Abstract
Objective
Given the high post-stroke mortality and disability and paucity of data on the quality of stroke care in Sub-Saharan Africa, we sought to characterize the implementation of stroke-focused treatments and 90-day outcomes of neuroimaging-confirmed stroke patients at the largest referral hospital in Tanzania.
Design
Prospective cohort study.
Setting
Muhimbili National Hospital (MNH) in Dar es Salaam, July 2016–March 2017.
Participants
Adults with new-onset stroke (<14 days), confirmed by head CT, admitted to MNH.
Main outcomes measures
Modified Rankin scale (mRS) and vital status.
Results
Of 149 subjects (mean age 57; 48% female; median NIH stroke scale (NIHSS) 19; 46% ischemic stroke; 54% hemorrhagic), implementation of treatments included: dysphagia screening (80%), deep venous thrombosis prophylaxis (0%), aspirin (83%), antihypertensives (89%) and statins (95%). There was limited ability to detect atrial fibrillation and carotid artery disease and no acute thrombolysis or thrombectomy. Of ischemic subjects, 19% died and 56% had severe disability (mRS 4–5) at discharge; 49% died by 90 days. Of hemorrhagic subjects, 33% died and 49% had severe disability at discharge; 50% died by 90 days. In a multivariable model, higher NIHSS score but not dysphagia, unconsciousness, or patient age was predictive of death by 90 days.
Conclusions
The 90-day mortality of stroke presenting at MNH is 50%, much higher than in higher income settings. Although severe stroke presentations are a major factor, efforts to improve the quality of care and prevent complications of stroke are urgently needed. Acute stroke interventions with low number needed to treat represent challenging long-term goals.
Keywords: ischemic stroke, intracranial hemorrhage, Africa, quality measurement, patient outcomes, needs assessment, equity in healthcare, developing countries, mortality
Introduction
It is widely recognized that stroke mortality in Sub-Saharan Africa (SSA) is high [1, 2]. However, detailed data from patients presenting with stroke in these settings are lacking, especially in regards to the quality and implementation of stroke-focused hospital care. Furthermore, due to resource limitations and the lack of uniform reporting, stroke severity assessments using the NIH Stroke Scale (NIHSS) score and head computed tomography (CT) imaging reviewed by trained neuroradiologists are uncommon. Dedicated stroke units and stroke registries are nearly absent, and uniform follow-up throughout a hospital stay and beyond discharge is infrequent in SSA [3].
In Tanzania, a low-income country in Eastern SSA, stroke outcomes are reported from rural (Hai) and urban (Dar es Salaam) districts. One study showed the age adjusted yearly stroke rates were 108.6 per 100 000 person-years in Hai but 315.9 per 100 000 in Dar es Salaam. The incidence in Hai was similar to that found in high-income countries, but the incidence in Dar es Salaam was much higher [4]. Given the high stroke incidence, high post-stroke mortality and disability [5], and the lack of detailed stroke care data in SSA, our objective was to characterize the implementation of clinical characteristics, treatments, and 90-day outcomes of patients with neuroimaging-confirmed stroke. Patients were assessed at Tanzania’s largest public referral hospital, Muhimbili National Hospital (MNH) in Dar es Salaam, with the goal of future interventional studies at MNH to improve stroke care and outcomes.
Methods
Ethics approval
The Ethics Committee of MNH, the National Institute of Medical Research of Tanzania and the Partners Healthcare institutional review board (Boston, USA) approved this study. Written informed consent was obtained, signed by the subject or next of kin.
Location
MNH serves as an academic teaching and public assistance hospital. MNH receives patients from throughout the country as well as transfers from smaller healthcare facilities. One male and one female neurology ward each hold approximately 25 beds. Although there is no dedicated stroke unit, most of the neurology ward is dedicated to stroke. The number of stroke patients admitted varies from 2 to 7 per day [6]. There are four adult neurologists working at MNH. MNH has 1 MRI machine, 2 CT machines, radiologists and radiology technicians.
Enrollment
At MNH, adults years of age with acute stroke (within 14 days) were enrolled prospectively by study physicians from July 2016 to March 2017. Subjects and next of kin were informed that they were participating in an observational study of stroke treatments and outcomes. The World Health Organization definition of stroke [7], ‘rapidly developing clinical signs of focal disturbance of cerebral function, lasting more than 24 h or leading to death with no apparent cause other than that of vascular origin,’ was used. Subjects were excluded if they had: transient ischemic attacks (symptoms <24 h and nonfatal), traumatic hemorrhage, primary subarachnoid hemorrhage, or were unable to provide consent and did not have a family member present. There were no payments to participants.
Clinical evaluation and care
Subjects’ demographic information, medical history, prior medications and history of presentation were recorded. Blood pressure, heart rate and temperature were recorded on arrival. An NIHSS score [8] was assessed within 48 h of arrival; all assessors were certified in the NIHSS. Subjects were also screened for dysphagia by nursing staff. If subjects were unconscious, they were considered to have ‘failed’ the screen. In addition, the implementation of the following stroke-focused care was recorded: deep venous thrombosis prophylaxis, antithrombotics, antihypertensives, statins, cardiac monitoring, vessel imaging, carotid endarterectomy, thrombolysis and thrombectomy.
Laboratory tests
Serum was collected at the time of enrollment. It was tested for HIV via rapid antibody test, and confirmed by ELISA and Uni-GoldTM tests. Serum sodium, creatinine, and glucose were also tested in the Emergency Department.
Neuroimaging
Stroke subtype was determined by head CT during hospitalization (Siemens, Somatom, Definition Flash, 128 axial slices, 0.5–10 mm thickness), which was interpreted by both a local radiologist and a Boston-based neuroradiologist. Subjects were excluded if the CT led to a diagnosis other than stroke (Fig. 1). For ischemic strokes, size was rated as ‘small’ for lacunes or small emboli, ‘medium’ for partial arterial territory and ‘large’ for complete territory involvement. Hemorrhagic stroke was classified by the presence or absence of intraventricular or subarachnoid extension of hemorrhage, and the presence or absence of hydrocephalus. For hemorrhages, size was rated as ‘small’ for petechial to ~10 cc, ‘medium’ for ~10–30 cc, and ‘large’ for >30 cc.
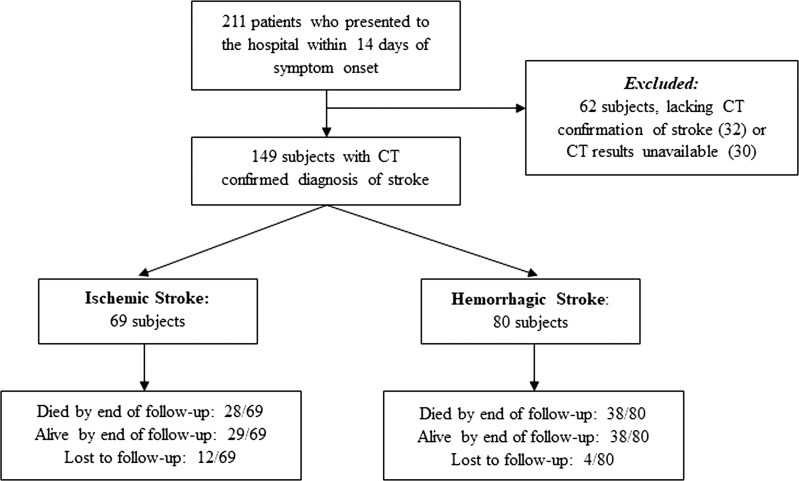
Figure 1.
Flow chart of subjects by the study’s inclusion and exclusion criteria. Computed tomography (CT).
Follow-up
A modified Rankin scale (mRS) score was ascribed, ranging from 0 (no disability) to 6 (death) [9], at the time of discharge and 90 days post-stroke onset. The subject or next of kin was contacted by telephone a minimum of three times before he or she was deemed ‘lost to follow-up.’ If a subject died, the date of death was inquired of the next of kin.
Statistical analyses
We summarized the data as counts and percentages, means and standard deviations, and medians and interquartile ranges. Percentages were calculated based on the number of non-missing responses. Outcomes of interest were mRS at hospital discharge and 90-day follow-up, calculated based on the total number enrolled. Survival curves were displayed using the Kaplan–Meier method. Cox proportional hazard regression models were constructed to assess predictors of the time to death. The model covariates were decided a priori based on clinical practice and knowledge. Associations were described with hazard ratios and their associated 95% confidence intervals. Two-tailed P-values of <0.05 were considered statistically significant. Analyses were performed using SAS software, version 9.4 (SAS Institute).
Results
Subjects
Of 211 subjects enrolled, 149 had head CT-confirmed stroke (69 ischemic and 80 hemorrhagic) (Fig. 1). No patient or proxy refused enrollment. Thirty-two subjects met the clinical definition of stroke but were later excluded due to a lack of evidence of stroke on CT. A further 30 were excluded due to unavailable CT for secondary review by the US neuroradiologist, mostly due to resource limitation issues. Sixteen subjects were lost to follow-up by 90 days.
Stroke presentation
Baseline features of the cohort are shown in Table 1. Eighty-five percentage of subjects had no insurance, and 15% had government insurance. Seventy-four percentage of subjects were transferred from another hospital to MNH. While 83% of subjects reported they had hypertension prior to stroke onset, only 49% were on antihypertensive agents. The prevalence of HIV infection was 6.0% for those with ischemic stroke and 10.4% for those with hemorrhagic. Median NIHSS was 19. The median hospitalization time was 5.5 days.
Table 1.
Demographic and clinical characteristics of subjects presenting with stroke. Data are counts (n) and percentages (%), means and standard deviations (SD), or medians and interquartile ranges (IQR).
| Ischemic stroke, n (%) | Hemorrhagic stroke, n (%) | Total, n (%) | |
|---|---|---|---|
| Sample size | 69 (46.3) | 80 (53.7) | 149 |
| Age in years, mean ± SD | 60.2 ± 13.6 | 54.7 ± 15.0 | 57.2 ± 14.6 |
| Female | 35 (50.7) | 37 (46.3) | 72 (48.3) |
| Employment status | |||
| Employed | 28 (40.6) | 34 (42.5) | 62 (41.6) |
| Peasant | 11 (15.9) | 5 (6.3) | 18 (12.1) |
| Homemaker | 9 (13.0) | 9 (11.3) | 43 (28.9) |
| Unemployed | 14 (20.3) | 29 (36.3) | 16 (10.7) |
| Retired | 7 (10.1) | 3 (3.8) | 10 (6.7) |
| Self-Reported Risk Factors | |||
| Hypertension | 56 (81.2) | 68 (85.0) | 124 (83.2) |
| Diabetes mellitus | 22 (31.9) | 6 (7.5) | 28 (18.8) |
| Obesity/Overweight | 8 (11.6) | 10 (12.5) | 18 (12.1) |
| Family history of stroke | 11 (15.9) | 20 (25.0) | 31 (20.8) |
| Prior stroke | 16 (23.2) | 8 (10.0) | 24 (16.1) |
| Smoking (current or prior) | 7 (10.1) | 6 (7.5) | 13 (8.7) |
| Illicit drug or alcohol use | 13 (18.8) | 9 (11.3) | 22 (14.8) |
| Prior Medicationsa | |||
| Aspirin | 13 (18.8) | 4 (5.0) | 17 (11.4) |
| Clopidogrel | 4 (5.8) | 4 (2.7) | |
| Antihypertensive | 36 (52.2) | 37 (46.3) | 73 (49.0) |
| Statin | 5 (7.2) | 1 (1.3) | 6 (4.0) |
| Oral contraceptive | 1 (1.4) | 2 (2.5) | 3 (2.0) |
| Hypoglycemic | 12 (17.4) | 1 (1.3) | 13 (8.7) |
| Initial examination | |||
| Limb weakness | 66 (95.7) | 73 (91.3) | 139 (93.3) |
| Altered level of consciousness | 29 (42.0) | 44 (55.0) | 73 (49.0) |
| Aphasia | 25 (36.2) | 29 (36.3) | 54 (36.2) |
| NIH stroke scale score, median (IQR) | 19 (13,25) | 19 (13,27) | 19 (13,26) |
| Intake Blood Pressure in mmHg, mean ± SD | |||
| Systolic | 150.8 ± 23.3 | 164.6 ± 25.6 | 158.2 ± 25.4 |
| Diastolic | 90.2 ± 18.4 | 99.1 ± 18.5 | 95.0 ± 18.9 |
| Intake Heart Rate, beats per minute, mean ± SD | 92.6 ± 20.3 | 86.6 ± 20.2 | 89.4 ± 20.4 |
| HIV infection | 4 (6.0) | 8 (10.4) | 12 (8.3) |
| Blood Glucose, mmol/l | 8.6 ± 4.0 | 7.8 ± 3.8 | 8.2 ± 3.9 |
| Serum Sodium, μmol/l | 135.8 ± 10.0 | 136.2 ± 11.6 | 136.0 ± 10.9 |
| Serum Creatinine, μmol/l | 117.6 ± 103.9 | 137.0 ± 99.2 | 127.7 ± 101.6 |
| Length of stay in days, median (IQR) | 5 (3,12), n = 55 | 6 (4,13), n = 53 | 5.5 (3,13), n = 108 |
| Received antibiotics | 21 (30.4) | 25 (31.3) | 47 (30.9) |
| Pneumonia | 16 (23.2) | 26 (31.3) | 42 (28.2) |
| Urinary tract infection | 5 (7.2) | 4 (5.0) | 9 (6.0) |
| Dysphagia screen | |||
| Completed | 52 (75.4) | 67 (83.8) | 119 (79.9) |
| Pass | 31 (59.6) | 30 (44.8) | 61 (51.3) |
*No subjects were taking anticoagulation
Implementation of interventions
Eighty percentage of subjects underwent dysphagia screening and none were treated with pharmacologic deep vein thrombosis (DVT) prophylaxis or sequential compression devices (SCDs) (Table 2). For secondary stroke prevention, 83% of ischemic stroke subjects were discharged on aspirin, 95% on a statin and 89% on an antihypertensive. No subject was monitored on continuous telemetry, prescribed 30-day heart monitors or underwent vessel imaging. About 50% of subjects with atrial fibrillation (AF) detected were started on warfarin and no subjects received carotid endarterectomy. None underwent endovascular thrombectomy or received thrombolysis.
Table 2.
Determining the implementation and largest impact of stroke interventions at Muhimbili National Hospital (MNH). GWTG (Get with the Guidelines, US-based registry), DVT (deep vein thrombosis), ppx (prophylaxis), SCD (sequential compression device), ASA (aspirin), ACEi (ACE inhibitor), AF (atrial fibrillation), CEA (carotid endarterectomy), tPA (tissue plasminogen activator), mRS (modified Rankin score), ET (endovascular thrombectomy).
| Ischemic stroke intervention* | % Implemented at MNH** | % Implemented in GWTG [Reference] | Trial [Reference] | Absolute risk reduction (%) | Number needed to treat |
|---|---|---|---|---|---|
| Early dysphagia screen (24/7), Pneumonia during admission | 80 | 68 [35] | EDS [11] | 12−3.8 = 8.2 | 12.8 |
| DVT ppx in 7 days, DVT during admission | 0 | 89 [35] | LMWH [39] | 28−4 = 24 | 4.2 |
| SCD in 12 h, DVT during admission | 0 | N/A | SCD [13] | 9.2−0.2 = 9 | 11.1 |
| ASA in 48 h, 14 days recurrent stroke | 83 | 92 [40] | IST [14] | 3.9−2.8 = 1.1 | 100 |
| ASA for stroke history, 3 years vascular event | 83 | 98 [40] | ATC [15] | 22−18 = 4 | 26.3 |
| Atorvastatin in 6 months, 5 years recurrent stroke | 95 | 83 [40] | SPARCL [16] | 13−11 = 2 | 52.6 |
| ACEi+/-diuretic after 2 weeks, 4 years recurrent stroke | 89 | 80 [40] | PROGRESS [17] | 14−10 = 4 | 26.7 |
| Warfarin-adjusted vs. ASA/Warfarin-fixed for AF history, 1 year recurrent stroke | 50 | 94 [40] | SPAF III [18] | 7.9−1.9 = 6 | 16.7 |
| CEA for carotid stenosis in 120 days, 2 years recurrent stroke | 0 | N/A | NASCET [20] | 26−9 = 17 | 5.9 |
| tPA in 3 h, 90 days mRS 2–6 | 0 | 60 [35] | NINDS [21] | 73−61 = 12 | 8.3 |
| tPA in 4.5 h, 90 days mRS 2–6 | 0 | 60 [35] | ECASS III [22] | 55−48 = 7 | 13.5 |
| ET in 6 h, 90 days mRS 2–6 | 0 | 27 [41] | HERMES [24] | 87−73 = 14 | 7.1 |
*Intervention, outcome.
**For implementation at MNH at the time of discharge, 54/65 were on an antiplatelet agent, 3/57 anticoagulation, 52/55 statin, 50/56 antihypertensive, 52/64 underwent dysphagia screen. References in brackets
Head CT findings
Forty-six percentage of subjects had ischemic stroke, and 54% had hemorrhagic stroke (Table 3). Sixty-eight percentage of ischemic strokes were medium to large, while 70% of hemorrhagic strokes were medium to large. Chronic microangiopathic white matter change was present in 57% of subjects with ischemic stroke and 63% of subjects with hemorrhagic. Among the hemorrhagic strokes, 50% had intraventricular extension and 34% had hydrocephalus. A high number of subjects (42%) with ischemic strokes had imaging evidence of prior ischemic stroke.
Table 3.
Computed tomography data of subjects presenting with stroke. Count (n), percentage (%)
| Ischemic stroke, n (%) | Hemorrhagic stroke, n (%) | Total, n (%) | |
|---|---|---|---|
| Stroke type | 69 (46.3) | 80 (53.7) | 149 (100) |
| Prior infarction | 29 (42.0) | 15 (18.8) | 44 (29.5) |
| Diffuse microangiopathic white matter change | 39 (56.5) | 50 (62.5) | 89 (59.7) |
| Hemorrhagic conversion | 4 (5.8) | 4 (2.7) | |
| Intraventricular extension | 40 (50.0) | 40 (26.8) | |
| Subarachnoid extension | 7 (8.8) | 7 (4.7) | |
| Hydrocephalus | 27 (33.8) | 27 (18.1) | |
| Size | |||
| Small | 22 (31.9) | 24 (30.0) | 46 (30.9) |
| Medium | 24 (34.8) | 53 (66.3) | 77 (51.7) |
| Large | 23 (33.3) | 3 (3.8) | 26 (17.4) |
| Structures | |||
| Frontal lobe | 42 (60.9) | 3 (3.8) | 45 (30.2) |
| Parietal lobe | 27 (39.1) | 6 (7.5) | 33 (22.1) |
| Temporal lobe | 15 (21.7) | 4 (5.0) | 19 (12.8) |
| Occipital lobe | 4 (5.8) | 2 (2.5) | 6 (4.0) |
| Putamen | 5 (7.2) | 34 (42.5) | 39 (26.2) |
| Caudate | 3 (4.3) | 1 (1.3) | 4 (2.7) |
| Internal capsule | 2 (2.9) | 2 (1.3) | |
| Thalamus | 5 (7.2) | 25 (31.3) | 30 (20.1) |
| Cerebellum | 4 (5.8) | 4 (5.0) | 8 (5.4) |
| Pons | 1 (1.4) | 4 (5.0) | 5 (3.4) |
Stroke outcomes
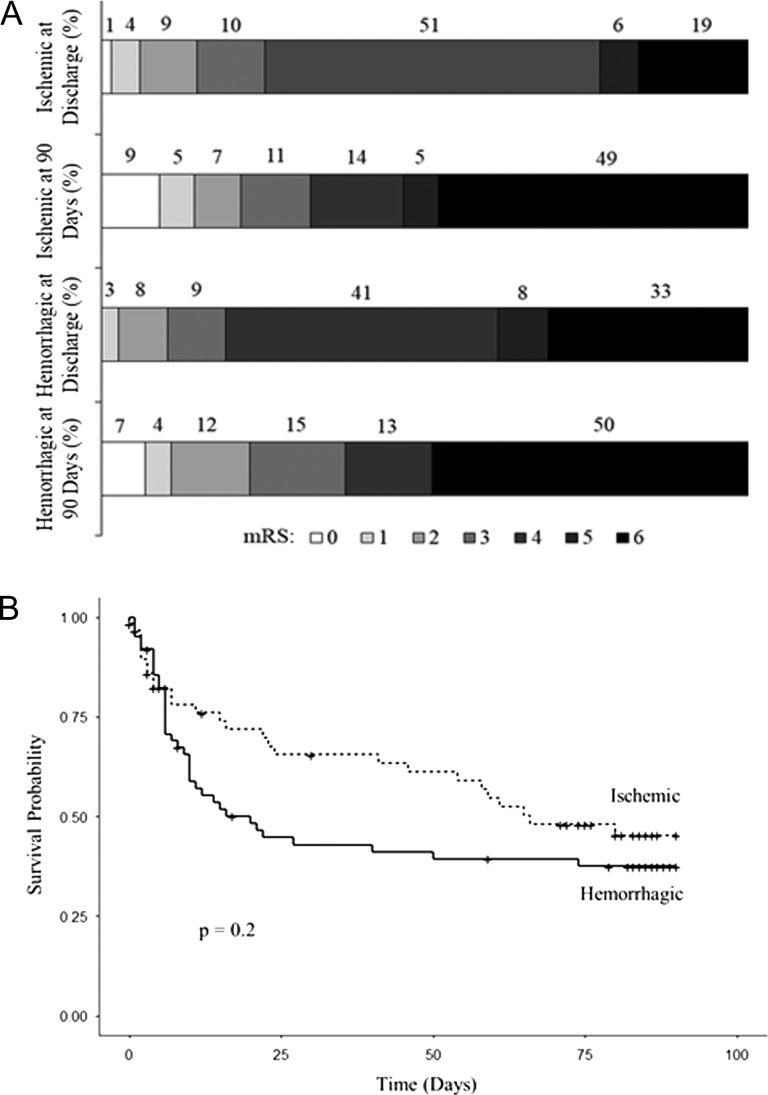
Among ischemic stroke subjects, 19% died and 56% had severe disability (mRS 4–5) during the hospitalization (Fig. 2A). At 90-day follow-up, 49% died. Among hemorrhagic, 33% died and 49% had severe disability during the hospitalization. At 90-day follow-up, 50% died. There was no difference comparing the survival curves of ischemic and hemorrhagic stroke subjects (Fig. 2B) or male and female subjects (not shown). For all strokes combined, older age, dysphagia, higher NIHSS and unconsciousness each predicted mortality in univariable analyses (Table 4). Of these variables, NIHSS at presentation was the only factor associated with time to death in a multivariable analysis.
Figure 2.
Modified Rankin Scale (mRS) scores (A) and Kaplan–Meier survival curves (B) of subjects by stroke type.
Table 4.
Cox proportional hazard models of survival over 90-day study for all stroke subjects. N = 148, Events = 66. HR (hazard ratio), CI (confidence interval), NIHSS (NIH Stroke Scale)
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Measure | HR (95%CI) | P-value | HR (95%CI) | P-value |
| Age | 1.019 (1.003–1.035) | 0.0232 | 0.997 (0.980–1.014) | 0.7115 |
| Dysphagia | 3.203 (1.797–5.708) | <0.0001 | 1.647 (0.871–3.112) | 0.1245 |
| NIHSS | 1.119 (1.085–1.153) | <0.0001 | 1.097 (1.056–1.139) | <0.0001 |
| Unconsciousness | 3.460 (2.023–5.915) | <0.0001 | 1.479 (0.810–2.701) | 0.2024 |
Discussion
Our data describe the quality of care, characterize the implementation of stroke-focused treatments, and confirm existing reports of poor outcomes following hospital admission for stroke in SSA. We elucidate several important characteristics of stroke patients at MNH that can lead to interventional research and programmatic planning. Our study is strengthened by the inclusion of well-characterized stroke subjects both clinically through NIHSS-certified physician assessors as well as duplicate review of head CTs acquired on hospital admission. It is also strengthened by prospectively enrolled participants with extended follow-up with a median of 90 days, allowing for a pragmatic understanding of the sample sizes needed for future clinical trials.
For the stroke-focused treatments for which we have recorded the prevalence of implementation, the number needed to treat (NNT) can help prioritize resources to improve the quality of care at MNH (Table 2). While the NNT may be higher for these treatments, an initial focus could include preventing post-stroke complications and improving secondary stroke prevention strategies. Others have shown incentivizing adherence to quality metrics makes substantial differences [10].
Prevention of post-stroke complications requires optimization at MNH. Eighty percentage of subjects underwent dysphagia screening. Early dysphagia screening has a NNT of 12.8 patients to prevent aspiration pneumonia [11]. Of subjects screened at MNH, 49% were reported to have dysphagia. Furthermore, 28% of all subjects were treated for pneumonia with antibiotics during admission. A recent study of stroke at MNH hypothesized that 54% of 30-day mortality was secondary to aspiration pneumonia [6]. Percutaneous endoscopic gastrostomy (PEG) tubes were also unavailable, and many subjects went home with nasogastric tubes. PEG tubes have been shown to increase survival and decrease aspiration [12]. In addition, no subjects were treated with pharmacologic DVT prophylaxis or SCDs. Pharmacologic DVT prophylaxis has a NNT of 4.2 to prevent DVT, and the addition of SCDs has a NNT of 11.1 [13]. There were no DVTs or pulmonary emboli documented in our study; this likely represents under diagnosis. Others have shown that up to 42% of stroke patients not treated with prophylaxis develop DVT and 10–20% experience pulmonary emboli [13].
Steps can also be made to improve secondary stroke prevention. Eighty-three percentage of ischemic stroke subjects were discharged on aspirin, 95% on a statin and 89% on an antihypertensive. Aspirin started within 48 h has a NNT of 100 to prevent recurrent stroke in 2 weeks [14]. A meta-analysis of antiplatelet therapy showed that for patients with history of stroke, the NNT was 50 to prevent a recurrent stroke and 91 to prevent vascular death after 3 years [15]. Statins, when started within 6 months, have a NNT of 52.6 to prevent recurrent stroke after 5 years [16]. An ACE inhibitor with or without a diuretic started 2 weeks after stroke has a NNT of 26.7 to prevent recurrent stroke after 4 years [17].
Several additional interventions represent moderately challenging goals. At the time of admission, no subjects had known history of AF. While an EKG was completed on most subjects, none were monitored on continuous telemetry or prescribed 30-day heart monitors at discharge. During the admission, 10% of ischemic stroke subjects were found to have AF. About 50% of these were started on anticoagulation at discharge, acknowledging that in some it may not have been safe due to hemorrhage risk. In patients with AF and at least one risk factor for stroke, warfarin has a NNT of 16.7 to prevent a stroke after 1 year of follow-up [18]. No subjects underwent imaging for carotid artery stenosis, and none were referred for carotid endarterectomy during the study period. While one study questions the utility of carotid imaging in SSA [19], endarterectomy in North America, when performed within 120 days of stroke, has a NNT of 5.9 to prevent any stroke and 9.4 to prevent major or fatal stroke over 2 years [20].
Acute stroke therapies represent challenging long-term goals, with large upfront costs and major system changes. No subjects received intravenous thrombolysis or endovascular thrombectomy in this study. While intravenous thrombolysis is technically available at MNH, patients rarely arrive at the hospital within the time window. To prevent disability at 90 days (mRS 2–6) from all ischemic strokes, thrombolysis when given in 3 h has a NNT of 8.3 [21] and when given in 4.5 h has a NNT of 13.5 [22]. With the exception of South Africa [23], thrombectomy is unavailable in SSA. A meta-analysis showed that thrombectomy for strokes secondary to large vessel occlusion has a NNT of 7.1 to prevent disability at 90 days (mRS 2–6) [24]. While this intervention is exceedingly costly upfront, one prospective study in the US demonstrated that the long-term cost is actually lower as significant disability is prevented [25].
There are also several interventions for which the NNT is not calculable or a study could not be completed for ethical reasons. Patients at MNH often have difficulty obtaining extraventricular drains for the treatment of post-stroke hydrocephalus after hemorrhagic stroke. Patients’ families must leave the hospital and find medical supply stores. Many cannot afford the kits. In the present cohort, 50% of subjects had intraventricular extension of blood and 34% developed hydrocephalus. While no randomized trial exists regarding the placement of drains for hydrocephalus, making drains readily available at MNH would make an impact. There is also limited physical therapy available and no inpatient rehabilitation available after discharge. This likely prevents patients from reaching their maximal recovery [26, 27].
Overall, we noted more hemorrhagic stokes and higher severity of strokes at MNH compared to other regions. Even though our study excluded traumatic and subarachnoid hemorrhages, intraparenchymal with or without intraventricular hemorrhagic strokes represented more than half of all strokes enrolled. An analysis of the Get with the Guidelines stroke registry showed that of stroke presentations to US hospitals, 60% are ischemic strokes and 11% are hemorrhagic [28]. Stroke subtype has been investigated in another study in Tanzania. In both Hai and Dar es Salaam, 82% of strokes were ischemic and 18% were hemorrhagic [29]. The strokes presenting to MNH were also severe in nature: the median NIHSS was 19 at MNH compared to 11 in a recent US-based hospital study (28). Furthermore, 68% of ischemic strokes were medium to large, while 70% of hemorrhagic were medium to large. The higher percentage of hemorrhagic strokes and large strokes in our study likely reflect referral bias; people with smaller infarcts likely do not present to the hospital. Given the severity of disease, this population stands to benefit substantially from careful analyses of the quality of care provided. The type and severity of stroke also have implications for resource utilization [30].
HIV infection in the present study approximated the general population prevalence [31], affecting 6.0% of subjects with ischemic stroke and 10.4% with hemorrhagic. In 2010, a study at MNH showed that the prevalence of HIV in stroke subjects was approximately 20% [32]. Previous studies have shown HIV infection is associated with a 5-fold increase in stroke risk and may worsen disability [33, 34].
Mortality at discharge at MNH was 19% for ischemic and 33% for hemorrhagic stroke subjects as compared to 6% and 25%, respectively, in the USA [35]. The 90-day mortality was approximately 50% for both stroke types at MNH. Post-stroke case fatality has been reported in Tanzania. One study found a 24% mortality within 28 days in a community-based sample. This increased to 60% at 3 years [36] and 82% at 7 years [37].
Limitations of this study include its single center recruitment, referral bias, and short duration of recruitment and follow-up. There was a lack of subject awareness of some stroke risk factors, and we depended on the next of kin’s report for patients with severe strokes. While some of the subjects lost to follow-up may have been unable to access telephones, it is likely that most died, and our mortality numbers are under-counted. We are uncertain of the degree to which our act of recording improved the measurement, documentation and potentially the outcomes in this study. Finally, we were unable to reliably categorize the cause of death in our subjects.
Our study joins a growing body of work in SSA that underscores the urgency of addressing the stroke epidemic. We provide additional insight into stroke subtypes and mortality and disability of patients admitted to MNH, with stroke confirmation by head CT. CT confirmation of stroke is lacking in many parts of SSA [38], and its inclusion in this study allows a clearer depiction of the critical nature of our stroke patients. Given the high proportion of hemorrhagic strokes and large-sized strokes, MNH provides a location to study and improve the care of some of the most critically ill stroke patients in SSA. Building upon other studies, we quantitatively show that patients admitted to MNH would benefit the most from interventions that are established as the standard of care in other parts of the world.
Acknowledgements
Ms. Geetha Iyer, SCM, for assistance with data organization and analysis.
Funding
This work is supported by National Institutes of Health [P30 AI060354 Subgrant of the Harvard Center for AIDS Research to FJM, R25 NS065743 Neuroscience Resident Research Program Grant to RWR]; Massachusetts General Hospital Global Health Travel Grant to RWR; and Partners Healthcare Centers of Expertise Global Health Grant to RWR.
Disclosures
None.
References
- 1. Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007;6:182–7. [DOI] [PubMed] [Google Scholar]
- 2. Feigin VL, Lawes CM, Bennett DA et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–69. [DOI] [PubMed] [Google Scholar]
- 3. Langhorne P, de Villiers L, Pandian JD. Applicability of stroke-unit care to low-income and middle-income countries. Lancet Neurol 2012;11:341–8. [DOI] [PubMed] [Google Scholar]
- 4. Walker R, Whiting D, Unwin N et al. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol 2010;9:786–92. [DOI] [PubMed] [Google Scholar]
- 5. Guida P, Iacoviello M, Passantino A et al. Intra-hospital correlations among 30-day mortality rates in 18 different clinical and surgical settings. Int J Qual Health Care 2016;28:793–801. [DOI] [PubMed] [Google Scholar]
- 6. Okeng’o K, Chillo P, Gray WK et al. Early mortality and associated factors among patients with stroke admitted to a large teaching hospital in Tanzania. J Stroke Cerebrovasc Dis 2016;26:871–8. [DOI] [PubMed] [Google Scholar]
- 7. Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 8. Brott T, Adams HP Jr, Olinger CP et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. [DOI] [PubMed] [Google Scholar]
- 9. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 1957;2:200–15. [DOI] [PubMed] [Google Scholar]
- 10. Yang JH, Kim SM, Han SJ et al. The impact of Value Incentive Program (VIP) on the quality of hospital care for acute stroke in Korea. Int J Qual Health Care 2016;28:580–5. [DOI] [PubMed] [Google Scholar]
- 11. Palli C, Fandler S, Doppelhofer K et al. Early dysphagia screening by trained nurses reduces pneumonia rate in stroke patients: a clinical intervention study. Stroke 2017;48:2583–5. [DOI] [PubMed] [Google Scholar]
- 12. Dwolatzky T, Berezovski S, Friedmann R et al. A prospective comparison of the use of nasogastric and percutaneous endoscopic gastrostomy tubes for long-term enteral feeding in older people. Clin Nutr 2001;20:535–40. [DOI] [PubMed] [Google Scholar]
- 13. Kamran SI, Downey D, Ruff RL. Pneumatic sequential compression reduces the risk of deep vein thrombosis in stroke patients. Neurology 1998;50:1683–8. [DOI] [PubMed] [Google Scholar]
- 14.Sandercock P, Collins R, Counsell C et al.; International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569–81. [PubMed] [Google Scholar]
- 15.Collins R, Peto R, Baigent C et al.; Antiplatelet Trialists' Collaboration. Collaborative overview of randomised trials of antiplatelet therapy-I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994;308:81–106. [PMC free article] [PubMed] [Google Scholar]
- 16. Amarenco P, Bogousslavsky J, Callahan A 3rd et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59. [DOI] [PubMed] [Google Scholar]
- 17. MacMahon S, Neal B, Tzourio C et al.; PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–41. [DOI] [PubMed] [Google Scholar]
- 18.Blackshear JL, Halperin JL, Hart RG et al.; Stroke Prevention in Atrial Fibrillation Investigators. Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996;348:633–8. [PubMed] [Google Scholar]
- 19. Jusabani A, Gray WK, Swai M et al. Post-stroke carotid ultrasound findings from an incident Tanzanian population. Neuroepidemiology 2011;37:245–8. [DOI] [PubMed] [Google Scholar]
- 20. Barnett HJM, Taylor DW, Taylor DW et al.; North American Symptomatic Carotid Endarterectomy Trial Collaborators.. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53. [DOI] [PubMed] [Google Scholar]
- 21. Marler JR et al.; National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–7. [DOI] [PubMed] [Google Scholar]
- 22. Hacke W, Kaste M, Bluhmki E et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008;359:1317–29. [DOI] [PubMed] [Google Scholar]
- 23. Taylor A, Le Feuvre D, Mngomezulu V et al. Advances in stroke treatment are within reach. S Afr Med J 2016;106:40–1. [DOI] [PubMed] [Google Scholar]
- 24. Goyal M, Menon BK, van Zwam WH et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. [DOI] [PubMed] [Google Scholar]
- 25. Shireman TI, Wang K, Saver JL et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke: results from the SWIFT-PRIME trial (Solitaire With the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke). Stroke 2017;48:379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke 2014;45:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang CY, Chen YR, Hong JP et al. Rehabilitative post-acute care for stroke patients delivered by per-diem payment system in different hospitalization paths: a Taiwan pilot study. Int J Qual Health Care 2017;29:779–84. [DOI] [PubMed] [Google Scholar]
- 28. Messe SR, Khatri P, Reeves MJ et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology 2016;87:1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker RW, Jusabani A, Aris E et al. A prospective study of stroke sub-type from within an incident population in Tanzania. S Afr Med J 2011;101:338–44. [DOI] [PubMed] [Google Scholar]
- 30. Murata K, Hinotsu S, Sadamasa N et al. Healthcare resource utilization and clinical outcomes associated with acute care and inpatient rehabilitation of stroke patients in Japan. Int J Qual Health Care 2017;29:26–31. [DOI] [PubMed] [Google Scholar]
- 31. UNAIDS United Republic of Tanzania: Country Factsheets. 2017; Available at: http://www.unaids.org/en/regionscountries/countries/unitedrepublicoftanzania Accessed 12/24, 2017.
- 32. Mlay M, Bakari M. The prevalence of HIV among patients admitted with stroke at the Muhimbili National Hospital, Dar es Salaam, Tanzania. Tanzan J Health Res 2010;12:105–13. [Google Scholar]
- 33. Benjamin LA, Bryer A, Emsley HC et al. HIV infection and stroke: current perspectives and future directions. Lancet Neurol 2012;11:878–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mateen FJ, Shinohara RT, Carone M et al. Neurologic disorders incidence in HIV+ vs HIV− men: Multicenter AIDS Cohort Study, 1996–2011. Neurology 2012;79:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fonarow GC, Reeves MJ, Smith EE et al. Characteristics, performance measures, and in-hospital outcomes of the first one million stroke and transient ischemic attack admissions in get with the guidelines-stroke. Circ Cardiovasc Qual Outcomes 2010;3:291–302. [DOI] [PubMed] [Google Scholar]
- 36. Walker RW, Jusabani A, Aris E et al. Post-stroke case fatality within an incident population in rural Tanzania. J Neurol Neurosurg Psychiatry 2011;82:1001–5. [DOI] [PubMed] [Google Scholar]
- 37. Walker RW, Wakefield K, Gray WK et al. Case-fatality and disability in the Tanzanian Stroke Incidence Project cohort. Acta Neurol Scand 2016;133:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kolapo KO, Ogun SA, Danesi MA et al. Validation study of the Siriraj Stroke score in African Nigerians and evaluation of the discriminant values of its parameters: a preliminary prospective CT scan study. Stroke 2006;37:1997–2000. [DOI] [PubMed] [Google Scholar]
- 39. Turpie AG, Levine MN, Hirsh J et al. Double-blind randomised trial of Org 10172 low-molecular-weight heparinoid in prevention of deep-vein thrombosis in thrombotic stroke. Lancet 1987;1:523–6. [DOI] [PubMed] [Google Scholar]
- 40. Bangalore S, Schwamm L, Smith EE et al. Secondary prevention after ischemic stroke or transient ischemic attack. Am J Med 2014;127:728–38. [DOI] [PubMed] [Google Scholar]
- 41. Smith EE, Saver JL, Cox M et al. Increase in endovascular therapy in get with the guidelines-stroke after the publication of pivotal trials. Circulation 2017;136:2303–10. [DOI] [PubMed] [Google Scholar]