Abstract
Early life experience and socioeconomic status (SES) are well-established predictors of health outcomes in people. Both factors likely influence health outcomes via hypothalamic-pituitary-adrenal (HPA) axis regulation. However, it is unclear how early experience and HPA axis activity influence adult social status. We studied differentially reared female rhesus monkeys (Macaca mulatta, N = 90) as models to test the hypothesis that chronic HPA axis activity assessed via hair cortisol concentrations (HCCs) mediated the relationship between early life experience and adult social rank. We found that mother-peer-reared (MPR) monkeys acquired higher social ranks than either of the two nursery-reared (NR) groups (peer-reared, PR, or surrogate-peer-reared, SPR monkeys) (β = −0.07, t(89) = −2.16, p = 0.034). We also found that MPR HCCs were lower during the juvenile period at 18 months (F(2,25) = 3.49, p = 0.047). Furthermore, for MPR but not NR monkeys, changes in HCCs from 18 to 24 months (r(s) = −0.627, p = 0.039) and adult HCCs (r(s) = −0.321, p = 0.03) were negatively correlated with adult social rank. These findings suggest that chronic HPA axis regulation in juvenility, and perhaps in adulthood, may influence adult social status for primates that experience typical early rearing. However, early life adversity may result in dissociation between neuroendocrine stress regulation and adult social competence, which may be risk factors for adverse health outcomes.
Keywords: Early experience, social rank, cortisol, HPA axis, Macaca mulatta
Introduction
It is well established that early life adversity negatively impacts health outcomes across the lifespan. Major childhood stressors, such as parental divorce, family dissolution, institutionalized care, child abuse and/or neglect, and natural disasters predict morbidity and mortality in adulthood (Miller, Chen, & Parker, 2011; Montez & Hayward, 2014; Power & Hertzman, 1997; Schwartz et al., 1995; Smith, Hart, Blane, Gillis, & Hawthorne, 1997). Another major predictor of adult health disparities is socioeconomic status (SES), which is consistently linked to disability and disease across the lifespan and worldwide (see Bahadori et al., 2015; Hackman, Farah, & Meaney, 2010; Marshall et al., 2015; Miller et al., 2011; National Center for Health Statistics, 2012; Langlois et al., 2015; Smith, 1996; for reviews).
One proposed mechanism for the relations between early life adversity, socioeconomic status (SES), and adult health is the neuroendocrine stress response system. It is known that chronic exposure to circulating glucocorticoids predisposes individuals at risk for developing health problems (McEwen, 2008), and many studies have shown that low SES individuals and those experiencing early life adversity are more prone to heightened and sometimes chronic hypothalamic-pituitary-adrenal (HPA) axis activity (Chen, Cohen, & Miller, 2010; Cohen, Doyle, & Baum, 2006; Gunnar, 2000; Hunter, Minnis, & Wilson, 2011; Li, Power, Kelly, Kirschbaum, & Hertzman, 2007; Luecken & Appelhans, 2006; Lupien, King, Meaney, & McEwen, 2001). In general, individuals exposed to more adversity early in childhood and/or living in low SES families exhibit greater physical and mental health disparities as well as dysregulated HPA axis activity across the lifespan (Browne & Jenkins, 2012; Bush, Obradović, Adler, & Boyce, 2011; Hackman, Betancourt, Brodsky, Hurt, & Farah, 2012; Lovallo, 2013; McLaughlin et al., 2011; Palmer et al., 2013). Importantly, some studies have indicated that, for those exposed to early life adversity, intervention programs can result in normalized cortisol reactivity (Brotman et al., 2007; Dozier, Peloso, Lewis, Laurenceau, & Levine, 2008; Fernald & Gunnar, 2009), and lower cortisol reactivity is associated with an improvement in health problems (Daubenmier et al., 2011; Ebrecht et al., 2004; McKinney, Antoni, Kumar, Tims, & McCabe, 1997).
Studies in animal models produce similar findings to those reported in the human literature. Rodents, non-human primates, and other mammals show similar risks of developing dysregulated HPA axis activity and suboptimal health when exposed to early life adversity (typically in the form of maternal separation; see Conti et al., 2012; Dettmer & Suomi, 2014; Hennessy, Kaiser & Sachser, 2009; McEwen, 2003). A rich body of literature has established the social buffering role that mothers play for their infants (see Hennessy et al., 2009 for a review): mothers mitigate their infants’ behavioral and HPA axis responses to disturbances such as temporary separation, novelty, and other threats (Hennessy et al., 2009; Smotherman, Hunt, McGinnis, & Levine, 1979; Wiener, Johnson, & Levine, 1987). Moreover, early life adversity in the form of nursery rearing (NR) reduces monkeys’ ability to regulate cortisol and behavioral responses to novelty in adolescence (Winslow, Noble, Lyons, Sterk, & Insel, 2003). Thus, the buffering effect of the mother lasts into adolescence, but it is unclear whether the effects of the lack of a maternal buffer against social stress in infancy continues to exert effects on stress regulation in the adult animal.
Animal models also exhibit dysregulated HPA axis activity and poorer health outcomes when occupying lower social ranks (DeVries, Glasper, & Detillion, 2003; Sapolsky, 2004, 2005; Sapolsky, Alberts, & Altmann, 1997). Social rank in animal models, particularly in non-human primates, is arguably a good proxy for SES in humans because in both cases, higher status is related to lower stress hormone expression, greater access to resources, and better health outcomes (see Cummins, 2005, for a review, as well as Kaplan, Manuck, Clarkson, Lusso, & Taub, 1982; Sapolsky, 2004; 2005; Shively, Laber-Laird, & Anton, 1997). An additional benefit to the use of animal models is their faster developmental time course and the ability to collect repeated biological and behavioral samples across the lifespan.
Rhesus macaques (Macaca mulatta) are good models for studying the influences of early life adversity on later social status and HPA axis regulation. In addition to their genetic, physiological, and neuroanatomical similarity to humans (Suomi, 1997), rhesus macaques 1) exhibit clear linear hierarchies (Balasubramaniam et al., 2012); 2) develop strong maternal-infant bonds, the disruption of which leads to behavioral and physiological irregularities across development (Dettmer & Suomi, 2014); and 3) demonstrate variations in maternal investment and care of offspring, ranging from very protective to downright abusive (McCormack, Sanchez, Bardi, & Maestripieri, 2006). These traits make macaques ideal for studying the long-term effects of early social buffering. To date, few macaque studies have examined the link between early life adversity and social rank, but the few that exist indicate that individuals exposed to early adversity occupy lower social ranks as juveniles and young adults (Bastian, Sponberg, Suomi, & Higley, 2003; Stevens, Leckman, Coplan, & Suomi, 2009), presumably because they also exhibit impaired social interactions (Andrews & Rosenblum, 1994; Hol, Van den Berg, Stevens et al., 2009; Van Ree, & Spruijt, 1999). What remains unclear is (1) how long the association between early experience and adult social rank persists, and (2) whether the HPA axis plays a role in this relationship. Elucidating such information may reveal an important relationship between early life adversity and later social status in humans and might also identify a mechanism by which these variables interact. Ultimately, studies answering these questions would add to the body of literature showing the downstream consequences of early social buffering by a competent caregiver.
To begin addressing these questions, we studied differentially reared rhesus monkeys in a cross-sectional study from adolescence through middle age. We tested the hypothesis that early life adversity (in the form of nursery rearing) influences later social rank, and that HPA axis activity relates to social rank differently for mother-peer-reared and nursery-reared monkeys. We also tested the hypothesis that HPA axis regulation in adolescence relates to adult social rank. We relied on hair cortisol concentration (HCCs) to assess HPA axis activity, as they reflect accumulated cortisol concentrations over several months (Meyer & Novak, 2012) and have shown to be a reliable biomarker of chronic stress in human and nonhuman primates (Novak, Hamel, Kelly, Dettmer, & Meyer, 2013; Russell, Koren, Rieder, & Van Uum, 2012). We predicted that mother-peer-reared (MPR) monkeys would occupy higher ranks as adults than nursery-reared monkeys. However, since to our knowledge the relation between early experience, HPA axis activity, and adult social rank has not been explored in nonhuman primates, we did not make specific predictions about the direction of this relationship.
Methods
Subjects, early life history, and housing
Subjects were 90 female rhesus macaques (Macaca mulatta) born and reared at the Laboratory of Comparative Ethology facilities at the NIH Animal Center in Poolesville, MD. Monkeys ranged in age from 3 to 11 years (mean ± SEM: 6.0 ± 0.2). All animals were randomly assigned to one of three rearing conditions according to established, published procedures for our laboratory (Dettmer, Novak, Meyer, & Suomi, 2014; Dettmer, Novak, Suomi, & Meyer, 2012; Shannon, Champoux, & Suomi, 1998): mother-peer-reared (MPR, n = 48), peer-reared (PR, n = 15), or surrogate-peer-reared (SPR, n = 27). Briefly, each birth season (spring), MPR monkeys were reared in social groups containing 1–2 adult males, 8–10 adult females, and 3–5 peers born in the same year. Each year, MPR infants were spread approximately evenly across five different social groups. PR and SPR monkeys were relocated to the nursery on the day of birth and reared by human caregivers for the first 37 days, then assigned to social groups with peers. PR monkeys were placed in a large cage with three other agemates while SPR monkeys lived in single cages with mobile, cloth-covered surrogates (Dettmer, Ruggiero, Novak, Meyer, & Suomi, 2008) and were given daily 2-h play session with three other peers. PR and SPR groups were sex-balanced. Monkeys remained in their rearing conditions until approximately 8 months of age, at which time they were relocated into a single social group comprised of all infants born in the same year (for details on housing specifications, see Dettmer et al., 2012). From 8 months onward, monkeys from each cohort lived together continuously and received identical treatment. At 3 years of age, males and females were separated to avoid unplanned pregnancies. Thus, the female monkeys in this study had all lived together from 8 months onward. Water was provided ad libitum and monkeys were fed Purina Monkey Chow (#5038, St. Louis, MO) and foraging enrichment twice daily.
Seven cohorts were studied, ranging in age from 3 to 9 years (although the 9-year-old cohort also had two 11-year-old females who had been part of the stable social group for many years). Because 3 years represents the transition to sexual maturity in female rhesus monkeys (Rawlins & Kessler, 1986), and because many females give birth to their first infant at 3 years (Blomquist, 2009; Dettmer, 2015), in this study we describe all cohorts as adults (3–9+ years). Table 1 gives a breakdown of each cohort by rearing condition.
Table 1.
Sample sizes for cohorts based on rearing condition.
| Rearing condition | ||||
|---|---|---|---|---|
| Cohort (year) | MPR | PR | SPR | Total |
| 3 | 7 | 3 | 3 | 13 |
| 4 | 8 | 2 | 4 | 14 |
| 5 | 6 | 3 | 4 | 13 |
| 6 | 4 | 3 | 5 | 12 |
| 7 | 9 | 0 | 3 | 12 |
| 8 | 8 | 2 | 4 | 14 |
| 9+ | 6 | 2 | 4 | 12 |
| Total | 48 | 15 | 27 | 90 |
Four monkeys from a later cohort (born in 2012) were born to mothers from an earlier cohort (born in 2006). The rearing condition of the mother-infant dyads (mother → infant) was as follows: two MPR → MPR, one MPR → SPR, and one PR → MPR.
Adult social rank
Dominance interactions
Researchers observed each cohort in bi-weekly, 30 min sessions for approximately 2 months. In these sessions, all instances of dominance interactions (i.e., displace, threat, chase, attack, and submissive) were coded ad libitum (Altmann, 1974), along with the individuals involved in the interactions. Each interaction was then entered into a spreadsheet and coded as a win (individual initiated a displacement, threat, chase, or attack, or received submissive behavior) or a loss (individual received a displacement, threat, chase, or attack, or initiated a submissive behavior such as a fear grimace). Inter-rater reliability for this coding was established at ≥85% agreement.
Dominance hierarchies
Dominance hierarchies were constructed from the dominance interactions via Elo-rating, a numerical system that tracks individual rank changes over time by regularly updating values based on wins and losses (Dettmer, Kaburu, et al., 2015; Elo, 1978; Neumann et al., 2011; Wooddell, Kaburu, Dettmer, & Suomi, 2015). Two major advantages of Elo-rating include the ability to track rank changes over time, and accommodating variations in social dynamics. Using the elo. sequence function in R software (v 3.1.2) provided by Neumann et al. (2011), Elo-ratings were generated by setting each animal’s initial rating at 1000, and the k factor (a constant weighted based on the probability of winning) at 200. An average Elo-rating was calculated for each individual after approximately 2 months of data collection. For monkeys aged 3–6 years old, Elo-ratings were calculated in April 2015; for all other monkeys Elo-ratings were calculated in September 2014. These times were chosen because all individuals were present in the social groups; after these dates some individuals were permanently removed for colony management reasons. The following interactions were analyzed for Elo-ratings: displace, threat, chase, attack, and fear grimace.
Elo-rating values fluctuate proportionally in relation to the frequency of aggression, which, in turn, can vary between groups (i.e., cohorts), making Elo-rating of monkeys from different cohorts not directly comparable. Therefore, because we aimed to examine predictors of adult rank within the social group, Elo-ratings were then used to calculate a relative rank for each individual within the social group. Within the group, monkeys were assigned ordinal ranks in reverse order from highest to lowest Elo-rating; then, that rank was divided by the total number of animals in the social group at the time of Elo-rating to calculate a relative rank (group size ranged from 12 to 14 individuals). Thus, relative rank within each group ranged from 0.07 (lowest ranking individual) to 1.0 (highest ranking individual). In this way, we could determine predictors of an individual’s relative rank within its group.
Hair cortisol collection and assay
Adult hair cortisol
Hair samples were collected in October 2014 and January 2015 according to established protocols (Dettmer et al., 2014; Dettmer, Rosenberg, et al., 2015) during routine health exams by shaving the back of each animal’s neck with commercial grooming clippers. These time points were selected as they represented the period of sample collection closest to the assessment of dominance ranks (April 2015 for 3–6 year old monkeys, September 2014 for older monkeys). These samples reflected long-term cortisol accumulation over the previous 3 months, as samples are routinely collected in our colony every 3 months during health exams. Samples were placed into a foil pouch and frozen at −80°C until shipment to the Hormone Assay Core Laboratory at the University of Massachusetts Amherst. Following Dettmer et al. (2014), Dettmer, Rosenberg, et al., (2015), samples were weighed and washed twice with isopropanol and dried for 5–7 days under a fume hood. Samples were then ground to a fine powder with a ball mill grinder (MM200; Retsch, Newtown, PA) and incubated in methanol for 24 h to extract cortisol from the samples. Aliquots of the methanol extract were dried down and reconstituted with assay buffer, then analyzed via enzyme immunoassay (EIA) using a salivary cortisol kit (#1–3002; Salimetrics, State College, PA). Resulting values (μg/dL) were converted to pg/mg for analysis. Inter- and intra-assay coefficients of variation were ≤5% based on aliquots of the same extracted pooled hair sample analyzed repeatedly across assays. Four subjects did not have hair samples; thus the sample size for this data set was n = 86 (out of 90 subjects). Three subjects missing hair samples were MPR and one was PR.
Juvenile hair cortisol
Some of these subjects (n = 26) were part of a larger study examining the influences of early life experience on chronic HPA axis activity across development. As such, hair samples were taken following procedures described above at months 6, 12, 18, and 24, representing HPA axis activity from infancy through the juvenile period. We previously found that early rearing experience results in differential HCCs at months 12 and 18 (Dettmer et al., 2012), the period during which the young monkeys are adapting to the mixed-group formation that occurred at 8 months, and that by 24 months of age HCCs were the same across rearing groups. For this study, we examined HCCs taken at 12 (n = 25; MPR/PR/SPR samples = 11/5/9, respectively), 18 (n = 26; MPR/PR/SPR samples = 11/6/9, respectively), and 24 (n = 26; MPR/PR/SPR samples = 11/6/9, respectively) months. Thirteen of these 26 subjects were part of the Dettmer et al. (2012) study.
Hair samples were analyzed for cortisol content according to the methods described above. Inter- and intra-assay coefficients of variation were <10%.
Data analysis
Regression analysis was used to examine whether early rearing experience (MPR, PR, or SPR) predicted adult rank, with relative rank as the dependent variable and cohort as a control variable. ANOVA was used to test for rearing effects on adult HCCs. To assess HPA axis regulation across the juvenile period, independent samples t-tests were used to test for rearing effects on HCCs at 12, 18, and 24 months, and for changes in HCCs from months 12 to 18 and 18 to 24 (calculated as percentage differences). This analysis was conducted for MPR vs. PR/SPR together (i.e., MPR vs. nursery-reared, or NR) owing to small samples sizes in the two NR groups.
To examine the extent to which individual HPA axis activity and/or regulation influences adult rank, owing to small samples sizes Spearman correlations were run for each rearing group separately to determine whether individuals with higher HCC values in adulthood or juvenility (to assess HPA axis activity), or with higher increases in HCCs across juvenility (to assess HPA axis reactivity), were associated with relative rank. A Bonferroni correction for multiple comparisons was made, resulting in an adjusted p ≤ 0.01.
IBM SPSS v.22 was used for all analyses, and an α < 0.05 was considered statistically significant.
Results
Early life history and adult social rank
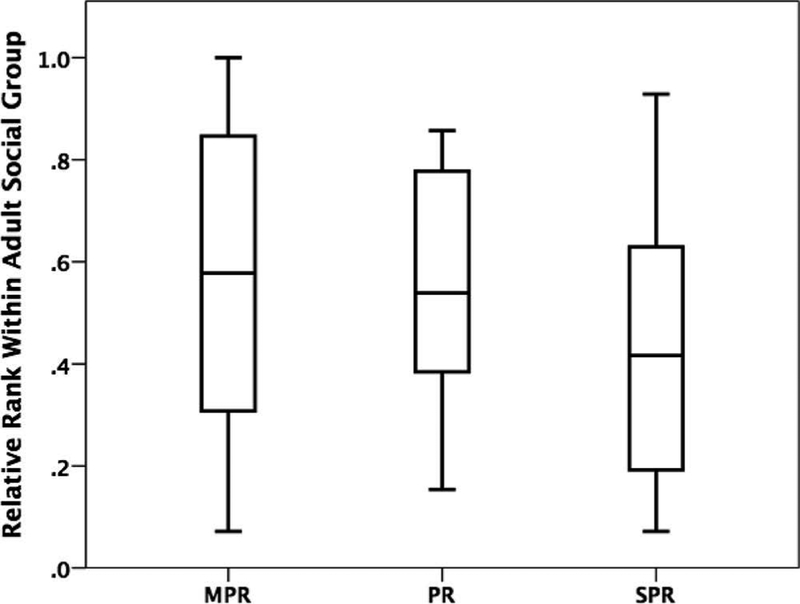
Regression analysis revealed that early rearing experience predicted adult social rank, such that MPR monkeys outranked PR and SPR monkeys, and PR monkeys outranked SPR monkeys (β = −0.067, t(89) = −2.03,p = 0.046; Figure 1). Early rearing also explained a significant proportion of variance in adult social rank (R2 = 0.05, F(1,85) = 4.11, p = 0.046).
Figure 1.
Relative rank within adult social groups for mother-peer-reared (MPR), peer-reared (PR), and surrogate-peer-reared (SPR) female rhesus monkeys (β = −0.067, t(89) = −2.03, p = 0.046).
Early life history and hair cortisol concentrations
Results from the t-tests revealed that the rearing groups (MPR and NR) differed in HCCs taken at 18 months (t(24) = −2.99, p = 0.008). No rearing group differences were revealed at any other time point in juvenility or adulthood. The t-tests did not reveal any rearing group differences (MPR vs. NR) for changes in HCCs across the juvenile period.
Adult hair cortisol and adult social rank
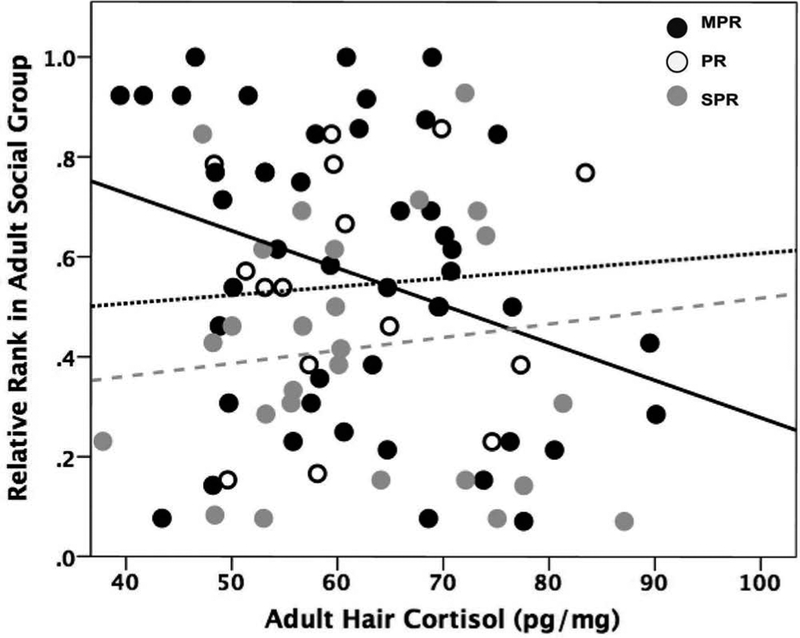
Spearman correlations revealed that, for MPR monkeys only, adult HCCs were negatively correlated with adult social rank (r(s) = −0.327, p = 0.03, n = 43; Figure 2). No such relation was evident for PR (r(s) = 0.288, p = 0.30, n = 15) or SPR monkeys (r(s) = −0.156, p = 0.45, n = 26).
Figure 2.
For mother-peer-reared (MPR) monkeys only (black circles), adult hair cortisol concentrations (HCCs) were negatively correlated with contemporaneous social rank (r(s) = −0.327, p = 0.03). Adult HCCs were not associated with rank in peer-reared (PR, white circles), or surrogate-peer-reared (SPR, gray circles) monkeys.
Juvenile hair cortisol and adult social rank
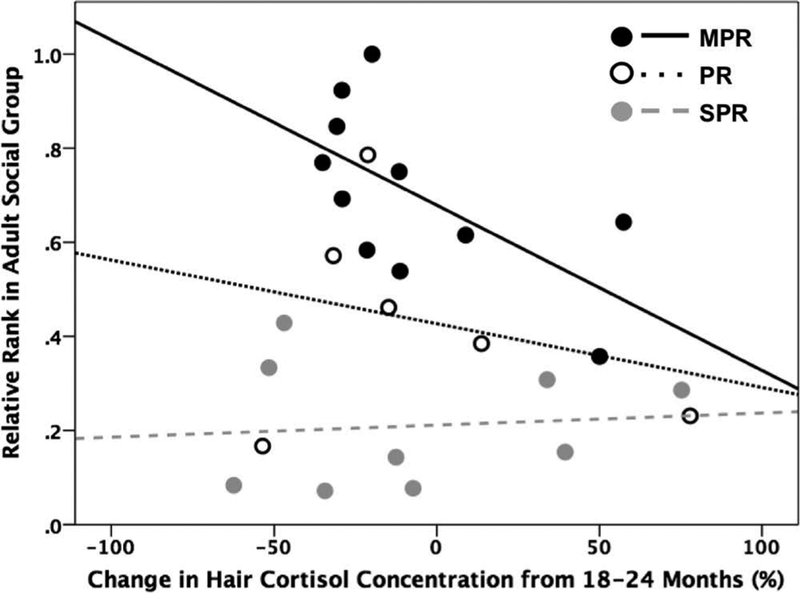
For MPR monkeys only, the change in HCCs from 18 to 24 months was significantly negatively correlated with adult social rank (r(s) = −0.627, p = 0.039, n = 11; Figure 3; Table 2). However, when Bonferroni corrections for multiple comparisons were made, these results did not reach significance with the adjusted p ≤ 0.01. No other significant correlations were found with any other changes in HCCs, or with any HCCs taken at specific time points (i.e., 12, 18, and 24 months).
Figure 3.
For mother-peer-reared (MPR) monkeys only, changes in hair cortisol concentrations (HCCs) in the juvenile period (18–24 months) tended to negatively correlated with adult social rank (r(s) = −0.627, p = 0.039). A subset of individuals (n = 26) had hair samples available during the juvenile period.
Table 2.
Correlations between hair cortisol concentrations (HCCs) and adult rank for mother-peer-reared (MPR) and nursery-reared (NR) monkeys.
| Rearing condition | Adult rank (na) | p-value |
|---|---|---|
| MPR | ||
| Adult HCCs | −0.327 (43) | 0.037 |
| Juvenile HCCs | ||
| 12 months | −0.518 (11) | 0.102 |
| 18 months | −0.227 (11) | 0.502 |
| 24 months | −0.573 (11) | 0.06 |
| Δ Juvenile HCCs | ||
| 12–18 months | 0.545 (11) | 0.08 |
| 18–24 months | −0.627 (11) | 0.039* |
| NR | ||
| Adult HCCsb | PR: 0.288 (15) | 0.30 |
| SPR: −0.156 (26) | 0.45 | |
| Juvenile HCCs | ||
| 12 months | 0.204 (14) | 0.483 |
| 18 months | 0.011 (15) | 0.970 |
| 24 months | 0.05 (15) | 0.860 |
| Δ Juvenile HCCs | ||
| 12–18 months | −0.341 (14) | 0.233 |
| 18–24 months | −0.007 (15) | 0.980 |
n = sample size for each correlation.
For adult HCCs, NR rearing conditions were examined separately:PR = peer-reared; SPR = surrogate-peer-reared.
After Bonferroni correction for multiple corrections, the correlation was not significant (p ≤ 0.01).
No significant correlations were evident between HCCs, or changes in HCCs, at any time in the juvenile period and adult social rank for NR monkeys (all r(s) values ranged between −0.34 and 0.20; all p-values ranged between 0.23 and 0.98; NR n = 15). The same was true when PR/SPR were examined separately (PR n = 6, SPR n = 9; Figure 3).
Discussion
To our knowledge, this is the first study in any primate linking early life adversity, chronic HPA axis activity, and adult social rank. Relying on nonhuman primate models of development, we studied female rhesus monkeys in a cross-sectional design across the lifespan and found that exposure to early life adversity in the form of nursery-rearing resulted in lower social status well into adulthood. Importantly, this is also the first study to show that early rearing predicts adult social rank from sexual maturity (3 years) through middle age (9–11 years), extending previous findings linking early rearing with juvenile and young adult social rank (Bastian et al., 2003). These findings indicate that early life experiences exert lifelong impacts on one’s social standing, which may have important implications for social status-related health outcomes.
We also identified a first link between chronic HPA axis activity, measured via HCCs, and adult social rank, but this relationship differed for MPR and PR/SPR monkeys. Only MPR monkeys showed a negative correlation between adult HCCs and contemporaneous social rank. They also tended to show negative correlations between changes in HCCs in the juvenile period (18–24 months) and adult social rank. However, these results should be interpreted with caution given the small sample size in these analyses, and future studies should aim to replicate these findings with larger cohorts. These results suggest that HPA axis activity/regulation beginning in adolescence, and possibly persisting into adulthood, may influence social rank later in life. As a consequence of their normative early experience, MPR monkeys likely experienced social buffering during a critical window of development that established HPA axis set points for this group that differ for those of NR monkeys (Gunnar, 2003). It is also possible that stress sensitivity conferred by nursery-rearing results in both hyperactivity of the HPA axis and behavioral responsivity to novelty or stress during adolescence that later influence adult rank. This notion is supported by the literature in numerous mammals (see Hennessy et al., 2009 for a review), and is especially supported by findings from Winslow et al. (2003) demonstrating that MPR monkeys exhibit more appropriate behavioral and neuroendocrine responses to novelty than NR monkeys. Future studies examining HPA axis reactivity, social behavior, and rank changes in the same individuals continually from infancy to adulthood are warranted to test this hypothesis directly.
Why might HPA axis regulation during the juvenile period in particular play such an important role in later social competence? It is possible that since the period of 18–24 months is a time of increasing agonistic behavior, particularly amongst juvenile females (Bernstein & Ehardt, 1985, 1986), NR monkeys—who have not witnessed typical adult social interactions—may take longer than MPR monkeys to figure out both the social dynamics and the regulation of stress during formation of dominance hierarchies. It is also possible that individual temperament, which may influence later social rank, influences HPA axis activity and regulation. However, the current study does not have the data to explore this hypothesis; such studies would add important information to current knowledge in the field. Another explanation is that the brain expression of receptors to which glucocorticoids bind during baseline and stressful conditions has been altered by early life experience (Hostinar, Sullivan, & Gunnar, 2014; Oitzl, Champagne, van der Veen, & de Kloet, 2010). Thus, by the time they are juveniles, NR monkeys exhibit an imbalance in glucocorticoid (GR) and mineralocorticoid receptors (MR) that put them at risk for ineffective regulation of stress responses, including behavioral responses (Oitzl et al., 2010). It is well established that epigenetic changes in the expression of MR:GR are induced by early life experiences in rodents (Meaney & Szyf, 2005; Oitzl et al., 2010), but future studies are necessary to elucidate similar mechanisms in macaques.
Our findings are consistent with studies of stable social groups of adult macaques showing that subordinate individuals exhibit higher short-term cortisol levels (i.e., plasma or urinary cortisol), especially when subjected to higher rates of social stressors (Abbott et al., 2003; Gust, Gordon, Hambright, & Wilson, 1993), such as the increased aggression recorded for low-ranking monkeys in the present study. That no relationship between HCCs and adult social rank was found for any NR monkeys indicates a dissociation between neuroendocrine stress regulation and adult social competence that appears to be present in MPR monkeys. However, the small sample sizes for the juvenile HCCs warrant further investigation of this potential mechanism. It is possible that, if housed in an iso-rearing group after relocation, NR monkeys may have exhibited a relationship between HCCs and social rank in the adult group, absent MR monkeys being present. The current study could not address this possibility, but future work will be important in elucidating the influences that MR monkeys exert on juvenile and adult social ranks.
Our current results showing increased HCCs in SPR monkeys at 18 months of age partially replicate our previous findings of higher HCCs in both SPR and PR monkeys, compared to MPR monkeys, at this age (Dettmer et al., 2012). The fact that in the present study, PR monkeys exhibited intermediary HCC values between SPR and MPR monkeys underscores the necessity of studying individual differences in stress responsivity (particularly as it relates to later behavior). It is possible that subtle individual variations in HCCs across puberty are present for different rearing groups across different cohorts, although the sample size in the present study was too small to test this hypothesis directly. Nevertheless, our collective findings (the present study and those of Dettmer et al., 2012) suggest not only early maternal contact, but the amount of exposure to peers early in life, may be a key factor in the regulation of long-term HPA axis activity.
One limitation of our study is that we only examined female rhesus monkeys, and our results may not be applicable to male primates. Clearly, research with larger, mixed-sex social groups is needed, though most primate research programs that employ nursery rearing do not maintain male monkeys beyond adolescence due to the high demand for these animals in biomedical research. Another limitation is the small sample sizes for the juvenile hair cortisol data. We are currently adding to this data set and will be able to determine whether the findings here are replicable in the near future, although it is equally important for these findings to be replicated in other primate facilities that house larger, mixed-sex social groups. Thus, an intriguing opportunity exists for researchers who study naturally occurring variations in maternal care (i.e., neglect, rejection, abuse; see Maestripieri, Hoffman, Anderson, Carter, & Higley, 2009) in large social settings to study chronic HPA axis activity as it relates to later social rank.
The precise mechanisms by which early social experience might influence juvenile and adult HPA axis activity, and adult social rank, are unclear. It is possible that early social experience results in higher chronic cortisol levels for some NR monkeys (Dettmer et al., 2012) owing to these monkeys’ lack of appropriate social behaviors as has been documented previously (Stevens et al., 2009; Suomi, 1997). However, it is also possible that altered HPA axis activity, especially in the juvenile period, makes certain individuals more likely to be hyper-fearful and less likely to be socially competent, and thus more likely to receive aggressive interactions, across development and into adulthood. It is also likely that differences in early caregiving experience are resulting in differential expression of receptors in the brain that bind glucocorticoids (Meaney & Szyf, 2005; Oitzl et al., 2010), thus resulting in differences in physiological and behavioral responses to stress. Future work should increase study population sizes, assess hair samples across the entire developmental period, and examine receptor expression to begin to elucidate these relationships.
Taken together, our findings suggest an intriguing association between early life experience, long-term HPA axis activity/regulation, and adult social status. Each of these variables is known to predict adult health outcomes. Our findings lay the groundwork for future studies examining how the association between these traits puts individuals at risk for physical and mental health disparities later in life, and suggest that nonhuman primates are an important translational model for such studies in humans.
Acknowledgements
We thank Ryan McNeill, Kristen Byers, and Ashley Murphy for assistance with hair sample collection and recordings of dominance interactions. This manuscript resulted from the participation of two of us (AMD and SJS) in the National Science Foundation Symposium, “The Neurodevelopment of Stress Regulation, Social Buffering, and Fear Learning: Integration and Crosstalk,” held in Washington, DC in May 2015 (funded by NSF Grant BSC-1439258 to Dr. Megan Gunnar).
Funding
This work was supported by the Division of Intramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and by the National Institutes of Health [Grant number OD011180].
Footnotes
This work was authored as part of the Contributor’s official duties as an Employee of the United States Government and is therefore a work of the United States Government. In accordance with 17 U.S.C. 105, no copyright protection is available for such works under U.S. Law.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA,Mendoza SP, Saltzman W,…Sapolsky RM (2003). Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior, 43(1), 67–82. doi: 10.1016/S0018-506X(02)00037-5 [DOI] [PubMed] [Google Scholar]
- Altmann J (1974). Observational study of behavior: Sampling methods. Behaviour, 49(3), 227–266. doi: 10.1163/156853974X00534 [DOI] [PubMed] [Google Scholar]
- Andrews MW, & Rosenblum LA (1994). The development of affiliative and agonistic social patterns in differentially reared monkeys. Child Development, 65(5), 1398–1404. doi: 10.2307/1131506 [DOI] [PubMed] [Google Scholar]
- Bahadori M, Sanaeinasab H, Ghanei M, Tavana AM,Ravangard R, & Karamali M (2015). The social determinants of health (SDH) in Iran: A systematic review article. Iranian Journal of Public Health, 44(6), 728. [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam KN, Dittmar K, Berman CM,Butovskaya M, Cooper MA, Majolo B,…De Waal FBM (2012). Hierarchical steepness, counter aggression, and macaque social style scale. American Journal of Primatology, 74(10), 915–925. doi: 10.1002/ajp.2012.74.issue-10 [DOI] [PubMed] [Google Scholar]
- Bastian ML, Sponberg AC, Suomi SJ, & Higley JD (2003). Long-term effects of infant rearing condition on the acquisition of dominance rank in juvenile and adult rhesus macaques (Macaca mulatta). Developmental Psychobiology, 42(1), 44–51. doi: 10.1002/dev.10091 [DOI] [PubMed] [Google Scholar]
- Bernstein IS, & Ehardt C (1986). The influence of kinship and socialization on aggressive behaviour in rhesus monkeys (Macaca mulatta). Animal Behaviour, 34(3), 739–747. doi: 10.1016/S0003-3472(86)80057-4 [DOI] [Google Scholar]
- Bernstein IS, & Ehardt CL (1985). Age-sex differences in the expression of agonistic behavior in rhesus monkey (Macaca mulatta) groups. Journal of Comparative Psychology, 99(2), 115–132. doi: 10.1037/0735-7036.99.2.115 [DOI] [PubMed] [Google Scholar]
- Blomquist GE (2009). Trade-off between age of first reproduction and survival in a female primate. Biology Letters, 5(3), 339–342. doi: 10.1098/rsbl.2009.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang K-Y, Kamboukos D,Fratto C, & Pine DS (2007). Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry, 64(10), 1172–1179. doi: 10.1001/archpsyc.64.10.1172 [DOI] [PubMed] [Google Scholar]
- Browne DT, & Jenkins JM (2012). Health across early childhood and socioeconomic status: Examining the moderating effects of differential parenting. Social Science & Medicine, 74(10), 1622–1629. doi: 10.1016/j.socscimed.2012.01.017 [DOI] [PubMed] [Google Scholar]
- Bush NR, Obradović J, Adler N, & Boyce WT (2011).Kindergarten stressors and cumulative adrenocortical activation: The “first straws” of allostatic load? Development and Psychopathology, 23(04), 1089–1106. doi: 10.1017/S0954579411000514 [DOI] [PubMed] [Google Scholar]
- Chen E, Cohen S, & Miller GE (2010). How low socioeconomic status affects 2 year hormonal trajectories in children. Psychological Science, 21(1), 31–37. doi: 10.1177/0956797609355566 [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, & Baum A (2006). Socioeconomic status is associated with stress hormones. Psychosomatic Medicine, 68(3), 414–420. doi: 10.1097/01.psy.0000221236.37158.b9 [DOI] [PubMed] [Google Scholar]
- Conti G, Hansman C, Heckman JJ, Novak MF, Ruggiero A, & Suomi SJ (2012). Primate evidence on the late health effects of early-life adversity. Proceedings of the National Academy of Sciences, 109(23), 8866–8871. doi: 10.1073/pnas.1205340109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins D (2005). Dominance, status, and social hierarchies In The handbook of evolutionary psychology (pp. 676–697). Hoboken, NJ: Wiley. [Google Scholar]
- Daubenmier J, Kristeller J, Hecht FM, Maninger N,Kuwata M, Jhaveri K,…Epel E (2011). Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: An exploratory randomized controlled study. Journal of Obesity, 2011, 1–13. doi: 10.1155/2011/651936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM (2015). Age at first reproduction in female rhesus monkeys at the LCE field station. Unpublished raw data. [Google Scholar]
- Dettmer AM, Kaburu SS, Byers KL, Murphy AM, Soneson E, Wooddell LJ, & Suomi SJ (2015). First-time rhesus monkey mothers, and mothers of sons, preferentially engage in face-to-face interactions with their infants. American Journal of Primatology. doi: 10.1002/ajp.22503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS, & Suomi SJ (2014).Population density dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology, 42, 59–67. doi: 10.1016/j.psyneuen.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Suomi SJ, & Meyer JS (2012).Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: Hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology, 37(2), 191–199. doi: 10.1016/j.psyneuen.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Rosenberg KL, Suomi SJ, Meyer JS, & Novak MA (2015). Associations between parity, hair hormone profiles during pregnancy and lactation, and infant development in rhesus monkeys (Macaca mulatta). Plos One, 10(7), e0131692. doi: 10.1371/journal.pone.0131692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, Ruggiero AM, Novak MA, Meyer JS, & Suomi SJ (2008). Surrogate mobility and orientation affect the early neurobehavioral development of infant rhesus macaques (Macaca mulatta). Developmental Psychobiology, 50(4), 418–422. doi: 10.1002/(ISSN)1098-2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer AM, & Suomi SJ (2014). Nonhuman primate models of neuropsychiatric disorders: Influences of early rearing, genetics, and epigenetics. ILAR Journal, 55(2), 361–370. doi: 10.1093/ilar/ilu025 [DOI] [PubMed] [Google Scholar]
- DeVries AC, Glasper ER, & Detillion CE (2003). Social modulation of stress responses. Physiology & Behavior, 79(3), 399–407. doi: 10.1016/S0031-9384(03)00152-5 [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau J-P, & Levine S(2008). Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology, 20(3), 845–859. doi: 10.1017/S0954579408000400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrecht M, Hextall J, Kirtley L-G, Taylor A, Dyson M, & Weinman J (2004). Perceived stress and cortisol levels predict speed of wound healing in healthy male adults. Psychoneuroendocrinology, 29(6), 798–809. doi: 10.1016/S0306-4530(03)00144-6 [DOI] [PubMed] [Google Scholar]
- Elo A (1978). The rating of chess players, past and present. New York, NY: Arco. [Google Scholar]
- Fernald LCH, & Gunnar MR (2009). Poverty-alleviation program participation and salivary cortisol in very low-income children. Social Science & Medicine, 68(12), 2180–2189. doi: 10.1016/j.socscimed.2009.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR (2000). Early adversity and the development of stress reactivity and regulation. The Effects of Early Adversity on Neurobehavioral Development, 31, 163–200. [Google Scholar]
- Gunnar MR (2003). Integrating neuroscience and psychological approaches in the study of early experiences. Proceedings of the National Academy of Sciences, 1008, 238–247. doi: 10.1196/annals.1301.024 [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK, & Wilson ME(1993). Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta). Hormones and Behavior, 27(3), 318–331. doi: 10.1006/hbeh.1993.1024 [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, & Farah MJ (2012). Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience, 6.doi: 10.3389/fnhum.2012.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, & Meaney MJ (2010).Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nature Reviews Neuroscience, 11(9), 651–659. doi: 10.1038/nrn2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, & Sachser N (2009). Social buffering of the stress response: Diversity, mechanisms, and functions. Frontiers in Neuroendocrinology, 30(4), 470–482. [DOI] [PubMed] [Google Scholar]
- Hol T, Van den Berg CL, Van Ree JM, & Spruijt BM(1999). Isolation during the play period in infancy decreases adult social interactions in rats. Behavioural Brain Research, 100(1–2), 91–97. doi: 10.1016/S0166-4328(98)00116-8 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014).Psychobiological mechanisms underlying the social buffering of the HPA axis: A review of animal models and human studies across development. Psychological Bulletin, 140(1), 256–282. doi: 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AL, Minnis H, & Wilson P (2011). Altered stress responses in children exposed to early adversity: A systematic review of salivary cortisol studies. Stress, 14(6), 614–626 [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, & Taub DM (1982). Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis, Thrombosis, and Vascular Biology, 2(5), 359–368. doi: 10.1161/01.ATV.2.5.359 [DOI] [PubMed] [Google Scholar]
- Langlois ÉV, Miszkurka M, Zunzunegui MV, Ghaffar A,Ziegler D, & Karp I (2015). Inequities in postnatal care in low-and middle-income countries: A systematic review and meta-analysis. Bulletin of the World Health Organization, 93(4), 259–270G. doi: 10.2471/BLT.14.140996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, & Hertzman C (2007).Life-time socio economic position and cortisol patterns in mid-life. Psychoneuroendocrinology, 32(7), 824–833. doi: 10.1016/j.psyneuen.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Lovallo WR (2013). Early life adversity reduces stress reactivity and enhances impulsive behavior: Implications for health behaviors. International Journal of Psychophysiology, 90(1), 8–16. doi: 10.1016/j.ijpsycho.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecken LJ, & Appelhans BM (2006). Early parental loss and salivary cortisol in young adulthood: The moderating role of family environment. Development and Psychopathology, 18(01), 295–308. doi: 10.1017/S0954579406060160 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, & McEwen BS (2001).Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Development and Psychopathology, 13(3), 653–676. doi: 10.1017/S0954579401003133 [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Hoffman CL, Anderson GM, Carter CS, & Higley JD (2009). Mother–infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiology & Behavior, 96(4–5), 613–619. doi: 10.1016/j.physbeh.2008.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall IJ, Wang Y, Crichton S, McKevitt C, Rudd AG, & Wolfe CD (2015). The effects of socioeconomic status on stroke risk and outcomes. The Lancet Neurology, 14(12), 1206–1218. doi: 10.1016/S1474-4422(15)00200-8 [DOI] [PubMed] [Google Scholar]
- McCormack K, Sanchez MM, Bardi M, & Maestripieri D(2006). Maternal care patterns and behavioral development of rhesus macaque abused infants in the first 6 months of life. Developmental Psychobiology, 48(7), 537–550. doi: 10.1002/(ISSN)1098-2302 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2003). Early life influences on life-long patterns of behavior and health. Mental Retardation and Developmental Disabilities Research Reviews, 9(3), 149–154. doi: 10.1002/(ISSN)1098-2779 [DOI] [PubMed] [Google Scholar]
- McEwen BS (2008). Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology, 583(2–3), 174–185. doi: 10.1016/j.ejphar.2007.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney CH, Antoni MH, Kumar M, Tims FC, & McCabe PM (1997). Effects of guided imagery and music (GIM) therapy on mood and cortisol in healthy adults. Health Psychology, 16(4), 390–400. doi: 10.1037/0278-6133.16.4.390 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Breslau J, Green JG, Lakoma MD,Sampson NA, Zaslavsky AM, & Kessler RC (2011). Childhood socio-economic status and the onset, persistence, and severity of DSM-IV mental disorders in a US national sample. Social Science & Medicine, 73(7), 1088–1096. doi: 10.1016/j.socscimed.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, & Szyf M (2005). Environmental programming of stress responses through DNA methylation: Life at the interface between a dynamic environment and a fixed genome. Dialogues in Clinical Neuroscience, 7(2), 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, & Novak MA (2012). Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153(9), 4120–4127. doi: 10.1210/en.2012-1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137(6), 959–997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez JK, & Hayward MD (2014). Cumulative childhood adversity, educational attainment, and active life expectancy among US adults. Demography, 51(2), 413–435. doi: 10.1007/s13524-013-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics (US). (2012). Health, United States, 2011: With special feature on socioeconomic status and health. Hyattsville, MD: Author. [PubMed] [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M,…Engelhardt A (2011). Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour, 82(4), 911–921. doi: 10.1016/j.anbehav.2011.07.016 [DOI] [Google Scholar]
- Novak MA, Hamel AF, Kelly BJ, Dettmer AM, & Meyer JS (2013). Stress the HPA axis, and nonhuman primate well-being: A review. Applied Animal Behaviour Science, 143 (2–4), 135–149. doi: 10.1016/j.applanim.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, Champagne DL, van der Veen R, & de Kloet ER (2010). Brain development under stress: Hypotheses of glucocorticoid actions revisited. Neuroscience & Biobehavioral Reviews, 34(6), 853–866. doi: 10.1016/j.neubiorev.2009.07.006 [DOI] [PubMed] [Google Scholar]
- Palmer FB, Anand KJ, Graff JC, Murphy LE, Qu Y,Völgyi E,…Tylavsky FA (2013). Early adversity, socio-emotional development, and stress in urban 1-year-old children. The Journal of Pediatrics, 163(6), 1733–1739. doi: 10.1016/j.jpeds.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Power C, & Hertzman C (1997). Social and biological pathways linking early life and adult disease. British Medical Bulletin, 53(1), 210–221. doi: 10.1093/oxfordjournals.bmb.a011601 [DOI] [PubMed] [Google Scholar]
- Rawlins RG, & Kessler MJ (1986). The Cayo Santiago macaques:History, behavior, and biology. Albany, NY: SUNY Press. [Google Scholar]
- Russell E, Koren G, Rieder M, & Van Uum S (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37(5), 589–601. doi: 10.1016/j.psyneuen.2011.09.009 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2004). Social status and health in humans and other animals. Annual Review of Anthropology, 33, 393–418. doi: 10.1146/annurev.anthro.33.070203.144000 [DOI] [Google Scholar]
- Sapolsky RM (2005). The influence of social hierarchy on primate health. Science, 308(5722), 648–652. doi: 10.1126/science.1106477 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Alberts SC, & Altmann J (1997).Hypercortisolism associated with social subordinance or social isolation among wild baboons. Archives of General Psychiatry, 54(12), 1137–1143. doi: 10.1001/archpsyc.1997.01830240097014 [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Friedman HS, Tucker JS, Tomlinson-Keasey C, Wingard DL, & Criqui MH (1995). Sociodemographic and psychosocial factors in childhood as predictors of adult mortality. American Journal of Public Health, 85(9), 1237–1245. doi: 10.2105/AJPH.85.9.1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Champoux M, & Suomi SJ (1998). Rearing condition and plasma cortisol in rhesus monkey infants. American Journal of Primatology, 46(4), 311–321. doi: 10.1002/(ISSN)1098-2345 [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, & Anton RF (1997). Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry, 41(8), 871–882. doi: 10.1016/S0006-3223(96)00185-0 [DOI] [PubMed] [Google Scholar]
- Smith GD (1996). Down at heart–the meaning and implications of social inequalities in cardiovascular disease. Journal of the Royal College of Physicians of London, 31 (4), 414–424. [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Hart C, Blane D, Gillis C, & Hawthorne V (1997). Lifetime socioeconomic position and mortality: Prospective observational study. Bmj, 314(7080), 547. doi: 10.1136/bmj.314.7080.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman WP, Hunt LE, McGinnis LM, & Levine S(1979). Mother-infant separation in group-living rhesus macaques: A hormonal analysis. Developmental Psychobiology, 12(3), 211–217. [DOI] [PubMed] [Google Scholar]
- Stevens HE, Leckman JF, Coplan JD, & Suomi SJ(2009). Risk and resilience: Early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. Journal of the American Academy of Child & Adolescent Psychiatry, 48(2), 114–127. doi: 10.1097/CHI.0b013e318193064c [DOI] [PubMed] [Google Scholar]
- Suomi SJ (1997). Early determinants of behaviour: Evidence from primate studies. British Medical Bulletin, 53(1), 170–184. doi: 10.1093/oxfordjournals.bmb.a011598 [DOI] [PubMed] [Google Scholar]
- Wiener SG, Johnson DF, & Levine S (1987). Influence of postnatal rearing conditions on the response of squirrel monkey infants to brief perturbations in mother-infant relationships. Physiology & Behavior, 39(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Lyons CK, Sterk SM, & Insel TR(2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology, 28(5), 910–918. [DOI] [PubMed] [Google Scholar]
- Wooddell LJ, Kaburu SK, Dettmer AM, & Suomi SJ (2015, November). Elo rating as a tool to measure rank changes and dominance stability in semi free ranging rhesus macaques. American Journal of Primatology, 77 (S1), 80. [Google Scholar]