Abstract
Objective(s):
Gallic acid is a natural phenolic compound found in several fruits and medicinal plants. It is reported to have several health-promoting effects. This review aims to summarize the pharmacological and biological activities of gallic acid in vitro and animal models to depict the pharmacological status of this compound for future studies.
Materials and Methods:
All relevant papers in the English language were collected up to June 2018. The keywords of gallic acid, antioxidant, anticancer, antimicrobial, gastrointestinal-, cardiovascular-, metabolic-, neuropsychological-, and miscellaneous- diseases were searched in Google Scholar, PubMed, and Scopus.
Results:
Several beneficial effects are reported for gallic acid, including antioxidant, anti-inflammatory, and antineoplastic properties. This compound has been reported to have therapeutic activities in gastrointestinal, neuropsychological, metabolic, and cardiovascular disorders.
Conclusion:
Current evidence confirms the pharmacological and therapeutic interventions of gallic acid in multiple health complications; however, available data are limited to just cellular and animal studies. Future investigations are essential to further define the safety and therapeutic efficacy of gallic acid in humans.
Key Words: Anticancer, Antioxidant, Gallic acid, Health benefits, Pharmacological effects
Introduction
The term “phytochemical” points to a vast range of biologically active natural compounds with valuable pharmaceutical and nutritional properties. Phenolic compounds are a group of phytochemicals with at least one hydroxylated benzene ring. The members of this large and diverse group of chemical compounds are usually classified based on the number of carbon atoms in their structures. Simple phenolics, phenolic acids, acetophenones, cinnamic acid derivatives, coumarins, chromones, chalcones, aurones, flavonoids, anthocyanins, betacyanins, benzophenones, xanthones, stilbenes, quinones, lignans, lignins, tannins, and phlobaphenes are the main subgroups of natural phenolic compounds (1).
Phenolic acids are an important and abundant subgroup of phenolic compounds with the basic chemical structure of C6-C1 (hydroxybenzoic acids) or C6-C3 (hydroxycinnamic acids), consisting of a phenolic ring and a carboxyl substituent. The shikimic acid or phenylpropanoid pathway of plant metabolism usually regulate the biosynthesis of phenolic acids. In some cases, phenolic acids are the precursor of other important phytochemicals, such as tannins, coumarins, benzoquinones, and naphthoquinones. Caffeic acid, ferulic acid, p-hydroxybenzoic acid, protocatechuic acid, vanillic acid, salicylic acid, and gallic acid are the most common members of phenolic acids (1, 2).
Today, foodstuff containing phenolic compounds and their metabolites are of the main interest due to their favorable effects on human health. In this case, the positive effect of red wine polyphenols on cardiac health or the protective role of flavonoids against various types of cancer and age-related diseases are important examples (2).
Gallic acid and its derivatives: from chemistry to medicine
Gallic acid or 3,4,5-trihydroxybenzoic acid (CAS No 149-91-7) is one of the most abundant phenolic acids in the plant kingdom. It is a colorless or slightly yellow crystalline compound, with extensive application in the food and pharmaceutical industries. Gallic acid has been isolated from different plant species such as Quercus spp. and Punica spp., via various chromatographical methods; however, from the industrial point of view, gallic acid is produced through the hydrolytic breakdown of tannic acid using a glycoprotein esterase, namely tannase (EC 3.1.1.20) (3).
Gallic acid and its derivatives such as lauryl gallate, propyl gallate, octyl gallate, tetradecyl gallate, and hexadecyl gallate, can inhibit the oxidation and rancidity of oils and fats ascribed to their free radical scavenging and antioxidant nature. Therefore, they can be useful as additives in the food industry (4).
Besides the edible uses of gallic acid and its ester derivatives as flavoring agents and preservatives in the food industry, there are diverse scientific reports on biological and pharmacological activities of these phytochemicals, with emphasis on antioxidant, antimicrobial, anti-inflammatory, anticancer, cardioprotective, gastroprotective, and neuroprotective effects (4). This paper reviews the rtant biological and pharmacological activities of gallic acid in order to provide a clear view of the therapeutic aspects of this valuable phenolic acid.
Therapeutic effects of gallic acid and its derivatives
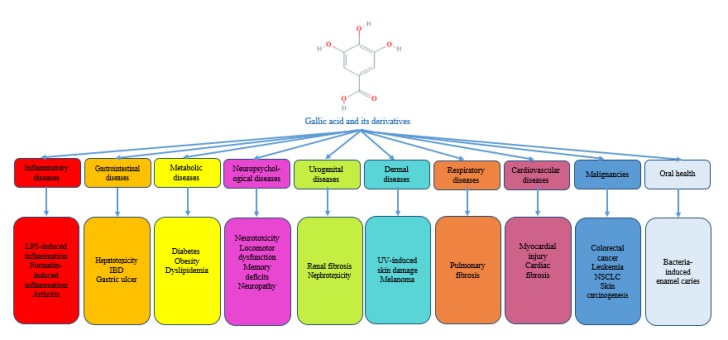
Figure 1 represents the most relevant pharmacological activities of gallic acid and related compounds.
Figure 1.
An overview of the pharmacological activities of gallic acid based on in vitro and in vivo studies
Antimicrobial activity
Structure-activity relationship studies of phenolic acids show that some parameters such as the basic chemical structure, the position, and the number of hydroxyl groups as well as their substituents on the phenolic ring, and the esterification of the carboxyl group, can affect the antimicrobial activity. Generally, hydroxycinnamic acids have higher antibacterial activity compared with hydroxybenzoic acids (5). Hydroxybenzoic acids with a lower degree of hydroxylation in phenol groups, highly methoxylated phenol groups, highly oxidized phenol groups, or ester derivatives with long alkyl chains showed higher antibacterial activities in comparison with their parent structures (5). On the other hand, hydroxybenzoic acids with more free –OH groups on the phenol ring were found more potent against the human immunodeficiency virus (HIV) and hepatitis C virus (HCV) (5-9).
From the mechanistic point of view, gallic acid can inhibit motility, adherence and biofilm formation of Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus mutans, Chromobacterium violaceum, and Listeria monocytogenes (10-12). The compound can also disrupt the integrity of the cell membrane in Gram-positive and Gram-negative bacteria and change the charge, hydrophobicity, and permeability of the membrane surface (13). Gallic acid can interfere with the membrane permeability of Campylobacter jejuni and elevate the antibiotic accumulation in the microorganism (14). Moreover, it can disintegrate the outer membrane of Gram-negative bacteria via chelation of divalent cations (15).
In addition to its effects on the bacterial cell membrane, there are some reports on the inhibitory activity of gallic acid against bacterial dihydrofolate reductase and its excitatory activity on topoisomerase IV-mediated DNA cleavage in different bacteria (16). Alkyl gallates can also penetrate the bacterial cell membrane and interfere with the electron transport chain and cellular respiration (17).
Some ester derivatives of gallic acid, i.e., octyl gallate, use the hydrophilic catechol part as a hook to bind to the polar surface of the cell membrane and enter the lipid bilayer using the hydrophobic alkyl part. Subsequently, they act as a nonionic surfactant and interfere with the selective permeability of cell membrane in fungi (17).
Gallic acid can inhibit HIV-1 integrase, HIV-1 transcriptase, HIV-1 protease dimerization (18-22), HCV attachment and penetration, HCV replication, HCV serine protease (23-26), the herpes simplex virus (HSV)-1 and HSV-2 attachment and penetration (22). It also causes disruption in Haemophilus influenza A and B particles (27).
In connection with protozoa, gallic acid can bind to the glutamate-gated chloride channels in the nervous system of Caenorhabditis elegans and initiates the hyperpolarization of the cell membranes and excitation of muscles. These events finally result in worm paralysis and death (28).
Gallic acid, alkyl gallates and chitosan-based formulations of gallic acid can potentiate the antimicrobial activity of other antibiotics, including erythromycin, gentamicin, norfloxacin, ciprofloxacin, ampicillin, penicillin, and oxacillin via synergism (29-34) (Table 1).
Pharmacological activities of gallic acid and its derivatives in different diseases
| Disease category | Compound name | Model | Effects | References |
|---|---|---|---|---|
| Anti-inflammatory |
Gallic acid |
In vitro: LPS-induced inflammation in A549 lung cancer cells In vivo: LPS-induced inflammation in mice |
In vitro: ↓HAT, ↓p300 & CBP acetyltransferase, p300-mediated RelA acetylation, NF-κB–regulated antiapoptotic & cell survival genes expression, p300-induced p65 acetylation, LPS-induced p65 translocation to the nucleus, ↑cytosolic IĸBα In vivo: ↓p65 acetylation, IFN-, IL-6, IL-1β & NF-κB–regulated antiapoptotic genes expression |
(72) |
| Gallic acid |
In vitro: zymosan-induced damage in human PMN In vivo: zymosan-induced acute food pad swelling in mice |
In vitro: interference with PMN function, ↓MPO & reduction rate of cytochrome c In vivo: ↓footpad swelling size |
(71) | |
| Gallic acid ethyl ester | Acetic acid-induced abdominal constriction, formalin-induced nociception, capsaicin-induced nociception, rat paw hyperalgesia induced by substance-P, bradykinin, PGE2 or carrageenan | ↓Acetic acid-induced abdominal constrictions, ↓formalin-induced licking, ↓hyperalgesia induced by substance P & bradykininNo significant change in capsaicin-induced nociception |
(70) | |
| Bergenin (C-glycoside of 4-O-methyl gallic acid) | Mycobacterium tuberculosis-induced inflammatory arthritis in mice | ↓Inflammatory arthritis, ↓IL-2, IFN-γ, TNF-α, IL-4 & IL-5 | (69) | |
| Gallic acid |
In vitro: AEGs- treated rabbit chondrocytes In vivo: collagenase-induced knee osteoarthritis in rabbit |
In vitro: ↓ROS, collagen II & aggrecan degradation, NO, i-NOS, COX-2, PGE2 ↑GSH, SOD In vivo: ↓knee Mankin’s score |
(73) | |
| Cardiovascular |
Gallic acid | ISO-induced myocardial infarction in rats | ↓Myocardial injury, ↓TC, TG, LDL-C, VLDL-C, MDA, ↑HDL-C, CAT & GPx, ↑membrane-bound Na+/K+, Ca2+ & Mg2+ ATPase | (85) |
| Gallic acid | ISO-induced cardiotoxicity in rats | ↓CK-MB & LDH, ↓lysosomal membrane damage, LPO, ↑GSH |
(86) | |
| Gallic acid | Lindane-induced cardiotoxicity | ↓CK, LDH & LPO, ↑GSH, SOD, GPx & GST, ↑membrane bound Na+/K+ & Mg2+ ATPase & ↓ Ca2+ATPase | (87) | |
| Gallic acid | Evaluation of antioxidant enzymes in the heart of male Sprague-Dawley rats | ↑Cardiac SOD, GPx, CuZnSOD, CAT, GSH/GSSG ratio, heme oxygenase-1 & Nrf2 | (88) | |
| Gallic acid | AGEs-induced cardiac remodeling in rats | ↓Cardiac fibrosis, ↓TNF-α, TGF-β, MMP-2 & MMP-9 | (89) | |
| Gallic acid | STZ-induced myocardial dysfunction in diabetic rats | ↓CK-MB, ↓LDH, LPO, LDL-C & VLDL-C, ↓MBP, SBP & bradycardia, collagen content, ↑CAT, SOD, GSH |
(90) | |
| Gallic acid | Isoproterenol-induced myocardial infarction in rats | ↓CK-MB, ↑SOD, CAT, GPx, GST, GSH, Vit C & E, ↓troponin-T, LDH-1 & LDH-2 | (58) | |
| Gallic acid | Fructose-enriched-diet-induced cardiac fibrosis | ↓BP, HOMA-IR, ↓NADPH oxidase subunits gp91 phox & p22 phox, ↓collagen I & osteopontin | (91) | |
| Gallic acid | Al2O3-induced myocardial injury | ↓LDH, CPK, CK-MB, TG, LDL, TNF-α & MDA, ↑HDL, GSH, SOD & CAT | (92) | |
| Gallic acid | Alloxan-induced diabetes & endothelial dysfunction | ↓MDA, ↑TAC & histamine vasodilatory response of mesenteric vascular bed | (93) | |
| Gallic acid | L-NAME-induced hypertension | ↓SBP, LV wall thickness & cardiac fibrosis, ↓hypertrophy markers, ↓HDAC1 & 2 | (94) | |
| Gallic acid | Cyclophosphamide-induced cardiorenal dysfunction | ↓MDA & H2O2, ↑CAT, GST, GSH & GPx | (95) | |
| Gastrointestinal |
Gallic acid | CCl4-induced hepatotoxicity in Charles Foster rats & Swiss albino mice | ↓Sleep time & paralysis time, ↓LPO, ↑hepatic amidopyrine-N-Demethylase, aniline & membrane-bound hepatic glucose-6-phosphatase activity, ↓hepatic TAG | (53) |
| Gallic acid | Hepatic ischemia & reperfusion injury in rats |
↓ALT, AST & LDH activities, ↑CAT & GPx, ↓MDA | (57) | |
| n-propyl gallate | Isolated perfused rat liver | ↓Gluconeogenesis, pyruvate carboxylation, glucose output inhibition |
(96) | |
| Gallic acid | Brush border disaccharidases inhibition in rats, LACA/L mice, BALB/c mice & rabbit | ↓Sucrase, maltase, trehalase & lactase activity | (97) | |
| Gallic acid | Primary HSC & hepatocytes | Cytotoxicity to HCS but not hepatocytes, ↑intracellular Ca2+ & calpain activity |
(98) | |
| Gallic acid | Ethanol-induced pancreatic injury in rats | ↑Cathepsin B activities, ↓cathepsin B & L enzymes release, cytosolic/lysosomal ratio of cathepsin B & L, pancreatic tissue injury | (99) | |
| Gallic acid | Ethanol-induced liver damage in rats | ↓AST, ALT, LDH activity, ↑paraoxonase & arylesterase activity | (54) | |
| Gallic acid | Gastric mucosal lesions caused by ischemia-reperfusion injury in rats | ↓Total area of gastric lesions, ↓caspase-3 & i-NOS | (47) | |
| Gallic acid |
In vitro: rat gastric epithelial cells In vivo: indomethacin & diclofenac-induced gastropathy |
In vitro: ↓mucosal cell death, ↑mitochondrial dehydrogenases In vivo: ↓mitochondrial protein carbonyl formation, ↓LPO & caspase-9 activation, ↑thiol content & MMP |
(48) | |
| Tryptamine-gallic acid |
In vitro: rat gastric epithelial cells In vivo: indomethacin-induced gastropathy |
In vitro: ↓intramitochondrial ROS generation In vivo: ↓mucosal cell death, gastropathy, mitochondrial protein carbonyl formation, ↓LPO, bcl-2 expression & caspase-9 activation, ↑thiol content & bax expression |
(100) | |
| Gallic acid | DSS-induced experimental colitis in mice | ↓DAI & colon shortening, ↓IL-21, IL-23, MDA, ↑SOD, GPx, CAT, GR, Nrf2, UDP-GT & NQO1 | (50) | |
| Gallic acid | DSS-induced colitis in mice | ↓MPO activity, i-NOS, COX-2, p65-NF-κB & IL-6/p-STAT3Y705 activation | (51) | |
| Gallic acid | Paracetamol-induced liver damage in mice | ↓ALT, AST, ALP, & ↓TNF-α, ↑SOD, CAT, GSH, GPx & GST | (101) | |
| Trimethylgallic acid esters | CCl4-induced liver damage in rats | ↓Vacuole formation, inflammation & necrosis, ↓AST, ALT, TG, TC, LPO & ↓TNF-α, ↑SOD, CAT & GSH | (102) | |
| Gallic Acid | Aspirin + pyrolus ligation-induced gastric ulcer in rats | ↓Ulcer index, gastric juice volume, free & total acidity, total protein, carbohydrates concentration, ↑SOD, CAT, GSH, GPx, GR & glucose-6-phosphate dehydrogenase | (103) | |
| Gallic acid | Bromobenzene-induced liver injury in rat | ↓Aniline hydroxylase & AMND activity, ↓LPO, ↑epoxide hydrolase activity |
(52) | |
| Gallic acid | CCl4-induced liver fibrosis in mice | ↓Liver fibrosis, HA, MDA, ALT, AST & GGT | (104) | |
| Gallic acid & piperine | Beryllium-induced hepatorenal dysfunction in rats | ↓Bilirubin, Cr, LDH, GGT, LPO, AST, ALT, ALP, ↑GSH, SOD & CAT | (105) | |
| Gallic acid | Lead-induced toxicity in blood, liver & kidney of rats | ↓LPO & carbonyl, prevention of body weight loss, ↑ALA-D activity, ↑SOD, CAT & GSH | (56) | |
| Gallic acid & ellagic acid | LPS-induced liver injury | ↓ALT, AST & i-NOS expression | (106) | |
| Gallic acid | CCl4-induced chronic liver injury in rats | ↓ALT, AST & MDA, ↑SOD, CAT, GSH, GR, GPx & GSH/GSST | (107) | |
| Gallic acid | Lindane-induced hepatorenal toxicity in rats | ↓ALT, AST, ALP, LPO, creatinine & urea, ↑GSH, CAT, SOD, GPx & GST | (108) | |
| Gallic acid | Beryllium-induced hepatorenal toxicity | ↓AST, ALT, ALP, LPO, AMND, ↑GSH, CAT, SOD, GPx & GST, ↓Cr & urea | (109) | |
| Gallic acid | Cyclophosphamide-induced hepatotoxicity in rats | ↓AST, ALT, MDA, ↑GSH, CAT, SOD & GST | (55) | |
| Gallic acid | Indomethacin-induced gastric ulcer in Swiss albino mice | ↑Ulcer healing, ↓PGE2 synthesis, ↑e-NOS/i-NOS ratio | (49) | |
| Metabolic | Gallic acid | Diet-induced obesity in mice | ↓TAG & FBS, ↓adipocyte size in the epididymal white adipose tissue, ↑PPAR expression, ↑Akt signaling pathway activity, ↓glucose tolerance & lipid metabolism | (59) |
| Gallic acid | High-fat-diet- & -STZ-induced type 2 diabetes in rats | ↓Body weight gain, FBS & FPI, ↑adipose tissue insulin sensitivity, Cytoprotective action on pancreatic β-cell, ↑PPARγ expression in treated tissue, liver & skeletal muscle, ↑insulin-dependent glucose transport, ↑interactions with the GLUT4, GLUT1, PI3K & p-Akt, ↓adipogenesis | (60) | |
| Gallic acid | High-fat-diet-induced dyslipidemia, hepatosteatosis & oxidative stress in rats | ↓Obesity, liver weight, peritoneal & epididymal adipose tissue weights, ↓serum TAG, phospholipid, TC, LDL-C, insulin & leptin, ↓lipid droplets size, ↓hepatic TAG & cholesterol, ↓oxidative stress & GSSG, ↑GSH, GPx, GR & GST | (61) | |
| Gallic acid | High-fructose-diet-induced diabetes | ↑Glucose uptake activity, ↓AUCglucose & HOMA-IR, ↓C-peptide, fructosamine & cardiovascular risk index, ↑IR, IR-1, PI3K, Akt/protein kinase B & GLUT-2, ↓F-1,6-BP, ↑hexokinase, PFK & aldolase | (62) | |
| Gallic acid | STZ-induced diabetic rats | ↑Vit C, ↓GSH, ↓LPO, ↑free radical scavenging property, Fe2+ chelating ability & Fe3+ reducing property, ↑CAT, GST, δ-aminolevulinic acid dehydratase & LDH, ↓purinergic enzymes | (63) | |
| Gallic acid | STZ-induced diabetic Wistar rats | ↓FBS, regeneration of β-cells, ↓TC, TAG, LDL-C, urea, uric acid, creatinine, ↑FPI, C-peptide & glucose tolerance restored the total protein, albumin & body weight | (110) | |
| Gallic acid | Fructose-induced metabolic syndrome & cardiac fibrosis in rats | ↓Insulin resistance, ROS & NADPH overproduction, collagen I & osteopontins | (98) | |
| Gallic acid |
In vitro: porcine pancreatic lipase kit In vivo: high-fat-diet-induced obesity in mice |
In vitro: ↓pancreatic lipase activity In vivo: ↓weight gain, ↑feces neutral fat |
(111) | |
| Gallic acid | STZ-induced diabetes in rats | ↑FPI, hepatic hexokinase activity, CAT, SOD, GPx, ↓FBS, HbA1C, G6PD & fructose-1, 6-bisphosphatase, LPO | (112) | |
| Gallic acid | STZ-induced diabetes in rats | ↓FBS, HbA1C, LPO, ↑FPI, Vit C, SOD, CAT, GSH, GR, GST, GPx, HMG-CoA reductase activity | (113) | |
| Gallic acid | Alloxan-induced diabetes in rats | ↓FBS, ↑FPI, GSH, GPx, CAT, SOD & osmotic fragility of RBCs | (114) | |
| Gallic acid | STZ-induced diabetes in rats | ↓FBS, brain LPO, SOD, CAT, GR, GST, GPx, brain lipids | (37) | |
| Gallic acid | Chromium-induced thyroid dysfunction | ↓SOD & GST up-regulation, ↓NO, i-NOS, TNF-α, IL-6 & COX-2 | (115) | |
| Gallic acid |
In vitro: high glucose toxicity in NRK 52E rat proximal tubular epithelial cells In vivo: high fat diet/STZ- induced diabetes in rats |
In vitro: ↓p38 MAPK, NF-κB activation In vivo: ↓FBS, HbA1C, HOMA-IR, body weight, Cr, Cr clearance, BUN, IL-1β, IL-6, TNF-α & ↓MDA, ↓renal p38 MAPK, NF-κB activation, TGF-β, fibronectin, ↑GSH, GSST, GSH/GSST ratio, GR, CAT, SOD & GPx |
(116) | |
| Gallic acid | STZ-induced diabetes & oxidative stress in rats | ↓ROS & lipid peroxidation, ↑SOD & δ-ALA-D, CAT, GST & vit C | (117) | |
| Neuropsychological |
Gallic acid | 6-Hydroxydopamine induced oxidative stress in rats | ↑Passive avoidance memory, ↑TTM, GPx, ↓MDA | (65) |
| Gallic acid | STZ-induced memory deficits & oxidative stress in rats | ↑Passive avoidance & spatial memory, performance, ↑TTM, SOD, GPx & CAT, ↓MDA | (118) | |
| Gallic acid | EPM in rats | ↑Time spent & entries in the open arms of EPM, ↓locomotor activity, involvement of 5-HT1A receptors | (119) | |
| Gallic acid | Sodium fluoride-induced oxidative stress in rat brain | ↓LPO, ↑SOD & GSH | (120) | |
| Gallic acid | STZ-induced oxidative damage in rat brain | ↓MDA, ↑TTM, CAT, SOD & GPx, ↑ Na+/K+, Ca2+ & Mg2+ ATPases activity | (121) | |
| Gallic acid | Spinal cord injury-induced oxidative stress in rat | ↓LPO, ROS, nitrite, NF-kB & COX-2 ↑GSH, CAT, SOD & GPx |
(122) | |
| Gallic acid (as chitosan nanoparticles) | Scopolamine-induced amnesia & locomotor activity | ↓Transfer latency in the EPM test, ↑spatial learning & memory in MWM, ↓AChE activity, | (66) | |
| Gallic acid | Tyrosine hydroxylase Gal4/UAS-X RNAi Drosophila melanogaster model of Parkinson's disease | ↑Locomotor activity, protection of dopaminergic neurons, ↑life span & climbing abilities | (123) | |
| Gallic acid | Cyclophosphamide-induced neurotoxicity in rats | ↓Neurotoxicity, ↓cerebellar & cerebral MDA & nitrite, ↑CAT, GST & SOD | (55) | |
| Gallic acid | Reserpine-induced vacuous chewing movements in rats | ↓Vacuous chewing movements | (124) | |
| Gallic acid | Lead-induced locomotor damage & brain oxidative stress in rats | ↑Locomotor & exploratory activities by attenuating crossing & rearing time, ↓brain levels of Pb, ↑SOD & ↑GSH | (125) | |
| Gallic acid | Sodium nitroprusside oxidative stress-induced mitochondrial impairment | ↓NO level, ↓mitochondrial protein tyrosine nitration, ↓LPO, ↓protein carbonyl, ↑GSH & ↓MPT | (126) | |
| Gallic acid |
In vitro: sodium hydrosulfite-induced mitochondrial dysfunctions in SH-SY5Y cells In vivo: cerebral ischemia/reperfusion-induced by middle cerebral artery occlusion |
In vitro: protects against cytotoxicity of SH-SY5Y cells, ↓mitochondrial dysfunction, ↓level of mitochondrial ROS by ↓MitoSOX-fluorescence intensity, ↓intracellular DCF-fluorescence intensity, ↓intracellular MDA, by modulating mitochondrial dysfunctions by ↑oxygen consumption In vivo: ↓total infarct volume |
(127) | |
| Gallic acid (as chitosan nanoparticles) | FST & TST in rat | ↓Immobility in FST & TST, ↓MAO-A activity & MDA, ↑GSH & CAT | (128) | |
| Gallic acid | Aβ-induced toxicity in cultured rat cortical neurons | ↓Apoptotic neuronal death, ↓(Ca2+)c elevation & ROS formation, ↑glutamate release | (64) | |
| Gallic acid | H2O2-induced apoptosis in rat pheochromocytoma PC12 cells | Gallic acid & EGCG: ↓cell viability methyl gallate: ↑cell viability, ↓mitochondrial depolarization, caspase-9 activation & DNA degradation |
(68) | |
| Gallic acid | Immobilization-induced Swiss male albino mice | ↓Plasma nitrite in both unstressed & stressed mice, ↓plasma corticosterone, ↓n-NOS activity, ↓anxiety in behavioral tests | (129) | |
| Gallic acid | Global ischemia/reperfusion in Wistar rats | ↑Gait performance, sensorimotor disorders, & hypoalgesia (high dose), ↑passive avoidance memory (low dose), improvement in behavioral motor activity tests | (130) | |
| Gallic acid | Experimental sciatic nerve crush in rats | Improved motor coordination & SNCV sciatic nerve conduction velocity, ↑delayed foot lifting | (131) | |
| Gallic acid | Aβ-induced AD in rats | Improved LTP amplitude & area under the curve, ↑PS Amp, ↓Aβ plaque |
(132) | |
| Gallic acid | H2O2-induced apoptosis in rat pheochromocytoma PC12 cells | ↓Cell viability, ↑PARP cleavage, ↑JNK phosphorylation, ↓Bcl-2 | (67) | |
| Gallic acid | STZ-induced cerebral oxidative stress in rats | ↑Weight loss, ↓hyperglycemia, HbA1C, LPO, AChE & purinergic enzymes, ↑radical scavenging & Fe2+ chelating ability, Vit C, GSH, CAT, GST, cerebral LDH & Na+/K+-ATPase activity | (133) | |
| Gallic acid |
In vitro: Aβ-induced neurotoxicity in murine microglial BV-2 cells & neuroblastoma Neuro-2A cells In vivo: Aβ-induced AD in ICR mice |
In vitro: ↓RelA acetylation & cytokine production, cell death, ↑viability of Neuro-2A, ↓memory deficits in Ab peptide-induced mice In vivo: ↓cytokine production, neuronal cell death, nuclear NF-κB & IL-1β |
(134) | |
| Gallic acid | Chronic cerebral hypoperfusion-induced cognitive deficit & brain oxidative damage in rats | ↑Spatial memory, ↑TTM & GPx, ↓LPO | (135) | |
| Gallic acid & its derivatives | 6-OHD-induced neurotoxicity in human SH-SY5Y neuroblastoma cells | ↓Neurotoxicity, ↑GSH, ↓GSSG, ↓elevation in (Ca2+)I | (136) | |
| Oral health | Gallic acid | Streptococcus sobrinus 6715-induced enamel caries in rats | ↑Remineralization of enamel caries lesions, residual first molar enamel volume & mineral density values, ↓severity of molar enamel caries | (137) |
| Radiation-induced toxicity | Gallic acid | Whole body γ-radiation exposure in mice | ↑Rate of DNA repair process in peripheral blood leukocytes, bone marrow cells, & splenocytes, ↑GPx, GSH, ↓mortality, weight loss & LPO | (82) |
| Gallic acid |
In vitro: rat liver microsomes & plasmid pBR322 DNA exposed to γ-irradiation In vivo: whole body γ-irradiation in mice |
In vitro: ↓LPO in rat liver microsomes, ↓DNA damage in plasmid In vivo: ↓DNA damage in leukocytes |
(81) | |
| Respiratory | Gallic acid | Bleomycin-induced pulmonary fibrosis in rats | ↓Lesions & fibrosis, collagen content, hydroxyproline accumulation, LPO, ↓TNF-α & IL-1β, ↑GPx activity & TTM | (80) |
| Urinary |
Gallic acid | Doxorubicin-induced chronic kidney disease in rats | ↑Albumin, ↓AST, ↓ALT, ↓TG, ↓cholesterol, ↓LPO, ↓BUN | (79) |
| Gallic acid | Glyoxal-induced renal fibrosis in rats | ↓Renal fibrosis, ↓BUN, ALP, collagen I & III, MMP-2 & -9, NOx & ROS, ↑SOD | (78) | |
| Gallic acid | Ferric nitriloacetic acid-induced renal toxicity in rats | ↓Renal toxicity & cell proliferation, BUN, H2O2, renal microsomal LPO & quinone reductase, ↑CAT, xanthine oxidase, GPx, GST & G6PD | (77) | |
| Gallic acid | Cisplatin-induced nephrotoxicity in rats | ↓LPO, ROS, Cr, urea, uric acid, arginase activity, ↑SOD, CAT, GSH & GPx | (75) | |
| Gallic acid | Experimental renal ischemia-reperfusion in rats | ↓BUN, Cr, MDA | (74) | |
| Urogenital | Gallic acid | Cyclophosphamide-induced toxicity in testis & epididymis of rats | ↓Reproductive toxicity, nitrite, H2O2 & MDA ↑SOD, GST, FSH, LH & testosterone | (55) |
| Gallic acid | Cyclophosphamide-induced toxicity in testis & epididymis of rats | ↓MDA, NO, H2O2, ↑GSH, GPx, SOD, CAT & testosterone | (76) | |
| Gallic acid | STZ-induced oxidative stress in testis of rats | ↑SOD & CAT, ↓MDA, TNF-α, VEGF & NOS2 | (138) | |
| Dermal | Gallic acid |
In vitro: normal human dermal fibroblasts exposed to UVB In vivo: hairless mice exposed to UVB |
In vitro: ↓transcription factor activation protein 1 activity In vivo: ↓dryness, skin thickness, wrinkle formation & MMP-1, ↑elastin, type I procollagen & TGF-β1 |
(84) |
| Gallic acid |
In vitro: murine melanoma B16F10 cells In vivo: zebrafish, UVB-induced hyperpigmentation in mice ear |
In vitro: ↓melanin production & tyrosinase activity, melanogenesis regulatory genes, activation of the ERK pathway, involvement of AKT/GSK3b & PKA/CREB signaling In vivo: ↓body pigmentation in zebrafish, ↓hyperpigmentation of ear skin, inflammation, melanocytes activation & melanogenic genes |
(83) | |
| Malignancy | Gallic acid | DMH-induced colon carcinogenesis in male Wistar rats | ↑SOD, GSH, GR, GPx, & CAT activity, LPO modification | (39) |
| Gallic acid | DMH-induced colon carcinogenesis | ↑Activity of phase I enzymes (cyt. P450 & cyt. b5), ↓activity of phase II enzymes (GST, DTD & GGT) | (139) | |
| Isobutyl gallate-3,5-dimethyl ether (IGDE) & methyl gallate-3,5-dimethyl ether (MGDE) |
In vitro: EAT & LLC1 cells In vivo: EAT cells /BALB/c mice & LLC1 cells /C57bl/6 mice |
In vitro: no significant cytotoxic effects In vivo: EAT cells ↑Survival (IGDE>MGDE), NK cells cytotoxicity In vivo (LLC1): ↓tumor size (IGDE>MGDE) |
(44) | |
| Gallic acid |
In vitro: HL-60 human promyelocytic leukemia In vivo: athymic nude mice model |
In vitro: induction of G1 cell cycle arrest, ↓cyclin D1, CDK4, cyclin E, CDK2, & cyclin A, ↑p27KIP expression In vivo: ↓Tumor progression |
(45) | |
| Gallic Acid | Diethylnitrosamine-induced hepatocellular carcinoma in rats | ↓Tumor size, AFP & CEA, ↓serum AST, ALT, ACP, ALP, LDH, GGT, ↓liver AgNORs & PCNA | (46) | |
| Gallic acid |
In vitro: human NCSLC NCI-H460 cells In vivo: mouse NCI-H460 xenograft model |
In vitro: ↓viability, induction of G2/M phase cell cycle arrest, ↑intracellular Ca2+, CDK1 activity, caspase-3, caspase-8 & caspase-9 activation, ↓ΔΨ In vivo: ↓tumor size |
(140) | |
| Gallic acid |
In vitro: LL-2 mouse lung cancer cells In vivo: LL-2 lung cancer cells transplanted in mice |
In vitro: ↓viability In vivo: ↓tumor size, ↑number or apoptotic cells in tumor, synergistic effects in combination with cisplatin |
(141) | |
| Gallic acid & methyl gallate | two-stage skin carcinogenesis in ICR mice | ↓average number of papillomas per mouse | (142) | |
| Gallic acid | 7,12-DMBA/croton oil- induced two-stage skin carcinogenesis in Swiss albino mice |
↓time of appearance & average number of papillomas per mouse, tumor incidence, ↓LDH total activity& LDH-isoenzymes, LPO, MMP-2 & MMP-9 activity & expression, ↑GST, SOD, CAT activity & GSH, synergistic effect with 5-FU | (40) | |
| Gallic acid |
In vitro: cell-free kinases, primary HUVECs, primary human dermal LECs, human HT29 colon carcinoma cells & MT-450 rat mammary carcinoma cells In vivo: MT-450 tumor-bearing rats |
In vitro: slight inhibition of RTKs, ↓VEGF-induced autophosphorylation of VEGFR-2 in HT29 cells, ↓proliferation & ↑apoptosis in all cell lines In vivo: ↓tumor angiogenesis, ↑metastasis |
(143) | |
| Pyrogallol |
In vitro: MCF10DCIS.com cells In vivo: xenograft mouse model of MCF10DCIS.com |
In vitro: induction of S phase cell cycle arrest ↑ROS In vivo: ↓tumor size, IR, IRS1, IGF-1R, p70S6K, & ERK phosphorylation, ↓IL-1β, involvement of AMPK & AKT/mTOR signaling |
(43) |
i-NOS: nitric oxide synthase; IL-2: interleukin-2; IFN-γ: interferon-γ; TNF-α: tumour necrosis factor-α; IL-4: interleukin-4; IL-5: interleukin-5; IL-1β: interleukin-1β; COX-2: cyclooxygenase-2; IL-6:i nterleukin-6, NO: nitric oxide; SOD: superoxide dismutase; GPx: glutathione peroxidase; VCAM-1: vascular cell adhesion molecule-1; HUVECs: human umbilical vein endothelial cells; TC: total cholesterol; TG: triglycerides; VLDL-C: very low density lipoprotein cholesterol; HDL-C: high density lipoprotein cholesterol; LDL: low-density lipoprotein; CAT: catalase; LPO: lipid peroxidation; GSH: glutathione; GST: glutathione-S-transferase; AGEs: advanced glycation end products; ECM: extracellular matrix; TGF-β: transforming growth factor- β; MMPs: matrix metalloproteinases; cTnT: cardiac troponin T; LDH: lactate dehydrogenase; ROS: reactive oxygen species; LAP: leucine aminopeptidase; y-GTP ;y-glutamyl transpeptidase; Bcl-2: B-cell lymphoma 2; IL-21: interleukin-21; IL-23: interleukin-23; UDP-GT: UDP glucuronosyltransferase; NQO1: NAD(P)H quinone dehydrogenase-1; MPO: myeloperoxidase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; CCl4: carbon tetrachloride, HA; hyaluronic acid; MDA: malondialdehyde; γ -GT: γ -glutamyl transferase; ALP: alkaline phosphatase; ALA-D: aminolevulinic acid dehydratase; PDX-1: pancreas/duodenum homeobox 1; PPAR-γ: peroxisome proliferator-activated receptor γ; TBARS: 2-thiobarbituric acid reactive substances; MFB: medial forebrain bundle; H2O2: hydrogen peroxide; MWM: Morris water maze; EPM: elevated plus maze; MPT: membrane permeability transition; LPS: lipopolysaccharide; BUN: blood urea nitrogen; AChE: acetyl cholinesterase; MAO-A: Monoamine oxidase-A; G6PD: glucose-6-phosphate dehydrogenase; MAPKs: mitogen-activated protein kinases; AEGs: Advanced glycation end products; GLUT1: glucose transporter protein 1, GLUT4: glucose transporter protein 4; PI3K: phosphatidylinositol 3-kinase; p-Akt: phosphorylated protein kinase B; TAG: triacylglycerol; GSSG: glutathione disulfide;, AUCglucose: area under the curve for glucose; HOMA-IR: homeostasis model assessment insulin resistance; IRS-1: insulin receptor substrate-1; IR: insulin receptor; GLUT-2: glucose transporter protein 2;; F-1,6-BP: fructose-1,6-bisphosphatase; PFK: phosphofructokinase; a-CN: a-casein; STZ: streptozotocin; β-LG: β-lactoglobulin; Aβ: amyloid β Protein; (Ca2+): cytosolic Ca2+ concentration; MTT: 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl-tetrazolium bromide; H2DCF-DA: Hoechst 33342 dye, fluo-4 AM & 2_,7_-dichlorodihydrofluorescin diacetate; n-NOS: neuronal nitric oxide synthase; SNCV: sciatic nerve conduction velocity; LTP: long-term potentiation; PS Amp: population spikes amplitude; AUC: area under curve; CA-1: region I of hippocampus proper; AD: Alzheimer disease; PC12: pheochromocytoma cells; Bcl-2: B-cell lymphoma 2; JNK: c-Jun N-terminal protein kinase; , ICR: institute of cancer research; peroxidase, MDA: malondialdehyde, k-CN: kappa-casein, DCF: dichlorofluorescin, ; PGE2: prostaglandin E2; e-NOS: endothelial nitric oxide synthase; HSCs: hepatic stellate cells; UVB: ultraviolet B; TAC: total antioxidant capacity; L-NAME: NG-nitro-L-argininemethyl ester; SBP: systolic blood pressure; LV: left ventricle; HDAC: histone deacetylase; VEGF: vascular endothelial growth factor.
Anticancer activity
In normal physiological conditions, the cells of a healthy organism are programmed for collaboration and coordination, thereby disruption in cells can evoke different life-threatening diseases, such as cancer. At the cellular level, cancer is defined as an unusual increase of cell division, the resistance of the produced cells to death, and their tendency to invade and metastasize.
The cancerous cells disturb the normal functions of other cells by invasion or metastasis. No matter where the origin of the problem is, the overall quality of life is overshadowed by cancer. According to the official reports of health- and wellness-related organizations, the magnitude of personal and social consequences of cancer is very significant and the investigation of new drugs to control this problem continues (35-38).
Gallic acid can exert its cytotoxic and antitumor effect via modulation of antioxidant/pro-oxidant balance. In some cases, the compound can control the reactive oxygen species (ROS)-induced carcinogenesis through increasing the activity of superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx) and/or by reducing the lipid peroxidation and ROS production. In other cases, gallic acid can induce the cell cycle arrest, autophagy, and apoptosis via activating the caspases pathway and ROS generation. In addition, it can inhibit the invasion and metastasis by decreasing the matrix metalloproteinase expression and activity (39-43).
Moreover, some derivatives of gallic acid, such as isobutyl gallate-3,5-dimethyl ether and methyl gallate-3,5-dimethyl ether, are able to reduce the tumor size and increase the survival rate in in vivo models of cancer (44). Gallic acid regulates the cell-cycle-related proteins such as cyclin A, cyclin D1, and cyclin E, and slow down the cell division by induction of the p27KIP enzyme and inhibition of CDK activity (45). In the case of hepatocellular carcinoma, gallic acid decreased the tumor size and the serum level of tumor marker enzymes such as aspartate transaminase (AST), alanine transaminase (ALT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT) by inhibiting the proliferation of hepatic cells (46) (Table 1).
Gastrointestinal diseases
Gallic acid protects the mucosal layer of the gastrointestinal tract from ulcer via different mechanisms by reducing the acid secretion, inducing the release of endogenous antioxidant agents and defensive factors (i.e. SOD, CAT, endothelial nitric oxide synthase (e-NOS) and prostaglandin E2 (PGE2)), as well as decreasing oxidative stress and lipid peroxidation. In addition, gallic acid has been associated with several other beneficial pathways including reduction of the expression of pro-inflammatory mediators (i.e., tumor necrosis factor (TNF)-α and inducible nitric oxide synthase (i-NOS)), up-regulation of the pro-angiogenesis factors (i.e., Von Willebrand factor (vWF) VIII, mucosal hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF)), promotion of angiogenesis, and inhibition of the expression of apoptosis parameters (i.e., caspase-3 and caspase-9) (47-49) (Table 1).
Gallic acid interferes with various intra-cellular inflammatory pathways that induce ulcerative colitis. The compound inhibits the expression of nuclear transcription factors, such as nuclear factor (NF)-κB and signal transducer and activator of transcription 3 (STAT3), and down-regulates their inflammatory downstream targets (50). It also reduces the expression and/or activity of pro-inflammatory cytokines and inflammatory proteins, including TNF-α, interferon-γ (INF-γ), interleukin (IL)-1β, IL-6, IL-17, IL-21, IL-23, cyclooxygenase (COX)-2, and i-NOS, and decreases the expression and infiltration of neutrophils and CD68+ macrophages into the colon (50-51).
Gallic acid inhibits the lipid peroxidation and malondialdehyde production by inducing transcription factors (i.e., Nrf2) and its cytoprotective downstream targets including NAD(P)H quinone dehydrogenase 1 (NQO1) and UDP-glucuronosyltransferase (UDP-GT) (50-51).
Beside the gastroprotective activity, gallic acid ameliorates the hepatotoxic effects of xenobiotic agents by acting as an antioxidant compound that scavenges free radicals, such as ROS, and improves the capacity of antioxidant defense systems including SOD, GST, GPx, CAT, GSH, and cytochrome P450-dependent detoxifying enzymes (52-57) (Table 1).
Cardiovascular diseases
Myocardial ischemia is defined as a condition that is caused by an imbalance between oxygen supply and demand of the myocardium, of which coronary artery atherosclerosis is known to be the main cause. To decrease the risk of myocardial infarction, the ischemia can be treated using different surgical methods and/or pharmacological agents.
Gallic acid pretreatment decreases the harmful oxidative consequences of myocardial infarction in the context of its antioxidant potency (58), either by increasing the activity of antioxidant enzymes, such as SOD, CAT, GST, and GPx (58) and/or by elevation of the level of non-enzymatic antioxidant agents, such as GSH, vitamin C, and vitamin E (58). All of these activities can inhibit the detrimental effects of free radicals on the integrity and function of myocytes membranes, and consequently, the concentration of serum cardiac biomarkers, including cardiac troponin T (cTnT) and creatine kinase-MB (CK-MB) decreases after infarction (35, 58) (Table 1).
Metabolic diseases
Obesity, diabetes mellitus, and hyperlipidemia are the most prevalent metabolic disorders among adults. The ability to store the excess energy in adipocytes and release it in the future is vital for survival. However, genetic susceptibility, excessive energy intake and sedentary lifestyle may provoke increased adipose storage and further cause metabolic disorders.
In metabolic disorders, gallic acid inhibits diet-induced hyperglycemia and hypertriglyceridemia, reduces the size of adipocytes, and protects pancreatic β-cells by inducing the expression of peroxisome proliferator-activated receptor-γ (PPAR-γ), a nuclear transcription factor that induces differentiation and insulin sensitivity in adipocytes (59). Gallic acid also increases the cellular glucose uptake via stimulation of the phosphatidylinositol 3-kinase (PI3K)/p-Akt signaling pathway and translocation of insulin-stimulated glucose transporters, such as GLUT4, GLUT2, and GLUT1 (59).
The compound prevents the diet-induced oxidative stress by stimulating various enzymatic and non-enzymatic antioxidant defenses (60). Gallic acid can up-regulate the hepatic glycolysis enzymes, such as hexokinase, aldolase, and phosphofructokinase, and down-regulate the hepatic gluconeogenesis enzyme, named fructose-1,6-bisphosphatase, in rodents fed a high fructose diet (59-63) (Table 1).
Neuropsychological diseases
Alzheimer’s disease is a cognitive neurodegenerative problem (35), which commonly results in dementia in elderly individuals. Insidious memory loss and progressive dementia over the years are the major clinical presentations of patients. In this disease, the atrophy of the brain starts from the temporal lobe and spreads to the parietal and frontal lobes. In the microscopic scale, plaques of amyloid-β (Aβ) molecules and fibrillary tangles of hyperphosphorylated tau filaments are visible in the nervous system (35).
The protective effect of gallic acid on nerve cells is a controversial issue. On the one hand, gallic acid decreases the Aβ-induced toxicity in cultured cortical neurons of rats via inhibiting Ca2+ release from the endoplasmic reticulum into the cytoplasm or Ca2+ influx, inhibiting ROS generation and apoptosis (64). The compound restores the streptozotocin (STZ)-induced cerebellar oxidative stress and cognitive impairment in rats by scavenging free radical molecules such as ROS, inhibiting lipid peroxidation, and stimulating the activity of endogenous antioxidant agents, such as SOD, CAT, and GPx (65). Gallic acid is also able to reverse the scopolamine-induced amnesia in mice, probably through inhibiting oxidative stress and decreasing acetylcholinesterase (AChE) enzyme activity in the brain (66).
On the other hand, gallic acid decreases the viability of PC-12 rat pheochromocytoma cells in the H2O2-induced toxicity model (67). In this manner, gallic acid increases the rate of apoptosis via stimulation of the c-Jun N-terminal kinase (JNK) protein, down-regulation of Bcl-2 protein, inducing poly (ADP-ribose) polymerase cleavage, or even increasing intracellular Ca2+ and ROS generation (67) (Table 1).
Miscellaneous diseases
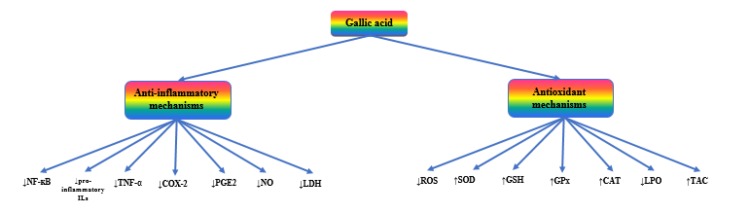
As shown in Figure 2, gallic acid can extinguish the flames of inflammation via different mechanisms. It decreases the expression and release of pro-inflammatory and inflammatory mediators, such as bradykinin, substance P, COX-2, NF-κB, IL-2, IL-4, IL-5, IFN-γ, and TNF-α. The compound also inhibits the phagocyte- or polymorphonuclear (PMN)-mediated inflammatory responses by scavenging ROS and decreasing the myeloperoxidase (MPO) activity (69-73).
Figure 2.
The most important mechanisms of gallic acid mediating its pharmacological activities
As mentioned earlier, gallic acid can partially neutralize the substance-induced toxicity in the liver and neural system. The beneficial and protective effects of gallic acid on substance- or radiation-induced toxicity in connective tissue, especially bone marrow, renal, reproductive, and respiratory systems have been proven. Almost all of the above-mentioned effects are linked to the antioxidant activity of gallic acid (74-82).
Topical application of gallic acid prevents the UV-B induced hyperpigmentation and photoaging of mice skin via down-regulating the melanogenic genes such as tyrosinase, increasing the skin hydration and transforming growth factor (TGF)-β1 induced production of procollagen type I and elastin, and decreasing ROS activation, wrinkle formation, and epidermal thickening (83, 84) (Table 1).
Conclusion
Studies presented here showed that the most important pharmacological properties of gallic acid are attributed to its antioxidant and anti-inflammatory potentials. In addition, gallic acid is involved in various signaling pathways that regulate the wide range of biological functions including pro- and inflammatory pathways, NO signaling pathway, intrinsic and extrinsic pathways of apoptosis, and NF-κB signaling pathway. Gallic acid and its derivatives demonstrated a broad range of beneficial effects in prevention and/or management of several disorders, also their acceptable safety and stability profiles, make them significant options to be introduced as dietary supplements.
Acknowledgment
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
All authors declare no potential conflicts of interest.
References
- 1.Pengelly A. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine. 2nd ed. CABI; 2004. [Google Scholar]
- 2.Siah M, Farzaei M, Ashrafi-Kooshk M, Adibi H, Arab S, Rashidi M, Khodarahmi R. Inhibition of guinea pig aldehyde oxidase activity by different flavonoid compounds: an in vitro study. Bioorg Chem. 2016;64:74–84. doi: 10.1016/j.bioorg.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Fernandes F, Salgado H. Gallic acid: review of the methods of determination and quantification. Crit Rev Anal Chem. 2016;46:257–265. doi: 10.1080/10408347.2015.1095064. [DOI] [PubMed] [Google Scholar]
- 4.Choubey S, Varughese L, Kumar V, Beniwal V. Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharm Pat Anal. 2015;4:305–315. doi: 10.4155/ppa.15.14. [DOI] [PubMed] [Google Scholar]
- 5.Borges A, Ferreira C, Saavedra M, Simoes M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist. 2013;19:256–265. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 6.Cueva C, Moreno-Arribas M, Martin-Alvarez P, Bills G, Francisca Vicente M, Basilio A, et al. Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria. Res Microbiol. 2010;161:372–382. doi: 10.1016/j.resmic.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Farag M, Al-Mahdy D, Salah El Dine R, Fahmy S, Yassin A, Porzel A, et al. Structure activity relationships of antimicrobial gallic acid derivatives from pomegranate and acacia fruit extracts against potato bacterial wilt pathogen. Chem Biodivers. 2015;12:955–962. doi: 10.1002/cbdv.201400194. [DOI] [PubMed] [Google Scholar]
- 8.Rivero-Buceta E, Carrero P, Doyagüez E, Madrona A, Quesada E, Camarasa M, et al. Linear and branched alkyl-esters and amides of gallic acid and other (mono-, di- and tri-) hydroxy benzoyl derivatives as promising anti-HCV inhibitors. Eur J Med Chem. 2015;92:656–671. doi: 10.1016/j.ejmech.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Maldonado A, Schieber A, Ganzle M. Structure–function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J Appl Microbiol. 2011;111:1176–1184. doi: 10.1111/j.1365-2672.2011.05141.x. [DOI] [PubMed] [Google Scholar]
- 10.Shao D, Li J, Li J, Tang R, Liu L, Shi J, et al. Inhibition of gallic acid on the growth and biofilm formation of Escherichia coli and Streptococcus mutans. J Food Sci. 2015;80:1299–1305. doi: 10.1111/1750-3841.12902. [DOI] [PubMed] [Google Scholar]
- 11.Borges A, Saavedra MJ, Simoes M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling. 2012;28:755–767. doi: 10.1080/08927014.2012.706751. [DOI] [PubMed] [Google Scholar]
- 12.Kang M, Oh J, Kang I, Hong S, Choi C. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J Microbiol. 2008;46:744–750. doi: 10.1007/s12275-008-0235-7. [DOI] [PubMed] [Google Scholar]
- 13.Teodoro G, Ellepola K, Seneviratne C. potential use of phenolic acids as anti-candida agents-a review. Front Microbiol. 2015;6:1420. doi: 10.3389/fmicb.2015.01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh B, Jeon E. Synergistic anti-Campylobacter jejuni activity of fluoroquinolone and macrolide antibiotics with phenolic compounds. Front Microbiol. 2015;13 doi: 10.3389/fmicb.2015.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nohynek L, Alakomi H, Kahkonen M, Heinonen M, Helander I, Oksman-Caldentey K, et al. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer. 2006;54:18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 16.Godstime C, Felix O, Augustina O, Christopher O. Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens. J Pharm Chem Biol Sci. 2014;2:77–85. [Google Scholar]
- 17.Kubo I, Fujita K, Nihei K, Masuoka N. Non-antibiotic antibacterial activity of dodecyl gallate. Bioorg Med Chem. 2003;11:573–580. doi: 10.1016/s0968-0896(02)00436-4. [DOI] [PubMed] [Google Scholar]
- 18.Modi M, Goel T, Das T, Malik S, Suri S, Rawat AK, et al. Ellagic acid & gallic acid from Lagerstroemia speciosa L inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian J Med Res. 2013;137:540–548. [PMC free article] [PubMed] [Google Scholar]
- 19.Singh A, Pal T. Docking analysis of gallic acid derivatives as HIV-1 protease inhibitors. Int J Bioinform Res Appl. 2015;11:540–546. doi: 10.1504/ijbra.2015.073239. [DOI] [PubMed] [Google Scholar]
- 20.Ahn C, Jung W, Park S, Kim Y, Kim W, Je J. Gallic acid-g-chitosan modulates inflammatory responses in LPS-stimulated RAW2647 cells via NF-kappaB, AP-1, and MAPK pathways. Inflammation. 2016;39:366–374. doi: 10.1007/s10753-015-0258-2. [DOI] [PubMed] [Google Scholar]
- 21.Flausino O, Dufau L, Regasini L, Petronio M, Silva D, Rose T, et al. Alkyl hydroxybenzoic acid derivatives that inhibit HIV-1 protease dimerization. Curr Med Chem. 2012;19:4534–4540. doi: 10.2174/092986712803251557. [DOI] [PubMed] [Google Scholar]
- 22.Kratz J, Andrighetti-Frohner C, Kolling D, Leal P, Cirne-Santos C, Yunes R, et al. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem Inst Oswaldo Cruz. 2008;103:437–442. doi: 10.1590/s0074-02762008000500005. [DOI] [PubMed] [Google Scholar]
- 23.Zuo G, Li Z, Chen L, Xu X. In vitro anti-HCV activities of Saxifraga melanocentra and its related polyphenolic compounds. Antivir Chem Chemother. 2005;16:393–398. doi: 10.1177/095632020501600606. [DOI] [PubMed] [Google Scholar]
- 24.Govea Salas M, Rivas Estilla A, Morlett Chávez J, Lozano Sepúlveda S, Rodríguez Herrera R, Aguilar González C. P420 gallic acid has antiviral effect against hepatitis C virus (HCV), which is mediated by its antioxidant activity. J Hepatol. 2014;60:S208. [Google Scholar]
- 25.Govea-Salas M, Rivas-Estilla A, Rodriguez-Herrera R, Lozano-Sepulveda S, Aguilar-Gonzalez C, Zugasti-Cruz A, et al. Gallic acid decreases hepatitis C virus expression through its antioxidant capacity. Exp Ther Med. 2016;11:619–624. doi: 10.3892/etm.2015.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu W, Chang S, Lin L, Li C, Richardson C, Lin C, et al. Limonium sinense and gallic acid suppress hepatitis C virus infection by blocking early viral entry. Antiviral Res. 2015;118:139–147. doi: 10.1016/j.antiviral.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Oh M, Seok J, Kim S, Lee D, Bae G, et al. Antiviral effects of black raspberry (Rubus coreanus) seed and its gallic acid against influenza virus infection. Viruses. 2016;6. pii doi: 10.3390/v8060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ndjonka D, Abladam E, Djafsia B, Ajonina-Ekoti I, Achukwi M, Liebau E. Anthelmintic activity of phenolic acids from the axlewood tree Anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J Helminthol. 2014;88:481–488. doi: 10.1017/S0022149X1300045X. [DOI] [PubMed] [Google Scholar]
- 29.Abouelhassan Y, Garrison A, Bai F, Norwood V, Nguyen M, Jin S, et al. A Phytochemical-halogenated quinoline combination therapy strategy for the treatment of pathogenic bacteria. Chem Med Chem. 2015;10:1157–1162. doi: 10.1002/cmdc.201500179. [DOI] [PubMed] [Google Scholar]
- 30.Li D, Liu Z, Yuan Y, Liu Y, Niu F. Green synthesis of gallic acid-coated silver nanoparticles with high antimicrobial activity and low cytotoxicity to normal cells. Process Biochem. 2015;50:357–366. [Google Scholar]
- 31.Lima V, Oliveira-Tintino C, Santos E, Morais L, Tintino S, Freitas T, et al. Antimicrobial and enhancement of the antibiotic activity by phenolic compounds: gallic acid, caffeic acid and pyrogallol. Microb Pathog. 2016;99:56–61. doi: 10.1016/j.micpath.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Lee D, Eom S, Kim Y, Kim H, Yim M, Lee S, et al. Antibacterial and synergic effects of gallic acid-grafted-chitosan with beta-lactams against methicillin-resistant Staphylococcus aureus (MRSA) Can J Microbiol. 2014;60:629–638. doi: 10.1139/cjm-2014-0286. [DOI] [PubMed] [Google Scholar]
- 33.Choi J, Kang O, Lee Y, Oh Y, Chae H, Jang H, et al. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J microbiol biotechnol. 2008;18:1848–1852. doi: 10.4014/jmb.0800.025. [DOI] [PubMed] [Google Scholar]
- 34.Shibata H, Kondo K, Katsuyama R, Kawazoe K, Sato Y, Murakami K, et al. Alkyl gallates, intensifiers of β-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob agents chemother. 2005;49:549–555. doi: 10.1128/AAC.49.2.549-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kasper D, Hauser S, Jameson J, Fauci A, Longo D, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. Mc Graw Hill; 2015. [Google Scholar]
- 36.Farzaei M, Bahramsoltani R, Rahimi R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr Pharm Des. 2016;22:4201–4218. doi: 10.2174/1381612822666160601100823. [DOI] [PubMed] [Google Scholar]
- 37.Shokoohinia Y, Jafari F, Mohammadi Z, Bazvandi L, Hosseinzadeh L, Chow N, et al. Potential anticancer properties of osthol: a comprehensive mechanistic review. Nutrients. 2018;10 doi: 10.3390/nu10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmedin J, Freddie B, Melissa M, Jacques F, Elizabeth W, David F. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 39.Giftson J, Jayanthi S, Nalini N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1, 2-dimethyl hydrazine induced rat colon carcinogenesis. Invest New Drugs. 2010;28:251–259. doi: 10.1007/s10637-009-9241-9. [DOI] [PubMed] [Google Scholar]
- 40.Subramanian V, Venkatesan B, Tumala A, Vellaichamy E. Topical application of gallic acid suppresses the 7, 12-DMBA/croton oil induced two-step skin carcinogenesis by modulating anti-oxidants and MMP-2/MMP-9 in Swiss albino mice. Food Chem Toxicol. 2014;66:44–55. doi: 10.1016/j.fct.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Balch C, Gershenwald J, Soong S, Thompson J, Atkins M, Byrd D, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao C, Lai K, Huang A, Yang J, Lin J, Wu S, et al. Gallic acid inhibits migration and invasion in human osteosarcoma U-2 OS cells through suppressing the matrix metalloproteinase-2/-9, protein kinase B (PKB) and PKC signaling pathways. Food Chem Toxicol. 2012;50:1734–1740. doi: 10.1016/j.fct.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 43.Nemec M, Kim H, Marciante A, Barnes R, Talcott S, Mertens-Talcott S. Pyrogallol, an absorbable microbial gallotannins-metabolite and mango polyphenols (Mangifera Indica L) suppress breast cancer ductal carcinoma in situ proliferation in vitro. Food Funct. 2016;7:3825–3833. doi: 10.1039/c6fo00636a. [DOI] [PubMed] [Google Scholar]
- 44.Da Silva S, Chaar J, Yano T. Chemotherapeutic potential of two gallic acid derivative compounds from leaves of Casearia sylvestris Sw (Flacourtiaceae) Eur J Pharmacol. 2009;608:76–83. doi: 10.1016/j.ejphar.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Huang P, Hseu Y, Lee M, Kumar K, Wu C, Hsu L, et al. In vitro and in vivo activity of gallic acid and Toona sinensis leaf extracts against HL-60 human premyelocytic leukemia. Food Chem Toxicol. 2012;50:3489–3497. doi: 10.1016/j.fct.2012.06.046. [DOI] [PubMed] [Google Scholar]
- 46.Jagan S, Ramakrishnan G, Anandakumar P, Kamaraj S, Devaki T. Antiproliferative potential of gallic acid against diethylnitrosamine-induced rat hepatocellular carcinoma. Mol Cell Biochem. 2008;319:51–59. doi: 10.1007/s11010-008-9876-4. [DOI] [PubMed] [Google Scholar]
- 47.Mard S, Mojadami S, Farbood Y, Gharib Naseri M. The anti-inflammatory and anti-apoptotic effects of gallic acid against mucosal inflammation- and erosions-induced by gastric ischemia-reperfusion in rats. Vet Res Forum. 2015;6:305–311. [PMC free article] [PubMed] [Google Scholar]
- 48.Pal C, Bindu S, Dey S, Alam A, Goyal M, Iqbal MS, et al. Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radicals Biol Med. 2010;49:258–267. doi: 10.1016/j.freeradbiomed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Chatterjee A, Chatterjee S, Biswas A, Bhattacharya S, Chattopadhyay S, Bandyopadhyay SK. Gallic acid enriched fraction of Phyllanthus emblica potentiates indomethacin-induced gastric ulcer healing via e-NOS-dependent pathway. Evid Based Complement Alternat Med. 2012;2012:487380. doi: 10.1155/2012/487380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandurangan A, Mohebali N, Norhaizan M, Looi C. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des Devel Ther . 2015b;9:3923–3934. doi: 10.2147/DDDT.S86345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pandurangan A, Mohebali N, Mohd Esa N, Looi C, Ismail S, Saadatdoust Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: possible mechanisms. Int Immunopharmacol . 2015a;28:1034–1043. doi: 10.1016/j.intimp.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 52.Park J, Han W, Park J, Choi S, Choi J. Changes in hepatic drug metabolizing enzymes and lipid peroxidation by methanol extract and major compound of Orostachys japonicus. J Ethnopharmacol. 2005;102:313–318. doi: 10.1016/j.jep.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 53.Anand K, Singh B, Saxena A, Chandan B, Gupta V, Bhardwaj V. 3,4,5-trihydroxy benzoic acid (gallic acid), the hepatoprotective principle in the fruits ofterminalia belerica-bioassay guided activity. Pharmacol Res. 1997;36:315–321. doi: 10.1006/phrs.1997.0236. [DOI] [PubMed] [Google Scholar]
- 54.Kartkaya K, Oglakci A, Senturk H, Bayramoglu G, Canbek M, Kanbak G. Investigation of the possible protective role of gallic acid on paraoxanase and arylesterase activities in livers of rats with acute alcohol intoxication. Cell Biochem Funct. 2013;31:208–213. doi: 10.1002/cbf.2874. [DOI] [PubMed] [Google Scholar]
- 55.Oyagbemi A, Omobowale O, Asenuga E, Akinleye A, Ogunsanwo R, Saba A. Cyclophosphamide-induced hepatotoxicity in wistar rats: the modulatory role of gallic acid as a hepatoprotective and chemopreventive phytochemical. Int J Prev Med. 2016;7:51–7802. doi: 10.4103/2008-7802.177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reckziegel P, Dias V, Benvegnú D, Boufleur N, Barcelos R, Segat H, et al. Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol Rep. 2016;3:351–356. doi: 10.1016/j.toxrep.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bayramoglu G, Kurt H, Bayramoglu A, Gunes H, Degirmenci I, Colak S. Preventive role of gallic acid on hepatic ischemia and reperfusion injury in rats. Cytotechnology. 2015;67:845–849. doi: 10.1007/s10616-014-9724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Priscilla D, Prince P. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in wistar rats. Chem Biol Interact. 2009;179:118–124. doi: 10.1016/j.cbi.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Gandhi G, Jothi G, Antony PJ, Balakrishna K, Paulraj M, Ignacimuthu S, et al. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARgamma in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur J Pharmacol. 2014;745:201–216. doi: 10.1016/j.ejphar.2014.10.044. [DOI] [PubMed] [Google Scholar]
- 60.Kade I, Ogunbolude Y, Kamdem J, Rocha J. Influence of gallic acid on oxidative stress-linked streptozotocin-induced pancreatic dysfunction in diabetic rats. J Basic Clin Physiol Pharmacol. 2014;25:35–45. doi: 10.1515/jbcpp-2012-0062. [DOI] [PubMed] [Google Scholar]
- 61.Bak E, Kim J, Jang S, Woo G, Yoon H, Yoo Y, et al. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand J Clin Lab Invest. 2013;73:607–614. doi: 10.3109/00365513.2013.831470. [DOI] [PubMed] [Google Scholar]
- 62.Hsu C, Yen G. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br J Nutr. 2007;98:727–735. doi: 10.1017/S000711450774686X. [DOI] [PubMed] [Google Scholar]
- 63.Huang D, Chang W, Wu J, Shih R, Shen S. Gallic acid ameliorates hyperglycemia and improves hepatic carbohydrate metabolism in rats fed a high-fructose diet. Nutr Res. 2016;36:150–160. doi: 10.1016/j.nutres.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 64.Ban J, Nguyen H, Lee H, Cho S, Ju H, Kim J. Neuroprotective properties of gallic acid from Sanguisorbae radix on amyloid beta protein (25-35)-induced toxicity in cultured rat cortical neurons. Biol Pharm Bull. 2008;31:149–153. doi: 10.1248/bpb.31.149. [DOI] [PubMed] [Google Scholar]
- 65.Mansouri M, Farbood Y, Sameri M, Sarkaki A, Naghizadeh B, Rafeirad M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food chem. 2013;138:1028–1033. doi: 10.1016/j.foodchem.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 66.Nagpal K, Singh S, Mishra D. Nanoparticle mediated brain targeted delivery of gallic acid: in vivo behavioral and biochemical studies for protection against scopolamine-induced amnesia. Drug Deliv. 2013;20:112–119. doi: 10.3109/10717544.2013.779330. [DOI] [PubMed] [Google Scholar]
- 67.Kang M, Kang N, Jang Y, Lee K, Lee H. Gallic acid induces neuronal cell death through activation of c-Jun N-terminal kinase and downregulation of Bcl-2. Ann N Y Acad Sci. 2009;1171:514–520. doi: 10.1111/j.1749-6632.2009.04728.x. [DOI] [PubMed] [Google Scholar]
- 68.Crispo J, Piché M, Ansell D, Eibl J, Tai I, Kumar A. Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. Biochem Biophys Res Commun. 2010;393:773–778. doi: 10.1016/j.bbrc.2010.02.079. [DOI] [PubMed] [Google Scholar]
- 69.Nazir N, Koul S, Qurishi M, Taneja S, Ahmad S, Bani S, et al. Immunomodulatory effect of bergenin and norbergenin against adjuvant-induced arthritis—a flowcytometric study. J Ethnopharmacol. 2007;112:401–405. doi: 10.1016/j.jep.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 70.Santos A, De Campos R, Miguel O, Cechinel-Filho V, Yunes R, Calixto J. The involvement of K+ channels and Gi/o protein in the antinociceptive action of the gallic acid ethyl ester. Eur J Pharmacol. 1999;379:7–17. doi: 10.1016/s0014-2999(99)00490-2. [DOI] [PubMed] [Google Scholar]
- 71.Kroes B, van den Berg A, Quarles van Ufford H, van Dijk H, Labadie R. Anti-inflammatory activity of gallic acid. Planta Med. 1992;58:499–504. doi: 10.1055/s-2006-961535. [DOI] [PubMed] [Google Scholar]
- 72.Choi K, Lee Y, Jung M, Kwon S, Kim M, Jun W, et al. Gallic acid suppresses lipopolysaccharide-induced nuclear factor-κB signaling by preventing RelA acetylation in A549 lung cancer cells. Mol Cancer Res. 2009;7:2011–2021. doi: 10.1158/1541-7786.MCR-09-0239. [DOI] [PubMed] [Google Scholar]
- 73.Wen L, Qu T, Zhai K, Ding J, Hai Y, Zhou J. Gallic acid can play a chondroprotective role against AGE-induced osteoarthritis progression. J Orthop Sci. 2015;20:734–741. doi: 10.1007/s00776-015-0718-4. [DOI] [PubMed] [Google Scholar]
- 74.Canbek M, Bayramoglu G, Senturk H, Oztopcu Vatan A, Uyanoglu M, Ceyhan E, et al. The examination of protective effects of gallic acid against damage of oxidative stress during induced-experimental renal ischemia-reperfusion in experiment. Bratisl Lek Listy. 2014;115:557–562. doi: 10.4149/bll_2014_108. [DOI] [PubMed] [Google Scholar]
- 75.Akomolafe S, Akinyemi A, Anadozie S. Phenolic acids (gallic and tannic acids) modulate antioxidant status and cisplatin induced nephrotoxicity in rats. Int Sch Res Notices. 2014;2014:984709. doi: 10.1155/2014/984709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olusoji M, Oyeyemi O, Asenuga E, Omobowale T, Ajayi O, Oyagbemi A. Protective effect of Gallic acid on doxorubicin-induced testicular and epididymal toxicity. Andrologia. 2016;49:10. doi: 10.1111/and.12635. [DOI] [PubMed] [Google Scholar]
- 77.Prasad L, Khan T, Jahangir T, Sultana S. Effect of gallic acid on renal biochemical alterations in male Wistar rats induced by ferric nitriloacetic acid. Hum Exp Toxicol. 2006;25:523–529. doi: 10.1191/0960327106het652oa. [DOI] [PubMed] [Google Scholar]
- 78.Yousuf M, Vellaichamy E. Protective activity of gallic acid against glyoxal -induced renal fibrosis in experimental rats. Toxicol Rep. 2015;2:1246–1254. doi: 10.1016/j.toxrep.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng C, Hsieh C, Wang H, Chung J, Chen K, Peng R. Ferulic acid is nephrodamaging while gallic acid is renal protective in long term treatment of chronic kidney disease. Clin Nutr. 2012;31:405–414. doi: 10.1016/j.clnu.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Nikbakht J, Hemmati A, Arzi A, Mansouri M, Rezaie A, Ghafourian M. Protective effect of gallic acid against bleomycin-induced pulmonary fibrosis in rats. Pharmacol Rep. 2015;67:1061–1067. doi: 10.1016/j.pharep.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 81.Gandhi N, Nair C. Protection of DNA and membrane from gamma radiation induced damage by gallic acid. Mol Cell Biochem. 2005;278:111–117. doi: 10.1007/s11010-005-6940-1. [DOI] [PubMed] [Google Scholar]
- 82.Nair G, Nair C. Radioprotective effects of gallic acid in mice. Biomed Res Int. 2013;2013:953079. doi: 10.1155/2013/953079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar K, Vani M, Wang S, Liao J, Hsu L, Yang H, et al. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: a novel skin lightening agent for hyperpigmentary skin diseases. Biofactors. 2013;39:259–270. doi: 10.1002/biof.1064. [DOI] [PubMed] [Google Scholar]
- 84.Hwang E, Park S, Lee H, Lee T, Sun Z, Yi T. Gallic acid regulates skin photoaging in UVB-exposed fibroblast and hairless mice. Phytother Res. 2014;28:1778–1788. doi: 10.1002/ptr.5198. [DOI] [PubMed] [Google Scholar]
- 85.Shaik A, Rasool S, Reddy A, Kareem M, Saayi Krushna G, Lakshmi Devi K. Cardioprotective effect of HPLC standardized ethanolic extract of Terminalia pallida fruits against isoproterenol-induced myocardial infarction in albino rats. J Ethnopharmacol. 2012;141:33–40. doi: 10.1016/j.jep.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 86.Prince P, Priscilla H, Devika P. Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in wistar rats. Eur J Pharmacol. 2009;615:139–143. doi: 10.1016/j.ejphar.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Padma V, Poornima P, Prakash C, Bhavani R. Oral treatment with gallic acid and quercetin alleviates lindane-induced cardiotoxicity in rats. Can J Physiol Pharmacol. 2013;91:134–140. doi: 10.1139/cjpp-2012-0279. [DOI] [PubMed] [Google Scholar]
- 88.Yeh C, Ching L, Yen G. Inducing gene expression of cardiac antioxidant enzymes by dietary phenolic acids in rats. J Nutr Biochem. 2009;20:163–171. doi: 10.1016/j.jnutbio.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 89.Umadevi S, Gopi V, Elangovan V. Regulatory mechanism of gallic acid against advanced glycation end products induced cardiac remodeling in experimental rats. Chem Biol Interact. 2014;208:28–36. doi: 10.1016/j.cbi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Patel S, Goyal R. Cardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in rats. Pharmacognosy Res. 2011;3:239–245. doi: 10.4103/0974-8490.89743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sutra T, Oiry C, Azay-Milhau J, Youl E, Magous R, Teissedre PL, et al. Preventive effects of nutritional doses of polyphenolic molecules on cardiac fibrosis associated with metabolic syndrome: involvement of osteopontin and oxidative stress. J Agric Food Chem. 2008;56:11683–11687. doi: 10.1021/jf802357g. [DOI] [PubMed] [Google Scholar]
- 92.El-Hussainy E, Hussein A, Abdel-Aziz A, El-Mehasseb I. Effects of aluminum oxide (Al2O3) nanoparticles on ECG, myocardial inflammatory cytokines, redox state, and connexin 43 and lipid profile in rats: possible cardioprotective effect of gallic acid. Can J Physiol Pharmacol. 2016;94:868–878. doi: 10.1139/cjpp-2015-0446. [DOI] [PubMed] [Google Scholar]
- 93.Badavi M, Sadeghi N, Dianat M, Samarbafzadeh A. Effects of gallic Acid and cyclosporine a on antioxidant capacity and cardiac markers of rat isolated heart after ischemia/reperfusion. Iran Red Crescent Med J. 2014;16:e16424. doi: 10.5812/ircmj.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin L, Lin M, Piao Z, Cho J, Kim G, Choi S, et al. Gallic acid attenuates hypertension, cardiac remodeling, and fibrosis in mice with NG-nitro-L-arginine methyl ester-induced hypertension via regulation of histone deacetylase 1 or histone deacetylase 2. J Hypertens. 2017;35:1502–1512. doi: 10.1097/HJH.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 95.Ogunsanwo O, Oyagbemi A, Omobowale T, Asenuga E, Saba A. Biochemical and electrocardiographic studies on the beneficial effects of gallic acid in cyclophosphamide-induced cardiorenal dysfunction. J Complement Integr Med. 2017;144 doi: 10.1515/jcim-2016-0161. oi: 10.1515/jcim-2016-0161. [DOI] [PubMed] [Google Scholar]
- 96.Eler G, Peralta R, Bracht A. The action of n-propyl gallate on gluconeogenesis and oxygen uptake in the rat liver. Chem Bio Interact. 2009;181:390–399. doi: 10.1016/j.cbi.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 97.Gupta N, Gupta S, Mahmood A. Gallic acid inhibits brush border disaccharidases in mammalian intestine. Nutr Res. 2007;27:230–235. [Google Scholar]
- 98.Hsieh S, Wu C, Wu C, Yen J, Liu M, Hsueh C. Gallic acid selectively induces the necrosis of activated hepatic stellate cells via a calcium-dependent calpain I activation pathway. Life Sci. 2014;102:55–64. doi: 10.1016/j.lfs.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 99.Kanbak G, Canbek M, Oglakci A, Kartkaya K, Senturk H, Bayramoglu G, et al. Preventive role of gallic acid on alcohol dependent and cysteine protease-mediated pancreas injury. Mol Biol Rep. 2012;39:10249–10255. doi: 10.1007/s11033-012-1901-8. [DOI] [PubMed] [Google Scholar]
- 100.Pal C, Bindu S, Dey S, Alam A, Goyal M, Iqbal M, et al. Tryptamine-gallic acid hybrid prevents non-steroidal anti-inflammatory drug-induced gastropathy: correction of mitochondrial dysfunction and inhibition of apoptosis in gastric mucosal cells. J Biol Chem. 2012;287:3495–3509. doi: 10.1074/jbc.M111.307199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 101.Rasool M, Sabina E, Ramya S, Preety P, Patel S, Mandal N, et al. Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J Pharm Pharmacol. 2010;62:638–643. doi: 10.1211/jpp.62.05.0012. [DOI] [PubMed] [Google Scholar]
- 102.Sachdeva M, Chadha R, Kumar A, Karan M, Singh T, Dhingra S. Hepatoprotective effect of trimethylgallic acid esters against carbon tetrachloride-induced liver injury in rats. Indian J Exp Biol. 2015;53:803–809. [PubMed] [Google Scholar]
- 103.Sen S, De B, Devanna N, Chakraborty R. Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb an Indian medicinal plant. Chin J Nat Med. 2013;11:149–157. doi: 10.1016/S1875-5364(13)60042-4. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Chung F, Lee S, Dykes G. Inhibition of attachment of oral bacteria to immortalized human gingival fibroblasts (HGF-1) by tea extracts and tea components. BMC Res Notes. 2013;6:143. doi: 10.1186/1756-0500-6-143. https://doi.org/10.1186/1756-0500-6-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao X, Wang Y, Sun Y. Quantitative and qualitative determination of Liuwei Dihuang tablets by HPLC-UV-MS-MS. J Chromatogr Sci. 2007;45:549–552. doi: 10.1093/chromsci/45.8.549. [DOI] [PubMed] [Google Scholar]
- 106.Sugimoto K, Sakamoto S, Nakagawa K, Hayashi S, Harada N, Yamaji R, et al. Suppression of inducible nitric oxide synthase expression and amelioration of lipopolysaccharide-induced liver injury by polyphenolic compounds in Eucalyptus globulus leaf extract. Food Chem. 2011;125:442–446. [Google Scholar]
- 107.Tung Y, Wu J, Hsieh C, Chen P, Chang S. Free radical-scavenging phytochemicals of hot water extracts of Acacia confusa leaves detected by an on-line screening method. Food Chem. 2009;115:1019–1024. [Google Scholar]
- 108.Padma V, Sowmya P, Felix T, Baskaran R, Poornima P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem Toxicol. 2011;49:991–998. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Nirala S, Li P, Bhadauria M, Guo G. Combined effects of gallic acid and propolis on beryllium-induced hepatorenal toxicity. Integr Zool. 2008;3:194–207. doi: 10.1111/j.1749-4877.2008.00090.x. [DOI] [PubMed] [Google Scholar]
- 110.Latha R, Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb in streptozotocin-induced diabetic rats. Chem Biol Interac. 2011;189:112–118. doi: 10.1016/j.cbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 111.Oi Y, Hou I, Fujita H, Yazawa K. Antiobesity effects of Chinese black tea (Pu-erh tea) extract and gallic acid. Phytother Res. 2012;26:475–481. doi: 10.1002/ptr.3602. [DOI] [PubMed] [Google Scholar]
- 112.Punithavathi V, Prince P, Kumar R, Selvakumari J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic wistar rats. Eur J Pharmacol. 2011;650:465–471. doi: 10.1016/j.ejphar.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 113.Punithavathi V, Prince P, Kumar M, Selvakumari C. Protective effects of gallic acid on hepatic lipid peroxide metabolism, glycoprotein components and lipids in streptozotocin-induced type II diabetic wistar rats. J Biochem Mol Toxicol. 2011;25:68–76. doi: 10.1002/jbt.20360. [DOI] [PubMed] [Google Scholar]
- 114.Ramkumar K, Vijayakumar R, Vanitha P, Suganya N, Manjula C, Rajaguru P, et al. Protective effect of gallic acid on alloxan-induced oxidative stress and osmotic fragility in rats. Hum Exp Toxicol. 2014;33:638–649. doi: 10.1177/0960327113504792. [DOI] [PubMed] [Google Scholar]
- 115.Mohammed A, Koorbanally N, Islam M. Phytochemistry, antioxidative activity and inhibition of key enzymes linked to type 2 diabetes by various parts of Aframomum meleguetain vitro. Acta Pol Pharm. 2016;73:403–417. [PubMed] [Google Scholar]
- 116.Ahad A, Ahsan H, Mujeeb M, Siddiqui W. Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells. Chem Bio Interact. 2015;240:292–303. doi: 10.1016/j.cbi.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 117.de Oliveira L, de Oliveira T, da Costa R, de Souza Gil E, Costa E, Passaglia C, et al. The vasorelaxant effect of gallic acid involves endothelium-dependent and -independent mechanisms. Vascul Pharmacol. 2016;81:69–74. doi: 10.1016/j.vph.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 118.Mansouri M, Naghizadeh B, Ghorbanzadeh B, Farbood Y, Sarkaki A, Bavarsad K. Gallic acid prevents memory deficits and oxidative stress induced by intracerebroventricular injection of streptozotocin in rats. Pharmacol Biochem Behav. 2013;111:90–96. doi: 10.1016/j.pbb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 119.Mansouri M, Soltani M, Naghizadeh B, Farbood Y, Mashak A, Sarkaki A. A possible mechanism for the anxiolytic-like effect of gallic acid in the rat elevated plus maze. Pharmacol Biochem Behav. 2014;117:40–46. doi: 10.1016/j.pbb.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 120.Nabavi S, Habtemariam S, Jafari M, Sureda A, Nabavi S. Protective role of gallic acid on sodium fluoride induced oxidative stress in rat brain. Bull Environ Contam Toxicol. 2012;89:73–77. doi: 10.1007/s00128-012-0645-4. [DOI] [PubMed] [Google Scholar]
- 121.Naghizadeh B, Mansouri M. Protective effects of gallic acid against streptozotocin-induced oxidative damage in rat striatum. Drug Res (Stuttg) 2015;65:515–520. doi: 10.1055/s-0034-1377012. [DOI] [PubMed] [Google Scholar]
- 122.Yang Y, Wang Z, Zheng J, Wang R. Protective effects of gallic acid against spinal cord injury-induced oxidative stress. Mol Med Rep. 2015;12:3017–3024. doi: 10.3892/mmr.2015.3738. [DOI] [PubMed] [Google Scholar]
- 123.Ortega-Arellano H, Jimenez-Del-Rio M, Velez-Pardo C. Dmp53, basket and drICE gene knockdown and polyphenol gallic acid increase life span and locomotor activity in a Drosophila Parkinson’s disease model. Genet Mol Biol. 2013;36:608–615. doi: 10.1590/S1415-47572013000400020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reckziegel P, Peroza L, Schaffer LF, Ferrari M, de Freitas C, Burger M, et al. Gallic acid decreases vacuous chewing movements induced by reserpine in rats. Pharmacol Biochem Behav. 2013;104:132–137. doi: 10.1016/j.pbb.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 125.Reckziegel P, Dias VT, Benvegnu D, Boufleur N, Barcelos R, Segat H, et al. Locomotor damage and brain oxidative stress induced by lead exposure are attenuated by gallic acid treatment. Toxicol Lett. 2011;203:74–81. doi: 10.1016/j.toxlet.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 126.Parihar P, Jat D, Ghafourifar P, Parihar M. Efficiency of mitochondrially targeted gallic acid in reducing brain mitochondrial oxidative damage. Cell Mol Biol. 2014;60:35–41. [PubMed] [Google Scholar]
- 127.Sun H, Zhang Y, Xie X, Che Y. Biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012;19:378–391. doi: 10.3109/10717544.2012.738437. [DOI] [PubMed] [Google Scholar]
- 129.Dhingra D, Chhillar R, Gupta A. Antianxiety-like activity of gallic acid in unstressed and stressed mice: possible involvement of nitriergic system. Neurochem Res. 2012;37:487–494. doi: 10.1007/s11064-011-0635-7. [DOI] [PubMed] [Google Scholar]
- 130.Farbood Y, Sarkaki A, Hashemi S, Mansouri M, Dianat M. The effects of gallic acid on pain and memory following transient global ischemia/reperfusion in wistar rats. Avicenna J Phytomed. 2013;3:329–340. [PMC free article] [PubMed] [Google Scholar]
- 131.Hajimoradi M, Fazilati M, Gharib-Naseri M, Sarkaki A. Gallic acid and exercise training improve motor function, nerve conduction velocity but not pain sense reflex after experimental sciatic nerve crush in male rats. Avicenna J Phytomed. 2015;5:288–297. [PMC free article] [PubMed] [Google Scholar]
- 132.Hajipour S, Sarkaki A, Farbood Y, Eidi A, Mortazavi P, Valizadeh Z. Effect of gallic acid on dementia type of Alzheimer disease in rats: electrophysiological and histological studies. Basic Clin Neurosci. 2016;7:97–106. doi: 10.15412/J.BCN.03070203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kade I, Rocha J. Gallic acid modulates cerebral oxidative stress conditions and activities of enzyme-dependent signaling systems in streptozotocin-treated rats. Neurochem Res. 2013;38:761–771. doi: 10.1007/s11064-013-0975-6. [DOI] [PubMed] [Google Scholar]
- 134.Kim M, Seong A, Yoo J, Jin C, Lee Y, Kim Y, et al. Gallic acid, a histone acetyltransferase inhibitor, suppresses beta-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol Nutr Food Res. 2011;55:1798–1808. doi: 10.1002/mnfr.201100262. [DOI] [PubMed] [Google Scholar]
- 135.Korani M, Farbood Y, Sarkaki A, Fathi Moghaddam H, Mansouri M. Protective effects of gallic acid against chronic cerebral hypoperfusion-induced cognitive deficit and brain oxidative damage in rats. Eur J Pharmacol. 2014;733:62–67. doi: 10.1016/j.ejphar.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 136.Lu Z, Nie G, Belton P, Tang H, Zhao B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem Int. 2006;48:263–274. doi: 10.1016/j.neuint.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Q, Chen W, Zhao J, Xi W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016;200:230–236. doi: 10.1016/j.foodchem.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 138.Yigitturk G, Acara A, Erbas O, Oltulu F, Yavasoglu N, Uysal A, et al. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed Pharmacother. 2017;87:240–246. doi: 10.1016/j.biopha.2016.12.102. [DOI] [PubMed] [Google Scholar]
- 139.Giftson Senapathy J, Jayanthi S, Viswanathan P, Umadevi P, Nalini N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1,2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats – a chemopreventive approach. Food Chem Toxicol. 2011;49:887–892. doi: 10.1016/j.fct.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 140.Ji B, Hsu W, Yang J, Hsia T, Lu C, Chiang J, et al. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J Agric Food Chem. 2009;57:7596–7604. doi: 10.1021/jf901308p. [DOI] [PubMed] [Google Scholar]
- 141.Kawada M, Ohno Y, Ri Y, Ikoma T, Yuugetu H, Asai T, et al. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anticancer Drugs. 2001;12:847–852. doi: 10.1097/00001813-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura E, Kurosaki F, Arisawa M, Mukainaka T, Takayasu J, Okuda M, et al. Cancer chemopreventive effects of a Brazilian folk medicine, Juca, on in vivo two-stage skin carcinogenesis. J Ethnopharmacol. 2002;81:135–137. doi: 10.1016/s0378-8741(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 143.Thiele W, Rothley M, Teller N, Jung N, Bulat B, Plaumann D, et al. Delphinidin is a novel inhibitor of lymphangiogenesis but promotes mammary tumor growth and metastasis formation in syngeneic experimental rats. Carcinogenesis. 2013;34:2804–2813. doi: 10.1093/carcin/bgt291. [DOI] [PubMed] [Google Scholar]