Abstract
Objective(s):
The effects of alpha linolenic acid (ALA) on tracheal responsiveness (TR), total protein (TP), phospholipase A2 (PLA2), immunoglobulin E (IgE), interleukin 4 (IL-4), interferon gamma (INF-γ) level and INF-γ/IL4 ratio in bronchoalveolar lavage fluid (BALF) of sensitized rats were examined.
Materials and Methods:
TR to methacholine and ovalbumin (OA), BALF levels of TP, PLA2 and IgE as well as IL-4, INF-γ and INF-γ/IL4 ratio were measured in control group (non-sensitized, group C), sensitized rats to OA (group S), S groups treated with two concentrations of ALA and dexamethasone group.
Results:
TR to methacholine and OA, BALF levels of TP, PLA2, IgE and IL-4 were significantly increased but BALF level of INF-γ and INF-γ/IL4 ratio decreased in group S compared to group C (P<0.001 for all cases). Treated S groups with dexamethasone and both concentrations of ALA lead to significant decrease in TR to methacholine and OA, BALF levels of TP, PLA2, IgE and IL-4 compared to group S (P<0.001 for all case). The effects of all concentrations of ALA on INF-γ, IL-4 and INF-γ/IL4 ratio and also the effect of its highest concentration on TP and IgE level were significantly higher than dexamethasone treatment (P<0.001 for all cases).
Conclusion:
Results showed an immune modulatory effect of the ALA that increased INF-γ, INF-γ/IL4 ratio (as an index of Th1/Th2) and decreased IL-4 in sensitized rats. ALA also showed preventive effect on inflammatory markers and tracheal responsiveness in sensitized animals comparable to the effect of dexamethasone.
Key Words: Asthma, Alpha linolenic acid, Inflammatory markers, Sensitized rats, Th1/Th2 balance, Tracheal responsiveness
Introduction
Asthma is a chronic lung inflammatory (1) with airway remodeling that associate with airway hyper-responsiveness (AHR) to pharmacological agonists and other stimuli (1). AHR is defined as airways constriction to small stimuli which do not induce airway constriction in normal subjects (2) which closely related to airway inflammation as the main underlying mechanisms of the disease (3).
In asthma, activated inflammatory cells, release phospholipase A2 (PLA2), (4) which resulted to synthesis of eicosanoids that play an important role in inflammatory process (4). Increased serum and bronchoalveolar lavage fluid (BALF) level of PLA2 (5) and total protein was shown in subjects with occupational asthma (6).
Airway inflammation in asthma is due to several inflammatory cells activation (7). In asthma, T helper 2 (Th2) is over-activated which its cytokines cause airway inflammation and mucus hyper-secretion (8). Th1 can inhibit Th2 responses and increased Th1/Th2 balance could be a treatment option of asthma (9). In allergic disease such as asthma and allergic rhinitis, interferon gamma/interleukin 4 (IFN-γ/IL-4) cytokines or Th1/Th2 balance shifted toward IL-4 or Th2 lymphocyte (10). Th2 interleukins such as IL-4 and interleukin 5 (IL-5), leads to immunoglobulin E (IgE) production and inducing of the growth of mucosal-type mast cells which can resulted in allergic response (11).
Alpha linolenic acid (ALA), an 18-carbon, essential omega-3 polyunsaturated fatty acid (PUFA) showed various pharmacological effects, such as anti-inflammatory (12-15), analgesic (16, 17), antibacterial (18), antimicrobial (19), antioxidant (20, 21) and neuro-protective properties (22-24). The relaxant effect of ALA on skeletal muscle (25, 26) and its effect on Th1/Th2 balances were demonstrated, suggesting its possible treatment effect in asthma which associated with decreased Th1/Th2 balance (27, 28). Therefore, in the present study, the effects of oral ALA on total protein, PLA2 and IgE level of lung lavage as well as Th1/Th2 balance and tracheal responsiveness in sensitized rats were examined.
Materials and Methods
Animals and studied groups
Twenty six male Wistar rats (weighted 220±50 g) were purchased from Animal House, School of Medicine, Mashhad University of Medical Sciences and were kept in an animal cage in an animal room with clean filtered air (Maximiser, Thorens Caging System Inc, Hazleton, PA, U.S.A) at 22±2 ºC on a 12 hr light/dark cycle and water and food available ad libitum (29). Animals were randomly divided in five groups according to the previous study (30) and Table 1.
Table 1.
Experimental animal groups
| Groups | Definition | Dose | Abbreviated name | n |
|---|---|---|---|---|
| Control | Non-sensitized rats | C | 6 | |
| Sensitized | Non-treated, OA sensitized rats | S | 6 | |
| Treated with alpha linolenic acid (ALA) | 0.2 mg/ml 0.4 mg/ml |
S+ALA 0.2 mg/ml S+ALA 0.4 mg/ml |
4 4 |
|
| Treated with Dexamethasone | 1.25 μg/ml | D | 6 | |
Alpha linolenic acid (Sigma-Aldrich, Darmstadt, Germany), and dexamethasone (Sigma, purity; 97%) were added in drinking water of animals during sensitization period. Each rat in all groups averagely drank 40 ml/day drinking water which wasn’t significant difference among different groups. The animals of groups C and S were given drinking water alone.
Animal sensitization method
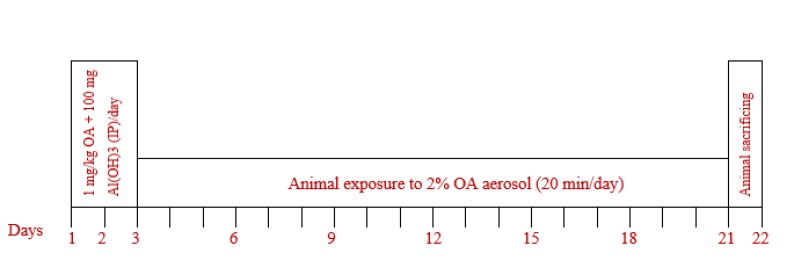
Sensitization of rats was done by intra-peritoneal injections of ovalbumin (OA) and Al(OH)3 and their exposure to OA aerosol according to previous studies (31, 32) as shown in Figure 1. Animal handeling were performed in compliance with the rulings of the Institute of Laboratory Animals Resources Commission on Life Sciences and the study was approved by the ethical Committee of the Mashhad University of Medical Sciences.
Figure 1.
Method of rat sensitization. Ovalbumin (OA), 1 mg/kg+100 mg Al(OH)3 as adjuvant in 0.9% sterile saline were intraperitonaly (IP) injected on days 1, 2 and 3. Then rats were exposed to 1% OA aerosol for 20 min on days 6, 9, 12, 15, 18 and 21 with animal normal-breathing. OA aerosol was produced by a DeVilbiss PulmoSonic nebulizer (DeVilbiss Health Care Ltd, Feltham, U.K) with an air flow of 8 lit/min in a 0.8 m3 chamber
BALF preparation
BALF was prepared exactly as described in previous study (33).
Measurement of BALF levels of total protein , PLA 2 , IgE, IL-4 and IFN-γ
Total protein, PLA2 and IgE levels were measured using the enzyme-linked immunosorbent assay (ELISA) sandwich method according to the manufacturer protocol with photometric method as previously described (34).
Tracheal tube preparations and measurement of racheal responsiveness (TR) to methacholine and ovalbumin
Tracheal tube containing 5–6 cartilaginous was prepared, mounted in organ bath containing Krebs-Henseliet solution (KHS) and equilibrated for one hour exactly as previously described (35).
TR to methacholine was measured by performing cumulative log concentration–response curves to methacholine hydrochloride (Sigma, purity; 98%) and calculation of the effective concentration of methacholine, causing 50% of maximum response (EC50) according the previously described method (35).
TR to OA was measured as its contractile response 10 min after producing 0.2% OA concentration in the organ bath according to the previously described method (35).
Statistical analysis
The results were quoted as means±SEM. Comparison of the data among different groups as well as those of two concentrations of ALA were performed using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test by InStat (GraphPad Software, Inc, La Jolla, USA). P values less than 0.05 was considered as statistical significance.
Results
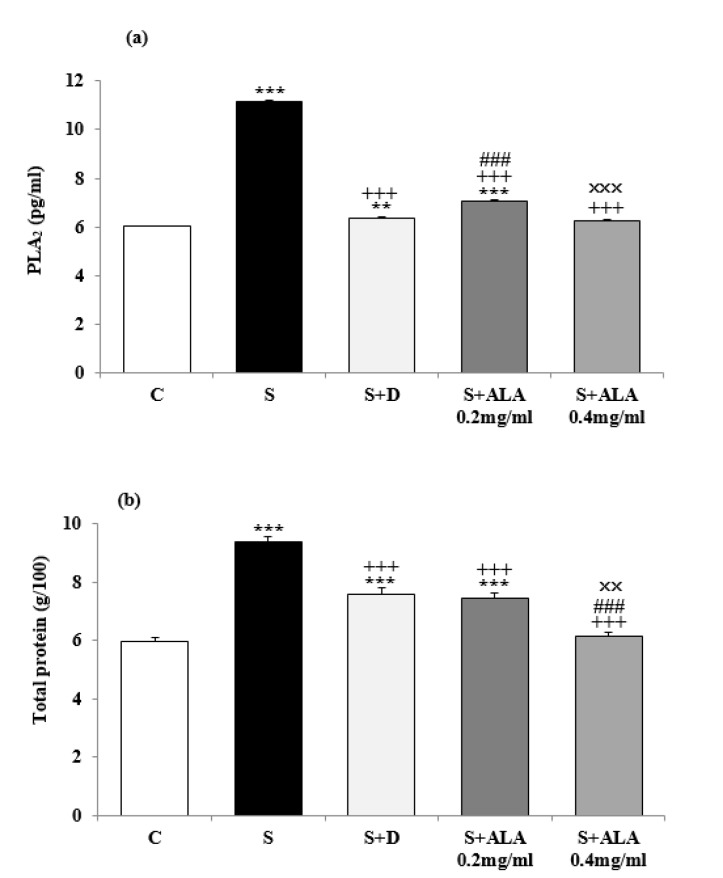
Significantly higher PLA2 and total protein levels in BALF were observed in group S compared to group C (P<0.001 for both cases, Figure 2) but their levels were significantly decreased in treated animals with both concentrations of ALA and dexamethasone compared to group S (P<0.001 for all cases, Figure 2). However, BALF levels of PLA2 and total protein in treated groups with dexamethasone and low concentration of ALA were significantly higher than group C (P<0.01 to P<0.001, Figure 2). The effect of low concentration of ALA on PLA2 level was significantly lower (P<0.001, Figure 2) but the effect of its high concentration (0.4 mg/mL) on total protein (TP) was significantly higher than dexamethasone (P<0.001, Figure 2). The effect of high ALA concentration on BALF levels of PLA2 and TP were significantly higher than the effect of its low concentration (0.2 mg/mL), (P<0.001 and P<0.01 for PLA2 and TP respectively, Figure 2).
Figure 2.
BALF levels (mean±SEM) of PLA2 (a) and total protein (b) in control rats (C), sensitized animals (S), S treated with dexamethasone (S+D) and two concentrations of alpha linolenic acid (S+ALA), (n=4 for ALA treated groups and n=6 for other groups)
Statistical differences between groups S, S+D and S+ALA versus group C; ** P<0.01, *** P<0.001. Statistical differences between groups S+D and S+ALA versus group; S +++ P<0.001
Statistical differences between groups S+ALA versus group S+D; ### P<0.001
Statistical differences between groups S+ALA 0.2 mg/ml versus S+ALA 0.4 mg/ml; ×× P<0.01, ××× P<0.001. The statistical comparisons were made using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test
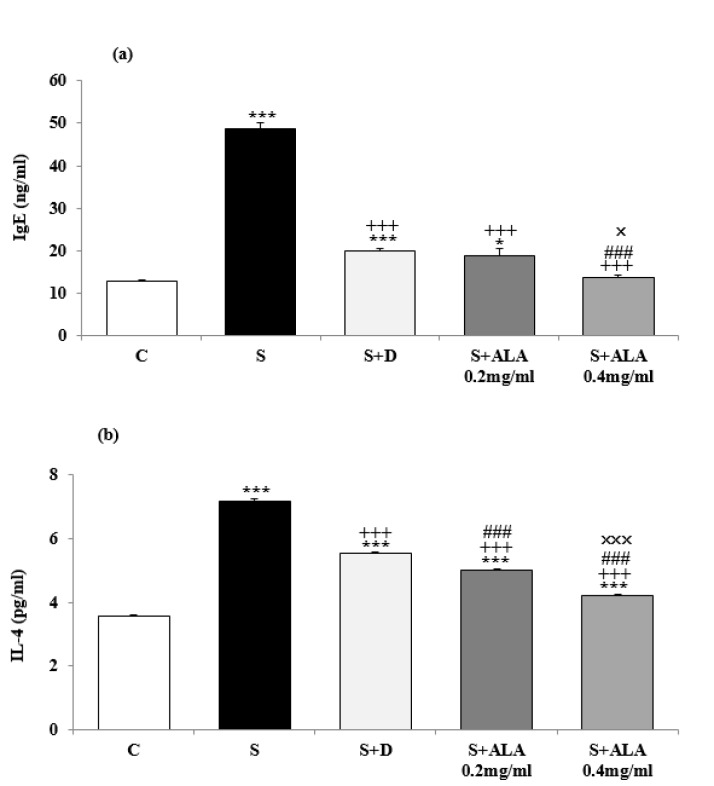
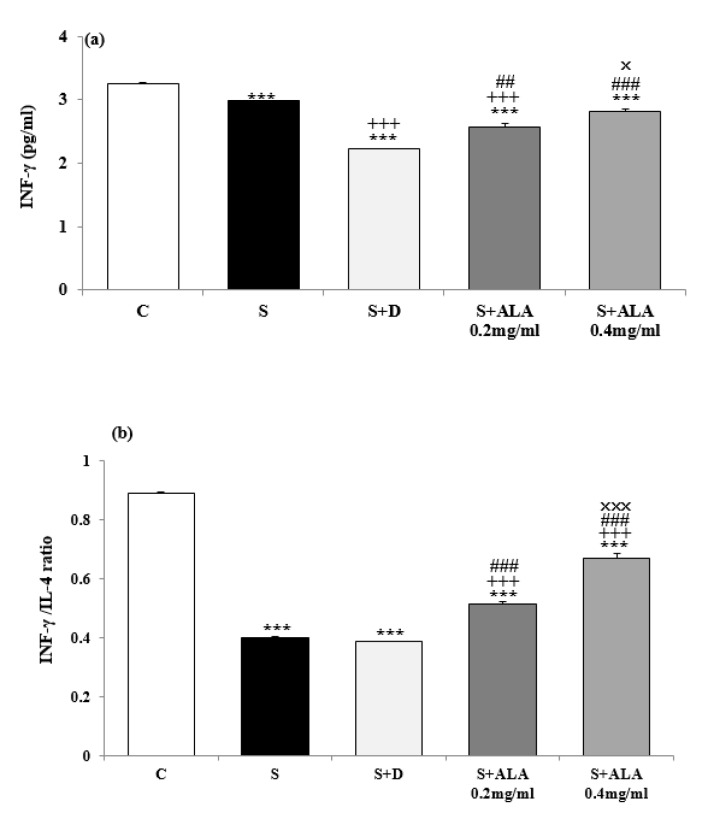
BALF level of IL-4 and IgE in group S were significantly higher but the level of IFN-γ and INF-γ/IL-4 ratio were significantly lower than group C (P<0.001 for all cases, Figures 3 and 4). IL-4 and IgE levels in treated groups with dexamethasone and both concentrations of ALA and IFN-γ levels in treated groups with dexamethasone and low concentration of ALA were significantly decreased compared to group S (P<0.001 for all cases, Figures 3 and 4). However, INF-γ/IL-4 ratio in treated groups with both concentrations of ALA were significantly increased compared to group S (P<0.001 for both cases, Figure 4). The effect of both concentrations of ALA on IL-4, IFN-γ and INF-γ/IL-4 ratio as well as its high concentration on IgE level were significantly higher than the effect of dexamethasone (P<0.001 for all cases, Figures 3 and 4). However, BALF levels of IgE and IL-4 were significantly higher but values of IFN-γ and INF-γ/IL-4 ratio were significantly lower in all treated groups than group C (P<0.001 for all cases, Figures 3 and 4).
Figure 3.
BALF levels (mean±SEM) of IgE (a) and IL-4 (b) in control rats (C), sensitized animals (S), S treated with dexamethasone (S+D) and two concentrations of alpha linolenic acid (S+ALA), (n=4 for ALA treated groups and n=6 for other groups)
Statistical differences between groups S, S+D and S+ALA versus group C; * P<0.05, *** P<0.001. Statistical differences between groups S+D and S+ALA versus group S; +++ P<0.001
Statistical differences between groups S+ALA versus group S+D; ### P<0.001
Statistical differences between groups S+ALA 0.2 mg/ml versus S+ALA 0.4 mg/ml; × P<0.05, ××× P<0.001. The statistical comparisons were made using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test
Figure 4.
BALF levels of INF-γ (a) and INF-γ/IL-4 ratio (b) (mean±SEM) in control rats (C), sensitized animals (S), S treated with dexamethasone (S+D) and two concentrations of alpha linolenic acid (S+ALA), (n=4 for ALA and n=6 for other groups)
Statistical differences between groups S, S+D and S+ALA versus group C; *** P<0.001
Statistical differences between groups S+D and S+ALA versus group S; +++ P<0.001
Statistical differences between groups S+ALA versus group S+D; ## P<0.01, ### P<0.001
Statistical differences between groups S+ALA 0.2 mg/ml versus S+ALA 0.4 mg/ml; × P<0.05, ××× P<0.001. The statistical comparisons were made using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test
The effect of high ALA concentration (0.4 mg/ml) on BALF levels of IgE, IL-4, IFN-γ and INF-γ/IL-4 ratio were significantly higher than the effect of its low concentration (0.2 mg/ml), (P<0.05 to P<0.001, Figures 2-4).
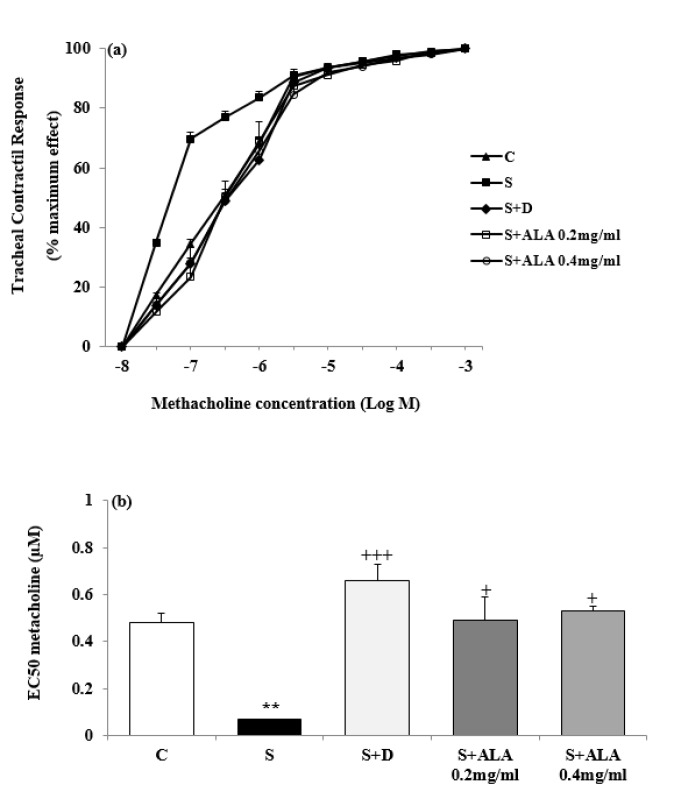
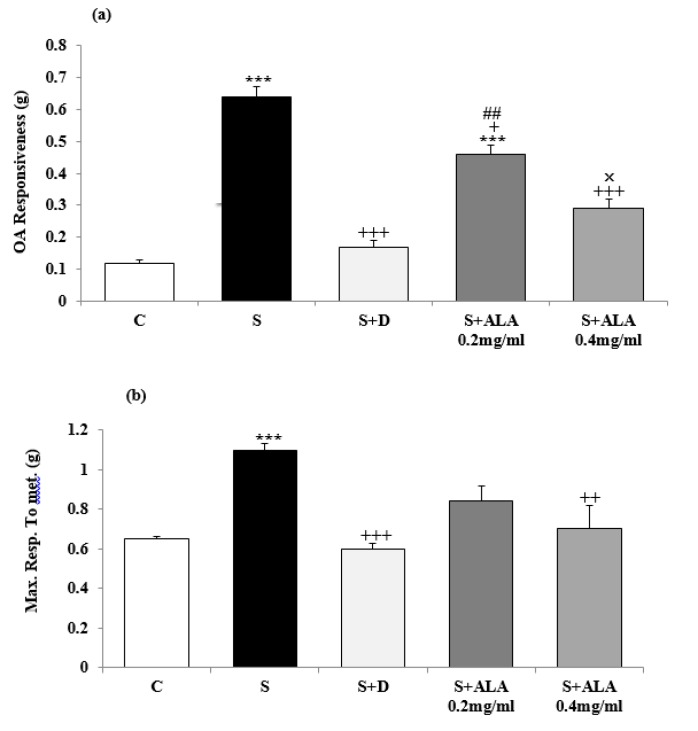
Concentration-response curves to methacholine in group S shifted to left compared to group C but in the treated groups with dexamethasone and both concentrations of ALA, right-ward shift of the curves were observed compared to group S (Figure 5). EC50 methacholine was significantly lower but maximum response to methacholine and TR to OA were higher in group S compared to group C (P<0.01 for EC50 and P<0.001 for maximum response and tracheal response to OA, Figures 5 and 6). However, EC50 and TR to OA in treated groups with dexamethasone and both concentrations of ALA as well as maximum response to methacholine in treated groups with dexamethasone and high concentration of ALA were significantly improved compared to the group S (P<0.05 to P<0.001, Figures 5 and 6). The effect of low concentration of ALA on TR to OA was significantly lower than dexamethasone (P<0.01, Figure 6). TR to OA in treated group with low concentration of ALA was still significantly higher than group C (P<0.001, Figure 6). The effects of high concentration of ALA (0.4 mg/ml) on tracheal response to OA were significantly higher than its low concentration (0.2 mg/ml), (P<0.05, Figure 6).
Figure 5.
Cumulative log concentration-response curves of methacholine induced contraction of isolated trachea (a) and values (mean±SEM) of EC50, (the effective concentration of methacholine, causing 50% of maximum response) (b) in control rats (C), sensitized animals (S), S treated with dexamethasone (S+D) and two concentrations of alpha linolenic acid (S+ALA), (n=4 for ALA and n=6 for other groups)
Statistical differences between groups S, S+D and S+ALA versus group C; ** P<0.01
Statistical differences between groups S+D and S+ALA versus group S; + P<0.05, +++ P<0.001. The statistical comparisons were made using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test
Figure 6.
Values (mean±SEM) of maximum response to methacholine (a) and tracheal response to ovalbumin (b) in control rats (C), sensitized animals (S), S treated with dexamethasone (S+D) and two concentrations of alpha linolenic acid (S+ALA), (n=4 for ALA and n=6 for other groups).
Statistical differences between groups S, S+D and S+ALA versus group C; *** P<0.001
Statistical differences between groups S+D and S+ALA versus group S; + P<0.05, ++ P<0.01, +++ P<0.001.
Statistical differences between groups S+ALA versus group S+D; ## P<0.01
Statistical differences between groups S+ALA 0.2 mg/ml versus S+ALA 0.4 mg/ml; × P<0.05
The statistical comparisons were made using one way analysis of variance (ANOVA) with Tukey-Kramer’s post-test
Discussion
In present study, BALF level of TP, PLA2, IgE and IL-4 were increased but, INF-γ and INF-γ/IL-4 ratio were decreased in sensitized compared to control animal. In addition, left-ward shift in concentration-response curve to methacholine, increased, maximum response to methacholine and decreased EC50 methacholine were also seen in sensitized animal which indicated increased non-specific TR. Increased TR to OA was also observed indicating specific airway hyper responsiveness in sensitized animals. All these results indicated sensitization process or induction of animal model of asthma in rat. Similar changes in inflammatory and immune markers and TR were also shown in previous studies using similar method of animal sensitization (36-38) which support the findings of the present study.
Treatment of sensitized animals with dexamethasone and both concentrations of the ALA caused significant reduction in BALF levels of PLA2, TP, IL-4 and IgE. Moreover, treatment by both concentrations of ALA significantly increased INF-γ/IL-4 ratio compare to sensitized group. Treatment with ALA and dexamethasone significantly increased EC50 and decreased maximum response to methacholine which indicated reduction of non-specific airway responsiveness in sensitized animals due to ALA and dexamethasone treatment. TR to OA as specific airway responsiveness also significantly decreased in sensitized animals treated with ALA and dexamethasone. The effect of ALA on decrease BALF levels of PLA2 and TP in sensitized rats indicates the preventing effect of ALA on lung inflammation of sensitized rats which is the most prominent lung change in asthma.
The suppressive effect of ALA on IL-4 but its INF-γ enhancement in the BALF of sensitized animals suggests the inhibitory effect of ALA on Th2 and stimulatory effect on Th1 helper cells. INF-γ/IL-4 ratio was also increased in sensitized rats treated with ALA which indicated increased Th1/Th2 balance due to ALA treatment in animal model of asthma. The effect of ALA treatment on TR to methacholine and OA showed that this agent is able to reduce both specific and non-specific airway responsiveness in an animal model of asthma. Previous studies also indicated inhibitory effect of ALA on the production of interleukin-1 and tumor necrosis factor (34). Anti-inflammatory (12-15) and immunomodulatory effects of ALA on lactating dairy cows (39) have been demonstrated previously. The effect of ALA on Th1/Th2 balances was also demonstrated (27,28). The effect of hydro-ethanolic extract of Portulaca oleracea containing ALA on Th1/Th2 balance in isolated human lymphocytes was also reported (40). All the above studies support the findings of the present study which may suggest its therapeutic value in asthma by anti-inflammatory, immunoregulatory and its effect on TR mechanisms.
Therapeutic effects of omega-3 PUFA in asthma and exercise-induced bronchoconstriction (EIB) was reported. In addition, fish oil supplementation, rich in omega-3 PUFA, reduced airway narrowing, medication use, and inflammatory mediators in non-atopic elite athletes with EIB (41). Significant decrease in human blood lymphocyte proliferation and delayed-type hypersensitivity response were also seen 6 weeks after administration of linseed oil (providing about 15 g ALA/day) added to a low-fat diet (total fat provided 29% energy) (42). ALA also caused significant reduction in BALF level of IgE probably due to decreased lymphocytes proliferation which was depends on the level of linoleic acid and the total PUFA content of the diet (43). The blood lipid-lowering and immune-modulatory effects of ALA in rats was also shown (44). High dose of ALA (approximately 15 g/day) can suppress human Th1-drived cytokines production (42) which is in line with the findings of the current study. Supplementation of the diet with fish-oil derived omega-3 PUFA (1.2–14 g/day) results in decreased lymphocyte proliferation, production of IFN-γ (45), PGE2 production and synthesize of eicosanoids by immune cells. Omega-3 fatty acids are therefore, able to reduce disease-promoting inflammatory responses (45).
In the present study, dexamethasone was used as a corticosteroid positive control drug. Previously also, dexamethasone showed the inhibitory effect on airway inflammation in asthmatic mice (46). The effect of ALA on most measured variables was higher or at least equal to the effect of dexamethasone in sensitized rats. The effects of high concentration of ALA on TP and IgE levels and its both concentrations on IL-4 and IFN-γ levels as well as IFN-γ/IL-4 ratio were significantly higher than dexamethasone. In addition INF-γ/IL-4 ratio was only increased due to ALA treatment. Therefore, ALA showed equal or higher anti-inflammatory and effect on TR but more specific effect on Th1/Th2 balance compared to dexamethasone in rat model of asthma.
Concentration-dependent effects of the ALA on most measured variables were observed in the current study. The effects of high concentration of ALA on BALF levels of TP, PLA2, IgE, IL-4, INF-γ, INF-γ/IL-4 ratio and tracheal response to OA were significantly higher than its low concentration. The concentration dependency effect of ALA was also a further evidence for it effect on various measured variables on an animal model of asthma.
Various effect of P. oleracea containing ALA on respiratory system including its relaxant effect on the tracheal smooth muscle (47,48), antitussive effect (49), bronchodilatory effect on asthmatic airways (50) as well as the possible mechanism(s) of the relaxant effect of the plant on tracheal smooth muscle such as its stimulatory effect on β-adrenoceptors (51) and anticholinergic property of this plant (52) were also demonstrated previously.
Conclusion
Therefore ALA may have therapeutic effect on asthma by both bronchodilatory as relieving drug and effect on airway inflammation, immunomodulation and airway responsiveness as preventive drug. However, further studies including clinical trials needed to prove this suggestion.
Acknowledgment
This study was financially supported by Research Department of Mashhad University of Medical Sciences, Mashhad, Iran. This paper is the results of a part of PhD thesis of Mahsa Kaveh.
Conflicts of Interest
The authors have no conflict of interests to declare.
References
- 1.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Tura M, et al. Airway inflammation in childhood asthma. Am J Respir Crit Care Med. 2003;168:798–803. doi: 10.1164/rccm.200305-650OC. [DOI] [PubMed] [Google Scholar]
- 2.Boulet LP. Physiopathology of airway hyperresponsiveness. Curr Allergy Asthma Rep . 2003;3:166–171. doi: 10.1007/s11882-003-0030-9. [DOI] [PubMed] [Google Scholar]
- 3.Brannan JD, Lougheed MD. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Front Physiol . 2012;3:1–11. doi: 10.3389/fphys.2012.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vadas P, Pruzanski W. Role of secretory phospholipase A2 in the pathology of disease. Lab Invest . 1986;55:391–404. [PubMed] [Google Scholar]
- 5.Kashima N, Nakajima H, Katsura T, Sugihara S, Fukaura A, Takahashi T. Study of serum phosholipase A2 activity in bronchial asthmatic patients. Arerugi . 1993;42:723–727. [PubMed] [Google Scholar]
- 6.Qureshi S, Memon SA, Laghari AJ, Ghanghro AB. Metabolic total protein investigation in local industry workers exposed to acid anhydrids. Sindh Univ Res J . 2009;41:63–66. [Google Scholar]
- 7.Kelly C, Ward C, Stenton CS, Bird G, Hendrick DJ, Walters EH. Numbers and activity of cells obtained at bronchoalveolar lavage in asthma, and their relationship to airway responsiveness. Thorax. 1998;43:684–692. doi: 10.1136/thx.43.9.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol . 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 9.Randolph DA, Stephens R, Carruthers CJLD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest . 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busse WW, Coffman RL, Gelfand EW, Kay AB, Rosenwasser LJ. Mechanisms of persistent airway inflammation in asthma: a role for T cells and T-cell products. Am J Respir Crit Care Med. 1995;152:388–393. doi: 10.1164/ajrccm.152.1.7599853. [DOI] [PubMed] [Google Scholar]
- 12.Ren J, Chung SH. Anti-inflammatory effect of α-linolenic acid and its mode of action through the inhibition of nitric oxide production and inducible nitric oxide synthase gene expression via NF-κB and mitogen-activated protein kinase pathways. J Agric Food Chem. 2007;55:5073–5080. doi: 10.1021/jf0702693. [DOI] [PubMed] [Google Scholar]
- 13.Anand R, Kaithwas G. Anti-inflammatory potential of alpha-linolenic acid mediated through selective COX inhibition: computational and experimental data. Inflammation. 2014;37:1297–1306. doi: 10.1007/s10753-014-9857-6. [DOI] [PubMed] [Google Scholar]
- 14.Erdinest N, Shmueli O, Grossman Y, Ovadia H, Solomon A. Anti-inflammatory effects of alpha linolenic acid on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2012;53:4396–4406. doi: 10.1167/iovs.12-9724. [DOI] [PubMed] [Google Scholar]
- 15.Wendell SG, Baffi C, Holguin F. Fatty acids, inflammation and asthma. J Allergy Clin Immunol . 2014;133:1255–1264. doi: 10.1016/j.jaci.2013.12.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaithwas G, Mukherjee A, Chaurasia AK, Majumdar DK. Anti-inflammatory, analgesic and antipyretic activities of Linum usitatissimum L (flaxseed/linseed) fixed oil. Indian J Exp Biol . 2011;49:932–938. [PubMed] [Google Scholar]
- 17.Francès H, Coudereau JP, Sandouk P, Clément M, Monier C, Bourre JM. Influence of a dietary alpha-linolenic acid deficiency on learning in the Morris water maze and on the effects of morphine. Eur J Pharmacol. 1996;18:217–225. doi: 10.1016/0014-2999(95)00825-x. [DOI] [PubMed] [Google Scholar]
- 18.Huang CB, Ebersole JL. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol Oral Microbiol. 2010;25:75–80. doi: 10.1111/j.2041-1014.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee JY, Kim YS, Shin DH. Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J Agric Food Chem . 2002;50:2193–2199. doi: 10.1021/jf011175a. [DOI] [PubMed] [Google Scholar]
- 20.Dhar P, Chattopadhyay K, Bhattacharyya D, Roychoudhury A, Biswas A, Ghosh S. Antioxidative effect of conjugated linolenic acid in diabetic and non-diabetic blood: an in vitro study. J Oleo Sci. 2006;56:19–24. doi: 10.5650/jos.56.19. [DOI] [PubMed] [Google Scholar]
- 21.Simopoulos AP. Omega-3 fatty acids and antioxidants in edible wild plants. Biol Res . 2004;37:263–277. doi: 10.4067/s0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- 22.Blondeau N, Lipsky RH, Bourourou M, Duncan MW, Gorelick PB, Marini AM. Alpha-linolenic acid: an omega-3 fatty acid with neuroprotective properties—ready for use in the stroke clinic? Biomed Res Int. 2015;2015:1–8. doi: 10.1155/2015/519830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan H, Hu XZ, Jacobowitz DM, Chen C, McDonough J, Van Shura K, et al. Alpha-linolenic acid is a potent neuroprotective agent against soman-induced neuropathology. Neurotoxicology. 2012;33:1219–1229. doi: 10.1016/j.neuro.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Pan H, Piermartiri TC, Chen J, McDonough J, Oppel C, Driwech W, et al. Repeated systemic administration of the nutraceutical alpha-linolenic acid exerts neuroprotective efficacy, an antidepressant effect and improves cognitive performance when given after soman exposure. Neurotoxicology. 2015;51:38–50. doi: 10.1016/j.neuro.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Jeromson S, Gallagher IJ, Galloway SDR, Hamilton DL. Omega-3 fatty acids and skeletal muscle health. Mar Drugs . 2015;13:6977–7004. doi: 10.3390/md13116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cornish SM, Chilibeck PD. Alpha-linolenic acid supplementation and resistance training in older adults. Appl Physiol Nutr Metab . 2009;34:49–59. doi: 10.1139/H08-136. [DOI] [PubMed] [Google Scholar]
- 27.Mizota T, Fujita-Kambara C, Matsuya N, Hamasaki S, Fukudome T, Goto H, et al. Effect of dietary fatty acid composition on Th1/Th2 polarization in lymphocytes. JPEN J Parenter Enteral Nutr. 2009;33:390–396. doi: 10.1177/0148607108325252. [DOI] [PubMed] [Google Scholar]
- 28.Kankaanpää P, Sütas Y, Salminen S, Lichtenstein A, Isolauri E. Dietary fatty acids and allergy. Ann Med. 1999;31:282–287. doi: 10.3109/07853899908995891. [DOI] [PubMed] [Google Scholar]
- 29.Salmon M, Walsh D, Huang T, Barnes P, Leonard T, Hay D, et al. Involvement of cysteinyl leukotrienes in airway smooth muscle cell DNA synthesis after repeated allergen exposure in sensitized Brown Norway rats. Br J Pharmacol . 1999;127:1151–1158. doi: 10.1038/sj.bjp.0702669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaveh M, Akram E, Nemati A, Boskabady MH. The extract of Portulaca oleracea and its constituent, alpha linolenic acid affects serum oxidantlevels and inflammatory cells in sensitized rats. Iran J Allergy Asthma Immunol. 2017;16:256–270. [PubMed] [Google Scholar]
- 31.Kianmeher M, Ghorani V, Boskabady MH. Animal model of asthma, various methods and measured parameters: a methodological review. Iran J Allergy Asthma Immunol . 2017;15:445–65. [PubMed] [Google Scholar]
- 32.Shakeri F, Soukhtanloo M, Boskabady MH. The effect of hydro-ethanolic extract of Curcuma longa rhizome and curcumin on total and differential WBC and serum oxidant, antioxidant biomarkers in rat model of asthma. Iran J Basic Med Sci. 2017;20:155–165. doi: 10.22038/ijbms.2017.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boskabady MH, Bayrami G. The effect of safranal on tracheal responsiveness and white blood cells count in lung lavage of sensitized guinea-pigs. Pharmacol Rep . 2014;66:56–61. doi: 10.1016/j.pharep.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Shakeri F, Boskabady MH. Anti-inflammatory, antioxidant and immunomodulatory effects of curcumin in ovalbumin-sensitized rat. BioFactors. 2017;43:567–576. doi: 10.1002/biof.1364. [DOI] [PubMed] [Google Scholar]
- 35.Keyhanmanesh R, Boskabady MH, Khamneh S, Ebrahimi MA. The effect of thymoquinone, the main constituent of Nigella sativa on tracheal responsiveness and WBC count in lung lavage of sensitized guinea-pigs. Planta Med . 2010;76:218–222. doi: 10.1055/s-0029-1186054. [DOI] [PubMed] [Google Scholar]
- 36.Boskabady MH, Ziaei T. Effect of ascorbic acid on airway responsiveness in ovalbumin sensitized guinea pigs. Respirology . 2003;8:473–478. doi: 10.1046/j.1440-1843.2003.00511.x. [DOI] [PubMed] [Google Scholar]
- 37.Boskabady MH, Mehrjardi SS, Rezaee A, Rafatpanah H, jalali S. The impactof Zataria multiflora Boiss extracton in vitro and in vivo Th1/Th2 cytokine (IFN-γ/IL4) balance. J Ethnopharmacpol. 2013;150:1024–1031. doi: 10.1016/j.jep.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, Van Der Meer JW, et al. The effect of dietary supplementation with n—3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med . 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 39.Sun P, Wang J, Yang G, Khas-Erdene , Liu Q. Effects of different doses of free alpha-linolenic acid infused to the duodenum on the immune function of lactating dairy cows. Arch Anim Nutr. 2010;64:504–513. doi: 10.1080/1745039X.2010.511517. [DOI] [PubMed] [Google Scholar]
- 40.Askari VR, Rezaei A, Boskabady MH, Sadeghnia H, Abnus K, Iranshahi M. The influence of hydro-ethanolic extract of Portulaca oleracea on Th1/Th2 balance in isolated human lymphocytes. J Ethnopharmacol . 2016;16:31626–31629. doi: 10.1016/j.jep.2016.10.082. [DOI] [PubMed] [Google Scholar]
- 41.Mickleborough TD, Rundell KW. Dietary polyunsaturated fatty acids in asthma-and exercise-induced bronchoconstriction. Eur J Clin Nutr . 2005;59:1335–1346. doi: 10.1038/sj.ejcn.1602250. [DOI] [PubMed] [Google Scholar]
- 42.Kelley DS, Branch LB, Love JE, Taylor PC, Rivera YM, Iacono JM. Dietary alpha-linolenic acid and immunocompetence in humans. Am J Clin Nutr. 1991;53:40–46. doi: 10.1093/ajcn/53.1.40. [DOI] [PubMed] [Google Scholar]
- 43.Jeffery NM, Newsholme EA, Calder PC. Level of polyunsaturated fatty acids and the n-6 to n-3 polyunsaturated fatty acid ratio in the rat diet alter serum lipid levels and lymphocyte functions. Prostaglandins Leukot Essent Fatty Acids . 1997;57:149–160. doi: 10.1016/s0952-3278(97)90005-3. [DOI] [PubMed] [Google Scholar]
- 44.Stark AH, Crawford MA, Reifen R. Update on alpha-linolenic acid. Nutr Rev . 2008;66:326–332. doi: 10.1111/j.1753-4887.2008.00040.x. [DOI] [PubMed] [Google Scholar]
- 45.Calder PC. Omega-3 polyunsaturated fatty acids, inflammation and immunity. World Rev Nutr Diet. 2001;88:109–116. doi: 10.1159/000059774. [DOI] [PubMed] [Google Scholar]
- 46.Tang XY, Yu HP, Deng HJ, Chen X, Fan HZ, Gong YX, et al. Pathogenic mechanism of CD8(+) CD28(-) T cell and the effect of dexamethasone in asthmatic mouse. Zhonghua Yi Xue Za Zhi . 2011;91:1861–1865. [PubMed] [Google Scholar]
- 47.Boskabady MH, Boroushaki M, Aslani MR. Relaxant effect of Portulaca oleraceae on guinea pig tracheal chains and its possible mechanism (s) of action. Med Hypotheses Res . 2004;1:139–147. [Google Scholar]
- 48.Parry O, Okwuasaba F, Ejike C. Effect of an aqueous extract of Portulaca oleracea leaves on smooth muscle and rat blood pressure. J Ethnopharmacol. 1988;22:33–44. doi: 10.1016/0378-8741(88)90228-0. [DOI] [PubMed] [Google Scholar]
- 49.Boroushaki MT, Boskabady MH, Maleka F. Antitussive effect of Portulaca oleracea L in guinea pigs. Iran J Pharm Res . 2010;3:187–190. [Google Scholar]
- 50.Malek F, Boskabady MH, Borushaki MT, Tohidi M. Bronchodilatory effect of Portulaca oleracea in airways of asthmatic patients. J Ethnopharmacol. 2004;93:57–62. doi: 10.1016/j.jep.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Boskabady MH, Hashemzehi M, Khazdair MR, Askari VR. Hydro-ethanolic extract of Portulaca oleracea affects beta-adrenoceptors of guinea pig tracheal smooth muscle. Iran J Pharm Res . 2016;15:867–874. [PMC free article] [PubMed] [Google Scholar]
- 52.Hashemzehi M, Khazdair MR, Kiyanmehr M, Askari VR, Boskabady MH. Portulaca oleracea affects muscarinic receptors of guinea pig tracheal smooth muscle. Ind J Pharmac Sci. 2016;78:388–394. [PMC free article] [PubMed] [Google Scholar]