Abstract
Caveolin1 (Cav1) is an important component of the plasmamembrane microdomains, such as caveolae/lipid rafts, that are associated with angiotensin II type 1 (AT1) and epidermal growth factor (EGF) receptors in certain cell types. The interactions of Cav1 with other signaling molecules that mediate AT1 receptor function were analyzed in angiotensin II (Ang II)- and EGF-stimulated hepatic C9 cells. This study demonstrated that cholesterol-rich domains mediate the actions of early up- stream signaling molecules such as Src and intracellular Ca2+in cells stimulated by Ang II, but not by EGF, and that Cav1 has a scaffolding role in the process of mitogen-activated protein kinase activation. Furthermore, Cav1 phosphorylation by Ang II and EGF was regulated by intracellular Ca2+ and Src, further indicating reciprocal interactions among Cav1, Src, and intra- cellular Ca2+ through the AT1 receptor. Phosphorylation of Cav1 and the EGF receptor by Ang II, but not of extracellular signal-regulated kinase 1/2, was dependent on intracellular Ca2+. The phosphatidylinositol 3-kinase inhibitors, 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride (LY294002) and wortmannin, differentially modulated both Cav1 and EGF receptor activation by Ang II through intracellular Ca2+. These findings further demonstrate the importance of Cav1 in conjunction with the receptor-mediated signaling path-ways involved in cell proliferation and survival. It is clear that differential signaling pathways are operative in Ang II- and EGF-stimulated C9 cells and that cholesterol-enriched microdomains are essential components in cellular signaling processes that are dependent on specific agonists and/or cell types.
Caveolin1 (Cav1) is an essential component of caveolae/lipid rafts, which are involved in cell differentiation, proliferation, and apoptosis (Cohen et al., 2004). Cav1 interacts with and modulates many signaling molecules in caveolae/lipid rafts, including receptor tyrosine kinases, G proteins, and serine/threonine protein kinases (Anderson, 1998; Shaul and Anderson, 1998). Ang II promotes Cav1 association with AT1 and EGF receptors, which undergo functional interactions in Cav1-enriched caveolae/lipid rafts (Ushio-Fukai et al., 2001; Cohen et al., 2004; Olivares-Reyes et al., 2005). Such associations are essential for EGFR transactivation in multiple cell types (Ushio-Fukai et al., 2001; Cohen et al., 2004). EGF receptor transactivation by G protein-coupled receptors (GPCRs) such as the AT1R is a central factor in cellular signaling processes (Eguchi and Inagami, 2000; Haendeler and Berk, 2000; Shah and Catt, 2003; Yin et al., 2005). Together, such studies have revealed that the functions and regulation of Cav1 can involve diverse signaling molecules, consistent with its regulatory role in the activation of cell signaling pathways.
Ang II and EGF are known to act as potent regulatory factors in the heart, kidney, and liver. Ang II and EGF initiate AT1 and EGF receptor signaling involved in cell growth, mitogenesis, and other functions (de Gasparo et al., 2000; Carver et al., 2002; Kiyatkin et al., 2006; Kumar et al., 2007). Binding of Ang II and EGF to their specific receptors stimulates the phosphorylation of caveolar targets such as signaling molecules including Cav1, Raf1, and MAP kinases (Mineo et al., 1996; Ushio-Fukai et al., 2001). The AT1 receptor is a typical GPCR, and its signaling characteristics reflect the molecular mechanisms operating in many cell types (de Gasparo et al., 2000). Previous studies have shown that both Ang II and EGF promote the association of Cav1 with the AT1R, and that MAPK phosphorylation may occur through differential signaling pathways in Ang II- and EGF-stimulated C9 cells (Shah et al., 2004; Olivares-Reyes et al., 2005). Relatively little is known about the manner in which Cav1 interacts with upstream and downstream signaling molecules in these signal pathways. Although there is evidence for the involvement of cholesterol-rich microdomains during ERK1/2 activation in Ang II-stimulated C9 cells (Olivares- Reyes et al., 2005), the mechanisms involved in such path- ways are not entirely understood. Phosphatidylinositol 3-kinase (PI3K) is known to promote a wide variety of cellular functions via activation of Akt, including growth, differentiation, and survival, which are initiated by Ang II and EGF (Cantrell, 2001; Cantley, 2002). PI3K/Akt signaling is also involved in phosphorylation of the AT1R but has no role in MAPK activation in Ang II- or EGF-stimulated C9 cells (Garcia-Caballero et al., 2001; Shah et al., 2004; Olivares-Reyes et al., 2005). In view of these data, we determined the extent to which PI3K/Akt also affects other signaling molecules, such as Cav1, during AT1R signaling in these cells.
The major purpose of this study was to delineate the interactions of Cav1 with other key components of signaling pathways activated by Ang II and EGF in hepatic C9 cells, such as Src, EGFR, PI3K/Akt, and MAPK. We also examined the influence of intracellular Ca2[H11001] and cholesterol-enriched microdomains on MAPK cascade activities and the subcellular targeting of key signaling molecules in hepatic C9 cells.
Materials and Methods
Antibodies and Reagents.
F-12K nutrient mixture (Kaighn’s modification), fetal bovine serum, and antibiotic solution were purchased from Invitrogen (Carlsbad, CA). Phospho-EGF receptor (Tyr1068), phospho-caveolin1 (Tyr14), phospho-Akt (Ser473), phospho-Src family (Tyr416), p42/44 MAPK, and phospho-ERK1/2 (Thr202/Tyr204) E10 antibodies were from Cell Signaling Technology (Danvers, MA); BAPTA-2/AM was from Calbiochem (San Diego, CA). Antibodies to RSK2, Akt, and caveolin1 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies [IRDye 800CW Conjugated Goat (polyclonal) anti-rabbit IgG, and IRDye 680 Conjugated Goat (polyclonal) anti-mouse IgG] were purchased from LI-COR Biosciences (Lincoln, NE). 125I-[Sar1,Ile8]Ang II was from PerkinElmer (Waltham, MA), and clone 9 rat liver cells were obtained from American Type Culture Collection (Manassas, VA). Silencer predesigned siRNA for rat caveolin1 (siRNA number 53737) and Silencer Negative Control Number 1 siRNA were purchased from Ambion (Austin, TX). DharmaFECT1 transfection reagent was obtained from Dharmacon RNA Technologies (Lafayette, CO). All other drugs and reagents were obtained from Sigma Chemical Co. (St. Louis, MO). Nystatin, filipin III, LY294002, and wortmannin were solubilized in dimethyl sulfoxide.
Cell Culture.
Hepatic clone 9 cells (C9 cells), which are derived from normal rat liver and retain an epithelial phenotype, were maintained in culture in F-12K nutrient mixture (Kaighn’s modification) supplemented with 10% (v/v) fetal calf serum, 100 [H9262]g/ml streptomycin, 100 IU/ml penicillin, and 250 [H9262]g/ml amphotericin B (Fungizone) and cultured in a humidified atmosphere of 5% CO2 in air at 37°C.
Cells were subcultured weekly as described previously (Shah and Catt, 2002). In this study, subconfluent C9 cells were used between passages 4 and 10, when these cells display maximum expression of their endogenous AT1 receptors.
AT1 Receptor Binding Assays.
AT1 receptor binding assays were performed according to methods described previously (Hunyady et al., 2002). C9 cells were cultured in 24-well plates, and the surface binding of the AT1R was determined at equilibrium by incubating the cells in the presence of 125I-[Sar1,Ile8]Ang II (0.05 μCi/well) and unlabeled [Sar1,Ile8]Ang II (2 nM) at 4°C overnight. Cells were washed twice with 1 ml of ice-cold Dulbecco’s PBS and then lysed with 0.5 M NaOH and 0.05% SDS at room temperature for 10 min before measurement of the bound radioactivity by [H9253] spectrometry.
Immunoblot Analysis.
For each experiment, cells were cultured in six-well plates with medium containing 10% fetal calf serum, and at 60 to 70% confluence, the culture medium was replaced by serum- free medium for 24 h before specific treatments and stimulation at 37°C. After the time periods indicated in the individual experiments, the media were aspirated, and cells were washed three times with ice-cold PBS and then lysed in 100 μl of Laemmli sample buffer for 10 min. Samples were harvested by scraping and were frozen at–70°C. The samples were sonicated briefly, heated at 95°C for 5 min, and centrifuged for 5 min at 4°C. For immu noblot analyses, cellular proteins were resolved by SDS-PAGE (8–16%) gradient gels and transferred to polyvinylidene difluoride membranes. Blots were then incubated overnight at 4°C with primary antibodies and washed three times with PBS containing 0.1% Tween 20 and then probed with secondary antibodies (LI-COR Biosciences) according to the manufacturer’s instructions. Densitometric analyses of the immunoblots were performed with an Odyssey Infrared Imager (LI-COR). In some cases, blots were stripped and reprobed with other antibodies.
Measurement of Intracellular Calcium.
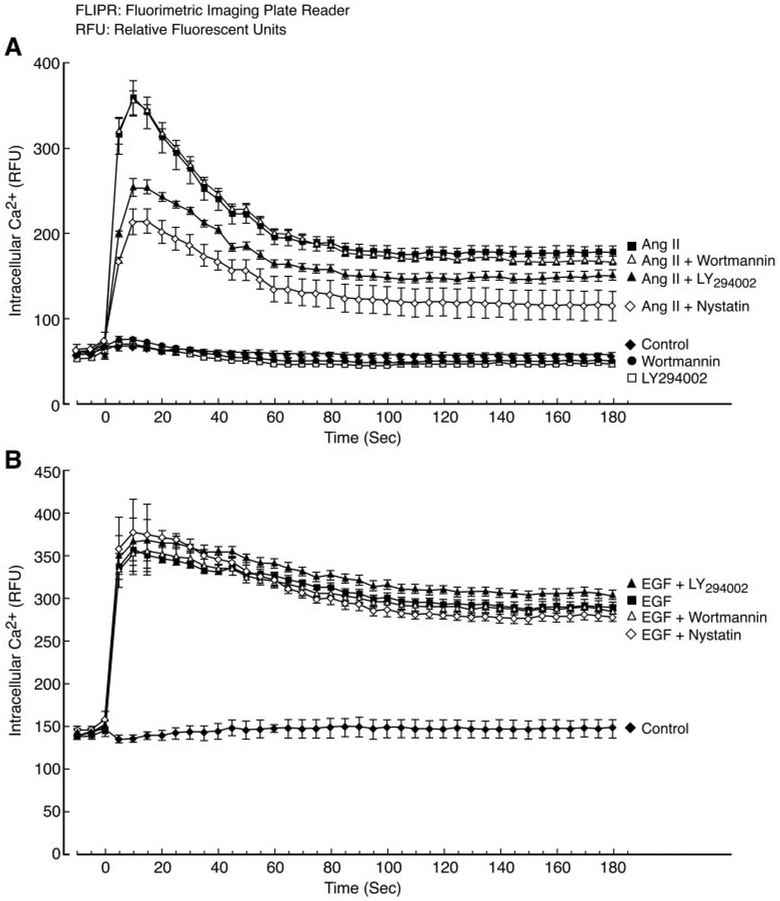
C9 cells (50,000 cells/well) were seeded in 96-well plates (Costar 3603; Corning Life Science, Acton, MA) and incubated overnight at 37°C in a humidified CO2 incubator. On the day of assay, the cells were washed once with loading buffer (Hanks’ balanced salt solution containing 20 mM HEPES, pH 7.4). Then, 30 [H9262]l of loading dye (FLIPR Calcium 3 Assay Kit; Molecular Devices, Sunnyvale, CA) supplemented with 2.5 mM probenecid was added to each well, followed by 45-min incubation at 37°C. After the incubation step, cells were treated as indicated in the legend to Fig. 11 at room temperature. Thereafter, intracellular calcium ([Ca2+i) changes were measured at room temperature via FLIPR (FLIPR TETRA, Molecular Devices; excitation wavelength, 470–495 nm; emission wavelength, 515–575 nm) essentially following the manufacturer’s instructions. In brief, cells in each well were simultaneously exposed to 100 nM Ang II or 20 ng/ml EGF for 5 min and pretreated with LY294002 or wortmannin. Fluorescence readings were recorded from each well each second for a total of 3 min and then every 3 s for 10 min. Time course data from FLIPR Ca2+ assay were expressed in terms of relative fluorescent units. Each tracing represents mean results ([H11006] S.E.M.) of four independent experiments.
Fig. 11.
Effects of LY294002, wortmannin, and nystatin on Ang II- or EGF-induced increases of [Ca2+]i. C9 cells were prepared and data were acquired as described under Materials and Methods. The cells were pretreated with LY294002 or wortmannin for 20 min or with nystatin for 30 min before stimulation with or without 100 nM Ang II or 20 ng/ml EGF. As shown in Fig. 11A, the maximum [Ca2+]i increase elicited by Ang II was significantly inhibited by pretreatment with LY294002 or nystatin but not wortmannin (P < 0.01 compared with Ang II alone). However, pretreatment with LY294002, nystatin, or wortmannin had no significant effect on the maximum [Ca2+]i increase elicited by EGF in Fig. 11B (compared with EGF alone). There are no significant differences among control and pretreatment with LY294002, wortmannin, or nystatin, with or without Ang II or EGF.
Caveolin1 siRNA Preparation and Transfection.
C9 cells cultured in six-well plates were transfected with the corresponding siRNA using DharmaFECT1 transfection reagent following the transfection protocol. In these experiments, each siRNA was used at a final concentration of 100 nM, and cells were studied 72 h after transfection. The specific caveolin1 siRNA sequences were as follows: sense, GGGACACACAGUUUCGACGtt; and antisense, CGUCGAAACUGUGUGUCCCtt. Transfection reagent and Silencer Negative Control Number 1 siRNA served as controls to assess the efficiency of transfection by visual inspection. The efficiency of caveolin1 knockdown was determined by immunoblotting. Expression of p42/44 MAPK was also examined to determine whether the siRNAs have nonspecific effects on protein expression and to control for gel loading.
Statistical Analysis.
Data represent the mean ± S.E. derived from at least three independent experiments. Statistical analyses were performed by one-way analysis of variance, and a P value of less than 0.05 was considered to be significant.
Results
Roles of Cholesterol-Rich Microdomains and Cav1 in Up- and Downstream Signaling Molecule Activities in Agonist-Stimulated C9 Cells.
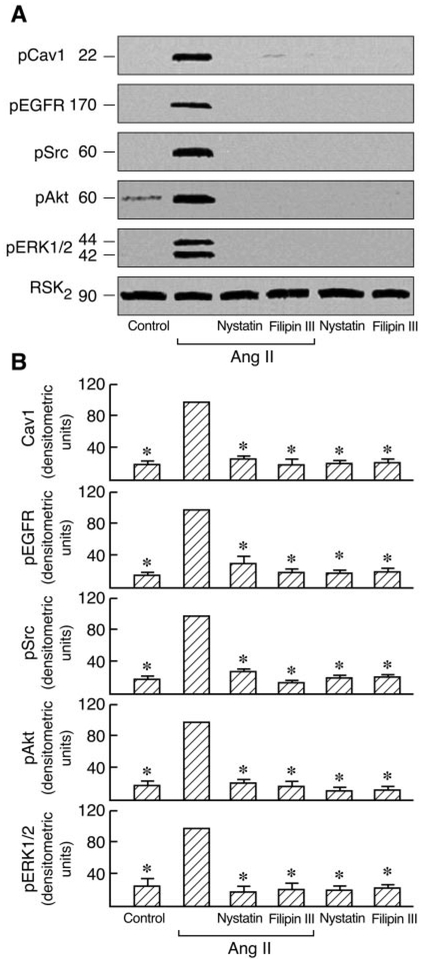
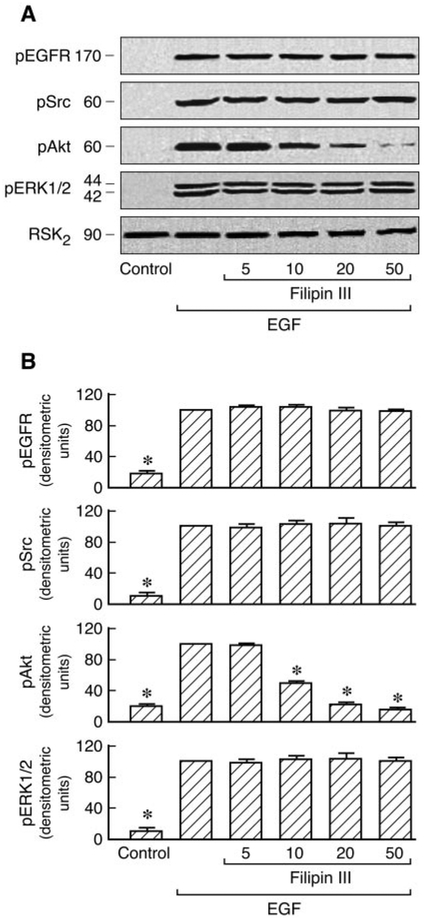
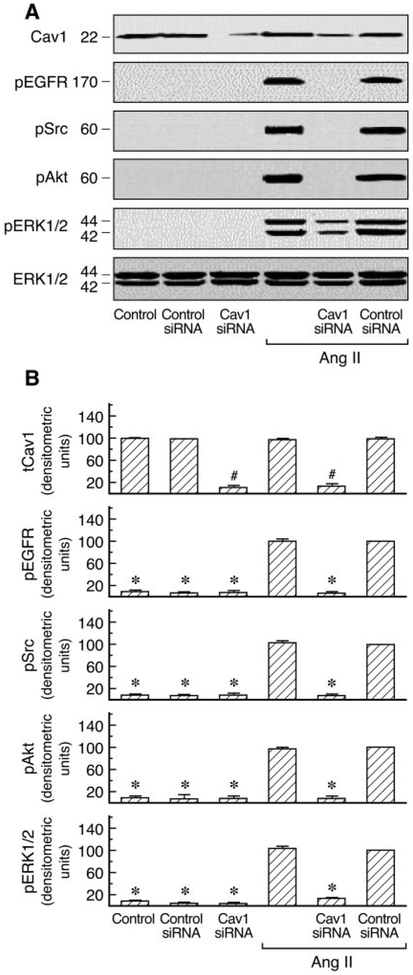
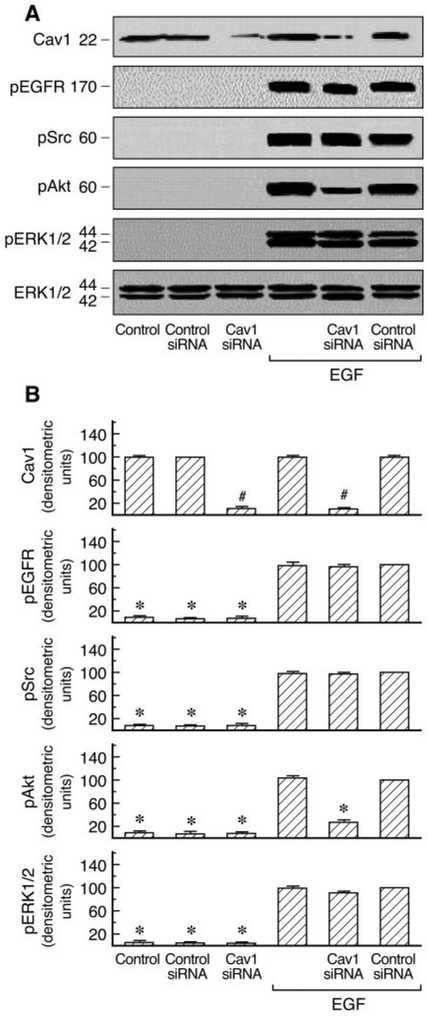
A number of signal transduction molecules, including the EGFR, Ras/Raf, and p42/44 ERK, are known to be concentrated in caveolae/lipid rafts. Cav1 has also been reported to regulate diverse signaling cascades by interaction with specific signaling molecules (Shaul and Anderson, 1998). AT1 receptor expression has been examined by binding assays in C9 cells (data not shown). To investigate the role of Cav1 in AT1R signaling, we first analyzed the effects of cholesterol-depleting agents (filipin III and nystatin), which disrupt caveolar structure and endocytosis, on Ang II-induced Cav1, EGFR, Src, Akt, and ERK1/2 phosphorylation in C9 cells. Preliminary experiments in C9 cells revealed that no significant changes were observed in the EGFR, Src, Akt ERK1/2, and RSK2 after treatment with 20 μM filipin III or 50 μM nystatin before agonist stimulation. These molecules were detected with antibodies directed against the total proteins (i.e., the sum of nonphosphorylated and phosphorylated proteins). The compartmentalization of signaling molecules in cholesterol-rich structures is specifically required for EGFR transactivation in AT1R signaling (Ushio-Fukai et al., 2001). As shown in Fig. 1, Ang II-induced EGFR, Src, Akt, and ERK1/2 phosphorylation were inhibited by 20 [H9262]M filipin III or 50 μM nystatin. EGF-induced Akt phosphorylation was also inhibited by filipin III in a dose-dependent manner; however, treatment with filipin III did not affect EGF-induced tyrosine phosphorylation of Src and phosphorylation of ERK1/2 and that of EGFR in C9 cells (Fig. 2). Similar results were obtained after pretreatment with increasing concentrations of nystatin (10–100 μM) in EGF-stimulated C9 cells (data not shown). These findings demonstrate that cholesterol-rich microdomains involve early up- and downstream regulatory molecules during cellular signaling in C9 cells. We also used siRNA-induced down-regulation of Cav1 expression by ~80% to explore its role in AT1R signaling. As shown in Figs. 3 and 4, cells treated with Cav1 siRNA and Ang II or EGF, respectively, showed a marked decrease in Akt phosphorylation relative to treatment with control and Ang II or EGF. Pre-treatment with Cav1 siRNA also significantly reduced EGFR, Src, and ERK1/2 phosphorylation in cells stimulated by Ang II but not by EGF. These data further confirm that Cav1 has a positive role in activation of the PI3K/Akt pathway in Ang II- or EGF-stimulated cells and a central role in the Ras/Raf/MAPK pathway in cells stimulated by Ang II but not by EGF.
Fig. 1.
Effects of nystatin and filipin III on the activation of phospho-EGFR, -Cav1, -Src, -Akt, and -ERK1/2 in Ang II-stimulated cells. A, C9 cells were pretreated with 50 [H9262]M nystatin or 20 μM filipin III for 30 min and then stimulated with or without 100 nM Ang II for 5 min. Cell lysates were separated by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-Cav1, phospho-Src, phospho-EGFR, phospho-Akt, phospho-ERK1/2, and total RSK2. B, pooled plot of signal intensity for each data point as determined by densitometry. *, P [H11021] 0.01 for decrease in phosphorylation by Ang II in the presence of inhibitors versus Ang II alone, shown as 100.
Fig. 2.
Effects of filipin III on EGFR, Src, Akt, and ERK1/2 phosphorylation in EGF-stimulated cells. A, C9 cells were pretreated for 30 min with filipin III (5–50 μM) before 20 ng/ml EGF stimulation for 5 min. After treatment, the cell lysates were separated by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-EGFR, phospho-Src, phospho-Akt, phospho-ERK1/2, and total RSK2. B, pooled data plot signal intensity of each data point as determined by densitometry. * P < 0.01 compared with EGF alone, taken as 100.
Fig. 3.
Effects of siRNA-mediated caveolin1 knockdown on the activation of phospho-EGFR, -Src, -Akt, and -ERK1/2 in Ang II-stimulated cells. A, an immunoblot prepared from C9 cells transfected with siRNA targeted against caveolin1 or control siRNA and treated with Ang II (100 nM) for 5 min. Cell lysates were separated by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-EGFR, phospho-Src, phospho-Akt, phospho-ERK1/2, and total ERK1/2 and caveolin1. B, pooled plot of signal intensity for each data point as determined by densitometry, showing the level of EGFR, Src, Akt, ERK1/2 phosphorylation and total caveolin1 in caveolin1 siRNA- or control siRNA-transfected cells in basal condition and after treatment with Ang II. #, P < 0.001 compared with control siRNA alone, taken as 100; *, P < 0.01 compared with control siRNA and Ang II, taken as 100.
Fig. 4.
Effects of siRNA-mediated caveolin1 knockdown on the activation of phospho-EGFR, -Src, -Akt, and -ERK1/2 in EGF-stimulated cells. A, an immunoblot prepared from C9 cells transfected with siRNA targeted against caveolin1 or control siRNA and treated with EGF (20 ng/ml) for 5 min. Cell lysates were separated by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-EGFR, phospho-Src, phospho-Akt, phospho-ERK1/2, and total ERK1/2 and caveolin1. B, pooled plot of signal intensity for each data point as determined by densitometry, showing the level of EGFR, Src, Akt, ERK1/2 phosphorylation, and total caveolin1 in caveolin1 siRNA- or control siRNA-transfected cells in basal condition and after treatment with EGF. #, P < 0.001 compared with control siRNA alone, taken as 100; *, P < 0.01 compared with control siRNA and EGF, taken as 100.
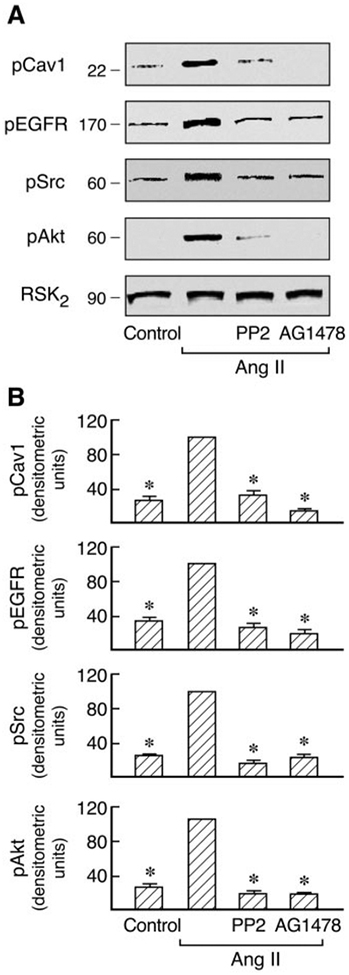
Regulation of Agonist-Induced Cav1 Activation by Src and EGF Receptor Kinase in C9 Cells.
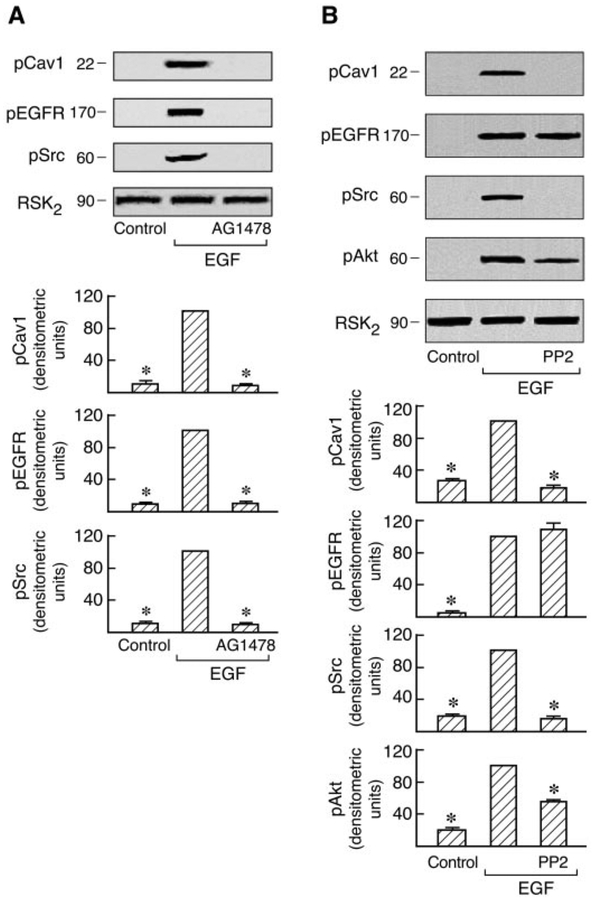
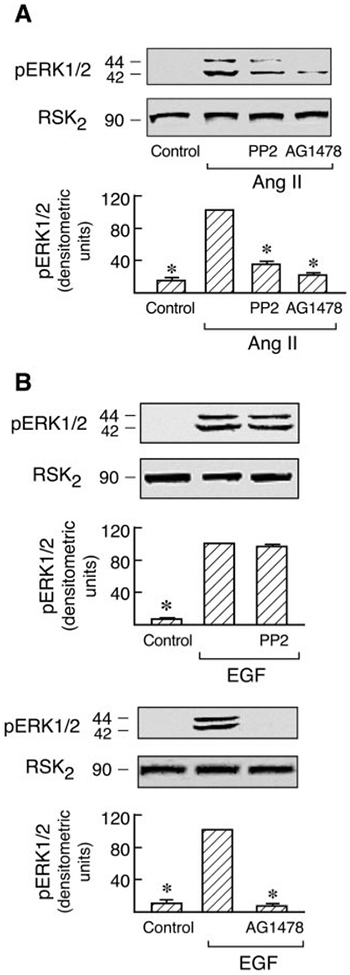
Recent studies have shown that agonist activation of AT1 and EGF receptors promotes their association with Cav1 and that the EGF receptor kinase inhibitor AG1478 prevents this association in C9 cells (Olivares-Reyes et al., 2005). Consistent with this, Ang II-induced EGFR, Src, Akt, and ERK1/2 phosphorylation are all dependent on Cav1 in C9 cells (Fig. 3). To determine whether Cav1 phosphorylation is also modulated by other signal molecules, the effects of the Src family kinase inhibitor (PP2) and EGFR kinase inhibitor (AG1478) on Cav1, Src, Akt, and EGFR phosphorylation induced by Ang II and EGF were examined in C9 cells. As shown in Figs. 5 and 6, pretreatment with AG1478 completely inhibited Ang II- and EGF-induced Src and Cav1 phosphorylation and caused almost complete loss of EGFR phosphorylation. Pretreatment with PP2 also abolished the Cav1 phosphorylation induced by Ang II and EGF, respectively, but had no inhibitory effect on EGF-induced phosphorylation of the EGFR. However, it did prevent Ang II-induced EGFR phosphorylation. Pretreatment with PP2 or AG1478 also completely inhibited Akt phosphorylation induced by Ang II or EGF. Because the data in Fig. 3 suggest that Cav1 activation by Ang II has a scaffolding role in MAP kinase signaling, the activation of signal molecules downstream of Cav1 and the EGFR after pretreatment with AG1478 or PP2 was examined. In cells treated with AG1478, activation of ERK1/2 by both EGF and Ang II was eliminated. However, pretreatment with PP2 only abolished ERK1/2 activation in response to Ang II but not to EGF (Fig. 7). These findings further indicate the reciprocal interaction of Cav1 with these signaling molecules in membrane domains. They also suggest that Src and Cav1 are involved in EGFR transactivation and the ERK1/2 cascade phosphorylation induced by Ang II but not by EGF. However, EGFR activation is consistently required for MAPK activation by Ang II or EGF in hepatic C9 cells.
Fig. 5.
Effects of inhibitors on Cav1, Src, EGF receptor, and Akt phosphorylation by Ang II. A, C9 cells were preincubated with PP2 (10 [H9262]M) or AG1478 (10 [H9262]M) for 20 min followed by treatment with 100 nM Ang II for 5 min. The cell lysates were resolved by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-Cav1, phospho-EGFR, phospho-Src, phospho-Akt, and RSK2. B, pooled data of densitometric analyses show the levels of Cav1, EGFR, Src, and Akt phosphorylation in cells with and without the above treatment. *, P < 0.01 for decrease in phosphorylation by Ang II in the presence of inhibitors versus Ang II alone, taken as 100.
Fig. 6.
Effects of inhibitors on Cav1, EGFR, Src, Akt, and ERK1/2 phosphorylation in EGF-stimulated cells. Serum-starved C9 cells were pretreated with PP2 (10 μM) or AG1478 (10 μM) for 20 min followed by stimulation with 20 ng/ml EGF for 5 min. After treatment, the cell lysates were separated by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-Cav1, phospho-Src, phospho-EGFR, phospho- Akt, phospho-ERK1/2, and total RSK2. Representative data are shown (A and B). * P < 0.001 compared with EGF alone, taken as 100.
Fig. 7.
Roles of EGF receptor and Src kinases in Ang II- and EGF-induced ERK1/2 phosphorylation. C9 cells were pretreated with PP2 (10 μM) or AG1478 (10 μM) for 20 min and then stimulated with 100 nM Ang II (A) or 20 ng/ml EGF (B) for 5 min. The cell lysates were resolved by SDS- PAGE and analyzed in immunoblots probed with antibodies against phospho-ERK1/2 and RSK2. B, pooled data plot signal intensity of each data point as determined by densitometry. *, P < 0.01 compared with Ang II or EGF alone, taken as 100.
Role of Intracellular Calcium in Cav1 and EGFR Phosphorylation Induced by Ang II and EGF in C9 Cells.
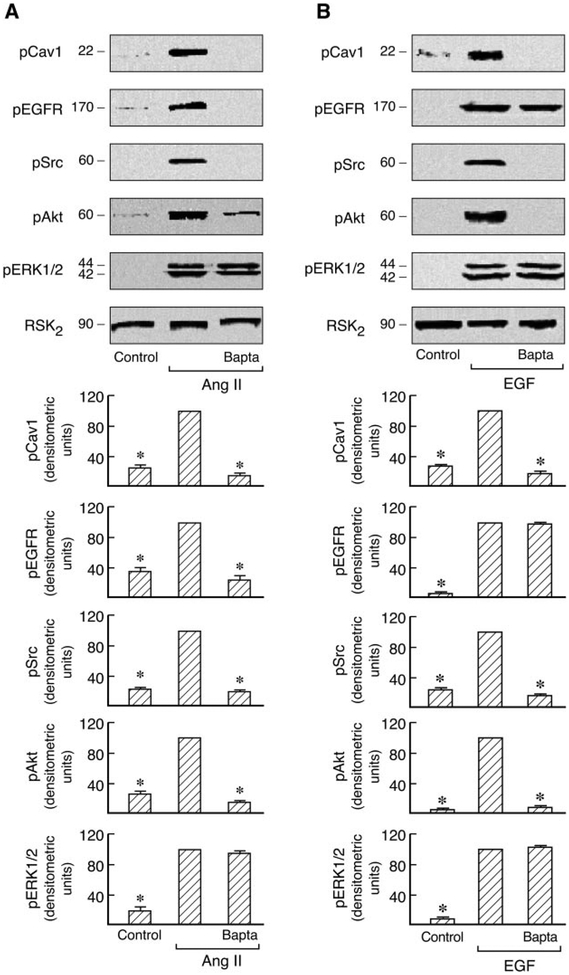
Ca2+ is a key signaling molecule in numerous cellular processes and mediates the actions of a variety of biological and pharmacological stimuli, including Ang II and EGF. Increases in intracellular Ca2+ trigger or regulate many cellular responses, including proliferation and survival (Clapham, 1995; Ghosh and Greenberg, 1995; Haendeler et al., 2003; Gifford et al., 2004; Mifune et al., 2005). To gain further insight into signaling of the AT1R by Cav1, we also explored the role of intracellular calcium as an upstream signaling molecule in the modulation of Cav1-dependent processes. The phosphorylation of signal proteins was analyzed in immunoblots probed with the respective phosphospecific antibodies after pretreatment with the intracellular calcium chelator BAPTA-2/AM in C9 cells stimulated by Ang II. As shown in Fig. 8, this caused significant inhibition of Cav1, EGFR, Src, and Akt phosphorylation. In contrast, pretreatment with BAPTA-2/AM did not markedly decrease ERK1/2 phosphorylation as observed previously (Shah and Catt, 2002). The EGF-induced phosphorylation of Cav1 and Akt, but not of the EGFR and ERK1/2, was also abolished by BAPTA-2/AM in these cells. These results indicate that intracellular Ca2+ has a major regulatory role in both Cav1 and EGFR phosphorylation in Ang II-stimulated C9 cells and that it regulates Cav1 but not EGFR activation in EGF- stimulated C9 cells.
Fig. 8.
Roles of intracellular calcium in Ras/Raf/MAPK and PI3K/Akt pathways in Ang II-and EGF-treated cells. Serum-starved C9 cells were pretreated with BAPTA-2/AM (10 μM) for 30 min and then stimulated with 100 nM Ang II (A) or 20 ng/ml EGF (B) for 5 min. Cells were lysed, and the cell lysates were separated by SDS-PAGE and analyzed in immunoblots probed with antibodies against phosphoCav1, phospho-EGFR, phospho-Src, phospho-Akt, phospho-ERK1/2, and RSK2. For each experiment, pooled data plot signal intensity of each data point as determined by densitometry. *, P < 0.01 compared with Ang II or EGF alone, taken as 100.
Effects of PI3K/Akt on MAP Kinase Cascade Phosphorylation Induced by Ang II or EGF in C9 Cells.
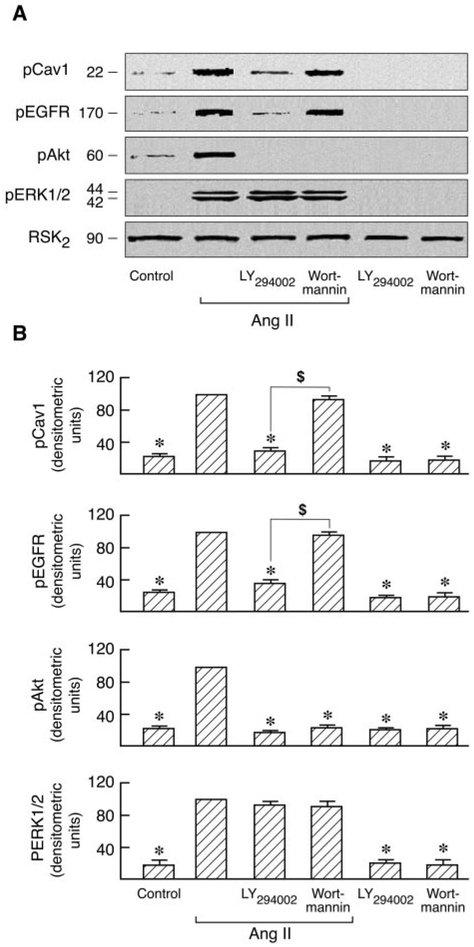
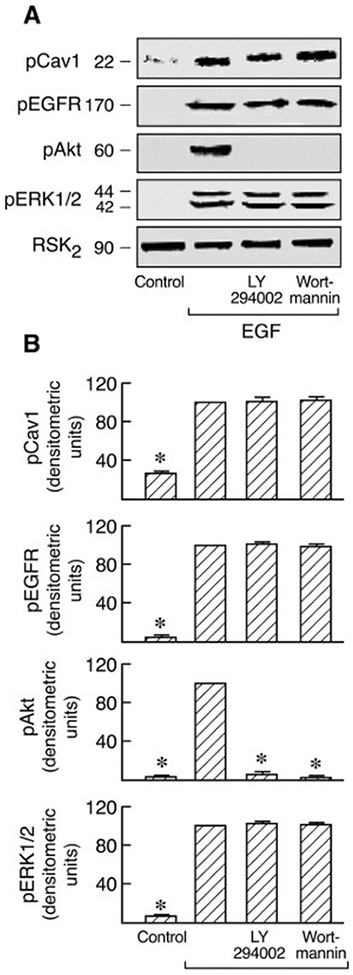
Activation of PI3K initiates a major signaling pathway via Akt that regulates a wide variety of cellular functions. Two PI3K inhibitors, LY294002 and wortmannin, have been used in defining PI3K-dependent biological functions. Both of these agents were used to determine whether PI3K influences the Ras/Raf/MAPK signal pathway in which Cav1 is involved (Fig. 4). It is interesting that similar to the effect of pretreatment with BAPTA-2/AM (Fig. 8), LY294002 blocked both Cav1 and EGFR activation but not ERK1/2 activation in Ang II-stimulated C9 cells whereas completely inhibiting Akt activation. In contrast, wortmannin had no effect on Cav1 and EGFR phosphorylation (Fig. 9). These agents have been reported to exert similar effects in multiple cell types (Brunn et al., 1996; Adi et al., 2001; Ethier and Madison, 2002). In addition, both inhibitors had no effect on Cav1, EGFR, and ERK1/2 phosphorylation in EGF-stimulated C9 cells during complete inhibition of Akt activation (Fig. 10). These findings indicate that LY294002 and wortmannin differentially regulate both Cav1 phosphorylation and EGFR activation in Ang II-stimulated C9 cells and that PI3K has no effect on Ras-p42/44 MAPK activation in Ang II- or EGF-stimulated C9 cells.
Fig. 9.
Effects of PI3K inhibitors on Ang II-induced phosphorylation of Cav1, EGFR, Akt, and ERK1/2. A, serum-starved C9 cells were pretreated with 50 μM LY294002 and 100 nM wortmannin for 20 min followed by stimulation with or without 100 nM Ang II for 5 min. Cells were lysed, and the cell lysates were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. The membranes were immunoblotted with antibodies against phospho-Cav1, phospho-EGFR, phospho-Akt, phospho-ERK1/2, and RSK2. B, for each experiment, pooled data plot signal intensity of each data point as determined by densitometry. *, P < 0.001 compared with Ang II alone, taken as 100; $, P < 0.01 for LY294002 versus wortmannin-pretreated cells.
Fig. 10.
Effects of PI3K inhibitors on EGF-induced phosphorylation of Cav1, EGFR, Akt, and ERK1/2. A, C9 cells were pretreated with 50 μM LY294002 and 100 nM wortmannin; 20 min after treatment, the cells were stimulated with EGF (20 ng/ml) for 5 min. The cell lysates were resolved by SDS-PAGE and analyzed in immunoblots probed with antibodies against phospho-Cav1, phospho-EGFR, phospho-Akt, phospho-ERK1/2, and RSK2. B, pooled plot of signal intensity for each data point as determined by densitometry. *, P < 0.01 compared with EGF alone, taken as 100.
Effects of Pretreatment with LY294002, Wortman- nin, and Nystatin on Agonist-Induced [Ca2+]i Re- sponses in C9 Cells.
Both Ang II and EGF can trigger increases of [Ca2+]i (de Gasparo et al., 2000; Eguchi et al., 1998; Zhang et al., 2002). LY294002 affected the phosphorylation by Ang II of Cav1 and EGFR like BAPTA-2/AM, and cholesterol-enriched microdomains were involved in activation of the early upstream signal molecule Src by Ang II. Further analysis of these events was conducted by examining the effects of pretreatment with 50 μM LY294002, 100 nM wortmannin, and 50 μM nystatin on [Ca2+]i measured by FLIPR in C9 cells. As shown in Fig. 11A, LY294002 and nystatin significantly reduced the Ang II-induced [Ca2+i increase in C9 cells, whereas wortmannin had no such effect. Similar results have been reported in NIH 3T3 fibroblasts, HepG2 cells, and airway smooth muscle cells (Ethier and Madison, 2002; Tolloczko et al., 2004). However, no such effects of pretreatment with LY294002 or nystatin were observed in EGF-stimulated C9 cells (Fig. 11B). These findings suggest that the two PI3K inhibitors have differential effects on Ang II-triggered intracellular calcium mobilization in C9 cells and indicate that cholesterol-rich domains mediate agonist-induced intracellular calcium regulation in Ang II- stimulated but not EGF-stimulated C9 cells.
Discussion
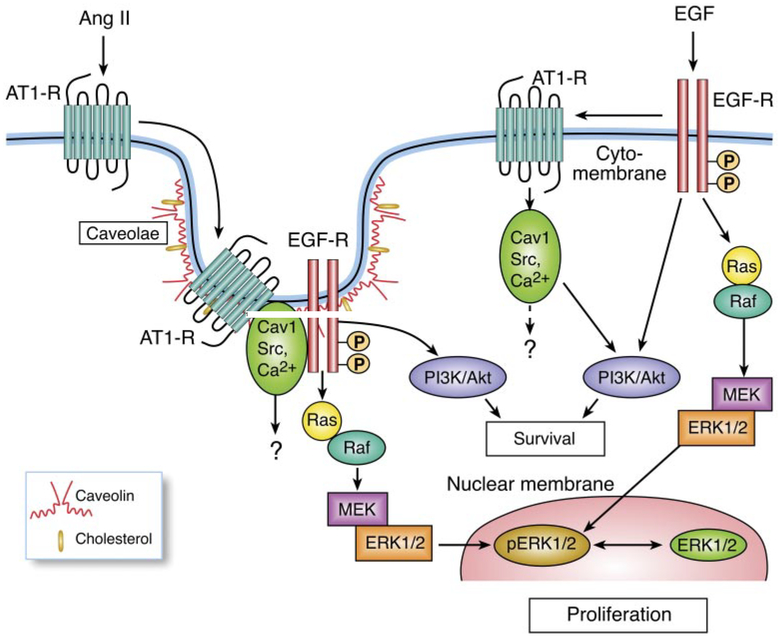
Recent studies have revealed that caveolins participate in cell differentiation, proliferation, and apoptosis through the association of Cav1 with EGFR and AT1 receptors (Ishizaka et al., 1998; Ushio-Fukai et al., 2001; Olivares-Reyes et al., 2005; Ushio-Fukai and Alexander, 2006). In particular, the role of caveolin in agonist-dependent interactions between endogenous AT1R and EGFR has been demonstrated in hepatic C9 cells (Olivares-Reyes et al., 2005). The present study has demonstrated apparent interactions of Cav1 with intracellular Ca2[H11001], Src, EGFR, Akt/protein kinase B, and ERK1/2 in hepatic C9 cells. Two different agonists, Ang II and EGF, activate MAPK through distinct cellular signaling pathways (Fig. 12). Ang II promotes the association of Cav1 with the AT1R and EGFR and reciprocal cross-talk among Cav1, Src, and intracellular Ca2[H11001] through the AT1R, which activates the EGFR through a matrix metalloproteinase (Shah et al., 2004), leading to MAPK and PI3K/Akt phosphorylation. Unlike Ang II, EGF promotes the association of the EGFR, AT1R, and Cav1 (Olivares-Reyes et al., 2005) and the interaction of Cav1 with Src and intracellular Ca2[H11001] as downstream signaling molecules of the EGFR, consistent with reports in other cell types (Che and Carmines, 2002; Fleming et al., 2006; Paugh et al., 2008). However, this interaction regulates PI3K/Akt cascade activities but not EGFR activity in C9 cells. We observed that when Ang II-induced Cav1 phosphorylation via the AT1 receptor was almost abolished by 20 [H9262]M filipin III or 50 [H9262]M nystatin, phosphorylation of Src, an early upstream signaling molecule, and of the EGFR, Akt, and MAP was also inhibited. The analysis of intracellular calcium changes by FLIPR in nystatin-treated C9 cells showed a similar inhibitory effect on the [Ca2+i in response to the other early upstream signal molecule, Ang II, but not in response to EGF. To our knowledge, this is the first report that in AT1 receptor signaling, cholesterol-rich microdomains involve both up- and downstream signaling molecules such as early upstream signaling molecule Src and intracellular calcium in C9 cells in contrast to observations in vascular smooth muscle cells (Griendling et al., 1986; Ushio-Fukai et al., 2001). Recently, others have also reported that such microdomains involve intracellular Ca2+ or Src in certain cell types (Minshall et al., 2000; Janas et al., 2005). However, no effects of filipin III and nystatin on tryosine phosphorylation of Src and phosphorylation of ERK1/2 were observed in EGF- treated C9 cells. This suggests that the involvement of cholesterol-enriched microdomains in the MAPK cascade and early upstream signaling molecule activities are probably dependent on specific agonists and/or cell types.
Fig. 12.
Schematic diagram of the signal transduction pathways used by Ang II and EGF to activate MAPK in hepatic C9 cells. Ang II promotes the association of Cav1 with the AT1R and EGFR and reciprocal cross-talk among Cav1, Src, and intracellular Ca2+ through the AT1R, which activates the EGFR leading to MAPK and PI3K/Akt phosphorylation. Unlike Ang II, EGF promotes the association of the EGFR, AT1R, and Cav1 and the interaction of Cav1 with Src and intracellular Ca2+ as downstream signaling molecules of the EGFR through the AT1R. This interaction regulates PI3K/Akt cascade activities but not EGFR activity in C9 cells.
Despite the fact that Cav1, as a key component of caveolae, has a positive regulatory role in MAPK cascade activities, its phosphorylation was also affected by the EGFR inhibitor AG1478, the Src inhibitor PP2, and the intracellular calcium chelator BAPTA-2/AM. In addition, Src activation, upstream of the EGFR, was significantly reduced by AG1478, probably through inhibition of the Cav1/AT1R phosphorylation induced by Ang II in C9 cells. The effects of PP2 and BAPTA-2/AM on Cav1 activation and that of AG1478 on Cav1 and Src activation were also observed in EGF-stimulated C9 cells. The studies on cells treated with Cav1 siRNA showed that Cav1 has a positive regulatory role in activation of the PI3K/Akt pathway in response to Ang II or EGF. This finding differs from a previous report in vascular smooth muscle cells (Ushio-Fukai et al., 2001) and indicates that the interaction of Cav1 with other signaling molecules could depend on the cell type. These findings suggest that Cav1 has close interactions with EGFR, AT1R, intracellular calcium, and Src in C9 cells. However, Cav1 siRNA had no effect on the MAP kinase cascade and activity of Src and EGFR in EGF-stimulated C9 cells. On the other hand, ERK1/2 phosphorylation by Ang II but not by EGF was reduced by PP2 though inhibition of ERK1/2 phosphorylation by AG1478 was consistently observed in Ang II- and EGF-stimulated C9 cells. Thus, Ang II causes ERK1/2 phosphorylation by activating Src, Cav1, and the EGFR. In contrast, EGF activates ERK1/2 only through the EGFR and without the participation of Cav1 and Src in C9 cells. In general, these events further indicate the close interaction of Cav1 with other signaling molecules in AT1 receptor signaling and suggest that different agonists specifically modify the pathways of communication among signaling molecules in C9 cells.
Changes in [Ca2+]i are known to be critical factors in many intracellular signaling processes. As shown in Fig. 8, BAPTA-2/AM markedly attenuated both Cav1 and EGFR phosphorylation by Ang II and blocked Cav1 but not EGFR phosphorylation by EGF. In addition, such pretreatment did not inhibit ERK1/2 phosphorylation by Ang II or EGF. This indicates a possible dissociation between ERK1/2 activation and the activation of signal molecules, such as that between ERK1/2 and EGFR activation by Ang II in these cells. Such a dissociation might be accounted for if ERK1/2 activation were induced by GPCRs through EGFR-independent pathways (Yin et al., 2005) and/or that BAPTA-2/AM blocks ERK1/2 dephosphorylation through inhibition of calcium-dependent phosphatase activation, such as MAPK phosphatase 1 (Maloney et al., 1999). Our findings clearly show that both Cav1 and EGFR activation by Ang II are dependent on intracellular calcium and further indicate differential interactions of signaling molecules between Ang II- and EGF-stimulated C9 cells.
Previous studies have suggested that PI3K/Akt is involved in Ang II- and EGF-induced AT1 receptor phosphorylation, although PI3K blockade has no effect on ERK1/2 activation in C9 cells (Garcia-Caballero et al., 2001; Olivares-Reyes et al., 2005). We used the two structurally distinct PI3K inhibitors LY294002 and wortmannin to examine the role of PI3K/Akt in signal pathways initiated by Ang II and EGF in C9 cells. It is interesting that LY294002 and wortmannin differentially blocked phosphorylation by Ang II but not by EGF of both Cav1 and EGFR, but neither compound affected the ERK1/2 phosphorylation induced by Ang II and EGF. Similar results have been reported in other cell types (Ethier and Madison, 2002; Tolloczko et al., 2004). It is possible that LY294002 has regulatory roles in both Cav1 and EGFR activation via intracellular Ca2+ in Ang II-stimulated C9 cells. Indeed, as shown in Fig. 11A, LY294002 but not wortmannin reduced the Ang II-induced rise of [Ca2+]i. This supports the hypothesis that in Ang II-stimulated C9 cells, LY294002 and wortmannin differentially regulate the activation of both Cav1 and EGFR via changes in [Ca2+]i that modulate the interactions of both Cav1 and the EGFR with other signaling proteins. Thus, it is possible that LY294002 has actions on Ca2+ signaling that are unrelated to its effect on PI3K in Ang II-stimulated C9 cells. However, their differential roles in the mobilization of intracellular Ca2+ were not observed in EGF- stimulated C9 cells, consistent with the distinct pathways of signaling molecules in Ang II- and EGF-stimulated C9 cells. The above data complement and extend earlier observations that both Ang II and EGF promote the association of AT1R with the EGFR and induce MAPK phosphorylation via protein kinase C-dependent/independent pathways in these cells (Shah et al., 2004; Olivares-Reyes et al., 2005).
In conclusion, these studies further demonstrate the interactions of Cav1 with crucial signaling molecules in Ang II- stimulated C9 cells. It is clear that 1) Cav1 serves as a platform protein to interact with other signaling molecules in caveolae/lipid rafts, subsequently resulting in cellular proliferation and survival; 2) intracellular calcium and Src are essential for both Cav1 and EGFR activation by Ang II; and 3) the PI3K inhibitors LY294002 and wortmannin differentially affect Cav1 and EGFR activation via changes of [Ca2+]i, further indicating the close association of Cav1 with the EGFR in AT1R signaling. This report also provides evidence that early upstream signaling molecules, such as intracellular Ca2+ and Src, are required for EGF-induced Cav1 activation, although they have no effect on EGFR and MAP kinase cascade activation in C9 cells. These findings suggest that differential pathways of communication among signaling molecules occur between Ang II- and EGF-stimulated C9 cells and that the roles of cholesterol-enriched microdomains in cellular signaling process are determined by specific agonists and/or cell types.
ABBREVIATIONS:
- Cav1
caveolin1
- C9 cells
hepatic clone 9 cells
- FLIPR
fluorometric imaging plate reader
- AT1
angiotensin II type 1
- Ang II
angiotensin II
- EGF
epidermal growth factor
- AT1R
angiotensin II type 1 receptor
- EGFR
epidermal growth factor receptor
- ERK1/2
extracellular signal-regulated kinase 1/2
- PBS
phosphate-buffered saline
- PI3K
phosphatidylinositol 3-kinase
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- RSK2
ribosomal S6 kinase 2
- GPCR
G-protein-coupled receptor
- siRNA
small interfering RNA
- PAGE
polyacrylamide gel electrophoresis
- BAPTA-2/AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4,d]pyrimidine
- AG1478
4-(3′-chloroanilino)-6,7-dimethoxy-quinazoline
- LY294002
2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride
References
- Adi S, Wu NY, and Rosenthal SM (2001) Growth factor-stimulated phosphorylation of Akt and p70(S6K) is differentially inhibited by LY294002 and Wortmannin. Endocrinology 142:498–501. [DOI] [PubMed] [Google Scholar]
- Anderson RG (1998) The caveolae membrane system. Annu Rev Biochem 67:199–225. [DOI] [PubMed] [Google Scholar]
- Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC Jr, and Abraham RT (1996) Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J 15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- Cantley LC (2002) The phosphoinositide 3-kinase pathway. Science 296:1655–1657. [DOI] [PubMed] [Google Scholar]
- Cantrell DA (2001) Phosphoinositide 3-kinase signalling pathways. J Cell Sci 114:1439–1445. [DOI] [PubMed] [Google Scholar]
- Carver RS, Stevenson MC, Scheving LA, and Russell WE (2002) Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology 123:2017–2027. [DOI] [PubMed] [Google Scholar]
- Che Q and Carmines PK (2002) Angiotensin II triggers EGFR tyrosine kinase-dependent Ca2+ influx in afferent arterioles. Hypertension 40:700–706. [DOI] [PubMed] [Google Scholar]
- Clapham DE (1995) Calcium signaling. Cell 80:259–268. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, and Lisanti MP (2004) Role of caveolae and caveolins in health and disease. Physiol Rev 84:1341–1379. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Catt KJ, Inagami T, Wright JW, and Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52: 415–472. [PubMed] [Google Scholar]
- Eguchi S and Inagami T (2000) Signal transduction of angiotensin II type 1 receptor through receptor tyrosine kinase. Regul Pept 91:13–20. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, et al. (1998) Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II- induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem 273:8890–8896. [DOI] [PubMed] [Google Scholar]
- Ethier MF and Madison JM (2002) LY294002, but not wortmannin, increases intracellular calcium and inhibits calcium transients in bovine and human airway smooth muscle cells. Cell Calcium 32:31–38. [DOI] [PubMed] [Google Scholar]
- Fleming JM, Desury G, Polanco TA, and Cohick WS (2006) Insulin growth factor-I and epidermal growth factor receptors recruit distinct upstream signaling molecules to enhance AKT activation in mammary epithelial cells. Endocrinology 147:6027–6035. [DOI] [PubMed] [Google Scholar]
- García-Caballero A, Olivares-Reyes JA, Catt KJ, and García-Saínz JA (2001) Angiotensin AT1 receptor phosphorylation and desensitization in a hepatic cell line. Roles of protein kinase C and phosphoinositide 3-kinase. Mol Pharmacol 59:576–585. [DOI] [PubMed] [Google Scholar]
- Ghosh A and Greenberg ME (1995) Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science 268:239–247. [DOI] [PubMed] [Google Scholar]
- Gifford SM, Grummer MA, Pierre SA, Austin JL, Zheng J, and Bird IM (2004) Functional characterization of HUVEC-CS: Ca2[H11001] signaling, ERK 1/2 activation, mitogenesis and vasodilator production. J Endocrinol 182:485–499. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Rittenhouse SE, Brock TA, Ekstein LS, Gimbrone MA Jr, and Alexander RW (1986) Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem 261:5901–5906. [PubMed] [Google Scholar]
- Haendeler J and Berk BC (2000) Angiotensin II mediated signal transduction. Important role of tyrosine kinases. Regul Pept 95:1–7. [DOI] [PubMed] [Google Scholar]
- Haendeler J, Yin G, Hojo Y, Saito Y, Melaragno M, Yan C, Sharma VK, Heller M, Aebersold R, and Berk BC (2003) GIT1 mediates Src-dependent activation of phospholipase C[H9253] by angiotensin II and epidermal growth factor. J Biol Chem 278:49936–49944. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, and Balla T (2002) Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 157:1211–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka N, Griendling KK, Lassègue B, and Alexander RW (1998) Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension 32:459–466. [DOI] [PubMed] [Google Scholar]
- Janas E, Priest R, Wilde JI, White JH, and Malhotra R (2005) Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin Exp Immunol 139:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin A, Aksamitiene E, Markevich NI, Borisov NM, Hoek JB, and Kholodenko BN (2006) Scaffolding protein Grb2-associated binder 1 sustains epidermal growth factor-induced mitogenic and survival signaling by multiple positive feedback loops. J Biol Chem 281:19925–19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Singh VP, and Baker KM (2007) The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab 18:208–214. [DOI] [PubMed] [Google Scholar]
- Maloney JA, Tsygankova OM, Yang L, Li Q, Szot A, Baysal K, and Williamson JR (1999) Activation of ERK by Ca2[H11001] store depletion in rat liver epithelial cells. Am J Physiol 276:C221–C230. [DOI] [PubMed] [Google Scholar]
- Mifune M, Ohtsu H, Suzuki H, Nakashima H, Brailoiu E, Dun NJ, Frank GD, Inagami T, Higashiyama S, Thomas WG, et al. (2005) G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem 280:26592–26599. [DOI] [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, and Anderson RG (1996) Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem 271:11930–11935. [DOI] [PubMed] [Google Scholar]
- Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, and Malik AB (2000) Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J Cell Biol 150:1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares-Reyes JA, Shah BH, Hernández-Aranda J, García-Caballero A, Farshori MP, García-Sáinz JA, and Catt KJ (2005) Agonist-induced interactions between angiotensin AT1 and epidermal growth factor receptors. Mol Pharmacol 68:356–364. [DOI] [PubMed] [Google Scholar]
- Paugh BS, Paugh SW, Bryan L, Kapitonov D, Wilczynska KM, Gopalan SM, Rokita H, Milstien S, Spiegel S, and Kordula T (2008) EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKC{delta}, and sphingosine kinase 1 in glioblastoma cells. FASEB J 22:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah BH and Catt KJ (2002) Calcium-independent activation of extracellularly regulated kinases 1 and 2 by angiotensin II in hepatic C9 cells: roles of protein kinase C[H9254], Src/proline-rich tyrosine kinase 2, and epidermal growth receptor trans-activation. Mol Pharmacol 61:343–351. [DOI] [PubMed] [Google Scholar]
- Shah BH and Catt KJ (2003) A central role of EGF receptor transactivation in angiotensin II-induced cardiac hypertrophy. Trends Pharmacol Sci 24:239–244. [DOI] [PubMed] [Google Scholar]
- Shah BH, Yesilkaya A, Olivares-Reyes JA, Chen HD, Hunyady L, and Catt KJ (2004) Differential pathways of angiotensin II-induced extracellularly regulated kinase 1/2 phosphorylation in specific cell types: role of heparin-binding epidermal growth factor. Mol Endocrinol 18:2035–2048. [DOI] [PubMed] [Google Scholar]
- Shaul PW and Anderson RG (1998) Role of plasmalemmal caveolae in signal transduction. Am J Physiol 275:L843–L851. [DOI] [PubMed] [Google Scholar]
- Tolloczko B, Turkewitsch P, Al-Chalabi M, and Martin JG (2004) LY-294002 [2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one] affects calcium signaling in airway smooth muscle cells independently of phosphoinositide 3-kinase inhibition. J Pharmacol Exp Ther 311:787–793. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M and Alexander RW (2006) Caveolin-dependent angiotensin II type 1 receptor signaling in vascular smooth muscle. Hypertension 48:797–803. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, and Alexander RW (2001). Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem 276:48269–48275. [DOI] [PubMed] [Google Scholar]
- Yin X, Polidano E, Faverdin C, and Marche P (2005) Role of L-type calcium channel blocking in epidermal growth factor receptor-independent activation of extracellular signal regulated kinase 1/2. J Hypertens 23:337–350. [DOI] [PubMed] [Google Scholar]
- Zhang BX, Ma X, Yeh CK, Lifschitz MD, Zhu MX, and Katz MS (2002) Epidermal growth factor-induced depletion of the intracellular Ca2[H11001] store fails to activate capacitative Ca2[H11001] entry in a human salivary cell line. J Biol Chem 277:48165–48171. [DOI] [PubMed] [Google Scholar]