Abstract
PURPOSE
Essential cancer medicine stock outs are occurring at an increasing frequency worldwide and represent a potential barrier to delivery of standard therapy in patients with cancer in low- and middle-income countries. The objective of this study was to measure the impact of cancer medicine stock outs on delivery of optimal therapy in Botswana.
METHODS
We conducted a retrospective analysis of patients with common solid tumor malignancies who received systemic cancer therapy in 2016 at Princess Marina Hospital, Gaborone, Botswana. Primary exposure was the duration of cancer medicine stock out during a treatment cycle interval, when the cancer therapy was intended to be administered. Mixed-effects univariable and multivariable logistic regression analyses were used to calculate the association of the primary exposure, with the primary outcome, suboptimal therapy delivery, defined as any dose reduction, dose delay, missed cycle, or switch in intended therapy.
RESULTS
A total of 378 patients met diagnostic criteria and received systemic chemotherapy in 2016. Of these, 76% received standard regimens consisting of 1,452 cycle intervals and were included in this analysis. Paclitaxel stock out affected the highest proportion of patients. In multivariable mixed-effects logistic regression, each week of any medicine stock out (odds ratio, 1.9; 95% CI, 1.7 to 2.13; P < .001) was independently associated with an increased risk of a suboptimal therapy delivery event.
CONCLUSION
Each week of cancer therapy stock out poses a substantial barrier to receipt of high-quality cancer therapy in low- and middle-income countries. A concerted effort between policymakers and cancer specialists is needed to design implementation strategies to build sustainable systems promoting a reliable supply of cancer medicines.
INTRODUCTION
Recent updates to the WHO Essential Medicines List (EML) have included an expansion of essential cancer medicines in an effort to increase access to cancer medicines, especially in low- and middle-income countries (LMICs).1 Despite these efforts, essential cancer medicine stock outs are occurring at a high frequency worldwide and represent a complex global issue.2-8 The Millennium Development Goal Gap Task Force report in 2015 called attention to low access to essential health products in LMICs, with on average 58.1% of generic medicines available in public sector and 66.6% available in private sector facilities.9
Research in sub-Saharan Africa (SSA) has identified weak infrastructure along the supply chain, including procurement and distribution,10 inadequate drug supply and lack of trained personnel,10,11 and inaccurate demand forecasting as mechanisms for stock outs.8,12 Previous studies have reported extended stock-out duration for essential medicines ranging from a mean of 1 month for cancer medicines, 6 months for antipneumonia and antimalarial therapy, and up to 76 days for combination antiretroviral therapies (ARTs).8,10,13 Among HIV-infected patients studied in Cote d’Ivoire, ART stock outs that resulted in treatment discontinuation were independently associated with a significantly higher risk of interruption in care or death13; in a small cross-sectional study in Nigeria, they were associated with significantly higher rates of drug resistance mutations.14 Furthermore, drug stock outs also resulted in an increase in the financial burden of care in a recent study in Tanzania showing that stock outs resulted in a 21% increase in the cost of care for malaria when compared with periods without stock outs.15
In Botswana and other LMICs where cancer incidence and mortality are increasing,16 chemotherapy stock outs potentially present a significant barrier to standard therapy delivery, leading to adverse disease outcomes (including cancer recurrence and survival), thereby impeding efforts to address the global cancer epidemic. Although stock outs of essential medicines for cancer are prevalent in SSA, the impact of these stock outs on the adequacy of therapy delivery and subsequent disease outcomes in patients with cancer in the region has not been studied. A prior study showed that more than 80% of the drugs included in the proposed 2015 WHO EML for cancer were also included in the Botswana national EML, and 40% of these drugs were out of stock for a median of 30 days.8 The objective of this study was to assess the impact of cancer medicine stock outs on delivery of optimal therapy for patients with cancer in Botswana.
METHODS
Study Design and Population
We conducted a retrospective cohort study of patients diagnosed with any of the 10 most commonly diagnosed and treatable solid tumor malignancies in Botswana: cervical, breast, prostate, esophageal, lung, uterine, ovarian, colorectal, and head and neck cancers and Kaposi sarcoma. Patients were included in the study if they were age 18 years or older and had been diagnosed with any of these solid malignancies, regardless of the date of diagnosis, and received at least one dose of systemic chemotherapy from January 1, 2016, to December 31, 2016, at the Princess Marina Hospital (PMH) in Gaborone, Botswana. This site was selected because it is the largest cancer care provider in the country. Institutional review and ethics boards at the University of Pennsylvania and Botswana Ministry of Health approved this study.
Measures and Definitions
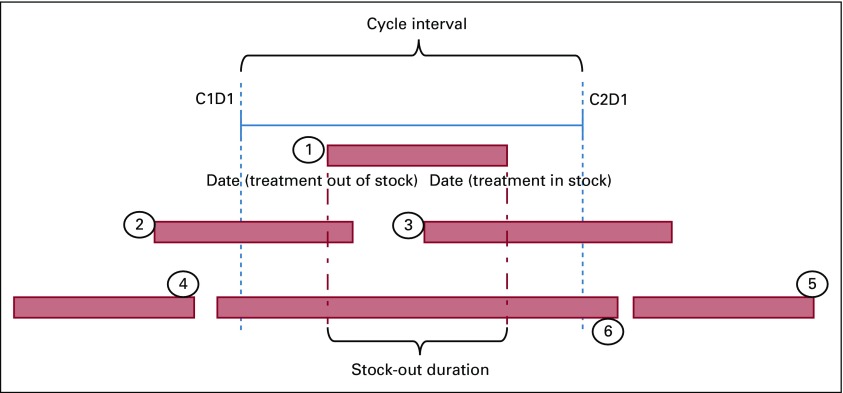
The measured exposure was chemotherapy stock out, quantified as the duration of chemotherapy stock out within a cycle interval (Figs 1 and 2). Dates of stock outs were obtained from drug stock and availability data prospectively reported by the Central Medical Stores (CMS) in Botswana, a semiautonomous agency responsible for tendering, procurement, and distribution of all medicines in the public sector. Stock-out duration was calculated by counting the days from the date the drug was out of stock to the date it was recorded as being back in stock. Complete chemotherapy administration data, including dates and doses administered, were obtained from patient records and a pharmacy log book with data on all chemotherapy administered in the hospital in 2016. The exposure window was calculated as the duration of chemotherapy stock out within a given chemotherapy cycle interval. We calculated the duration of a stock out by generating a code for six different permutations of possible patterns of chemotherapy stock out in association with a given cycle (Fig 2). Pattern 1 represents a stock out occurring after cycle 1 (C1) and before C2. Patterns 2 and 4, occurring before C1, were not captured as part of our exposure, because we were unable to determine whether therapy was initiated on time or delayed; therefore, our analysis was limited to the exposure window once therapy was initiated. Patterns 3 and 6 represent periods where CMS drug stock outs are reported; however, the local pharmacy may still have some supply in stock, and therefore, patient care may not be affected despite the central stock outs. Pattern 5 was not included in our exposure for C2, day 1 (C2D1), because it occurs after C2D1 has been administered. Delineating these patterns was designed to ensure that the duration of stock out was calculated specific to a cycle interval to more accurately assess association between the duration of stock out and the therapy delivery event within that cycle. The codes were executed in STATA software (STATA, College Station, TX) to generate the number of days of cancer medicine stock out per given cycle interval (Fig 2). If more than one medicine stock out occurred during a cycle, the greater number of stock-out days was assigned as the exposure.
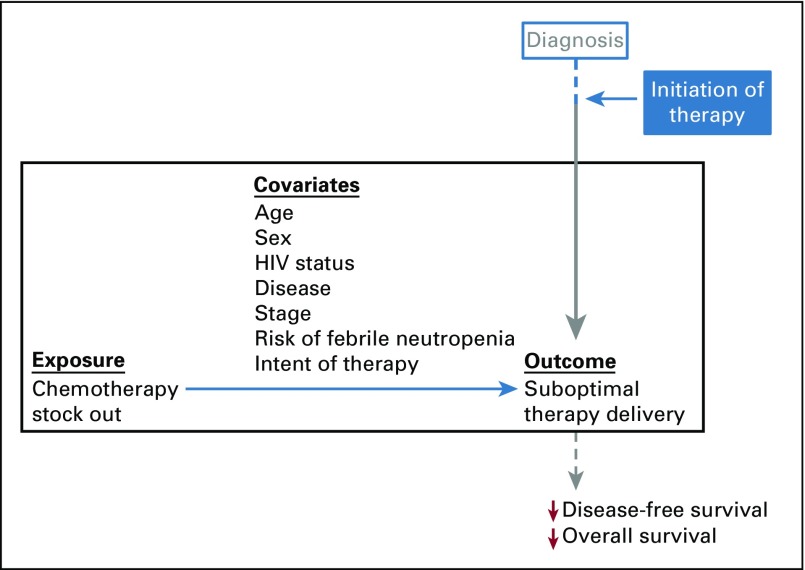
FIG 1.
Study schematic highlighting exposure, measured covariates, and suboptimal therapy delivery, along the treatment pathway from diagnosis to survival outcomes.
FIG 2.
Suboptimal therapy delivery and stock-out metrics. C1D1 represents cycle 1 day 1, and C2D2 represents cycle 2 day 2 of a given regimen. C1D1 to C2D1 represents the cycle interval between cycles 1 and 2. The numbered scenarios represent different ways in which stock out can occur during a given cycle: (1) midinterval stock out; (2) stock out occurring before and extending during the cycle interval; (3) stock out occurring during the cycle and extending post cycle interval; (4) stock out occurring prior to the cycle; (5) stock out occurring after the cycle; and (6) stock out affecting the entire duration of the cycle interval.
The primary outcome, suboptimal therapy delivery, was defined as any of the following events: any dose reduction, at least 1-week delay in receipt of therapy, any missed dose, and any switch in intended therapy. We used the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology and the WHO supplemental guidelines to define standard chemotherapy regimens.17,18
We assessed several covariates, including patient demographics (age, sex), medical comorbidities (HIV, diabetes, hypertension, tuberculosis, cardiac disease), and cancer characteristics (primary diagnosis, stage at diagnosis, molecular phenotype [estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 status for patients with breast cancer]), which were extracted from patient paper and electronic medical records where available, and indication for current therapy (adjuvant v metastatic setting). Neutropenia, a serious complication of systemic chemotherapy that can lead to clinically indicated delays in treatment, was not routinely documented in the patient records. Therefore, we implemented a surrogate measure of this potential confounding factor using the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology to identify regimens with high risk for febrile neutropenia (> 20%).17
Statistical Analysis
Descriptive statistics were used to summarize baseline characteristics and covariates for all patients. Two-sample t test and analysis of variance were used to test the difference in mean stock out per cycle for the overall group and stratified by the selected covariates. Mixed-effects logistic regression was used to analyze the association between duration of specific cancer medicine stock outs, covariates, and risk of suboptimal therapy. The covariates associated with both exposure and outcome in the univariable analyses with a P value of less than .1 were included in the mixed-effects multivariable logistic regression model to adjust for possible confounding.19 We developed the multivariable modeling using a forward regression analysis. Age was included in our regression analysis as a dichotomous variable using the age groups of younger than 65 years and 65 years or older, because age 65 years or older has been listed in prior studies as a predictor of low dose-intensity cancer therapy.20 The regimens were coded based on whether they were associated with a high risk for febrile neutropenia and included as a covariate in our analysis.21
RESULTS
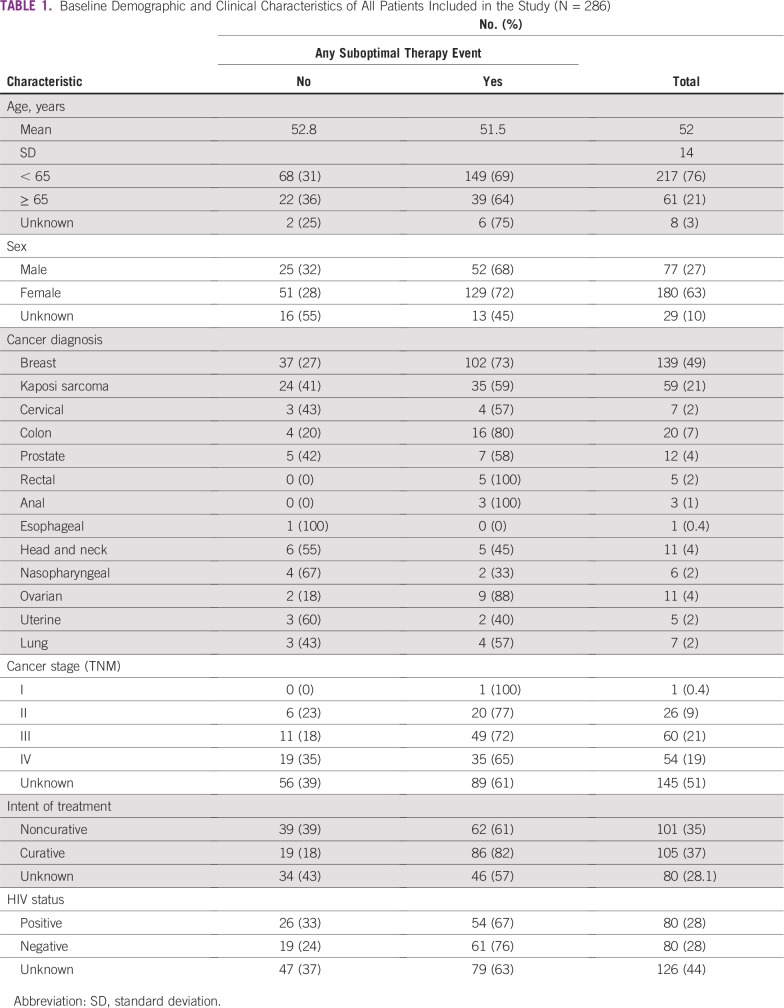
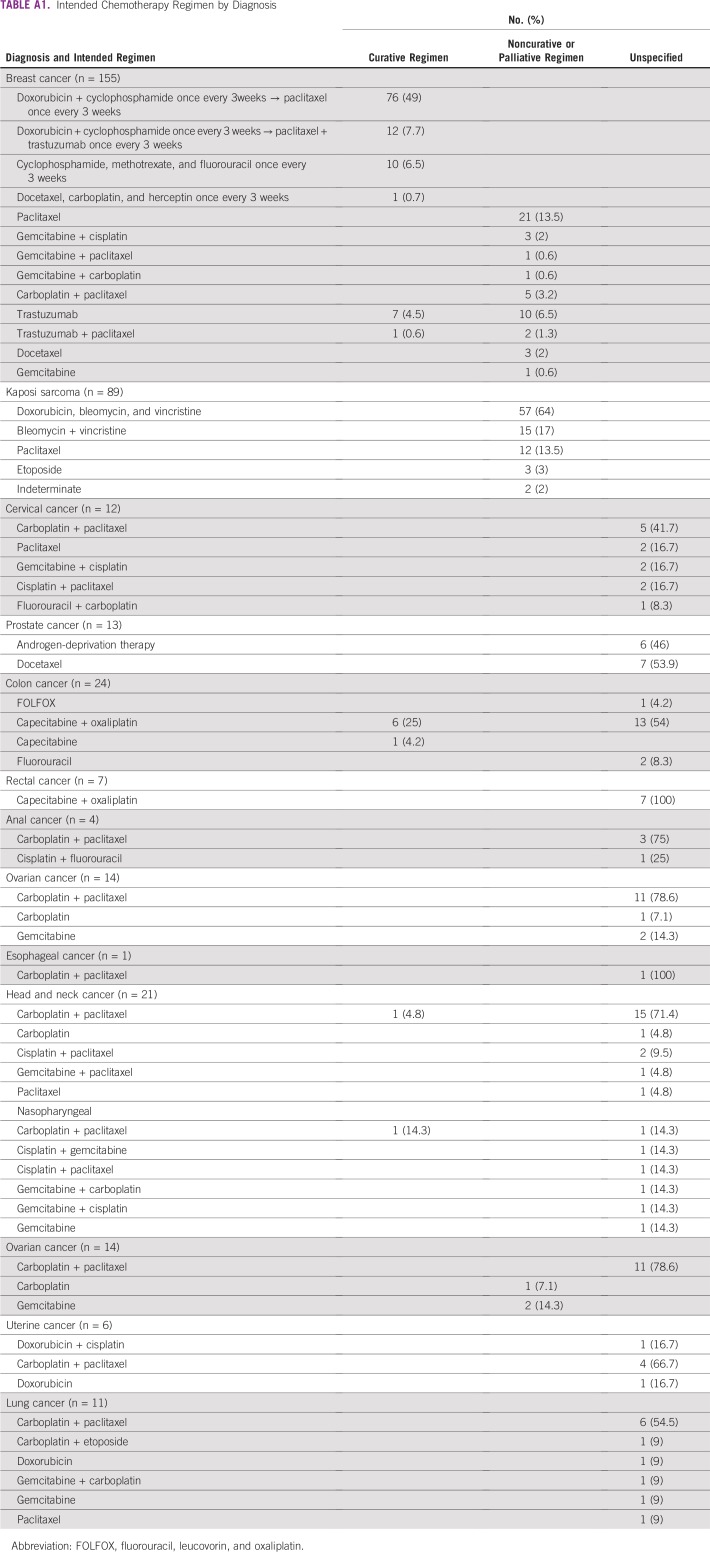
We identified 378 patients who met diagnostic and age criteria for our study and who had received at least one dose of systemic chemotherapy at PMH between January 1, 2016, and December 31, 2016. Of these, 286 (76%) were administered therapy on a standard regimen consisting of 1,452 cycle intervals and were included in our receipt of optimal therapy analysis (Table 1; Appendix Table A1). The median age at diagnosis for our sample was 51.8 years. More than 70% of our patients were younger than 65 years. Additionally, almost half of the patients included in our analysis had a diagnosis of breast cancer. A majority of the patients with stage information had either stage III or IV disease. Of patients with known intent of treatment, 51% were receiving curative regimens and 49% were receiving noncurative regimens. Of those who had information regarding HIV status (57% of patients in our analysis), 51% were HIV positive. The patient medical records had limited data on other medical comorbid illnesses.
TABLE 1.
Baseline Demographic and Clinical Characteristics of All Patients Included in the Study (N = 286)
Cancer Medicine Stock-Out Analysis
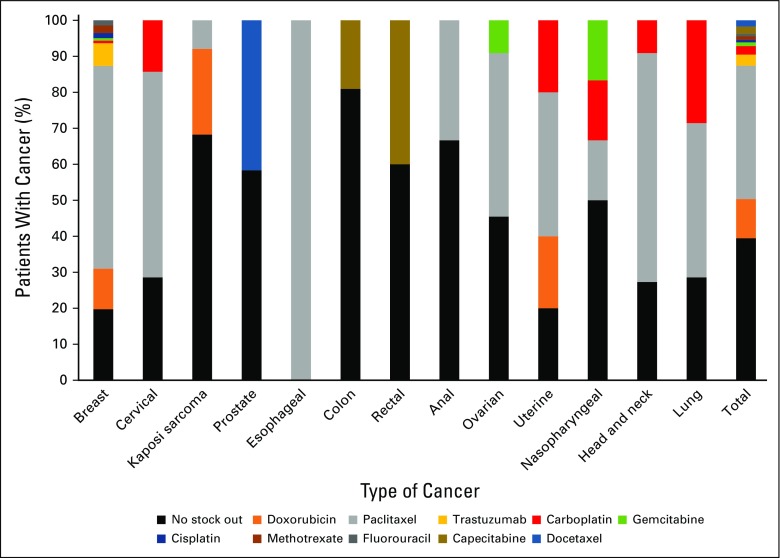
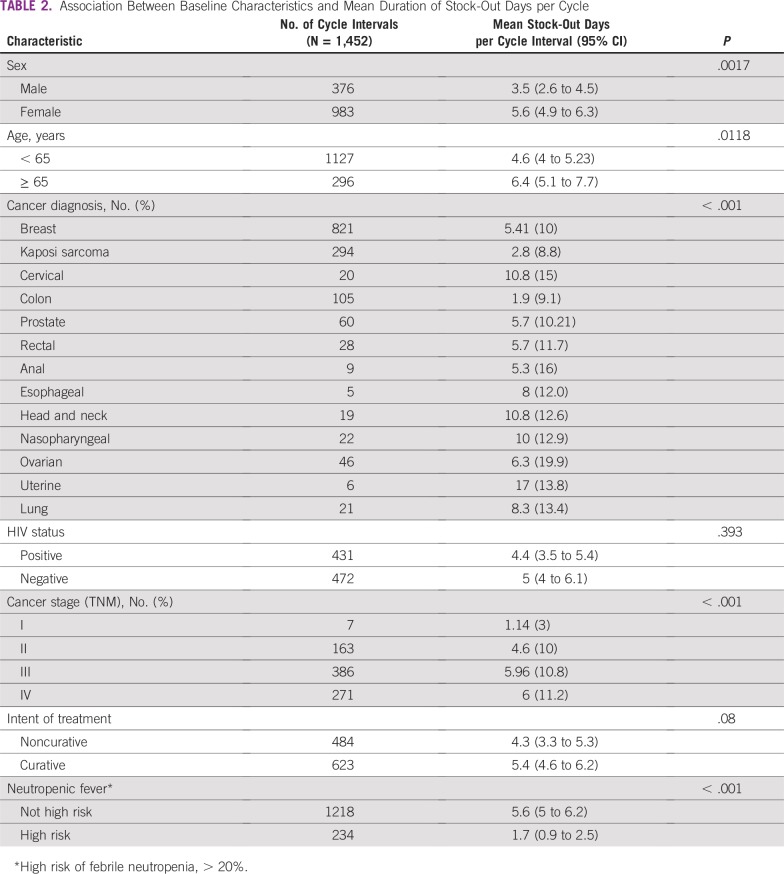
Thirty-nine percent of patients had no cancer therapy stock out of their cancer regimen drugs during the course of their treatment (Fig 3). Capecitabine, carboplatin, cisplatin, docetaxel, doxorubicin, gemcitabine, fluorouracil, methotrexate, and trastuzumab stock outs affected patients receiving therapy in 2016 (Fig 3). Paclitaxel was out of stock during the treatment cycle for 37% of patients for whom it was prescribed. Of all patients who experienced a medicine stock out during treatment, 64% had a diagnosis of breast cancer. Of adjuvant chemotherapy cycle intervals that resulted in a suboptimal therapy delivery event, 42% occurred when there was a cancer medicine stock out, compared with 41% in the metastatic setting. The median and mean durations of stock out per treatment cycle interval for cancer medicines that were out of stock were 16 and 18 days, respectively (standard deviation, 13 days), with a range of 1 to 122 days. In stratified analyses of stock-out duration by measured covariates, women, older patients, those receiving regimens used in the metastatic setting, and those receiving non–high-risk febrile neutropenia regimens were affected by medicines that had significantly longer durations of stock out (Table 2).
FIG 3.
Proportions of patients with cancer affected by specific cancer medicine stock outs.
TABLE 2.
Association Between Baseline Characteristics and Mean Duration of Stock-Out Days per Cycle
Impact on Therapy Delivery
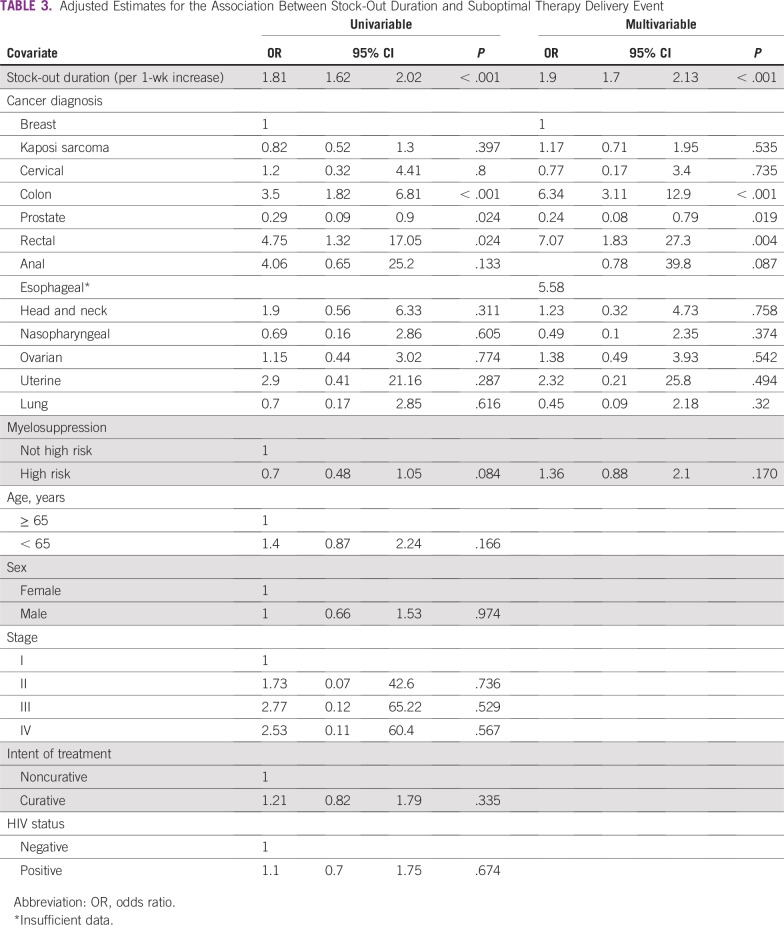
In unadjusted analyses, each week of stock out (odds ratio [OR], 1.81; 95% CI, 1.62 to 2.02) was strongly associated with a suboptimal therapy delivery event. Additionally, risk of febrile neutropenia and cancer type were associated with a suboptimal therapy event at P < .1 and were included in our adjusted analysis. In contrast, there was no significant association between age, sex, stage, HIV status, intent of therapy, and suboptimal therapy delivery event.
As summarized in Table 3, after adjustment for covariates, stock-out duration remained independently associated with a higher risk of a suboptimal therapy delivery event during the course of prescribed treatment. Every week of stock-out duration was associated with an almost two-fold increased risk of a suboptimal therapy delivery event (OR, 1.9; 95% CI, 1.7 to 2.13; P < .001). Our model also suggested that patients receiving treatment regimens for colon (OR, 6.34; 95% CI, 3.11 to 12.9; P < .001) or rectal cancer (OR, 7.07; 95% CI, 1.83 to 27.3; P = .004) were at the highest risk of an event after adjusting for stock out, whereas those with prostate cancer were less likely than their counterparts to experience a suboptimal therapy delivery event (adjusted OR, 0.24; 95% CI, 0.08 to 0.79; P = .019; Table 3).
TABLE 3.
Adjusted Estimates for the Association Between Stock-Out Duration and Suboptimal Therapy Delivery Event
DISCUSSION
Our study identified a high rate of cancer medicine stock outs affecting standard regimens for commonly treated cancers in Botswana. Those with breast cancer were the highest proportion of patients affected by cancer medicine stock outs. We found that a majority of patients experienced a cancer medicine stock out during their therapy, and each week of chemotherapy stock out conferred a 1.9-fold increased risk of experiencing suboptimal cancer treatment. Adjusting for risk of febrile neutropenia and type of cancer had no impact on the strong risk of experiencing a suboptimal treatment event.
Similar findings from SSA among HIV patients have identified ART stock outs as a barrier to initiation of therapy and retention in care among patients treated in Tanzania22 and Cote d’Ivoire.13 In cancer treatment as well, given that most cancer regimens are dosed every 2, 3, or 4 weeks, interruptions in cancer medicine supplies leading to erratic stock outs lead to significant gaps in adequate therapy delivery, as demonstrated by the results of our study. Outside the context of LMICs, other studies based on provider perspectives in the United States have reported that cancer drug shortages resulted in delays in chemotherapy administration or changes in regimens for patients seen in their respective institutions and also affected the conduct of clinical trials at 44% of the institutions surveyed.23
Paclitaxel stock outs adversely affected therapy delivery for a significant proportion of patients with cancer, most of whom had breast cancer. Paclitaxel stock outs also confer potentially high financial costs to the health care system. A study from a New York City university hospital comparing periods of low drug shortages in 2010 with high drug shortages in 2011 showed a 69% significant decrease in paclitaxel use and an 80% increase in docetaxel use resulting from stock outs (P = .009 and .024, respectively), which resulted in an estimated 1,704% increase in cost, from $47.49 for paclitaxel to $858.39 for docetaxel substitution per patient for a complete regimen.24
Regimens for colon and rectal cancers were independently associated with an increased risk of suboptimal therapy, whereas the converse was noted for regimens for prostate cancer. This association might be explained by the increased dosing frequency and once-every-two-weeks infusion visits required for some colon cancer regimens, compared with patients with prostate cancer receiving androgen-deprivation therapy, who receive doses every 1 or 3 months depending on the luteinizing hormone-releasing hormone agonist prescribed. However, not many patients in these groups were included in our analysis (7%, 2%, and 4% for colon, rectal, and prostate cancers, respectively), limiting our ability to draw any firm conclusions about this association.
Our analysis had several limitations. First, we present data on risk of suboptimal treatment per cycle, which does not convey the overall clinical impact per patient. However, our study builds upon previous work highlighting that these events lead to reduced relative dose-intensity and worse survival outcomes.20,25-28 Our results highlight that patients with breast cancer comprise a majority of patients receiving systemic therapy at PMH and the highest proportion of patients affected by stock outs.
Second, the data on chemotherapy stock outs are based on stock data available at the country’s CMS. If a drug reported out of stock at CMS were still available at PMH pharmacy, we would have misclassified the interval as having a stock-out exposure. In general, this misclassification bias would result in bias toward the null, which means the actual risk for suboptimal treatment may have been even greater than we found in our analysis.
Third, the population of patients studied only reflects those receiving systemic chemotherapy at PMH. Therefore, although cervical cancer is the most common cancer diagnosed among women in Botswana, these patients are underrepresented in our study, because a majority of these patients are referred to a private facility for concurrent chemoradiotherapy. Therefore, our data do not fully represent the impact of stock outs for patients diagnosed with cervical cancer in Botswana. Furthermore, our data are reflective only of patients who engage in therapy and therefore represent only a proportion of the disease prevalence of these cancers.
Finally, our analysis is limited by the inability to sufficiently adjust for certain covariates, such as sex, HIV status, and intent of therapy, because of the collinearity between female sex and breast and cervical cancers and male sex and prostate cancer; this is similar for palliative intent and HIV-positive status and Kaposi sarcoma, because all treatment regimens for Kaposi sarcoma are palliative. To address the issue of multicollinearity among covariates, only one of these variables was included in our adjusted analysis. For instance, sex and HIV status were not included in our multivariable analysis but were included the primary cancer diagnoses. As a result of multicollinearity, we could potentially have missed additional independent predictors of suboptimal therapy delivery, such as HIV status, in our analysis.
Our study has several strengths. Although some studies have highlighted the magnitude of the essential medicine shortages in developing countries,29 most of these have been conducted among patients with communicable diseases. Additionally, prior studies on stock outs and cancer therapy delivery globally have been survey-based perspectives of medical providers and oncologists in developed countries and have not quantified individual patient risk per treatment cycle when there is a specific cancer medicine stock out. In contrast, our analysis considered different types of stock out that may or may not have affected therapy delivery by analyzing the specific pattern of cancer medicine stock out in relation to a given cycle interval. These data were also critical in estimating that 1-week duration of cancer medicine stock out may be deemed clinically meaningful and is correlated with a significantly high risk of inadequate therapy delivery.
The findings reported in our study are likely generalizable to other countries in SSA where breast cancer is either the most common or second most common cancer diagnosed among women and represents a vulnerable population most affected by stock outs. Specifically, our study may be generalizable to other countries where cancer medicines are on respective national EMLs and provided free of charge to patients through the public sector but frequently experience stock outs. In settings where the cost of cancer treatment is out of pocket, cost may be a more significant barrier to care, or the effect may be multiplicative. For instance, studies have shown that stock outs not only pose a barrier to care but also subsequently increase the cost of care for patients seeking malaria treatment.15
Our analysis of the impact of essential cancer medicine stock outs in an understudied population provides critical data and an essential framework for evaluating barriers to receipt of timely and high-quality cancer treatment, especially in LMICs, where current efforts to scale up access to cancer medicines are under way. Our results show that stock outs undermine investments in health for cancer treatment and have potentially adverse effects on clinical outcomes. An analysis of how cancer medicine stock outs affect survival outcomes will be performed in future studies. Our research adds to the current literature on the magnitude and impact of cancer medicine stock outs in LMICs and raises awareness of similar challenges faced in the continuum of care for patients with cancer. Current systems and innovative interventions in LMICs that have shown success in minimizing stock outs in other disease areas should be scaled up to address stock outs of cancer medicines. However, given the complexity of chemotherapy ordering for different cancer types, a concerted effort between policymakers and cancer specialists is needed to design implementation strategies to build sustainable systems promoting a reliable supply of cancer medicines.
Appendix
TABLE A1.
Intended Chemotherapy Regimen by Diagnosis
AUTHOR CONTRIBUTIONS
Conception and design: Yehoda M. Martei, Surbhi Grover, Warren B. Bilker, Barati Monare, Robert Gross, Lawrence N. Shulman, Angela DeMichele
Financial support: Lawrence N. Shulman
Administrative support: Surbhi Grover, Barati Monare, Lawrence N. Shulman
Provision of study material or patients: Surbhi Grover, Barati Monare, Dipho I. Setlhako
Collection and assembly of data: Yehoda M. Martei, Surbhi Grover, Barati Monare, Dipho I. Setlhako, Patrick Manshimba, Angela DeMichele
Data analysis and interpretation: Yehoda M. Martei, Warren B. Bilker, Tlotlo B. Ralefala, Robert Gross, Lawrence N. Shulman, Angela DeMichele
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Essential Medicine Stock Outs on Cancer Therapy Delivery in a Resource-Limited Setting
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Yehoda Martei
Research Funding: Celgene (Inst)
Warren B. Bilker
Consulting or Advisory Role: Genentech
Tlotlo B. Ralefala
Travel, Accommodations, Expenses: Roche
Robert Gross
Consulting or Advisory Role: Pfizer
Angela DeMichele
Honoraria: Pfizer
Consulting or Advisory Role: Calithera Biosciences, Novartis, Context Therapeutics, Pfizer (I)
Research Funding: Pfizer (Inst), Genentech (Inst), Incyte (Inst), Millennium Pharmaceuticals (Inst), Bayer HealthCare Pharmaceuticals (Inst), Veridex (Inst), Calithera Biosciences (Inst), GlaxoSmithKline (Inst), Wyeth (Inst)
Travel, Accommodations, Expenses: Pfizer, Calithera Biosciences, Novartis, Pfizer
No other potential conflicts of interest were reported.
REFERENCES
- 1.Shulman LN, Wagner CM, Barr R, et al. Proposing essential medicines to treat cancer: methodologies, processes, and outcomes. J Clin Oncol. 2016;34:69–75. doi: 10.1200/JCO.2015.61.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatesman ML, Smith TJ. The shortage of essential chemotherapy drugs in the United States. N Engl J Med. 2011;365:1653–1655. doi: 10.1056/NEJMp1109772. [DOI] [PubMed] [Google Scholar]
- 3.Gray A, Manasse HR., Jr Shortages of medicines: A complex global challenge. Bull World Health Organ. 2012;90:158–158A. doi: 10.2471/BLT.11.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox ER, Sweet B V., Jensen V. Drug shortages: A complex health care crisis. Mayo Clin Proc. 2014;89:361–373. doi: 10.1016/j.mayocp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Gehrett BK. A prescription for drug shortages. JAMA. 2012;307:153–154. doi: 10.1001/jama.2011.2000. [DOI] [PubMed] [Google Scholar]
- 6.Link MP, Hagerty K, Kantarjian HM. Chemotherapy drug shortages in the United States: Genesis and potential solutions. J Clin Oncol. 2012;30:692–694. doi: 10.1200/JCO.2011.41.0936. [DOI] [PubMed] [Google Scholar]
- 7.Tirelli U, Berretta M, Spina M, et al. Oncologic drug shortages also in Italy. Eur Rev Med Pharmacol Sci. 2012;16:138–139. [PubMed] [Google Scholar]
- 8.Martei YM, Chiyapo S, Grover S, et al. Availability of WHO essential medicines for cancer treatment in Botswana. J Glob Oncol. 2018;4:1–8. doi: 10.1200/JGO.17.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. UN Economic Analysis & Policy Division: MDG Gap Task Force Report 2015: Taking Stock of the Global Partnership for Development. http://www.un.org/en/development/desa/policy/mdg_gap.
- 10.Lufesi NN, Andrew M, Aursnes I. Deficient supplies of drugs for life threatening diseases in an African community. BMC Health Serv Res. 2007;7:86. doi: 10.1186/1472-6963-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mikkelsen-Lopez I, Shango W, Barrington J, et al. The challenge to avoid anti-malarial medicine stock-outs in an era of funding partners: The case of Tanzania. Malar J. 2014;13:181. doi: 10.1186/1475-2875-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung N-HZ, Chen A, Yadav P, et al. The impact of inventory management on stock-outs of essential drugs in sub-Saharan Africa: Secondary analysis of a field experiment in Zambia. PLoS One. 2016;11:e0156026. doi: 10.1371/journal.pone.0156026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasquet A, Messou E, Gabillard D, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Côte d’Ivoire. PLoS One. 2010;5:e13414. doi: 10.1371/journal.pone.0013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meloni ST, Chaplin B, Idoko J, et al. Drug resistance patterns following pharmacy stock shortage in Nigerian Antiretroviral Treatment Program. AIDS Res Ther. 2017;14:58. doi: 10.1186/s12981-017-0184-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikkelsen-Lopez I, Tediosi F, Abdallah G, et al. Beyond antimalarial stock-outs: implications of health provider compliance on out-of-pocket expenditure during care-seeking for fever in South East Tanzania. BMC Health Serv Res. 2013;13:444. doi: 10.1186/1472-6963-13-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012 v1.0. IARC Press; Lyon, France: 2013. [Google Scholar]
- 17. National Comprehensive Cancer Network: NCCN evidence-based cancer guidelines, oncology drug compendium, oncology continuing medical education. https://www.nccn.org.
- 18. World Health Organization: The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2015 (including the 19th WHO Model List of Essential Medicines and the 5th WHO Model List of Essential Medicines for Children). http://apps.who.int/medicinedocs/documents/s22190en/s22190en.pdf.
- 19. Breslow NE, Clayton DG: Approximate inference in generalized linear mixed models. J Am Stat Assoc 88:9-25, 1993. [Google Scholar]
- 20.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: A nationwide study of community practices. J Clin Oncol. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 21. National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Myeloid growth factors. https://www.nccn.org.
- 22.Layer EH, Kennedy CE, Beckham SW, et al. Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One. 2014;9:e104961. doi: 10.1371/journal.pone.0104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride A, Holle LM, Westendorf C, et al. National survey on the effect of oncology drug shortages on cancer care. Am J Health Syst Pharm. 2013;70:609–617. doi: 10.2146/ajhp120563. [DOI] [PubMed] [Google Scholar]
- 24.Becker DJ, Talwar S, Levy BP, et al. Impact of oncology drug shortages on patient therapy: Unplanned treatment changes. J Oncol Pract. 2013;9:e122–e128. doi: 10.1200/JOP.2012.000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna RK, Poniewierski MS, Laskey RA, et al. Predictors of reduced relative dose intensity and its relationship to mortality in women receiving multi-agent chemotherapy for epithelial ovarian cancer. Gynecol Oncol. 2013;129:74–80. doi: 10.1016/j.ygyno.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7:99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 27.Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009;114:479–484. doi: 10.1007/s10549-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 28.Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. J Natl Cancer Inst. 1998;90:1205–1211. doi: 10.1093/jnci/90.16.1205. [DOI] [PubMed] [Google Scholar]
- 29.Yifru S, Muluye D. Childhood cancer in Gondar University Hospital, Northwest Ethiopia. BMC Res Notes. 2015;8:474. doi: 10.1186/s13104-015-1440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]