Abstract
Face-to-face interactions between mothers and infants occur in both human and non-human primates, but there is large variability in the occurrence of these behaviors and the reason for this variability remains largely unexplored. Other types of maternal investment have been shown to be dependent on infant sex (e.g. milk production and maternal responsiveness) and maternal experience (e.g. symmetrical communication). Thus, we sought to determine whether variability in face-to-face interactions, that is, mutual gazing (MG), which are hypothesized to be important for later socio-cognitive development, could be explained by these variables. We studied 28 semi-free ranging rhesus monkey (Macaca mulatta) mother-infant dyads (6 primiparous; 12 male infants) born and reared at the Laboratory of Comparative Ethology field station at the NIH Animal Center in Poolesville, MD, across the first 90 postnatal days. Infant sex (i.e. male) was a significant predictor of maternal grooming (β ± SE = 0.359 ± 0.164, Z = 2.19, P = 0.029) whereas both parity (i.e. first time mothers) and infant sex (i.e. male) significantly predicted MG (parity: β ± SE = −0.735 ± 0.223, Z = −3.30, P < 0.0001; infant sex: β ± SE = 0.436 ± 0.201, Z = 2.17, P = 0.029). Separation from the mother (outside of arm’s reach) was not influenced by parity or infant sex. Together with existing literature, these findings point toward differential maternal investment for sons versus daughters. Mothers may be investing differentially in sons, behaviorally, to ensure their future social competence and thus later reproductive success. Collectively, our findings add to the literature that is beginning to identify early life experiences that may lead to sex differences in neurological and behavioral development.
Keywords: Macaca mulatta, mother–infant interaction, parity, infant sex, mutual gaze
INTRODUCTION
Face-to-face interactions between mothers and their newborns are known to occur in human and non-human primates [Bard et al., 2005; Blehar et al., 1977; Ehardt and Blount, 1984; Ferrari et al., 2009]. Studies in humans have suggested that these facial interactions facilitate the development of emotion regulation in infants [Feldman, 2007; Tronick, 1989], increase bonding and closeness between infant and mother [Trevarthen, 1998], improve infants’ cognitive skills [Murray et al., 1996], and influence infants’ physiological regulation [Feldman et al., 2009]. However, in these documented cases of face-to-face interactions (e.g. mutual gazing, facial expressions, play), there is often large inter-individual variability in their occurrence. The reasons for this variability remain largely unexplored.
Mothers are known to differentially engage with their infants in other ways depending on their own experience as well as their infant’s sex. For example, first-time rhesus macaque mothers tend to be more protective of their offspring [Hooley & Simpson, 1981], show higher anxious behaviors towards their infant [Mitchell & Stevens, 1968], produce milk with higher cortisol, which “programs” later infant temperament [Hinde et al., 2015], and provide their sons with richer milk [Hinde, 2009]. First-time chimpanzee (Pan troglodytes) mothers nurse, groom, and play with their infants more than experienced mothers [Stanton et al., 2014]. Similarly, in humans (Homo sapiens) first-time mothers engage in more social and caretaking behavior with their first child than with their second child [Jacobs & Moss, 1976] and are more likely to maintain symmetrical communication (i.e. mutually coordinated actions) for longer periods of time before transitioning to asymmetrical communication [i.e. one active and one passive partner; Hsu & Fogel, 2003]. In addition, human mothers are more responsive [Lewis, 1972], and engage in more physical play [MacDonald & Parke, 1986] with their sons than with daughters. In chimpanzees, mothers with sons are more gregarious and spend more time in parties containing males compared to mothers of daughters especially in the first six months of life, probably as a way to influence their sons’ social environment in a male-bonded society [Murray et al., 2014].
A wide variety of studies have demonstrated how variations in mother–infant interactions can influence offspring development at a genetic [reviewed in Meaney, 2001], cognitive [Murray et al., 1996; Olson et al., 1986], physiological [Feldman, 2012] and behavioral level [Mitchell & Stevens, 1968]. For example, pups of high licking/grooming-arched-back nursing (LG-ABN) rat mothers show reduced physiological and behavioral reactivity to stressful situations, and are themselves better mothers than low LG-ABN mothers [Meaney, 2001]. Most of the literature concerns naturally occurring variations in physical aspects of maternal care, yet little is known about the influences of less obvious aspects of care such as face-to-face communication.
Given the potential for mother-infant face-to-face communication to also exert downstream socio-cognitive effects, and the fact that other types of mother–infant interaction are experience- and sex-dependent, we tested the hypothesis that these variables would also influence the occurrence of a particularly salient form of face-to-face communication, mutual gazing (MG), in rhesus monkeys (Macaca mulatta). We predicted that first-time mothers, and mothers of sons, would engage in MG more frequently than experienced (i.e., multiparous) mothers or mothers of daughters. We also compared rates of grooming (GR) and mother-infant proximity (SEP), which are more hands-on indicators of maternal care, to determine if rates of these behaviors differed based on parity and infant sex. In rhesus macaques, infants in the first week of life (i.e. newborns) stay almost exclusively in ventral contact with their mothers. Infants regularly separate from their mothers for short distances and brief periods of time starting in the second week of life, although locomotor skills are strong by 6 weeks of age [Lindburg, 1971]. Although infant rhesus macaques can start eating solid food at 2 weeks of age, it is at about 4 months that mothers start rejecting infants’ attempts to nurse, while full weaning is reached by the birth of the next sibling, at approximately 1 year of age [Fooden, 2000]. Based on these developmental milestones, we expected to see consistent decreases in all three behaviors across the first three months of life as infants became more independent of their mothers.
METHODS
Subjects and Housing
Rhesus monkey mother-infant dyads (N = 28; n = 12 male infants; n 6 primiparous mothers; see Table I) were born and raised at the Laboratory of Comparative Ethology’s 5-acre field station at the NIH Animal Center near Poolesville, MD. Dyads were studied in the spring and summer of 2013 and 2014. Mothers ranged in age from 4 to 16 years (mean ± SEM: 7.6 ± 0.5), and all infants were carried to term without complications. Twenty-three individual mothers were represented in this sample; thus, five mothers gave birth in both 2013 and 2014. This semi-free ranging population of rhesus monkeys has been well characterized [Dettmer et al., 2014, 2015], and a small sample of this population (n = 6 dyads) has previously been confirmed to exhibit some of the face-to-face interactions described previously [Ferrari et al., 2009] and studied here. Monkeys were fed twice daily (Purina High Protein Monkey Chow #5038, St. Louis, MO), and given fresh fruit or foraging items (e.g., seeds, nuts) daily. Water was available ad libitum.
TABLE I.
Breakdown of Subjects by Parity and Infant Sex
| Female infant | Male infant | Total | |
|---|---|---|---|
| Primiparous mother | 2 | 4 | 6 |
| Multiparous mother | 14 | 8 | 22 |
| Total | 16 | 12 | 28 |
Importantly, mothers and infants were undisturbed for the duration of the study; that is, infants were never removed from their mothers. In previous studies of MG in nonhuman primates, infants were separated briefly from their mother for behavioral testing (five times during the first 30 days of life) [Ferrari et al., 2009], which may account for at least some of the rates of gazing observed [Bard et al., 2005].
Social Rank
Because dominance status has been associated with aspects of maternal behavior [Berman, 1992; Schino et al., 1999], we quantified each mother’s social rank to determine whether high or low social status varied by parity or infant sex. We used Elo-rating [Elo, 1978], a recently proposed method in behavioral research [Neumann et al., 2011], which has several advantages over conventional matrix-based analyses including the ability to detect changes in rank dynamics [Neumann et al., 2011; Wooddell et al., 2015]. A total of 3,567 ad libitum [Altmann, 1974] agonistic (supplants, threats, chases, attacks) and submissive (fear grimaces) interactions were collected between February 2013 and April 2015. All agonistic interactions between 93 troop individuals were entered into a database. Using R software (v3.1.2), Elo-ratings were generated after each sequential interaction using the elo. sequence function devised by Neumann et al. [2011]. At the end of the 2-year observation period, average Elo-ratings were generated for each of the 23 mothers (range: 524–1,481). A median split (= 940) then divided the Elo-ratings into low (N = 12) or high (N = 11) dominance rank. High-ranking females were those who rarely received agonistic behaviors from others and instead directed much of the agonistic behaviors (thus reflecting higher Elo-ratings), and lower ranking monkeys rarely directed aggressive behaviors, but most often received these behaviors.
Mother–Infant Interactions
Monkeys were observed by five different observers, who were blind to the aim of the research (as to avoid any bias during data collection), according to previously published procedures for this species [Ferrari et al., 2009]. Live focal animal observations [Altmann, 1974] were conducted between 900 and 1,700, 1–2 times per day, 5 days per week for the first 30 days of the infant’s life; 3 times per week during days 31–60; and once per week during days 61–90. A total of 649 observations were collected (mean ± SEM per focal: 20.9 ± 0.8). Data collection began only if both the mother and infant had their eyes open and were alert [Ferrari et al., 2009]. If the dyad moved out of sight or if the mother or infant fell asleep for more than 50% of the session, the session was terminated. Sessions were 15 min long (verified with a stopwatch) and were coded from the infant’s perspective. Frequencies of the following behaviors in each 15 min session were recorded: gazes (initiated, received, and mutual), lipsmacking (initiated, received, and mutual), grooming (received), and separate from mother (within arm’s reach and outside of arm’s reach). Each bout (i.e. behavior lasting at least 3 sec) was recorded once, and the end of a bout occurred when the behavior ceased for approximately 3 sec or longer. For gazing, lipsmacking, and grooming, the social partner (mother, adult female, adult male, juvenile, or infant) was recorded. For this study, only interactions between the mother and infant were analyzed. Table II presents an ethogram for all behaviors.
TABLE II.
Ethogram of Behaviors for This Study
| Behaviora | Definition |
|---|---|
| Gazing | Infant looks at the face of another monkey, or another monkey looks at infant’s face, within 1m. Coded as mutual gaze if one subject reciprocated the gaze of another. |
| Lipsmacking | Rapid movement of the lips directed toward another monkey. |
| Grooming | One monkey picks at and sweeps the hair of another monkey. |
| Separation from mother | Infant moves off of the mother’s ventrum to within or outside an arm’s distance, or mother puts infant down within arm’s distance, or mother walks away from infant. |
In this study, only interactions between the mother and infant were analyzed.
Of the 649 observations, 61 (9.4%) were coded by two or more observers to establish reliability. We calculated Gwet’s AC1 coefficient to assess inter-rater reliability [Gwet, 2014] using the function gwet. ac1.raw implemented in R 3.1.2. This method is more robust than Cohen’s κ, as it is not sensitive to infrequent behaviors (such as MG), which can result in high inter-observer agreement (most probably due to chance, given the high probability of having zeros) but low k values [Gwet, 2002a,b, 2014; Lang et al., 2014; Wongpakaran et al., 2013]. Since we aimed to assess the presence of MG, GR, and SEP, we calculated inter-rater reliability on the basis of the number of bouts each rater observed for each behavior. Lipsmacking was observed so rarely that it was not included in this dataset. We found moderate agreement for GR (AC1: 0.57, SE: 0.068, P < 0.001), substantial agreement for SEP (AC1: 0.72, SE: 0.062, P < 0.001), and almost perfect agreement for MG (as defined by Lang et al., 2014; AC1: 0.84, SE: 0.051, P < 0.001). In instances in which MG was recorded by both observers, the agreement on the identity of the initiator of MG (i.e. whether it was the mother or the infant) was almost perfect (AC1: 0.89, SE: 0.04, P < 0.001).
Data Analysis
In order to determine whether rank should be included in all following analyses, we used chi-square analysis to assess whether social rank (high and low) was evenly distributed across parity (primiparous and multiparous) and infant sex (male and female), and Spearman’s rank correlation to test whether mean rates of MG, GR, and SEP significantly correlated to mother’s dominance rank.
For MG, GR, and SEP, we calculated the mean frequency across three consecutive days in the first 30 days of life [Ferrari et al., 2009], then weekly thereafter, resulting in a mean frequency per 15 min session for each dyad for days 0–2, 3–5, 6–8, 9–11, 12–14, 15–17, 18–20, 21–23, 24–26, and 27–30, and for weeks 5, 6, 7, 8, 9, 10, 11, and 12.
Across the entire study period, average rates of MG, GR, and SEP were calculated for each mother and Spearman correlation was used to determine if the occurrence of these behaviors was correlated. We then assessed whether mean frequencies of these behaviors varied between age groups using a polynomial contrast analysis, with LSD post-hoc test to assess whether adjacent ages significantly differed in the frequencies of those behaviors. In addition, we tested the effects of infant age, infant sex, mother parity and their interactions on the frequency of MG, GR, and SEP using Generalized Linear Mixed Model analysis (GLMM), in order to account for multiple sampling of the same mother-infant dyads across multiple time points. We used the glmmadmb function [Bolker et al., 2012] with Gaussian distribution implemented in R 3.1.2 as this function handles zero-inflated data sets, and we had some days in which mother-infant dyads were not observed engaging in mutual gazing or grooming, or infants were not recorded to be outside of arm’s reach. The data were square root transformed to more closely approach a Gaussian distribution. Rates of MG, GR, and SEP were entered as dependent variables with continuous distribution while mother parity (binary) and infant sex (binary), as well as their interactions with age (continuous) were set as fixed factors, with both mother’s and infant’s identity included as random factors with nested structure. Age was entered in these models by assigning to the age groups described above a cardinal number in ascending order from 1 to 18.
The Kruskal–Wallis test was used to examine the proportion of MG initiated by the mother versus by the offspring for each dyad across the study period. This test was completed using parity × sex (e.g., primiparous-male, primiparous-female, multiparous-male, multiparous-female) as the grouping variable to determine the influence of each partner on this behavior.
This research adhered to the American Society of Primatologists principles for the ethical treatment of primates. All procedures had prior approval from the NICHD Animal Care and Use Committee, and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
RESULTS
Social Rank and Correlation of Behaviors
Mothers did not differ in rank based on parity (χ2 = 0.07, P = 0.79) or infant sex (χ2 = 0.41, P = 0.52). Additionally, none of the behaviors examined was significantly related to mother’s dominance rank (Spearman’s rank correlation test, MG: N 23, rs = 0.081, P = 0.714; GR: N = 23, rs = −0.291, P = 0.179; SEP: N = 23, rs = −0.162, P = 0.460).
Mutual Gazing (MG)
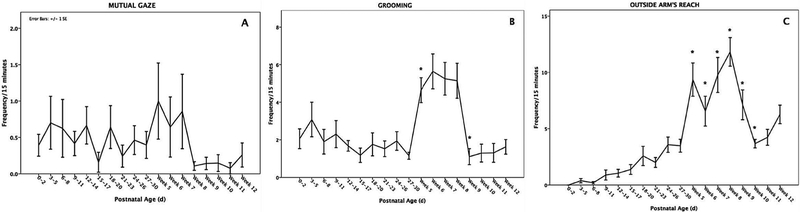
The polynomial contrast test revealed that there was no significant difference in means across the different age groups (F(1,16) = 1.534, P = 0.079, η2 = 0.058, Fig. 1A).
Fig. 1.
Changes in the average frequencies (±SEM) of mutual gaze (MG, panel A), grooming by mother (GR, panel B), and outside of mother’s arm’s reach (SEP, panel C) across the first three postnatal months. *Indicates significant difference from the previous time point, P < 0.05.
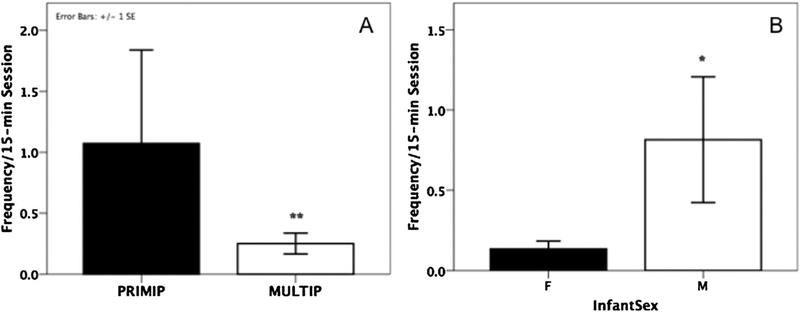
Rates of MG were significantly predicted by both parity and infant sex (GLMM, parity: β ± SE = −0.735 ± 0.223, Z = −3.30, P < infant sex: β ± SE = 0.436 ± 0.201, Z = 2.17, P = 0.029; Table III) although the interaction between the two variables was not significant (β ± SE = 0.239 ± 0.0442, Z = −0.54, P = 0.588). Primiparous females engaged more frequently in MG with their infants (mean ± SE = 1.31 + 0.27 per session) than multiparous mothers (mean ± SE = 0.22 ± 0.04 per session; Fig. 2A), and MG occurred more frequently with sons (mean ± SE = 0.81 ± 0.15 per session) than with daughters (mean ± SE = 0.19 ± 0.04 per session; Fig. 2B). No significant interaction was found for infant sex and age (β ± SE = 0.002 ± 0.018, Z = 0.13, P = 0.899), or for parity and age, although there was a trend for the latter (β ± SE = −0.042 ± 0.022, Z = −1.90, P = 0.058). MG decreased over time for multiparous females (β ± SE = −0.029 ± 0.011, Z = 2.49, P = 0.013), while age did not predict frequencies MG for primiparous females (β ± SE = −0.011 ± 0.019, Z = −0.60, P = 0.547). Finally, there was no significant effect of the interaction between parity, infant sex and infant age (β ± SE = −0.001 ± 0.045, Z = −0.03, P = 0.97).
TABLE III.
Summary of General Linear Mixed Model (GLMM) Results for Mutual Gaze (MG), Grooming (GR), and Separation From Mother (SEP)
| Behaviors | |||
|---|---|---|---|
| Predictors | MG | GR | SEP |
| S | 0.436 ± 0.201* | 0.359 ± 0.164* | 0.046 ± 0.149 |
| P | 0.735 ± 0.223*** | 0.119 ± 0.215 | −0.099 ± 0.176 |
| P × S | 0.239 ± 0.442 | −0.185 ± 0.410 | 0.561 ± 0.361 |
| P × A | −0.042 ± 0.022 | −0.078 ± 0.032* | 0.008 ± 0.027 |
| S × A | 0.002 ± 0.018 | 0.058 ± 0.022** | 0.016 ± 0.02 |
| P × S × A | −0.001 ± 0.045 | −0.057 ± 0.056 | 0.039 ± 0.056 |
S, infant sex; P, mother parity; A, infant Age.
P < 0.05,
P < 0.01,
P < 0.001.
Fig. 2.
Mutual gaze (MG) was higher in primiparous mothers (**P < 0.001; A) and mothers of sons (*P < 0.05; B).
Grooming
We found a significant difference in rates of GR between the different age groups (F(1,17) = 6.236, P < 0.001, η2 = 0.199, Fig. 1B), with a quadratic relationship (F(1,15) = 6.583; P = 0.011) between grooming and infant’s age. Rates of GR significantly increased from weeks 5 to 8 before returning to levels seen from days 0 to 30 (Fig. 3).
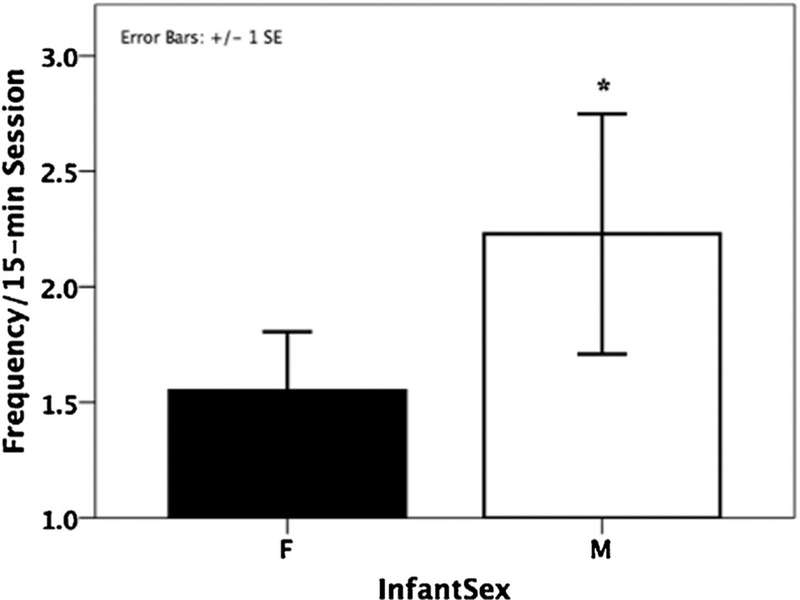
Fig. 3.
Mothers of sons groomed their infants more than did mothers of daughters (*P < 0.05).
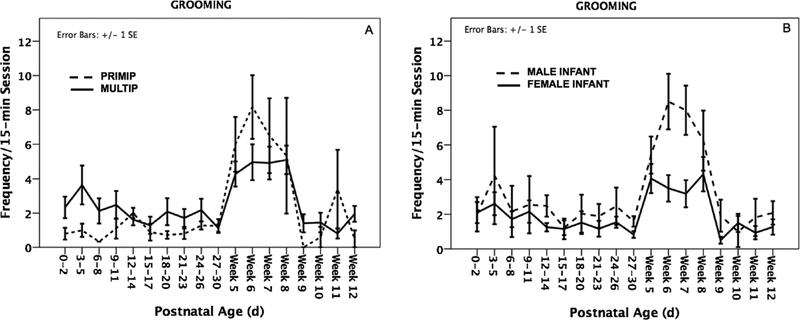
The GLMM analysis showed that whereas parity did not have an effect on frequencies of GR (β ± SE = 0.119 ± 0.215, Z = 0.55, P = 0.58; Table III), the latter was significantly predicted by infant sex (β ± SE = 0.359 ± 0.164, Z = 2.19, P = 0.029), with sons receiving significantly more GR (mean ± SE: 3.37 ± 0.33) than daughters (mean ± SE: 2.03 ± 0.16; Fig. 3). No significant interaction between parity and infant sex was found (β ± SE = 0.185 ± 0.410, Z = 0.45, P = 0.651). We did find a significant interaction between parity and infant age (β ± SE = −0.078 ± 0.032, Z = 2.42, P = 0.015; Fig. 4A), with primiparous mothers significantly increasing rates of GR at weeks 5–8 (β ± SE = 0.119 ± 0.024, Z = 5.03, P < 0.001; Fig. 4A). This interaction was not significant for multiparous mothers (β ± SE = 0.021 ± 0.013, Z = 1.62, P = 0.11; Fig. 4A). We also found a significant interaction between infant sex and age for GR (β ± SE = 0.058 ± 0.022, Z = 2.67, P = 0.008; Fig. 4B), whereby sons received increasingly more GR at weeks 5–8 (β ± SE = 0.052 ± 0.024, Z = 2.14, P = 0.032; Fig. 4B)but no such pattern was among daughters (β ± SE = 0.020 ± 0.014, Z = 1.45, P = 0.15; Fig. 4B). We did not find any significant interaction between parity, infant sex, and infant age (β ± SE = −0.057 ± 0.056, Z = −1.01, P = 0.314).
Fig. 4.
Primiparous mothers (A) and mothers of sons (B) increased rates of infant grooming (GR) as the infant aged.
Separation From Mother
Mean frequencies of SEP varied with infant’s age (F(1,17) = 15.285, P < 0.001, η2 = 0.389) with a quadratic relationship (F(1,15) = 17.893, P < 0.001): they steadily rose from birth through 30 days, peaked from weeks 5–8, then dropped to 30-day levels thereafter (Fig. 1C). No effect of parity or infant sex on SEP was found (Table III; parity: β ± SE = −0.099±0.176, Z =−0.56, P = 0.57; infant sex: β± SE = −0.046 ± 0.149, Z =−0.31, P = 0.76), nor was their interaction (β ± SE = 0.561 ± 0.361, Z = 1.55, P = 0.12). Interactions between parity and infant age and between infant sex and age were not statistically significant (parity × infant age: β ± SE = 0.008 ± 0.027, Z = 0.32, P = 0.75; infant sex × infant age: β ± SE = 0.016 ± 0.02, Z = 0.71, P = 0.48), so was not the interaction between parity, infant sex, and infant age (parity × infant sex × infant age: β ± SE = 0.039 ± 0.056, Z = 0.70, P = 0.48).
Initiation of Mutual Gazing
Five of the 28 dyads never engaged in MG (three multiparous mothers of females, two multiparous mothers of males). Across the first month of life, dyads did not differ in the proportion of MGs initiated by the mother versus by the infant (K(25) =0.190, P = 0.98).
DISCUSSION
We sought to determine whether some of the variability in the observed face-to-face interactions between macaque mothers and their newborn infants could be explained by maternal history and infant sex and age, as has been the case for other types of mother–infant interactions in both human and nonhuman primates [Lewis, 1972; Mitchell & Stevens, 1968]. Our study of semi-free ranging rhesus monkeys afforded us the opportunity to study these interactions in a naturalistic environment without the possible confound of human caregivers or interactions influencing these behaviors. In addition, our large sample of dyads was not subjected to repeated separations as in earlier studies [Ferrari et al., 2009]. We found that first-time mothers engaged more frequently in MG with their infants, as did mothers of sons, although our methodological approach did not allow us to assess whether there were also significant differences in the duration of the behaviors in relation to females’ parity or infant’s sex and age. That our primiparous mothers engage in MG with their infants more frequently than experienced mothers may simply be due to the fact that multiparous mothers have multiple offspring and thus less time to devote to each. This study could not address this question directly, as we did not study the amount of time mothers spent grooming, gazing, or providing other types of care for their older offspring. Our future work will be able to incorporate these variables. We also found a negative (though not significant) trend in the relationship between rates of MG and infant age only among multiparous but not primiparous mothers. It is possible that first-time mothers and their infants continued to engage in this form of face-to-face communication over time without decreasing in frequency as infants developed, though our small sample size of primiparous mothers (n = 6) warrants further investigation of first-time mother-hood on infant face-to-face interactions.
Another possible explanation for the high MG in primiparous mothers is that with subsequent off-spring, the “novelty” of the first infant wears off and mothers become less preoccupied with their infants. First-time human parents experience heightened preoccupation compared to experienced parents [Kim et al., 2013], and first-time mothers and their infants maintain symmetrical communication for longer periods of time than experienced mothers [Hsu & Fogel, 2003]. Moreover, experienced mothers feel more effective at parenting [Fish & Stifter, 1993] and thus may not feel the need to employ MG in order to regulate their infants’ attention or affect. Although the “novelty hypothesis” is only speculation at this point and requires further study, we may be observing a similar phenomenon in our macaque mothers, as evidenced by the higher rates of MG (1.3 per 15 min session) by our primiparous mothers compared to our multiparous mothers (0.2 per 15 min session). Our findings are consistent with previous reports in macaques showing that first-time mothers are more protective of their infants [Hooley & Simpson, 1981], and with those in chimpanzees [Stanton et al., 2014] showing that first-time mothers nurse, groom, and play with their infants more. Interestingly, the highest average rates of MG we found (1 MG per 15 min session at week 5, Fig. 1A) are substantially lower than the average MG rates reported by Ferrari et al. [2009; see Fig. 1A]. This difference in MG rates is probably due to the repeated, brief mother-infant separations that Ferrari et al. [2009]’s monkey colony was subjected to. Mothers and infants tend to have increased affiliative interactions after a period of separation, which can account for at least part of the higher rates of MG reported by Ferrari et al. [2009]. Hinde & McGinnis [1977] showed, for instance, that mother and infants who had been separated for 13 days displayed higher levels of physical contact compared to dyads that were not separated. The large inter-dyad variation in MG within and between populations highlights the flexibility of face-to-face interactions in response to the amount of daily contact between mothers and infants.
Perhaps infants of primiparous mothers initiate MG with their mothers more frequently, and this difference may be driving our results. However, we found that dyads did not differ in the proportion of MG initiated by the mother, which means that they also did not differ in the proportion of MG initiated by the infant. Thus, it appears that MG is a behavior that relies equally on both partners in the mother-infant dyad. It is still unclear why first-time mothers, and mothers of sons, engage in MG more frequently. Mothers may initiate MG more frequently very early in the infant’s life, and the infant then becomes the primary initiator after having received this particular type of attention from its mother. That is, some mothers (first-time mothers and mothers of sons) may “teach” their infants to engage in and initiate this behavior. Future studies could explore in more detail the sequential nature of this behavior to test this hypothesis directly.
We observed that mothers of sons engaged in more frequent MG and also increased the rates of GR (especially in the second month of life) they directed to their infants over time. Nonhuman primates show other forms of sex-biased investment [Berkovitch et al., 2002], such as differential social interactions [Murray et al., 2014] and milk production [Hinde, 2009; Hinde et al., 2015] for sons versus daughters. It is possible that the lower volume of richer milk provided for sons encourages more frequent nursing, and more frequent nursing encourages more MG. However, this hypothesis could not be directly tested in this study, as we did not assess bouts of nursing. For macaques, sex-specific maternal care may be due to the fact that males emigrate to join new troops during puberty and must be physically healthy enough and socially savvy in order to be accepted. Hinde [2007, 2009] have hypothesized that mothers of sons in particular may be using lactation to signal to their infants that they should prioritize growth during the critical newborn period [Hinde et al., 2015], which may place them in good standing for later emigration. Additionally, in primate societies, social grooming is important for the maintenance of social bonds [see Dunbar & Shultz, 2010 for a review; Nakamichi & Yamada, 2007; Schino et al., 2007], which are in turn critical for survival [Archie et al., 2014; Silk et al., 2010] and fitness [Silk, 2007; Silk et al., 2003, 2009]. It is also known that more socially competent adult male primates enjoy greater reproductive success [Kaburu & Newton-Fisher, 2015; Langergraber et al., 2013; Schülke et al., 2010], but that males are at a greater risk for mortality across the lifespan, particularly when they emigrate from the troop [Fedigan & Zohar, 1997; Isbell et al., 1993; Small & Smith, 1986]. Thus, if mothers can “prime” their young sons for adaptive social engagement early in life, they may be providing them with an advantage later in life that makes them more likely to survive their emigration and integration into a new troop. Future work following the development of young males into adulthood as they join new troops will be valuable in addressing this possibility.
Bard et al. [2005] suggested that, in chimpanzees, MG may be in part interchangeable with tactile forms of mutual engagement, for example, cradling. In fact, they found that cradling was inversely related with MG. Bard et al. [2005] compare this interchangeable relationship in chimpanzees with that in humans, emphasizing that in Western societies, mutual engagement between mothers and infants is more often visual as a result of reduced physical contact. This idea is supported by studies of tribal cultures in Africa. In particular, the Gusii, a minority tribe living in densely populated highlands of southwestern Kenya, engage in very little gazing overall, and mothers rarely look at their infants [Dixon et al., 1981]. One likely reason for this is that mothers hold their infants less than half the time after 5 months of age, and most of this holding is on the hip or on the back [Dixon et al., 1981], thus allowing for very little face-to-face interaction but increasing the amount of communication that occurs through touch.
Whether or not face-to-face interactions such as MG do indeed influence an infant’s later social and emotional development remains to be determined. There is some evidence that firstborn humans, who tend to receive greater care from the parents than siblings, are more sociable [Lees, 1952]. Further, we know that in humans early face-to-face interactions are predictive of later mother-infant attachment [Belsky et al., 1984; Blehar et al., 1977], and that maternal sensitivity (but not face-to-face interactions per se) during mother–infant interactions is predictive of infant cognitive development [Murray et al., 1996; Olson et al., 1986]. Whether or not infants who engage in more MG (or similar types of face-to-face interactions) are also more social later in development remains to be determined. We have preliminary data indicating that this may indeed be the case [Dettmer & Suomi, 2015], and we are now systematically studying this in current and future cohorts in our laboratory.
Collectively, our data along with other studies showing effects of maternal experience and infant sex on maternal investment are identifying early life experiences that may lead to later sex differences in neurological and behavioral development. These studies point toward an important window for development for both infants and mothers, and give us a greater understanding of the changes that mothers undergo as they transition to first-time motherhood and, subsequently, to experienced motherhood. Such information will be invaluable to understanding the complexities surrounding social development across the lifespan.
ACKNOWLEDGMENTS
This research was supported by the Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and by NICHD grant PO1HD064653. We are grateful to Samantha Haynie and Denisse Guitarra for assistance with data collection, to Dru Corbeille for assistance with database management, and to two anonymous reviewers for their insightful comments on a previous draft of the article.
Contract grant sponsor: Division of Intramural Research; contract grant sponsor: National Institute of Child Health and Human Development; contract grant number: PO1HD064653.
REFERENCES
- Altmann J 1974. Observational study of behaviour: sampling methods. Behaviour 49:227–265. [DOI] [PubMed] [Google Scholar]
- Archie E, Tung J, Clark M, Altmann J, Alberts SC. 2014. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proceedings of the Royal Society B: Biological Sciences 281:20141261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard K, Myowa-Yamakoshi M, Tomonaga M, et al. 2005. Group differences in the mutual gaze of chimpanzees (Pan troglodytes). Developmental Psychology 41:616–624. [DOI] [PubMed] [Google Scholar]
- Belsky J, Rovine M, Taylor DG. 1984. The pennsylvania infant and family development project, III: the origins of individual differences in infant-mother attachment: maternal and infant contributions. Child Development 55:718–728. [PubMed] [Google Scholar]
- Berkovitch FB, Widdig A, Nurnberg P. 2002. Maternal investment in rhesus macaques (Macaca mulatta): reproductive costs and consequences of raising sons. Behavioral Ecology and Sociobiology 48:1–11. [Google Scholar]
- Berman CM. 1992. Immature siblings and mother infant relationships among free ranging rhesus on Cayo Santiago. Animal Behaviour 44:247–258. [Google Scholar]
- Blehar MC, Lieberman AF, Ainsworth MDS. 1977. Early face-to-face interaction and its relation to later infant-mother attachment. Child Development 48:182–194. [Google Scholar]
- Bolker B, Skaug H, Magnusson A, Nielson A. 2012. Getting started with the glmmADMB package, http://glmmadmb.r-forge.r-project.org/glmmADMB.html
- Dettmer AM, Novak MA, Meyer JS, Suomi SJ. 2014. Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 42:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Suomi SJ. 2015. Mother-infant face-to-face interactions predict later social behavior in infant rhesus monkeys. Society for Research in Child Development Annual Meeting, Philadelphia, PA. [Google Scholar]
- Dettmer AM, Woodward RA, Suomi SJ. 2015. Reproductive consequences of a matrilineal overthrow in rhesus monkeys. American Journal of Primatology 77:346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon S, Tronick E, Keefer C, Brazelton TB. 1981. Mother-infant interaction among the Gussi of Kenya In: Field TM, Sostek AM, Vietze P, Leiderman PH, editors. Culture and early interactions. New York: Psychology Press; p 149–168. [Google Scholar]
- Dunbar R, Shultz S. 2010. Bondedness and sociality. Behaviour 147:775–803. [Google Scholar]
- Ehardt C, Blount BG. 1984. Mother-infant visual interaction in Japanese macaques. Developmental Psychobiology 17:391–405. [DOI] [PubMed] [Google Scholar]
- Elo AE. 1978. The rating of chess players, past and present. Arco; New York. [Google Scholar]
- Fedigan LM, Zohar S. 1997. Sex differences in mortality of Japanese macaques: twenty-one years of data from the Arashiyama West population. American Journal of Physical Anthropology 102:161–175. [DOI] [PubMed] [Google Scholar]
- Feldman R, Granat A, Pariente C, et al. 2009. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. Journal of the American Academy of Child & Adolescent Psychiatry 48:919–927. [DOI] [PubMed] [Google Scholar]
- Feldman R 2007. Parent-infant synchrony biological foundations and developmental outcomes. Current directions in psychological science 16:340–345. [Google Scholar]
- Feldman R 2012. Oxytocin and social affiliation in humans. Hormones and Behavior 61:380–391. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Paukner A, Ionica C, Suomi SJ. 2009. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Current Biology 19:1768–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish M, Stifter CA. 1993. Mother parity as a main and moderating influence on early mother-infant interaction. Journal of Applied Developmental Psychology 14:557–572. [Google Scholar]
- Fooden J 2000. Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). [Google Scholar]
- Gwet K 2002a. Inter-rater reliability: dependency on trait prevalence and marginal homogeneity. Statistical Methods for Inter-Rater Reliability Assessment Series 2:1–9. [Google Scholar]
- Gwet K 2002b. Kappa statistic is not satisfactory for assessing the extent of agreement between raters. Statistical Methods for Inter-Rater Reliability Assessment 1:1–6. [Google Scholar]
- Gwet KL. 2014. Handbook of inter-rater reliability: the definitive guide to measuring the extent of agreement among raters: Advanced Analytics, LLC. [Google Scholar]
- Hinde K, Skibiel AL, Foster AB, et al. 2015. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K 2007. First-time macaque mothers bias milk composition in favor of sons. Current Biology 17:R958–R959. [DOI] [PubMed] [Google Scholar]
- Hinde K 2009. Richer milk for sons but more milk for daughters: sex-biased investment during lactation varies with maternal life history in rhesus macaques. American Journal of Human Biology 21:512–519. [DOI] [PubMed] [Google Scholar]
- Hinde R, McGinnis L. 1977. Some factors influencing the effects of temporary mother-infant separation: some experiments with rhesus monkeys. Psychological Medicine 7:197–212. [DOI] [PubMed] [Google Scholar]
- Hooley JM, Simpson MJA. 1981. A comparison of primiparous and multiparous mother-infant dyads in Macaca mulatta. Primates 22:379–392. [Google Scholar]
- Hsu H-C, Fogel A. 2003. Stability and transitions in mother-infant face-to-face communication during the first 6 months: a microhistorical approach. Developmental Psychology 39:1061–1082. [DOI] [PubMed] [Google Scholar]
- Isbell LA, Cheney DL, Seyfarth RM. 1993. Are immigrant vervet monkeys, Cercopithecus aethiops, at greater risk of mortality than residents? Animal Behaviour 45:729–734. [Google Scholar]
- Jacobs BS, Moss H. 1976. Birth order and sex of sibling as determinants of mother-infant interaction. Child Development 47:315–322. [PubMed] [Google Scholar]
- Kaburu SSK, Newton-Fisher NE. 2015. Trading or coercion? Variation in male mating strategies between two communities of East African chimpanzees. Behavioral Ecology and Sociobiology 69:1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Mayes L, Feldman R, Leckman JF, Swain JE. 2013. Early postpartum parental preoccupation and positive parenting thoughts: relationship with parent-infant interaction. Infant Mental Health Journal 34:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AT, Sturm MS, Koch T, et al. 2014. The accuracy of a patient or parent-administered bleeding assessment tool administered in a paediatric haematology clinic. Haemophilia 20:807–813. [DOI] [PubMed] [Google Scholar]
- Langergraber KE, Mitani JC, Watts DP, Vigilant L. 2013. Male-female socio-spatial relationships and reproduction in wild chimpanzees. Behavioural Ecology and Sociobiology 67:861–873. [Google Scholar]
- Lees JP. 1952. The social mobility of a group of eldest-born and intermidate adult males. British Journal of Psychology 43:210–221. [Google Scholar]
- Lewis M 1972. State as an infant-environment interaction: an analysis of mother-infant interaction as a function of sex. Merrill-Palmer Quarterly of Behavior and Development 18:95–121. [Google Scholar]
- Lindburg DG, 1971. The rhesus monkey in North India: an ecological and behavioral study In: Roseblum LA, editor. Primate behavior: developments in field and laboratory research. New York: Academic Press; p 2–106. [Google Scholar]
- MacDonald K, Parke RD. 1986. Parent-child physical play: the effects of sex and age of children and parents. Sex Roles 15:367–378. [Google Scholar]
- Meaney MJ. 2001. Maternal care, gene expression, and the trasmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience 24:1161–1192. [DOI] [PubMed] [Google Scholar]
- Mitchell G, Stevens CW. 1968. Primiparous and multiparous monkey mothers in a mildly stressful social situation: first three months. Developmental Psychobiology 1:280–286. [Google Scholar]
- Murray CM, Lonsdorf EV, Stanton MA, et al. 2014. Early social exposure in wild chimpanzees: mothers with sons are more gregarious than mothers with daughters. Proceedings of the National Academy of Sciences 111:201409507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. 1996. The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Development 67:2512–2526. [PubMed] [Google Scholar]
- Nakamichi M, Yamada K. 2007. Long-term grooming partnerships between unrelated adult females in a free-ranging group of Japanese Monkeys (Macaca fuscata). American Journal of Primatology 69:652–663. [DOI] [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, et al. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour 82:911–921. [Google Scholar]
- Olson SL, Bayles K, Bates JE. 1986. Mother-child interaction and children’s speech progress: a longitudinal study of the first two years (1982-). Merrill-Palmer Quarterly 32:1–20. [Google Scholar]
- Schino G, Cozzolino R, Troisi A. 1999. Social rank and sex-biased maternal investment in captive Japanese macaques: behavioural and reproductive data. Folia Primatologica 70:254–263. [DOI] [PubMed] [Google Scholar]
- Schino G, di Sorrentino EP, Tiddi B. 2007. Grooming and coalitions in Japanese macaques (Macaca fuscata): partner choice and the time frame reciprocation. Journal of Comparative Psychology 121:181–188. [DOI] [PubMed] [Google Scholar]
- Schülke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Current Biology 20:2207–2210. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302:1231–1234. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, et al. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings of the Royal Society of London B 7:3099–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, et al. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Current Biology 20:1359–1361. [DOI] [PubMed] [Google Scholar]
- Silk JB. 2007. Social components of fitness in primate groups. Science 317:1347–1351. [DOI] [PubMed] [Google Scholar]
- Small MF, Smith DG. 1986. The Influence of birth timing upon infant growth and survival in captive rhesus macaques (Macaca mulatta). International Journal of Primatology 7:289–304. [Google Scholar]
- Stanton MA, Lonsdorf EV, Pusey AE, Goodall J, Murray CM. 2014. Maternal behavior by birth order in wild chimpanzees (Pan troglodytes): increased investment by first-time mothers. Current Anthropology 55:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarthen C 1998. The concept and foundations of infant intersubjectivity In: Braten S, editor. Intersubjective communication and emotion in early ontogeny. Cambridge, MA: Cambridge University Press; p 15–46. [Google Scholar]
- Tronick EZ. 1989. Emotions and emotional communication in infants. American Psychologist 44:112–119. [DOI] [PubMed] [Google Scholar]
- Wongpakaran N, Wongpakaran T, Wedding D, Gwet KL. 2013. A comparison of Cohen’s Kappa and Gwet’s AC1 when calculating inter-rater reliability coefficients: a study conducted with personality disorder samples. BMC Medical Research Methodology 13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooddell L, Kaburu SSK, Dettmer A, Suomi S. 2015. Elo-rating as a tool to measure rank changes and dominance stability in semi-free ranging rhesus macaques. American Journal of Primatology 77:80–80. [Google Scholar]