Abstract
Purpose
Treatment options are limited for patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL). Tumor cells can exploit the programmed death-1 checkpoint pathway to evade immune surveillance. In the current study, we evaluated the efficacy and safety of programmed death-1 blockade by nivolumab in patients with relapsed/refractory DLBCL.
Methods
In this phase II, open-label study, patients with relapsed/refractory DLBCL who were ineligible for autologous hematopoietic cell transplantation (auto-HCT) or who had experienced failure with auto-HCT received nivolumab 3 mg/kg every 2 weeks. We assessed the efficacy and safety of nivolumab as well as genetic alterations of 9p24.1.
Results
Among 121 treated patients, patients in the auto-HCT–failed cohort (n = 87) received a median of four nivolumab doses and a median of three doses were administered to those in the auto-HCT–ineligible cohort (n = 34). At a median follow-up of 9 months in the auto-HCT–failed cohort and 6 months in the auto-HCT–ineligible cohort, independently assessed objective response rates were 10% and 3%, and median durations of response were 11 and 8 months, respectively. Median progression-free survival and overall survival were 1.9 and 12.2 months in the auto-HCT–failed cohort and 1.4 and 5.8 months in the auto-HCT–ineligible cohort respectively. All three patients with complete remission—3% of the auto-HCT–failed cohort—had durable response (11 or more, 14 or more, and 17 months). Treatment-related grade 3 and 4 adverse events were reported in 24% of patients. The most common were neutropenia (4%), thrombocytopenia (3%), and increased lipase (3%). Of all evaluable samples for 9p24.1 analysis, 16% exhibited low-level copy gain and 3% had amplification.
Conclusion
Nivolumab monotherapy is associated with a favorable safety profile but a low overall response rate among patients with DLBCL who are ineligible for auto-HCT or who experienced failure with auto-HCT. Genetic alterations of 9p24.1 are infrequent in DLBCL.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma worldwide.1 With standard front-line therapy, approximately two thirds of adult patients achieve long-term remission and others who experience chemosensitive relapse may benefit from autologous hematopoietic cell transplantation (auto-HCT).2,3 However, patients with refractory DLBCL or those who are unsuitable for or who have experienced relapse after auto-HCT have limited treatment options,4 with a median survival of only 6 to 10 months from progression.3,5 Early results of chimeric antigen receptor T-cell therapy in small numbers of selected patients with refractory DLBCL are promising, but the technology is costly and long-term data are not available.6 Accessible treatments that provide durable responses and improved outcomes are needed in this setting.
Programmed death-1 (PD-1) and its ligands PD-L1/PD-L2 are immune checkpoints that, in healthy populations, downregulate immune response and are crucial for maintaining self-tolerance and preventing autoimmunity.7,8 Genes that encode PD-L1/PD-L2 are located on chromosome 9p24.1.9 Genetic alterations at the locus lead to ligand overexpression, which is common in Hodgkin lymphoma (HL) but are yet to be fully characterized in DLBCL.10-12 Overexpression of PD-L1 by tumor cells and on tumor-infiltrating nonmalignant cells in the tumor microenvironment has the potential to interact with PD-1–expressing T cells and B cells, which results in the inhibition of antitumor immune response.7,8 Increased PD-L1 expression has been found in certain defined subtypes of large B-cell lymphoma (LBCL), such as primary mediastinal B-cell lymphoma and Epstein-Barr virus (EBV)–positive and select non–germinal center cell DLBCLs, and is associated with inferior overall survival (OS).8,10,12,13 Thus, blockade of the PD-1/PD-L1 pathway may have the potential to exert antitumor effects in certain subsets of DLBCL.
Nivolumab is a fully human immunoglobulin G4 anti–PD-1 monoclonal antibody that blocks tumor cell signaling via the PD-1 pathway, which releases T cells from the inhibitory effects of tumor cells and restores T-cell–mediated antitumor immune responses.14 In clinical trials, nivolumab monotherapy has demonstrated activity in solid tumors, including melanoma, non–small-cell lung cancer, renal cell cancer, and bladder cancer, among others,15-18 and in relapsed/refractory classic HL (cHL).19,20 In a phase I dose-escalation study of nivolumab monotherapy in patients with relapsed/refractory hematologic malignancies, four of 11 patients with DLBCL demonstrated objective responses.21 These preliminary results led to this phase II study, which evaluated the efficacy and safety of nivolumab monotherapy in patients with relapsed/refractory DLBCL after auto-HCT or who were not candidates for auto-HCT.
METHODS
Study Design and Patients
This was a multicenter, single-arm, open-label, phase II study conducted in accordance with Good Clinical Practice and the Declaration of Helsinki and approved by the institutional review board and independent ethics committee. All patients provided written informed consent before trial enrollment.
Eligibility criteria included age 18 years or older and Eastern Cooperative Oncology Group performance status of 0 or 1. Patients had de novo DLBCL or transformed lymphoma (confirmed by biopsy before initiation of the study drug) that had either relapsed after high-dose conditioning chemotherapy and auto-HCT or was relapsed/refractory after two or more prior multiagent chemotherapy regimens if auto-HCT ineligible. Key exclusion criteria included prior therapy with any an antibody or drug specifically targeting T-cell costimulation or checkpoint pathways, prior allogeneic HCT, known CNS lymphoma, and history of interstitial lung disease.
Treatment
Patients received nivolumab 3 mg/kg intravenously over 60 minutes every 2 weeks until disease progression, unacceptable toxicity, or withdrawal from the study. Dose delays were permitted for drug-related adverse events (AEs), but dose escalations or reductions were not. Treatment beyond investigator-assessed disease progression was permitted in patients who did not experience rapid disease progression and who had investigator-determined clinical benefit from nivolumab and stable performance status.
Assessments
Efficacy.
Patients were evaluated for tumor response according to the 2007 International Working Group response criteria for malignant lymphoma22 using spiral computed tomography/magnetic resonance imaging. Evaluation was performed at baseline, beginning at week 9 and continuing every 8 weeks through the first 8 months of treatment, every 12 weeks during months 9 to 24, and then every 6 months thereafter until disease progression or until the patient initiated a preparative regimen for allogeneic or auto-HCT. Tumor assessments via fluorodeoxyglucose–positron emission tomography were performed at baseline and were required to confirm complete remission (CR). Baseline and all subsequent scans were submitted to an independent radiology review committee (IRC) for assessment.
Safety.
Safety evaluations included assessment of AEs, clinical laboratory tests, and physical examination with assessment of Eastern Cooperative Oncology Group performance status. Local laboratory assessments were performed within 72 hours before dosing. After discontinuation from the study, safety evaluations were scheduled at the first follow-up visit (35 days from the last dose) and the second follow-up (80 days from the first follow-up visit). Patients were then observed every 3 months for ongoing treatment-related AEs and survival.
Biomarkers.
In patients with archival tumor biopsies, 9p24.1 genetic alterations were evaluated using a fluorescence in situ hybridization (FISH) assay. Probes encompassed CD274 (PD-L1) or PDCD1LG2 (PD-L2) and included a centromeric control. Copy number alterations were defined as previously described11,23 on the basis of the target:control signal ratio in 50 analyzed tumor cells per DLBCL. Nuclei with a target:control signal ratio of 3 or more:1 were defined as coamplified for PD-L1 and PD-L2, and those with a signal ratio of more than 1:1 but less than 3:1 were classified as having relative copy gain. Nuclei with a signal ratio of 1:1 were defined as either polysomic if more than two copies per probe or disomic if there were exactly two copies of the target and control probes.11 For each patient, we noted the percentage and magnitude of 9p24.1 amplification, copy gain, polysomy, and disomy. Patients were classified by the highest observed level of 9p24.1 genetic alteration. Those with 9p24.1 copy gain lacked amplification and those with 9p polysomy lacked 9p24.1 copy gain or amplification. Chromosomal rearrangements that involved PD-L1 or PD-L2 were also detected with this assay.
We performed dual immunohistochemical staining of PD-L1 (clone 405.9A11)24 and PAX5 (24/Pax-5; BD Biosciences, San Jose, CA) to delineate PD-L1 expression on tumor biopsies, as previously described.19,23 H-score (0 to 300) was calculated by multiplying the percentage of malignant (PAX5dim+) cells with PD-L1–positive staining and the average intensity of staining (0 to 3 or more on 50 malignant cells).
Cell-of-origin (COO) assay by gene expression profiling was performed at Bristol-Myers Squibb by targeted expression directly from formalin-fixed, paraffin-embedded sections as previously described.25 The HTG EdgeSeq DLBCL Cell of Origin Assay comprised probes that targeted 93 genes commonly assessed in lymphomas. Cell Of Origin was assigned using HTG proprietary software (HTG Molecular Diagnostics, Tucson, AZ).
In situ hybridization was performed for EBV-encoded RNA-1 (EBER-1) using formalin-fixed, paraffin-embedded tissue samples. We tested RNA preservation using a nonspecific positive control, and background was assessed with a negative control. Scoring was performed for EBV/EBER if RNA was preserved and score was greater than background. Scores were EBV/EBER positive nuclear staining of any intensity greater than background in tumor cells.
Outcomes
The primary end point was objective response rate (ORR), defined as either partial remission (PR) or CR, as assessed by IRC using the 2007 revised International Working Group criteria. Secondary objectives included duration of response (DOR) on the basis of IRC assessments, IRC-assessed CR rate and duration of CR, IRC-assessed PR rate and duration of PR (DOPR), IRC-assessed progression-free survival (PFS), and investigator-assessed ORR. OS, safety and tolerability, and biomarker assessment were among additional exploratory end points.
Statistical Analyses
Planned sample size was approximately 120 treated patients, divided into two treatment groups on the basis of prior auto-HCT failure (n = 90) or auto-HCT ineligibility (n = 30). The primary analysis population for safety and efficacy was all patients who had received one or more dose of nivolumab. The protocol had an interim analysis that allowed for discontinuation of the study as a result of a lack of efficacy; however, accrual was rapid and the protocol allowed continued enrollment while the interim analysis was performed. This resulted in complete accrual before the interim analysis was concluded. For the auto-HCT–failed cohort, the null hypothesis that the true ORR was 20% or less would be rejected if 25 or more responses were observed in 90 treated patients. For the auto-HCT–ineligible cohort, the sample size of 30 treated patients was determined such that if the observed number of patients with objective response was 10, the true ORR was larger than 17% with 95% confidence.
IRC-assessed ORR, CR, and PR rates, duration of CR, DOPR, and investigator-assessed ORR were summarized for both cohorts separately by binomial response rates and their corresponding two-sided 95% CIs. IRC-assessed DOR, PFS, and OS were analyzed by cohort using the Kaplan-Meier product-limit method with median values and two-sided CI. Survival rate at 6 months was estimated using Kaplan-Meier values from the survival curve. AEs were coded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.26 All on-study AEs, serious AEs (SAEs), and treatment-related AEs and SAEs were tabulated according to worst grade per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0, by system organ class and Medical Dictionary for Regulatory Activities preferred term.
RESULTS
Patient Baseline Characteristics and Disposition
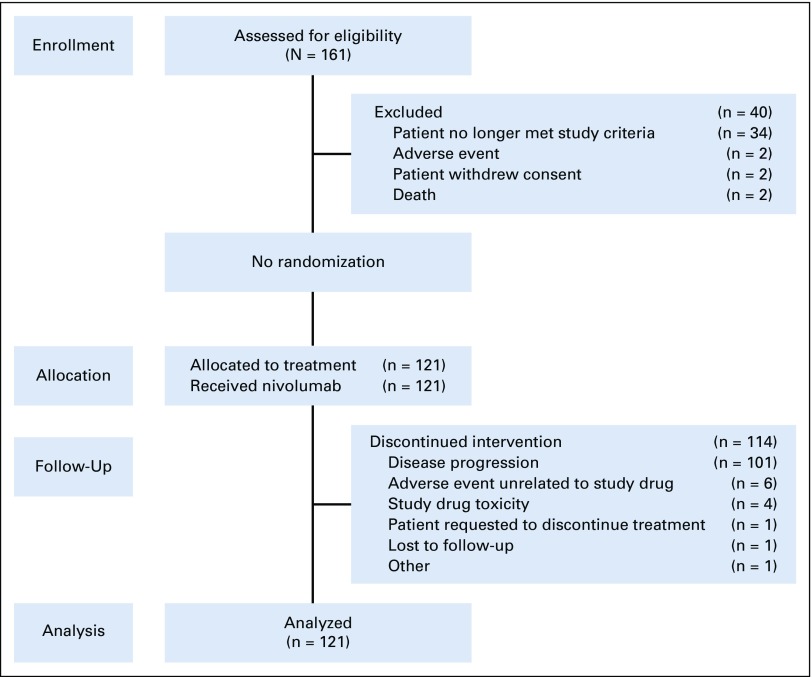
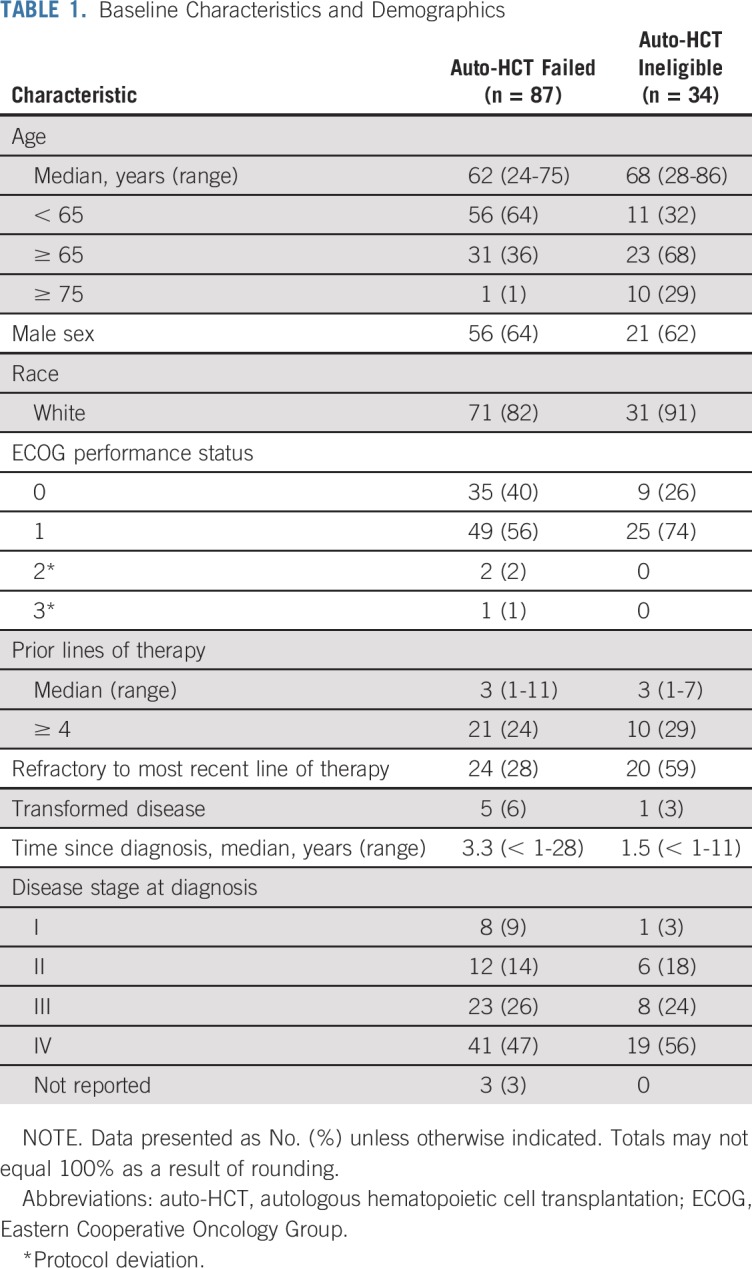
Patients were enrolled between March 2014 and August 2015 across 45 sites globally (Appendix Fig A1, online only). Of 121 patients treated, 87 had relapsed/refractory disease after auto-HCT and 34 were auto-HCT ineligible. Baseline characteristics of the two groups are listed in Table 1. In general, patients in the auto-HCT–failed group were younger and had better baseline performance status and longer time since diagnosis than did patients who were ineligible for auto-HCT. Patients had received a median of three prior systemic therapies in each cohort. In the auto-HCT–failed cohort, 40 patients (46%) did not receive any additional lines of systemic or radiation therapy between auto-HCT and the initiation of nivolumab. In the auto-HCT–failed group, 40% of patients had germinal center B-cell–like (GCB) subtype and 32% had non-GCB subtype disease, and in the auto-HCT–ineligible group, 56% had GCB and 18% non-GCB subtype disease. Data were missing in the remainder. All but one patient who had evaluable samples were EBV negative (70 of 121 patients), one patient was EBV positive, and the remainder (n = 50) had either nonevaluable data, mixed results (more than one sample), or missing data.
TABLE 1.
Baseline Characteristics and Demographics
Median number of doses of nivolumab received were four (range, one to 44 doses) in the auto-HCT–failed cohort and three (range, one to 22 doses) in the auto-HCT–ineligible cohort. At the time of analysis, seven patients (8%) in the auto-HCT–failed group were still on treatment, for whom the median duration of treatment was 14 months from the first study dose. No patients in the auto-HCT–ineligible cohort were still on treatment. Disease progression was the primary reason for discontinuing treatment (83%).
Efficacy
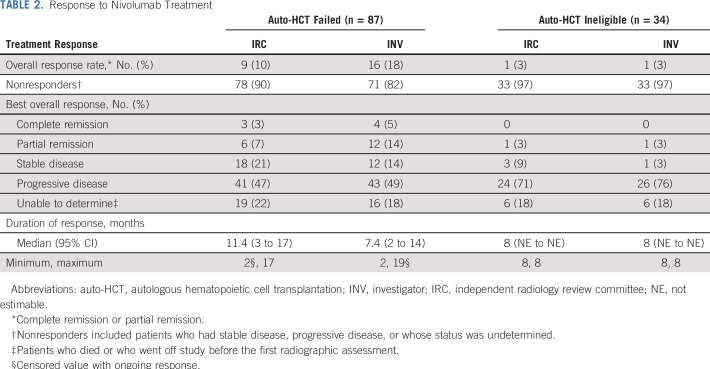
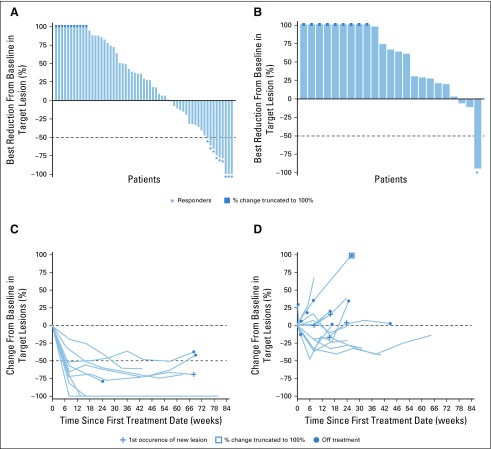
At a median follow-up of 9 months (range, 0.1 to 25 months) in the auto-HCT–failed cohort and 6 months (0.2 to 24 months) in the auto-HCT–ineligible cohort, IRC-assessed ORRs were 10% and 3%, respectively (Table 2). Nearly one third of patients (31%) in the auto-HCT–failed cohort had IRC-assessed stable disease or better response (Fig 1A). Of the nine responders in the auto-HCT–failed cohort, CR occurred in three, with durable response and DOR of 11 or more months, 14 or more months, and 17 months at data cutoff. Of these three patients who achieved CR, two were still on treatment and one developed myelodysplastic syndrome unrelated to study drug and subsequently died. The other six patients achieved PR, with a median DOPR of 7 months (95% CI, 3 to 11 months). The one patient with IRC-assessed PR in the auto-HCT–ineligible group had a DOR of 8.3 months. Patients who achieved CR or PR had early responses, with a median time to response of 1.9 months (Fig 1C). Responses were observed in both the GCB subtype (CR in one and PR in four patients) and non-GCB subtype (CR in one and PR in three patients). Among responders, eight were EBV negative, one was not evaluable, and one had missing data (Appendix Table A1, online only). One patient with PR had transformed lymphoma. Among the seven patients who were still on treatment, two had CR, two PR, and three stable disease. Nineteen patients in the auto-HCT–failed cohort and five patients in the auto-HCT–ineligible cohort were treated with nivolumab beyond disease progression—none achieved response after progression.
TABLE 2.
Response to Nivolumab Treatment
FIG 1.
Change in target lesion burden per independent radiology review committee by best overall response in all response-evaluable patients. Best reduction in target lesion in patients (A) who experienced failure with autologous hematopoietic cell transplantation (auto-HCT) and (B) who were ineligible for auto-HCT. Tumor burden change over time in patients (n = 9) of the auto-HCT–failed cohort who had (C) complete remission (CR) or partial remission (PR) and (D) stable disease (SD; n = 15). Horizontal reference line indicates 50% reduction consistent with a response per revised 2007 International Working Group criteria. Response-evaluable patients had assessments at baseline and at one or more postbaseline time point.
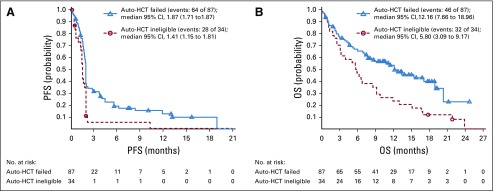
Median PFS was 1.9 months in the auto-HCT–failed cohort and 1.4 months in the auto-HCT–ineligible cohort, with a 6-month IRC-assessed PFS rate of 19.1% and 5.2%, respectively (Fig 2A). Median OS was 12.2 months in the auto-HCT–failed cohort and 5.8 months in the auto-HCT–ineligible cohort, and 6-month OS rate was 67% and 47%, respectively (Fig 2B).
FIG 2.
(A) Progression-free survival (PFS) and (B) overall survival (OS) as assessed by independent radiology review committee. Symbols represent censored observations. auto-HCT, autologous hematopoietic cell transplantation.
Safety
Grade 3 or 4 AEs were reported in 62% of all treated patients, of which 24% were deemed to be treatment related; none had a frequency of more than 5% (Table 3). The most common treatment-related AEs (TRAEs) were nausea (17%), fatigue (17%), and diarrhea (10%) —these were generally not severe (majority grade 1 to 2). Treatment-related SAEs were reported in 14 patients (12%). Four patients (3%) had TRAEs as the primary cause of discontinuation of treatment. TRAEs that contributed to treatment discontinuation included neutropenia, thrombocytopenia, diarrhea, pancreatitis, lipase increase, and dermatitis psoriasiform.
TABLE 3.
Drug-Related Adverse Events With an Incidence of ≥ 5%
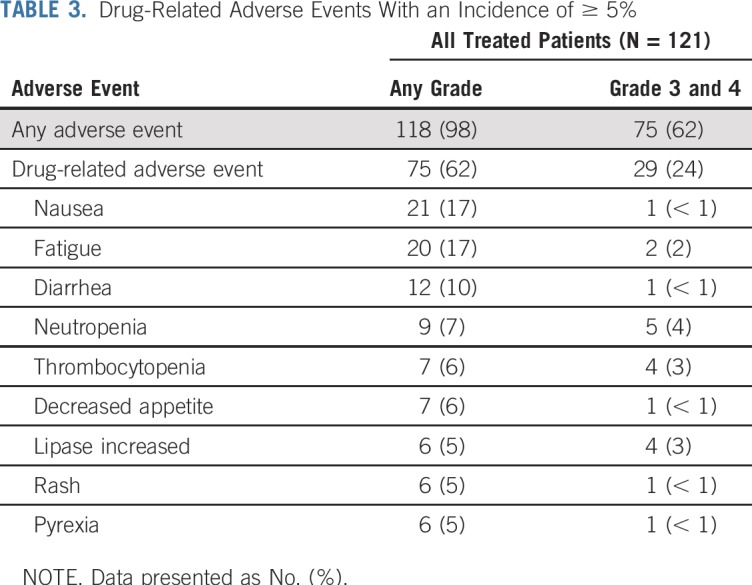
Rates of immune-mediated AEs were low in all treated patients with extended follow-up. The most common grade 3 to 4 immune-mediated AEs were nephritis and renal dysfunction (4%), hepatitis and hepatic dysfunction (3%), diarrhea (3%), and rash (2%).
Overall, 78 patients died. The primary reason for death was disease progression in 39 (45%) and 29 (85%) patients in the auto-HCT–failed and auto-HCT–ineligible cohorts, respectively. No death was attributable to study drug toxicity.
FISH and Immunohistochemistry
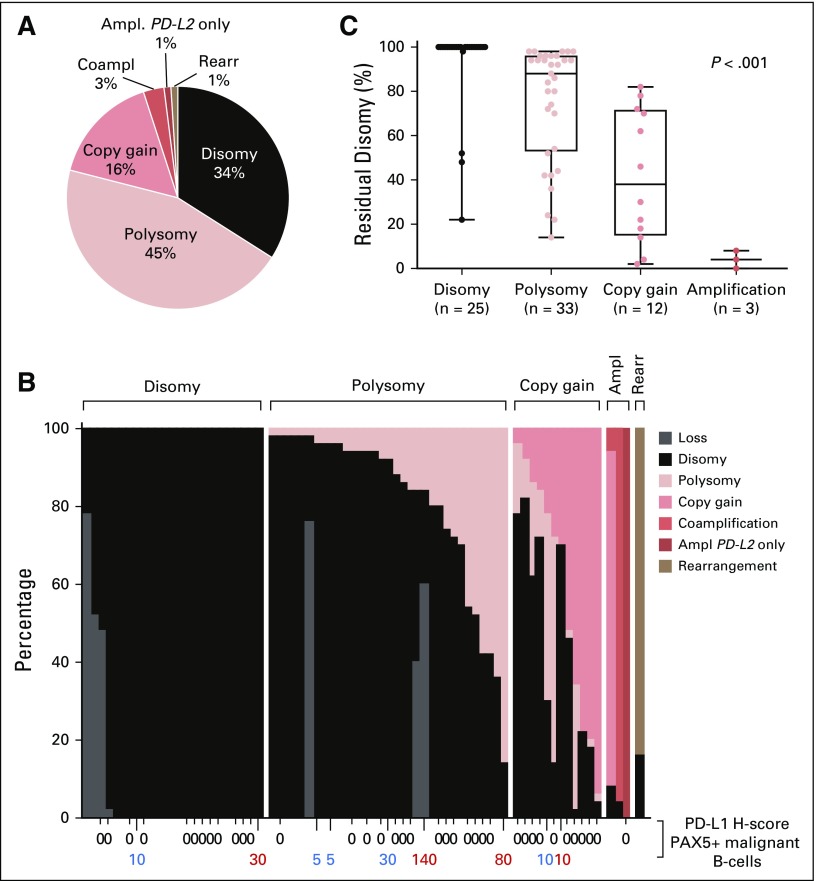
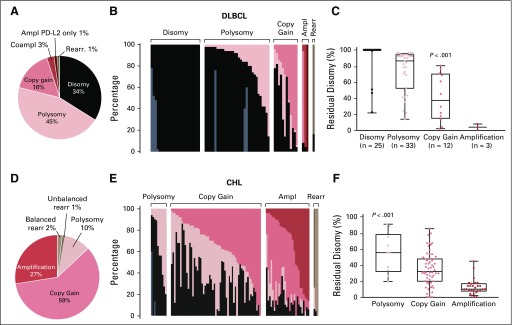
In total, 74 of 112 patients had evaluable tumor biopsy specimens for 9p24.1 FISH; 34% of DLBCL specimens were disomic and 45% were polysomic at PD-L1/PD-L2. PD-L1/PD-L2 polysomy in these DLBCLs was low level, largely only three copies in these tumors. Only 16% of DLBCLs exhibited copy gain, which was also low level in the majority of cases (three copies in nine of 13 cases). An additional 3% of tumors had amplification, including PD-L2–selective amplification in one patient. Patients were classified by the highest-level 9p24.1 alterations (Figs 3A and 3B), as previously described.11,23 DLBCLs with 9p24.1 amplification had additional tumor cells with copy gain (0% to 86%) and/or disomy (4% to 8%). Tumors with relative copy gain had additional cells with polysomy (2% to 58%) and/or disomy (2% to 82%), and DLBCLs that were categorized as polysomic had additional disomic tumor cells (14% to 98%; Fig 3B). The percentage of residual disomic cells was lowest in DLBCLs with amplification, intermediate in tumors with copy gain, and highest in tumors with polysomy (P < .001; Fig 3C), which is consistent with an ordered spectrum of 9p24.1 genetic alterations.
FIG 3.
Prevalence and spectrum of 9p24.1 genetic alterations and association of residual disomy with 9p24.1 genetic categories. (A) Prevalence of 9p24.1 genetic alterations in evaluated diffuse large B-cell lymphomas (DLBCLs). (B) Spectrum of 9p24.1 alterations in evaluated DLBCLs. Each patient is classified by the highest observed level of 9p24.1 alteration in tumor cells: polysomy, copy gain, amplification (Ampl), or rearrangement (Rearr). Individual patients are visualized as columns on the x-axis. Percentages of tumor cells with monosomy/relative copy loss (gray), disomy (black), polysomy (light red), copy gain (medium red), amplification (dark red), and rearrangement (brown) are depicted on the y-axis. In cases with evaluable 9p24.1 status and PD-L1 immunohistochemistry (n = 46), programmed death ligand 1 (PD-L1) expression (H-score) on PAX5-positive malignant B cells is indicated below the x-axis (membranous PD-L1 in red, cytoplasmic PD-L1 in blue). (C) Percentage of tumor cells with residual 9p24.1 disomy in DLBCLs classified by 9p24.1 genetic categories (P < .001 from ordinary one-way analysis of variance of unpaired t test data). Coampl, coamplification; PD-L2, programmed death ligand 2.
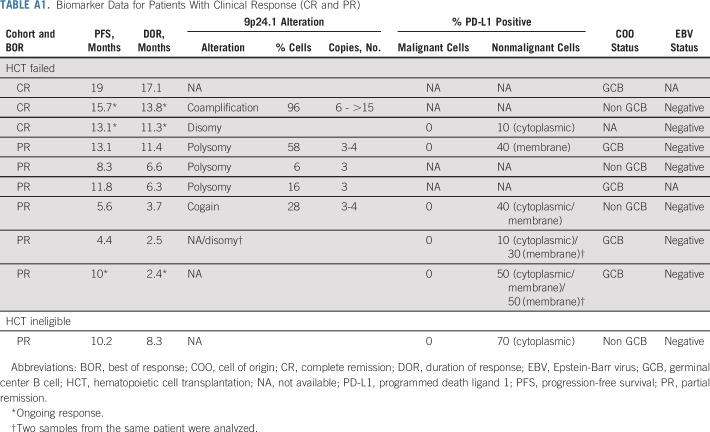
In this series of DLBCLs with infrequent low-level 9p24.1 alterations, membranous PD-L1 expression was only detected in four (9%) of 46 evaluable cases. One of the three patients who achieved CR had high-level 9p24.1 amplification, whereas the other two had normal 9p24.1 copy number or an unavailable biopsy specimen. Of the seven patients who achieved PR, five had available biopsy specimens and either normal 9p24.1 copy numbers (one patient), low-level polysomy (three patients), or copy gain (one patient). The five evaluable patients with PR had no detectable PD-L1 expression on tumor cells (Appendix Table A1).
DISCUSSION
In this phase II study, nivolumab monotherapy was associated with a low incidence of objective response in patients with relapsed/refractory DLBCL, and the study thus did not meet the primary end point. No new safety concerns were identified compared with previous studies that involved patients with solid tumors, HL, and non-HL, acknowledging that most patients received a limited number of doses.19,21,27
ORR was lower in the current phase II study than in the phase I study of patients with DLBCL who had received nivolumab monotherapy.21 This result may be attributed, in part, to differences in study design and patient population. We observed a higher response rate by investigator assessment than by IRC assessment in the phase II study population (Table 2). Centralized IRC assessment on the basis of imaging scans offers standardized and more objective evaluation. In addition, the number of patients with relapsed/refractory DLBCL (n = 11) was low in the phase I study, leading to wide CIs around observed response rates.21
Although ORR was low in this study, three patients who achieved CR had DORs of 17 months, 11 or more months, and 14 or more months; none of them had experienced progression at the data cutoff. Two were still alive at 30 and 38 months from first dose with their most recent survival status; however, the number of responders was too low to permit analysis of OS in this group. Whereas PD-L1/PD-L2 amplification in malignant cells was observed for one of these patients, the biologic basis for response in the other two patients is unclear.
Median number of nivolumab doses received (four in the auto-HCT–failed group and three in the auto-HCT–ineligible group) by patients with DLBCL was much lower than those received (16 and 17 doses) in studies that involved patients with relapsed/refractory HL,19,20 as the majority of the patients with DLBCL stopped treatment because of disease progression. Although it is possible that the rapid progression of DLBCL did not allow enough time for nivolumab to show an effect, we did not observe clear improvement of responses in the limited number of patients who had been treated beyond disease progression.
The study protocol included a prespecified stopping rule for futility but, on the basis of the safety profile of nivolumab and promising efficacy results observed in the phase I study, allowed enrollment to continue while the interim analysis result was pending. As a result of rapid accrual and delays related to IRC, the planned enrollment was reached before the futility analysis was concluded. Nevertheless, the majority of patients received only a short duration of nivolumab treatment and were allowed to switch to other cancer therapies upon discontinuation of the study treatment; there was no new safety signal observed. In the future, we shall ensure that study procedures are closely monitored to avoid potential delays in the timely completion of the interim analysis.
Previously reported data suggest that PD-L1 expression is associated with poor prognosis in patients with DLBCL8; however, the prevalence of PD-L1 expression on DLBCL tumor cells seemed to be low (11%; with a 30% threshold) in that series,8 as well as in a EBV-negative DLBCL series (11%; with a 5% threshold).10 The 9p24.1 FISH analyses on a large number of DLBCL trial cases in this study indicate that the incidence and magnitude of 9p24.1 alterations are significantly lower in DLBCL than in cHL, as is the level of PD-L1 expression20,23 (H-score; Appendix Fig A2, online only). These observations are consistent with the observed differences in the efficacy of PD-1 blockade in DLBCL and cHL and provide a possible explanation for why only a small proportion of patients with relapsed/refractory DLBCL benefit from nivolumab monotherapy. As a result of the low number of responders, there were insufficient data to accurately assess double-hit or c-Myc association with responses in this study.
Whereas the single-agent activity of PD-1 blockade was low in this unselected series of patients with DLBCL, it may be useful to evaluate the approach in select LBCL subtypes with increased PD-L1 expression, such as primary mediastinal B-cell lymphoma, EBV-positive LBCL, and T-cell/histiocyte-rich LBCL.10 Recent reports suggest that combination treatment strategies, such as chimeric antigen receptor T cells and nivolumab28 and PD-1 inhibition with concomitant radiotherapy,29 might also be considered.
In conclusion, the results of this phase II study indicate that nivolumab monotherapy has a low response rate but may provide benefit in a small number of patients with DLBCL who have experienced failure with auto-HCT or who are ineligible for auto-HCT and is associated with a favorable safety profile. Additional research on the influence of biologic factors, including the tumor microenvironment, is warranted to characterize whether a subset of patients with DLBCL may be more likely to respond to nivolumab-based treatment, if combination therapy can improve the therapeutic activity of PD-1 blockade, or if earlier use of these agents before intensive immunoablative chemotherapy may be more effective.
ACKNOWLEDGMENT
The authors thank all coinvestigators and the patients and families who participated in the trial. Writing assistance was provided by Janice Zhou, PhD, at Caudex, under the direction of the authors, and was funded by Bristol-Myers Squibb.
Appendix
FIG A1.
Flow diagram.
FIG A2.
Comparison of the incidence and magnitude of 9p24.1 alterations in diffuse large B-cell lymphoma (DLBCL; this study) and classic Hodgkin lymphoma (cHL).23 (A) Prevalence and (B) spectrum of 9p24.1 alterations in DLBCL. (C) Percentage of tumor cells with residual 9p24.1 disomy in DLBCLs classified by 9p24.1 genetic categories. (D) Prevalence and (E) spectrum of 9p24.1 alterations in cHL.23 (F) Percentage of tumor cells with residual 9p24.1 disomy in cHLs classified by 9p24.1 genetic categories.23 As noted, DLBCLs have a much lower incidence of 9p24.1 copy gain and amplification than cHLs. In addition, DLBCLs with low-level 9p24.1 alterations have a higher percentage of residual disomy than cHLs. Ampl, amplification; Coampl, coamplification; PD-L2, programmed death ligand 2; Rearr, rearrangement. [Reprinted with permission. © 2018 American Society of Clinical Oncology.]
TABLE A1.
Biomarker Data for Patients With Clinical Response (CR and PR)
Footnotes
Supported by Bristol-Myers Squibb, which also funded medical writing support. Also supported by the Harold and Virginia Lash Foundation (P.A.); US National Institutes of Health Grant No. R01CA161026 and the Miller Fund (M.A.S.); the Center for Immuno-Oncology of the Dana-Farber Cancer Institute (S.J.R.); and the American Society of Hematology and Lymphoma Research Foundation (388017; J.B.C.).
The views expressed in this article are the authors’ own and not an official position of Bristol-Myers Squibb or their respective institutions.
Clinical trials information: NCT02038933.
AUTHOR CONTRIBUTIONS
Conception and design: Stephen M. Ansell, Peter Johnson, Philippe Armand, Margaret A. Shipp, Scott J. Rodig, Kazunobu Kato
Administrative support: Nishitha Reddy
Provision of study materials or patients: Monique C. Minnema, Peter Johnson, Nishitha Reddy, Jonathon B. Cohen, Sarit Assouline, Michelle Poon, Selda Samakoglu
Collection and assembly of data: Stephen M. Ansell, Monique C. Minnema, Peter Johnson, John M. Timmerman, Philippe Armand, Margaret A. Shipp, Scott J. Rodig, Margaretha G.M. Roemer, Nishitha Reddy, Jonathon B. Cohen, Sarit Assouline, Michelle Poon, Manish Sharma, Andrew Grigg
Data analysis and interpretation: Stephen M. Ansell, Monique C. Minnema, Peter Johnson, John M. Timmerman, Margaret A. Shipp, Scott J. Rodig, Azra H. Ligon, Margaretha G.M. Roemer, Nishitha Reddy, Sarit Assouline, Manish Sharma, Kazunobu Kato, Selda Samakoglu, Anne Sumbul, Andrew Grigg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab for Relapsed/Refractory Diffuse Large B-Cell Lymphoma in Patients Ineligible for or Having Failed Autologous Transplantation: A Single-Arm, Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Stephen M. Ansell
Honoraria: WebMD, Research to Practice
Research Funding: Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Affimed Therapeutics (Inst), Regeneron (Inst), Pfizer (Inst), LAM Therapeutics (Inst), Trillium Therapeutics (Inst)
Monique C. Minnema
Consulting or Advisory Role: Servier, Takeda, Amgen, Janssen-Cilag
Research Funding: Celgene
Travel, Accommodations, Expenses: Roche, Amgen
Peter Johnson
Honoraria: Takeda, Bristol-Myers Squibb, Novartis, Celgene, Kite Pharma, Genmab, Incyte
Consulting or Advisory Role: Janssen Pharmaceuticals, Epizyme, Boehringer Ingelheim
Research Funding: Epizyme (Inst), Janssen Pharmaceuticals (Inst)
Patents, Royalties, Other Intellectual Property: Combined use of Fc gamma RIIb (CD32b) and CD20, specific antibodies, WO Patent, PCT/GB2011/051572; EU11760819.0
Travel, Accommodations, Expenses: Zenyaku Kogyo
John M. Timmerman
Stock and Other Ownership Interests: Biomarin
Honoraria: Kite Pharma, Gilead Sciences
Consulting or Advisory Role: Celgene, Seattle Genetics, Genmab
Research Funding: Bristol-Myers Squibb, ImmunGene, Kite Pharma, Merck
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Phillippe Armand
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Pfizer, Affimed Therapeutics, Adaptive Biotechnologies
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Affimed Therapeutics (Inst), Adaptive Biotechnologies (Inst), Genentech (Inst), Tensha Therapeutics (Inst), Otsuka (Inst), Sigma-Tau (Inst)
Travel, Accommodations, Expenses: Genmab
Margaret A. Shipp
Honoraria: Bristol-Myers Squibb, AstraZeneca
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst), Bayer (Inst), Merck (Inst)
Scott J. Rodig
Honoraria: PerkinElmer, Bristol-Myers Squibb
Consulting or Advisory Role: AstraZeneca, PerkinElmer
Research Funding: Bristol-Myers Squibb, Merck, Affimed Therapeutics, Kite Pharma
Patents, Royalties, Other Intellectual Property: Patent pending for use of anti-galectin1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche, Bristol-Myers Squibb
Azra H. Ligon
Leadership: Travera (I)
Stock and Other Ownership Interests: Travera (I)
Consulting or Advisory Role: Travera (I)
Nishitha Reddy
Consulting or Advisory Role: Celgene, AbbVie, Bristol-Myers Squibb, Adaptive Biotechnologies
Speakers' Bureau: Gilead Sciences
Research Funding: Bristol-Myers Squibb (Inst)
Jonathon B. Cohen
Consulting or Advisory Role: Pharmacyclics, Celgene, Millennium Pharmaceuticals, Seattle Genetics, Novartis, Infinity Pharmaceuticals, AbbVie
Research Funding: Bristol-Myers Squibb, Janssen Pharmaceuticals, Novartis, Takeda
Sarit Assouline
Stock and Other Ownership Interests: Knight Pharmaceuticals
Honoraria: Janssen Oncology, Pfizer
Speakers' Bureau: Pfizer, Janssen Oncology, Bristol-Myers Squibb
Research Funding: Roche Canada, Takeda, Epizyme, Gilead Sciences, Astex Pharmaceuticals, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Roche Canada
Michelle Poon
Consulting or Advisory Role: Takeda, Janssen Pharmaceuticals, AbbVie
Travel, Accommodations, Expenses: Roche
Manish Sharma
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
Kazunobu Kato
Employment: Bristol-Myers Squibb, Celgene
Stock and Other Ownership Interests: Bristol-Myers Squibb, Celgene
Anne Sumbul
Employment: Bristol-Myers Squibb
Andrew Grigg
Honoraria: Gilead Sciences, MSD Oncology
Consulting or Advisory Role: Gilead Sciences, Janssen-Cilag, Roche, Takeda, MSD Oncology
Expert Testimony: MSD Oncology
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported
REFERENCES
- 1.International Agency for Research on Cancer : World Cancer Report 2014: Diffuse Large B-cell Lymphoma. Geneva, Switzerland, WHO Press, 2014 [Google Scholar]
- 2.Friedberg JW: Relapsed/refractory diffuse large B-cell lymphoma. Hematology (Am Soc Hematol Educ Program) 2011:498-505, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Crump M, Neelapu SS, Farooq U, et al. : Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 130:1800-1808, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network : NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s lymphomas, version 4.2014. https://www.nccn.org/about/nhl.pdf
- 5.Nagle SJ, Woo K, Schuster SJ, et al. : Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 88:890-894, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Neelapu SS, Locke FL, Bartlett NL, et al. : Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377:2531-2544, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman GJ, Long AJ, Iwai Y, et al. : Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192:1027-1034, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiyasu J, Miyoshi H, Hirata A, et al. : Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood 126:2193-2201, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green MR, Monti S, Rodig SJ, et al. : Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116:3268-3277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen BJ, Chapuy B, Ouyang J, et al. : PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res 19:3462-3473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roemer MG, Advani RH, Ligon AH, et al. : PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 34:2690-2697, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lenz G, Wright GW, Emre NC, et al. : Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci USA 105:13520-13525, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andorsky DJ, Yamada RE, Said J, et al. : Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res 17:4232-4244, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Bristol-Myers Squibb : Nivolumab (Opdivo) prescribing information. https://packageinserts.bms.com/pi/pi_opdivo.pdf
- 15.Weber JS, D’Angelo SP, Minor D, et al. : Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375-384, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Callahan MK, Bono P, et al. : Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): A multicentre, open-label, two-stage, multi-arm, phase 1/2 trial. Lancet Oncol 17:1590-1598, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansell SM, Lesokhin AM, Borrello I, et al. : PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372:311-319, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younes A, Santoro A, Shipp M, et al. : Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17:1283-1294, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesokhin AM, Ansell SM, Armand P, et al. : Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase Ib study. J Clin Oncol 34:2698-2704, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, et al. : Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Roemer MGM, Redd RA, Cader FZ, et al. : Major histocompatibility complex class II and programmed death ligand 1 expression predict outcome after programmed death 1 blockade in classic Hodgkin lymphoma. J Clin Oncol 36:942-950, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahoney KM, Sun H, Liao X, et al. : PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol Res 3:1308-1315, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu-Monette ZY, Thompson D, LaFleur B, et al. : Diffuse large B-cell lymphoma cell of origin determination from formalin-fixed paraffin-embedded tissues. Blood 126:5052, 2015. (suppl) [Google Scholar]
- 26.US National Cancer Institute : Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Bethesda, MD, National Institutes of Health, 2009 [Google Scholar]
- 27.Topalian SL, Hodi FS, Brahmer JR, et al. : Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443-2454, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill BT, Roberts ZJ, Rossi JM, et al. : Marked re-expansion of chimeric antigen receptor (CAR) T cells and tumor regression following nivolumab treatment in a patient treated with axicabtagene ciloleucel (axi-cel; KTE-C19) for refractory diffuse large B cell lymphoma (DLBCL). Blood 130:2825, 2017. (suppl) [Google Scholar]
- 29.Wight JC, Hawkes EA, Berlangieri SU, et al. : An abscopal effect may augment PD-1 inhibition in refractory classical Hodgkin lymphoma. Leuk Lymphoma 10.1080/10428194.2018.1452217 [epub ahead of print on March 23, 2018] [DOI] [PubMed] [Google Scholar]