Abstract
Milk provides not only the building blocks for somatic development but also the hormonal signals that contribute to the biopsychological organization of the infant. Among mammals, glucocorticoids (GCs) in mother’s milk have been associated with infant temperament. This study extended prior work to investigate rhesus monkey (Macaca mulatta) mother–infant dyads (N = 34) from birth through 8 months postpartum. Regression analysis revealed that cortisol concentrations in milk during the neonatal period predicted impulsivity on a cognitive task, but not global social behaviors, months later. During this time period, sex-differentiated social behavior emerged. For female infants, milk cortisol concentrations predicted total frequency of play. Collectively, these findings support and extend the “lactational programming” hypothesis on the impact of maternal-origin hormones ingested via milk.
Mother’s milk contains nutritional, immunological, and signaling factors that organize infant physiological and neurobiological development (reviewed in Neville et al., 2012). Among these bioactive factors are hormones, and interrogating the hormonal compartment of milk is an accelerating area of research (Bernstein & Hinde, 2016). Maternal-origin hormones ingested via milk influence offspring metabolism, growth, reproduction, and biopsychology. For examples, relaxin ingested via milk is instrumental in the development of reproductive tissues in the hours and days after birth in suids (Bartol, Wiley, & Bagnell, 2008; Miller, Wiley, Chen, Bagnell, & Bartol, 2013), metabolic hormones in milk—leptin, ghrelin, adiponectin, growth factors, glucocorticoids (GCs)—are implicated in infant growth (Hinde et al., 2015; Power & Schulkin, 2013; Savino, Liguori, Fissore, & Oggero, 2009), and maternal-origin GCs additionally affect the biopsy-chology of offspring (reviewed in Catalani, Alemà, Cinque, Zuena, & Casolini, 2011). Collectively, the shaping of offspring phenotype via the transmission of biological components in milk has been termed “lactocrine programming” (Bartol et al., 2008) and “lactational programming” (Hinde, 2013).
Catalani et al. (2011) have described a “mother–offspring pituitary–adrenal interrelationship” during the postnatal period facilitated by mother’s milk. Biologically active GCs transfer from maternal circulation into milk (Grosvenor, Picciano, & Baumrucker, 1993; Kato, Hsu, Raymoure, & Kuhn, 1985). Glucocorticoid concentrations in milk are correlated with maternal plasma concentrations (Sullivan, Hinde, Mendoza, & Capitanio, 2011) and reflect local regulation within the mammary gland (reviewed in Hinde et al., 2015). Glucocorticoid concentrations in mother’s milk are not associated with depression or perceived stress in humans (Grey,Davis, Sandman, & Glynn, 2013) or social rank in rhesus monkeys (Hinde et al., 2015) but have been associated with maternal parity and body mass (Hinde et al., 2015). During the neonatal period, maternal-origin GCs ingested via milk retain bioactivity in the neonate and can be absorbed in the intestinal tract due to low stomach acidity (Falling-borg, 1999) and gut permeability in early life (reviewed in Catalani et al., 2011). Experimental elevation of maternal GCs, administered to rodent dams via water, increases plasma GC concentrations in pups (Catalani et al., 2011). Importantly, GC receptors in the intestinal tract are characterized by high density and expression during infancy (Donovan et al., 2000; reviewed in Hinde, 2013; Pàcha, 2000). Indeed consumption of breast milk increases GC receptor expression 5.5-fold over formula feeding (Donovan et al., 2000). Exposure to elevated maternal-origin GCs ingested via milk increased GC receptor expression within neural regions that influence hypothalamic–pituitary–adrenal (HPA) regulation (Catalani et al., 2011). Maternal-origin GCs have the potential to influence offspring physiology via translocation to target tissues, signaling along the gut–brain axis, and interactions with microbial communities within the intestinal tract (Allen-Blevins, Sela, & Hinde, 2015; Catalani et al., 2011). Taken together, the growing body of evidence demonstrates that maternal physiological signaling extends into the postnatal period to influence infant outcomes in mammals (Bartol et al., 2008; Grey et al., 2013; Hinde, 2013; Power & Schulkin, 2013).
GCs in milk have been repeatedly demonstrated to affect or be associated with biopsychological development in human and animal studies (Catalani et al., 2011; Hinde et al., 2015; Peaker & Neville, 1991). Decades of elegant experimental investigations within rodent model systems have revealed that adult progeny exposed to elevated GC in milk demonstrate reduced anxiety, better coping, hyporeactivity of the HPA axis, and enhanced spatial learning (Catalani et al., 2011). In both human and nonhuman primates, cortisol in milk predicts temperament in infants aged 3–4 months (Grey et al., 2013; Hinde et al., 2015; Sullivan et al., 2011). Higher concentrations of cortisol in milk were associated with a more nervous and less confident temperament in monkeys and with negative affect in humans. In these studies, however, effects were mediated in part by infant sex (or depending on one’s scholarly discipline, infant gender). For example, Grey et al. (2013) found that higher concentrations of cortisol in milk were associated with higher scores for negative affect—evincing more fear, anger, and frustration, but less able to be soothed—but only for daughters. Hinde et al. (2015) found in macaques that the effects of milk cortisol were present for both daughters and sons but that sons were more sensitive than daughters. Moreover, critical windows of sensitivity diverged for sons and daughters (Hinde et al., 2015). Experimental manipulation of milk GCs in rodents reveals the opposite pattern found in human and nonhuman primates, namely that elevated milk GCs enhance memory and reduce behavioral and physiological anxiety, in part, along sex-differentiated pathways (reviewed in Catalani et al., 2011). These sex differences may reflect adaptive differences in sensitivity or may be a by-product of other aspects of sex-differentiated developmental trajectories (Bernstein & Hinde, 2016; Hinde et al., 2015).
Our understanding of maternal effects, the ways in which the experiences and condition of the mother impacts offspring outcomes within and across species, is enhanced by incorporating life-history theory. Maternal physiology provides the building blocks, fuel, and signal for the developing mammalian organism that likely co-organizes multiple systems—metabolism, neurobiology, commensal microbial communities, immunity, stress physiology, and temperament (Allen-Blevins et al., 2015; Hinde et al., 2015; Neville et al., 2012)—and the behavioral manifestations of their aggregate influences such as social behavior and cognition. Across humans and animals, mothers encounter constraints and make tradeoffs for allocating resources across offspring while also protecting their own condition (Clutton-Brock, 1991; Dettmer, Rosenberg, Menard, et al., 2015; Stearns, 1992). Insofar as local ecology influences maternal constraints and natural selection has shaped maternal tradeoffs, maternal investment affects and informs infant outcomes. Phenomena demonstrated in laboratory mice and rats likely reflect effects more representative of taxa characterized by fast developmental trajectories in which conditions during early life are highly predictive of adult ecology, circumstances under which selection is more likely to favor the evolution of predictive adaptive responses (Nettle, Frankenhuis, & Rickard, 2013). For long-lived primates, in which development is protracted over years, early life experiences are expected to have reduced predictive power for adulthood (Nettle et al., 2013; Wells, 2007). Instead, it has been hypothesized that maternal cues calibrate infant developmental trajectories to maternal resources for investment (Wells, 2014). Maternal input may influence offspring to prioritize survival and growth over behavioral activity, via programming of less exploratory and active temperament (Hinde & Capitanio, 2010; Hinde et al., 2015). In these ways, a life-history perspective is integral for understanding developmental trajectories.
While previous studies have identified a role for GCs in the early postnatal period in shaping temperament later in development, the extent to which GCs influence infants beyond temperament or how such effects persist across time remains unexplored in humans and nonhuman primates. This study evaluated the hypotheses that mother’s milk in the neonatal period is associated with later infant cognitive and social development. Relying on a rhesus monkey model of development, we predicted that infants whose mothers produced milk with higher cortisol concentrations would perform differently than those whose mothers produced milk with lower concentrations on a cognitive task measuring impulsivity, as well as in social settings with other monkeys. These predictions derive from previous research demonstrated that: (a) the first weeks of life represent a time of marked physiological development in the gut and brain of infant primates (Clarke, O΄Mahony, Dinan, & Cryan, 2014), and (b) between 4 and 8 months of age, infant macaques increase their independence from mothers and show concomitant development of the neural networks underlying complex social interactions with others (reviewed in Machado, 2013). In humans, this developmental window corresponds to roughly 2–2.5 years of age (i.e., toddlerhood; Harlow & Mears, 1983), a period during which children increase social interactions with peers (Parker, Rubin, Erath, Wojslawowicz, & Buskirk, 2006). Similarly, rhesus monkeys between 4 and 8 months of age increase the amount of time spent away from their mothers and engaging in social play and social contact with peers. During this transition to juvenility, rhesus monkeys begin to practice the social behaviors that will lay the foundation for successful navigation of social interactions in adolescence and adulthood (Machado, 2013). However, because scant literature exists regarding hormones in milk in the first weeks of life and implications for later infant development, we did not make predictions about the direction of these differences. We also studied the relation between milk cortisol and infant development in the infants’ social living environment, as the previous monkey studies examined infant temperament while the infant was separated from its mother for a 25-hr biobehavioral assessment (see Hinde & Capitanio, 2010; Hinde et al., 2015).
The present study further evaluated the extent to which mother’s milk was associated with infant social behavior as a function of infant sex. Across mammalian taxa, sex differences in social behaviors are evident early in life with males of many species engaging in play behaviors more frequently and more vigorously than females, particularly well-established among primates (Meredith, 2013, 2015; Brown & Dixson, 2000), and females engaging in social grooming more frequently than males (Mitchell & Tokunaga, 1976). Although androgens and social experiences are known to influence sex-differentiated behaviors (Meredith, 2013, 2015), the extent to which other proximate causes, like mother’s milk, organize sex differences in social behaviors is unknown. Because male and female infant monkeys have shown differential sensitivity to milk cortisol with respect to temperament (Hinde et al., 2015), we predicted that a similar pattern of sex-differentiation would emerge for social behaviors as well.
Mothers with higher milk cortisol may be experiencing more energetic or psychosocial stressors, and this physiological biomarker of maternal experience underlies, in part, maternal organization of offspring biobehavioral processes. Substantial variations in both milk cortisol (Sullivan et al., 2011) and hair cortisol concentrations (a long-term measure of HPA axis activity; Meyer & Novak, 2012) have been reported for macaque mothers (Dettmer, Rosenberg, Suomi, Meyer, & Novak, 2015). Previous reports in macaques have demonstrated differential maternal investment by mothers with higher hair cortisol profiles, dependent on body condition (Dettmer, Rosenberg, Menard et al., 2015), as well as differential neurological development for infants of mothers with higher hair cortisol profiles, dependent on life history (i.e., maternal experience; Dettmer, Rosenberg, Menard et al., 2015). Importantly, cortisol concentrations in milk are positively correlated with cortisol concentrations in maternal plasma in mammals (Patacchioli et al., 1992; Sullivan et al., 2011), but to date the relation between cortisol concentrations in milk and chronic HPA axis activity as measured in hair is unknown. Hair samples provide insights into integrated GC secretion across weeks and months; if some mothers had higher GCs in their milk due to chronic activation of their stress response system, we would expect a positive association between milk and hair cortisol concentrations. As such, we tested the hypothesis that milk cortisol is related to maternal hair cortisol concentrations across pregnancy and lactation.
Method
Ethics Statement
All procedures were approved by the NICHD Animal Care and Use Committee and were conducted in accordance with the American Psychological Association’s Ethical Principles of Psychologists and Code of Conduct (American Psychological Association, 2002).
Subjects and Housing
Rhesus monkey (Macaca mulatta) mother–infant dyads (N = 34; Table 1), representing approximately 25% of the colony and equally representing high, middle, and low social rank, were studied at a primate facility in rural Maryland across three birth cohorts (rhesus monkeys are seasonal breeders that give birth each spring). Monkeys were studied in 2013 (n = 21; nine female infants), 2014 (n = 4; one female infant), and 2015 (n = 9; three female infants) from birth through 8 months of age. Per standard protocols (Dettmer, Novak, Suomi, & Meyer, 2012), infant monkeys were born into large social groups containing 8–10 adult females, 1 adult male, and other peers from the same birth cohort. Monkeys lived in indoor–outdoor pens measuring 2.44 m × 3.05 m × 2.21 m (inside) and 2.44 m × 3.0 m × 2.44 m (outside), and were given free access between the two portions except when temporarily contained in one pen for cleaning. Inside lighting was maintained on a 12:12-hr cycle (07:00–19:00), and the outdoor portion was exposed to ambient lighting. Monkeys were fed Purina High Protein Monkey Chow (St. Louis, MO, #5038) twice daily and received water ad libitum. Supplemental fruit and other foraging materials were provided daily.
Table 1.
Subject Characteristics for This Study
| Birth cohort | Total subjects (female:male) | Cognitive data available (n) | Social data available (n) |
|---|---|---|---|
| 2013 | 21 (9:12) | 4 | 20 |
| 2014 | 4 (1:3) | 3 | 4 |
| 2015 | 9 (3:6) | 5 | 9 |
| Total (female:male) | 34 (13:21) | 12 (4:8) | 33 (14:21) |
Milk Collection
Milk collection occurred on days when infants underwent a routine assessment (the Primate Neonatal Nursery Assessment; Schneider, Champoux, & Moore, 2006) for neonatal neurological development based on the Brazelton assessment (Brazelton, Nugent, & Lester, 1987). The neonatal assessment occurred on postnatal days 7, 14, 21, and 30 in 2013 and, owing to protocol modifications, on days 14 and 30 only in 2014 and 2015. In 2013, milk collection occurred for mothers either on days 7 and 21 (n = 8; 4 female, 4 male infants) or on days 14 and 30 (n = 13; 7 female, 6 male infants) so as to minimize disruption of the behavioral biology of lactation of the mother–infant dyads. In 2014 and 2015, milk collection occurred on days 14 and 30. Neonatal assessment analysis was outside the scope of the present study examining the influences of neonatal milk GCs on later social and cognitive functioning and is not included here.
Mother–infant dyads were separated from their social runs beginning at 11:00 each testing day. The mother was anesthetized (intramuscular ketamine HCl, 10 mg/kg) and the infant was temporarily relocated at approximately 11:15 to the neonatal nursery to conduct the neonatal assessment. While the mother was still anesthetized, she was weighed, measured for crown-rump length, and outfitted with a mesh jacket to prevent nursing by the infant once the dyad was reunited after the neonatal assessment. Jacketing allowed milk accumulation for a standardized period of time (2.5 hr) and subsequent evacuation of the entire mammary gland(s) for hormonal assay (Hinde, Power, & Oftedal, 2009). The infant was reunited with his or her mother at approximately 12:30 and was allowed to remain in contact in a temporary holding cage away from the social group until milk collection began between 13:45 and 14:00. At this time, the mother was lightly sedated with a half dose of intramuscular ketamine HCl based on the dose given at 11:00 (~ 5 mg/kg). Once the mother was immobilized, the infant was separated and held by a researcher to provide contact comfort while two other researchers collected milk. According to standardized procedures (Hinde et al., 2009), the mother’s nipples were trimmed of hair and wiped with alcohol-soaked gauze (70% isopropanol), then the mother was administered intramuscular oxytocin HCl (0.05 mg/kg; 20 UPS/ml, 0.05 ml/kg; VetOne, Ontario, Canada) to stimulate milk let down. Milk collection began within 30 s via gentle manual stripping of the nipples into a 15 ml tube, which was weighed before and immediately after to calculate milk yield (see Hinde et al., 2009). Collection ended when each nipple slowed milk production to isolated droplets with no streaming milk (Hinde et al., 2009). Tubes were placed on wet ice and transferred to the laboratory for processing, where samples were vortexed on low speed for 3–5 s, aliquoted into 2 ml cryotubes, and frozen at −80°C.
Immediately following milk collection, the mother was returned with her infant to the temporary holding cage and monitored until both were fully awake and alert. At approximately 15:00, the dyad was returned to the social run. Thus, for this study, mothers and infants were removed from their social run twice for 4 hr, being separated for only 75 min for the neonatal assessment and for 10 min for milk collection.
Milk Cortisol Assay
Frozen samples were shipped to the Hormone Assay Core Laboratory at the University of Massachusetts Amherst, for analysis of cortisol content via radioimmunoassay (RIA) according to established procedures (Hinde et al., 2015). Briefly, milk samples were thawed and 200 μl of sample was diluted with 600 μl of deionized water (1:4 dilution). Cortisol concentrations were then estimated in duplicate using commercial coated tube RIA kits. For the 2013 samples, Coat-a-Count RIA kits from Siemens Medical Solutions Diagnostics (Los Angeles, CA) were used, and for the 2014 and 2015 samples, MP Biomedicals (Santa Ana, CA) were used after Siemens discontinued their kits. MP Biomedicals was chosen as it gives the closest results to the Siemens kit (MP Biomedicals, unpublished data). The correlation for nine samples collected in 2013 that were run on both kits was r(7) = .96, p < .01. Nevertheless, a categorical variable for assay approach was entered into analyses as a covariate. All samples from a given year were run in a single assay, so no interassay coefficients of variation were obtained. Intra-assay coefficients of variation were5.13% (2013), 3.21% (2014), and 4.3% (2015).
Maternal Hair Cortisol Sampling and Assay
The monkeys in this study were part of a larger study examining the effects of maternal GCs across pregnancy and lactation on infant development. The hair sample collection and cortisol assay for rhesus monkey mothers has been described in detail previously (Dettmer, Rosenberg, Menard et al., 2015). The time points at which hair samples were collected corresponded to midpregnancy, the perinatal period, and peak lactation.
Infant Social Behavior
Observation data on social behavior data were available for 33 of the 34 infants (Table 1). Infants were observed in their social runs twice weekly for 10 min each using focal animal sampling and one-zero scoring of behaviors (Martin & Bateson, 1993). Each 10-min session was divided into ten 1 min intervals. For each interval, the occurrence (yes or no) of each of the following behaviors was recorded: contact play (e.g., wrestling), noncontact play (e.g., chasing), social grooming, and mounting. Infants were observed from approximately 4–8 months of age. For each of the social behaviors (play, social grooming, mounting), the focal animal’s role as initiator or receiver, and its social partner, was recorded (Dettmer, Rosenberg, Guitarra, et al., 2015). Interrater reliability was established when two raters achieved ≥ 85% agreement across three consecutive observation sessions, whereby raters scored the same infant and agreement scores were calculated across the entire session. Reliability calculations included initiate and receive for each behavior.
Infant Cognitive Function
A subset of infants (n = 12; Table 1) was administered a task to assess inhibitory control and impulsivity using an apparatus and methods developed specifically for testing socially housed, mother–peer-reared infant monkeys (Dettmer, Murphy, & Suomi, 2015). Cognitive data were available from only 12 infants for this study due to the substantial time investment required to implement cognitive testing for these infants, combined with limited research staff availability. Infants were tested from approximately 6–8 months of age in their social runs; at 8 months they were removed from their mother’s social group and relocated to live in a large social group with all other infants born in the same birth year (Dettmer et al., 2012) to allow for the next year’s births. This study utilized an object detour-reach task presented to the infants at 192.00 days of age (SE = 8.5) as part of a larger cognitive assessment battery. The object detour-reach task measures impulse control by assessing the monkeys’ ability to inhibit the previously learned response of reaching straight ahead to retrieve a reward and instead reach around a transparent barrier to obtain the reward (Dettmer, Murphy et al., 2015; Parker, Buckmaster, Justus, Schatzberg, & Lyons, 2005). The apparatus for the object detour-reach task was a 61.7-cm × 17.5-cm opaque Plexiglas board fastened onto the home cage with chains and clips. In the center of the board was a 8-cm3 clear plastic box with one side open (Parker et al., 2005). The box was attached to the board so that the experimenter could rotate the opening but the monkey could not. A removable, opaque occluder (roughly 60-cm long) was also placed in front of the testing board to block the infant’s view in between each trial.
The procedure we developed for our laboratory is based on published procedures for administering this task with nonhuman primates (Dettmer, Murphy et al., 2015; Parker et al., 2005). Briefly, infants were trained on over 100 trials to reach straight into the box to retrieve a reward (e.g., marshmallow). They were then tested on 140 trials with box opening alternating straight, right, left, repeatedly across trials (Parker et al., 2005). In all trials, infants were given 30 s to retrieve the reward inside. For each trial, we recorded the frequency and direction of reaches (i.e., straight-line or side reaches) and the latency to retrieve the reward. For all trials, the experimenter sat behind the center of the testing apparatus, facing forward, with her eyes directed toward the center of the testing apparatus so as to reduce the likelihood that she would cue the subject.
Data Analysis
We constructed regression models to evaluate milk parameters and infant characteristics on outcome measures using Akaike′s information criterion (AIC) to avoid overfitting models (Akaike, 1974). AIC model selection processes retain predictors within the model that appreciably contribute to explaining the phenomena of outcome measures independent of conventional and arbitrary thresholds of significance (Mattison, Wander, & Hinde, 2015). Initial models included average milk cortisol and average milk yield value in the first postnatal month (continuous parameter), infant sex (nominal parameter), and birth cohort 2013–2015 (ordinal parameter). The independent variable was milk cortisol, which was calculated by averaging the cortisol values across the two time periods for each mother. No differences in milk cortisol were revealed for mothers whose milk was collected on days 7 or 21 versus on days 14 or 30, t(32) = [C0]1.43, p = .16. Milk yield accounts for approximately 95% of available milk energy (Hinde, 2009a), which has previously been reported to predict temperament in rhesus macaques (Hinde & Capitanio, 2010; Hinde et al., 2015). Birth cohort effectively binned calendar year, cohort size, and cortisol assay kit, capturing several phenomena that could not be effectively disentangled due to high covariance and small sample size.
Models were constructed to evaluate predictors for social behavior, cognitive function, and hair cortisol concentration. To assess the predictive power of milk during the neonatal period on social behavior, initial regression models included average neonatal milk cortisol, average neonatal milk yield volume, and birth cohort. The dependent variables were average percentage of intervals overall in which the infant initiated separate social behaviors (grooming, play, and mounting) and engaged in all social behaviors combined. Several mothers (n = 4) had two infants represented in the social behavior data set. Because infant sex effects were previously reported for milk cortisol and temperament with sex-differentiated sensitive windows during development (Hinde et al., 2015; Sullivan et al., 2011), after constructing models for all subjects, we constructed regression models for male and female infants separately for social behavior. All four mothers with two infants in the data set coincidentally had a son and a daughter. As such, maternal ID was not included in the sex-specific model construction. For cognitive function, initial regression models included average neonatal milk cortisol, average neonatal milk yield volume, and birth cohort on measures of erroneous impulsivity: the immediate straight-line reach when the box was turned to the side (percent “bonk”) and latency to retrieve the award (seconds) in this cognitive task (Dettmer, Rosenberg, Suomi et al., 2015). The small sample size for cognitive outcomes precluded evaluation of infant sex in these models. Once models were finalized, we reran them after removing the milk parameter (if the milk parameter had been retained with other covariates) to report the change in the adjusted R-squared (ΔadjR2) of the model to evaluate the contribution to explained variance provided by the milk parameter. To test the hypothesis that milk cortisol is related to maternal hair cortisol concentrations, regression analyses were again used with average milk cortisol as the dependent variable and hair cortisol concentrations at each time point (April, July, October) as the independent variables. Statistical analyses were conducted using statistical software JMP 12 (SAS Cary, NC, United States) and SPSS (IBM Armonk, NY, United States) v22.
Results
Descriptive statistics for all independent and dependent variables are presented in Table 2, and correlations between the dependent variables are presented in Table 3.
Table 2.
Sizes (N), Mean Values (M), Standard Deviations (SD) and Ranges for All Independent Dependent Variables
| Milk Cortisol (μg/dL) | Milk yield volume (g) | Latency to retrieve reward on cognitive task (s) | Impulsive responses on cognitive task (% trials) | Infant-initiated grooming (% intervals) | Infant-initiated mounting (% intervals) | Infant-initiated play (% intervals) | All social behavior (% intervals) | Maternal hair cortisol: perinatal (pg/mg) | Maternal hair cortisol: perinatal (pg/mg) | Maternal hair cortisol: lactation (pg/mg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 31 | 31 | 12 | 12 | 33 | 33 | 33 | 33 | 33 | 25 | 25 |
| M | 5.57 | 6.35 | 3.56 | 21.97 | 0.49 | 0 31 | 11.10 | 35.23 | 76.85 | 88.75 | 80.37 |
| SD | 2.27 | 5.41 | 1.69 | 16.50 | 0.64 | 0.64 | 4.14 | 10.02 | 19.93 | 29.12 | 17.27 |
| Range | 2.3–12.6 | 1.9–27.3 | 2.1–7.6 | 8.9–68.8 | 0–2.2 | 0–2.4 | 4.4–20.2 | 14.5–56.4 | 48.2–138.5 | 39.5–184.5 | 62.5–125.5 |
Table 3.
Correlations Between Dependent Variables
| 1 Impulsive responses on cognitive task (%) |
2 All social behavior (% intervals) |
3 Infant-initiated play (% intervals) |
4 Infant-initiated grooming (% intervals) |
5 Infant-initiated mounting (% intervals) |
6 Maternal hair cortisol: pregnancy (pg/mg) |
7 Maternal hair cortisol: perinatal (pg/mg) |
8 Maternal hair cortisol: lactation (pg/mg) |
||
|---|---|---|---|---|---|---|---|---|---|
| Latency to retrieve reward on cognitive task (s) | r | .642* | −.328 | .472 | −.098 | .748** | .386 | −.062 | .184 |
| N | 12 | 11 | 11 | 11 | 11 | 12 | 7 | 7 | |
| 1 | r | 1 | −.180 | .612* | .338 | .066 | .129 | .317 | −.247 |
| N | 11 | 11 | 11 | 11 | 12 | 7 | 7 | ||
| 2 | r | 1 | .257 | −.408* | −.102 | −.105 | .118 | .061 | |
| N | 33 | 33 | 33 | 32 | 24 | 24 | |||
| 3 | r | 1 | .088 | .377* | −.118 | −.083 | .035 | ||
| N | 33 | 33 | 32 | 24 | 24 | ||||
| 4 | r | 1 | −.033 | .290 | −.194 | −.050 | |||
| N | 33 | 32 | 24 | 24 | |||||
| 5 | r | 1 | .065 | .259 | .276 | ||||
| N | 32 | 24 | 24 | ||||||
| 6 | r | 1 | .178 | .049 | |||||
| N | 24 | 24 | |||||||
| 7 | r | 1 | .066 | ||||||
| N | 25 | ||||||||
| 8 | r | I | |||||||
| N |
Note: Bold indicates statistical significance.
p < .05.
p < .01.
Mother’s Milk and Infant Social Behavior
Milk cortisol in the first month of life did not significantly predict either the frequency of social behaviors the infants initiated or the frequency of total social behaviors (initiated + received). Cortisol concentrations in milk varied among individuals during the 1st month after parturition (M = 5.83, SD = 1.99 μg/dL, range = 2.4–10.5) as did milk yield (M = 6.42, SD = 5.4 ml, range = 1.9–27.3). During AIC model construction, cortisol concentrations in milk were retained only for frequency of mounting behavior, along with infant sex (see section below) and cohort, full AIC-selected model: adjR2 = .42, F(3, 18) = 8.79, p < .001. Infants whose mothers produced higher cortisol concentrations in milk engaged in less mounting behavior at 4–8 months of age, standard estimate standard error: b = −0.071, SE = 0.042, t(30) =–1.71, p =.098. Removing the milkcortisol parameter from the multiple regression model reduced the model fit (model without milk parameter: adjR2 = .38) and precipitated lack of fit. Milk yield was not retained in any AIC-selected models to evaluate the predictive power of parameters for social behavior of all infants pooled together.
Sex-Differentiated Social Behavior and Mother’s Milk
Male and female infants differed in their social behavior at 4–8 months of age. Male infants were significantly more likely to initiate social interactions (males: M = 13.5, SD = 4.8; females: M = 9.7, SD = 3.1% of intervals across session), t(31) = 2.55, p = .02, including play behavior (males: M = 12.5, SD = 4.3; females: M = 9.1, SD = 3.0% of intervals across session), t(31) = 2.52, p = .02, than were female infants. Infant sex was additionally retained during AIC model selection to assess parameters associated with frequency of play behavior and total social behavior, indicating that though nonsignificant, infant sex appreciably contributed to explaining variation. Male infants more frequently engaged in play behavior from 4 to 8 months of age than did females (males: M = 24.3, SD = 5.7; females: M = 20.0, SD = 6.1% of intervals across session), t(31) = 2.04, p = .05.
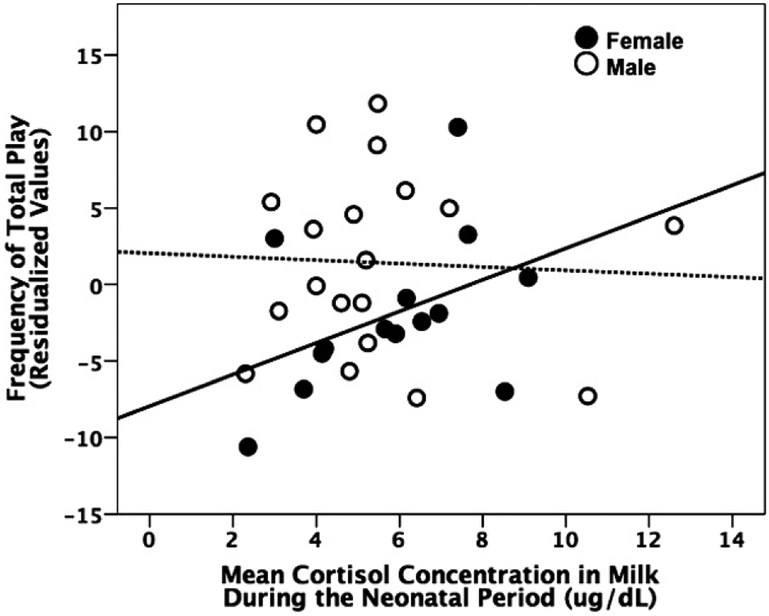
Independently constructing models for male and female infants, however, revealed that milk parameters importantly explained variance in social behavior, especially for female infants. Together, milk cortisol concentration and cohort predicted total frequency of play behavior for female infants, full AIC-selected model: adjR2 = .48, F(2, 13) = 6.90, p = .01 (Figure 1). Female infants whose mothers produced higher milk cortisol concentrations in the first weeks of lactation more frequently played at 4–8 months of age, b = 1.5, SE = 0.64, t(11) = 2.38, p = .037. Removing the milk cortisol parameter from the multiple regression model substantially reduced the model fit, model without milk parameter: adjR2 = .27, F(1, 13) = 5.88, p = .032. Similarly, together milk cortisol concentration and cohort were retained in model construction for total frequency of social behavior for female infants, full AIC-selected model: adjR2 = .69, F(2, 13) = 15.53, p < .001. Female infants whose mothers produced higher milk cortisol concentrations in the first weeks of lactation more frequently engaged in social behavior at 4–8 months of age, b = 1.29,SE = 0.81, t(11) = 1.59, p = .14. Removing the milk cortisol parameter from the multiple regression model slightly reduced the model fit (model without milk parameter: adjR2 = .65) indicating that milk cortisol concentration accounts for ~ 4% of the variance. Although female mounting was rare, higher milk yield in early infancy predicted more mounting behavior at 4–8 months of age. Only milk yield was retained during AIC model selection for mounting behavior in female infants, full AIC-selected model: adjR2 = .53, F(1, 13) = 15.86, t(12) =3.98, p = .002, b = 0.04, SE = 0.01. Together, milk cortisol concentration and cohort predicted total frequency of mounting behavior for male infants, full AIC-selected model: adjR2 = .52, F(2, 18) = 10.70, p = .001. Male infants whose mothers produced higher milk cortisol concentrations in the first weeks of lactation less frequently engaged in mounting behavior at 4–8 months of age, b = [C0]0.08, SE = 0.05, t(15) = −1.44, p = .17. Removing the milk cortisol parameter from the multiple regression model only slightly reduced the model fit (model without milk parameter: adjR2 = .49).
Figure 1.
Female infants that ingested milk with higher cortisol concentrations in the first months of life engaged in social play more frequently from 4 to 8 months of age (full Akaike’s information criterion-selected model: adjR2=0.48, df = 2, N = 14, F-ratio = 6.9, p = .011).
Milk parameters did not predict initiation of play or social behavior, or frequency of grooming for either male or female infants. Frequency of play behavior and total social behavior was not associated with milk parameters for male infants. Importantly, cortisol concentrations in milk did not differ in the milk produced for daughters and sons (females: M = 5.81, SD = 2.1; males: M = 5.47, SD = 2.49), t(31) = 0.41, p = .68, but milk yield was on average ~ 40% higher for daughters (females: M = 7.62, SD = 7.17; males: M = 5.58, SD = 3.76), t(31) = 1.06, p = .30. Although not significant, infant sex was retained by AIC model selection for appreciably explaining variance in milk yield.
Milk and Infant Cognition
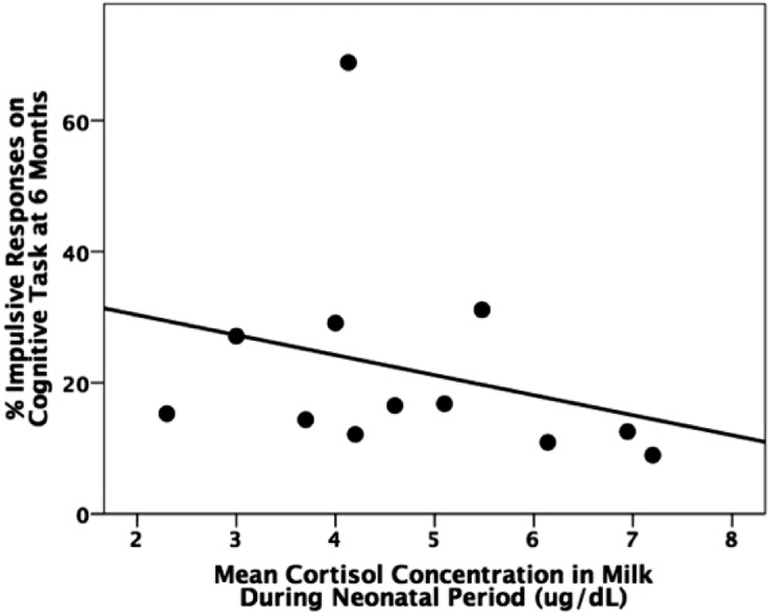
Mother′s milk cortisol and milk yield in the neonatal period were predictive of cognitive performance. Together, milk yield and cohort strongly predicted latency to success on the cognitive task, full AIC-selected model: adjR2 = .70, F(2, 11) = 14.13, p = .002. Infants whose mothers produced higher milk yield in the first weeks of lactation were quicker to retrieve the food reward in the object detour task, b = −0.15, SE = 0.07, t(8) = −2.22, p = .05. Together, cortisol concentration in milk and cohort predicted 30% of the variance in impulsive responses on the object detour-reach task, full AIC-selected model: adjR2 = .30, F(2, 11) = 3.39, p = .079; Figure 2. Infants whose mothers produced higher concentrations of cortisol in milk were less inclined to make impulsive, direct reaches in the object detour task,b = −6.45, SE = 3.11, t(8) = −2.07, p = .068. Remov ing the milk cortisol parameter from the multiple regression model substantially reduced the model fit, model without milk parameter: adjR2 = .074, F(1,11) = 1.88, p = .20. Small sample size prevented evaluation of differentiation of effects or sensitivity as a function of infant sex.
Figure 2.
Infants that ingested milk with higher cortisol concentrations in the first month of life made fewer impulsive errors(i.e., fewer straight-reach errors on side presentations) on the object detour-reach task at 6 months of age (full Akaike’s information criterion-selected model: adjR2 = 0.30, df = 2, N = 12, F-ratio = 3.39, p = .079).
Milk Cortisol and Maternal Hair Cortisol Concentrations
Maternal hair cortisol concentrations did not predict milk cortisol at any time period (pregnancy: R2 = .003, p = .79; perinatal: R2 = .047, ΔR2 = .04, p = .34; lactation: R2 = .05, ΔR2 = .003, p = .81).
Discussion
Our prospective, longitudinal study of rhesus monkeys revealed that cortisol concentrations in mother’s milk in the neonatal period (2–4 weeks postnatal) predicted cognitive and social behavior in infants months later. Although we observed no effects of milk cortisol on global social behavioral patterns, sons and daughters exhibited differential sensitivity to mother’s milk in the expression of particular social behaviors. Infants whose mothers produced milk with higher GC concentrations performed less impulsively on a cognitive task at 6 months of age. To our knowledge, this represents the first study in any mammal to assess downstream social behavior and cognition as a function of natural variation in milk cortisol in the neonatal period. Our findings refine and extend our insights into how hormones in milk may be shaping certain aspects of infant brain development.
We found no effects of milk cortisol during the neonatal period on global social behavior 3–7 months later. Previous research by Hinde et al. (2015) found that higher and increasing concentrations of milk cortisol across lactation were predictive of a more nervous, less confident temperament during an experimental separation from the mother and social group. Although we did not study changes in milk cortisol in the present study or directly assess temperament, our study importantly examined social behavior in a naturalistic context and found no differences in overall social behavior patterns as a function of milk cortisol concentrations in the neonatal period.
Earlier research in humans and monkeys found that female and male infants were differentially sensitive to maternal-origin GCs (Grey et al., 2013; Hinde et al., 2015), and within monkeys, that the window of sensitivity was at younger ages for females (Hinde et al., 2015). Mean GC concentration in milk did not differ between mothers rearing a son and mother rearing a daughter; rather there was evidence of differences in sensitivity as found elsewhere (Hinde et al., 2015). Among rhesus macaques, cortisol concentrations in milk from 4 to 5 weeks postpartum were most predictive of daughters’ nervous temperament, whereas sons were more sensitive to milk GCs later in infancy (Hinde et al., 2015). A previous study among rhesus macaques further substantiated that sons may be more sensitive to maternal-origin GCs later in infancy (Sullivan et al., 2011). Among humans, GCs in milk are implicated in greater negative affect for daughters, but not sons, but only a single time point was assessed preventing evaluation of sex-differentiated critical windows of sensitivity (Grey et al., 2013). In the present study, we found that maternal-origin cortisol in milk during the first neonatal weeks was more frequently implicated in the social behavior of daughters, most notably that higher concentrations of cortisol was predictive of more frequent play behavior. This relationship was largely unexpected, in part, because if maternal-origin GCs are influencing a nervous temperament or negative affect, we would predict less social behavior. However, milk GCs were not associated with greater initiation of play behavior but rather total play behavior, suggesting that more cautious, less impulsive female infants may represent a temperament that disproportionately attracts and productively reciprocates social behavior from conspecifics (Weinstein & Capitanio, 2008). Future integrative research should address temperament and social behavior simultaneously to investigate whether temperaments shaped by milk hormones influence partner preferences or other social adjustments not captured by our aggregated social behavior measures here. Caution is warranted for interpreting sex differences at low sample sizes (Brown & Silk, 2002), but we are reassured by the consistencies of sex-differentiated infant behavior (Brown & Dixson, 2000), sensitivity (Hinde et al., 2015), and higher milk yield for daughters (Hinde, 2009b) described previously for other macaque populations.
Among humans and macaques, maternal prenatal cortisol (as measured in amniotic fluid, saliva, plasma, and/or feces) is associated with infant cognition (Bergman, Sarkar, Glover, & O’Connor, 2010) and temperament (Bardi & Huffman, 2006; de Weerth, van Hees, & Buitelaar, 2003). Recent studies in some of these same macaque subjects revealed that higher maternal hair cortisol concentrations in the neonatal period were positively correlated with infant irritability and negatively correlated with sensorimotor reflex scores in the 1st month of life (Dettmer, Rosenberg, Suomi et al., 2015). Here we showed that postnatal maternal-origin GCs are associated with more cautious cognitive performance and are consistent with the “conception through weaning” model of early developmental programming (da Cunha, Leite, & de Almeida, 2015). Our findings that milk cortisol appreciably explained variance in the model of impulsive responses on the cognitive task are consistent with earlier findings of greater nervous affect in macaques whose mothers produce milk with higher cortisol content at the California National Primate Research Center (Hinde et al., 2015). More nervous or cautious infants are expected to be less impulsive than are more confident ones. Notably, we were unable to test for sex-specific sensitivity to cortisol in cognitive performance due to low sample sizes on this assessment. We predict that a larger sample size may reveal such sensitivity, as has been demonstrated with IQ in human infants given fatty acid supplementation favoring the performance of daughters (Isaacs et al., 2011).
Cortisol concentrations in milk represented dynamic physiological signaling to the infant. We found no relation between milk cortisol and maternal hair cortisol concentrations. Thus, it does not appear that mothers with higher cortisol concentrations in milk are necessarily more chronically stressed. These findings are not altogether surprising given that in previous work, cortisol concentrations in milk were not associated with maternal social rank. High-, middle-, and low-ranking mothers produced similar milk cortisol concentrations (Hinde et al., 2015). Binding of cortisol to GC receptors in mammary tissue may result in different GC concentrations in milk and hair (Tucker & Schwalm, 1977). Notably, the GC concentrations in milk postpregnancy, and in hair during pregnancy, are elevated in primiparous (first-time) compared to multiparous mothers (Dettmer, Rosenberg, Suomi et al., 2015; Hinde et al., 2015). Young, primiparous mothers are particularly handicapped in nourishing offspring: they produce lower milk volume. Similarly maternal body mass, indicative of somatic reserves to sustain lactation, has been associated with GC concentrations in milk (Hinde et al., 2015). Elsewhere, Hinde and colleagues have speculated that cortisol in milk programs an energetically conservative infant phenotype that prioritizes growth (Hinde et al., 2015). Energetically conservative infants would be expected to be less exploratory, playful, and active (and rather more cautious) and more food motivated. Our results are only partially consistent with those predictions, as higher milk cortisol was associated with better performance on a task with a food reward. Supporting the perspective that the organization of behavioral phenotype is sensitive to energetic conditions during development, milk yield—a proxy for available milk energy —was implicated in total social behavior in the present study. However, some findings were inconsistent, specifically that increased GC concentration in milk was associated with increased play and social behavior in daughters.
Our study represents an early exploration of these complex interactions and has limitations of sample size, absence of other important parameters such as maternal behavior, and evaluation of milk only during the neonatal period. First, the sample size for the cognitive study was small (n = 12), and as such our findings should be interpreted with caution. Because of the intensive nature of cognitive testing for socially housed mother–peer-reared infant monkeys (Dettmer, Murphy et al., 2015), only a handful of monkeys can be tested each year. We are adding to our data set annually and collaborating with other primate centers to implement our cognitive testing protocol. Importantly, the intersections of cognition and temperament will be essential to understand the extent to which performance on cognitive tasks reflect affective state versus cognitive architecture (Wolfe, Zhang, Kim-Spoon, & Bell, 2014). Another limitation is that maternal GC (either in hair or in milk) may be correlated with maternal temperament and/or parenting style, and this may explain in part our findings relating milk cortisol with infant cognitive performance. Unfortunately, maternal behavioral observations were not conducted for the cohorts in the present study. However, we have recently begun collecting data on maternal–infant interactions in these groups that will help disentangle these relationships in the future. Finally, our evaluation of GC concentrations in mother’s milk was restricted to the neonatal period in which the infant’s intestinal tract is characterized by low stomach acidity (Fallingborg, 1999) and gut permeability (reviewed in Catalani et al., 2011), although ingested GCs can continue to influence infant physiology even after the neonatal period (Donovan et al., 2000).
The results presented here have important implications for management of infant health in neonatal intensive care units (NICU) and infant feeding decisions at home. GCs are “the most extensively used pharmacological intervention used in the perinatal period′′ (Kalhan & Wilson-Costello, 2013, p. 9), with a single course given prenatally being effective in reducing morbidity and mortality after premature birth with few immediate side effects. However, perinatal supraphysiological GC treatments may have long-term consequences that should be considered by doctors who use these treatments (Yates & Newell, 2011). Future epidemiological research should explore ways to evaluate potential cognitive and biopsychological effects of exogenous GC administration, to the extent that such outcomes can be disentangled from effects of prematurity and other early life morbidities. There may be opportunities for more precise approaches for dosing, administration, and effect of exogenous GCs when considered in conjunction with NICU nutrition.
Currently, donor milk and artificial breast milk, due to processing and manufacturing, respectively, do not provide bioactive GCs that infants are adapted to ingest. Indeed, the GC receptor expression in human infant intestines is fivefold higher in breastfed than in formula-fed babies (Donovan et al., 2000). The extent to which the absence of ingested GCs affects neonatal health, in the NICU or at home, remains to be explored. Hormones that are too elevated, too low, or absent may lead to physiological dysregulation, and given that GCs can affect most tissues and orchestrate cellular metabolism and neurobehavioral function (Hinde, 2013), systematic studies in animal models and randomized trials may yield important tactics for health management (Neville et al., 2012). However, the results presented here suggest that natural variation in maternal-origin GCs is not associated with detrimental effects. During emergencies or humanitarian crises, some mothers suspend breastfeeding out of concern that their experience of stress is negatively affecting milk production and composition and therefore the infant (Gribble, 2013). Overwhelming scientific evidence supports continued breastfeeding under such circumstances (Gribble, 2013), and these data here should reassure parents, clinicians, and public health scientists about the relatively minor and seemingly nondetrimental effects of ingesting maternal-origin GCs via breast milk.
Collectively, these results support emerging evidence that mother’s milk may be signaling to infants to prioritize moderate behavioral inhibition that allows for maximal growth (Hinde et al., 2015). By producing infants with cautious temperaments (Hinde et al., 2015) and less cognitive impulsivity, milk cortisol may be calibrating infants to available resources, which enable them to better meet the demands of the environment (Catalani et al., 2011), as is evidenced by the fact that we found no deficits to overall social functioning. Future work will be able to draw upon these studies to determine the long-term effects of hormonal signals in mother’s milk. Our results are the first to reveal an association of cortisol in milk with infant cognitive and social measures within a primate social group, in concordance with the emerging conceptual model that maternal GCs co-organize infant physiological, neurobiological, immunological, and biobehavioral developmental trajectories (Allen-Blevins et al., 2015; Hinde, 2013; Love, McGowan, & Sheriff, 2013).
Acknowledgments
This research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Division of Intramural Research (HD001106 to Stephen J. Suomi) and by the National Institutes of Health (OD011180 to Melinda A. Novak). We thank the animal care and veterinary staff at the NIH Animal Center for their assistance with milk collection. The hair cortisol data included in this study were published in an article in July 2015 as part of a larger study examining influences of maternal glucocorticoids (GCs) on infant development.
References
- Akaike H (1974). A new look at the statistical model identification. IEEE Transactions on Automatic Control, 19, 716–723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Allen-Blevins CR, Sela DA, & Hinde K (2015). Milk bioactives may manipulate microbes to mediate parent–offspring conflict. Evolution, Medicine, and Public Health, 2015, 106–121. doi: 10.1093/emph/eov007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychological Association. (2002). Ethical principles of psychologists and code of conduct. American Psychologist, 57, 1060–1073. doi: 10.1037/0003-066X.57.12.1060 [DOI] [PubMed] [Google Scholar]
- Bardi M, & Huffman MA (2006). Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Developmental Psychobiology, 48, 1–9. doi: 10.1002/dev.20111 [DOI] [PubMed] [Google Scholar]
- Bartol FF, Wiley AA, & Bagnell CA (2008). Epigenetic programming of porcine endometrial function and the lactocrine hypothesis. Reproduction in Domestic Animals, 43, 273–279. doi: 10.1111/j.1439-0531.2008.01174.x [DOI] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, Glover V, & O’Connor TG (2010). Maternal prenatal cortisol and infant cognitive development: Moderation by infant–mother attachment. Biological Psychiatry, 67, 1026–1032. doi: 10.1016/j.biopsych.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein RM, & Hinde K (2016). Bioactive factors in milk across lactation: Maternal effects and influence on infant growth in rhesus macaques (Macaca mulatta). American Journal of Primatology, 78, 838–850. doi: 10.1002/ajp.22544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton TB, Nugent JK, & Lester BM (1987). Neonatal behavioral assessment scale In Osofsky JD (Ed.), Handbook of infant development (pp. 780–817). Oxford, UK: Wiley. [Google Scholar]
- Brown GR, & Dixson AF (2000). The development of behavioural sex differences in infant rhesus macaques (Macaca mulatta). Primates, 41, 63–77. doi: 10.1007/BF02557462 [DOI] [PubMed] [Google Scholar]
- Brown GR, & Silk JB (2002). Reconsidering the null hypothesis: Is maternal rank associated with birth sex ratios in primate groups? Proceedings of the National Academy of Sciences of the United States of America, 99, 11252–11255. doi: 10.1073/pnas.162360599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani A, Alemà GS, Cinque C, Zuena AR, & Casolini P (2011). Maternal corticosterone effects on hypothalamus pituitary–adrenal axis regulation and behavior of the offspring in rodents. Neuroscience and Biobehavioral Reviews, 35, 1502–1517. doi: 10.1016/j.neubiorev.2010.10.017 [DOI] [PubMed] [Google Scholar]
- Clarke G, O’Mahony SM, Dinan TG, & Cryan JF (2014). Priming for health: Gut microbiota acquired in early life regulates physiology, brain and behaviour. Acta Paediatrica, 103, 812–819. doi: 10.1111/apa.12674 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH (1991). The evolution of parental care. Princeton, NJ: Princeton University Press. [Google Scholar]
- da Cunha AJLA, Leite AJM, & de Almeida IS (2015). The pediatrician’s role in the first thousand days of the child: The pursuit of healthy nutrition and development. Jornal de Pediatria, 91, S1. doi: 10.1016/j.jped.2015.07.002 [DOI] [PubMed] [Google Scholar]
- de Weerth C, van Hees Y, & Buitelaar JK (2003). Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Human Development, 74, 139–151. doi: 10.1016/S0378-3782(03)00088-4 [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Murphy AM, & Suomi SJ (2015). Development of a cognitive testing apparatus for socially housed mother-peer-reared infant rhesus monkeys. Developmental Psychobiology, 57, 349–355. doi: 10.1002/dev.21285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, & Meyer JS (2012). Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology, 37, 191–199. doi: 10.1016/j.psyneuen.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Rosenberg K, Guitarra D, Novak MA, Meyer JS, & Suomi SJ (2015). Parity and infant sex interact to influence maternal cortisol in pregnancy and infant social behavior in rhesus monkeys. Developmental Psychobiology, 57, S11. doi: 10.1002/dev.21261 [DOI] [Google Scholar]
- Dettmer AM, Rosenberg K, Menard MT, El-Mallah SN, Woodward RA, Suomi SJ, & Meyer JS (2015). Differential relationships between chronic hormone profiles in pregnancy and maternal investment in rhesus monkey mothers with hair loss in the neonatal period. American Journal of Primatology, 79, 1–8. doi: 10.1002/ajp.22489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Rosenberg KL, Suomi SJ, Meyer JS, & Novak MA (2015). Associations between parity, hair hormone profiles during pregnancy and lactation, and infant development in rhesus monkeys (Macaca mulatta). PLoS One, 10, e0131692. doi: 10.1371/journal.pone.0131692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan SM, Wang M, Monaco MH, Martin CR,Davidson LA, Ivanov I, … Callanan C (2000). Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clinical Science, 98, 137–142. doi: 10.1042/cs0980137 [DOI] [PubMed] [Google Scholar]
- Fallingborg J (1999). Intraluminal pH of the human gastrointestinal tract. Danish Medical Bulletin, 46, 183–196. [PubMed] [Google Scholar]
- Grey KR, Davis EP, Sandman CA, & Glynn LM (2013). Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology, 38, 1178–1185. doi: 10.1016/j.psyneuen.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble K (2013). Formula feeding in emergencies In Preedy VR, Watson RR, & Zibadi S (Eds.), Handbook of dietary and nutritional aspects of bottle feeding (pp. 143–161). Wageningen, the Netherlands: Wageningen Academic. doi: 10.3920/978-908686-777-6 [DOI] [Google Scholar]
- Grosvenor CE, Picciano MF, & Baumrucker CR (1993). Hormones and growth factors in milk. Endocrine Reviews, 14, 710–728. doi: 10.1210/er.14.6.710 [DOI] [PubMed] [Google Scholar]
- Harlow HF, & Mears CE (1983). Emotional sequences and consequences In Plutchik R & Kellerman H (Eds.), Emotion: Theory, research and experience (Vol. 2). Emotions in early development (pp. 171–198). New York, NY: Academic Press. [Google Scholar]
- Hinde K (2009a). [Milk yield, energy density, and available milk energy]. Unpublished raw data. [Google Scholar]
- Hinde K (2009b). Richer milk for sons but more milk for daughters: Sex-biased investment during lactation varies with maternal life history in rhesus macaques. American Journal of Human Biology, 21, 512–519. doi: 10.1002/ajhb.20917 [DOI] [PubMed] [Google Scholar]
- Hinde K (2013). Lactational programming of infant behavioral phenotype In Clancy KBH, Hinde K, & Rutherford JN (Eds.), Building babies (pp. 187–207). New York, NY: Springer. doi: 10.1007/978146144060-4_9 [DOI] [Google Scholar]
- Hinde K, & Capitanio JP (2010). Lactational programming? Mother’s milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta). American Journal of Primatology, 72, 522–529. doi: 10.1002/ajp.20806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K, Power ML, & Oftedal OT (2009). Rhesus macaque milk: Magnitude, sources, and consequences of individual variation over lactation. American Journal of Physical Anthropology, 138, 148–157. doi: 10.1002/ajpa.20911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, & Capitanio JP (2015). Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behavioral Ecology, 26, 269–281. doi: 10.1093/beheco/aru186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs EB, Ross S, Kennedy K, Weaver LT, Lucas A, & Fewtrell MS (2011). 10-year cognition in pre-terms after random assignment to fatty acid supplementation in infancy. Pediatrics, 128, e890–e898. doi: 10.1542/peds.2010-3153 [DOI] [PubMed] [Google Scholar]
- Kalhan SC, & Wilson-Costello D (2013). Prematurity and programming: Contribution of neonatal intensive care unit interventions. Journal of Developmental Origins of Health and Disease, 4, 121–133. doi: 10.1017/S204017441200061X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato EA, Hsu BR, Raymoure WJ, & Kuhn RW(1985). Evidence for the direct transfer of corticosteroid-binding globulin from plasma to whey in the guinea pig. Endocrinology, 117, 1404–1408. doi: 10.1210/endo-117-4-1404 [DOI] [PubMed] [Google Scholar]
- Love OP, McGowan PO, & Sheriff MJ (2013). Maternal adversity and ecological stressors in natural populations: The role of stress axis programming in individuals, with implications for populations and communities. Functional Ecology, 27, 81–92. doi: 10.1111/j.1365-2435.2012.02040.x [DOI] [Google Scholar]
- Machado CJ (2013). Maternal influences on social and neural development in macaque monkeys In Clancy KBH, Hinde K, & Rutherford JN (Eds.), Building babies (pp. 259–279). New York, NY: Springer. doi: 10.1007/978-14614-4060-4_12 [DOI] [Google Scholar]
- Martin P, & Bateson P (1993). Measuring behaviour:An introductory guide (2nd ed.). Cambridge, UK: Cambridge University Press. doi: 10.1017/cbo9781139168342 [DOI] [Google Scholar]
- Mattison SM, Wander K, & Hinde K (2015). Breast-feeding over two years is associated with longer birth intervals, but not measures of growth or health, among children in Kilimanjaro, TZ. American Journal of Human Biology, 27, 807–815. doi: 10.1002/ajhb.22729 [DOI] [PubMed] [Google Scholar]
- Meredith SL (2013). Identifying proximate and ultimate causation in the development of primate sex-typed social behavior In Clancy KBH, Hinde K, & Rutherford JN (Eds.), Building babies (pp. 411–433). New York, NY: Springer. [Google Scholar]
- Meredith SL (2015). Comparative perspectives on human gender development and evolution. American Journal of Physical Anthropology, 156, 72–97. doi: 10.1002/ajpa.22660 [DOI] [PubMed] [Google Scholar]
- Meyer JS, & Novak MA (2012). Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153, 4120–4127. doi: 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Wiley AA, Chen JC, Bagnell CA, & Bartol FF (2013). Nursing for 48 hours from birth supports porcine uterine gland development and endometrial cell compartment-specific gene expression. Biology of Reproduction, 88, 4. doi: 10.1095/biolreprod.112.105056 [DOI] [PubMed] [Google Scholar]
- Mitchell G, & Tokunaga DH (1976). Sex differences in nonhuman primate grooming. Behavioural Processes, 1, 335–345. doi: 10.1016/0376-6357(76)90015-2 [DOI] [PubMed] [Google Scholar]
- Nettle D, Frankenhuis WE, & Rickard IJ (2013). The evolution of predictive adaptive responses in human life history. Proceedings of the Royal Society of London B: Biological Sciences, 280, 20131343. doi: 10.1098/rspb.2013.1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville MC, Anderson SM, McManaman JL, Badger TM, Bunik M, Contractor N, … Williamson P (2012). Lactation and neonatal nutrition: Defining and refining the critical questions. Journal of Mammary Gland Biology and Neoplasia, 17, 167–188. doi: 10.1007/s10911-012-9261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pàcha J (2000). Development of intestinal transport function in mammals. Physiological Reviews, 80, 1633–1667. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Justus KR, Schatzberg AF, & Lyons DM (2005). Mild early life stress enhances prefrontal dependent response inhibition in monkeys. Biological Psychiatry, 57, 848–855. doi: 10.1016/j.biopsych.2004.12.024 [DOI] [PubMed] [Google Scholar]
- Parker JG, Rubin KH, Erath SA, Wojslawowicz JC, & Buskirk AA (2006). Peer relationships, child development, and adjustment: A developmental psychopathology perspective In Cicchetti D & Cohen DJ (Eds.), Developmental psychopathology, theory and method (pp. 419–493). Hoboken, NJ: Wiley. doi: 10.1002/9780470939383.ch12 [DOI] [Google Scholar]
- Patacchioli FR, Cigliana G, Cilumbriello A, Perrone G, Capri O, Alema GS, … Angelucci L (1992). Maternal plasma and milk free cortisol during the first 3 days of breast-feeding following spontaneous delivery or elective cesarean section. Gynecologic and Obstetric Investigation, 34, 159–163. doi: 10.1159/000292751 [DOI] [PubMed] [Google Scholar]
- Peaker M, & Neville MC (1991). Hormones in milk: Chemical signals to the offspring? Journal of Endocrinology, 131, 1–3. doi: 10.1677/joe.0.1310001 [DOI] [PubMed] [Google Scholar]
- Power ML, & Schulkin J (2013). Maternal regulation of offspring development in mammals is an ancient adaptation tied to lactation. Applied and Translational Genomics, 2, 55–63. doi: 10.1016/j.atg.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F, Liguori SA, Fissore MF, & Oggero R(2009). Breast milk hormones and their protective effect on obesity. International Journal of Pediatric Endocrinology, 2009, 327505, 1–8. doi: 10.1155/2009/327505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ML, Champoux M, & Moore CF (2006). Neurobehavioral assessment of nonhuman primate neonates In Sackett GP, Ruppenthal GC, & Elias K (Eds.), Nursery rearing of nonhuman primates in the 21st century (pp. 215–247). New York, NY: Springer. doi: 10.1007/978-0-387-25640-5_12 [DOI] [Google Scholar]
- Stearns SC (Ed.) (1992). The evolution of life histories(Vol. 249). Oxford, UK: Oxford University Press. [Google Scholar]
- Sullivan EC, Hinde K, Mendoza SP, & Capitanio JP (2011). Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Developmental Psychobiology, 53, 96–104. doi: 10.1002/dev.20483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker HA, & Schwalm JW (1977). Glucocorticoids in mammary tissue and milk. Journal of Animal Science, 45, 627–634. doi: 10.2134/jas1977.453627x [DOI] [PubMed] [Google Scholar]
- Weinstein TA, & Capitanio JP (2008). Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Animal Behaviour, 76, 455–465. doi: 10.1016/j.anbehav.2008.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JC (2007). Flaws in the theory of predictive adaptive responses. Trends in Endocrinology & Metabolism, 18, 331–337. doi: 10.1016/j.tem.2007.07.006 [DOI] [PubMed] [Google Scholar]
- Wells JC (2014). Adaptive variability in the duration of critical windows of plasticity: Implications for the programming of obesity. Evolution, Medicine, and Public Health, 2014, 109–121. doi: 10.1093/emph/eou019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe CD, Zhang J, Kim-Spoon J, & Bell MA(2014). A longitudinal perspective on the association between cognition and temperamental shyness. International Journal Of Behavioral Development, 38, 266–276. doi: 10.1177/0165025413516257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates HL, & Newell SJ (2011). Postnatal intravenous steroids and long-term neurological outcome: Recommendations from meta-analyses. Archives of Disease in Childhood-Fetal and Neonatal Edition, 97, F299–F303. doi: 10.1136/adc.2010.208868 [DOI] [PubMed] [Google Scholar]