Abstract
The biological basis for documented in vivo bone-protective effects of turmeric-derived curcumin is unclear since curcumin is barely detectable in serum, being rapidly conjugated to form what is thought to be an inactive glucuronide. Studies were therefore undertaken to test the postulate that anti-resorptive effects of curcumin require deconjugation within bone to form the bioactive aglycone, and that ß-glucuronidase (GUSB), a deconjugating enzyme expressed by hematopoietic marrow cells, facilitates this site-specific transformation. Consistent with this postulate, aglycone, but not glucuronidated, curcumin inhibited RANKL-stimulated osteoclastogenesis, a key curcumin target in bone. Aglycone curcumin, expressed relative to total curcumin, was higher in bone marrow than in serum of curcumin-treated C57BL/6J mice, while remaining a minor component. Ex vivo, under conditions preventing further metabolism of the unstable aglycone, the majority of curcumin-glucuronide delivered to marrow in vivo was hydrolyzed to the aglycone, a process that was inhibited by treatment with saccharolactone, a GUSB inhibitor, or in mice having reduced (C3H/HeJ) or absent (mps/mps) GUSB activity. These findings suggest that curcumin, despite low systemic bioavailability, may be enzymatically activated (deconjugated) within GUSB-enriched bone to exert protective effects, a metabolic process that could also contribute to bone-protective effects of other highly glucuronidated dietary polyphenols.
Graphical Abstract
INTRODUCTION
For millennia, the turmeric rhizome (Curcuma longa L.) has been a staple of Ayurvedic medicine, especially for the treatment of inflammatory disorders, including arthritis1,2. In more recent decades, bone protective effects of curcumin, the most abundant turmeric polyphenol, have been documented in vivo in humans3,4 and in pre-clinical models of arthritis and other common bone resorptive disorders, including osteoporosis and osteolytic bone metastases5–11. These effects have been attributed to local actions of curcumin within the bone microenvironment that inhibit the formation of bone-resorbing osteoclasts5,10,12,13. Contrary to this observed efficacy in vivo, circulating levels of the administered aglycone curcumin (compound 1), are nearly undetectable in both rodents and humans14–19. Rather, curcumin-glucuronide (compound 2), a polar phase II metabolite targeted for excretion thought to lack biological activity20–22, is the predominant measurable serum metabolite14,17,19.
It has previously been postulated, but not proven, that site-specific deconjugation may reconcile observed curcumin bioactivity with low aglycone bioavailability23. Evidence supporting of this general concept comes from pre-clinical studies reporting the deconjugation of other glucuronidated “pro-drug” compounds at sites of inflammation, a process believed to be mediated by extracellular β-glucuronidase (GUSB), a deconjugating enzyme released from infiltrating inflammatory cells24–27. Because normal bone marrow is enriched with a full spectrum of mature and immature hematopoietic cells that are known to express GUSB28,29, this suggests that, in the context of bone protection, curcumin-glucuronide may act as a prodrug that is activated (deconjugated) within bone by GUSB. Local, GUSB-mediated aglycone curcumin production could then prevent bone loss in a range of disease states by inhibiting osteoclastogenesis, a postulate that will be tested here. However, GUSB is not the sole mammalian enzyme capable of deglucuronidation30, as α-klotho (KL) and heparanase (HPSE) also exhibit substrate-specific deconjugation activity31–33. Moreover, while our laboratory has recently demonstrated the ability of aglycone curcumin, but not curcumin-glucuronide, to inhibit osteoclast formation indirectly in osteolytic bone metastasis models20, direct effects of aglycone vs glucuronidated curcumin on osteoclast formation have, to our knowledge, not been previously reported. Therefore, experiments were undertaken to 1) determine whether inhibitory effects of curcumin on receptor activator of NF-κB ligand (RANKL)-induced osteoclastogenesis, the master regulator of bone resorption6, are similarly attributable to aglycone, but not glucuronidated curcumin, 2) to examine for the first time the bone-specific pharmacokinetics of curcumin in mice, including an investigation of the capacity of bone marrow to hydrolyze curcumin-glucuronide delivered in vivo to the normal bone microenvironment, and 3) the enzyme dependence of such a process.
RESULTS AND DISCUSSION
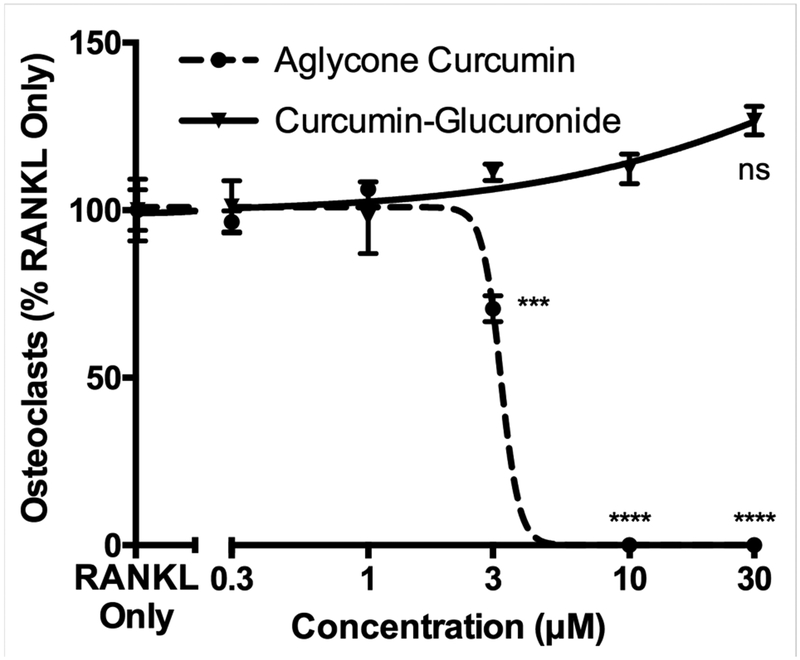
Effects of Aglycone Curcumin and Curcumin-Glucuronide on RANKL-Stimulated Osteoclast Formation.
Consistent with our hypothesis and previous demonstration of an inhibitory effect of aglycone, but not glucuronidated, curcumin in blocking tumoral drivers of osteoclastogenesis, thus indirectly blocking osteoclastogenesis20, aglycone curcumin directly inhibited RANKL-simulated osteoclast formation with an IC50 of approximately 3 μM (Fig. 1), a dose that did not alter RAW 264.7 cell viability (data not shown). In contrast, curcumin-glucuronide was without effect, even at 10-fold higher doses (Fig. 1).
Figure 1. Inhibition of RANKL-stimulated osteoclastogenesis by aglycone curcumin vs curcumin-glucuronide.
Murine RAW 264.7 cells were pretreated with aglycone curcumin or curcumin-glucuronide (GC) for 4 hours followed by 72 hours of RANKL stimulation. The number of TRAP-positive, multinucleated (n ≥ 3) osteoclasts formed were quantified. Data expressed as mean ± SEM (n=4/group). *** p < 0.001, **** p < 0.0001 vs GC. ns, not significantly different from RANKL control.
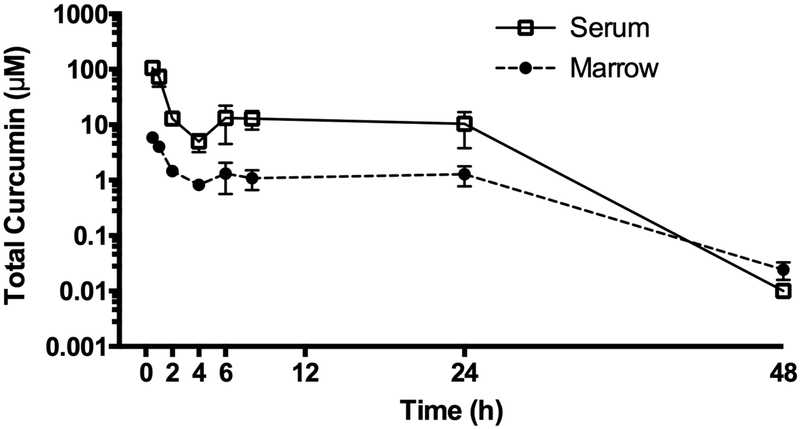
Pharmacokinetic Disposition of Curcumin in Bone Following Oral Administration.
Bone-specific pharmacokinetics of curcumin were determined in wild type C57BL/6J (BL/6J) mice following a single oral curcumin dose and compared to serum, a compartment that, in contrast to bone, has been well studied14–17,34–36. The AUC for total curcumin in bone marrow supernatants was 10-fold lower than in serum, but with a prolonged half-life (Fig. 2 and Table 1). The Cmax value for total curcumin was similar to the IC50 for inhibition of osteoclast formation by aglycone curcumin (Fig. 1 and Table 1). When comparing the relative amounts of aglycone vs. glucuronidated curcumin in serum, as has been previously reported, aglycone curcumin was a minor component (0.24% ± 0.07% of total curcumin [n=11], t= 30 min post-dose)14–17. Aglycone curcumin was also a minor constituent in lyophilized whole bone marrow, albeit at levels that were 3-fold higher relative to perfusing serum (0.77% ± 0.21% of total curcumin [n = 8], p < 0.05).
Figure 2. Pharmacokinetics of total curcumin in serum and marrow after acute curcumin treatment.
Total curcumin levels (sum of aglycone and glucuronidated curcumin) in serum (□) and marrow (●) were determined by LC/MS after oral gavage with 500 mg/kg curcumin in female 4-week C57BL/6J mice at the indicated times. Data expressed as mean ± SEM (n=4/group).
Table 1.
Pharmacokinetic analysis of total curcumin in murine serum and marrow following gavage administration of 500 mg/kg curcumina
| Parameter (Units) | Total Curcumin | |
|---|---|---|
| Serum | Marrow | |
| T1/2 (h) | 4.4 | 7.8 |
| Tmax (h) | 0.5 | 0.5 |
| Cmax (μmol/L) | 106.1 | 5.9 |
| AUC0–48h (h × μmol/L) | 487.4 | 48.0 |
| AUC0–∞ (h × μmol/L) | 487.5 | 48.3 |
Values are expressed as mean (n =4/time point)
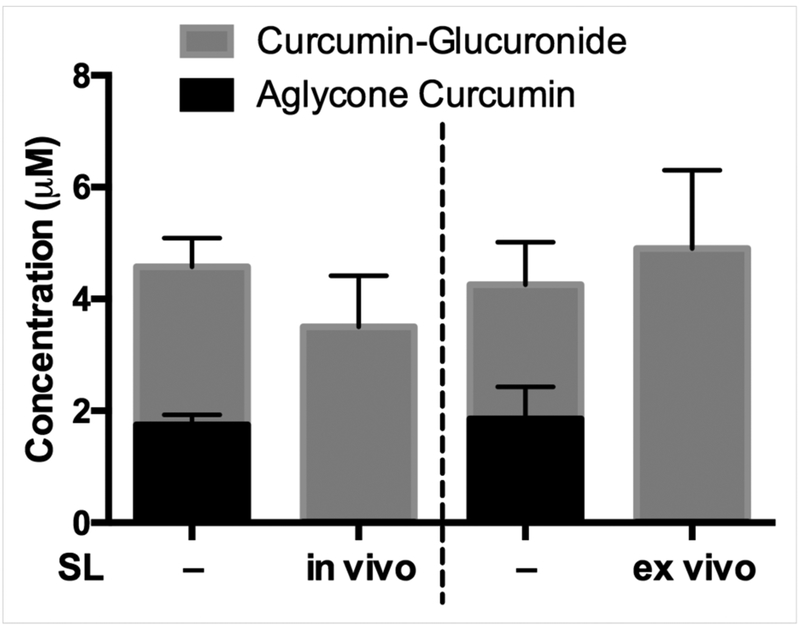
Curcumin-Glucuronide Hydrolyzing Capacity of Bone Marrow.
Because aglycone curcumin, once formed, is not stable and can be further metabolized via oxidative and reductive pathways37, ex vivo experiments were performed under conditions that stabilize aglycone curcumin to assess the capacity of bone marrow to hydrolyze curcumin-glucuronide distributing to bone following in vivo curcumin treatment. When bone marrow samples obtained from curcumin-treated mice 30 min post-dose were resuspended in 50 mM pH 5 sodium acetate buffer and incubated for 2 hours on ice (Fig.3, first and third bars)37, aglycone curcumin comprised 30–44% of total curcumin in the sample (vs. 0.1–0.2% in perfusing serum), indicating efficient hydrolysis. When oral curcumin dosing was preceded by in vivo treatment with saccharolactone (SL), a GUSB inhibitor38, serum levels of aglycone curcumin were unchanged (data not shown), while aglycone curcumin levels in marrow suspensions were no longer detectable (Fig. 3, second bar). Similarly, when SL was instead added ex vivo to the marrow suspensions, aglycone curcumin was also not detected (Fig. 3, fourth bar). These data suggest that normal bone marrow has the capacity to hydrolyze significant amounts of curcumin-glucuronide that distributes to bone following oral curcumin treatment, a process that may be mediated by GUSB, an enzyme whose activity is also optimized at the acidic pH used to stabilize aglycone curcumin in the bone marrow suspensions27,39.
Figure 3. Capacity of marrow to hydrolyze curcumin-glucuronide distributing to bone following oral administration of curcumin.
Bone marrow harvested 30 min after in vivo treatment of female, 4 wk C57BL/6J mice with curcumin (500 mg/kg by oral gavage) was resuspended in sodium acetate buffer as described for assay of aglycone and glucuronidated curcumin. For in vivo saccharolactone (SL) treatments, which did not alter serum aglycone curcumin levels (data not shown), SL (1 g/kg vs. normal saline) was administered via intraperitoneal injection 1 hour prior to curcumin treatment (left 2 bars). For ex vivo SL experiments, bone marrow from curcumin-treated mice was resuspended in sodium acetate buffer with or without 10 mM SL (right 2 bars). Data expressed as mean ± SEM (n=3–4/group).
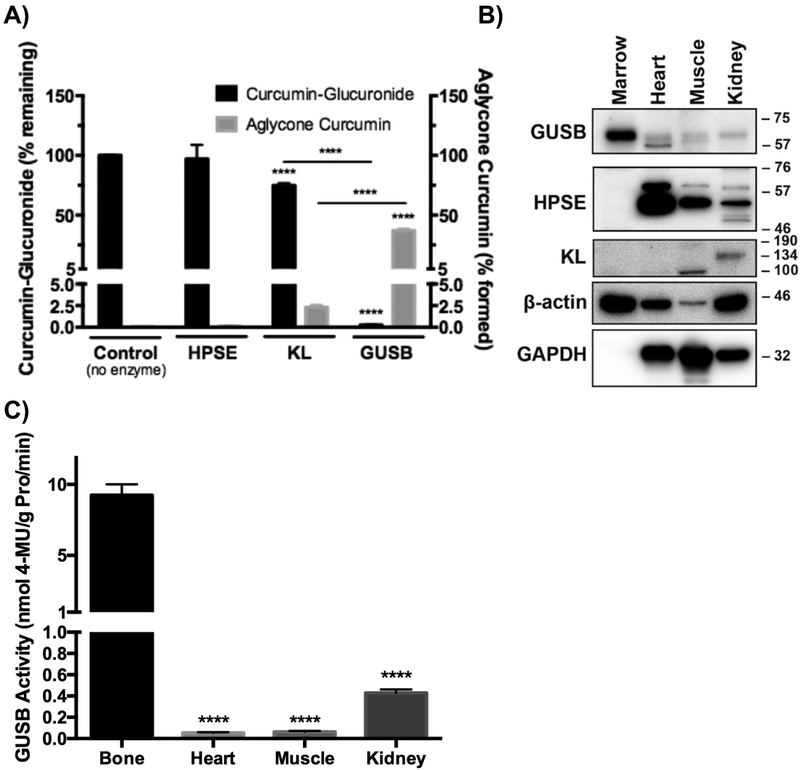
β-Glucuronidase is the Only Enzyme in Bone Capable of Deconjugating Curcumin-Glucuronide.
To further test the postulate that the curcumin-glucuronide hydrolyzing capacity of bone marrow is enzyme-mediated, the substrate specificity of mammalian enzymes with reported deglucuronidation activity (GUSB, α-klotho [KL], and heparanase [HPSE])30–33 vis-a-vis curcumin-glucuronide hydrolysis was assessed, and their expression levels in bone marrow were determined. Of these three enzymes, only GUSB and KL were able to deconjugate curcumin glucuronide, albeit at very different rates (Fig. 4A); using recombinant human enzymes, GUSB metabolized >99% of curcumin glucuronide, while 75% still remained after 24 hours of treatment with 3-fold greater amounts of KL. This agrees with known substrate specificity requirements for each enzyme, as GUSB requires an exposed β-glucuronic acid at the non-reducing end of glycosaminoglycans30, as occurs with curcumin-glucuronide, while KL preferentially deconjugates steroid β-glucuronides33 and HPSE specifically cleaves internal β-glucuronide bonds (e.g., heparan sulfate)31,32. Within bone, GUSB protein was abundantly expressed in comparison with other non-hematopoietic tissues (Fig. 4B), while KL was undetectable (Fig. 4B) and HPSE was either undetectable (Fig. 4B) or very low (Fig. 6B). Thus, GUSB was the only enzyme in bone capable of deconjugating curcumin-glucuronide. Importantly, and consistent with a possible role for GUSB in mediating curcumin-glucuronide deconjugation within bone, GUSB activity was much higher in bone marrow than in other tissues tested (Fig. 4C), including kidney, an organ with one of the highest reported levels of GUSB activity in prior multi-organ surveys that excluded bone24,40,41. These data further support the postulate that the capacity of bone marrow to hydrolyze curcumin-glucuronide distributing to bone after oral dosing is GUSB-dependent and is consistent with the relatively high levels of GUSB activity present within normal hematopoietic bone marrow.
Figure 4. Tissue specific expression and activity of deconjugating enzymes (GUSB, HPSE, KL) in C57BL/6J mice.
A) Curcumin-glucuronide specific deconjugation activity of GUSB, HPSE, or KL was determined by quantitation of curcumin-glucuronide substrate and curcumin product after incubating recombinant human enzymes with curcumin-glucuronide (n=3–6/group). **** p < 0.0001 vs control (or as specified). B) Immunoblot of GUSB, HPSE, and KL expression in indicated tissues from female, 4-week, C57BL/6J mice (representative of n=4 blots). C) Tissue-specific GUSB enzyme activity using 4-MUG in 4-week female C57BL/6J mice (n=4–5/group). ****p < 0.0001 vs marrow.
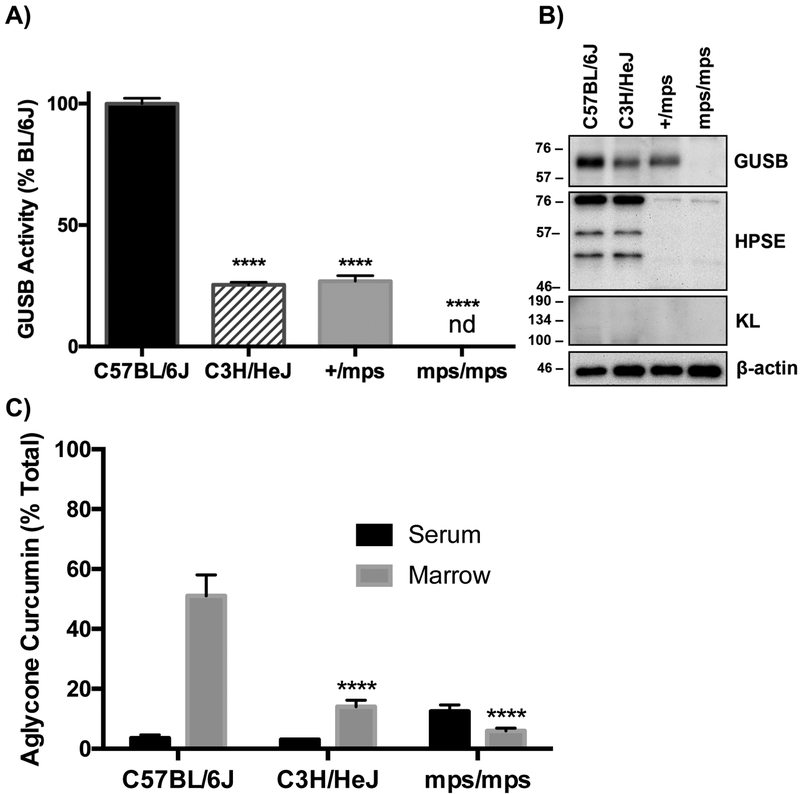
Figure 6. Comparison of GUSB expression and activity in marrow from wild type vs. GUSB mutant mice and the capacity of marrow to hydrolyze curcumin glucuronide distributing to bones following in vivo curcumin treatment.
A) GUSB enzyme activity in marrow (normalized to wild-type BL/6J) in 15-week male mice (n=3–5/group). B) Immunoblot of GUSB, HPSE, and KL expression in marrow from 15-week female wild-type C57BL/6J and partial loss-of-function C3H/HeJ mice, and 15-week male hetero- (+/mps) and homozygous (mps/mps) GUSB deficient mice (representative of n=3 biological replicate blots). C) Aglycone curcumin levels 20 minutes after IP curcumin administration (100 mg/kg) to 15-week female BL/6J, female HeJ mice and male mps/mps mice (n=3–5/group), expressed as % of total curcumin in serum vs. marrow suspensions. There were no significant differences in GUSB activity or aglycone curcumin content based on sex (data not shown). ****p < 0.0001 vs C57BL/6J. nd = not detectable
Curcumin Enrichment in Bone is Dependent on GUSB Expression and Activity.
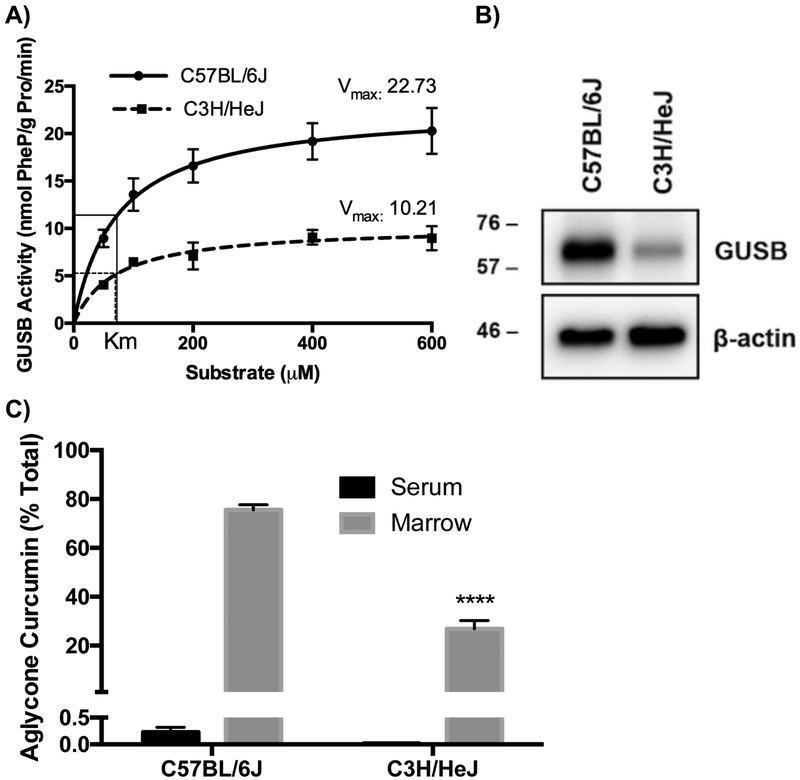
To test GUSB-dependency directly, effects of GUSB mutations on the capacity of bone marrow to deconjugate curcumin-glucuronide distributing to bone after oral dosing was assessed. C3H/HeJ (HeJ) mice possessing a hypomorphic GUSB allele (Gush) that is associated, for unclear reasons42–44, with reduced GUSB activity, were studied in comparison to BL/6J mice, a typical “wild type” (Gusb) control45–50. GUSB– specific enzyme kinetics in HeJ (vs BL/6J) bone marrow demonstrated normal substrate affinity (Km 69.6 μM vs 72.7 μM, respectively), but a 55% lower Vmax (Fig. 5A), which, when coupled with evidence of lower GUSB protein expression (Fig. 5B; 21.9% ± 2.6% of BL/6J, p < 0.0001, n=3), confirms a postulate that decreased GUSB activity in HeJ mice is attributable to lower levels of functional GUSB protein42–44. Key to these studies, this 55–78% decrease in GUSB activity/protein was associated with a decrease in the capacity of HeJ bone marrow to hydrolyze curcumin-glucuronide (Figure 5C and Table 2); while curcumin metabolites in serum and total curcumin in bone marrow were unchanged in curcumin-treated HeJ mice, absolute (p < 0.001) and relative amounts (p < 0.0001) of marrow aglycone curcumin were 59–65% lower (Table 2 and Fig. 5C). Even more strikingly, were changes in the hydrolytic capacity of marrow derived from mps/mps mice with a complete loss of function Gus mutation that results in a phenotype attributed to tissue accumulation of glycosaminoglycans, modeling a rare human GUSB-deficiency disorder, mucopolysaccharidosis (MPS) VII51,52. GUSB enzyme activity (Fig. 6A) and protein expression (Fig. 6B) in phenotypically normal heterozygotes (+/mps) were similar to HeJ mice (low), but completely undetectable in homozygous GUSB-null (mps/mps) mice. Although compensatory increases in non-deglucuronidating enzymes have been reported in GUSB-null models53, HPSE and KL protein levels in bone were not altered in +/mps or mps/mps mice (Fig. 6B). Because skeletal abnormalities in mps/mps mice made oral gavage difficult (e.g. shortened snouts)51,54, curcumin was dosed IP in experiments comparing mps/mps to HeJ or BL/6J mice, resulting in higher circulating levels of aglycone curcumin in wildtype BL/6J mice. However, aglycone curcumin remained a minor product in BL/6J serum, accounting for <4% of circulating total curcumin, and aglycone curcumin remained the major product in ex vivo bone marrow suspensions (51% of total) (Fig. 6C). In GUSB mutants, bone marrow aglycone curcumin, expressed as % of total curcumin, was 73% lower in HeJ mice (Fig. 6C, p < 0.0001) and lower still in mps/mps mice (88% decrease, p < 0.001 vs BL/6J), although this decrease was not statistically different from HeJ levels. Notably, circulating curcumin concentrations were significantly elevated in mps/mps mice (3.2-fold increase vs. BL/6J, p < 0.05), which may have contributed to aglycone curcumin content in GUSB-null bone marrow (Table 3). At the same time, blockade of curcumin-glucuronide deconjugation within the GUSB-null bone microenvironment may explain the notable 4-fold increase in bone marrow curcumin-glucuronide concentrations documented in mps/mps (vs. BL/6J) mice (p < 0.05), despite statistically similar circulating curcuminglucuronide levels (Table 3). In toto, these findings in GUSB-deficient or null mice demonstrate that the capacity of marrow to deconjugate curcumin-glucuronide is GUSB-dependent, and that even a partial (≥55%) loss of GUSB activity is sufficient to alter marrow’s deconjugation capacity.
Figure 5. Effect of a naturally occurring loss of function GUSB mutation in C3H/HeJ mice (vs C57BL/6J mice) on marrow GUSB expression and activity and the capacity of marrow to hydrolyze curcumin-glucuronide distributing to bones following in vivo curcumin treatment.
A) GUSB enzyme kinetics using phenolphthalein-glucuronide in marrow from female 4-week C57BL/6J vs. C3H/HeJ mice (n = 3/group). B) Immunoblot of GUSB protein in marrow from female 4-week C57BL/6J vs. C3H/HeJ mice (representative of n=4 biological replicate blots). C) Aglycone curcumin levels 30 minutes after oral gavage with 500 mg/kg curcumin in male 15-week C57BL/6J vs C3H/HeJ mice, expressed as % of total curcumin in serum vs. marrow suspensions. There were no significant differences in GUSB activity based on sex or age (data not shown). ****p < 0.0001 vs C57BL/6J.
Table 2.
Concentrations of curcumin and curcumin-glucuronide in serum and marrow of C57BL/6J and C3H/HeJ mice (mean ± SEM).
| C57BL/6J | C3H/HeJ | |
|---|---|---|
| Curcumin-Glucuronide (μM) | ||
| Serum | 82.7 (5.7) | 80.0 (9.3) |
| Marrow | 0.36 (0.04) | 1.19 (0.22) |
| Aglycone Curcumin (μM) | ||
| Serum | 0.17 (0.06) | nd (≤0.01) |
| Marrow | 1.14 (0.13) | 0.47 (0.13)a |
Tissues harvested 30 minutes after oral treatment with 500 mg/kg curcumin (n=3–4 mice/group), with marow suspension in sodium acetate buffer.
p < 0.001 vs C57BL/6J. nd = not detectable.
Table 3.
Concentrations of curcumin and curcumin-glucuronide in serum and marrow of wild-type and GUSB mutant mice (mean ± SEM).
| C57BL/6J | C3H/HeJ | mps/mps | |
|---|---|---|---|
| Curcumin-Glucuronide (μM) | |||
| Serum | 92.7 (25.1) | 92.1 (27.7) | 151.0 (19.3) |
| Marrow | 1.3 (0.4) | 2.7 (0.8) | 5.2 (1.4)a |
| Aglycone Curcumin (μM) | |||
| Serum | 7.2 (3.0) | 2.8 (1.0) | 22.8 (6.9)a,b |
| Marrow | 1.9 (0.5) | 0.5 (0.2) | 0.4 (0.1) |
Tissues harvested 20 minutes after IP treatment with 100 mg/kg curcumin (n=3–4 mice/group) with marow suspension in sodium acetate buffer.
p < 0.05 vs C57BL/6J.
p < 0.05 vs C3H/HeJ.
Role of Extracellular GUSB in the Bone Microenvironment
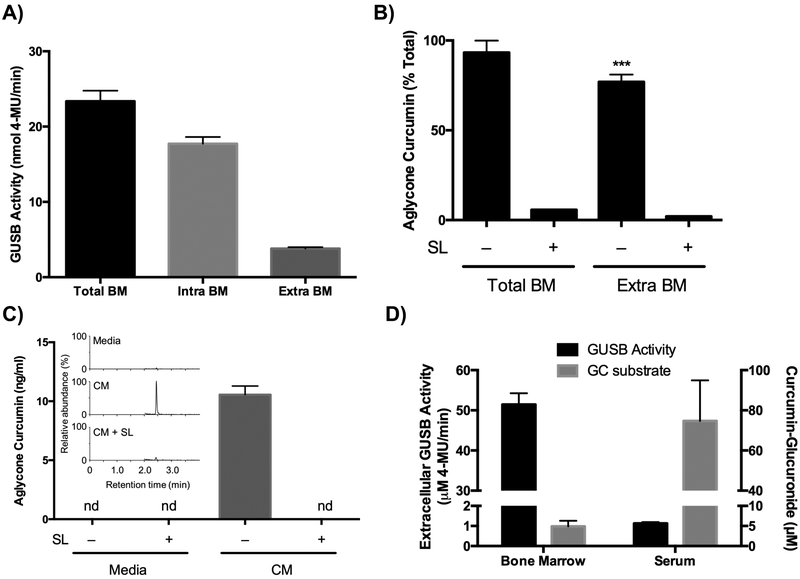
Because extracellular GUSB has been postulated to play a major role in deconjugating other glucuronidated compounds at sites of inflammation24–26, extracellular vs cell-associated GUSB activity in marrow was assessed. Soluble GUSB activity in cell-free marrow fractions (extracellular GUSB), while only representing 18% of total GUSB activity in marrow (Fig. 7A), was still sufficient to deconjugate curcumin-glucuronide added to cell-free fractions ex vivo, such that aglycone curcumin levels were only slightly lower than those formed under similar conditions by whole marrow suspensions (Figure 7B). Both of these effects were blocked by the GUSB inhibitor, SL (Fig. 7B). Because the acidic pH of the acetate buffer used to resuspend marrow both stabilizes aglycone curcumin and optimizes GUSB activity, the relatively higher abundance of aglycone curcumin formed ex vivo in marrow suspensions vs. markedly lower levels detected in lyophilized marrow suggests that: 1) optimal conditions for GUSB activity may not be present in vivo (i.e., limited or lack of microenvironments with acidic extracellular pH, or regional mismatch in the relative distributions of GUSB and substrate within the bone microenvironment), and/or 2) a lack of stability of aglycone curcumin formed in vivo limits its utility as a marker of deconjugation. In addressing the first postulate, it is notable that conditioned media from freshly isolated bone marrow cells cultured at a physiologic temperature and pH maintained the ability to deconjugate GC added in vitro, forming detectable aglycone curcumin in a process that remained saccharolactone inhibitable (Fig. 7C). This finding supports, while not proving, a physiologic role for extracellular, marrow cell-derived GUSB in deconjugating (activating) curcumin-glucuronide within the bone microenvironment. Moreover, it is notable that the ratio of extracellular enzyme activity to substrate (curcumin-glucuronide) is almost 800-fold higher in bone as compared to the circulation (Fig. 7D). Given previous evidence of an important role for extracellular GUSB in deconjugating glucuronidated pro-drugs at sites of inflammation, this finding suggests that normal bone marrow is also primed to deconjugate curcumin-glucuronide within bone due to its high extracellular GUSB content.
Figure 7. The role of extracellular bone marrow cell-derived GUSB in deconjugation of curcumin-glucuronide.
A) GUSB activity in total bone marrow (BM), cell-associated (intracellular) BM fraction, and cell-free (extracellular) BM fraction from female 4-week C57BL/6J (n=3/group) B) Aglycone curcumin levels, expressed as % of total curcumin, after incubating total BM or extracellular BM fraction for 2 hours in sodium acetate buffer (50 mM, pH 5) after ex vivo addition of curcumin-glucuronide (0.5 μM) using female 4-week C57BL/6J (n=3/group). C) Aglycone curcumin levels produced after incubating 10 μM curcumin-glucuronide in fresh media + 10% FBS + 1% pen/strep or marrow-conditioned media (CM), from female 4-week C57BL/6NJ, with or without SL (10 mM) for 2 hours at 37°C (n=4–8/group). Inset is a representative chromatogram. D) Comparison of GUSB enzyme activity in bone marrow (vs. serum) and curcumin glucuronide substrate concentrations in bone marrow (collected ex vivo in acetate buffer containing SL [10mM]) vs serum collected 30 minutes after gavage of female 4-week C57BL/6J mice with 500 mg/kg curcumin. Data expressed as mean ± SEM.
Conclusion
While definitive proof of in vivo deconjugation of curcumin-glucuronide by GUSB within bone is currently lacking, the microenvironment of bone, which has one of the highest GUSB activities of all organs assayed, appears to have a large capacity for GUSB-mediated deconjugation of a significant fraction of the curcumin-glucuronide that distributes to bone following oral curcumin administration, a transformation that appears to be required for anti-resorptive activity. These findings help reconcile prior in vivo evidence of bone protective effects of curcumin with its well-documented, rapid transformation into an inactive glucuronide conjugate, and may also help to explain turmeric’s traditional use to quell inflammation in tissues with inflammatory infiltrates that are rich in GUSB-expressing cells. Within bone, optimal conditions for GUSB-mediated deconjugation may vary with disease states. For example, the formation of multiple, highly acidic resorption pits by active osteoclasts55 during states of high turnover (i.e. bone development) or resorption (i.e. post-menopausal osteoporosis) could facilitate localized deconjugation within specific areas of the bone microenvironment, including metabolically active trabecular areas with high risk of fracture56. It should also be noted that, in addition to glucuronidation, curcumin may also undergo oxidative, reductive, and degradative metabolism57. However, our preliminary studies have not detected oxidative metabolites in serum or bone, and reduction is thought to be minor metabolic pathway in vivo16,18. Furthermore, our previous published58 and ongoing studies do not support a role for these metabolites mediating effects of curcumin in bone. While additional studies detailing bone specific metabolism of curcumin are ongoing, this current demonstration of possible pharmacologic consequences of even partial deficiencies in GUSB, a highly polymorphic gene in humans59, in “activating” ingested curcumin serves as a reminder, in this era of personalized medicine, that just as an individual’s genetics can determine their response to pharmaceutical drugs, the same may be true when considering the metabolic fate and effects of medicinal natural products.
EXPERIMENTAL SECTION
Materials
Curcumin (218580100, Fisher; 80.6% curcumin, 13.5% demethoxycurcumin, and 2.4% bisdemethoxycurcumin by weight) and curcuminglucuronide were purchased, with content/purity verified using standard LC/MS methods (see below)11,60 with stock solutions prepared in DMSO. Murine RAW 264.7 cells were obtained from American Type Culture Collection (#TIB-71), ATCC) and used within 10 passages. Primary antibodies against GUSB (ARP44234_T100, Aviva Systems Biology), HPSE (bs-1541R, Bioss USA), KL (ab181373, Abcam) and HRP secondary antibodies (7074, Cell Signaling) were purchased. Phenolphthalein-glucuronide (PhePG; P0501), 4-methylumbelliferyl-glucuronide (4-MUG; M9130), D-Saccharic acid 1,4-lactone monohydrate (saccharolactone, S0375), and RIPA lysis buffer (R0278) were purchased from Sigma Aldrich. Supersignal West Femto ECL (34095) and RestorePlus stripping buffer (46430) were purchased from ThermoFisher. Recombinant human (rh)-GUSB (6144-GH), rh-HPSE (7570-GH), and rh-KL (5334-KL), and recombinant mouse receptor activator of NFkB ligand (RANKL, 462-TEC) were purchased from R&D Systems. Mini-PROTEAN TGX-PAGE gels (4568046) were purchased from BioRad and PVDF membranes (IPFL0010) from Millipore.
Animals
All animal procedures were approved by the University of Arizona Institutional Animal Care and Use Committee (IACUC). GUSB-null (“mps/mps”) mice homozygous on a C57BL6J background for a spontaneous Gus base pair deletion causing a Gus frameshift mutation and premature stop codon (Gusmps), which models a rare human GUSB-deficiency syndrome (mucopolysaccharidosis type VII (MPS VII), were a kind gift of Dr. John H. Wolfe51,52. Phenotypically normal heterozygous (+/mps) mice were also provided by Dr. Wolfe from the same B6.C-H2bm1/ByBir-Gusmps/J, MPS VII (JAX strain 000256) colony maintained at the Children’s Hospital of Pennsylvania. Wild type C57BL/6J mice were obtained directly from Jackson labs (JAX strain 000664), as were C3H/HeJ mice (JAX strain 000659), phenotypically normal mice commonly used for GUSB research that exhibit decreased GUSB activity due to difference in GUSB gene complex alleles (vs. C57BL/6J) that may alter enzyme stability and rates of synthesis50. Mice were provided water and diet (NIH-31 7913, Teklad) ad libitum while maintained at the temperature and humidity-controlled University of Arizona central animal facility under a 14/10-hour light/dark cycle.
Osteoclastogenesis Assay
RAW 264.7 cells were plated at 5×103 cells/well in 48-well plates in DMEM containing 10% FBS and 1% penicillin/streptomycin and allowed to adhere overnight at 37°C and 5% CO2. Cells were pretreated with either aglycone curcumin or curcumin-glucuronide for 4 hours followed by induction of osteoclastogenesis by addition of RANKL61 (50 ng/mL, final concentration) and further incubation at 37°C for 72 hours before quantification of the formation of tartrate resistant acid phosphatase (TRAP)-positive (#387A, Sigma), multinucleated (n>3) osteoclasts (n = 4/group).
In Vivo Curcumin Treatment for Pharmacokinetic Analyses
Mice were administered curcumin, as indicated, via oral gavage (500 mg/kg in DMSO, 2.5 g human equivalent dose [HED]62) or intraperitoneal (IP) injection (100 mg/kg in DMSO, 0.5 g HED). Blood and bone marrow were obtained under isoflurane anesthesia at the designated times for pharmacokinetic analysis or at approximate times of maximal reported concentrations (30 minutes for oral gavage and 20 minutes for IP)14,15,63. Blood was allowed to clot for 10 minutes at room temperature and serum collected by centrifugation at 8,000 g for 10 minutes prior to storage at −80°C. After animal sacrifice by cervical dislocation, bone marrow was collected by centrifugation of legs, stripped of soft tissue, at 10,000 g for 15 seconds64. Bone marrow pellets were then 1) snap frozen in LN2, for later lyophilization, or 2) collected under conditions that stabilize aglycone curcumin by resuspending in sodium acetate buffer (50 mM, pH 5) and incubated for 2 hours on ice prior to centrifugation to obtain supernatants that were stored at −80°C for later analysis. The effects of a GUSB inhibitor, saccharolactone (SL), on curcuminglucuronide metabolism was determined either by: 1) pre-treatment of mice with SL (1 g/kg in saline by IP injection38) 1 hour prior to curcumin administration, or 2) resuspension of marrow from curcumin-treated mice in sodium acetate buffer ± SL (10 mM). Aglycone curcumin and curcumin-glucuronide were later quantified, as indicated, in serum, lyophilized marrow, or supernatants from bone marrow suspensions by LCMS.
Ex Vivo Curcumin-Glucuronide Treatment for Pharmacokinetic Analysis
To determine the deconjugation capacity of whole bone marrow vs. cell-free marrow supernatants, marrow from untreated mice was resuspended in sodium acetate buffer (50 mM, pH 5) ± SL (10 mM), with incubation on ice for 2 hours after addition of curcumin-glucuronide (0.5 μM) to: 1) whole suspensions or 2) supernatant alone (cell-free fraction) obtained from immediate centrifugation of suspensions. Following incubations, supernatants were snap-frozen for later analysis. Curcumin-glucuronide deconjugation capacity of bone marrow was also determined at physiologic temperature and pH; marrow from untreated mice was resuspended and incubated in DMEM + 10% FBS + 1% pen/strep at 1×106 cells/ml for 2 hours at 37°C and 5% CO2 to generate conditioned media. Aliquots (300 μl per well) of fresh vs conditioned media, centrifuged to remove cells, were then transferred to a 24 well plate and incubated with curcuminglucuronide (10 μM) ± saccharolactone (10 mM) for 2 hours at 37°C and 5% CO2. All samples were centrifuged prior to snap-freezing of supernatants for later analysis of aglycone and glucuronidated curcumin by LC/MS.
LC/MS Determination of Aglycone Curcumin and Curcumin-Glucuronide
Serum was acidified to pH 5 using HCl (1 M) and bone marrow supernatants were diluted in 20 mM sodium acetate pH 5 prior to loading on 30-mg Waters HLB (hydrophilic-lipophilic balance) cartridges, washing with water, and elution with methanol. Eluates were evaporated under a stream of nitrogen and dissolved in water/acetonitrile (50:50). Snap-frozen marrow pellets were lyophilized overnight prior to extraction using water/acetonitrile (30:70), with addition of 10 mM saccharolactone. Samples were sonicated and filtered before analysis. LC-MS analyses were performed using a Thermo Finnigan TSQ Vantage triple stage quadrupole mass spectrometer equipped with an electrospray interface operated in the positive ion mode. For chromatography a Waters Symmetry Shield C18 column (2.1 × 50 mm, 1.8 μm) was eluted at room temperature with a gradient of acetonitrile in water/0.1% formic acid changed from 15% acetonitrile to 85% in 3 min and further increased to 95% in 1 min at a flow rate of 0.4 ml/min. The SRM transitions were for curcumin m/z 369 → 177, d6-curcumin m/z 375 → 180, curcumin-glucuronide m/z 545 → 369 and d6-curcumin-glucuronide m/z 551 → 375. The limits of detection for curcumin and curcumin-glucuronide were 14.9 nM and 2.9 nM, respectively. Aglycone curcumin, curcumin-glucuronide or total curcumin (sum of aglycone and glucuronidated curcumin) content is expressed, as indicated, either as an absolute concentration or a percentage of total curcumin content. Absolute concentrations were adjusted to the original volume of the marrow pellet (i.e., dilution of sample with sodium acetate buffer), utilizing pellet mass and assuming a tissue density of 1.06 g/ml.
In Vitro Determination of Mammalian Deconjugating Enzyme Specificity for Curcumin-Glucuronide
Recombinant human (rh)-GUSB, rh-HPSE, or rh-KL enzymes (5 μg/mL, corresponding to 15 nM, 100 nM and 45 nM of active enzyme complex, respectively)30,31,33, were incubated for 24 hours at 37°C with synthesized curcuminglucuronide60 (459 nM) at pH 5 in buffers optimized for each enzyme. The reaction was stopped by addition of HCl, extracted with HLB cartridge, curcumin-glucuronide remaining and aglycone curcumin formed were quantified by LC/MS. Molar concentrations are expressed as % of control, normalized to unreacted curcuminglucuronide assayed in control samples lacking enzyme (n=3/group).
GUSB-Specific Enzymatic Activity Assay
GUSB-specific deconjugation activity was determined using standard enzymatic assay methods65,66 and GUSB-specific substrates. Briefly, to determine deconjugation activity, serum, bone marrow supernatants, lysed tissues/cells, or recombinant enzymes were normalized to total protein or molarity, as indicated, and incubated for 30 to 60 minutes at 37°C in 50 mM sodium acetate buffer (pH 5) with glucuronides of 4-methylumbelliferone [4-MU] or phenolphthalein [PheP]), using substrate concentrations exceeding Km values (1.4 mM and 0.6 mM, respectively), before reaction quenching and quantification of product formation in comparison to 4-MU or PheP standard curves using fluorescence (360nm/470nm, Ex/Em) or visual absorbance (540 nm), respectively. The GUSB-specificity for deconjugation of these substrates was determined by incubation with recombinant human GUSB, HPSE or KL enzymes and quantification of product formation; GUSB cleaved glucuronidated 4-MU at a rate of 2.64 nmol 4-MU/pmol enzyme/min, while HPSE and KL were without effect (< 0.007 nmol 4-MU/nmol enzyme/min), and glucuronidated PheP was cleaved by GUSB at a rate of 23.5 vs. 1.0 or 0.14 nmol PheP/nmol enzyme/min for KL and HPSE, respectively.
Western Blots
Tissue-specific protein expression for the three enzymes with reported glucuronide deconjugating activity (GUSB, HPSE, and KL) were determined by immunoblot of protein lysates isolated from indicated tissues as previously described20. Blots were probed with primary antibodies raised against KL, HPSE, and GUSB followed by HRP-conjugated secondary antibody. Expression was visualized by chemiluminescence. Blots were stripped and re-probed for house-keeping genes. Because housekeeping gene expression was tissue-specific, equal protein loading was also confirmed by visualization of total protein content, using BioRad Stain-Free labeling67,68. For quantitative analyses comparing enzyme expression in bones from different strains, densitometry units were quantitated using ImageJ (NIH) with values normalized to β-actin expression.
Statistical Analysis
All statistical analyses were performed using Prism software (v. 6.0h, GraphPad), including Lineweaver-Burke enzyme kinetics. Unless otherwise specified, values are presented as mean ± SEM. Significant differences were determined by t-test or one-way/two-way ANOVA with Tukey or Sidak post-hoc test, as appropriate. Pharmacokinetic analysis was performed with PKSolver 2.0, using noncompartmental analysis after extravascular administration69.
STRUCTURES
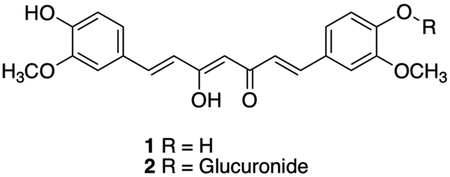
ACKNOWLEDGEMENTS
We kindly thank Dr. John H. Wolfe (University of Pennsylvania) for providing B6.C-H2bm1/ByBir-Gusmps/J mice. This work was supported by the National Cancer Institute (NCI), the National Center for Complementary and Integrative Health (NCCIH), and the Office of Dietary Supplements (ODS) at the National Institutes of Health (NIH) (R01CA174926 and R34 AT007837 to JLF, R01AT006896 to CS and F31AT009938 to AK); the United States Department of Agriculture (2014-38420-21799 National Needs Fellowship to AK); and the American Heart Association (16POST27250138 postdoctoral fellowship to PBL). Mass spectrometric analyses were performed in part through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404 Core Scholarship. Pharmacokinetic analyses were performed in part through the University of Arizona Cancer Center’s Analytical Chemistry Shared Resource supported by NCI Cancer Center Support Grant P30CA023074.
Footnotes
Dedicated to Dr. Barbara N. Timmerman, University of Kansas, for her pioneering work on bioactive natural products.
REFERENCES
- (1).Velayudhan KC; Dikshit N; Abdul Nizar M Ethnobotany of Turmeric (Curcuma Longa L.). Indian J. Tradit. Knowl 2012, 11 (4), 607–614. [Google Scholar]
- (2).Fadus MC; Lau C; Bikhchandani J; Lynch HT Curcumin: An Age-Old Anti-Inflammatory and Anti-Neoplastic Agent. J. Tradit. Complement. Med 2017, 7 (3), 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hatefi M; Ahmadi MRH; Rahmani A; Dastjerdi MM; Asadollahi K Effects of Curcumin on Bone Loss and Biochemical Markers of Bone Turnover in Patients with Spinal Cord Injury. World Neurosurg. 2018, 114, e785–e791. [DOI] [PubMed] [Google Scholar]
- (4).Khanizadeh F; Rahmani A; Asadollahi K; Ahmadi MRH Combination Therapy of Curcumin and Alendronate Modulates Bone Turnover Markers and Enhances Bone Mineral Density in Postmenopausal Women with Osteoporosis. Arch. Endocrinol. Metab 2018, 62 (4), 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wright LE; Frye JB; Timmermann BN; Funk JL Protection of Trabecular Bone in Ovariectomized Rats by Turmeric (Curcuma Longa L.) Is Dependent on Extract Composition. J. Agric. Food Chem. 2010, 58 (17), 9498–9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Funk JL; Frye JB; Oyarzo JN; Kuscuoglu N; Wilson J; McCaffrey G; Stafford G; Chen G; Lantz RC; Jolad SD; et al. Efficacy and Mechanism of Action of Turmeric Supplements in the Treatment of Experimental Arthritis. Arthritis Rheum. 2006, 54 (11), 3452–3464. [DOI] [PubMed] [Google Scholar]
- (7).Wright LE; Frye JB; Lukefahr AL; Timmermann BN; Mohammad KS; Guise TA; Funk JL Curcuminoids Block TGF-β Signaling In Human Breast Cancer Cells And Limit Osteolysis In A Murine Model Of Breast Cancer Bone Metastasis. J. Nat. Prod 2013, 76 (3), 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).French DL; Muir JM; Webber CE The Ovariectomized, Mature Rat Model of Postmenopausal Osteoporosis: An Assessment of the Bone Sparing Effects of Curcumin. Phytomedicine 2008, 15 (12), 1069–1078. [DOI] [PubMed] [Google Scholar]
- (9).Hie M; Yamazaki M; Tsukamoto I Curcumin Suppresses Increased Bone Resorption by Inhibiting Osteoclastogenesis in Rats with Streptozotocin-Induced Diabetes. Eur. J. Pharmacol 2009, 621 (1–3), 1–9. [DOI] [PubMed] [Google Scholar]
- (10).Kim WK; Ke K; Sul OJ; Kim HJ; Kim SH; Lee MH; Kim HJ; Kim SY; Chung HT; Choi HS Curcumin Protects against Ovariectomy-Induced Bone Loss and Decreases Osteoclastogenesis. J. Cell. Biochem 2011, 112 (11), 3159–3166. [DOI] [PubMed] [Google Scholar]
- (11).Funk JL; Oyarzo JN; Frye JB; Chen G; Clark R; Jolad SD; Sólyom AM; Timmermann BN Turmeric Extracts Containing Curcuminoids Prevent Experimental Rheumatoid Arthritis. J. Nat. Prod 2006, 69 (3), 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Shang W; Zhao LJ; Dong XL; Zhao ZM; Li J; Zhang BB; Cai H Curcumin Inhibits Osteoclastogenic Potential in PBMCs from Rheumatoid Arthritis Patients via the Suppression of MAPK/RANK/c-Fos/NFATc1 Signaling Pathways. Mol. Med. Rep 2016, 14 (4), 3620–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).An S; Han F; Hu Y; Liu Y; Li J; Wang L Curcumin Inhibits Polyethylene-Induced Osteolysis via Repressing NF-KB Signaling Pathway Activation. Cell. Physiol. Biochem 2018, 50 (3), 1100–1112. [DOI] [PubMed] [Google Scholar]
- (14).Pan M; Huang T; Lin J Biotransformation of Curcumin Through Reduction and Glucuronidation in Mice. Drug Metab. Dispos 1999, 27 (1), 486–494. [PubMed] [Google Scholar]
- (15).Shoba G; Joy D; Joseph T; Majeed M; Rajendran R; Srinivas P Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers. Planta Med. 1998, 64 (5), 353–356. [DOI] [PubMed] [Google Scholar]
- (16).Ireson C; Orr S; Jones DJL; Verschoyle R; Lim C-K; Luo J; Howells L; Plummer S; Jukes R; Williams M; et al. Characterization of Metabolites of the Chemopreventive Agent Curcumin in Human and Rat Hepatocytes and in the Rat in Vivo, and Evaluation of Their Ability to Inhibit Phorbol Ester-Induced Prostaglandin E2 Production. Cancer Res. 2001, 61, 1058–1064. [PubMed] [Google Scholar]
- (17).Garcea G; Jones DJL; Singh R; Dennison AR; Farmer PB; Sharma RA; Steward WP; Gescher AJ; Berry DP Detection of Curcumin and Its Metabolites in Hepatic Tissue and Portal Blood of Patients Following Oral Administration. Br. J. Cancer 2004, 90 (5), 1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Zhongfa L; Chiu M; Wang J; Chen W; Yen W; Fan-Havard P; Yee LD; Chan KK Enhancement of Curcumin Oral Absorption and Pharmacokinetics of Curcuminoids and Curcumin Metabolites in Mice. Cancer Chemother. Pharmacol 2012, 69 (3), 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Asai A; Miyazawa T Occurrence of Orally Administered Curcuminoid as Glucuronide and Glucuronide/Sulfate Conjugates in Rat Plasma. Life Sci. 2000, 67 (23), 2785–2793. [DOI] [PubMed] [Google Scholar]
- (20).Kunihiro AG; Brickey JA; Frye JB; Luis PB; Schneider C; Funk JL Curcumin, but Not Curcumin-Glucuronide, Inhibits Smad-Signaling in TGFβ-Dependent Bone Metastatic Breast Cancer Cells and Is Enriched in Bone Compared to Other Tissues. J. Nutr. Biochem No. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shoji M; Nakagawa K; Watanabe A; Tsuduki T; Yamada T; Kuwahara S; Kimura F; Miyazawa T Comparison of the Effects of Curcumin and Curcumin Glucuronide in Human Hepatocellular Carcinoma HepG2 Cells. Food Chem. 2014, 151, 126–132. [DOI] [PubMed] [Google Scholar]
- (22).Choudhury AK; Raja S; Mahapatra S; Nagabhushanam K; Majeed M Synthesis and Evaluation of the Anti-Oxidant Capacity of Curcumin Glucuronides, the Major Curcumin Metabolites. Antioxidants 2015, 4, 750–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang J; Zhang J; Zhang CJ; Wong YK; Lim TK; Hua ZC; Liu B; Tannenbaum SR; Shen HM; Lin Q In Situ Proteomic Profiling of Curcumin Targets in HCT116 Colon Cancer Cell Line. Sci. Rep 2016, 6 (22146), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ishisaka A; Kawabata K; Miki S; Shiba Y; Minekawa S; Nishikawa T; Mukai R; Terao J; Kawai Y Mitochondrial Dysfunction Leads to Deconjugation of Quercetin Glucuronides in Inflammatory Macrophages. PLoS One 2013, 8 (11), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kawabata K; Mukai R; Ishisaka A Quercetin and Related Polyphenols: New Insights and Implications for Their Bioactivity and Bioavailability. Food Funct. 2015, 6 (5), 1399–1417. [DOI] [PubMed] [Google Scholar]
- (26).Joubert N; Denevault-Sabourin C; Bryden F; Viaud-Massuard MC Towards Antibody-Drug Conjugates and Prodrug Strategies with Extracellular Stimuli-Responsive Drug Delivery in the Tumor Microenvironment for Cancer Therapy. Eur. J. Med. Chem 2017, 142, 393–415. [DOI] [PubMed] [Google Scholar]
- (27).Chen KC; Cheng TL; Leu YL; Prijovich ZM; Chuang CH; Chen BM; Roffler SR Membrane-Localized Activation of Glucuronide Prodrugs by β-Glucuronidase Enzymes. Cancer Gene Ther. 2007, 14 (2), 187–200. [DOI] [PubMed] [Google Scholar]
- (28).Lorbacher P; Yam LT; Mitus WJ Cytochemical Demonstration of β-Glucuronidase Activity in Blood and Bone Marrow Cells. J. Histochem. Cytochem 1967, 5 (11), 680–687. [DOI] [PubMed] [Google Scholar]
- (29).de Graaf CA; Choi J; Baldwin TM; Bolden JE; Fairfax KA; Robinson AJ; Biben C; Morgan C; Ramsay K; Ng AP; et al. Haemopedia: An Expression Atlas of Murine Hematopoietic Cells. Stem Cell Reports 2016, 7 (3), 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Sperker B; Backman JT; Kroemer HK The Role of β-Glucuronidase in Drug Disposition and Drug Targeting in Humans. Clin. Pharmacokinet 1997, 33 (1), 18–31. [DOI] [PubMed] [Google Scholar]
- (31).Peterson SB; Liu J Unraveling the Specificity of Heparanase Utilizing Synthetic Substrates. J. Biol. Chem 2010, 285 (19), 14504–14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Pikas DS; Li JP; Vlodavsky I; Lindahl U Substrate Specificity of Heparanases from Human Hepatoma and Platelets. J. Biol. Chem 1998, 273 (30), 18770–18777. [DOI] [PubMed] [Google Scholar]
- (33).Tohyama O; Imura A; Iwano A; Freund JN; Henrissat B; Fujimori T; Nabeshima YI Klotho Is a Novel β-Glucuronidase Capable of Hydrolyzing Steroid β-Glucuronides. J. Biol. Chem 2004, 279 (11), 9777–9784. [DOI] [PubMed] [Google Scholar]
- (34).Marczylo TH; Verschoyle RD; Cooke DN; Morazzoni P; Steward WP; Gescher AJ Comparison of Systemic Availability of Curcumin with That of Curcumin Formulated with Phosphatidylcholine. Cancer Chemother. Pharmacol 2007, 60 (2), 171–177. [DOI] [PubMed] [Google Scholar]
- (35).Sharma RA; Mclelland HR; Hill KA; Sharma RA; Mclelland HR; Hill KA; Ireson CR; Euden SA; Manson MM; Pirmohamed M; et al. Pharmacodynamic and Pharmacokinetic Study of Oral Curcuma Extract in Patients with Colorectal Cancer. Clin. Cancer Res 2001, 7 (July), 1894–1900. [PubMed] [Google Scholar]
- (36).Vareed SK; Kakarala M; Ruffin MT; Crowell JA; Normolle DP; Djuric Z; Brenner DE Pharmacokinetics of Curcumin Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomarkers Prev 2008, 17 (6), 1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Griesser M; Pistis V; Suzuki T; Tejera N; Pratt DA; Schneider C Autoxidative and Cyclooxygenase-2 Catalyzed Transformation of the Dietary Chemopreventive Agent Curcumin. J. Biol. Chem 2011, 286 (2), 1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Tzou S-C; Roffler S; Chuang K-H; Yeh H-P; Su Y-C; Cheng C-M; Tseng W-L; Shiea J; Harm I-H; Cheng K-W; et al. Micro-PET Imaging of Beta-Glucuronidase Activity by the Hydrophobic Conversion of a Glucuronide Probe. Radiology 2009, 252 (3), 754–762. [DOI] [PubMed] [Google Scholar]
- (39).Ho K-J Human β-Glucuronidase. Studies on the Effects of PH and Bile Acids in Regard to Its Role in the Pathogenesis of Cholelithiasis. Biochimica et Biophysica Acta. 1985, pp 197–206. [DOI] [PubMed] [Google Scholar]
- (40).Johnson WG; Hong JL; Knights SM Variation in Ten Lysosomal Hydrolase Enzyme Activities in Inbred Mouse Strains. Biochem. Genet 1986, 24 (11–12), 891–909. [DOI] [PubMed] [Google Scholar]
- (41).Macsai CE; Derrick-Roberts ALK; Ding X; Zarrinkalam KH; McIntyre C; Anderson PH; Anson DS; Byers S Skeletal Response to Lentiviral Mediated Gene Therapy in a Mouse Model of MPS VII. Mol. Genet. Metab 2012, 106 (2), 202–213. [DOI] [PubMed] [Google Scholar]
- (42).Ganschow R Simultaneous Genetic Control of the Structure and Rate of Synthesis of Murine Glucuronidase. Isozymes Curr. Top. Biol. Med. Res 1975, IV Genetic, 633–647. [Google Scholar]
- (43).Wawrzyniak CJ; Gallagher PM; D’Amore M. a; Carter JE; Lund SD; Rinchik EM; Ganschow RE DNA Determinants of Structural and Regulatory Variation within the Murine Beta-Glucuronidase Gene Complex. Mol. Cell. Biol 1989, 9 (9), 4074–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wawrzyniak CJ; Meredith SA; Ganschow RE Two Genetic Elements Regulate Murine Beta-Glucuronidase Synthesis Following Transcript Accumulation. Genetics 1989, 121 (1), 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Morrow AG; Greenspan EM; Carrol DM Comparative Studies of Liver Glucuronidase Activity in Inbred Mice. J. Natl. Cancer Inst 1950, 10 (5), 1199–1203. [PubMed] [Google Scholar]
- (46).Ganschow R; Paigen K Glucuronidase Phenotypes of Inbred Mouse Strains. Genetics 1968, 59 (July), 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Fishman WH; Farmelant MH Effects of Androgens and Estrogens on β-Glucuronidase in Inbred Mice. Endocrinology 1953, 52 (5), 536–545. [DOI] [PubMed] [Google Scholar]
- (48).Pfister K; Paigen K; Watson G; Chapman V Expression of β-Glucuronidase Haplotypes in Prototype and Congenic Mouse Strains. Biochem. Genet 1982, 20 (5–6), 519–536. [DOI] [PubMed] [Google Scholar]
- (49).Morrow AG; Greenspan EM; Carrol DM Liver-Glucuronidase Activity of Inbred Mouse Strains. J. Natl. Cancer Inst. 1949, 10 (3), 657–661. [PubMed] [Google Scholar]
- (50).Gwynn B; Lueders K; Sands MS; Birkenmeier EH Intracisternal A-Particle Element Transposition into the Murine Beta-Glucuronidase Gene Correlates with Loss of Enzyme Activity: A New Model for β-Glucuronidase Deficiency in the C3H Mouse. Mol. Cell. Biol 1998, 18 (11), 6474–6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Birkenmeier EH; Davisson MT; Beamer WG; Ganschow RE; Vogler CA; Gwynn B; Lyford KA; Maltais LM; Wawrzyniak CJ Murine Mucopolysaccharidosis Type VII. Characterization of a Mouse with Beta-Glucuronidase Deficiency. J Clin Invest 1989, 83 (4), 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Wolfe JH; Sands MS; Barker JE; Gwynn B; Rowe LB; Vogler CA; Birkenmeier EH Reversal of Pathology in Murine Mucopolysaccharidosis Type VII by Somatic Cell Gene Transfer. Nature 1992, 360 (6406), 749–753. [DOI] [PubMed] [Google Scholar]
- (53).Tomatsu S; Orii KO; Vogler C; Grubb JH; Snella EM; Gutierrez M; Dieter T; Holden CC; Sukegawa K; Orii T; et al. Production of MPS VII Mouse (Gustm(He540A-ME536A)Sly) Doubly Tolerant to Human and Mouse β-Glucuronidase. Hum. Mol. Genet 2003, 12 (9), 961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Vogler C; Birkenmeier EH; Sly WS; Levy B; Pegors C; Kyle JW; Beamer WG A Murine Model of Mucopolysaccharidosis VII. Gross and Microscopic Findings in Beta-Glucuronidase-Deficient Mice. Am. J. Pathol 1990, 136 (1), 207–217. [PMC free article] [PubMed] [Google Scholar]
- (55).Ross FP Osteoclast Biology and Bone Resorption In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Rosen CJ, Ed.; Wiley-Blackwell: Ames, IA, 2013; pp 25–33. [Google Scholar]
- (56).Griffith JF; Adams JE; Genant HK Diagnosis and Classificatin of Vertebral Fracture In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism; Rosen CJ, Ed.; Wiley-Blackwell: Ames, IA, 2013; pp 317–335. [Google Scholar]
- (57).Gordon ON; Luis PB; Sintim HO; Schneider C Unraveling Curcumin Degradation: Autoxidation Proceeds through Spiroepoxide and Vinylether Intermediates En Route to the Main Bicyclopentadione. J. Biol. Chem 2015, 290 (8), 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Wright LE; Frye JB; Gorti B; Timmermann BN; Funk JL Bioactivity of Turmeric-Derived Curcuminoids and Related Metabolites in Breast Cancer. Curr. Pharm. Des 2013, 19 (34), 6218–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Bramwell KKC; Ma Y; Weis JH; Chen X; Zachary JF; Teuscher C; Weis JJ Lysosomal β-Glucuronidase Regulates Lyme and Rheumatoid Arthritis Severity. J. Clin. Invest 2014, 124 (1), 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Luis PB; Gordon ON; Nakashima F; Joseph AI; Shibata T; Uchida K; Schneider C Oxidative Metabolism of Curcumin-Glucuronide by Peroxidases and Isolated Human Leukocytes. Biochem. Pharmacol 2017, 132, 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Collin-Osdoby P; Osdoby P RANKL-Mediated Osteoclast Formation from Murine RAW 264.7 Cells In Bone Research Protocols; Helfrich MH, Ralston SH, Eds.; Springer: New York, 2012; Vol. 816, pp 187–202. [DOI] [PubMed] [Google Scholar]
- (62).Nair AB; Jacob S A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm 2016, 7 (2), 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Ramalingam P; Ko YT Enhanced Oral Delivery of Curcumin from N-Trimethyl Chitosan Surface-Modified Solid Lipid Nanoparticles: Pharmacokinetic and Brain Distribution Evaluations. Pharm. Res 2015, 32 (2), 389–402. [DOI] [PubMed] [Google Scholar]
- (64).Amend SR; Valkenburg KC; Pienta KJ Murine Hind Limb Long Bone Dissection and Bone Marrow Isolation. J. Vis. Exp 2016, 110 (e53936). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Glaser J; Sly WS β-Glucuronidase Deficiency Mucopolysaccharidosis: Methods for Enzymatic Diagnosis. J. Lab. Clin. Med 1973, 82 (6), 969–977. [PubMed] [Google Scholar]
- (66).Talalay P; Fishman W; Huggins C Chromogenic Substrates: Phenolphthalein Glucuronic Acid as Substrate for the Assay of Glucuronidase Activity. J. Biol. Chem 1946, 166 (2), 757–772. [PubMed] [Google Scholar]
- (67).McDonald K Overcoming the coomassie blues http://www.biorad.com/webroot/web/pdf/lsr/literature/Bulletin_5939.pdf (accessed Jul 10, 2018).
- (68).Aldridge GM; Podrebarac DM; Greenough WT; Weiler IJ The Use of Total Protein Stains as Loading Controls: An Alternative to High-Abundance Single Protein Controls in Semi-Quantitative Immunoblotting. J. Neurosci. Methods 2009, 172 (2), 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Zhang Y; Huo M; Zhou J; Xie S PKSolver: An Add-in Program for Pharmacokinetic and Pharmacodynamic Data Analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99 (3), 306–314. [DOI] [PubMed] [Google Scholar]