Abstract
BACKGROUND.
We have previously demonstrated Ang II type 2 (AT2-) receptor-mediated inhibition of EGF-induced prostate cancer cell growth in androgen-dependent (LNCaP) and independent (PC3) prostate cancer cell lines.
METHODS.
To explore the signaling pathways involved in this inhibitory effect, we examined the interaction of the AT2-receptor with its novel regulatory partner ATIP using real time PCR, over-expression, siRNA and [3H]thymidine incorporation assays.
RESULTS.
The results in human prostate cancer cell lines demonstrate the presence of ATIP in both cell lines examined, and suggest that (i) the AT2-receptor through an interaction with ATIP mediates an anti-growth factor effect in both androgen-dependent and androgen-independent cell lines; (ii) ATIP expression decreases as the rate of cell growth and androgen-independence increase; and (iii) EGF may act on cell growth in part by reducing the content of ATIP present in the cells.
CONCLUSIONS.
The results support our earlier proposal in normal cell lines that ATIP is an important component of the cellular response to AT2-receptor activation. The results further suggest that a critical level of ATIP is required to mediate the effect of AT2-receptor activation to inhibit EGF mediated increases in cell growth. They also suggest that EGF may in part induce cell growth by suppressing the level of ATIP expression.
Keywords: AT2-receptor, AT2-receptor interacting protein, prostate cancer cell line, epidermal growth factor
INTRODUCTION
The renin-angiotensin system (RAS) has important regulatory actions on growth factors and cell growth. There is emerging evidence that the incidence of cancer is reduced in patients undergoing long-term treatment with drugs that inhibit the RAS [1–6], with reports suggesting that Ang II directly stimulates cell growth via the AT1-receptor and that ACE inhibition or AT1-receptor blockade inhibits the growth of a range of tumors including prostate cancer [1–9].
All components of the RAS have been identified in the prostate [8,10–15] including Ang II, which has been localized to the basal epithelial cells in normal human prostate and to malignant epithelial cells in prostate cancer biopsies [10]. More recently, we demonstrated the existence of functional AT2-receptors, which inhibit the proliferative effects of EGF on DNA synthesis and MAPK phosphorylation, in both early stage, androgen-dependent, LNCaP, and late stage, androgen-independent, PC3 prostate cancer cell lines [7]. Therefore, it is possible that the reported anti-proliferative action of AT1-receptor blockers in prostate cancer [8,16], is not due solely to the blockade of AT1-receptor stimulation, but may also be in part attributable to endogenous Ang II selectively activating the AT2-receptor in the presence of AT1-blockade.
The signaling pathways of the AT1-receptor are well characterized [17–19], but the same cannot be said for the AT2-receptor. Although it is a member of the G-protein coupled receptor super-family, the AT2-receptor induces atypical signal transduction path- ways that include at least three intracellular cascades involving activation of protein phosphatases, regulation of nitric oxide and stimulation of phospholipase A2 [20]. Other unconventional AT2-receptor signaling pathways have also been identified and involve direct interaction with scaffolding or transducing molecules, such as ErbB3 [21] or PLZF [22].
Recently, a novel AT2-receptor interacting protein (ATIP) has been identified [23], also known as MTUS1, MTSG1, GK1, and ATBP50 [24–26], which interacts with the C-terminal tail of the AT2-receptor [23,26–28]. Five different ATIP isoforms have been identified, numbered 1, 2, 3A, 3B, and 4 and are derived from a single gene by alternate promoter utilization and exon/ intron splicing [23]. All share a common 118 amino acid AT2-receptor interacting domain (ID), suggesting that all 5 ATIP isoforms may interact with the AT2-receptor [27]. The ID forms part of a larger 395 amino acid homologous region, a sequence that is common to all ATIP members, which will be referred to as ATIP in this report. Transfection of either ATIP1 or ATIP-ID into CHO cells expressing the AT2-receptor inhibited the effects of bFGF and insulin on ERK2 phosphorylation in these cells [23] and transfection of MTSG1 (i.e., ATIP1) slowed the growth of pancreatic cancer cell lines [25].
Following our demonstration that the AT2-receptor has an inhibitory role in the growth of prostate cancer cell lines [7] we hypothesized that this involved an interaction with EGF. We report studies of the interaction between EGF, Ang II, ATIP and the AT2-receptor in controlling cell growth in the LNCaP and PC3 prostate cancer cell lines.
MATERIALS ANDMETHODS
Cell Culture
Two human prostate cancer cell lines, PC3 [29] and LNCaP [30], were grown in RPMI 1640 media supplemented with 2 mM glutamine and 10% FCS, and were maintained at 378C in a humidified atmosphere of 5% CO2/95% air.
ATIP mRNA Expression in LNCaP and PC3 Cells
Primers and TaqMan probes for ATIP, which identify all 5 ATIP isoforms, and ATIP1, were designed by Ms Josefa Pete (Baker Institute, Australia) with the help of Primer Express v2.0 (Applied Biosystems, CA).
LNCaP and PC3 cells were cultured in 12-well plates at 4 × 105 and 1 × 105 cells/well, respectively, in RPMI 1640 supplemented with 10% FCS and 2 mM L-glutamine and incubated overnight at 378C/5% CO2. As the cells had differing rates of growth different numbers of each were cultured so that the cells were of a similar level of confluence the following day. The cells were then approximately 70% confluent and hence assumed to be growing at an exponential rate. Total RNA was then isolated from the cells as previously described [7]. Samples of cDNA (n = 10–17) were analyzed using an Applied Biosystems 7500 Real-time PCR system (Applied Biosystems) according to our previously published method using VIC labeled 18sRNA (Applied Biosystems) as an internal control [7].
The sequences for the QPCR probes and primers used are given in Table I. It must be noted that an abnormal amplification profile was identified with the ATIP probe for cDNA generated from PC3 cells. On sequencing the ATIP probe and primer annealing site, a single point mutation in the ATIP probe binding site was found (this mutation did not affect the amino acid composition of ATIP in this cell line). However, for consistency in determining ATIP expression a new probe was synthesized that accounted for this dif- ference (Table I).
TABLE I.
Sequences of the Primers and Probes Used in Real-Time PCR
| Gene | Sequence (5′−3′) |
|---|---|
| ATIP | |
| Probe | CTCCGCCATCCCTa (for PC3 cells CTCTGCCATCCCT) |
| Forward | CCCAAGAGATCCCCCACAT |
| Reverse | GCCCGAATTCCTTGGTGACT |
| ATIP1 | |
| Probe | TGTCTCCCAAATTC |
| Forward | ACCTGCTCCGAAGACATGTTG |
| Reverse | GTCGTATGTGAATGGTGGATAAGG |
We identified a single point mutation in the ATIP probe annealing site in PC3 cells therefore a new probe that allowed for this mutation was designed for PC3 cell cDNA.
ATIP and ATIP1 mRNA Regulation in Prostate Cancer Cell Lines
LNCaP and PC3 cells were cultured in 12-well plates at 2 × 105 and 1 × 105 cells/well, respectively, in RPMI 1640 supplemented with 10% FCS + 2 mM L-glutamine. The following day, cells were treated in the presence or absence of a range of doses of Ang II (100 nM to 1 μM), a non-selective AT-receptor agonist, and EGF (0.1–10 ng/ml) concentrations. Treatments were diluted in RPMI 1640 supplemented with 5% charcoal-stripped FCS for LNCaP and 5% charcoal-stripped FCS and 2 mM L-glutamine for PC3 cells. At 72 hr post- treatment, RNA was extracted from cells and converted to cDNA; real-time PCR was performed for the gene of interest.
ATIP siRNA Knock-Down in LNCaP and PC3 Cells
Small interfering RNA (siRNA) for ATIP was purchased from Qiagen (MD). The ATIP siRNA duplex was a 19mer and the sense and antisense sequences contained 3′ terminal dTdT and dGdA overhangs respectively (Table II).
TABLE II.
Sequences of the Negative Control and ATIP RNA Interference Duplexes
| Gene | Sequence (5′−3′) |
|---|---|
| ATIP | |
| Target | TCC GTA TTA CGT GAC CGG CAA |
| Antisense | CGU AUU ACG UGA CCG GCA A dTdT |
| Sense | UUG CCG GUC ACG AA UAC dGdA |
| Negative control (modified with Alexa Fluor 488) | |
| Target | AAT TCT CCG AAC GTG TCA CGT |
| Antisense | UUC UCC GAA CGY GUC ACG U dTdT |
| Sense | ACG UGA CAC GUU CGG AGA A dTdT |
Preliminary studies showed that knock-down was maintained for at least 72 hr post-transfection (data not shown). The day before transfection, cells were plated in 12-well plates at 1 × 105 cells/well in RPMI 1640 supplemented with 10% FCS and 2 mM L-glutamine. Cells were transfected with either 5 nM ATIP siRNA or negative control (Alexa Fluor) probes (Qiagen), using HiPerfect™ transfection reagent (Qiagen) in RPMI 1640 supplemented with 10% FCS 2 mM L-glutamine, according to the manufacturer’s instructions. Cells were incubated in 378C/5% CO2 for up to 72 hr, RNA was isolated and converted to cDNA, and protein lysates were prepared [7]. In addition, 72 hr post- transfection, images of the transfected cells were taken using a Nikon Eclipse TE2000-E inverted microscope (Nikon Corporation, Tokyo, Japan) and NIS-Elements Advanced Research (Version 2.1) (Nikon Corporation) at 100 × magnification. Real-time PCR was then performed using ATIP probe/primers to determine the level of RNA knock-down.
The morphology of ATIP-silenced prostate cancer cells was investigated to determine whether ATIP is crucial for cell adhesion, development, and survival. Transient transfection of ATIP siRNA had little effect on the morphology of any of the three prostate cancer cell lines (data not shown).
[3H]Thymidine Incorporation Assay in ATIP siRNA Transfected Cells
Cells were cultured in six-well plates at 3.5 × 105 cells/well in RPMI 1640 supplemented with 10% FCS and 2 mM L-glutamine, and incubated overnight in 378C/5%CO2. The following day the cells were transfected with ATIP siRNA or negative control siRNA probe using Hiperfect according to the manufacturer’s instructions. Twenty four hours post- transfection, cells were detached and aliquoted into 48-well plates at a density of 1.5 × 104 in RPMI 1640 supplemented with 1% FCS and 2 mM L-glutamine, and then incubated overnight at 378C/5% CO2. The following day, the cells were treated in the presence or absence of a range of CGP42112A, an AT2-receptor specific agonist (Sigma–Aldrich Pty Ltd4, Australia), Ang II (0.1–1 μM) and EGF (0.1–10 ng/ml) concentrations. At 96 hr post-treatment, which had been established in earlier experiments [7] as the minimum time necessary to identify differences in thymidine incorporation, 2 μCi [3H]thymidine was added per well. After 6 hr, media were aspirated and cells washed with ice-cold PBS, then lysed in 0.5 M NaOH and 0.5% SDS and transferred into 24-well beta counter plates. Following addition of 200 μl Optiphase Supermix scintillant (PerkinElmer, USA), plates were counted for 2 min on a Wallac Hilux 1450 Microbeta counter (PerkinElmer). Preliminary studies (data not shown) indicated that the effects of Ang II and EGF plateaued at 500 nM and 10 ng/ml respectively [7], therefore, all subsequent studies were conducted at these concentrations. Data are expressed as mean and standard error of the mean of 4–6 independent experiments.
Studies of ERK2 Phosphorylation in PC3 Cells Transiently Over-Expressing ATIP1
In the PC3 cell line the [3H]thymidine incorporation assay is not overly sensitive and in these and our previous studies [7] we have had to incubate cells for a minimum of 96 hr to obtain robust and statistically significant differences between the treatment groups. In preliminary studies examining the transient transfection of ATIP1 we identified that high levels of ATIP1 over-expression were maintained for a maximum of 48 hr post-transfection (data not shown) and by 72 hr ATIP1 expression had returned to basal levels. Under these conditions it was not possible to obtain meaningful differences in [3H]thymidine incorporation and we proceeded to use the more sensitive ERK2 phosphorylation assay [7] to measure the activation of cell growth pathways in the transiently ATIP1 over- expressing PC3 cells.
The day prior to transfection PC3 cells were cultured in four 6-well plates at 1 × 105 cells/well in RPMI 1640 supplemented with 10% FCS and 2 mM L-glutamine, and incubated overnight in 37°C/5% CO2.
The following day PC3 cells were transfected with 1 mg of a pcDNA3 construct containing human ATIP1 [23], or an empty pcDNA3 vector as a negative control, using Lipofectin® (Invitrogen, CA) according to the manufacturer’s instructions.
Twenty four hours post-transfection, cells were starved overnight in serum-free RPMI 1640 and the following morning (approximately 42 hr post-transfection) mRNA was generated from two 6-well plates to identify the level of ATIP1 mRNA expression in the ATIP1 transfected cells compared to the pcDNA3 cells using real-time PCR techniques as described above.
In the remaining two plates, ERK2 phosphorylation was examined using our previously described method [7]. Briefly, ATIP1 and pcDNA3 transfected cells were stimulated for 5 min in the presence or absence of 10 ng/ml EGF, 500 nM CGP42112A and 1 μM Ang II. Cells were washed twice and lysed in ice-cold buffer consisting of 100 mM DTT, 40% glycerol, 250 mM Tris HCl (pH 6.8), 1 mM sodium-orthovanadate and 1.6% SDS. Samples were denatured at 95°C for 5 min and then separated on a 10% (w/v)-polyacrylamide gel containing 0.66% (w/v) bisacrylamide using the Bio- Rad Mini Protean II Cell System, and transferred onto nitrocellulose Hybond™ paper using the Mini Trans-Blot Electrophoretic Transfer Cell System. Membrane blots were then incubated with a 1:2,000 dilution of anti-diphospho ERK-1 and 2 antibody (Sigma–Aldrich Pty Ltd) overnight at 4°C and bands were visualized with an enhanced chemiluminescence kit (ECL Western blotting analysis system, Amersham Pharmacia Biotech UK Ltd, UK). Membrane blots were stripped and rehybridized with an anti-ERK2 antibody (Sigma–Aldrich Pty Ltd) as a control for protein added. Band intensities were measured using the Histogram function in Adobe Photoshop CS Version 8.0. The mean ratio of phospho-ERK2 to ERK2 was generated for 6–8 separate experiments.
Statistical Analysis
Real-time PCR, [3H]thymidine incorporation and ERK2 phosphorylation results were analyzed for statistical significance by ANOVA or Student’s t-test using GraphPad InStat® v3.06 (GraphPad Softwares, Inc., CA). Data are expressed as the mean ± SEM. The level of significance was set at P < 0.05, P < 0.01, and P < 0.001.
RESULTS
ATIP and ATIP1 Expression in Prostate Cancer Cell Lines
ATIP and ATIP1 mRNA expression was identified in both androgen-dependent LNCaP and androgen-independent PC3 cells. When cells grown under the same conditions were approximately 70% confluent, ATIP mRNA expression was significantly higher (30-fold) in LNCaP cells than in PC3 cells (Table III). Similarly, ATIP1 mRNA expression was significantly higher in LNCaP than PC3 cells, however, the magnitude of the difference in expression was lower (approximately 4.6-fold). Doubling time was more than 2-fold longer in LNCaP than PC3 cells suggesting that an inverse correlation existed between the rate-of-growth and the level of ATIP mRNA expression (Table III).
TABLE III.
ATIP and ATIP1 mRNA Expression in Prostate Cancer Cells, Which Were 70% Confluent at the Time of Harvesting, Grown Under Identical Conditions; N =10 – 17 Independent Samples
| Cell line | ΔCt | Doubling time (hr) | |
|---|---|---|---|
| ATIP | ATIP1 | ||
| LNCaP | 15.6 ± 0.4 | 18.7 ± 0.2 | 46 |
| PC3 | 20.5 ± 0.5 (30-fold)a | 20.9 ± 0.1 (4.6-fold)a | 22 |
Values are expressed as ΔCt expression. Values in brackets represent the fold changes in expression relative to LNCaP.
Denotes that there is significantly lower expression in PC3 cells compared to LNCaP cells (P < 0.001). Each value represents the mean.± SEM of 10–17 independent values.
Regulation of ATIP Expression by EGF and Angiotensin II
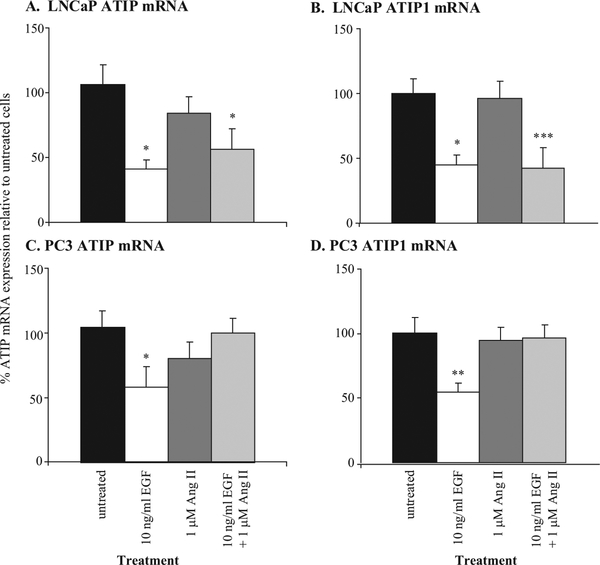
The effects on ATIP and ATIP1 mRNA expression of treating LNCaP and PC3 cells with 1 μM Ang II and/or 10 ng/ml EGF are summarized in Figure 1. Treatment with Ang II reduced ATIP mRNA expression 22% and 25% and ATIP1 mRNA expression 4%and 6% in LNCaP and PC3 cells, respectively. These decreases did not reach statistical significance.
Fig. 1.
Effects of EGF and Ang II on (A) ATIP and (B) ATIP1 mRNA expression in LNCaP prostate cancer cells and (C) ATIP and (D) ATIP1 mRNA expression in PC3 cells grown to approximately 70% confluence.Values are expressed as % fold difference relative to untreated (control) expression (100%).*,**,*** denote a significant decrease in ATIP mRNA expression in treated compared to untreated cells at P < 0.05, P < 0.01, and P < 0.001, respectively. Statistics were calculated using parametric ANOVAs with Tukey’spost-tests on ΔCt values from seven to nine independent determinations.
Treatment with EGF significantly decreased ATIP and ATIP1 mRNA expression by 55% and 65% in LNCaP cells and 45% and 47% in PC3 cells (P < 0.05, parametric ANOVA with Tukey’s post-test). When given in combination with EGF, Ang II did not significantly modify EGF-induced down-regulation of ATIP and ATIP1 mRNA in LNCaP cells, where it acts predominantly via AT1-receptors, whereas it blocked the ability of EGF to down-regulate ATIP and ATIP1 mRNA expression in PC3 cells, where its predominant action is via AT2-receptors [7].
Functional Consequences of Changes in ATIP Expression
Transient ATIP knock-down in LNCaP cells.
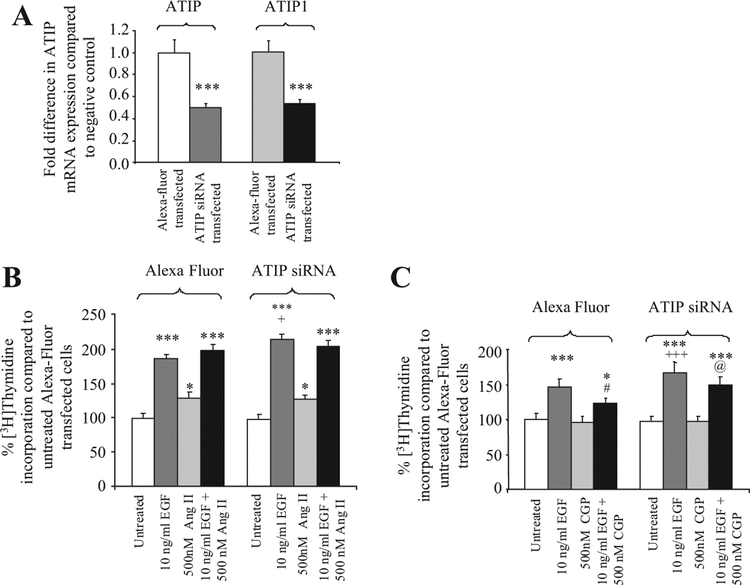
ATIP and ATIP1 mRNA expression was significantly downregulated by 50% and 47%, respectively (P < 0.001) in LNCaP cells transfected with siRNA specific for ATIP (Fig. 2A). The approximate 50% decrease in ATIP did not affect the basal growth rates of the transiently ATIP- silenced LNCaP cells compared to the negative control transfected cells, nor did it modify the stimulatory effect of Ang II (Fig. 2B) or the lack of effect of the selective AT2-receptor agonist, CGP42112A, on DNA synthesis seen in Alexa-fluor transfected cells (Fig. 2C).
Fig. 2.
A : Fold-decrease in ATIP and ATIP1 mRNA expression following transfection of ATIP siRNA into LNCaP cells.***indicatesa significant decrease in ATIP mRNA expression, P < 0.001. B : % Change in DNA synthesis in LNCaP cells transiently transfected with either ATIP siRNA or Alexa Fluor (negative control, reported as 100%) in the presence or absence of 10 ng/ml EGF and 500 nM Ang II or the combination of EGF and Ang II.C : 10 ng/ml EGF and 500 nM CGP42112A or the combination of EGF and CGP42112A for 72 hr.*and***denote that there are significant decreases in ATIP or ATIP1or increases in DNA synthesis compared to untreated LNCaP cells transfected with the same plasmid (P < 0.05 and 0.001, respectively, repeated-measures ANOVA test with Tukey post-test).+and+++ denote that in cells treated with EGF there is a significant increase in DNA synthesis in cells transfected with siRNA compared to cells transfected with Alexa fluor P < 0.05 and 0.001, respectively.# denotes that there is significantly less DNA synthesis in Alexa-fluor transfected cells treated with the combination of EGF and CGP42112A than in cells treated with EGF P < 0.05.@ indicates that in cells treated with EGF and CGP42112A there is significantly higher DNA synthesis in cells transfected with siRNA compared to cells transfected with Alexa-fluor P < 0.05. Each value represents the mean standard error of the mean of four independent experiments. Statistics were calculated using a repeated measures ANOVA, with aTukey-Kramer Multiple Comparisons Test post-test.
The effect of EGF (10 ng/ml) on DNA synthesis following ATIP knock-down was, however, increased almost 30% (P < 0.05) compared to its effect in Alexa- fluor transfected control cells. Unlike control transfected cells, the increase in DNA synthesis induced by EGF in silenced cells was not significantly affected by co-administration of CGP42112A, indicating that the ability of CGP42112A to inhibit EGF-induced DNA synthesis, which is mediated by AT2-receptors [7], seen in negative control transfected LNCaP cells was lost (Fig. 2C).
Finally, the decrease in ATIP mRNA expression resulting from transfection of ATIP siRNA had no effect on EGF mRNA expression, which was not significantly different from expression in the Alexa Fluor-transfected negative control cells (mean ΔCt ± SEM = 17.4 ± 0.2 and 17.4 ± 0.2, respectively, P 0.76). It also did not affect the expression of mRNA for the AT1-(mean ΔCt ± SEM 25.5 ± 0.4 and 26.1 ± 0.5, respectively, P =0.46 or AT2 –receptor (mean ΔCt ± SEM = 26.2 ± 0.4 and 25.9 ± 0.3, respectively, P =0.44).
Transient ATIP knock-down in PC3 cells.
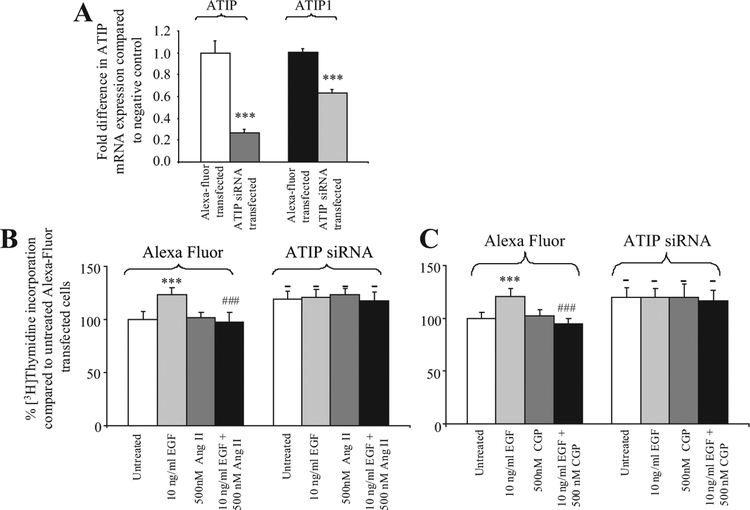
In PC3 cells transfected with siRNA specific for ATIP, both ATIP and ATIP1 mRNA were significantly down- regulated by 73% and 37% (P < 0.001) , respectively (Fig. 3A). In contrast to LNCaP cells, the transient decrease of ATIP expression in PC3 cells resulted in a significant increase in basal [3H]thymidine incorporation, compared to the negative control transfected cells (approximately 20% increase P < 0.01, Fig. 3B,C). However, in the ATIP-silenced PC3 cells 10 ng/ml EGF, unlike its effects in control transfected PC3 cells and LNCaP silenced cells, did not further increase [3H]thymidine incorporation above the elevated levels seen in untreated silenced PC3 cells, suggesting that this pathway may already be fully activated or that higher doses of EGF are required to further stimulate DNA synthesis. Treatment of the silenced cells with 500 nM Ang II (Fig. 3B) or CGP42112A (Fig. 3C) did not modify the effect of ATIP silencing on the increased basal levels of DNA synthesis or the lack of effect of EGF in silenced cells.
Fig. 3.
A : Fold-decreasein ATIP and ATIP1mRNA expression following transfection of ATIP siRNA into PC3 cells.*** indicates a significant decreasein ATIP mRNA expression, P < 0.001.B,C : % Change in DNA synthesis in PC3 cells transiently transfected with either ATIP siRNA or Alexa Fluor (negative control) in the presence or absence of (B) 10 ng/ml EGF and 500 nM Ang II and the combination of EGF and Ang II; or (C) 10 ng/mlEGF and 500 nM CGP42112A and the combination of EGF and CGP42112A for 72 hr.***denotes that there is a significant increase in DNA synthesis compared to untreated PC3 cells transfected with the same plasmid(P < 0.001, repeated measures ANOVA test with Tukey post-test).- denotes that there is a significant difference in DNA synthesis between ATIP silenced and untreated control cells.### denotes that there is significantly less DNA synthesisin Alexa Fluor-transfected cells treated with the combination of EGF and Ang II or EGF and CGP42112A thanin cells treated with EGF P < 0.001.Each value represents the mean + standard error of the mean of four independent experiments.
In PC3, as in LNCaP cells, a decrease in ATIP mRNA expression resulting from transfection of ATIP siRNA was not associated with any significant effect on EGF (mean ΔCt ± SEM = 21.1 ± 0.3 and 20.9 ± 0.3, respectively), AT1- (mean ΔCt ± SEM = 25.5 ± 0.7 and 25.6 ± 0.2, respectively or AT2-receptor (mean ΔCt SEM = 27.1 ± 0.6 and 26.7 ± 0.8, respectively) mRNA) expression compared to transfection of the Alexa Fluor negative control.
Transient over-expression of ATIP1 in PC3 cells.
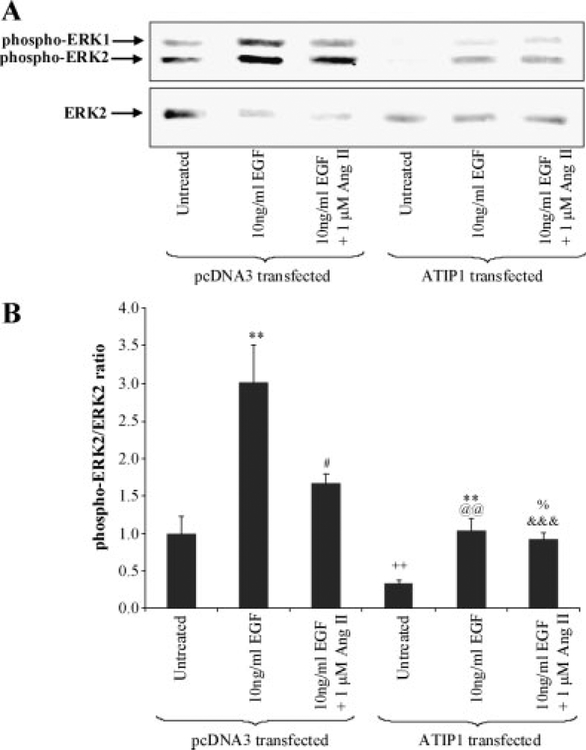
At 48 hr post-transfection, ATIP1 mRNA expression dramatically increased (approximately 130-fold) com- pared to control transfected (pcDNA3) cells (data not shown) and significantly reduced phospho-ERK2 and the phospho-ERK2/unphosphorylated ERK2 ratio, indicating significant inhibition of growth (Fig. 4A).
Fig. 4.
A: ERK1 and ERK2 phosphorylation (phospho-ERK1 and phospho-ERK2, respectively) after 5 min stimulation with the indicated dose of EGF and Ang II in PC3 cells transiently transfected with pcDNA3 or ATIP1 plasmid in a single representative experiment.Unphosphorylated ERK2 (ERK2) in the same nitrocelluloseblot. B : Summary of ERK2 phosphorylation in PC3 cells transfected with either pcDNA3 or pcDNA3-ATIP1 following a 5 min stimulation with EGF alone or in the presence of Ang II. Values are expressed as themean and standard error ofmean ofphospho- ERK2relativeto theamountofunphosphorylatedERK2.**indicates that there is a significant increase in ERK2 phosphorylation in EGF-treated compared to untreated PC3 cells transfected with the same plasmid at P < 0.01 (Friedman Nonparametric Repeated Measures ANOVA with Dunn’s post-test).# denotes that in pcDNA3-transfected PC3 cells ERK2 phosphorylation is significantly less in cells co-administered EGF and Ang II than in cells treated with EGF alone, P < 0.05.++ indicates that there is significantly less ERK2 phosphorylation in untreated ATIP1-transfected than pcDNA3 transfected PC3 cells, P < 0.01.@@ indicates that there is significantly less ERK2 phosphorylation in EGF-treated ATIP1-transfected than EGF-treated pcDNA3 transfected PC3 cells, P < 0.01.% denotes that there is a significant increase in ERK2 phosphorylation in EGF + Ang II-treated compared to untreated PC3 cells transfected with ATIP1 P < 0.05.&&& indicates that there is a significant decrease in ERK2 phosphorylation in EGF + Ang II treated ATIP1-transfected cells when compared to pcDNA3 transfected PC3 cells treated in the same way, P < 0.001 (Students t-test).
In pcDNA3-transfected PC3 cells (negative control), 10 ng/ml EGF significantly increased ERK2 phosphorylation (P < 0.05) (represented by an increase in phospho-ERK2 in Fig. 4A). When 1 μM Ang II was given in combination with EGF to the negative control transfected cells, ERK2 phosphorylation returned towards basal levels (Fig. 4A,B). By contrast, in ATIP1-transfected PC3 cells, where basal ERK phosphorylation was significantly reduced by 74% (P < 0.05) compared to pcDNA3-transfected cells, EGF still induced ERK phosphorylation. However, its effects were significantly reduced (65% decrease, P < 0.01). Under these conditions the addition of Ang II in combination with EGF did not reverse the effects of EGF in the ATIP1 over-expressing cells.
We also examined the effect of ATIP1 over-expression on a number of other genes, including EGF, the EGF receptor (EGFR), and the AT1- and AT2-receptors, to determine whether ATIP up-regulation induced changes in the mRNA expression of these other genes. However, no differences in the mRNA expression of genes other than ATIP1 were detected (data not shown).
Effect of AT2-Receptor Activation on Basal ERK2 Phosphorylation
In our report [7], as in our studies of the overexpression of ATIP1 in PC3 cells, we observed that the ERK2 phosphorylation assay was more sensitive than the [3H]thymidine incorporation assay for assessing the growth effects of various treatments. Therefore, we also examined the effects of CGP42112A on ERK2 phosphorylation using western blot techniques in both the LNCaP and PC3 cell lines. CGP42112A significantly depressed ERK2 phosphorylation when given alone in PC3 cells (1.00 ± 0.11 and 0.49 ± 0.11, P 0.02, in untreated and CGP 42112A-treated PC3 cells, respectively), but did not in LNCaP cells (1.01 ± 0.09 and 1.19 ± 0.17, P = 0.35, in untreated and CGP 42112A-treated LNCaP cells, respectively).
DISCUSSION
These studies identified the presence of ATIP and ATIP1 mRNA in two prostate cancer cell lines and examined the interaction between EGF and ATIP, a novel AT2-receptor-interacting protein and candidate tumor suppressor gene [27]. ATIP is localized to chromosome 8p22, a region where a frequent loss of heterozygosity exists in tumors of the prostate, bladder, breast, ovary, colon, liver and head and neck [27]. The five isoforms of ATIP share a common interacting domain and this region appears to be responsible for its anti-growth effects [23]. Most ATIP-related studies published to date have focused on ATIP1. Ectopic expression of this isoform mimics the effects of AT2-receptor activation and has been shown to inhibit activation of ERK2 and cell growth [23,26]. Reports of reduced ATIP1 mRNA levels in an undifferentiated pancreas tumor biopsy and in the poorly differentiated MIA PaCa-2 pancreatic tumor cell line, suggest that loss of ATIP1 expression could have a role in tumor progression in the pancreas [25]. Recombinant expression of ATIP1 in the MIA PaCa-2 cells inhibited their proliferation and there was a reverse correlation between ATIP1 mRNA expression and cellular differentiation and proliferation in a range of pancreatic cancer cell lines [25]. In addition, studies using gene silencing techniques have revealed that loss of ATIP expression is associated with increased cell proliferation [26]. These results suggest that loss of ATIP may contribute to the rate of progression of some malignant tumors and recent studies examining ATIP3 in breast cancer support this possibility [31].
In the present studies, two commonly used prostate cancer cell lines were used as models of relatively slow- growing, androgen-sensitive prostate cancer (LNCaP cells) and late stage, fast-growing, androgen-independent prostate cancer (PC3). Previously, we demonstrated that EGF, and to a lesser extent Ang II, both significantly stimulated cell growth in LNCaP cells but only EGF stimulated growth in PC3,where AT1-receptorsappear to be non-functional [7,8] (see also Figs. 2B and 3B). We also demonstrated the existence of functional AT2-receptors in both LNCaP and PC3 cells and that activation of the AT2-receptor in both cell lines by CGP42112A inhibited EGF- induced [3H]thymidine incorporation and ERK2 phosphorylation [7]. Ang II had a similar effect in PC3 cells, where its action is predominantly via the AT2-receptor, but not in LNCaP cells where its predominant action is on AT1-receptors [7].
We now report the presence of mRNA expression of the putative AT2-receptor interacting protein, ATIP, and its ATIP1 isoform in both cell lines and demonstrate that it is a necessary component of the growth inhibitory response to AT2-receptor activation. In the PC3 cell line ATIP and ATIP1 mRNA expression were approximately 30- and 4.6-fold lower, respectively, than in the more slowly growing, androgen-sensitive LNCaP cells (Table III) and as previously reported in pancreatic and breast cancer ATIP expression was inversely correlated with rate of proliferation in the two prostate cancer cell lines [25,31].
As part of this series of experiments we examined the possibility that EGF may promote growth in these cells, at least in part, by reducing the amount of ATIP present in the cell. In both cell lines the increased growth rate associated with 10 ng/ml EGF was associated with a significant down-regulation of ATIP and ATIP1 mRNA expression (Fig. 1). By contrast, neither Ang II or CGP42112A produced any significant change in ATIP mRNA expression in the LNCaP or PC3 cell lines (Fig. 1). However, Ang II inhibited EGF mediated down-regulation of both ATIP and ATIP1 mRNA in PC3 cells (Fig. 1) and the AT2-receptor agonist, CGP42112A, inhibited EGF mediated down- regulation of ATIP mRNA in both cell lines (data not shown).
These results are consistent with our hypothesis that EGF can act at least in part by reducing the amounts of ATIP and ATIP1 present in the cells, and that activation of the AT2-receptor may protect against this down-regulation and reduce the pro-growth effect of EGF. The results also demonstrate that the growth rate response to maximal doses of Ang II in LNCaP is considerably less than to EGF. Although this may in part reflect an AT2-receptor antagonist action of Ang II in this cell line it is also consistent with proposals that activation of the AT1-receptor transactivates the EGFR[32] or potentiates the action of EGF on the EGFR [33] without inducing full activation of the ERK2 pathway. We have reported the presence of Ang II in pre-malignant and malignant epithelial cells in high grade prostatic intra-epithelial neoplasia and prostate cancer and it is interesting to speculate that the presence of endogenous Ang II in these cells may contribute to activation of the EGFR in prostate cancer [10].
The mechanism via which AT2-receptor activation prevents EGF mediated down-regulation of ATIP is currently unknown, however, activation of the AT2-receptor has been shown to result in the formation of an ATIP/SHP-1 (Src homology 2 domain-containing protein-tyrosine phosphatase 1) complex, which translocates to the cell nucleus [34]. SHP-1 is a well-known inhibitor of activation-promoting signaling cascades [35]. Recently, it was demonstrated that SHP-1 was expressed at higher levels in LNCaP than in PC3 cells and that silencing of SHP-1 expression in LNCaP cells led to an increased rate of proliferation, whereas, transfection and over expression of SHP-1 in PC3 cells decreased proliferation [36]. The results of our studies examining the knock-down and over-expression of ATIP in these two cell lines closely resemble those reported for SHP-1, except that the degree of silencing achieved with the LNCaP cell line was associated with an increase in sensitivity to EGF rather than an increase in absolute growth rate. Taken together with the PC3 results, these data suggest ATIP expression in prostate cancer cells resembles the expression of SHP-1 and is consistent with the suggestion that formation of the ATIP/SHP-1 complex may protect against the down- regulation of ATIP.
The results also support the ability of activation of the AT2-receptor to inhibit EGF-induced increases in growth rate. This was clearly demonstrated in [3H]thymidine studies in both LNCaP and PC3 cells where the AT2-receptor agonist reversed EGF-induced cell proliferation in both cell lines. The inability of CGP42112A to decrease basal cell growth in LNCaP cells is not surprising as it has been previously reported that the p42/ERK2 pathway is not constitutively activated in this cell line [37], whereas, in the PC3 cell line, which is constitutively activated [38], although the addition of CGP42112A did not significantly reduce basal [3H]thymidine incorporation it did significantly reduce basal ERK2 phosphorylation.
The level of constitutive activation in the two cell lines may also in part explain the relative sensitivity of the two cell lines to exogenous EGF and Ang II. In LNCaP, where constitutive activation of the EGFR does not exist, exogenous EGF induces a robust response, increasing [3H]thymidine incorporation by up to approximately 80% in wild-type LNCaP cells and Ang II, which transactivates the EGF receptor, has a smaller effect (Fig. 2B). By contrast in PC3 cells, exogenous activation with the same dose of EGF induces a smaller increase in DNA synthesis of approximately 24% and Ang II has no effect (Fig. 3B).
The importance of the EGF/ATIP interaction was further documented in the ATIP silencing and ATIP over expression studies. In the LNCaP and PC3 cell lines, silencing reduced ATIP mRNA expression by approximately 50% and 73%, and ATIP1 by 50% and 37% respectively (Figs. 2A and 3A). In the former cell line, the reduction was to levels that were still higher than those measured in wild-type PC3 and in contrast to the silenced PC3 cells, this level of silencing was not associated with an increase in the basal level of [3H]thymidine incorporation (Figs. 2B and 3B). This may reflect specific differences between the cells, but is also consistent with the idea that ATIP levels must fall to a critical level before the basal rate of cell growth is influenced. The data also suggest that the initial effect of lowering ATIP expression may be to increase the sensitivity to EGF. Thus, in silenced LNCaP cells although the approximate 50% decrease in ATIP expression did not significantly modify the basal rate of growth it did result in a significant increase in the growth-promoting effects of EGF. By contrast, in PC3 cells where the endogenous ATIP levels were already low, a further reduction in ATIP resulted in an increased basal growth rate, but interestingly at these very low levels high doses of EGF appeared not to stimulate growth further, providing support for the idea that an important component of the EGF growth response is through its ability to lower ATIP expression and that with ATIP silencing the dose response to EGF is moved to the right. In addition, in the LNCaP cell line ATIP silencing abolished the ability of the AT2-receptor agonist, CGP42112A, to reverse the stimulatory effects of EGF on cell growth (Figs. 2C and 3C). In PC3-silenced cells, neither CGP42112A nor Ang II modified the increased growth rate. The results in both cell lines are consistent with ATIP being required to mediate the actions of AT2-receptor activation.
We then examined the effect of an increase in ATIP expression using the ratio of phospho-ERK2/ unphosphorylated ERK2 as a measure of prostate cancer cell growth For these studies, we chose the PC3 cell line, where the endogenous expression of ATIP is relatively low (Table II). ATIP1 over-expression (~ 130-fold increase in mRNA) significantly inhibited both basal ERK2 activation, and the ability of EGF to stimulate ERK2 phosphorylation in PC3 cells indicating a significant inhibition of growth (Fig. 4). These results are in line with our earlier studies in which ATIP1 over-expression inhibited growth factor-induced ERK2 activation in COS and CHO cells, two normal mammalian cell lines [23]. Moreover, in contrast to pcDNA3 transfected and wild-type PC3 cells, neither CGP42112A or Ang II had an inhibitory effect on EGF-induced PC3 cell growth, suggesting that at this level of transfection-induced ATIP over- expression, the anti-growth pathway mediated by the AT2-receptor/ATIP pathway in transfected cells may already be fully activated and cannot be stimulated further.
Although we have evidence that EGF acts in part by reducing ATIP and ATIP1 expression in prostate cancer cells, the converse is not true, at least for ATIP1. ATIP1 does not act by reducing the levels of EGF or EGFR as over-expression of this isoform had no effect on EGF mRNA expression in PC3 cells (data not shown). Therefore, it would appear, as we suggested above, that ATIP1 may act, at least in this cell line, at some other point on the growth factor signaling cascade, possibly via an interaction with SHP-1 [34].
Overall, the results suggest that the effect of ATIP down-regulation varies with the cell line studied, and that the initial response to a reduction in ATIP expression in cells may be an increase in sensitivity to EGF, followed by an increase in basal growth rates. At very low levels of ATIP expression the ability of EGF to stimulate growth further is either lost or higher doses of EGF are required. Moreover, at the extremes of ATIP expression (both high and low) the ability of AT2-receptor activation to further modify growth rate is also lost. These results in prostate cancer cell lines therefore not only suggest a role for ATIP in the action of EGF and prostate cancer cell growth but are also consistent with our earlier proposal in normal cell lines that ATIP is an important component of the cellular response to AT2-receptor activation [23], and further suggest that a critical level of ATIP is required to mediate the response to AT2-receptor activation.
ACKNOWLEDGMENTS
This work has been kindly supported by the INSERM/NH&MRC, Sir Edward Dunlop Medical Research Foundation, the Austin Hospital Medical Research Foundation and the University of Melbourne. The authors would also like to thank Dr. Clara Nahmias and Professor Pam Russell for their help in preparing this manuscript.
The authors declare that this is original work and has never been published elsewhere before. In addition, all authors of this manuscript have directly participated in its writing and all authors of this paper have read and approved the final version submitted. The contents of this manuscript have not been copyrighted or published previously and are not now under consideration for publication elsewhere. The contents of this manuscript will not be copyrighted, submitted, or published elsewhere while acceptance by the Journal is under consideration. There are no directly related manuscripts or abstracts, published or unpublished, by any author(s) of this paper.
Abbreviations:
- RAS
renin angiotensin system
- Ang II
Angiotensin II
- AT2-receptor
Ang II type 2 receptor
- ATIP
AT2-receptor interacting protein
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERK1
mitogen-activated protein kinase 1
- SHP-1
Src homology 2 domain-containing protein-tyrosine phosphatase 1
REFERENCES
- 1.Abali H, Gullu I, Engin H, Haznedaroglu I, Erman M, Tekuzman G. Old antihypertensives as novel antineoplastics: Angiotensin- I-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Med Hypotheses 2002;59:344–348. [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto Y, Sasaki T, Tsuchida A, Chayama K. Angiotensin II type 1 receptor expression in human pancreatic cancer and growth inhibition by angiotensin II type 1 receptor antagonist. FEBS Lett 2001;495:197–200. [DOI] [PubMed] [Google Scholar]
- 3.Fujita M, Hayashi I, Yamashina S, Itoman M, Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun 2002;294:441–447. [DOI] [PubMed] [Google Scholar]
- 4.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 1998;352:179–184. [DOI] [PubMed] [Google Scholar]
- 5.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. The angiotensin-I- converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: Possible role of the vascular endothelial growth factor. Clin Cancer Res 2001;7:1073–1078. [PubMed] [Google Scholar]
- 6.Deshayes F, Nahmias C. Angiotensin receptors: A new role in cancer? Trends Endocrinol Metab 2005;16:293–299. [DOI] [PubMed] [Google Scholar]
- 7.Chow L, Rezmann L, Immamura K, Wang L, Catt K, Tikellis C, Louis WJ, Frauman AG, Louis SNS. Functional angiotensin II type 2 receptors inhibit growth factor signalling in LNCaP and PC3 prostate cancer cell lines. Prostate 2008;68:651–660. [DOI] [PubMed] [Google Scholar]
- 8.Uemura H, Ishiguro H, Nakaigawa N, Nagashima Y, Miyoshi Y, Fujinami K, Sakaguchi A, Kubota Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: A possibility of tyrosine kinase inhibitor of growth factor. Mol Cancer Ther 2003;2:1139–1147. [PubMed] [Google Scholar]
- 9.Uemura H, Ishiguro H, Kubota Y. Pharmacology and new perspectives of angiotensin II receptor blocker in prostate cancer treatment. Int J Urol 2008;15:19–26. [DOI] [PubMed] [Google Scholar]
- 10.Louis SN, Wang L, Chow L, Rezmann LA, Imamura K, MacGregor DP, Casely D, Catt KJ, Frauman AG, Louis WJ. Appearance of angiotensin II expression in non-basal epithelial cells is an early feature of malignant change in human prostate. Cancer Detect Prev 2007;31:391–395. [DOI] [PubMed] [Google Scholar]
- 11.Dinh DT, Frauman AG, Somers GR, Ohishi M, Zhou J, Casley DJ, Johnston CI, Fabiani ME. Evidence for activation of the renin- angiotensin system in the human prostate: Increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol 2002;196:213–219. [DOI] [PubMed] [Google Scholar]
- 12.Dinh DT, Frauman AG, Sourial M, Casley DJ, Johnston CI, Fabiani ME. Identification, distribution, and expression of angiotensin II receptors in the normal human prostate and benign prostatic hyperplasia. Endocrinology 2001;142:1349–1356. [DOI] [PubMed] [Google Scholar]
- 13.Nassis L, Frauman AG, Ohishi M, Zhuo J, Casley DJ, Johnston CI, Fabiani ME. Localization of angiotensin-converting enzyme in the human prostate: Pathological expression in benign prostatic hyperplasia. J Pathol 2001;195:571–579. [DOI] [PubMed] [Google Scholar]
- 14.O’Mahony OA, Barker S, Puddefoot JR, Vinson GP. Synthesis and secretion of angiotensin II by the prostate gland in vitro. Endocrinology 2005;146:392–398. [DOI] [PubMed] [Google Scholar]
- 15.Uemura H, Ishiguro H, Nagashima Y, Sasaki T, Nakaigawa N, Hasumi H, Kato S, Kubota Y. Antiproliferative activity of angiotensin II receptor blocker through cross-talk between stromal and epithelial prostate cancer cells. Mol Cancer Ther 2005;4:1699–1709. [DOI] [PubMed] [Google Scholar]
- 16.Uemura H, Hasumi H, Kawahara T, Sugiura S, Miyoshi Y, Nakaigawa N, Teranishi J, Noguchi K, Ishiguro H, Kubota Y. Pilot study of angiotensin II receptor blocker in advanced hormone-refractory prostate cancer. Int J Clin Oncol 2005;10: 405–410. [DOI] [PubMed] [Google Scholar]
- 17.Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: Potential roles in cardiovascular and renal regulation. Endocr Rev 2003;24:261–271. [DOI] [PubMed] [Google Scholar]
- 18.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 2000;52:415–472. [PubMed] [Google Scholar]
- 19.Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: Distribution, signalling and function. Clin Sci (Lond) 2001;100:481–492. [PubMed] [Google Scholar]
- 20.Nouet S, Nahmias C. Signal transduction from the angiotensin II AT2 receptor. Trends Endocrinol Metab 2000;11:1–6. [DOI] [PubMed] [Google Scholar]
- 21.Knowle D, Ahmed S, Pulakat L. Identification of an interaction between the angiotensin II receptor sub-type AT2 and the ErbB3 receptor, a member of the epidermal growth factor receptor family. Regul Pept 2000;87:73–82. [DOI] [PubMed] [Google Scholar]
- 22.Senbonmatsu T, Saito T, Landon EJ, Watanabe O, Price E Jr, Roberts RL, Imboden H, Fitzgerald TG, Gaffney FA, Inagami T. A novel angiotensin II type 2 receptor signaling pathway: Possible role in cardiac hypertrophy. EMBO J 2003;22:6471–6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nouet S, Amzallag N, Li JM, Louis S, Seitz I, Cui TX, Alleaume AM, Di Benedetto M, Boden C, Masson M, Strosberg AD, Horiuchi M, Couraud PO, Nahmias C. Trans-inactivation of receptor tyrosine kinases by novel angiotensin II AT2 receptor- interacting protein, ATIP. J Biol Chem 2004;279:28989–28997. [DOI] [PubMed] [Google Scholar]
- 24.Kinjo T, Isomura M, Iwamasa T, Nakamura Y. Molecular cloning and characterization of two novel genes on chromosome 8p21.3. J Hum Genet 2000;45:12–17. [DOI] [PubMed] [Google Scholar]
- 25.Seibold S, Rudroff C, Weber M, Galle J, Wanner C, Marx M. Identification of a new tumor suppressor gene located at chromosome 8p21.3–22. FASEB J 2003;17:1180–1182. [DOI] [PubMed] [Google Scholar]
- 26.Wruck CJ, Funke-Kaiser H, Pufe T, Kusserow H, Menk M, Schefe JH, Kruse ML, Stoll M, Unger T. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol 2005;25:57–64. [DOI] [PubMed] [Google Scholar]
- 27.Di Benedetto M, Bieche I, Deshayes F, Vacher S, Nouet S, Collura V, Seitz I, Louis S, Pineau P, Amsellem-Ouazana D, Couraud PO, Strosberg AD, Stoppa-Lyonnet D, Lidereau R, Nahmias C. Structural organization and expression of human MTUS1, a candidate 8p22 tumor suppressor gene encoding a family of angiotensin II AT2 receptor-interacting proteins, ATIP. Gene 2006;380:127–136. [DOI] [PubMed] [Google Scholar]
- 28.Di Benedetto M, Pineau P, Nouet S, Berhouet S, Seitz I, Louis S, Dejean A, Couraud PO, Strosberg AD, Stoppa-Lyonnet D, Nahmias C. Mutation analysis of the 8p22 candidate tumor suppressor gene ATIP/MTUS1 in hepatocellular carcinoma. Mol Cell Endocrinol 2006;252:207–215. [DOI] [PubMed] [Google Scholar]
- 29.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979;17:16–23. [PubMed] [Google Scholar]
- 30.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res 1983;43:1809–1818. [PubMed] [Google Scholar]
- 31.Rodrigues-Ferreira S, Di Tommaso A, Dimitrov A, Cazaubon S, Gruel N, Colasson H, Nicolas A, Chaverot N, Molinie V, Reyal F, Sigal-Zafrani B, Terris B, Delattre O, Radvanyi F, Perez F, Vincent-Salomon A, Nahmias C. 8p22 MTUS1 gene product ATIP3 is a novel anti-mitotic protein underexpressed in invasive breast carcinoma of poor prognosis. PloS one 2009; 4:e7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murasawa S, Mori Y, Nozawa Y, Gotoh N, Shibuya M, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibazaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H. Angiotensin II type 1 receptor-induced extracellular signal-regulated protein kinase activation is mediated by Ca2 /calmodulin-dependent trans- activation of epidermal growthþfactor receptor. Circ Res 1998; 82:1338–1348. [DOI] [PubMed] [Google Scholar]
- 33.Norman J, Badie-Dezfooly B, Nord EP, Kurtz I, Schlosser J, Chaudhari A, Fine LG. EGF-induced mitogenesis in proximal tubular cells: Potentiation by angiotensin II. Am J Physiol 1987; 253:F299–309. [DOI] [PubMed] [Google Scholar]
- 34.Li JM, Mogi M, Tsukuda K, Tomochika H, Iwanami J, Min LJ, Nahmias C, Iwai M, Horiuchi M. Angiotensin II-induced neural differentiation via angiotensin II type 2 (AT2) receptor- MMS2 cascade involving interaction between AT2 receptor- interacting protein and Src homology 2 domain-containing protein-tyrosine phosphatase 1. Mol Endocrinol 2007;21:499–511. [DOI] [PubMed] [Google Scholar]
- 35.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: Diversified control of cell growth, inflammation, and injury. Histol Histopathol 2007;22: 1251–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tassidis H, Brokken LJ, Jirstrom K, Ehrnstrom R, Ponten F, Ulmert D, Bjartell A, Harkonen P, Wingren AG. Immunohis- tochemical detection of tyrosine phosphatase SHP-1 predicts outcome after radical prostatectomy for localized prostate cancer. Int J Cancer 2009. [DOI] [PubMed] [Google Scholar]
- 37.Putz T, Culig Z, Eder IE, Nessler-Menardi C, Bartsch G, Grunicke H, Uberall F, Klocker H. Epidermal growth factor (EGF) receptor blockade inhibits the action of EGF, insulin-like growth factor I, and a protein kinase A activator on the mitogen-activated protein kinase pathway in prostate cancer cell lines. Cancer Res 1999;59:227–233. [PubMed] [Google Scholar]
- 38.Fong CJ, Sherwood ER, Mendelsohn J, Lee C, Kozlowski JM. Epidermal growth factor receptor monoclonal antibody inhibits constitutive receptor phosphorylation, reduces autonomous growth, and sensitizes androgen-independent prostatic carcinoma cells to tumor necrosis factor alpha. Cancer Res 1992; 52:5887–5892. [PubMed] [Google Scholar]