Abstract
Objective
To compare clinical characteristics of patients suffering from neovascular age-related macular degeneration (nAMD) with mature and immature choroidal neovascularisation (CNV) as assessed by optical coherence tomography angiography (OCTA). To explore the effect of total anti-vascular endothelial growth factor exposure on the occurrence of mature CNV when correcting for potential confounders.
Methods and analysis
In this retrospective case series, we included 40 eyes of 36 patients with nAMD with CNV assessed by OCTA at the Manchester Eye Hospital between June 2016 and June 2017. A retinal specialist masked to patient information graded CNV depicted on OCTA scans. For statistical comparisons, we used t-tests, Fisher’s exact tests and a mixed-effects logistic regression model.
Results
18 patients (20 eyes) were treatment naïve, and the mean number of intravitreal injections (IVI) in the remaining eyes was 18.4 (range 2–71). The mean duration of nAMD was 19.3 months (range 0–87.4). 25 eyes (62.5%) exhibited mature CNV. Eyes with mature CNV did not differ from those with immature CNV regarding age (+2.8 years; p=0.288) or duration of disease (+9.4 months; p=0.061). However, they had a higher number of IVIs (+3.1; p=0.035). Among eyes with best corrected visual acuity over 25 letters, there was a strong association between the number of IVIs (0 vs 1–20: OR 68.01 [95% CI 1.30 to 3546.99; p=0.036], 0 vs >20 IVI: OR 380.01 [95% CI 2.60 to 55 464.89; p=0.019]) and maturity status when correcting for potential confounders.
Conclusion
Maturity status of CNV as assessed by OCTA may indicate treatment exposure of CNV in nAMD.
Keywords: neovascularisation, treatment medical, retina
Key messages.
What is already known about this subject?
In neovascular age-related macular degeneration (nAMD), optical coherence tomography angiography (OCTA) reveals distinct microvascular patterns thought to relate to disease activity.
What are the new findings?
Maturity status of choroidal neovascular membranes, as visualised by OCTA, was directly related to anti-vascular endothelial growth factor (VEGF) exposure regardless of disease duration, visual acuity and age.
How might these results change the focus of research or clinical practice?
Phenotypical maturation in nAMD, as illustrated by OCTA, is a useful structural biomarker demonstrating response to anti-VEGF therapy.
Introduction
Optical coherence tomography (OCT) is a non-invasive imaging technique that measures backscattered light to generate cross-sectional scans of biological tissues.1 In ophthalmology, OCT has contributed novel insights into choroidal and retinal anatomy significantly changing how we manage patients suffering from retinal disease, that is, neovascular age-related macular degeneration (nAMD).2 The most recent advance in OCT, so-called OCT angiography (OCTA), leverages improved acquisition speed and motion contrast techniques in order to detect choroidal and retinal blood flow.3–5 In contrast to fluorescein angiography, which masks the microvascular anatomy of choroidal neovascularisation (CNV) by leakage of fluorescent dye, OCTA allows investigating their phenotypical evolution in detail and in a non-invasive manner.
In CNV secondary to nAMD, OCTA has revealed two major microvascular patterns, each associated with distinct disease activity and the requirement for antiangiogenic treatment.6–9 Immature CNV lesions exhibit high vessel density and branching index, capillary sprouting, and anastomoses, and have been reported to be more active and accordingly require a higher number of anti-vascular endothelial growth factor (VEGF) intravitreal injections (IVI).6–9 In contrast, mature CNV lesions show an increase in vessel diameter with a paucity of capillaries and branching points.10 11 CNV has been described to undergo cyclic regression (ie, reduction of flow area and pruning of anastomoses) in response to IVI and subsequent reproliferation.10 11 With continued anti-VEGF treatment, reproliferating CNV undergoes phenotypical maturation with distinctive vascular remodelling (so-called ‘vascular abnormalisation’) and concomitant decrease in leakage activity.9 12
This preliminary evidence for an association between microvascular phenotype and disease activity has prompted a discussion about whether OCTA could inform anti-VEGF management decisions, such as selection of appropriate treatment intervals in a treat-and-extend anti-VEGF regimen or identifying when IVI ought to be stopped.11
However, it is still unclear whether the phenotypical maturation of CNV truly reflects their response to anti-VEGF treatment or it is an effect of the natural disease course and inherent features of the membrane type. The association of anti-VEGF treatment exposure and disease duration has impeded the investigation of this problem.
In this exploratory study, we therefore sought to compare the clinical aspects of patients showing mature CNV with those with immature CNV and to examine the association of anti-VEGF treatment exposure with the maturity status of the CNV when correcting for potential confounders including AMD disease duration.
Materials and methods
Study design and setting, patient recruitment and enrolment
This is a cross-sectional case series of consecutive patients with nAMD with an OCTA scan presenting to the injection clinic of the Manchester Royal Eye Hospital between June 2016 and June 2017. As this study involved a retrospective analysis of deidentified retinal images obtained during routine clinical care, informed consent was not required.
Inclusion and exclusion criteria
We included all patients with fluorescein angiography confirmed diagnosis of nAMD that presented to the injection clinic of the Manchester Royal Eye Hospital, either newly diagnosed (treatment naïve) or treated with anti-VEGF agents (ranibizumab [Lucentis] or aflibercept [Eylea]) and had an OCTA scan, either acquired at or after the date of first nAMD diagnosis. Ranibizumab was given on a fixed monthly dosing for the first 3 months followed by pro re nata. In line with the licensed posology, aflibercept was given monthly for the first 3 months followed by two monthly until 1 year from commencement. This was then followed by either pro re nata or treat-and-extend regimes.
We excluded patients with nAMD who had OCTA scans without visible CNV.
Swept-source OCTA
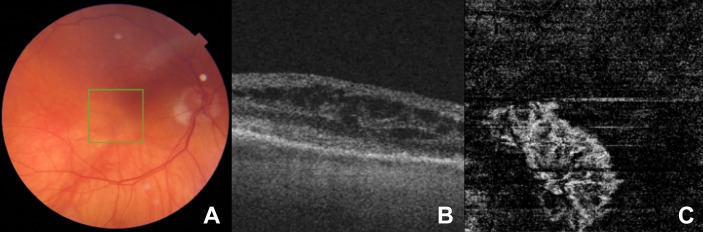
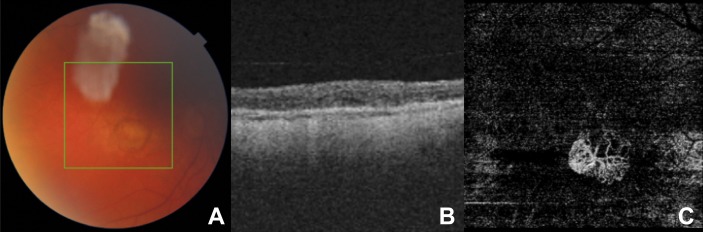
In each case, we used a swept-source OCTA system (Topcon Triton, Topcon, Tokyo, Japan) for image acquisition. Manual segmentation of the OCTA image fine-tuned the depth of imaging to ensure optimal visualisation of the CNV in each case. An experienced and board-certified Medical Retina specialist (KB) graded and classified morphological features of CNV. Following the observations of Al-Sheikh and colleagues, he classified the CNV as immature if they harboured a dense vascular network with a high branching index, high number of anastomoses and a smooth outline8 (figure 1). We classified the CNV as mature if the vascular network involved loosely packed and larger (trunk) vessels, with a small branching index and loss of the capillary fringe12 (figure 2).
Figure 1.
(A) A colour fundus image, (B) a cross-sectional foveal optical coherence tomography (OCT) image with intraretinal fluid (IRF), and (C) the corresponding en face OCT angiography (OCTA) image depicting outer avascular retina and choriocapillaris slab of a treatment-naïve patient with neovascular age-related macular degeneration (nAMD) with immature choroidal neovascularisation (CNV). Note the CNV in panel C demonstrates a dense vascular morphology with high branching index and anastomoses in conjunction with a smooth well-demarcated outline.
Figure 2.
(A) A colour fundus image, (B) a cross-sectional foveal optical coherence tomography (OCT) image, and (C) the corresponding en face OCT angiography (OCTA) image depicting outer avascular retina and choriocapillaris slab of a patient with neovascular age-related macular degeneration (nAMD) with immature choroidal neovascularisation (CNV) treated by 16 anti-vascular endothelial growth factor (VEGF) intravitreal injections (IVI). In contrast to figure 1C, the CNV vascular network is more loosely packed with larger trunk vessels seen and a loss of the capillary fringe.
The retinal specialist graded the CNV without the knowledge of the corresponding treatment exposure or disease course. We extracted possible confounders including patients’ age, disease duration, number of injections at the time of OCTA imaging, best corrected visual acuity (BCVA), presence of subretinal fluid (SRF) and presence of intraretinal fluid (IRF). We operationalised disease duration by the time difference between the date of first nAMD diagnosis and OCTA imaging. It was standard practice for OCTA to be done prior to IVI if both were on the same day.
Statistical analysis
Descriptive statistics
We summarised continuous variables with means and SDs and dichotomous variables as percentages. Differences in population characteristics between patients with immature and mature CNV were tested using parametric or non-parametric tests as appropriate. A p value of less than 5% was considered statistically significant.
To assess the association between the number of IVIs and the occurrence of mature CNV, we categorised the number of injections into three groups (no IVI, ≤20 IVI, >20 IVI) using two indicator variates. To correct for possible confounding we fitted a multivariable, mixed-effects logistic regression model using an indicator variate for mature state of CNV (yes/no) as the dependent variate, and categories of IVI along with the possible confounders stated above into the model. A mixed-effects model with an indicator variate for patient ID as a random factor was chosen to account for the fact that four subjects provided data on both eyes. For this analysis, we excluded seven eyes with very low BCVA (≤25 letters) as these represented extreme cases who did not receive standard care according to the UK National Institute for Health and Care Excellence (NICE) guidelines on nAMD and therefore the number of injections in these cases did not reflect any standardised treatment regimen.13
Analyses were done using the Stata V.14.2 statistical software package (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp).
Results
Patients’ descriptive statistics
A total of 40 eyes (25 eyes with mature and 15 eyes with immature CNV) were studied. Four patients provided both eyes to the analysis. In those four patients, we classified CNV of one patient contributing two eyes, as immature in one and as mature in the other eye. Mean age was 80.1 years (SD ±5.82), and 19 patients (51.4%) were female. Twenty eyes were treatment naïve, the mean number of IVIs in the remaining eyes was 18.4 (range 2–71). The mean disease duration was 19.3 months (range 0–87.4).
Immature versus mature neovascular membranes
Eyes with mature CNV did not significantly differ from those with immature CNV regarding age (+2.8 years; p=0.288) or duration of disease (+9.4 months; p=0.061). However, eyes with mature CNV were more often treated with anti-VEGF (14/22 vs 4/15; p=0.045). They also had a significantly higher median number of total IVIs (+6, IQR 0–18, p=0.042) and included a smaller proportion of treatment-naïve eyes (36.0% vs 73.3%, p=0.048). However, within the subgroup of treated eyes, treatment frequency and the median number of total IVIs were comparable. The mean BCVA in eyes with immature CNV was higher (58.27 letters, SD ±17.19) than in patients with mature membranes (44.48 letters, SD ±24.09, p=0.081). Eyes with immature CNV also showed SRF in more cases than eyes with mature membranes (46.7% vs 12.0%, p=0.014). However, the occurrence of IRF was comparable in both groups (20.0% vs 16.0%, p=0.747).
Table 1 shows the characteristics and comparisons of patients with immature versus mature CNV. When excluding seven eyes with very low BCVA (≤25 letters), there was a strong association between the number of IVIs (0 vs 1–20 IVI: OR 68.01 [95% CI 1.30 to 3546.99; p=0.036], 0 vs >20 IVI: OR 380.01 [95% CI 2.60 to 55 464.89; p=0.019]) and maturity status when correcting for potential confounding.
Table 1.
Characteristics and comparisons of patients with immature versus mature membranes
| Immature (15 eyes) | Mature (25 eyes) | P value | |
| Mean age in years (SD) | 81.80 (5.82) | 79.04 (8.81) | 0.288 |
| Female (proportion) | 7/15 (46.7%) | 14/25 (56.0%) | 0.745 |
| Treatment-naïve eyes (proportion) | 11/15 (73.3%) | 9/25 (36.0%) | 0.048† |
| Median number of IVI (IQR) | 0 (0–8) | 6 (0–18) | 0.042*† |
| Subgroup of treated eyes | 15 (11–43.5) | 17 (8.5–23) | 0.850 |
| IVI groups (injections, n) | |||
| 0 | 10 | 5 | |
| ≤20 | 3 | 9 | |
| >20 | 1 | 5 | |
| Overall | 0.035† | ||
| Mean IVI per month (SD) | 0.18 (0.36) | 0.33 (0.33) | 0.045† |
| Subgroup of treated eyes | 0.69 (0.39) | 0.52 (0.28) | 0.329 |
| Anti-VEGF type | |||
| Ranibizumab | 3 (20.0%) | 6 (24.0%) | |
| Aflibercept | 1 (6.7%) | 6 (24.0%) | |
| Both | 1 (6.7%) | 4 (16.0%) | |
| Overall | 0.875 | ||
| Mean disease duration in months (SD) | 13.39 (28.14) | 22.77 (26.41) | 0.061* |
| Mean BCVA in letters (SD) | 58.27 (17.19) | 44.48 (24.09) | 0.081* |
| Presence of IRF (proportion) | 3/15 (20.0%) | 4/25 (16.0%) | 0.747 |
| Presence of SRF (proportion) | 7/15 (46.7%) | 3/25 (12.0%) | 0.014† |
*Wilcoxon rank-sum (Mann-Whitney) test.
†Statistically significant
BCVA, best corrected visual acuity; IRF, intraretinal fluid; IVI, intravitreal injection; SRF, subretinal fluid; VEGF, vascular endothelial growth factor.
Subgroup of patients with very low visual acuity
Seven out of 40 eyes (17.5%) had very low visual acuity (≤25 letters). The mean time of follow-up in these eyes was 1 month (range 0–5.4 months). Among them, one treatment-naïve female eye with 20 letters BCVA showed no signs of maturity. Two male eyes (mean age: 83.5 years, mean BCVA: 5.5 letters, mean number of IVIs: 11.5) and four treatment-naïve female eyes (mean age: 81.0 years, mean BCVA: 13 letters) showed signs of maturity. Compared with the group with higher visual acuity, there was no significant difference in age, female gender and mean number of IVIs. While the frequency of SRF was not different between these two groups, we found a trend for a higher frequency of IRF (3/7 vs 4/33; Fisher’s exact p=0.088) in eyes with very low visual acuity.
Figures 1–4 show colour fundus images, cross-sectional foveal OCT images and corresponding en face OCTA images of example cases for mature and immature CNV, with and without anti-VEGF exposure.
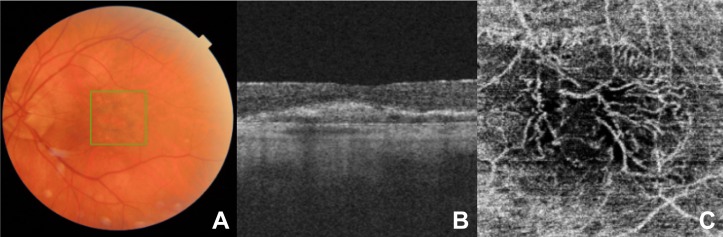
Figure 3.
(A) A colour fundus image, (B) a cross-sectional foveal optical coherence tomography (OCT) image, (C) and the corresponding en face OCT angiography (OCTA) image showing the choriocapillaris slab of a patient with neovascular age-related macular degeneration (nAMD) with mature choroidal neovascularisation (CNV) treated by 41 anti-vascular endothelial growth factor (VEGF) intravitreal injection (IVI).
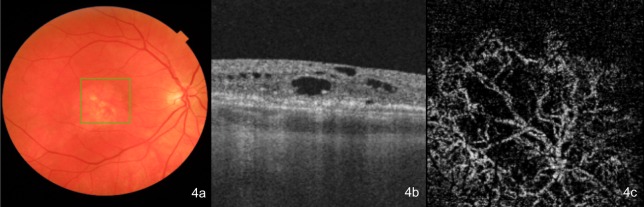
Figure 4.
(A) A colour fundus image, (B) a cross-sectional foveal optical coherence tomography (OCT) image with chronic intraretinal fluid (IRF), and (C) the corresponding en face OCT angiography (OCTA) image depicting the outer avascular retina and choriocapillaris slab of a treatment-naïve patient with neovascular age-related macular degeneration (nAMD) with mature choroidal neovascularisation (CNV) (a long-standing case that presented late).
Discussion
Main findings
In this retrospective case series of limited size, we found that the phenotypical maturation of CNV, as assessed by OCTA and graded by a retinal specialist, correlates with anti-VEGF treatment exposure after correcting for potential confounding, namely BCVA, age, SRF and disease duration.
Results in context
To our knowledge, this is the first study to explore the association between anti-VEGF treatment exposure and the maturity status of CNV while correcting for nAMD disease duration.
The design of this study was based on Spaide’s work that first introduced the concept of vascular abnormalisation in neovascular membranes hypothesising that the treatment-induced morphological changes may result from recurrent vascular pruning.12 Sulzbacher and colleagues’ study showed that in treatment-naïve eyes, most of the CNV included a dense vascular network, whereas in eyes exposed to anti-VEGF, membranes were also harbouring loose networks in one-third of cases. Furthermore, in line with our study, they correlated longer disease duration with the loosening of the membrane networks. In contrast to our approach, where we entered disease duration as a covariate in the regression analysis, they did not investigate it as a potential confounder for this association.7 Kuehlewein and colleagues found that the main trunk (‘feeder vessel’) of type 2 neovascularisations remained unchanged after regular IVI, hypothesising that this large vessel may be a part of a mature neovascular complex associated with chronicity of the disease.14 Lumbroso and colleagues investigated the morphologic evolution of treatment-naïve CNV in response to repeated anti-VEGF IVI and found increased blood flow in the feeding vessel after treatment associated with pruning of small vessels.10 15 They concluded that OCTA might offer additional guidance on the selection of the time interval between IVIs. Huang et al found diminished blood flow in CNV in response to anti-VEGF within the first 2 weeks followed by reappearing vessels at 4 weeks’ follow-up time.11 In a recent study, Wylęgała and colleagues found significant changes in vessel density, but non-significant changes in ramification ratio of CNV when exposed to anti-VEGF treatment.16 Xu et al showed that type 1 CNV often continues to grow in size while under anti-VEGF treatment, but our study did not assess CNV size.17 Finally, this analysis also allowed interrogation of the CNV development in the absence of anti-VEGF exposure.
Strengths and limitations
We conducted this study according to current guidelines for case series.18
We refrained from performing a formal sample size analysis, given that this study is of exploratory nature. We were not able to investigate subgroups of different types of neovascular membranes and other stratified analyses due to the small study size. We excluded post hoc seven eyes that fell beyond anti-VEGF treatment indication according to the NICE guidelines from the exploratory analysis, which further reduced the study size.
As in every study using real-world clinical data, we faced the problem of potential patient selection due to various reasons including, that is, variability in patient referral or availability of clinical resources for upstream evaluations. This might have introduced selection bias to an unknown extent. In view that these data should stimulate further evaluation of the biological phenomenon of CNV maturation in treated nAMD, we believe that our data will inform this discussion. It is also noted that the CNV membrane may progress between morphological phases following anti-VEGF injection. Future work visualising the evolution of CNV structural change following anti-VEGF would be helpful. Finally, while grading was performed twice for the majority of cases with no change in CNV maturity status resulting, we did not formally assess intragrader variability.
In this study, we demonstrate an association between anti-VEGF treatment exposure and CNV morphology as visualised on OCTA, after accounting for confounding factors including disease duration. We show a strong association between mature CNV phenotype with an increasing number of anti-VEGF injections.
Implications for research
Given the results presented in this case series, evaluations in a prospective cohort study of reasonable size enrolling patients who are presenting with suspected nAMD should be performed. The analysis should consider assessing several clinical subgroups including the different membrane types, the type of anti-VEGF drug and clinical activity.
Implications for practice and conclusions
This study further highlights the potential of OCTA to have a substantial impact on patient care. For instance, the response to anti-VEGF therapy in nAMD may be assessed by phenotypical maturation, thereby influencing clinical decision-making. In conclusion, the assessment of the maturity status of CNV in nAMD by OCTA may be a complementary way to follow the response to anti-VEGF therapy, providing additional information for treatment decisions.
Footnotes
Contributors: LF, LMB: substantial contributions to the analysis and interpretation of data, drafting of the work. ZA: substantial contributions to the conception or design of the work and the acquisition of data. SW, PJP, MKS, NW, PAK: substantial contributions to the interpretation of data. KB, DJF: substantial contributions to the conception and design of the work, the acquisition and interpretation of data. All authors revised the work critically for important intellectual content and gave final approval of the version published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PAK has received speaker fees from Heidelberg Engineering, Topcon, Carl Zeiss Meditec, Haag-Streit, Allergan, Novartis and Bayer. He has served on advisory boards for Novartis and Bayer and has been an external consultant for DeepMind and Optos.
Patient consent for publication: Not required.
Ethics approval: The Institutional Review Board of the Manchester Royal Eye Hospital, UK has approved this study (R03712).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Huang D, Swanson EA, Lin CP, et al. . Optical coherence tomography. Science 1991;254:1178–81. 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keane PA, Patel PJ, Liakopoulos S, et al. . Evaluation of age-related macular degeneration with optical coherence tomography. Surv Ophthalmol 2012;57:389–414. 10.1016/j.survophthal.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 3. Jia Y, Tan O, Tokayer J, et al. . Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express 2012;20:4710–25. 10.1364/OE.20.004710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zotter S, Pircher M, Torzicky T, et al. . Visualization of microvasculature by dual-beam phase-resolved Doppler optical coherence tomography. Opt Express 2011;19:1217–27. 10.1364/OE.19.001217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makita S, Hong Y, Yamanari M, et al. . Optical coherence angiography. Opt Express 2006;14:7821–40. 10.1364/OE.14.007821 [DOI] [PubMed] [Google Scholar]
- 6. Coscas GJ, Lupidi M, Coscas F, et al. . Optical coherence tomography angiography versus traditional multimodal imaging in assessing the activity of exudative age-related macular degeneration: a new diagnostic challenge. Retina 2015;35:2219–28. 10.1097/IAE.0000000000000766 [DOI] [PubMed] [Google Scholar]
- 7. Sulzbacher F, Pollreisz A, Kaider A, et al. . Identification and clinical role of choroidal neovascularization characteristics based on optical coherence tomography angiography. Acta Ophthalmol 2017;95:414–20. 10.1111/aos.13364 [DOI] [PubMed] [Google Scholar]
- 8. Al-Sheikh M, Iafe NA, Phasukkijwatana N, et al. . Biomarkers of neovascular activity in age-related macular degeneration using optical coherence tomography angiography. Retina 2018;38:220–30. 10.1097/IAE.0000000000001628 [DOI] [PubMed] [Google Scholar]
- 9. Miere A, Butori P, Cohen SY, et al. . Vascular remodeling of choroidal neovascularization after anti-vascular endothelial growth factor therapy visualized on optical coherence tomography angiography. Retina 2017. [DOI] [PubMed] [Google Scholar]
- 10. Lumbroso B, Rispoli M, Savastano MC. Longitudinal optical coherence tomography-angiography study of type 2 naive choroidal neovascularization early response after treatment. Retina 2015;35:2242–51. 10.1097/IAE.0000000000000879 [DOI] [PubMed] [Google Scholar]
- 11. Huang D, Jia Y, Rispoli M, et al. . Optical coherence tomography angiography of time course of choroidal neovascularization in response to anti-angiogenic treatment. Retina 2015;35:2260–4. 10.1097/IAE.0000000000000846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spaide RF. Optical coherence tomography angiography signs of vascular abnormalization with antiangiogenic therapy for choroidal neovascularization. Am J Ophthalmol 2015;160:6–16. 10.1016/j.ajo.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 13. National Institute for Health and Care Excellence Pharmacological management of AMD, antiangiogenic therapies (NICE guideline 82). Available: https://www.nice.org.uk/guidance/ng82/chapter/Recommendations2018 [Accessed 15 Aug 2018].
- 14. Kuehlewein L, Sadda SR, Sarraf D. OCT angiography and sequential quantitative analysis of type 2 neovascularization after ranibizumab therapy. Eye 2015;29:932–5. 10.1038/eye.2015.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakravarthy U, Williams M. The Royal College of ophthalmologists guidelines on AMD: Executive summary. Eye 2013;27:1429–31. 10.1038/eye.2013.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wylęgała A, Wylęgała F, Wylęgała E. Aflibercept treatment leads to vascular Abnormalization of the choroidal neovascularization. J Healthc Eng 2018;2018:1–5. 10.1155/2018/8595278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu D, Dávila JP, Rahimi M, et al. . Long-term progression of type 1 neovascularization in age-related macular degeneration using optical coherence tomography angiography. Am J Ophthalmol 2018;187:10–20. 10.1016/j.ajo.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 18. Jabs DA. Improving the reporting of clinical case series. Am J Ophthalmol 2005;139:900–5. 10.1016/j.ajo.2004.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]