Abstract
In humans, temperament plays an important role in socialization and personality. Some temperaments, such as behavioral inhibition are associated with an increased risk for psychopathology. Nonhuman primates can serve as a model for neurobiological and developmental contributions to emotional development and several recent studies have begun to investigate temperament in nonhuman primates. In rhesus monkeys, dominance rank is inherited from the mother and is associated with social and emotional tendencies that resemble differences in temperament. The current study assessed differences in temperament in infant rhesus monkeys as a function of maternal dominance rank. Temperament was assessed in 26 infants (13 males) from birth until 6 months of age with a battery that included Brazelton test, human intruder test, human intruder-startle, cortisol stress reactivity, and home cage observations of interactions with peers and the mother. Throughout testing, infants lived with their mothers and a small group of other monkeys in indoor/outdoor runs. Dominance rank of the mothers within each run was rated as either low/middle (N = 18, 9 male) or high/alpha (N = 8, 4 female). Infants of high-ranking mothers displayed more intruder-directed aggression and reduced startle potentiation in the human intruder tests. Dominant offspring also had reduced levels cortisol and startle across development and spent more time away from mothers in the interaction tests. These results suggest that dominance of the mother may be reflected in behavioral reactivity of infants early in life. These findings set up future studies, which may focus on contributing factors to both dominance and temperament such as genetics, rearing, and socialization. Such factors are likely to interact across development in meaningful ways. These results also suggest future human-based studies of a similar relationship may be warranted, although social dominance is clearly more complex in human than macaque societies.
Keywords: development, dominance, hierarchy, infant, temperament
INTRODUCTION
Dominance hierarchies are widely observed among socially living species [Rowell, 1974; Wilson, 1975]. Hierarchies serve to stabilize social structures and reduce competitive exchanges and aggression within a group by establishing individually tailored entitlements and ritualized patterns of signals to guide interactive behavior [de Waal, 1986; Drea & Wallen, 1999; Wilson, 1975].
The factors that contribute to an individuals’ dominance rank vary across species though in many instances dominance rank is not simply a function of physical factors such as size and strength [Bastian et al., 2003; Dewsbury, 1990; Masur & Benedito, 1974; Sallet et al., 2011; Sapolsky, 2005; Wang et al., 2011]. In the rhesus macaque and several other matrilineal organized primate species, dominance rank of infants and juveniles is largely a function of mothers’ status in the group [Fedigan, 1982; Hausfater et al., 1982; Sade et al., 1988]. Infant and juvenile rhesus monkeys are ranked just below their mothers. This status typically persists for females after puberty because they remain with the troop but for males who emigrate at puberty, dominance status must be reestablished within a new group.
In mature animals, dominance rank is associated with differential patterns of physiological and emotional reactivity. Animals lower on the dominance hierarchy generally have higher levels of circulating cortisol, lower levels of gonadal steroids, exhibit higher rates of anxious- and depressive-like behavior, and are more likely to be in poor health [Gesquiere et al., 2011; Onyango et al., 2008; Sapolsky, 2005; Shively et al., 2005]. However, it is unclear when in development such dominance-related differences in physiology, emotion, and behavior might emerge. Since dominance status is conferred at birth (via the mothers’ status) rather than acquired via interaction, one might expect at least some of these differences to be heritable. Indeed in some species, dominance-related behavior patterns have been shown to be largely heritable [Dewsbury, 1990; Masur & Benedito, 1974] and it has been suggested that in humans dominance–submissiveness is a temperamental trait associated with control and influence that is expressed throughout life [Mehrabian, 1996]. Furthermore, in some species including humans who do not display the typical dominance heritability patterns, stable social dominance structures have been reported in children as young as 2 [Frankel & Arabel, 1980]. On the other hand in primates, factors other than maternal rank can influence dominance status, as it has been shown that manipulation of early environment can alter dominance status [Bastianet al., 2003; Sallet et al., 2011], in addition to other factors that may affect social competence of animals [de Waal, 1986].
To the extent of our knowledge, this is the first study that studies the relationship between infant-mother dominance rank and while much is known about behaviors associated with dominance and trans-generational patterns of transmission in rhesus macaques, the manner in which dominance is expressed in infants and the mechanisms by which dominance status are conferred are poorly understood. For example, during early life, dominance could be the result of heritable emotional and personality characteristics that are expressed in the animal and confer dominance status. Alternatively, dominance could be a function of the degree of deferential behavior afforded to an individual animal as a function of his or her lineage, or a function of differential parental behavior expressed by dominant relative to subordinate mothers.
In order to better understand the factors that lead to the expression of dominance in rhesus monkeys, we first wanted to establish when in development dominance-related differences in emotional style or temperament are present. In the current study, we focused on the patterns of emotional behavior and temperament in both social and nonsocial contexts during the first 6 months of life. Several laboratory-based paradigms have recently been used to probe temperament in infant macaques [Bethea et al., 2004; Kalin & Shelton, 2003; Rogers et al., 2008; Schneider et al., 1991a; Sussman & Ha, 2011]. Because of the strong association between tempera mental profile and dominance status as well as the transmission of dominance status from mother to offspring, we predicted temperamental behavior patterns of infants would also differ as a function of maternal dominance rank. Specifically, we hypothesized that offspring of dominant mothers would be more resistant to stress, less inhibited, and more adventurous in social and threatening environments across the first 6 months of life than offspring of less dominant mothers.
METHODS
Subjects
Twenty-six infant rhesus monkeys (Macaca mulatta), 13 males, from a single birth-year cohort served as subjects. Data were collected between April and December 2010 in Poolesville, MD. All monkeys were bred and subsequently housed in an indoor/outdoor “run” at the NIH animal facility, Poolesville, MD. Each run contained one adult male and six-to-eight females and their offspring. Animals were fed daily with monkey chow and had ad libitum access to water. An animal caretaker checked runs daily for birth and overall health status of monkeys. All procedures performed were approved by the institutional animal care and use committee of the National Institute of Health, and adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates and all the prevailing legal requirements of the United States.
The dominance status of the mother of each subject was characterized as either low/middle (N = 18, 9 males) or high/alpha (N = 8, 4 males) rank by three independent observers, (animal care staff) using behaviors observed in their home cage such as aggression, physical displacement, and food intake [Bastian et al., 2003; Sapolsky, 2005]. We achieved 100% concurrence among the three raters of this bimodal categorization. Importantly, all raters were blind to outcome of behavioral assessments performed on the infants and all raters were blind to dominance rankings prior to data analysis.
Procedures
Assessment during first month
At postnatal days 7, 14, 21, and 30, infants were removed from the run and the mother and an adaptation of the Brazelton neonatal assessment was performed. The details of this procedure have been described previously [Schneider et al., 1991b; Schneider & Suomi, 1992]. Briefly, over the course of approximately 15 min, a series of probes of motor, emotional, and perceptual development were performed on the infant by a trained human experimenter and levels of response for each item were tabulated on a 0–2 point Likert scale at the time of assessment. Previous cluster analyses of this procedure indicated four major factors: orientation, motor development, locomotor activity, and emotional state control as well as several other individual items that did not cluster with any factor [Schneider et al., 1991b; Schneider & Suomi, 1992]. For the present study, we focused on the emotional aspects of the battery including the state control composite and individual items of response to restraint, fear, and startle. The state control composite is comprised of four separate items for irritability, consolability, struggle, and predominate arousal state during assessment. Irritability was defined as the amount of anger or frustration gauged by the experimenter during all probes. Consolability was defined as the ease of consoling the infant after distress. Struggle was measured as the amount of difficulty or effort required to perform all the tests. Predominate state was measured as the arousal level of the infant during testing (going from awake and alert to agitated). Restraint was an assessment of the amount of resistance generated by a 10-sec test in which the infant is pinned on its back. Fear was defined as the amount of nervousness or trepidation expressed during the entire assessment, and startle was a subjective index of the magnitude of startle generated by a loud auditory probe. At the end of this intensive and interactive assessment battery, the separation response was assessed by placing the infant alone and undisturbed in an incubator cage with a toy and blanket for an additional 5 min. The duration of active locomotor behavior and frequency of vocalizations (e.g. coo, bark, screech, squeal) expressed during this isolation period were recorded by a human observer.
Assessments at 3 and 6 months of age
At 3 and 6 months of age, the monkeys were again removed from the run and underwent testing in the human intruder paradigm (HIP) [Kalin & Shelton, 2003], and human intruder-startle (HIS) paradigm. These tests were performed twice at each age with a day in between test sessions. Both the HIP and HIS paradigms were designed to assess the infant’s emotional response to direct and indirect threats. The HIP is comprised of four phases: During the initial acclimation period, the subject was alone in a test cage. After the acclimation period was a profile phase in which a human “intruder” entered the room and presented their facial profile to the subject without making direct eye contact. This was followed by a second alone phase and finally a stare condition in which the same intruder returned and made direct eye contact with the subject. Each phase lasted 10 min and was videotaped for subsequent analysis.
The HIS paradigm was similar to the HIP but the dependent variable was the startle response rather than behavior and each phase was shorter. In the HIS paradigm, the infants were placed alone in a small mesh restraint box for a 6-min acclimation period, which was followed by two 3-min periods in which a human intruder presented either a profile or direct eye contact with the subject. In the HIS, the acclimation was always conducted first but unlike in the HIP, the order of the profile and stare conditions was counterbalanced across sessions. The restraint box was fixed to the accelerometer platform of a primate startle chamber. For 3-month-old infants the restraint box was 6″ height × 11″ long × 6″ depth and for 6-month-old infants 7″ height × 12″ Long × 7″ depth. This box was designed to minimize movement but did not fully restrain the animals. The startle chamber was a sound attenuating wooden chamber equipped with a ventilation fan that produced an ambient noise of approximately 65 dB. The HIS conditions were always conducted with the door to this chamber open to allow infants to see the human intruder. The human intruder presented profile and stare from a chair located approximately 2 m from the chamber. Two wall-mounted, high-frequency audio speakers (range 5–40 kHz) were located laterally inside the chamber and delivered computer-generated, 40-msec broadband acoustic pulses of 110 dB. Three startle probes were presented in each of the three test conditions (acclimation, profile, and stare). Movement of the restraint box, resulting from a whole-body startle response, displaced the accelerometer (Endevco, San Juan Capistrano, CA), and produced a voltage signal proportional to the chamber’s displacement velocity. Startle response measurement was defined as the maximal peak accelerometer output during the first 600 msec after the startle-eliciting noise onset. The analog output of this signal was amplified by a gain of 100 and then digitized using an InstruNet device, Model 100B (GW Instruments, Somerville, MA) interfaced with a Macintosh G3 (Apple, Cupertino, CA) computer system.
Blood Collection and Cortisol Assay
Immediately after the HIP test (and immediately before the HIS test), subjects were manually restrained and 50 μl of blood was collected from femoral vein for cortisol assay. On the day between tests, infants and mothers were captured together in the run and blood was taken from the infants while they were in physical contact with their mothers. This second-day blood was designed to be a control condition, without maternal separation or human intruder stare conditions. Care was taken to minimize all effects of experimenter contacting this “baseline” sample by ensuring that capture and sample were taken within 15 min of entry into the run. Because we only had a single baseline measure at each age, the values from the two sessions collected after the HIP test at 3 and 6 months of age were averaged to generate a mean “stress” response at each age. Blood samples were centrifuged in microtubes containing 1.3-ml K3E, 1.6-mg EDTA per milliliter blood. Cortisol concentration was determined by enzyme-linked immunosorbent assay (ELISA) assay performed by a commercial lab (Yerkes National Primate Research Center at Emory University). All cortisol assays were measured in duplicate in a single run, and intra assay CV was <20%.
Assessments at 4 and 5 months of age
At 4 and 5 months of age, infants were observed in their home environment for a 30-min period using a time sampling procedure. The focal animal was observed for the entire 30-min session by a trained human observer sitting approximately 1 m from the home cage. Every 30 sec during the 30-min observation period, the presence or absence of a series of behaviors was recorded. Tabulated behaviors included locomotor behavior scored if subject was actively moving about the cage; passive behavior, recorded if subject was sitting passively and not engaged in any other activities; environmental explore, scored if the subject was actively manipulating nonsocial object in the cage; social behavior recorded if subject was engaged in play, grooming, or other affiliative behavior with other monkeys or with the mother; and vocalizations (Voc) recorded if subject was actively vocalizing. Finally at each time point, it was indicated if the infant was on or off the mother while engaged in these activities. These data were tabulated at the end of the 30-min session.
Statistical Analysis
All statistical analyses were performed with SPSS software (IBM SPSS Statistics 19.0) with threshold set to P < 0.05. For the neonatal assessments, a mean was generated across the four assessment points and differences were compared between dominant and nondominant offspring on each measure with multivariate analysis of variance (ANOVA), where gender was included as a nuisance covariate. Because of the number of tests performed and the exploratory nature of this study, we opted not to employ Bonferonni correction because this would impose an overly stringent correction factor. Therefore our results from this analysis should be viewed as preliminary and in need of replication. Repeated measures ANOVA was performed on behavior collected at the 3–6 month intervals (HIP, HIS, cortisol, and home cage) with factors for group, gender, age, and specific test conditions as detailed in the results section.
RESULTS
Neonatal Assessments
The mean scores for the emotional portions of the Brazelton assessments are depicted in Table I. A two-factor ANOVA (gender and dominance group) revealed that infants of higher ranking mothers had significantly higher scores on the composite category of state control, F (1, 18) = 4.91, P < 0.05, and a trend toward higher irritability ratings, F (18) = 3.70, P = 0.071. No significant group differences were found on other measures and no main or interaction effects were found for gender on any measure. This indicates that while dominant offspring did not differ on the majority of measures of development or emotional temperament during the infantile period, some differences on emotional variables that are highly relevant for social interaction may be apparent even during the first month of life.
TABLE I.
Mean (+SEM) Scores Obtained in the Brazelton Neonatal Assessments Performed at Four Points during the First Month of Life
| Group | State control | Startle | Fear | Restrain | Isolation locomotion | Isolation vocalizations |
|---|---|---|---|---|---|---|
| Low | 1.3 (0.09) | 0.6 (0.1) | 0.2 (0.08) | 0.2 (0.06) | 29.6 (7.1) | 173.5 (21.1) |
| High | 1.4 (0.06)a | 0.5 (0.1) | 0.07 (0.03) | 0.4 (0.1) | 41.4 (14.2) | 187.6 (34.7) |
P < M.
Three- and Six-Month Assessments
Human intruder paradigm
The four behaviors that were consistently expressed across animals in the HIP test were locomotion, freeze, coo, and bark vocalizations. These measures, broken down by experimental condition, are depicted in Table II. Each behavior was subjected to a 2 (maternal dominance) × 2 (gender) × 2 (age) × 4 (test condition) mixed-design ANOVA. For locomotion, freeze, and coo, although main and interaction effects were found for age and test condition, no main or interaction effects were found for maternal dominance group or gender. For the bark vocalization, there was a highly significant effect for test condition, F (3, 72) = 12.72, P < 0.000 with more barks occurring during the stare than any other condition, and a significant dominance group by condition interaction, F (3, 72) = 2.78, P < 0.05. No main or interaction effects were found for gender.
TABLE II.
Mean (+SEM) Duration (Locomotion and Freeze) and Frequency (Bark and Coo) of Behaviors Expressed by the Offspring of Low- and High-Dominance Mothers in the HIP
| Behavior | Group | Acclimattableion | Profile | Alone | Stare |
|---|---|---|---|---|---|
| Locomotion | Low | 485.3 (20.5) | 405.6 (29.8) | 482.4 (24.4) | 358.4 (25.9) |
| High | 511.7 (13.4) | 436.7 (34.3) | 481.1 (25.1) | 333.1 (42.9) | |
| Freeze | Low | 1.5 (0.7) | 18.0 (10.5) | 0.03 (0.03) | 6.1 (6.1) |
| High | 0.9 (0.9) | 7.7 (5.7) | 0.0 (0.0) | 0.0 (0.0) | |
| Barka | Low | 2.3 (0.8) | 3.3 (1.4) | 2.5 (1.3) | 7.2 (2.5) |
| High | 4.3 (2.1) | 4.8 (2.2) | 2.3 (1.6) | 16.1 (5.9) | |
| Coo | Low | 72.5 (10.3) | 83.4 (11.2) | 88. 1 (11.4) | 102.5 (8.7) |
| High | 71.2 (12.5) | 93.2 (14.8) | 90.6 (13.7) | 113.3 (8.8) |
Group by condition interaction P < 0.05.
Human intruder-startle
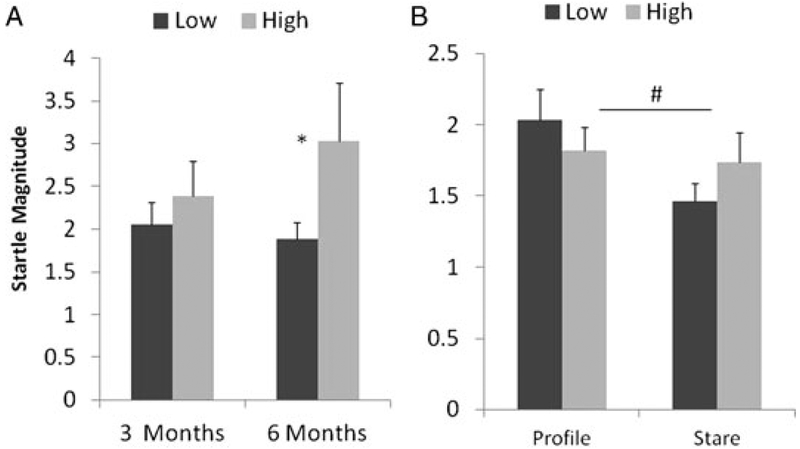
The mean startle amplitude was analyzed with a 2 (maternal dominance) × 2 (gender) × 2 (age) × 3 (test condition) mixed-design ANOVA that revealed a significant main effect of test condition, F (2, 48) = 8.02, P < 0.001. A lager startle response amplitude drove this effect in the alone condition across dominance groups. In addition, this omnibus ANOVA revealed a significant two-way interaction for condition by rank, F (2, 48) = 3.35, P < 0.05, and three-way interaction for age by condition by rank, F (2, 48) = 4.93, P < 0.05. In order to decompose this interaction, we separated our analysis into two groups. Because the alone condition was always performed first and the profile/stare conditions both contained an additional stimulus (a human intruder), we separated the data into these two groups and performed two separate repeated measures analyses. A dominance group by development analysis performed on the alone condition revealed no main or interaction effects as a function of dominance (Fig. 1A). However, the dominance group by development by condition analysis performed on the stare and profile conditions did reveal two significant effects related to dominance. First, a two-way dominance by condition interaction was found, F (1, 24) = 4.39, P < 0.05, that was driven by a significant difference in startle magnitude between the profile and stare conditions in the subordinate (paired t (17) = 4.45, P < 0.001) but not dominant (paired t (7) = 0.41, ns) offspring (Fig. 1B). This analysis also revealed a development by rank interaction, F (1, 24) = 7.88, P < 0.01, which was driven by overall reductions in startle in the dominant and overall increases in the subordinate offspring across development. No main or interaction effects of subject gender were found.
Fig. 1.
The mean (+SEM) startle amplitude for offspring of low- (dark) and high-ranking mothers (light) in the HIS paradigm. Startle response during the alone condition at both 3 and 6 months of age are depicted (A) at left and responses during the profile and eye contact conditions are shown (B) at right. In Figure 1, startle response is depicted by maternal dominance group and condition and collapsed across age. (* significant difference between groups, # significant difference within group across time points).
Plasma cortisol concentration
Cortisol values were subjected to a 2 (group) × 2 (gender) × 2 (age) × 2 (stress vs. basal) ANOVA. This analysis revealed a main effect of stress, F (1, 24) = 226.91, P < 0.001, an effect of age, F (1, 24) = 5.38, P < 0.05, and an interaction between age and rank, F (1, 24) = 6.72, P < 0.01. A three-way interaction of rank by sex by age, F (1, 22) = 4.61, P < 0.05 was also found. Across all conditions, cortisol was markedly higher after the HIP tests than during the “baseline” condition when infants were with their mothers (see Fig. 2). The rank by age interaction was driven by a reduction in cortisol levels in the dominant offspring and an increase in cortisol levels in the subordinate offspring between 3 and 6 months of age collapsed across conditions. However, there was no interaction with stress level (see Fig. 2). In order to decompose the gender effect, we performed a separate gender × age × stress analysis in each rank. In the dominant offspring, we found a three-way interaction between sex, age, and stress condition, F (1, 22) = 59.62, P = 0.001, which was driven by an elevation of cortisol in the baseline condition of females at the 3-month time point. No sex differences emerged in the nondominant group.
Fig. 2.
The mean (+SEM) plasma cortisol levels at 3 and 6 months of age. (A) “Baseline” and post HIP (post stress) conditions averaged across both dominance group and development. The baseline sample was taken on the second test day when infants were removed from the run along with their mothers, and the post HIP sample was taken on days 1 and 3 of testing after infants had undergone HIP testing. (B) Plasma cortisol levels of the two dominance groups as a function of age collapsed across baseline and stress conditions.
Four- and Five-Month Assessments
Home cage observations
In the home observations, the five most common behaviors were locomotion, passive, environmental exploration, social affiliative behavior, and vocalization. Each of these behaviors was analyzed with a 2 (maternal dominance group) × 2 (gender) × 2 (age) ANOVA. In addition, a composite measure of the total amount of time spent on mother versus off mother while engaged in all behavior was tabulated for each subject and likewise subject to a 2 × 2 × 2 ANOVA. Since there was no group by age interactions, all data were averaged across development and are depicted in Table III. Significant main effects of maternal dominance status were found for three of these behaviors. Dominant infants spent more time off mom than nondominant during the 4- to 5-month average home observations, F (1, 20) = 7.51, P < 0.05. A significant main effect of maternal dominance was also found for locomotion, F (1, 20) = 4.33, P < 0.05, and environmental explore, F (1, 20) = 8.01, P < 0.05.
TABLE III.
Mean (+SEM) Number of Home Observation at Intervals in which Offspring of Low- and High- Dominance Mothers were Engaged in Behavior during Home Cage Observations
| Group | Locomotion | Passive | Explore | Social mom | Social No mom | Vocal | Off mom |
|---|---|---|---|---|---|---|---|
| Low | 6.7 (0.9) | 2.2 (0.4) | 8.7 (1.0) | 10.4 (1.5) | 6.8 (12) | 0.3 (0.1) | 32.7 (4.6) |
| High | 10.0 (1.4)a | 2.3 (0.4) | 14.1 (1.6)a | 11.0 (1.9) | 8.9 (1.7) | 0.1 (0.6) | 55.3 (6.9)a |
Group difference P < 0.05.
DISCUSSION
This is the first study we are aware of to compare the emotional profile of infant offspring from dominant and subordinate adult rhesus monkeys. Dominance status in rhesus monkeys co-varies across infant and mother. As soon as they are mobile, infants of dominant mothers gain access to more desired resources than infants from lower ranking mothers. This study assessed the extent to which maternal dominance rank is also associated with differences in temperament in the infants, and whether emotionality and temperament may develop along different trajectories in dominant versus nondominant monkeys. While infants of high and mid/low rank were similar on most measures, we did find some noteworthy differences in emotional behavior across the first 6 months of life.
In general, infants of dominant mothers were more reactive to adverse environmental stimuli. During the first month of life, infants of high-ranking mothers were significantly more irritable, and trended toward higher struggle scores. At 3 and 6 months of life high-ranking infants displayed more aggressive bark vocalizations in the provocative stare condition of the human intruder test and they showed less potentiation of startle in the profile relative to the stare condition. This pattern of results indicates that when confronted with adverse threatening or unpleasant conditions, offspring of dominant mothers are more likely to generate overt behavioral reactions than to retreat or inhibit. This same response pattern is characteristic of adult dominant animals.
The startle response in the HIS was particularly complex and deserves further comment. Because we were concerned about carryover effects, the alone condition was always conducted before the human intruder entered the room and the two human intruder conditions were counterbalanced. Contrary to expectations, a highly significant reduction in startle was found across all subjects in the stare condition relative to both the profile condition and the alone condition. However, direct comparisons between both human intruder condition and the alone condition is problematic because of potential order effects. Because of this, we conducted separate analyses on the alone and human intruder conditions. When separated in this way, startle magnitude did not vary as a function of dominance status in the alone condition, but maternal dominance status did result in two interactions in the human intruder analyses. A number of studies have demonstrated that the stare condition in the HIP paradigm is a provocative stimulus that tends to generate overt behavior patterns such as cage shake, vocalization, and locomotor agitation, while the profile condition induces freezing and locomotor reduction [Kalin & Shelton, 2003]. Indeed, we observed this same behavioral pattern as well in the standard HIP (see Table II). However, the inhibition of behavior in the profile condition is not considered a passive response but rather one of hiding and preparation for rapid escape [Kalin & Shelton, 2003]. In rodent-conditioned aversion tests, such active freezing is generally associated with a potentiated startle response [Luyten et al., 2011] (although startle and freezing are dissociable under some conditions [McNish et al., 2000; Silva et al., 2004]). As such, we expected to observe a pattern across all animals of greatest startle response in the profile condition. In rats and monkeys, freezing and startle potentiation are thought to involve hyperactivation of amygdala and related circuitry [Antoniadis et al., 2009; Kalin et al., 2004; Orsini & Maren, 2012]. Here, we did observe a potentiated startle response during the profile condition relative to the stare condition in the offspring of low dominant monkeys suggesting hyperactivation of the amygdala-related circuitry in this condition but only in the offspring of low dominant mothers.
The other pattern that emerged in both the startle and the cortisol data were what appeared to be different developmental trajectories in the two groups collapsed across stimulus condition. In the startle response, this was evidenced by a diminishing startle response across development in the dominant offspring and an increase in the nondominant offspring. In the cortisol data, we found that high-ranking monkeys began to develop a pattern of lower cortisol across development while lower ranking monkeys remained mostly unchanged. This pattern is consistent with a number of studies that have found an inverse relationship between dominance rank and cortisol levels in both mature human and nonhuman primates [Gesquiere et al., 2011; Onyango et al., 2008; Sapolsky, 2005; Shively et al., 2005]. Although in some circumstances, the most dominant alpha male may in fact express higher cortisol levels than other group members [Gesquiere et al., 2011; Sapolsky, 2005]. In addition to the overall developmental patterns of cortisol in the two groups, the interaction between dominance rank and stress across development is also informative. While stress-reactive cortisol response diminishes across age in the dominant offspring, the opposite pattern can be seen in the lower ranking infants. The present data suggest that this pattern of high dominance and low cortisol levels may develop early in life while a profile more consistent with behavioral inhibition and anxiety develops in lower ranking juveniles [Gunnar & Cheatham, 2003; Hirshfeld-Becker et al., 2003; Miller & Coll, 2007]. While this pattern has been observed previously in humans, the fact that it is present prior to puberty in rhesus monkeys is noteworthy.
Finally, the observations in the home cage revealed that at 4 and 5 months of age, high-ranking infants spent less time in physical contact with the mother than low-ranking infants. This indicates that they are spending more time exploring, playing, and foraging away from the mother, a pattern that suggests less fear [Stevens et al., 2009; Suomi, 1997] more self-confidence and less social wariness [Bohlin & Hagekull, 2009]. We also observed a higher frequency of environmental explore and higher frequency of locomotion in high-ranking infants, findings that are likely related to spending more time away from their mothers. Surprisingly, other than these differences, we found no other differences in social interaction across groups. This could be explained since most of their social interactions, in both groups, is still mostly with their mothers.
In sum, these findings indicate that although similar on many measures, infants of dominant and subordinate mothers do differ on a variety of measures of emotional reactivity across the first 6 months of life. Infants of high-ranking mothers display an emotional pattern that is more reactive and context-dependent than infants of low-ranking mothers. This pattern suggests that a more engaged or exuberant temperament may be associated with high maternal dominance and a more reserved or inhibited temperament may be associated with lower dominance rank. In general, these patterns did not vary by gender of subject, although this study was not designed for that purpose and was clearly underpowered to detect gender effects.
Although the results from the present study are provocative, they clearly await replication. Due to the novel aspect of this study, many exploratory analyses were run without correction for multiple comparisons leading to the potential for type II errors. Furthermore, most of the analyses conducted here were based on a bimodal comparison of high-versus middle- and low-ranking offspring, while hierarchies are generally linear. We restricted our analyses to this bimodal approach because of the small sample size and relative difficulty of accurately characterizing midlevel points in the hierarchy. However, future studies should explore the extent to which dominance-related variance in temperament is linear. In addition, a recent study showed that not all captive societies in rhesus monkeys are clearly linear [Fushing et al., 2011]. It is worth noting that during the blood collection, we had a 15-min window and therefore the baseline measure might not reflect a true baseline. However, a significant difference was seen between our “baseline” and after testing sample. Finally, the present study did not address the origin of dominance differences. Although present early in life, the behavioral profiles we observed here could have arisen as a function of heredity, parenting style, or differential treatment by other group members, for example, though it seems most likely that such emotional profiles emerge as an interaction of these factors.
ACKNOWLEDGMENTS
We thank all the intruder participants, as well as the Animal Care staff, Shop staff, and Veterinary Staff for their invaluable help with this project. All procedures performed were approved by the institutional animal care and use committee of the National Institute of Health, and adhered to the American Society of Primatologists Principles for the Ethical Treatment of Nonhuman Primates and all the prevailing legal requirements of the United States. The authors have no conflict of interest to declare.
Contract grant sponsor: NIMH Intramural Research Program.
Footnotes
This article is a U.S. government work and, as such, is in the public domain in the United States of America.
REFERENCES
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. 2009. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biol Psychiatry 65:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian ML, Sponberg AC, Suomi SJ, Higley JD. 2003. Longterm effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta). Dev Psychobiol 42:44–51. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK, Moessner R, Cameron JL. 2004. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR). Behav Genet 34:295–307. [DOI] [PubMed] [Google Scholar]
- Bohlin G, Hagekull B. 2009. Socio-emotional development: from infancy to young adulthood. Scand J Psychol 50:592 601. [DOI] [PubMed] [Google Scholar]
- de Waal FB. 1986. The integration of dominance and social bonding in primates. Q Rev Biol 61:459–479. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. 1990. Fathers and sons: genetic factors and social dominance in deer mice, Peromyscus maniculatus. Anim Behav 39:284–289. [Google Scholar]
- Drea CM, Wallen K. 1999. Low-status monkeys “play dumb” when learning in mixed social groups. Proc Natl Acad Sci USA 96:12965–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedigan LM. 1982. Primate paradigms. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Frankel DG, Arabel T. 1980. Group formation by two-year olds. Intl J Behav Dev 3:287–298. [Google Scholar]
- Fushing H, McAssey MP, Beisner B, McCowan B. 2011. Ranking network of a captive rhesus macaque society: a sophisticated corporative kingdom. PLoS One 6:e17817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MC, Onyango PO, Alberts SC, Altmann J. 2011. Life at the top: rank and stress in wild male baboons. Science 333:357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Cheatham CL. 2003. Brain and behavior interface: stress and the developing brain. Infant Ment Health J 24:195–211. [Google Scholar]
- Hausfater G, Altmann J, Altmann S. 1982. Long-term consistency of dominance relations among female baboons (Papio cynocephalus). Science 217:752–755. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Calltharp S, Rosenbaum ED, Faraone SV, Rosenbaum JF. 2003. Behavioral inhibition and disinhibition as hypothesized precursors to psychopathology: implications for pediatric bipolar disorder. Biol Psychiatry 53:985–999. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. 2003. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci 1008:189–200. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. 2004. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci 24:5506–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B. 2011. Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behav Brain Res 222:357–362. [DOI] [PubMed] [Google Scholar]
- Masur J, Benedito MA. 1974. Genetic selection of winner and loser rats in a competitive situation. Nature 249:284. [DOI] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. 2000. Disruption of contextual freezing, but not contextual blocking of fear-potentiated startle, after lesions of the dorsal hippocampus. Behav Neurosci 114:64–76. [DOI] [PubMed] [Google Scholar]
- Mehrabian A 1996. Pleasure-arousal-dominance: a general framework for describing and meansuring individual differences in temperament. Curr Psychol 14:261–292. [Google Scholar]
- Miller SR, Coll E. 2007. From social withdrawal to social confidence: evidence for possible pathways. Curr Psychol 26:86 101. [Google Scholar]
- Onyango PO, Gesquiere LR, Wango EO, Alberts SC, Altmann J. 2008. Persistence of maternal effects in baboons: mother’s dominance rank at son’s conception predicts stress hormone levels in subadult males. Horm Behav 54:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. 2012. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36:1773–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. 2008. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav 7:463469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell TE. 1974. The concept of social dominance. Behav Biol 11:131–154. [DOI] [PubMed] [Google Scholar]
- Sade DS, Altmann M, Loy J, Hausfater G, Breuggeman JA. 1988. Sociometrics of Macaca mulatta: II. Decoupling centrality and dominance in rhesus monkey social networks. Am J Phys Anthropol 77:409–425. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MF. 2011. Social network size affects neural circuits in macaques. Science 334:697–700. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. 2005. The influence of social hierarchy on primate health. Science 308:648–652. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Suomi SJ, Champoux M. 1991a. Laboratory assessment of temperament and environmental enrichment in rhesus monkey infants. Am J Primatol 25:137–155. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Suomi SJ, Champoux M. 1991b. Laboratory assessment of temperament and environmental enrichment in rhesus monkey infants (Macaca mulatta). Am J Primatol 25:137–155. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Suomi SJ. 1992. Neurobehavioral assessment in rhesus monkey neonates (Macaca mulatta): developmental changes, behavioral stability, and early experience. Infant Behav Dev 15:155–177. [Google Scholar]
- Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. 2005. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis). Biol Psychol 69:67–84. [DOI] [PubMed] [Google Scholar]
- Silva RC, Gargaro AC, Brandao ML. 2004. Differential regulation of the expression of contextual freezing and fear-potentiated startle by 5-HT mechanisms of the median raphe nucleus. Behav Brain Res 151:93–101. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, Suomi SJ. 2009. Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. J Am Acad Child Adolesc Psychiatry 48:114127. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. 1997. Early determinants of behaviour: evidence from primate studies. Br Med Bull 53:170–184. [DOI] [PubMed] [Google Scholar]
- Sussman A, Ha J. 2011. Developmental and cross-situational stability in infant pigtailed macaque temperament. Dev Psychol 47:781–791. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin Z, Hu H. 2011. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334:693697. [DOI] [PubMed] [Google Scholar]
- Wilson EO. 1975. Sociobiology: the new synthesis. Cambridge, MA: Harvard University Press. [Google Scholar]