Abstract
Objective:
To define inflammatory pathways in youth living with HIV infection (YLWH), assessments of biomarkers associated with lymphocyte and macrophage activation, vascular injury, or bone metabolism were performed in YLWH in comparison to healthy controls (HC).
Design:
Longitudinal multi-center study comparing biomarkers in YLWH suppressed on antiretroviral therapy (ART), those with ongoing viral replication, and HC were compared using single blood samples obtained at end of study.
Methods:
Twenty-three plasma proteins were measured by ELISA or multiplex assays. Principle components analysis (PCA) was used to define contributions of individual biomarkers to define outcome groups.
Results:
The study cohort included 129 predominantly African American, male participants, 21–25 years old at entry. Nine biomarkers of lymphocyte and macrophage activation and cardiovascular injury differed between HC and YLWH. Significant positive correlations were identified between lymphocyte and macrophage activation biomarkers among HC and YLWH. Correlations distinct to YLWH were predominantly between biomarkers of macrophage and vascular inflammation. PCA of outcome groups showed HC and suppressed YLWH clustering together for lymphocyte activation biomarkers while macrophage activation markers showed all YLWH clustering distinct from HC. Cardiovascular biomarkers were indistinguishable across groups. Averaged variable importance projection to assess single biomarkers that maximally contribute to discriminate among outcome groups identified soluble CD27, CD14, and CD163 as the three most important with TNFα and LPS also highly relevant in providing separation.
Conclusions:
Soluble inflammatory and lymphocyte biomarkers sufficiently distinguish YLWH from HC. Persistent macrophage activation biomarkers may provide a means to monitor consequences of HIV-infection in fully suppressed YLWH.
Keywords: HIV, adolescents, macrophage, biomarker, inflammation, cytokines, chemokines, lymphocytes
Introduction
HIV infection perturbs inflammatory networks before the loss of CD4 T cells and development of immune deficiency [1]. Chronic lymphocyte and macrophage activation are implicated in the increase in non-AIDS related morbidity and mortality among chronically HIV-infected adults [2–5]. In contrast, few studies have focused on the inflammatory pathways and biomarkers associated with early infection in youth living with HIV infection (YLWH). YLWH, ages 13 to 24, represent over 25% of newly acquired HIV infections in the United States and are the most rapidly expanding population of infected individuals worldwide [6]. Recent changes in treatment recommendations for the initiation of combination antiretroviral therapy (ART) will lead to treatment of all YLWH independent of CD4 T cell counts or extent of HIV-associated disease[7, 8].
In general, at diagnosis YLWH have higher CD4 T cell counts, fewer co-morbidities, and less advanced disease progression than their adult counterparts [9, 10]. YLWH who initiate ART prior to CD4 T cell decline have a high capacity to reconstitute functional T cell immunity [11], although macrophage and T cell activation, osteopenia, and biomarkers of vascular injury persist, even when viral replication is optimally controlled and CD4 T cell counts are normal[11–13]. For example, effective ART lowers plasma levels of biomarkers for lymphocyte activation, soluble CD27 (sCD27), and macrophage activation, soluble CD163 (sCD163), compared to untreated individuals [14, 15], even though biomarker levels among virally suppressed individuals remain elevated compared to HIV-uninfected youth [11]. A comprehensive, integrated assessment of biomarkers associated with lymphocyte, macrophage, vascular, and bone metabolism in YLWH compared with uninfected youth is lacking. The goal of the current study was to examine multiple biomarkers associated with distinct inflammatory pathways altered by HIV infection in YLWH using novel biostatistical modeling to identify inflammatory profiles among YLWH. Understanding the relationship between inflammatory pathways and disease progression in YLWH is critical to designing unique treatment interventions for this population.
Methods
Study Subjects.
YLWH were enrolled in the Adolescent Trials Network (ATN) for HIV/AIDS Interventions protocol 071/101, Assessment of Inflammatory Markers Associated with Neurocognitive Impairment in HIV-infected Adolescents, a sub-study of ATN 071, Neurocognitive Assessment in Youth Initiating ART, a 3-year longitudinal study, initiated in April 2008 with the last participant completing the study in 2013 (ClinicalTrials.gov Identifier NCT00683579) [16]. Enrollment was at 22 sites throughout the United States and Puerto Rico; procedures included routine monitoring of clinical HIV disease, neurocognitive assessments and self-reported substance use[16]. A single blood sample to assess biomarkers was obtained at week 144. Inclusion criteria included age ≥18 and <25 years at enrollment, HIV-infection acquired via behavioral means, and no previous ART. Exclusion criteria included current pregnancy, substance use that would interfere with the ability to complete the study, and significant non-HIV-related cognitive or motor impairment. Participants were stratified at entry based on CD4 T cell counts into three study arms; 1. CD4 T cell counts > 350 cells/μl who at entry initiated ART consisting of a protease inhibitor plus two nucleotide reverse transcriptase inhibitors; 2. CD4 T cell counts > 350 cells/μl who remained untreated until CD4 counts declined to <350 cells/μl; and 3. CD4 T cell counts <350 cells/μl who initiated ART at entry [16]. Plasma viral levels, CD4 counts, diagnoses, and medications were abstracted from clinic records at study visits. At the end of study participants were classified based on HIV RNA viral levels as viral load negative (VL−) (<50 copies/mL) or viral load positive (VL+) (> 50 copies/mL).
Age, gender, and race-balanced self-declared healthy control (HC) participants, with no acute illnesses at the time of enrollment or immunizations during the past three months, were enrolled at the University of South Florida. Blood samples were obtained at a single time point. Clinical, demographic, and laboratory data, similar to ATN 071/101 data, were collected [17]. IRB approval was obtained at each study site and from the University of Florida and University of South Florida. Informed consent was obtained from each participant.
ELISA and multiplex assays for plasma cytokine/chemokine.
Biomarkers included cytokines, chemokines, growth factors, enzymes, proteins, or soluble receptors associated with lymphocyte activation, macrophage activation, or markers of vascular or bone injury impacted by HIV infection in different cohorts/studies [13–15, 18–25]. Six biomarkers associated with lymphocyte activation (sCD27, IFNγ, sCD25, IL-10, IL-2, and IL-5) [14, 19, 24], twelve associated with macrophage activation (sCD163, sCD14, TNFα, TNF-β, GM-CSF, MCP-1, IL-1β, IL-1α, IL-6, IL-8, LPS, and MMP-2) [4, 14, 15, 18, 20, 23], four with vascular injury (MPO, sICAM-1, sVCAM-1, and CRP), and one with bone metabolism (osteopontin) were selected[13, 21, 22, 25]. ELISA assays were performed per manufacturer’s instructions: sCD14, sCD163, matrix metalloprotease-2 (MMP-2), osteopontin (R&D Systems, Minneapolis, MN) and sCD27 (eBioscience, San Diego, CA). Three distinct multiplex analyses were performed using the Luminex platform (Millipore, Darmstadt, Germany): a 13-plex assay for IFNγ, IL-2, IL-5, IL-10, sCD25 (sIL-2Rα), CCL2 (MCP-1), TNFα, TNFβ, IL-1α, IL-1β, IL-6, IL-8, and GMCSF; a 3-plex assay for sICAM-1, sVCAM-1, and myeloperoxidase (MPO); and a 1-plex assay for C reactive protein (CRP). Plasma was assayed undiluted for the 13-plex panel, and diluted 1:200 or 1:2000 per manufacturer’s instructions for 3- or 1-plex panels. To normalize for lower limits of detection, standard curves from each assay were combined to provide one least detectable value. Imputation process and sensitivity analysis were performed to provide a robust data set. Samples below the level of detection were recoded to one significant figure below the lowest detectable value for each biomarker. Plasma LPS was measured after 1:2 dilution in 0.15 M NaCl and heat inactivated at 65° C, using the limulus amebocyte assay (LAL) Chromogenic Endpoint Assay [14, 18] . Because over 40% of HC had levels of IL-2, IL-5, TNF-β, or IL-1α below detection in their respective assays these biomarkers were excluded from further analysis, leaving 19 biomarkers.
Data Processing.
Raw values for each biomarker were used for correlation analysis. Natural log-transformed values were used for group comparison on single biomarkers and multiple biomarkers as a bioprofile. Natural log-transformed values were standardized to a mean of zero and standard deviation of one to use for principal component analysis (PCA) or partial least squares discriminant analysis (PLS-DA).
Statistical Analysis.
Chi-square test compared gender and race distribution, while Wilcoxon rank-sum test compared distribution of age, total CD4 count, and CD4 percent between healthy controls and HIV-infected VL+ and VL- participants. Linear mixed models, with adjustment for clustering resulting from a shared assay plate, were fit to test the effect of HIV-1 infection, therapy, or virus on each individual biomarker. Multivariate analysis of variance (MANOVA) was conducted to examine the effect of age, gender, ethnicity, HIV infection, ART and viral levels on bioprofiles consisting of full or refined biomarker panels. Semiparametric Spearman’s rank correlation coefficient was used to quantify statistical dependence between pairs of biomarkers in HIV-infected subjects and healthy controls, separately. Heatmaps were produced to visualize the strength of the correlations. PCA biplots were generated to visualize two-dimensional representation of bioprofiles among groups, while the supervised partial least squares-discriminant analysis (PLS-DA) was applied to identify biomarkers that provide the most information in separating of designated groups. Q2 criterion of leave-one-out cross-validation was used to select the optimal number of PLS-DA components. Identified important factors were ordered using averaged variable importance in projection (VIP) scores for the selected number of components. VIP scores above 1 were considered relevant for group separation. Bonferroni correction was considered to adjust for multiple comparisons based on biomarker categories and control the overall Type I error rate at 0.05. All statistical analysis results were generated using R 3.1.2 and SAS ® 9.4 software.
Results
Demographics of the study cohort.
YLWH were 85% male and 67% African American. At entry, average length of infection was 10.75 months based on medical history (Table 1). At week 144, median age of the 129 YLWH was 24 years median, while CD4 T cell count was 651cells/μl (34%) (Table 1) Most YLWH, 110 /129 (85%), initiated ART while on study. One subject had missing CD4 and viral load data and was not included in the analysis for Tables 1 and 2. Of those receiving ART, 79/110 (72%) with viral suppression were classified as VL− while those with detectable plasma virus (28%) were classified as VL+. Among the HC, median CD4 T cell percentage was 46.1%, significantly higher than YLWH, but absolute CD4 T cell counts were similar (723 cells/μl).
Table 1.
Demographics of the study population.
| Healthy Control | HIV+ | p-value | HIV VL- <50 copies/ml |
HIV VL+ >50 copies/ml |
p-value | |
|---|---|---|---|---|---|---|
| N | 56 | 129 | 80 | 48 | ||
| ARTa | NA | 110 (85.3%) | 79 (98.75%) | 31 (64.58%) | ||
| Age in yearsb,d | 22 (18,25) | 24 (21,28) | <0.001* | 24 (21,28) | 24 (21,28) | 0.870 |
| Gendera,c | ||||||
| Male | 41 (73.2%) | 110 (85.3%) | 0.082 | 68 (86%) | 41 (85.4%) | >0.999 |
| Female | 15 (26.8%) | 19 (14.7%) | 11 (14%) | 7 (14.6%) | ||
| Racea,c | ||||||
| African American | 38 (67.9%) | 87 (67.4%) | >0.999 | 52 (65.8%) | 33 (68.75%) | 0.884 |
| other | 18 (32.1%) | 42 (32.6%) | 27 (34.2%) | 15 (31.25%) | ||
| CD4%b,d | 46.1 (27.4, 64.3) | 34 (7, 53) | <0.001* | 35.5(11,52) | 30.5(7, 50) | <0.005* |
| CD4 (cells/ul)b,d | 723 (192, 1416) | 651 (80, 1320) | 0.149 | 668 (231,1280) | 564.5 (80, 1320) | 0.038 |
| Viral load (copies/ml)b | NA | <50 (<50 to 500,000) | <50 | 7856 (65,500000) | ||
number of subjects (%),
median (range),
Chi-square test,
Wilcoxon rank-sum test.
indicate statistical significance.
Table 2.
Comparison of biomarker levels across outcome groups.
| Group comparison | HC | HIV | VL+ | VL- |
|---|---|---|---|---|
| N | 56 | 129 | 48 | 80 |
| Lymphocyte biomarkers | ||||
| sCD27 (U/ml) | 23.18 (11.54)a | 57.10 (36.90)* | 75.69 (46.63)* | 45.45 (23.33)* |
| IFN- (pg/ml) | 23.27 (33.50) | 12.43 (36.05)* | 20.67 (49.20) | 7.62 (24.47) |
| sCD25 (pg/ml) | 50.06 (34.30) | 67.40 (80.26) | 106.72 (116.17)* | 43.90 (30.15) |
| IL-10 (pg/ml) | 12.61 (18.65) | 5.99 (13.08) | 9.59 (20.03) | 3.73 (4.98) |
| Monocyte/Macrophage biomarkers | ||||
| sCD163 (ng/ml) | 351.14 (168.21) | 546.48 (304.55)* | 711.58 (401.55)* | 446.99 (166.58)* |
| sCD14 (ng/ml) | 991.05 (501.97) | 1463.09 (422.77)* | 1428.84 (450.53)* | 1482.36 (409.40)* |
| LPS (EU/ml) | 0.16 (0.07) | 0.21 (0.08)* | 0.23 (0.06)* | 0.20 (0.09) |
| TNF (pg/ml) | 4.68 (3.26) | 11.31 (25.62)* | 13.71 (24.80)* | 9.95 (26.29) |
| GM-CSF (pg/ml) | 2.60 (4.60) | 0.90 (1.99)* | 0.62 (1.33)* | 1.08 (2.30) |
| CCL2 (pg/ml) | 142.88 (98.34) | 208.88 (303.25) | 273.27 (468.82) | 167.80 (116.30) |
| IL-1 (pg/ml) | 3.82 (7.58) | 28.10 (101.36) | 57.17 (151.15) | 10.99 (47.26) |
| IL-6 (pg/ml) | 6.68 (10.83) | 13.80 (66.69) | 30.44 (106.95) | 3.98 (11.56) |
| IL-8 (pg/ml) | 7.93 (7.92) | 64.22 (290.41) | 128.28 (464.51) | 21.61 (50.32) |
| MMP-2 (ng/ml) | 206.03 (35.14) | 195.61 (39.13) | 196.38 (39.28) | 194.29 (38.75) |
| Cardiovascular biomarkers | ||||
| MPO (ng/ml) | 181.13 (211.48) | 280.54 (269.37)* | 314.82 (256.49)* | 260.98 (277.92) |
| slCAM-1 (ng/ml) | 1780.94 (9454.89) | 214.33 (302.62)* | 201.15 (243.60) | 219.32 (335.03)* |
| sVCAM-1 (ng/ml) | 633.28 (202.84) | 597.08 (238.86) | 718.78 (286.44) | 522.98 (170.22)* |
| CRP (ng/ml) | 1987.63 (6700.38) | 1417.70 (5496.65) | 1016.86 (3651.08) | 1675.71 (6390.02) |
| Bone related | ||||
| Osteopontin (ng/ml) | 60.50 (34.58) | 51.53 (21.21) | 48.88 (20.93)* | 53.68 (20.85) |
all values shown are mean (standard deviation).
indicate statistical significance compared with HC. P-values are calculated using mixed model after log transformation.
Bonferroni-corrected significance cut-off to adjust for multiple comparisons: p<0.002 for lymphocyte and cadiovascular biomarkers, p<0.00083 for monocyte/macrophage biomarkers, p<0.0083 for bone related biomarker.
HIV infection disrupts normal biomarker levels.
There were no significant pairwise differences between HC and YLWH for 10 biomarkers, including osteopontin, lymphocyte markers (sCD25 and IL-10), macrophage (CCL2, IL-1β, IL-6, IL-8, and MMP-2), and cardiovascular (sVCAM-1, and CRP). (Table 2) Plasma levels of nine biomarkers, including lymphocyte (sCD27 and IFNγ), monocyte/macrophage (sCD163, sCD14, LPS, TNFα and GM-CSF) and cardiovascular markers (MPO and sICAM-1), were significantly different between HC and YLWH.
To identify the impact of persistent viral replication, biomarkers were evaluated between HC and VL+ YLWH (Table 2). Five monocyte/macrophage biomarkers, as well as sCD27 (lymphocyte) and MPO (cardiovascular), differed between HC and VL+, similar to the comparison between HC and all YLWH. In addition, VL+ youth differed from HC in levels of sCD25 (lymphocyte biomarker) and osteopontin (bone). When the VL− group was compared to HC, five biomarkers (sCD27, sCD163, sCD14, sVCAM, and sICAM) remained different, while sCD25, LPS, TNFα, GM-CSF, and MPO were no longer significantly different (Table 2). The potential effect of ART, independent of viral replication, was assessed, but no significant difference for any biomarkers between the groups was detected (Table S1). No clear confounding variables such as gender, age, ethnicity, or substance use fully provided an explanation for pairwise differences between groups and biomarkers (data not shown).
Impact of HIV infection on biomarker networks.
To evaluate the relationships among biomarkers within either the HC or YLWH groups, pairwise Spearman correlations between all 19 biomarkers were performed (Figure 1 and Table S2). Among HC, significant positive correlations with rho values ranging from 0.485 to 0.822 (p<0.0002) were identified for 8 lymphocyte and monocyte/macrophage biomarkers (Figure 1A). Biomarkers of lymphocyte and Monocyte/macrophage activation markers TNFα, IL-6, and IL-8 were positively correlated with IFNγ, IL-8 positively correlated with TNFα and IL-6, IL-10 with IL-1β, and CD25 with CCL2. With the exception of TNFα with IFNγ and sCD25 with CCL2 all of these biomarkers also positively correlated with YLWH (Figure 1B and 1C).
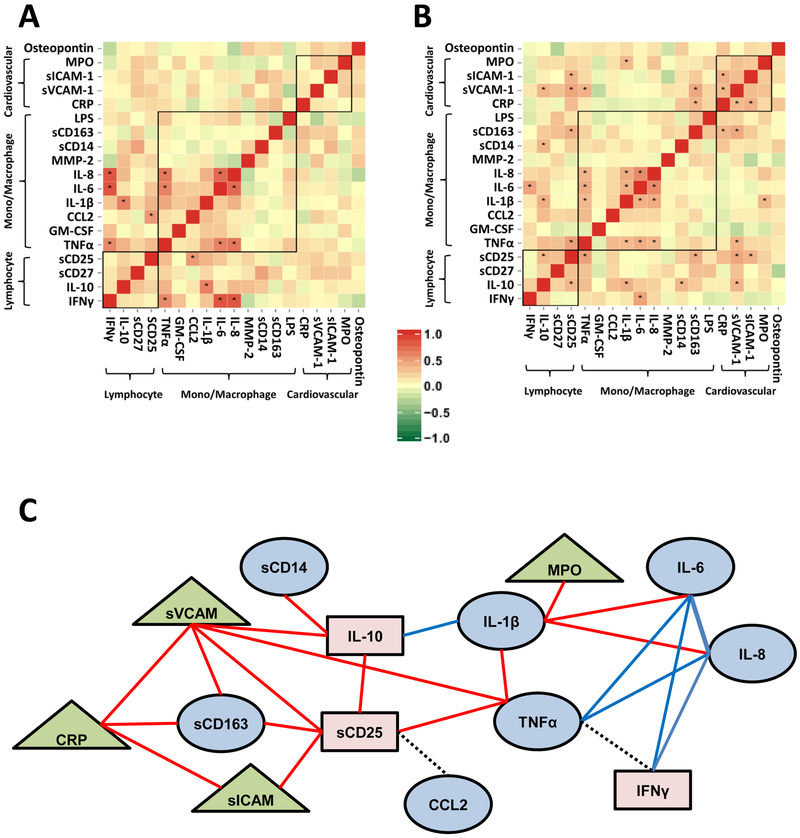
Figure 1: Biomarker Correlation Networks.
Heatmap of correlation matrix for HC (Panel A) and YLWH (Panel B). Biomarkers by classification are clustered top to bottom and right to left. Asterisks within black box indicate significant correlations (p< 0.0002 based on Bonferroni correction). Heat map colors and respective ρ are shown in the legend; positive red, negative green. Panel C shows significant pairwise correlations indicated by lines connecting biomarkers. Red lines indicate correlations found only in YLWH, blue lines indicate correlations found only in both YLWH and HC, and black dashed lines indicate correlations found in HC only. Monocyte/macrophage related biomarkers are depicted in blue ovals, lymphocyte biomarkers are pink rectangles, and cardiovascular biomarkers are green triangles.
Among the 129 YLWH 16 significant relationships with rho values ranging from 0.334–0.570 were observed (Figure 1B and Table S2). Most correlations were distinct to YLWH, including activation biomarkers for lymphocytes (TNFα and IL-10 with sCD25), macrophages (IL-1β with IL-6 or IL-8 and TNFα), and vascular inflammation biomarkers (CRP significantly correlated with sVCAM-1 and sICAM-1). Macrophage biomarkers sCD163 correlated with sCD25, while sCD14 correlated with IL-10. Macrophage and vascular inflammation biomarkers also significantly correlated within YLWH as shown by correlations between IL-1β with MPO, sVCAM-1 with TNFα, sCD163, sCD25, IL-10, and CRP. A summary of the biomarker correlation networks across lymphocyte, macrophage, and cardiovascular markers combined data from both HC and YLWH (Figure 1C). An unexpected finding was lack of significant correlations between many of the lymphocyte and macrophage activation markers, including sCD163 with sCD14 or IL-6; LPS with sCD14; or IFNγ with sCD27.
Macrophage and lymphocyte related cytokines contribute to bioprofiles unique to YLWH.
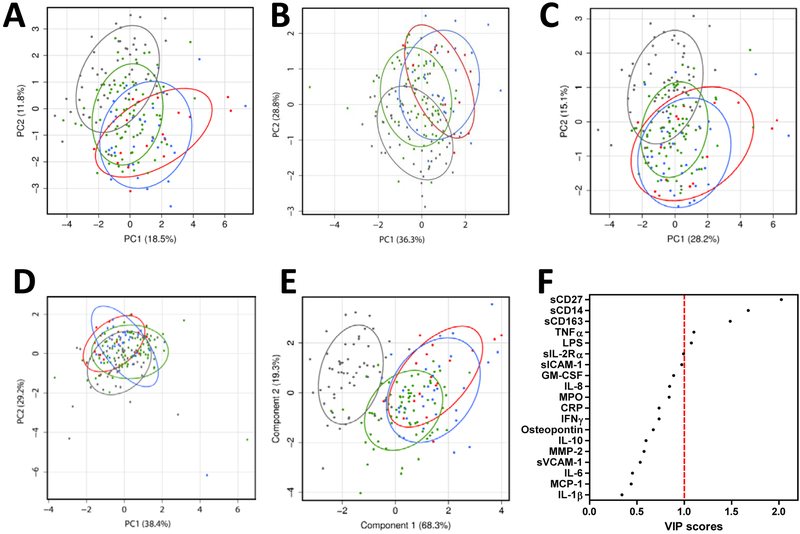
PCA was performed to reduce 19 biomarkers into a smaller set of components while retaining most of the variation. Biomarkers were evaluated within four outcome groups; HC, HIV+ ART+ VL−, HIV+ ART+ VL+, and HIV+ ART− VL+. Principal component (PC)1 vs. PC2 for all 19 biomarkers explained over 30% of the total variance (Figure 2A). Although the HC group was generally distinct from YLWH, considerable overlap between HC and virally suppressed (ART+ VL−) groups was apparent, while VL+ groups clustered together independent of treatment. Similar results occurred when the analysis was limited to the subset of biomarkers of lymphocyte activation (Figure 2B). In contrast, the PCA biplot of monocyte/macrophage activation markers showed overlap among all YLWH, independent of treatment or viral load, all distinct from HC. (Figure 2C) Based on cardiovascular biomarker-specific PCA biplot, all outcome groups clustered and were indistinguishable from each other (Figure 2D). PCA analysis of the 19 biomarkers based on CD4% did not distinguish among groups of YLWH (data not shown). Overall, clustering the biomarkers into specific inflammatory pathways revealed trends toward differences among the outcome groups.
Figure 2. Effect of HIV on bioprofile visualization.
PCA biplots from first and second principal components utilizing on all 19 biomarkers (Panel A), lymphocyte biomarkers (Panel B), monocyte/macrophage biomarkers (Panel C), cardiovascular biomarkers (Panel D) are shown. Percentage shown on the X and Y axis indicates percentage of total variance explained by the components. In Panel E all 19 biomarkers were utilized to create PLS-DA plot maximizing separation among four groups using first and second components. Percentage shown on the X and Y axis indicates percentage of intergroup variance explained by the components. Panel F shows a VIP plot depicting relative contribution of all 19 soluble factors to four group classification. Healthy controls are represented by grey dots and ellipses, ART+VL- are green dots and ellipses, ART+VL+ are blue dots and ellipses, and ART-VL+ are red dots and ellipses. Ellipses indicate normal contour line within one standard deviation away from mean.
sCD27, sCD14, and sCD163 are important contributors to separating biomarker bioprofiles in YLWH.
To identify biomarkers unique to YLWH a PLS-DA was applied. Components in PLS-DA, designed to separate groups of interest, were applied to visualize the bioprofile based on 19 biomarkers with maximum segregation between HC and the 3 HIV-infected groups. Cross-validation results suggested two components were optimal for classification; PLS scores for the first two components of the group separation explained 88% of the intergroup variance (Figure 2E).
Averaged VIP scores for the first two components identified the single biomarkers sCD27, sCD14 and sCD163 as most critical for the discrimination between the outcome group classification, while contributions to the separation by TNFα and LPS (Figure 2F). Taken together, these results indicate that a subset of markers from lymphocyte and macrophage activation domains contributed to discrimination between HIV+ (with or without viral suppression) and HC bioprofiles.
Key biomarker bioprofiles distinguishing YLWH.
To determine if bioprofiles containing all 19 biomarkers were significantly altered by HIV infection, therapy, or viral load a MANOVA was conducted using all participants (Table 3). Information deduced from 19 biomarkers generated unique bioprofiles distinguishing HC from YLWH regardless of ART or VL outcome (p<0.0001). This bioprofile also identified significant differences within HIV-infected outcome groups based on whether virus was fully suppressed or detectable. However, the 19-factor bioprofile was unable to distinguish a therapy effect alone, as untreated YLWH were not significantly different from those on ART. Similar results were obtained when the bioprofile was limited to only sCD27, sCD14, and sCD163, the biomarkers identified as top contributors in the PLS-DA. In this case the 3 biomarkers distinguished HC from all HIV+, VL+ or ART+ VL−. In addition, the 3-biomarker bioprofile was significantly different when VL− and VL+ YLWH with or without consideration of ART were compared. No effect of age, gender, or ethnicity was observed for any of the biomarkers in YLWH or HC. Overall, the application of sCD27, sCD14, and sCD163 provided the most discrimination between YLWH from HC.
Table 3.
Group comparison of bioprofiles utilizing all 19 biomarkers or 3 biomarkers with highest VIP scores.
| Bioprofile comparison | N | 19 biomarker | 3 biomarker |
|---|---|---|---|
| HC vs HIV | 56 vs 129 | <0.0001* | <0.0001* |
| HC vs VL+ | 56 vs 48 | <0.0001* | <0.0001* |
| HC vs ART VL- | 56 vs 79 | <0.0001* | <0.0001* |
| VL- vs VL+ | 80 vs 48 | <0.0001* | <0.0001* |
| ART VL- vs ART VL+ | 79 vs 31 | 0.0026* | 0.0002* |
| ART- vs ART+ | 18 vs 111 | 0.0533 | 0.0055* |
| ART- VL+ vs ART+ VL+ | 17 vs 31 | 0.5314 | 0.5039 |
indicate statistical significance.
Bonferroni-corrected significance cutoff: p<0.007.
Discussion
HIV perturbs multiple inflammatory pathways through effects on both macrophages and lymphocyte function. As a result biomarkers associated with HIV pathogenesis and complications vary greatly based on extent of CD4 T cell decline, viral load, age, length of infection, and infectious co-morbidities [1]. Biomarkers specific for YLWH are needed for the early identification HIV-associated co-morbidities when initiating ART before CD4 T cell decline. These biomarkers provide biological distinctions among individuals with normal CD4 T cells with, or without, suppressed virus. Interpretation of the relevance of inflammatory biomarkers in HIV-infected individuals is often hampered by the lack of appropriate HIV-uninfected control groups balanced for age, gender, ethnicity and other confounding variables. A unique aspect of the current study is inclusion of a balanced population of HIV-uninfected predominantly African American young men allowing comparisons between HC and YLWH with optimally suppressed or ongoing viral replication. [26] Comparisons among YLWH with and without viral suppression were enhanced because both groups were similar with respect to their CD4 T cells counts, demographics, and disease stage. While the impact of gender, ethnicity, and race could not be thoroughly evaluated due to the relatively homogenous make up the study population, nonetheless biomarker profiles emerged specific to YLWH with normal CD4 T cells many of whom initiated ART prior to CD4 decline. The findings of this study contrast to biomarker assessments within populations of chronically HIV-infected adults who have advanced disease where biomarkers such as IL-6 are associated with disease progression and morbidity [3, 23, 27, 28]. This is one of few studies of HIV-associated biomarkers among recently infected youth and are highly relevant because they identify the earliest inflammatory pathways perturbed by HIV. The distinct bioprofiles among YLWH are likely to enhance longitudinal assessments of interventions designed to attenuate long term metabolic, inflammatory, cardiovascular, and neurological complications of HIV.
Assessment of multiple biomarkers associated with different inflammatory pathways identified factors that normalized with ART when compared to HC, improved with ART but remain elevated relative to HC, or remained abnormal in spite of optimal ART. Differences among outcome groups identified inflammatory mechanisms independent or dependent on viral replication. For example, it was expected that effective ART would result in improved sCD27, sCD163 and TNF α levels [14, 15, 24], but unexpected that even with prolonged viral suppression these biomarkers would remain significantly higher in YLWH compared to HC. In contrast, effective viral suppression by ART normalized sCD25, TNFα, GM-CSF, and MPO. The results indicate that early use of ART and prolonged viral suppression can reverse many but not all of the inflammatory pathways impacted by HIV in other cohorts. Previous studies show that control of viral replication by ART has no impact on sCD14 levels, a biomarker of macrophage activation primarily mediated by microbial translocation [11, 14]. This inflammatory pathway remained unchanged even after years of viral suppression and confirms the persistence of macrophage activation due to gastrointestinal microbial translocation and LPS binding to TLR4/CD14 [18].
Biomarker measurements have a high degree of variability due to quantitative differences in assay methods, variation in plasma half-life, and overall production levels [29]. Several plasma biomarkers related to key immune pathways were below detectable levels of the assay in a significant proportion of the participants preventing statistical comparisons and therefore not included in the study. However, correlations between inflammatory biomarkers were identified. Associations between biomarkers of lymphocyte and macrophage activation revealed similarities and sharp contrasts between YLWH and HC. Highly associated biomarkers in HC generally involved factors associated with acute immune activation such as IFNγ, TNFα, IL-8, IL-6, IL-1β, and IL-10. These biomarkers are driven in general by viral and bacterial infections and inflammation and are not unique to HIV [30–36]. In contrast, associations among biomarker networks involved in chronic macrophage activation and vascular injury were seen among YLWH. sCD163 was associated with higher levels of sCD25, a known biomarker of macrophage, activation syndrome, and was also associated with sVCAM and CRP in conditions associated with vascular injury in both HIV and non-HIV associated conditions such as autoimmune disease [13, 23, 26, 37–40]. While both sCD14 and sCD163 are associated with macrophage activation, the markers represent distinct pathways impacted by HIV; therefore, the lack of associations between these two biomarkers is not surprising but indicate complex effects by HIV infection on multiple innate immunity pathways [41, 42].
Complex relationships between HIV-related inflammatory pathways, some related to direct viral replication and others indirect, complicates the assessment of biomarkers distinct to YLWH. PCA enabled better identification of the biomarkers unique to YLWH compared to HC. When aggregated, all biomarkers discriminate HC from YLWH. Comparisons of biomarkers associated with lymphocyte activation identified clear differences based on viral outcomes. HC and YLWH who suppressed viral replication clustered together and were distinct from YLWH who were VL+ whether receiving ART or not. In contrast, PCA of biomarkers of macrophage activation showed all YLWH clustering together regardless of viral outcome and distinct from HC. There were no distinguishing factors that differentiated HIV outcome groups and HC in the assessment of vascular markers. Simultaneous assessments of multiple biomarkers identify individual bioprofiles that normalize relative to HC or remain perturbed independent of viral replication. Although PCA provides an aggregate view of differences among outcome groups, the analysis does not reveal the extent of the contribution of specific biomarkers in defining individual groups.
Supervised PLS-DA provided distinct separation between HC and YLWH and revealed three biomarkers; sCD27, sCD14, and sCD163 showed the greatest separation between infected and uninfected youth. Refined bioprofile analysis provides sufficient information to recognize immune normalization and will be helpful in the identification of YLWH whose biomarker profiles show optimal responses to interventions designed to decrease viral replication and HIV-associated inflammation. Conversely, biomarker profiles showing persistent immune activation in spite of optimal viral suppression, may become apparent. However, they do provide accurate appraisals of the pathways most perturbed by HIV infection. This is the first study to simultaneously evaluate perturbations in inflammation and lymphocyte activation among YLWH who are in the early stages of infection. The pathways identified in YLWH can be compared to case controlled studies of HIV-infected adults who have developed HIV-associated comorbidities such as myocardial infarction and stoke [27]. Plasma biomarkers similar to both groups include TNF and sCD14 while IL-6 is more associated as a biomarkers of HIV comorbidities in older adults [4, 27, 39].
The current study identifies similarities and differences among viral outcome groups and is unique in its focus on YLWH who are still healthy and early in disease. YLWH ages 18 to 24 years are the predominant populations for newly infected youth. [43] The exclusion of younger adolescents under 18 years limits the applicability of the findings to all YLWH. However, examining changes in inflammatory biomarkers over time provides an opportunity to create novel models to assess the development of HIV-associated co-morbidities across the life span. These biomarkers can serve as intermediate outcomes for interventions to attenuate HIV complications. Now that initiation of ART is recommended for all infected individuals most YLWH should start therapy prior to CD4 T cell decline [44]. This study reveals interventions targeting inflammation mediated by macrophages are needed for YLWH to prevent long-term complications of HIV [45].
Supplementary Material
Acknowledgements:
This work was supported in part by funding from the National Institute on Drug Abuse (DA031017) and the Adolescent Trials Network for HIV/AIDS Interventions (ATN) from the National Institutes of Health (U01 HD040533 and U01 HD040474) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with supplemental funding from the National Institute on Drug Abuse and the National Institute for Mental Health. The study was co-endorsed by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group. Support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (U01 A1068632). Further support was provided by the Stephany W. Holloway Endowed University of Florida (UF) Chair for AIDS Research, the UF Center for Research in Pediatric Immune Deficiency, and the UF Laura McClamma Fellowship Endowment. This publication was also supported in part by funding from Duke University Center for AIDS Research (CFAR), (NIH 5P30 AI064518).
The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilberman, Julian), Children’s Hospital of Los Angeles (Belzer, Flores, Tucker), University of Southern California at Los Angeles (Kovacs, Homans, Lozano), Childrens National Medical Center (D’Angelo, Hagler, Trexler), Children’s Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California-San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children’s Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude’s Children’s Research Hospital (Flynn, Dillard), and Children’s Memorial (Garofalo, Brennan, Flanagan).
The following IMPAACT sites participated in the study: Children’s Hospital of Michigan – Wayne State (Moore, Rongkavilit, Hancock), Duke University Medical Center Pediatric CRS (Cunningham, Wilson), Johns Hopkins University (Ellen, Chang, Noletto), New Jersey Medical School CTU/CRS (Dieudonne, Bettica, Monti), St. Jude/Memphis CTU/CRS (Flynn, Dillard, McKinley), University of Colorado School of Medicine/The Children’s Hospital (Reirden, Kahn, Witte) University of Southern California Medical Center (Homans, Lozano), Howard University Hospital (Rana, Deressa),
Four of the ATN and IMPAACT sites utilized their General Clinical Research Center (GCRC)/Pediatric Clinical Research Center (PCRC) for the study. The centers were supported by grants from the General Clinical Research Center Program of the National Center for Research Resources (NCRR), National Institutes of Health, Department of Health and Human Services as follows: Children’s National Medical Center, M01RR020359; Howard University Hospital, MO1-RR010284; University of California at San Francisco, UL1 RR024131; and University of Colorado School of Medicine/Children’s Hospital, UL1 RR025780. The University of Pennsylvania/Children’s Hospital of Philadelphia utilized its Institutional Clinical and Translational Science Award Research Center (CTRC), supported by grant UL1 RR024134 from NCRR. The Tulane University Health Sciences Center utilized its Clinical and Translational Research Center (CTRC) for the study which was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP (RC/EEP - 06). The authors thank Kai-fen Chang, Guglielmo Venturi, Bernard Fischer, and Susan Lukas for technical support and are grateful to all of the youth who agreed to participate in the study.
Sources of support: NIH grants (DA031017, U01 HD040533, U01 HD040474, U01 AI068632, 5P30 AI064518, UO1 DA044571), Duke University Center for AIDS Research (CFAR), (NIH 5P30 AI064518), Stephany W. Holloway Endowed University of Florida (UF) Chair for AIDS Research, the UF Center for Research in Pediatric Immune Deficiency, and the UF Laura McClamma Fellowship Endowment.
REFERENCES
- 1.Hunt PW (2012) HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 9, 139–47. [DOI] [PubMed] [Google Scholar]
- 2.Rodger AJ, Lodwick R, Schechter M, Deeks S, Amin J, Gilson R, Paredes R, Bakowska E, Engsig FN, Phillips A, INSIGHT SMART, E. S. P. R. S. G. (2013) Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 27, 973–9. [DOI] [PubMed] [Google Scholar]
- 3.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, Neaton JD, Brenchley JM, Deeks SG, Sereti I, Douek DC, Group, I. S. S. (2011) Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 203, 780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson EM, Singh A, Hullsiek KH, Gibson D, Henry WK, Lichtenstein K, Önen NF, Kojic E, Patel P, Brooks JT, Sereti I, Baker JV, Investigators, S. t. U. t. N. H. o. H. A. i. t. E. o. E. T. S. S. (2014) Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis 210, 1396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, Lederman MM, McComsey GA (2013) Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med 14, 385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC (2014) HIV Among Youth. In HIV/AIDS. [Google Scholar]
- 7.(2016) What’s New in the Guidelines? | Adult and Adolescent ARV Guidelines | [Google Scholar]
- 8.WHO (2015) Policy brief: consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: what’s new. [Google Scholar]
- 9.Rudy BJ, Lindsey JC, Flynn PM, Bosch RJ, Wilson CM, Hughes ME, Douglas SD, Team, P. A. C. T. G. S. (2006) Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses 22, 213–21. [DOI] [PubMed] [Google Scholar]
- 10.Rudy BJ, Crowley-Nowick PA, Douglas SD (2001) Immunology and the REACH study: HIV immunology and preliminary findings. Reaching for Excellence in Adolescent Care and Health. J Adolesc Health 29, 39–48. [DOI] [PubMed] [Google Scholar]
- 11.Rudy BJ, Kapogiannis BG, Worrell C, Squires K, Bethel J, Li S, Wilson CM, Agwu A, Emmanuel P, Price G, Hudey S, Goodenow MM, Sleasman JW, Interventions, A. T. N. f. H. (2015) Immune Reconstitution but Persistent Activation After 48 Weeks of Antiretroviral Therapy in Youth With Pre-Therapy CD4 >350 in ATN 061. J Acquir Immune Defic Syndr 69, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan K, Harris DR, Emmanuel P, Fielding RA, Worrell C, Kapogiannis BG, Monte D, Sleasman J, Wilson CM, Aldrovandi GM, team, A. P. (2012) Low bone mass in behaviorally HIV-infected young men on antiretroviral therapy: Adolescent Trials Network Study 021B. Clin Infect Dis 55, 461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syed SS, Balluz RS, Kabagambe EK, Meyer WA, Lukas S, Wilson CM, Kapogiannis BG, Nachman SA, Sleasman JW (2013) Assessment of biomarkers of cardiovascular risk among HIV type 1-infected adolescents: role of soluble vascular cell adhesion molecule as an early indicator of endothelial inflammation. AIDS Res Hum Retroviruses 29, 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallet MA, Rodriguez CA, Yin L, Saporta S, Chinratanapisit S, Hou W, Sleasman JW, Goodenow MM (2010) Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS 24, 1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burdo TH, Lentz MR, Autissier P, Krishnan A, Halpern E, Letendre S, Rosenberg ES, Ellis RJ, Williams KC (2011) Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J Infect Dis 204, 154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols SL, Bethel J, Kapogiannis BG, Li T, Woods SP, Patton ED, Ren W, Thornton SE, Major-Wilson HO, Puga AM, Sleasman JW, Rudy BJ, Wilson CM, Garvie PA, Interventions, a. t. A. M. T. N. f. H. A. (2015) Antiretroviral treatment initiation does not differentially alter neurocognitive functioning over time in youth with behaviorally acquired HIV. J Neurovirol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nichols SL, Lowe A, Zhang X, Garvie PA, Thornton S, Goldberger BA, Hou W, Goodenow MM, Sleasman JW (2014) Concordance between self-reported substance use and toxicology among HIV-infected and uninfected at risk youth. Drug Alcohol Depend 134, 376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12, 1365–71. [DOI] [PubMed] [Google Scholar]
- 19.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, Martínez-Maza O, Bream JH (2015) The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 29, 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Wu Y, Keating SM, Du H, Sammet CL, Zadikoff C, Mahadevia R, Epstein LG, Ragin AB (2013) Matrix metalloproteinase levels in early HIV infection and relation to in vivo brain status. J Neurovirol 19, 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borato DC, Parabocz GC, Ribas SR, Kalva-Filho CA, Borba LM, Ito CA, Bail L, dos Santos FA, Vellosa JC (2012) Changes of metabolic and inflammatory markers in HIV infection: glucose, lipids, serum Hs-CRP and myeloperoxidase. Metabolism 61, 1353–60. [DOI] [PubMed] [Google Scholar]
- 22.Fourie CM, Schutte AE, Smith W, Kruger A, van Rooyen JM (2015) Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis 240, 154–60. [DOI] [PubMed] [Google Scholar]
- 23.Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, Bohjanen PR, Novak RM, Neaton JD, Sereti I, Group, I. S. (2011) Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis 203, 1637–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Milito A, Aleman S, Marenzi R, Sonnerborg A, Fuchs D, Zazzi M, Chiodi F, D F (2002) Plasma levels of soluble CD27: a simple marker to monitor immune activation during potent antiretroviral therapy in HIV-1-infected subjects. Clin Exp Immunol 127, 486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q, Lifson JD, Duan L, Schacker TW, Reilly C, Carlis J, Estes JD, Haase AT (2005) Potential roles of follicular dendritic cell-associated osteopontin in lymphoid follicle pathology and repair and in B cell regulation in HIV-1 and SIV infection. J Infect Dis 192, 1269–76. [DOI] [PubMed] [Google Scholar]
- 26.Enkhmaa B, Anuurad E, Zhang W, Kim K, Berglund L (2015) Diverging trajectory patterns of systemic versus vascular inflammation over age in healthy Caucasians and African-Americans. Atherosclerosis 239, 509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, Lederman MM, Landay AL (2014) Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 210, 1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, McLaughlin B, Landay AL, Adeyemi O, Gilman LE, Clagett B, Rodriguez B, Martin JN, Schacker TW, Shacklett BL, Palmer S, Lederman MM, Deeks SG (2013) The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 121, 4635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong HL, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS (2008) Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol Biomarkers Prev 17, 3450–6. [DOI] [PubMed] [Google Scholar]
- 30.Sachdeva N, Yoon HS, Oshima K, Garcia D, Goodkin K, Asthana D (2007) Biochip array-based analysis of plasma cytokines in HIV patients with immunological and virological discordance. Scandinavian journal of immunology 65, 549–54. [DOI] [PubMed] [Google Scholar]
- 31.Feng X, Scheinberg P, Wu CO, Samsel L, Nunez O, Prince C, Ganetzky RD, McCoy JP, Maciejewski JP, Young NS (2011) Cytokine signature profiles in acquired aplastic anemia and myelodysplastic syndromes. Haematologica 96, 602–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung R, Proitsi P, Simmons A, Lunnon K, Güntert A, Kronenberg D, Pritchard M, Tsolaki M, Mecocci P, Kloszewska I, Vellas B, Soininen H, Wahlund LO, Lovestone S (2013) Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PLoS One 8, e64971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacerda HR, Falcão M.a. C., de Albuquerque VM, Zírpoli JC, Miranda-Filho D. e. B., de Albuquerque M. e. F., Montarroyos U, Ximenes RA (2014) Association of inflammatory cytokines and endothelial adhesion molecules with immunological, virological, and cardiometabolic disease in HIV-infected individuals. J Interferon Cytokine Res 34, 385–93. [DOI] [PubMed] [Google Scholar]
- 34.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, Lederman MM, Storer N, Labbato DE, McComsey GA (2014) Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 28, 969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sturgill J, McGee E, Menzies V (2014) Unique cytokine signature in the plasma of patients with fibromyalgia. J Immunol Res 2014, 938576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Zhang X, Zhao B, Wang J, Zhu Z, Teng Z, Shao J, Shen J, Gao Y, Yuan Z, Wu F (2011) Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One 6, e28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy VV, Myles A, Cheekatla SS, Singh S, Aggarwal A (2014) Soluble CD25 in serum: a potential marker for subclinical macrophage activation syndrome in patients with active systemic onset juvenile idiopathic arthritis. Int J Rheum Dis 17, 261–7. [DOI] [PubMed] [Google Scholar]
- 38.Zakynthinos E and Pappa N (2009) Inflammatory biomarkers in coronary artery disease. J Cardiol 53, 317–33. [DOI] [PubMed] [Google Scholar]
- 39.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D (2012) Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr 60, 234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cassol E, Misra V, Morgello S, Gabuzda D (2013) Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J Neuroimmune Pharmacol 8, 1087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenchley JM and Douek DC (2012) Microbial translocation across the GI tract. Annu Rev Immunol 30, 149–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown JN, Kohler JJ, Coberley CR, Sleasman JW, Goodenow MM (2008) HIV-1 activates macrophages independent of Toll-like receptors. PLoS One 3, e3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC (2015) HIV Surveillance Report. 27. [Google Scholar]
- 44.Tabernilla A and Poveda E (2015) The START Trial: Definitive Evidence to Treat All HIV-Positive Persons Regardless of CD4 Counts. AIDS Rev 17, 187. [PubMed] [Google Scholar]
- 45.Zanoni BC and Mayer KH (2014) The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 28, 128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.