Abstract
Importance
Bipolar disorder (BD) is difficult to distinguish from other psychiatric disorders. Neuroimaging studies can identify objective markers of BD risk.
Objective
To identify neuroimaging measures in emotion processing and regulation neural circuitries, and their relationships with symptoms, specific to youth at risk for BD.
Design
Cross-sectional (August 2011-July 2017) and longitudinal (February 2013-November 2017) neuroimaging study.
Setting
Academic Medical Center: University of Pittsburgh.
Participants
Referred sample of offspring of bipolar parents (OBP;n=31) and offspring of comparison parents with non-BD psychopathology (OCP;n=28) from the Bipolar Offspring Study and offspring of healthy parents (OHP;n=21) from the Longitudinal Assessment of Manic Symptoms Study.
Main Outcomes and Measures
Elastic net regressions and ANOVAs examined group differences in activity and functional connectivity (FC) during emotional face processing and n-back task performance in amygdala, dorsolateral and ventrolateral prefrontal cortices (PFC), caudal (cACC) and rostral (rACC) anterior cingulate cortices. Correlation analyses examined relationships among neuroimaging measures showing between-group differences and symptom severity (anxiety, affective lability, depression, mania). We hypothesized: elevated amygdala activity and/or lower PFC activity and abnormal amygdala-PFC FC would distinguish OBP from OCP and OHP, and magnitudes of these abnormalities would positively correlate with elevated symptom severity. We explored relationships between changes in neuroimaging and symptom measures over follow-up (mean(SD)=2.88(1.37) years) in a subset of participants (n=30).
Results
Eighty participants were included (mean(SD) age=14.17(2.06), 35 female). Twelve neuroimaging measures explained 51% of the variance in group. Of these, seven showed significant effects of group (P<.05, corrected). Of these, two showed significant relationships with symptoms. OBP had greater right rACC activity when regulating attention to happy faces versus OCP (mean(SD) difference=.744(.249), 95%CI=.134–1.354, P=.011), which positively correlated with affective lability severity (ρ=.304, P=.006, uncorrected). OBP had greater amygdala-left cACC FC when regulating attention to fearful faces versus OCP (mean(SD) difference=.493(.169), 95%CI=.079-.908, P=.014). Increases in this measure positively correlated with increases in affective lability over follow-up (r=.541, P=.003).
Conclusions and Relevance
Greater anterior cingulate cortex activity and FC during emotion regulation tasks may be specific markers of BD risk. These findings highlight potential neural targets to aid earlier identification of, and guide new treatment developments for, BD.
Background
Bipolar Disorder (BD), a serious, recurrent illness, often emerges during adolescence1–3. 15–28% of adults with BD experience illness onset before age 13 years and 50–66% before age 194-6. Approximately 5.6% of adolescents have subthreshold manic, hypomanic, or depressive symptoms, while some symptoms of BD overlap with other disorders, such as Major Depressive Disorder (MDD), Attention Deficit/Hyperactive Disorder (ADHD), or Anxiety Disorders, making it difficult to diagnose BD7,8. It is thus important to identify objective biological markers to help differentiate BD from other disorders.
BD has a heritability of 59–87%, placing first-degree relatives at high risk for BD9. Compared with children of parents without psychiatric illness, offspring of bipolar parents (OBP) are at increased risk of BD and other mood and anxiety disorders10. Studying OBP, and comparing OBP with offspring of healthy parents (OHP), can identify early phenotypes associated with BD risk. An additional comparison group is necessary to determine whether risk markers are specific to BD or to general psychopathology, however. In a recent study, 23% of OBP developed a bipolar spectrum disorder by age 21 compared with 3.2% in offspring of comparison parents (OCP) with a non-BD diagnosis11. Including OCP can thus control for risk for non-BD psychiatric disorders and for environmental effects of living with a parent with psychiatric illness12. The Bipolar Offspring Study (BIOS) is a longitudinal study that aims to identify objective neural markers of BD risk by comparing emotion processing and regulation neural circuitries in OBP and OCP13. Two previous BIOS studies examined activity and functional connectivity (FC) using emotion processing and regulation tasks, separately14,15. No studies examined how measures of activity and FC in emotion processing and emotional regulation neural circuitries distinguish OBP from control groups.
Neural regions implicated in emotion processing16 and regulation17 include the amygdala, anterior cingulate cortex (ACC), and dorsolateral (dlPFC) and ventrolateral (vlPFC) prefrontal cortex. Functional abnormalities in these circuitries in youth and adults with BD18 include elevated amygdala activity to emotional stimuli19,20, lower prefrontal cortical (PFC) activity during emotion regulation16,21,22, and lower amygdala-vlPFC FC23–28. Cross-sectional studies of BD at-risk youth reported mixed results. Compared with OHP, OBP showed greater vlPFC activity to happy faces and reduced amygdala-vlPFC FC to fearful faces during emotional regulation15, greater amygdala activity to fearful faces during emotion processing29, and abnormal PFC-subcortical resting state FC30. Comparing all three groups during emotional face processing, OBP and OCP showed greater right amygdala activity to all emotional faces versus OHP, while OBP showed lower positive right amygdala-ACC FC to all emotional faces and more positive right amygdala-left vlPFC FC to happy faces than OCP and OHP14. More studies are needed to identify abnormalities in emotion processing and regulation neural circuitries specific to OBP.
Relationships between neuroimaging measures and symptoms associated with BD risk remain relatively unexamined. Significant symptoms of anxiety, affective lability, depression, and mania are the strongest dimensions of psychopathology associated with BD risk31. In emotionally dysregulated youth, worsening affective lability and depression severity correlated with increased right amygdala and left vlPFC activity, worsening anxiety with decreased right amygdala and increased left vlPFC activity, and worsening mania with increased right amygdala and decreased left vlPFC activity over time32. In OCP, right amygdala-ACC FC positively correlated with affective lability, depression, and anxiety severity14. Such studies have yet to find significant relationships between functioning in emotion processing and regulation neural circuitries and symptom severity in OBP, however. Examining these relationships can improve understanding of BD development in youth, and may enhance early identification of BD risk in, and guide novel interventions for, OBP.
Given studies showing differences between OBP and both OCP and OHP in emotion processing and regulation neural circuitries, and the importance of relating these measures to symptoms associated with BD risk, we hypothesized: elevated amygdala and/or lower PFC activity and abnormal amygdala-PFC FC in emotion processing and regulation neural circuitries would distinguish OBP from OCP and OHP; and magnitudes of these abnormal neuroimaging measures would be positively associated with elevated anxiety, affective lability, depression, and/or mania severity in OBP versus other youth. In exploratory analyses, we examined whether changes in neuroimaging measures over time were significantly associated with changes in symptom severity in all offspring.
Methods
Participants
Thirty-one OBP (mean(SD) age=13.87(2.42), 15 female) and twenty-eight OCP (mean(SD) age=14.48(2.01), 10 female) were recruited from BIOS33, and twenty-one OHP (mean(SD) age=14.20(1.48), 10 female) from BIOS and the Longitudinal Assessment of Manic Symptoms Study (Table 1)34,35. Participants were matched for age, sex, IQ, and socioeconomic status (SES). Twenty-six OBP, twenty-one OCP, and nineteen OHP were included in Manelis et al., 201514.
Table 1.
Comparison of OBP, OCP, and OHP.
| OBP N=31 M(SD) or Total |
OCP N=28 M(SD) or Total |
OHP N=21 M(SD) or Total |
Statistic | P = | |
|---|---|---|---|---|---|
| Demographic Information | |||||
| Age | 13.87(2.42) | 14.48(2.01) | 14.20(1.48) | F = 0.648 | .53 |
| Sex (females) | 15 | 10 | 10 | χ2 = 1.133 | .57 |
| IQ | 101.13(15.55) | 101.75(14.84) | 105.71(12.18) | F = 0.692 | .50 |
| Socioeconomic Status | χ2 = 13.986 | .08 | |||
| Very Low (8–19) | 7 | 5 | 1 | ||
| Low (20–29) | 8 | 1 | 4 | ||
| Medium (30–39) | 6 | 4 | 1 | ||
| High (40–54) | 7 | 10 | 9 | ||
| Very High (55–66) | 3 | 8 | 6 | ||
| Handedness | χ2 = 5.050 | .28 | |||
| Right | 26 | 26 | 19 | ||
| Left | 2 | 2 | 2 | ||
| Mixed | 3 | 0 | 0 | ||
| Highest Parental Education | χ2 = 5.960 | .43 | |||
| High School Graduate or Lower | 5 | 1 | 4 | ||
| Partial College or Specialized Training | 13 | 8 | 8 | ||
| Standard College or University Graduate | 7 | 11 | 5 | ||
| Graduate Professional Training | 6 | 8 | 4 | ||
| Clinical Measures | |||||
| Diagnosis | 12 | 14 | 0 | F = 8.569 | <.01a |
| Major Depressive Disorder | 3 | 3 | 0 | F = 1.156 | .32 |
| Anxiety Disorder | 3 | 5 | 0 | F = 2.164 | .12 |
| Attention Deficit/Hyperactivity Disorder | 5 | 8 | 0 | F = 3.807 | .03a |
| Oppositional Defiant or Conduct Disorder | 1 | 3 | 0 | F = 1.623 | .20 |
| Obsessive Compulsive Disorder | 0 | 1 | 0 | F = 0.927 | .40 |
| Eating Disorder | 1 | 0 | 0 | F = 0.786 | .46 |
| Psychotropic Medication Use | 5 | 6 | 0 | F = 2.608 | .08 |
| Scan Day Assessments | |||||
| SCARED-P | 9.84(6.92) | 9.50(11.14) | 4.62(4.69) | F = 2.932 | .06 |
| SCARED-C | 12.81(14.95) | 8.00(12.16) | 9.36(11.86) | F = 1.029 | .36 |
| CALS-P | 8.19(9.19) | 4.64(4.84) | 1.62(2.71) | F = 6.464 | <.01a |
| CALS-C | 10.32(12.48) | 5.32(8.76) | 6.19(13.96) | F = 1.504 | .23 |
| MFQ-P | 6.55(9.08) | 4.48(5.00) | 1.38(2.13) | F = 3.909 | .02a |
| MFQ-C | 8.41(10.87) | 7.84(10.95) | 5.29(11.03) | F = 0.536 | .59 |
| Assessment Closest to Scan | |||||
| KMRS | 1.77(2.69) | 0.54(1.04) | 0.05(0.23) | F = 6.223 | <.01a |
| KDRS | 2.58(5.26) | 2.00(3.74) | 0.26(0.56) | F = 2.005 | .14 |
Abbreviations: =significant at P=.05; F=ANOVA test statistical value; χ2=chi-squared test statistical value; OBP=Offspring of Bipolar Parents; OCP=Offspring of Comparison Parents; OHP=Offspring of Healthy Parents; IQ=Intelligence Quotient Wechsler Intelligence Test; -P=Parent Rating; -C=Child Rating; SCARED=Screen for Child Anxiety Related Emotional Disorders; CALS=Children’s Affective Lability Sale; MFQ=Mood and Feelings Questionnaire; KMRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale; KDRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Depression Rating Scale.
OBP had at least one parent with BD; OCP had at least one parent with a non-BD disorder: MDD, ADHD, and/or an Anxiety Disorder. Exclusion criteria included: history of serious medical illness, head injury, or neurological disorder; IQ<70, assessed with Wechsler Abbreviate Scale of Intelligence36; BD, autism, or schizophrenia; magnetic resonance imaging (MRI) contraindication (e.g., pregnancy, metal in the body); substance abuse on the day of the scan or substance abuse disorder in the last three months; and task accuracy<70%. For OHP, additional exclusion criteria included history of DSM-5 disorder. Before participation, parents and guardians provided written informed consent, and youth provided written informed assent. Participants received monetary compensation. (Supplementary Material for recruitment and exclusion criteria).
Psychiatric diagnoses were confirmed by a licensed psychiatrist or psychologist before scanning using the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS)-Present and Lifetime Version37 for offspring, and the Structural Clinical Interview for DSM-IV38 for parents. Symptom assessments included the Screen for Child Anxiety Related Disorders (SCARED)39,40, Children’s Affective Lability Sale (CALS)41, Mood and Feelings Questionnaire (MFQ)42, and K-SADS Mania (KMRS)43 and Depression (KDRS)37 Rating Scales. Parent-reported (-P) and child-reported (-C) SCARED, CALS, and MFQ were administered on the scan day; summary KMRS and KDRS interviews, based on both parent and child information, were administered, on average, two months after the scan.
Five OBP and six OCP took antidepressant, antipsychotic, stimulant, and/or non-stimulant medications for non-BD diagnoses. Medicated OBP had greater CALS-P severity than unmedicated OBP (mean(SD) difference=8.853(3.916), 95%CI=.845–16.862, t(29)=−2.261, P=.031, uncorrected).
Neuroimaging Data Acquisition
All scan 1 and fifteen scan 2 images (mean(SD)=2.88(1.37) year inter-scan interval) were acquired on a Siemens Magnetom TrimTrio 3T scanner. Fifteen scan 2 images were acquired on a Siemens Magnetom Prisma scanner. Participants completed an emotional face processing task, the dynamic faces task (DFT), during functional MRI (fMRI) to assess implicit emotional processing21,44–46, and an emotional face n-back task, with 0-back (EF-0-BACK) and 2-back (EF-2-BACK) conditions, to examine neural regions implicated in emotional regulation, during redirection of attention away from emotionally-salient distracters during a working memory task47. (Supplementary Material)
Neuroimaging Data Analyses
(Supplementary Material for preprocessing). Generalized psychophysiological interaction analyses assessed task-related connectivity between a bilateral amygdala seed and regions of interest (ROIs). Task stimulus contrasts included, separately: happy, sad, angry, and fearful faces versus shapes for DFT; fearful, happy, and neutral versus no faces, and fearful and happy versus neutral faces, for EF-0-BACK and EF-2-BACK; and EF-2-BACK versus EF-0-BACK for fearful, happy, neutral, and no faces. ROIs, anatomically defined using FreeSurfer Center for Morphometric Analysis standard labels, included bilateral amygdala, caudal ACC (cACC), rostral ACC (rACC), dlPFC, and vlPFC. Individual-level averaged Blood-Oxygen-Level Dependent waveforms to the onset of each stimulus type were extracted in native space from anatomic ROIs to main stimulus contrasts per task.
Primary Hypotheses
A single elastic net regression analysis with k=10-fold cross-validation and alpha=0.5 was used for data selection and reduction using GLMNET in R48. This one model contained 2 dummy-coded outcome variables: BD risk (OBP versus OCP/OHP) and general psychiatric disorders risk (OBP/OCP versus OHP), and 336 predictor variables: demographics (age, sex, IQ, SES (assessed with Hollingshead Four Factor Index of Social Status49), handedness, highest parental education); FC between bilateral amygdala and each ROI (left/right cACC, rACC, dlPFC, vlPFC) and activity in each ROI (left/right amygdala, cACC, rACC, dlPFC, vlPFC) for each contrast and task. (Supplementary Material)
Post-hoc pseudo r-squared analyses examined the proportion of variance in dependent variables explained by the non-zero predictor variables observed with elastic net. ANOVAs and post-hoc t-tests examined between-group differences in neuroimaging measures for all non-zero predictors and symptom measures. Correlation analyses examined relationships among neuroimaging and symptom measures.
Exploratory Analyses
In nine OBP, seven OCP, and fourteen OHP with second scans, correlation and linear regression analyses examined relationships between changes in symptoms and changes in neuroimaging measures showing between group differences in the above analyses. All analyses were repeated removing medicated youth. (Supplementary Material)
Results
Hypothesis Testing
Of the initial 336 predictors, 12 variables, together, optimized model fit (ΔAICc=1.811, λ=0.553; Figure 1). A pseudo r-squared, calculated containing 12 predictors from the model versus an intercept-only model, indicated that 51.39% of the variance in group was explained by these predictors. All predictors were neuroimaging variables (Table 2). Post-hoc t-tests, Bonferroni-corrected for three between-group parallel tests, examined all twelve neuroimaging measures that were selected as non-zero predictors of group (Figure 2). Compared with OHP, OBP had lower DFT left dlPFC activity to angry faces versus shapes (mean(SD) difference=.108(.033), 95%CI=.027-.189, P=.005). Compared with OCP, OBP had greater EF-2-BACK amygdala-left cACC FC to fearful (mean(SD) difference=.493(.169), 95%CI=.079-.908, P=.014), happy (mean(SD) difference=.516(.148), 95%CI=.155-.877, P=.002), and neutral (mean(SD) difference=.604(.159), 95%CI=.215-.992, P=.001) versus no faces, and greater EF-2-BACK right rACC activity to happy versus no faces (mean(SD) difference=.744(.249), 95%CI=.134–1.354, P=.011). Compared with OHP, OCP had lower EF-0-BACK left (mean(SD) difference=.802(.241), 95%CI=.212–1.391, P=.004) and right (mean(SD) difference=.691(.236), 95%CI=.113–1.269, P=.014) rACC activity to happy versus neutral faces, and OBP had lower EF-0-BACK right rACC activity to happy versus neutral faces (mean(SD) difference=.626(.231), 95%CI=.060–1.192, P=.025). No significant group differences were found for the remaining measures.
Figure 1. Elastic Net Plots Generated in GLMNET.
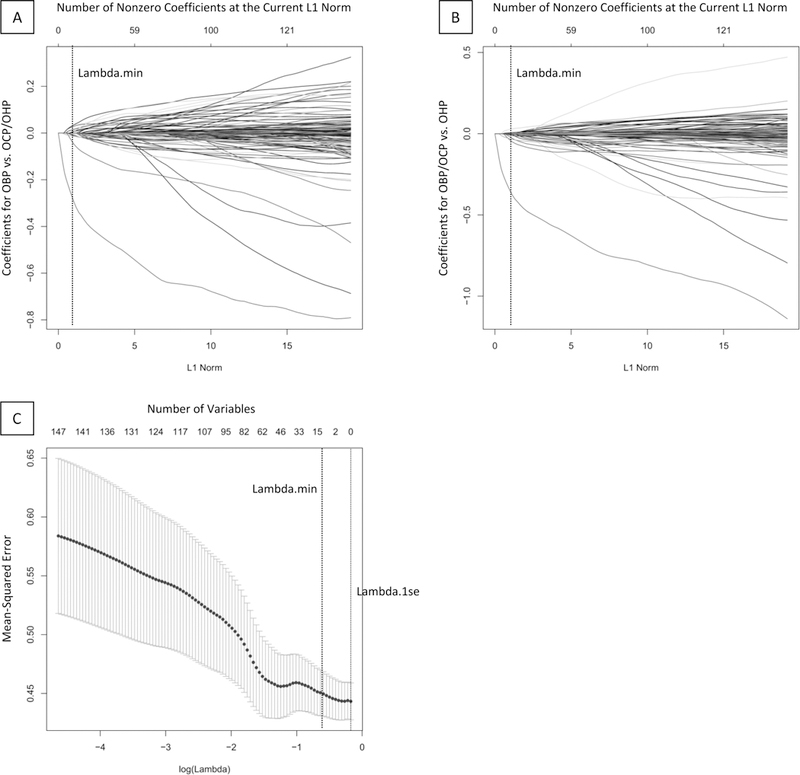
A-B. Plots of variable fit for BD risk group (OBP versus OCP and OHP, A) and general risk group (OBP and OCP versus OHP, B). Each curve corresponds to an independent variable in the full model prior to optimization. Curves indicate the path of each variable coefficient as λ varies. Lambda.min (λ=0.553) corresponds to the λ which corresponds to the selected model with 12 predictor variables. C. Plot of non-zero variable fit after cross validation. Representation of the 10-fold cross validation performed for the elastic net regression that chooses the optimal λ. Lambda.min corresponds to the λ which minimizes mean squared error. Lambda.1se corresponds to the λ that is one standard error from the lambda.min.
Abbreviations: Bipolar Disorder (BD); Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Offspring of Healthy Parents (OHP).
Table 2.
Between-Group Differences in Neuroimaging and Symptom Measures.
| Neuroimaging Measure | ANOVA | Bonferroni Sig. Test for Multiple Comparisons | |||
|---|---|---|---|---|---|
| F = | P = | OBP vs. OCP P = |
OBP vs. OHP P = |
OCP vs. OHP P = |
|
| DFT: Amygdala-Left dlPFC FC to Sad Faces vs. Shapes | 3.010 | .055 | .768 | .049 | .526 |
| DFT: Left dlPFC Activity to Angry Faces vs. Shapes | 5.522 | .006a | 1.00 | .005a | .057 |
| EF-2-BACK: Amygdala-Left cACC FC to Fearful vs. No Faces | 4.352 | .016a | .014a | .289 | .985 |
| EF-2-BACK: Amygdala-Left cACC FC to Happy vs. No Faces | 6.110 | .003a | .002a | .337 | .352 |
| EF-2-BACK Task: Amygdala-Left cACC FC to Neutral vs. No Faces | 7.413 | .001a | .001a | .784 | .068 |
| EF-2-BACK: Amygdala-Right vlPFC FC to Happy vs. Neutral Faces | 2.007 | .141 | .822 | .152 | 1.00 |
| EF-2-BACK: Right rACC Activity to Happy vs. No Faces | 4.458 | .015a | .011a | .591 | .477 |
| EF-0-BACK: Amygdala-Left dlPFC FC to Happy vs. No Faces | 3.368 | .040a | 1.00 | .100 | .053 |
| EF-0-BACK: Amygdala-Left rACC FC to Happy vs. Neutral Faces | 2.254 | .112 | .551 | .126 | 1.00 |
| EF-0-BACK: Left rACC Activity to Happy vs. Neutral Faces | 5.643 | .005a | .716 | .071 | .004a |
| EF-0-BACK: Right rACC Activity to Happy vs. Neutral Faces | 5.039 | .009a | 1.00 | .025a | .014a |
| EF-2-BACK vs. EF-0-BACK: Amygdala-Left rACC FC to Happy Faces | 3.247 | .044a | .074 | .146 | 1.00 |
| Symptom Measure | ANOVAb | Bonferroni Sig. Test for Multiple Comparisons | |||
|---|---|---|---|---|---|
| F = | P = | OBP vs. OCP P = |
OBP vs. OHP P = |
OCP vs. OHP P = |
|
| SCARED-P | 2.932 | .059 | 1.00 | .084 | .131 |
| SCARED-C | 1.029 | .362 | .504 | 1.00 | 1.00 |
| CALS-P | 6.464 | .003a (.024a) | .123 | .002a | .343 |
| CALS-C | 1.504 | .229 | .320 | .652 | 1.00 |
| MFQ-P | 3.909 | .024a (.192) | .730 | .020 | .328 |
| MFQ-C | 0.536 | .588 | 1.00 | .966 | 1.00 |
| KMRS | 6.223 | .003a (.024a) | .032a | .005a | 1.00 |
| KDRS | 2.005 | .142 | 1.00 | .155 | .451 |
Abbreviations: =significant at P=.05;
=additional Bonferroni corrections presented in parentheses; F=ANOVA test statistical value; OBP=Offspring of Bipolar Parents; OCP=Offspring of Comparison Parents; OHP=Offspring of Healthy Parents; DFT=Dynamic Faces Task; EF-2-BACK=Emotional 2-Back Task; EF-0-BACK=Emotional 0-Back Task; FC=Functional Connectivity; dlPFC=Dorsolateral Prefrontal Cortex; cACC=Caudal Anterior Cingulate Cortex; vlPFC=Ventrolateral Prefrontal Cortex; rACC=Rostral Anterior Cingulate Cortex; -P=Parent Rating; -C=Child Rating; SCARED=Screen for Child Anxiety Related Emotional Disorders; CALS=Children’s Affective Lability Sale; MFQ=Mood and Feelings Questionnaire; KMRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale; KDRS=Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Depression Rating Scale.
Figure 2. Group Differences in Neuroimaging Measures.
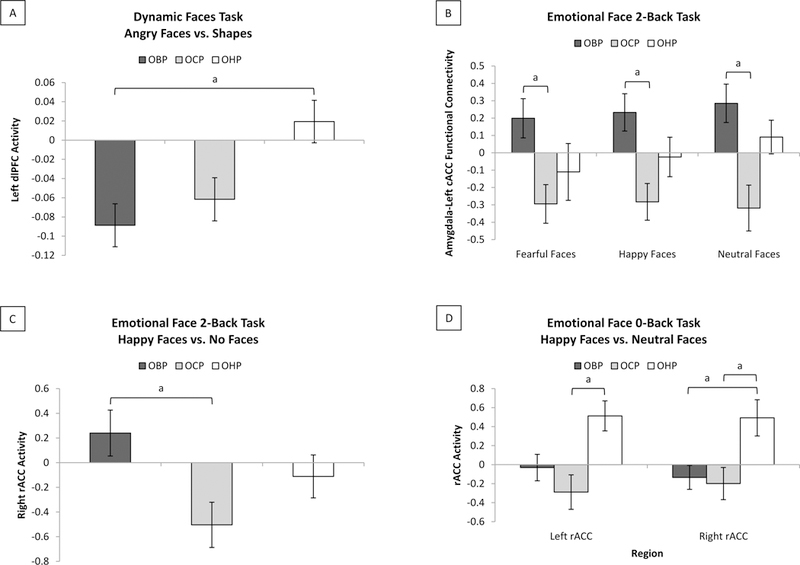
Bonferroni-corrected group comparisons in non-zero predictor neuroimaging measures. A. For the dynamic faces task, compared with OHP, OBP had significantly lower left dlPFC activity to angry faces versus shapes (mean(SD) difference=.108(.033), 95%CI=.027-.189, P=.005). B. For the emotional face 2-back task, compared with OCP, OBP had significantly greater left cACC-amygdala FC to fearful (mean(SD) difference=.493(.169), 95%CI=.079-.908, P=.014), happy (mean(SD) difference=.516(.148), 95%CI=.155-.877, P=.002), and neutral (mean(SD) difference=.604(.159), 95%CI=.215-.992, P=.001) versus no faces. C. For the emotional face 2-back task, compared with OCP, OBP had significantly greater right rACC activity to happy versus no faces (mean(SD) difference=.744(.249), 95%CI=.134–1.354, P=.011). D. For the emotional face 0-back task, compared with OHP, OCP had significantly lower left (mean(SD) difference=.802(.241), 95%CI=.212–1.391, P=.004) and right (mean(SD) difference=.691(.236), 95%CI=.113–1.269, P=.014) rACC activity to happy versus neutral faces; compared with OHP, OBP had significantly lower right rACC activity to happy versus neutral faces (mean(SD) difference=.626(.231), 95%CI=.060–1.192, P=.025).
Abbreviations: a=significant at P=.05; Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Offspring of Healthy Parents (OHP); Dorsolateral Prefrontal Cortex (dlPFC); Caudal Anterior Cingulate Cortex (cACC); Functional Connectivity (FC); Rostral Anterior Cingulate Cortex (rACC).
ANOVAs examined effects of group on all symptoms (Table 2). Bonferroni corrections for eight parallel tests revealed two significant findings: CALS-P (F(2,77)=6.464, P=.003(.024, corrected)) and KMRS (F(2,75)=6.223, P=.003(.024, corrected)). Bonferroni-corrected post-hoc t-tests revealed that OBP had greater CALS-P severity than OHP (mean(SD) difference=6.575(1.853), 95%CI=2.04–11.11, P=.002), and greater KMRS severity than OHP (mean(SD) difference=1.722(.529), 95%CI=.43–3.02, P=.005) and OCP (mean(SD) difference=1.238(.473), 95%CI=.08–2.40, P=.032) (Figure 3A).
Figure 3. Relationships between Symptoms and Neuroimaging Measures.
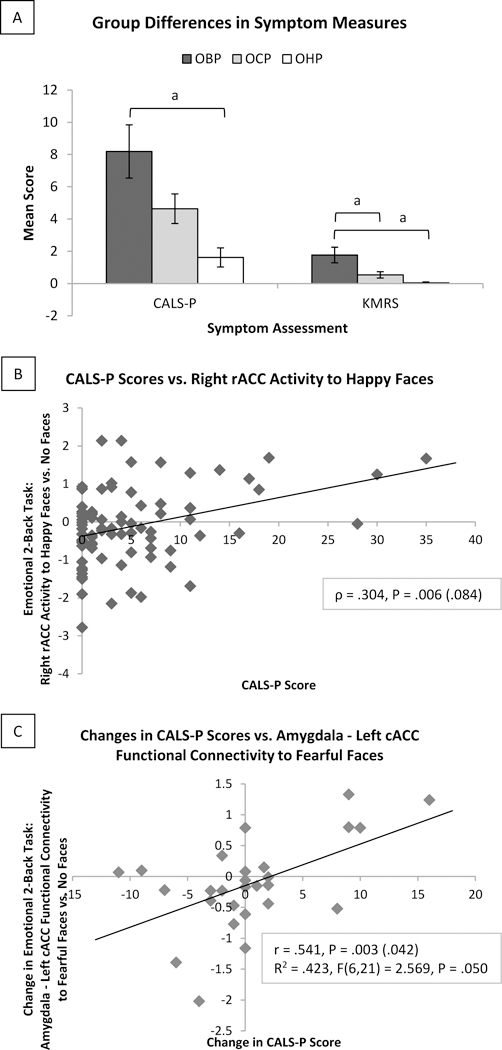
Bonferroni-corrected group comparisons in symptom measures. A. Bonferroni corrections for eight parallel tests revealed two significant findings: CALS-P (F(2,77)=6.464, P=.003(.024, corrected)) and KMRS (F(2,75)=6.223, P=.003(.024, corrected)). Bonferroni-corrected post-hoc t-tests revealed that OBP had greater CALS-P severity than OHP (mean(SD) difference=6.575(1.853), 95%CI=2.04–11.11, P=.002), and greater KMRS severity than OHP (mean(SD) difference=1.722(.529), 95%CI=.43–3.02, P=.005) and OCP (mean(SD) difference=1.238(.473), 95%CI=.08–2.40, P=.032). B. Across all subjects, baseline CALS-P severity positively correlated with emotional face 2-back task right rACC activity to happy faces (ρ=.304, P=.006, uncorrected). C. Follow-up analyses comparing changes in symptom and neuroimaging measures in a subset of thirty subjects. Across all thirty subjects, changes in CALS-P scores were positively correlated with changes in emotional face 2-back task amygdala-left cACC FC to fearful faces (r=.541, P=.003(.042, corrected)). Changes in CALS-P scores, with age, gender, IQ, time between scans, and scanner, significantly predicted changes in emotional face 2-back task amygdala-left cACC FC to fearful faces (R2=.423, F(6,21)=2.569, P=.050).
Abbreviations: a=significant at P=.05; Offspring of Bipolar Parents (OBP); Offspring of Comparison Parents (OCP); Offspring of Healthy Parents (OHP); Parent-Reported Children’s Affective Lability Sale (CALS-P); Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale (KMRS); Rostral Anterior Cingulate Cortex (rACC); Caudal Anterior Cingulate Cortex (cACC); Functional Connectivity (FC).
Bivariate correlation analyses examined relationships among all seven neuroimaging and two symptom measures showing significant group differences, above. Across all subjects, one significant relationship was found: baseline CALS-P severity positively correlated with EF-2-BACK right rACC activity to happy faces (ρ=.304, P=.006; Figure 3B). This just missed significance using Bonferroni corrections for fourteen tests (P<.004).
Exploratory Analyses
Follow-up analyses were in nine OBP (mean(SD) age=15.17(1.89)), seven OCP (mean(SD) age=16.94(1.66)), and fourteen OHP (mean(SD) age=15.78(1.34)). One OBP and two OCP took medications. Bivariate correlation analyses examined relationships among changes in all seven neuroimaging and two symptom measures showing significant group differences, above. Across all thirty subjects, one significant (Bonferroni-corrected) relationship was found: increase in CALS-P severity significantly positively correlated with increase in EF-2-BACK amygdala-left cACC FC to fearful faces (r=.541, P=.003(.042, corrected); Figure 3C). A linear regression, with covariates: age, gender, IQ, time between scans, and scanner, showed that change in CALS-P scores significantly predicted change in amygdala-left cACC FC to fearful faces (R2=.423, F(6,21)=2.569, P=.050).
When analyses were repeated removing medicated youth, OBP no longer had significantly greater right rACC activity to EF-2-BACK happy faces (mean(SD) difference=.408(.275), 95%CI=−.269–1.085, P=.432) and showed borderline significantly greater amygdala-left cACC FC to fearful faces (mean(SD) difference=.454(.188), 95%CI=−.009-.917, P=.056) versus OCP. In follow-up analyses, the relationship between change in CALS-P score and change in EF-2-BACK amygdala-left cACC FC to fearful faces remained significant (r=.597, P=.002); the linear regression model just missed significance (R2=.442, F(6,18)=2.378, P=.072). No other findings changed by removing medicated youth.
Conclusions
To identify neural markers of future BD risk in OBP, we examined measures of activity and FC in amygdala-PFC circuitry during emotion processing and regulation that distinguished OBP from OCP and OHP, and the extent to which these measures were associated with symptom severity.
OBP showed greater right rACC activity to happy faces during EF-2-BACK performance than OCP. The rACC is the “affective division” of the ACC with connections to affective neural regions (e.g. amygdala)50,51 and roles in processing emotional conflict and integrating emotion and cognition52–56. rACC recruitment may help resolve emotional conflict by suppressing amygdala activity, leading to reduced emotional responsivity and blunted sympathetic autonomic responses to incongruent emotional distracters57. Greater right rACC activity to happy faces positively correlated with greater parent-reported affective lability severity, a precursor of BD in OBP, however31, and may reflect inefficient recruitment of rACC to downregulate amygdala activity, leading to affective lability, and risk for future BD in OBP. The relationship with parent-reported, versus child-reported, affective lability may reflect the greater reliability of parental reports of child symptoms, as these are considered more useful than child reports in diagnosing BD in children58.
OBP and OCP showed lower rACC activity than OHP to happy faces during EF-0-BACK performance. Similarly, OBP had lower dlPFC activity than OHP to angry faces during the DFT, another face emotion processing task with no working memory component. These findings suggest that OBP and OCP fail to recruit, to a normal extent, PFC regions important for emotional regulation when processing or attending to emotional stimuli, while OBP recruit the rACC inefficiently when required to distract attention away from positive emotional stimuli. Differential patterns of aberrant recruitment of PFC regions to emotional stimuli in different contexts is thus a potential neural mechanism distinguishing OBP from OCP and conferring risk for BD in OBP.
OBP also showed greater amygdala-left cACC FC to fearful, happy, and neutral faces during EF-2-BACK performance than OCP. Changes in amygdala-left cACC FC to fearful faces positively correlated with changes in parent-reported affective lability severity over time. Along with the rACC, the cACC is implicated in implicit emotional regulation59–63. The cACC is part of the central executive control network and has a more specific role than the rACC in attentional task performance64–66. Our findings thus suggest that greater amygdala-left cACC FC to emotional face distracters, and increasing amygdala-left cACC FC over time to fearful face distracters, may reflect a compensatory, but inefficient, neural mechanism to redirect attention away from emotional face distracters during attentional tasks, which, in turn, may predispose to increasing affective lability and BD in youth.
Removing medicated youth reduced the significance of the differences between right rACC activity to happy faces and amygdala-left cACC FC to fearful faces during EF-2-BACK performance in OBP versus OCP, as well as the relationship between change in the latter measure and change in affective lability during follow-up. Medicated OBP had greater affective lability severity than unmedicated OBP, however, and thus reflected a particularly high-risk subset of OBP. Furthermore, removing medicated youth from analyses affected the significance only of neuroimaging measures showing significant relationships with affective lability severity. Additionally, medication was not a predictor of group in an additional elastic net regression model including medication and all clinical variables, as well as all neuroimaging and demographic measures, as predictors (Supplementary Material). Thus, greater right rACC activity to happy faces, and greater amygdala-left cACC FC to fearful faces, during EF-2-BACK performance may represent markers of BD risk in higher-risk OBP who are more affectively labile and more likely to be medicated, but psychotropic medication in itself is not a predictor of risk for BD in youth.
Previous studies reported that OBP show greater amygdala and PFC activity during emotion processing and regulation14,15,29. While OBP showed greater right rACC activity to happy faces during EF-2-BACK versus OCP, OBP also showed lower left dlPFC activity to angry faces and lower right rACC activity to EF-0-BACK happy faces versus OHP. This is consistent with studies of patients with BD showing reduced activity in PFC regions supporting emotion regulation16,21,22. Previous studies also reported mixed results of either elevated14 or reduced15,23–28 amygdala-PFC FC in OBP. Our findings implicate amygdala-cACC FC, while other studies focused on the vlPFC, however. Additionally, our DFT findings differ from those in BIOS showing greater amygdala activity, lower amygdala-ACC FC, and more positive amygdala-vlPFC FC in OBP versus OHP14. Unlike this previous study, the present study employed emotional regulation and processing tasks, with most findings pertaining to emotional regulation. Together, our findings suggest differential patterns of functional abnormalities in circuitries associated with these two tasks in OBP (and OCP) versus OHP.
This study had limitations. Sample size was limited, particularly for follow-up data. Future studies should replicate and validate our findings with larger sample sizes. We focused on activity and FC in emotion processing and regulation neural circuitries; analyzing gray matter volume and cortical thickness may enhance understanding of BD risk. We assumed linear models between neuroimaging and symptom measures, while nonlinear models could be considered. Interpreting findings based on non-linear models is significantly limited in studies with such complex designs, however67. While age, which significantly correlated with pubertal development (Supplementary Material), did not significantly affect neuroimaging measures, pubertal development cannot be ruled out as a contributing factor in our results. Additionally, recent studies have debated the possible inflation of predictions in neuroimaging studies in individuals with psychiatric disorders68. We used a well-validated approach that penalizes complex models using regularization, cross-validation, and sparsity enforcement in model fit. While medication impacted some findings, these effects may, in fact, reflect the medicated status of the most affectively labile and high-risk OBP. Furthermore, medication was not a predictor of group in additional elastic net regression analyses. Further study is needed to determine relationships between medications and emotional regulation neural circuitry functioning.
This is the first study to employ both cross-sectional and longitudinal analyses of emotion processing and regulation neural circuitries in youth at risk for BD versus comparative at-risk and healthy control groups. We show that greater right rACC activity to happy faces and greater amygdala-left cACC FC to fearful faces during attentional task performance with high-memory load conditions significantly distinguish OBP from OCP, at the group level, and these measures have significant relationships with affective lability, a precursor of BD. We conclude that greater right rACC activity and greater amygdala-cACC FC during emotional regulation are candidate objective markers of BD risk in youth. Our findings are important steps toward identifying neural markers of BD risk to aid in enhanced early identification, and guide interventions for, BD at-risk youth.
Supplementary Material
Key Points.
Question
Are there specific abnormalities in activity and functional connectivity in emotion processing and regulation neural circuitries in offspring at risk for Bipolar Disorder?
Findings
Relative to offspring of healthy parents, offspring of bipolar parents had significantly greater right rostral anterior cingulate cortex activity when regulating attention away from happy faces. This measure was significantly positively correlated with affective lability symptom severity. Additionally, offspring of bipolar parents had significantly greater left caudal anterior cingulate cortex-amygdala functional connectivity to fearful faces relative to offspring of non-bipolar, psychiatric disorder comparison parents, and increases in this measure over follow-up (mean 2.88 years) was significantly positively correlated with increases in affective lability severity.
Meaning
Greater activity and functional connectivity during emotion regulation tasks in the anterior cingulate cortex may help distinguish youth at risk for bipolar disorder from healthy youth and from youth at risk for other psychiatric disorders.
Acknowledegements
We would like to acknowledge the participants and their families for their contributions to this study.
Dr. Axelson has served as a consultant for Janssen Research and received royalties from UpToDate.
Dr. Tina Goldstein receives research funding from NIMH, AFSP, and the Brain and Behavior Foundation and receives royalties from Guilford Press.
Dr. Sakolsky serves as an editorial board member of Child & Adolescent Psychopharmacology News and specialty consultant for the Prescriber’s Letter. She has received a consultant fee of $300 from L.E.K. Consulting in 2015. She currently receives research funding from NIMH and has received funding from NARSAD, the World’s Leading Charity Dedicated to Mental Health Research.
Dr. Birmaher receives or will receive royalties for publications from Random House, Inc. (New Hope for Children and Teens with Bipolar Disorder), Lippincott Williams & Wilkins (Treating Child and Adolescent Depression), and UpToDate. He is employed by the University of Pittsburgh and the University of Pittsburgh Medical Center/Western Psychiatric Institute and Clinic and receives research funding from NIMH.
Funding/Support
This work was supported by the National Institute of Mental Health (B.B. and M.L.P., grant number R01 MH060952–16), (H.E.A., grant number F30 MH111102–01A1).
Role of Funder/Sponsor Statement: The funders/sponsors B.B., M.L.P., and H.E.A. all did have a role in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Non-author Contributions to Data Collection, Analysis, or Writing/Editing Assistance
Longitudinal Assessment of Manic Symptoms (LAMS) Consortium
Compensation was not received for any person named below.
L. Eugene Arnold, MD, Department of Psychiatry, Ohio State University, Columbus, OH: Study Design and Clinical Assessment
Mary A. Fristad, PhD, ABPP, Department of Psychiatry, Ohio State University, Columbus, OH: Study Design and Clinical Assessment
Genna Bebko, PhD, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, PA: Data Analyses
Mary Kay Gill, MS, RN, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, PA: Participant Recruitment
Claudiu Schirda, PhD, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, PA: Data Acquisition
Michael Travis, MD, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, PA: Data Acquisition
Vaibhav A. Diwadkar, PhD, Department of Psychiatry and Behavioral Neuroscience, Wayne State University, Detroit, MI: Data Analyses
Robert L. Findling, MD, MBA, Department of Psychiatry, Johns Hopkins University, Baltimore, MD: Study Design and Clinical Assessment
Scott K. Holland, PhD, Department of Radiology, University Hospitals Case Medical Center/Case Western Reserve University, Cleveland, OH: Data Acquisition
Sarah M. Horwitz, PhD, Department of Child and Adolescent Psychiatry, New York University School of Medicine, New York City, NY: Study Design and Clinical Assessment
Robert A. Kowatch, MD, PhD, Research Institute at Nationwide Children’s Hospital, Columbus, OH: Study Design
Jeffrey L. Sunshine, MD, PhD, University Hospitals Case Medical Center/Case Western Reserve University, Cleveland, OH: Data Acquisition
Eric A. Youngstrom, PhD, Department of Psychology, University of North Carolina at Chapel Hill, Chapel Hill, NC: Statistical Analyses
Access to Data and Data Analysis
Heather Acuff had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of Interest Disclosure
Acuff, Dr. Versace, Dr. Bertocci, Dr. Hanford, Dr. Ladouceur, Dr. Manelis, Dr. Monk, Dr. Bonar, Dr. McCaffrey, Dr. Goldstein, and Dr. Phillips have no financial interests or potential conflicts of interest.
References
- 1.Kowatch RA, Fristad M, Birmaher B, Wagner KD, Findling RL, Hellander M. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(3):213–235. [DOI] [PubMed] [Google Scholar]
- 2.Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar disorders. 2005;7(6):483–496. [DOI] [PubMed] [Google Scholar]
- 3.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44(9):846–871. [DOI] [PubMed] [Google Scholar]
- 4.Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. J Clin Psychiatry. 2003;64(5):506–515. [DOI] [PubMed] [Google Scholar]
- 5.Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51(4):288–297. [DOI] [PubMed] [Google Scholar]
- 6.Perlis RH, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2004;55(9):875–881. [DOI] [PubMed] [Google Scholar]
- 7.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34(4):454–463. [PubMed] [Google Scholar]
- 8.Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Development and psychopathology. 2006;18(4):1023–1035. [DOI] [PubMed] [Google Scholar]
- 9.Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am J Med Genet C Semin Med Genet. 2003;123C(1):48–58. [DOI] [PubMed] [Google Scholar]
- 10.Chang KD, Steiner H, Ketter TA. Psychiatric phenomenology of child and adolescent bipolar offspring. J Am Acad Child Adolesc Psychiatry. 2000;39(4):453–460. [DOI] [PubMed] [Google Scholar]
- 11.Axelson D, Goldstein B, Goldstein T, et al. Diagnostic Precursors to Bipolar Disorder in Offspring of Parents With Bipolar Disorder: A Longitudinal Study. The American journal of psychiatry. 2015;172(7):638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein TR, Birmaher B, Axelson D, et al. History of suicide attempts in pediatric bipolar disorder: factors associated with increased risk. Bipolar disorders. 2005;7(6):525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Archives of general psychiatry. 2009;66(3):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manelis A, Ladouceur CD, Graur S, et al. Altered amygdala-prefrontal response to facial emotion in offspring of parents with bipolar disorder. Brain : a journal of neurology. 2015;138(Pt 9):2777–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladouceur CD, Diwadkar VA, White R, et al. Fronto-limbic function in unaffected offspring at familial risk for bipolar disorder during an emotional working memory paradigm. Dev Cogn Neurosci. 2013;5:185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. [DOI] [PubMed] [Google Scholar]
- 17.Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion-cognition interactions: A review of evidence from brain imaging investigations. J Cogn Psychol (Hove). 2011;23(6):669–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. The American journal of psychiatry. 2014;171(8):829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55(6):578–587. [DOI] [PubMed] [Google Scholar]
- 20.Blumberg HP, Donegan NH, Sanislow CA, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl). 2005;183(3):308–313. [DOI] [PubMed] [Google Scholar]
- 21.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hafeman DM, Bebko G, Bertocci MA, et al. Abnormal deactivation of the inferior frontal gyrus during implicit emotion processing in youth with bipolar disorder: attenuated by medication. J Psychiatr Res. 2014;58:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusslock R, Almeida JR, Forbes EE, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14(3):249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermpohl F, Kahnt T, Dalanay U, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp. 2010;31(7):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linke J, King AV, Rietschel M, et al. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry. 2012;169(3):316–325. [DOI] [PubMed] [Google Scholar]
- 26.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33(9):2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15(8):839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170(5):533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsavsky AK, Brotman MA, Rutenberg JG, et al. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(3):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh MK, Chang KD, Kelley RG, Saggar M, Reiss AL, Gotlib IH. Early signs of anomalous neural functional connectivity in healthy offspring of parents with bipolar disorder. Bipolar disorders. 2014;16(7):678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hafeman DM, Merranko J, Axelson D, et al. Toward the Definition of a Bipolar Prodrome: Dimensional Predictors of Bipolar Spectrum Disorders in At-Risk Youths. The American journal of psychiatry. 2016;173(7):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertocci MA, Bebko G, Dwojak A, et al. Longitudinal relationships among activity in attention redirection neural circuitry and symptom severity in youth. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(4):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Arch Gen Psychiatry. 2009;66(3):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Findling RL, Youngstrom EA, Fristad MA, et al. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. J Clin Psychiatry. 2010;71(12):1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horwitz SM, Demeter CA, Pagano ME, et al. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. J Clin Psychiatry. 2010;71(11):1511–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wechsler D Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 37.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 38.First MB, SPitzer RL, Gibbon M, Williams J Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press; 1996. [Google Scholar]
- 39.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36(4):545–553. [DOI] [PubMed] [Google Scholar]
- 40.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–1236. [DOI] [PubMed] [Google Scholar]
- 41.Gerson AC, Gerring JP, Freund L, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. 1996;65(3):189–198. [DOI] [PubMed] [Google Scholar]
- 42.Sund AM, Larsson B, Wichstrom L. Depressive symptoms among young Norwegian adolescents as measured by the Mood and Feelings Questionnaire (MFQ). Eur Child Adolesc Psychiatry. 2001;10(4):222–229. [DOI] [PubMed] [Google Scholar]
- 43.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463–470. [DOI] [PubMed] [Google Scholar]
- 44.Almeida JR, Kronhaus DM, Sibille EL, et al. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: a potential neural mechanism of female MDD. Front Psychiatry. 2011;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perlman SB, Almeida JR, Kronhaus DM, et al. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar disorders. 2012;14(2):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ladouceur CD, Silk JS, Dahl RE, Ostapenko L, Kronhaus DM, Phillips ML. Fearful faces influence attentional control processes in anxious youth and adults. Emotion. 2009;9(6):855–864. [DOI] [PubMed] [Google Scholar]
- 48.Friedman J, Hastie, T., Simon, N. Tibshirani, R. GLMNET. 2014.
- 49.Hollingshead AB. Four factor index of social status. 1975. [Google Scholar]
- 50.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. The Journal of comparative neurology. 1995;363(4):615–641. [DOI] [PubMed] [Google Scholar]
- 51.Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex Neurobiology of cingulate cortex and limbic thalamus: Springer; 1993:249–284. [Google Scholar]
- 52.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature neuroscience. 2004;7(2):184. [DOI] [PubMed] [Google Scholar]
- 53.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4(6):215–222. [DOI] [PubMed] [Google Scholar]
- 54.Bissière S, Plachta N, Hoyer D, et al. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biological psychiatry. 2008;63(9):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain : a journal of neurology. 1995;118(1):279–306. [DOI] [PubMed] [Google Scholar]
- 56.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cerebral cortex. 1992;2(6):435–443. [DOI] [PubMed] [Google Scholar]
- 57.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. [DOI] [PubMed] [Google Scholar]
- 58.Youngstrom EA, Findling RL, Calabrese JR, et al. Comparing the diagnostic accuracy of six potential screening instruments for bipolar disorder in youths aged 5 to 17 years. J Am Acad Child Adolesc Psychiatry. 2004;43(7):847–858. [DOI] [PubMed] [Google Scholar]
- 59.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frank DW, Dewitt M, Hudgens-Haney M, et al. Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev. 2014;45:202–211. [DOI] [PubMed] [Google Scholar]
- 61.Goodkind M, Gyurak A, Etkin A. Functional neurocircuitry and neuroimaging studies of anxiety disorders Neurobiology of Mental Illness: Oxford University Press, New York, NY; 2013:606–620. [Google Scholar]
- 62.Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223(2):403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular psychiatry. 2008;13(9):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of cognitive neuroscience. 2002;14(4):593–602. [DOI] [PubMed] [Google Scholar]
- 65.Haas BW, Omura K, Constable RT, Canli T. Interference produced by emotional conflict associated with anterior cingulate activation. Cognitive, affective & behavioral neuroscience. 2006;6(2):152–156. [DOI] [PubMed] [Google Scholar]
- 66.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37(2):579–588. [DOI] [PubMed] [Google Scholar]
- 67.Marsh L, McGlynn M, Chakraborty D. Interpreting complex nonlinear models. Paper presented at: Proceedings of SAS User’s Group International1994. [Google Scholar]
- 68.Whelan R, Garavan H. When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2014;75(9):746–748. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


