH, Tarasov K, Schlessinger D, Uda M, Lakatta EG. Associations of large artery structure and function with adiposity: effects of age, gender, and hypertension. The SardiNIA Study. Atherosclerosis 2012; 221:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scuteri A, Tesauro M, Rizza S, Iantorno M, Federici M, Lauro D, et al. Endothelial dysfunction and arterial stiffness in normotensive normoglycemic first degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis 2008; 18:349–356. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension 2009; 54:3–10. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson PM, Boutouyrie P, Cunha P, Kotsis V, Narkiewicz K, Parati G, et al. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens 2013; 8:1517–1526. [DOI] [PubMed] [Google Scholar]
- 14.Cunha PG, Cotter J, Oliveira P, Vila I, Boutouyrie P, Laurent S, Peter M. Pulse wave velocity distribution in a cohort study: from arterial stiffness to early vascular aging. J Hypertens 2015; 33:1438–1445. [DOI] [PubMed] [Google Scholar]
- 15.Olsen MH, Angell SY, Asma S, Boutouryie P, Burger D, Chirinos JA, et al. A call to action and a life course strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 2016; 388: 2665–2712. [DOI] [PubMed] [Google Scholar]
- 16.Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Prevalence, correlates, and prognosis of healthy vascular aging in a western community–dwelling cohort: the Framingham Heart Study. Hypertension 2017; 70:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J 2010; 31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whelton SP, Silverman MG, McEvoy JW, Budoff MJ, Blankstein R, Eng J, et al. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA Study. JACC Cardiovasc Imaging 2015; 8:1393–1400. [DOI] [PubMed] [Google Scholar]
- 19.Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Mañas LR, et al. , Metabolic Syndrome and Arteries Research (MARE) Consortium. Metabolic syndrome across Europe: different clusters of risk factors. Eur J Prev Cardiol 2015; 22:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadi SF, Streja E, Zahmatkesh G, Streja D, Kashyap M, Moradi H, et al. Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc 2015; 16:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension 2018; 71:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Currie G, Delles C. Healthy vascular aging. Hypertension 2017;70:229–231. [DOI] [PubMed] [Google Scholar]
- 23.Scuteri A, Lattanzio F, Bernabei R. Life-course approach to chronic disease: the active and healthy aging perspective. J Am Geriatr Soc 2016; 64:e59–e61. [DOI] [PubMed] [Google Scholar]
- 24.Ji H, Teliewubai J, Lu Y, Xiong J, Yu S, Chi C, et al. Vascular aging and preclinical target organ damage in community-dwelling elderly: the Northern Shanghai Study. J Hypertens 2018; 36:1391–1398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.