Abstract
Pax4 is a homeobox gene encoding Pax4, a transcription factor that is essential for embryonic development of the endocrine pancreas. In the pancreas, Pax4 counters the effects of the related transcription factor, Pax6, which is known to be essential for eye morphogenesis. In this study, we have discovered that Pax4 is strongly expressed in retinal photoreceptors of the rat. Pax4 expression is not detectable in the foetal eye; however, postnatal Pax4 transcript levels rapidly increase. In contrast, Pax6 exhibits an inverse developmental pattern of expression being more strongly expressed in the foetal eye. Histological analysis revealed that Pax4 mRNA is exclusively expressed in the retinal photoreceptors, whereas Pax6 mRNA and protein are present in the inner nuclear layer and in the ganglion cell layer of the mature retina. In the adult retina, Pax4 transcripts exhibit a diurnal rhythm with maximal levels occurring during the light period, whereas retinal Pax6 transcript levels do not change throughout the day. The daily changes in Pax4 expression may contribute to daily changes in function in the differentiated retinal photoreceptor.
Keywords: Pax4, Pax6, retina, development, diurnal rhythm
Members of the Pax gene family encode highly conserved transcription factors involved in organogenesis throughout the animal kingdom (reviewed by Chi and Epstein 2002). Based on sequence similarities, the homeobox-containing Pax4 and Pax6 constitute a well-defined subgroup among mammalian Pax genes.
Pax6 is known to be involved in eye morphogenesis (reviewed by Ashery-Padan and Gruss 2001; Gehring 1998, 2005); the gene is widely expressed in the developing murine eye (Walther and Gruss 1991) and is required for gross eye development (Hill et al. 1991; Grindley et al. 1995). Pax6 has been assigned several specific roles during early retinal development, including proliferation of retinal cell progenitors (Xu et al. 2007), maintenance of multipotent progenitor potential (Marquardt et al. 2001) and regulation of timing of differentiation and cell fate determination (Philips et al. 2005).
In addition to controlling development of the eye, Pax6 is also involved in development of pancreatic α-cells (St-Onge et al. 1997), whereas the related Pax4 gene is essential for development of pancreatic β-cells (Sosa-Pineda et al. 1997).Pax4 is highly expressed in pancreatic islets of the foetal rodent (Smith et al. 1999; Wang et al. 2004), but is only detectable at low levels in mature islets (Brun et al. 2004). Interestingly, Pax4 is known to act as a transcriptional repressor (Fujitani et al. 1999; Kalousová et al. 1999; Smith et al. 1999), suppressing the expression of genes that are otherwise activated by Pax6 in the pancreas (Campbell et al. 1999; Smith et al. 1999; Petersen et al. 2000; Ritz-Laser et al. 2002).
The current study on retinal Pax4 and Pax6 was stimulated by the discovery that Pax4 is expressed in the pineal gland (Rath et al. 2008) together with evidence that the pinealocyte and the retinal photoreceptor evolved from a common ancestral photoreceptor as indicated by ultrastructural similarities (Collin 1971) and expression in both tissues of a set of genes dedicated to phototransduction and melatonin synthesis (reviewed by Klein 2004). Moreover, the molecular identity of these cell types seems to be governed by a common set of transcription factors, including members of the orthodenticle family of homeobox genes, Otx2 and Crx (Furukawa et al. 1999; Nishida et al. 2003; Rath et al. 2006, 2007), and Pax6 (Gehring 1998, 2005; Estivill-Torrús et al. 2001). The results of our studies reveal that Pax4 is expressed in retina and that it exhibits an inverse spatial and temporal relationship to Pax6, with marked differences in the ontogenetic and 24-hour patterns of expression.
Materials and methods
Animals
For the developmental series, Sprague–Dawley rats were obtained from timed-pregnant mothers (Charles River, Sulzfeld, Germany); the animals were housed under a 12 : 12 light/dark schedule and decapitated at zeitgeber time (ZT) 6. Eyeballs were fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and cryoprotected in 25% sucrose before freezing on crushed solid CO2.
For the day-night radiochemical in situ hybridization experiments and western blot analysis, adult male Sprague–Dawley rats (200–300 g; Charles River) were housed under a 12 : 12 light/dark schedule. Bilateral superior cervical ganglionectomy was performed 10 days prior to killing (Møller et al. 1997). Animals were decapitated at ZT6 and ZT18; eyeballs or retinae and spinal cords were removed immediately and frozen on crushed solid CO2. For the quantitative real-time PCR (qRT-PCR) and northern blot analysis, female Sprague–Dawley rats (150–200 g; Taconic Farms, Germantown, NY, USA) were housed under a 14 : 10 light/dark schedule and killed by decapitation at time points indicated in the figures and figure legends. Retinae were immediately frozen on crushed solid CO2 and stored at −80°C.
All experiments with animals were performed in accordance with the guidelines of EU Directive 86/609/EEC (approved by the Danish Council for Animal Experiments) and the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Radiochemical in situ hybridization
Cryostat sections (12 μm, adult; 14 μm, developmental series) were hybridized with 38-mer [35S]dATP-labelled DNA probes as previously described (Møller et al. 1997; Rath et al. 2006). For hybridization, the following anti-sense probes as well as corresponding sense control probes were used:
5′-TCCAATCAGATGATGCACAGGATGGGTGGTGAGGCAGG-3′, anti-sense, position 1053–1016 on rat Pax4 mRNA (NM_031799)
5′-GCATCCTTAGTTTATCATACATGCCGTCTGCGCCCATC-3′, anti-sense, position 605–568 on rat Pax6 mRNA (NM_ 013001.2).
After hybridization and washing, the sections were exposed to an X-ray film for 2 weeks and developed. Images on the X-ray film were transferred to a computer by use of a CCD camera and quantified (Image 1.42; Wayne Rasband, NIH, Bethesda, MD, USA). Optical densities were converted to dpm/mg tissue by using simultaneously exposed 14C-standards calibrated by comparison with 35S-tissue paste standards. Sections were subsequently dipped in a photographic emulsion (Amersham, Hillerød, Denmark) at 40°C and exposed for 3 weeks. After exposure, the autoradiographs were developed in amidol and fixed in thiosulphate. The sections were counter-stained in cresyl violet and photographed in a microscope in both transmitted light and dark field mode.
Quantitative real-time PCR
Total RNA was isolated using a RiboPure RNA isolation kit (Ambion, Austin, TX, USA) and total RNA was subject to DNase treatment using TURBO DNA-free (Ambion). cDNA production was performed following the Superscript II protocol (Invitrogen, Carlsbad, CA, USA) using 1 μg of DNase-treated total RNA as starting material. Experiments were performed using a LightCycler 2.0 (Roche Diagnostics, Indianapolis, IN, USA). Reactions (25 μL) contained 0.5 μM primers, RT Real-Time SYBR Green master mix (SuperArray Bioscience, Frederick, MD, USA) and cDNA according to the manufacturer’s instructions. Primer sequences are given in Table 1. Assays included an initial denaturation step at 95°C for 10 min, proceeded by 40 cycles of 95°C denaturation for 15 s, 30 s annealing at 63°C then extension at 72°C for 30 s. Product specificity was confirmed by agarose gel electrophoresis of the amplified products and thereafter during every qRT-PCR run by melting curve analysis. Transcript number was determined using internal standards; these were prepared by cloning the target PCR products into pGEM-T Easy vectors (Promega, Madison, WI, USA). Clone verification was performed by direct sequence analysis. For each experiment, a set of 100-fold serial dilutions of each internal standard (101–107 copies/1 μL) was prepared and used to generate standard curves; values were normalized per 1000 copies of glyceraldehyde 3-phosphate dehydrogenase.
Table 1.
Sequences of primers used for quantitative RT-PCR analyses
| Transcript | Forward primer 5′-3′ | Reverse primer 5′-3′ |
|---|---|---|
| Pax4 | 5′-AGATGTTCCAGTGACACCACA-3′ | 5′-CACAGGAAGGAGGGAGTGG-3′ |
| Aanat | 5′-TGCTGTGGCGATACCTTCACCA-3′ | 5′-CAGCTCAGTGAAGGTGAGAGAT-3′ |
| Pax6 | 5′-AACAGCGACGAAAGAGAGGA-3′ | 5′-CACTCTTTGAATAGAAGATCTCACACA-3′ |
| Gapdh | 5′-TGGTGAAGGTCGGTGTGAACGGAT-3′ | 5′-TCCATGGTGGTGAAGACGCCAGTA-3′ |
Aanat, arylalkylamine N-acetyltransferase; Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Northern blot analysis
Total RNA was prepared from frozen tissues using Trizol (Invitrogen). Eight micrograms of total RNA was loaded per lane in a 1% agarose/0.7 M formaldehyde gel and separated by electrophoresis in a 1× 3-(N-Morpholino) propanesulfonic acid (50 mM) buffer (Quality Biological, Gaithersburg, MD, USA). Membrane transfer was performed in 20× saline sodium citrate transfer buffer by use of the TurboBlotter system (Schleicher and Schuell, Keene, NH, USA). DNA probes corresponding to position 1–436 on rat Pax4 (NM_031799) and position 78–387 on rat glyceraldehyde-3-phosphate dehydrogenase mRNA (BC059110) were generated as previously described (Rath et al. 2008) and labelled with 32P by random priming (Amersham Biosciences, Piscataway, NJ, USA). Hybridization, imaging and stripping of blots were performed as previously described (Rath et al. 2006, 2008). Transcript sizes were determined by comparison with standard RNA markers (Invitrogen).
Immunohistochemistry
Cryostat sections, 14 μm in thickness, were cut and mounted on Superfrost Plus slides (Menzel, Braunschweig, Germany). For blocking of endogenous peroxidase activity, the sections were pre-incubated in 1% H2O2 in phosphate-buffered saline (PBS) for 10 min. Sections were washed in PBS for 3 × 5 min and incubated in 5% normal swine serum diluted in PBS for 30 min. This was followed by incubation in rabbit anti-Pax6 polyclonal antibody (Millipore, Copenhagen, Denmark) diluted 1 : 500 in PBS with 1% bovine serum albumin and 0.3% Triton X-100 for 16 h at 4°C. Absorption control was performed by pre-incubating the diluted antibody in the presence of 100 μg/mL blocking peptide (Millipore) for 3 days at 4°C. Sections were washed 3 × 10 min in PBS with 0.25% bovine serum albumin and 0.1% Triton X-100 followed by incubation for 1 h in biotinylated swine anti-rabbit IgG (Dako, Glostrup, Denmark) diluted 1 : 500 in the same buffer. The sections were washed 3 × 5 min in PBS with 0.1% Triton X-100 and incubated for 45 min in ABC-Vectastain solution (Vector Laboratories, Burlingame, CA, USA) diluted 1 : 100 in the same buffer. After washing for 2 × 5 min in PBS and 0.1% Triton X-100 and for 5 min in 0.05 M Tris (pH 7.6), chromogenic development was performed in 1.4 mM diaminobenzidine and 0.01% H2O2 in 0.05 M Tris (pH 7.6) for 15 min. The reaction was terminated by extensive washing of the sections in deionized water. Finally, the sections were dried and embedded in Pertex® (Histolab, Gothenburg, Sweden).
Western blot analysis
Samples were homogenized in a 2× Laemmli buffer containing 72% (v/v) 155 mM Tris buffer (pH 8.3), 9% (w/v) sodium dodecyl sulfate, 16 mM bromphenol blue, 18% (v/v) glycerol and 10% (v/v) 2-mercaptoethanol (0.1 g tissue/mL buffer). Samples were boiled and centrifuged at 13 000 g for 1 h at 4°C. Protein content of the supernatants was determined by use of the RC DC Protein Assay (BioRad, Copenhagen, Denmark). A protein amount of 50 μg per lane was run in a NuPAGE® Bis-Tris 12% Gel (Invitrogen, Taastrup, Denmark) and transferred to a nitrocellulose membrane by use of the XCell® Surelock Mini-Cell system (Invitrogen). The membrane was blocked in 0.5% skim milk in PBS and incubated in the same rabbit anti-Pax6 polyclonal antibody (Millipore) used for immunohistochemistry, diluted 1 : 1000 in 0.5% skim milk in PBS for 16 h at 4°C. Absorption control was performed by pre-incubating the diluted antibody in the presence of 100 μg/ml blocking peptide (Millipore) for 5 days at 4°C. The membrane was washed in PBS and subsequently incubated in biotinylated swine anti-rabbit IgG (Dako) diluted 1 : 500 in blocking solution for 1 h and washed. Chromogenic development was performed as described for immunohistochemistry. Protein size was estimated by comparison with standard protein molecular weight markers (BioRad).
Statistical analysis
Two-tailed Student’s t-test, one-way ANOVA, two-way ANOVA or Tukey’s multiple comparison test were used for comparing means of in situ hybridization signals (dpm/mg tissue) or the copy numbers obtained by qRT-PCR. A p-value of <0.05 was considered to represent a statistically significant difference.
Results
Expression of Pax4 in the developing retina
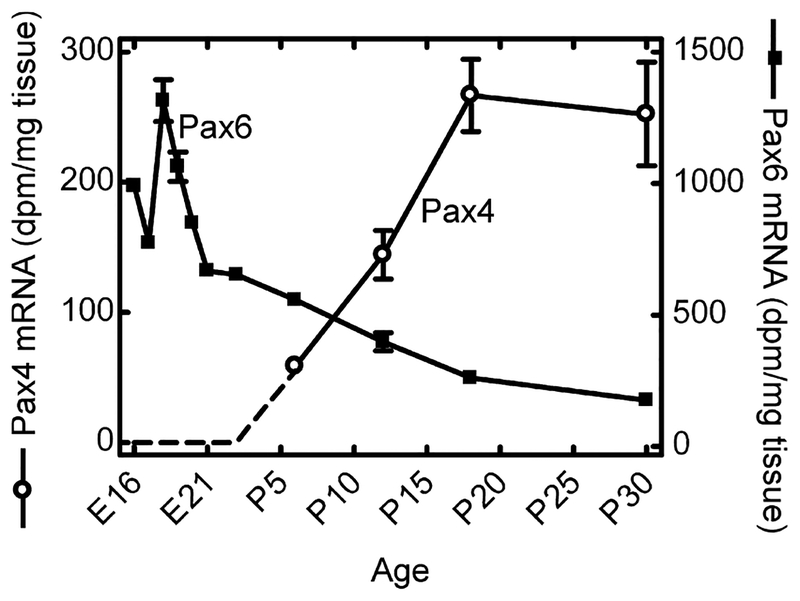
Pax4 mRNA was not detectable by radiochemical in situ hybridization in the embryonic or neonatal retina (data not shown). However, at postnatal day (P) 6, a weak signal was observed in the outer part of the neural retina (Fig. 1; Fig. S1); thereafter, a distinct signal was observed in the outer nuclear layer and in the inner segments of the photoreceptors (Figs 1 and 2). Accordingly, within the retina, Pax4 appears to be specifically expressed in the photoreceptor cells. Densitometric quantification of Pax4 expression in the developing retina (Fig. 3) revealed that expression changed in a time-dependent manner (p < 0.01, one-way ANOVA): a low transcript level detected at P6 was followed by an increase thereafter, with maximal levels at P18 and P30 (p < 0.05, Tukey’s test).
Fig. 1.
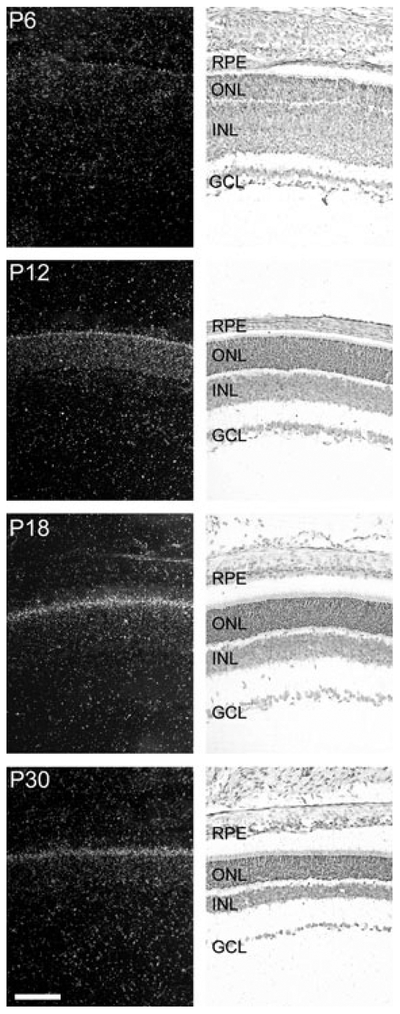
Ontogenetic expression of Pax4 in the rat retina. Radiochemical in situ hybridization for detection of Pax4 mRNA in sections of the rat retina. The last four stages, postnatal day 6 (P6), P12, P18 and P30, of a developmental series ranging from embryonic day 16 (E16) to P30 are presented. Left column: dark field photomicrographs of hybridized sections dipped in a photographic emulsion. Right column: bright field photomicrographs of the same hybridized sections. Photomicrographs of a section hybridized with a sense control probe are provided in Fig. S1. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Scale bar, 100 μm.
Fig. 2.
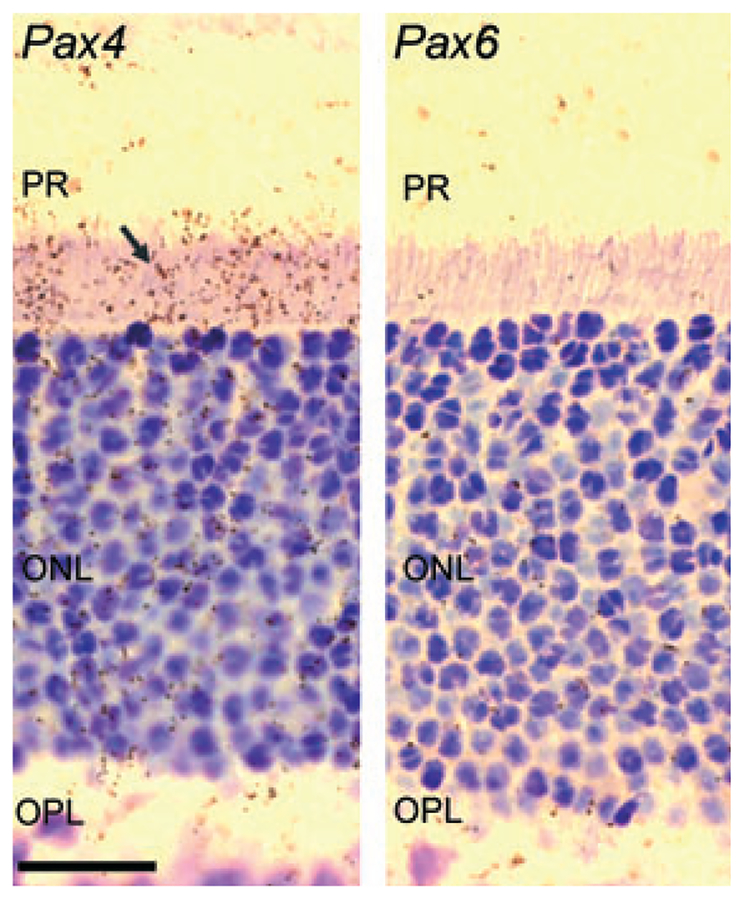
Radiochemical in situ hybridization for detection of Pax4 (left) and Pax6 mRNA (right) in the outer part of the neural retina of the rat at postnatal day 30 (P30). The arrow indicates the positive hybridization signal in the inner segments of the photoreceptors in the section hybridized for detection of Pax4 mRNA. Photomicrographs of a section hybridized with a sense control probe are provided in Fig. S1. Pax6 mRNA is not detected in the photoreceptor cells at P30. ONL, outer nuclear layer; OPL, outer plexiform layer; PR, photoreceptors. Scale bar, 20 μm.
Fig. 3.
Densitometric quantification of in situ hybridization autoradiographs of ontogenetic Pax4 and Pax6 gene expression in the rat retina. In the earliest stages, a Pax4 signal above background was not detected (dotted line). Values on graphs represent the mean ± SEM of three animals at each developmental stage examined. Significant differences in Pax4 mRNA levels occurred during development (one-way ANOVA, F3,8 = 14.2, p = 0.0015) with maximal levels detected at postnatal day 18 (P18) and P30 (Tukey’s multiple comparison test, p-values < 0.05). Significant differences in Pax6 mRNA levels during development were also detected (one-way ANOVA, F10,21 = 130.8, p < 0.0001) with maximal levels obtained at embryonic day 18 (E18) (Tukey’s multiple comparison test, p-values < 0.001).
Expression of Pax6 in the developing retina
Pax6 is known to be expressed in the murine retina (Walther and Gruss 1991). We here compared the developmental expression pattern of Pax6 to that of Pax4 in the rat retina (Fig. 4; Fig. S1). At embryonic day (E) 16, Pax6 expression was detected in the whole retina. During later embryonic stages, the Pax6 mRNA became progressively restricted to the inner part of the retina; from P6, retinal Pax6 mRNA was confined to the inner nuclear layer and the ganglion cell layer (Figs 2 and 4). Non-retinal Pax6 mRNA was detected in the cornea, lens and ciliary body (data not shown; Walther and Gruss 1991; Koroma et al. 1997). Densitometric quantification of Pax6 expression in the rat retina revealed developmental differences in Pax6 mRNA levels (p < 0.0001, one-way ANOVA) with a prenatal peak at E18 (p < 0.001, Tukey’s test) followed by a perinatal decline (Fig. 3). Postnatally, lower but sustained Pax6 expression was detected. Accordingly, retinal Pax6 expression exhibits an inverse temporal relationship to that of Pax4 in this tissue.
Fig. 4.
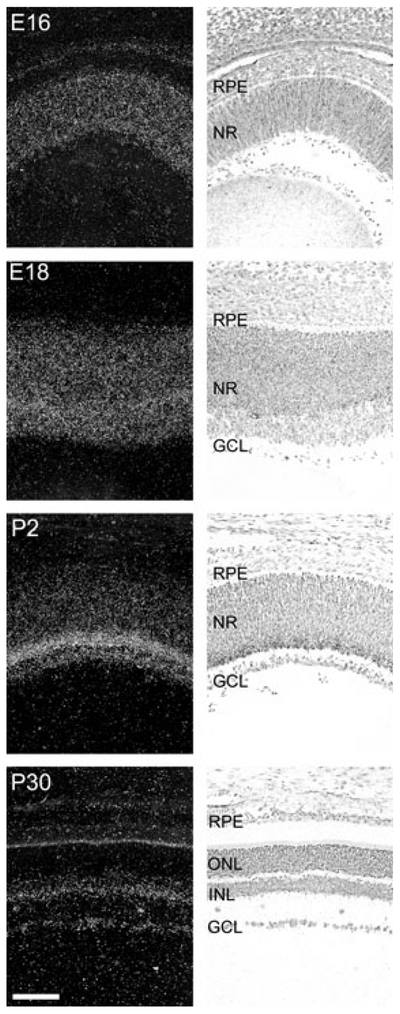
Ontogenetic expression of Pax6 in the rat retina. Radiochemical in situ hybridization for detection of Pax6 mRNA in sections of the rat retina. Images of representative stages of a developmental series ranging from embryonic day (E16) to postnatal day (P30) are displayed. Left column: dark field photomicrographs of hybridized sections dipped in a photographic emulsion. The bright line corresponding to the inner segments of the photoreceptors on the P30 image is because of an endogenous optic scattering of light in this layer and does not represent a hybridization signal. Right column: bright field photomicrographs of the same hybridized sections. Photomicrographs of a section hybridized with a sense control probe are provided in Fig. S1. GCL, ganglion cell layer; INL, inner nuclear layer; NR, neural retina; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Scale bar, 100 μm.
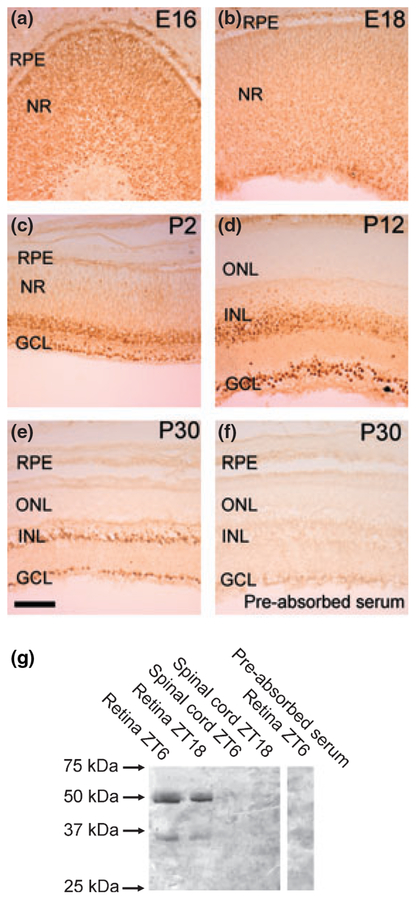
The distribution of Pax6 protein in the developing rat retina was examined by use of immunohistochemistry (Fig. 5). In the early stages, uniform cellular staining of the whole retina was detected (Fig. 5a); this was proceeded by a gradual decline in staining intensity in the outer part of the retina at later embryonic stages (Fig. 5b and c) followed by a clear restriction of immunoreactivity to most of the cells of the inner nuclear layer and the ganglion cell layer from P6 (Fig. 5d and e). Nuclear localization of the protein was observed at all stages examined with a weak immunostaining of the cytoplasm. Western blot analysis further revealed the presence in retinal extracts of an immunopositive band (c. 50 kDa) similar to the predicted mass of Pax6 protein (46.8 kDa; Fig. 5g).
Fig. 5.
Pax6 protein in the rat retina. (a–e) Immunohistochemical analysis of Pax6 protein in the developing rat retina. Photomicrographs of representative developmental stages, as indicated in the upper right corner of each photomicrograph, are displayed. Note the strong immunoreactivity in the nuclei of immunoreactive cells. (f) Immunohistochemical pre-absorption control. GCL, ganglion cell layer; INL, inner nuclear layer; NR, neural retina; ONL, outer nuclear layer; RPE, retinal pigment epithelium. Scale bar, 100 μm. (g) Western blot analysis of the presence of Pax6 protein in the retina and spinal cord of adult rats killed at zeitgeber time (ZT6) and ZT18, respectively. The right hand part of the blot displays a pre-absorption control. Rat Pax6 protein (NP_037133) has a predicted molecular weight of 46.8 kDa. Arrows indicate molecular weight markers. P, postnatal day; E, embryonic day.
Pax4 exhibits a diurnal rhythm in the adult retina
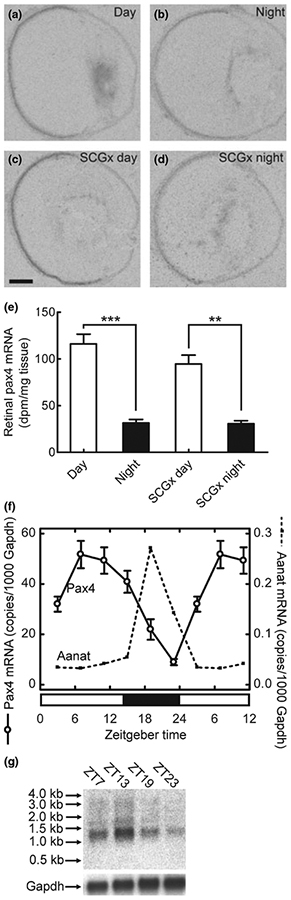
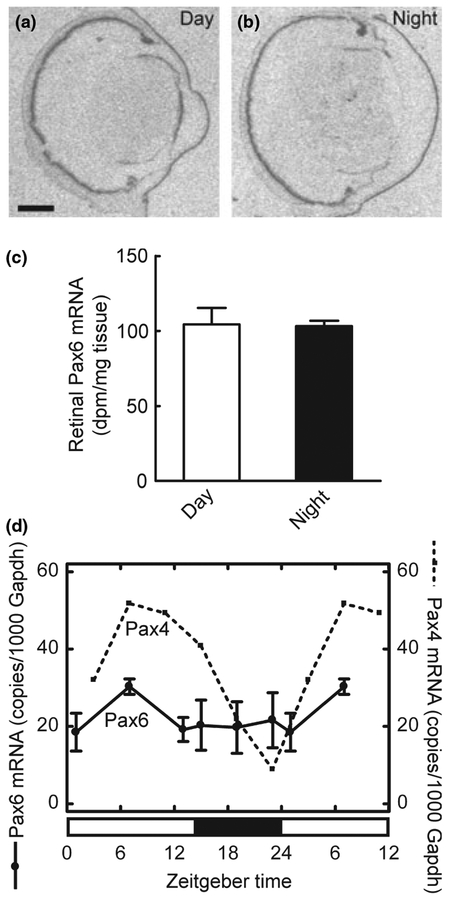
We investigated whether Pax4 expression exhibits a 24-hour rhythm in the retina using in situ hybridization combined with densitometric quantification. This effort revealed a day-night difference in retinal Pax4 expression levels with highest levels of mRNA during daytime (p < 0.001, two-tailed Student’s t-test; Fig. 6a, b and e); a day-night ratio of 3.7 ± 0.5 was detected (Fig. 6e). It has been found that removal of the superior cervical ganglia abolishes the day-night rhythm in pineal Pax4 expression (Rath et al. 2008). In contrast, the diurnal pattern of Pax4 expression in the retina was not influenced by this surgery (p > 0.05, two-way ANOVA; Fig. 6c–e).
Fig. 6.
Diurnal expression of Pax4 in the retina of the adult rat. (a–e) Quantitative radiochemical in situ hybridization analysis of a diurnal variation in expression of Pax4 in the retina of the adult rat housed under a 12 : 12 (light : dark) schedule. (a) Autoradiograph of a section of the eyeball from an animal killed at midday zeitgeber time (ZT6). (b) Autoradiograph of a section of the eyeball from an animal killed at midnight (ZT18). (c) Autoradiograph of a section of the eyeball from a superior cervical ganglionectomized animal killed at midday (ZT6). (d) Autoradiograph of a section of the eyeball from a superior cervical ganglionectomized animal killed at midnight (ZT18). (e) Densitometric quantification of Pax4 mRNA in the rat retina. Values on the bar graph represent the mean ± SEM of four animals in each experimental group. Time of sampling was found to significantly influence retinal Pax4 mRNA levels (two-way ANOVA, F1,12 = 102.5, p < 0.0001), whereas an effect of surgery was not detected (two-way ANOVA, F1,12 = 2.3, p = 0.15). Pair-wise comparison revealed a significant difference in retinal Pax4 expression between day and night in intact animals (two-tailed Student’s t-test, t6 = 7.7, p = 0.00025) and in ganglionectomized animals (two-tailed Student’s t-test, t5 = 5.6, p = 0.0024). (f) Quantitative RT-PCR analysis of diurnal expression ofPax4 and Aanat in the retina of adult rats housed under a 14 : 10 light/dark schedule. Nine animals were killed at each of six time points throughout the 24-hour period. Values on graphs represent the mean ± SEM of three different pools of retinae at each time point. Pax4 mRNA levels changed significantly during the 24-hour period (one-way ANOVA, F5,12 = 15.9, p < 0.0001) with transcript levels at ZT7 being higher than levels at ZT19 (two-tailed Student’s t-test, t4 = 4.4, p = 0.012). Aanat mRNA levels changed significantly during the 24-hour period (one-way ANOVA, F5,12 = 33.1, p < 0.0001) with levels at ZT19 being higher than levels at ZT7 (two-tailed Student’s t-test, t4 = 7.2, p = 0.002). (g) Northern blot analysis of Pax4 expression in the retina of adult rats housed under a 14 : 10 light/dark schedule. Four to five animals were killed at each time point (ZT7, ZT13, ZT19 and ZT23). Arrows on the upper image indicate molecular weight markers. The lower image displays the same blot hybridized for detection of Gapdh mRNA. **p < 0.01; ***p < 0.001. Scale bar, 1 mm. Gapdh, glyceraldehyde-3-phosphate dehydrogenase; Aanat, arylalkylamine N-acetyltransferase; SCGx, superior cervical ganglionectomy.
The daily expression pattern of Pax4 was examined in detail using qRT-PCR. This analysis confirmed diurnal changes in retinal Pax4 mRNA levels (p < 0.001, one-way ANOVA; Fig. 6f); an increase in Pax4 mRNA levels occurred in the retina during the light period, whereas expression declined gradually during the dark period (Fig. 6f). For comparison, the arylalkylamine N-acetyltransferase (Aanat) mRNA levels were determined (Fig. 6f); Aanat mRNA encodes the penultimate enzyme in melatonin synthesis and is known to exhibit a large increase in expression at night in the retina (Sakamoto and Ishida 1998). Comparing mid-day and midnight values confirmed that Pax4 mRNA levels were highest at daytime (p < 0.05; two-tailed Student’s t-test), whereas Aanat levels were highest during the dark period (p < 0.001; two-tailed Student’s t-test): These results show that diurnal expression patterns of Pax4 and Aanat are out of phase (Fig. 6f).
Using northern blotting, we confirmed that the daily rhythm in Pax4 mRNA revealed by qRT-PCR reflected daily changes in a 1.3 kb Pax4 transcript (Fig. 6g). This size corresponds to full-length Pax4 mRNA, referred to as Pax4a (Tokuyama et al. 1998). Diagnostic reverse transcription-PCR confirmed the presence of Pax4a mRNA and also the truncated isoform Pax4c in rat retina (Fig. S2; Table S1); however, Pax4c mRNA was not detected by northern blot analysis suggesting that Pax4a is the major isoform in the retina.
In contrast to the rhythmic nature of Pax4, daily changes in the abundance of retinal Pax6 mRNA were not detected by in situ hybridization (p > 0.05, two-tailed Student’s t-test; Fig. 7a–c) or by qRT-PCR (p > 0.05, one-way ANOVA; Fig. 7d). This is in agreement with the presence of similar levels of Pax6 protein in daytime and night-time retinal extracts (Fig. 5g).
Fig. 7.
Daily expression of Pax6 in the retina of the adult rat. (a–c) Quantitative radiochemical in situ hybridization analysis of a diurnal expression of Pax6 in the retina of the adult rat housed under a 12 : 12 light/dark schedule. (a) Autoradiograph of a section of the eyeball from an animal killed at mid-day zeitgeber time (ZT6). (b) Autoradiograph of a section of the eyeball from an animal killed at midnight (ZT18). Scale bar, 1 mm. (c) Densitometric quantification of Pax6 mRNA in the rat retina. Values on the bar graph represent the mean ± SEM of four animals in each group. No day-night differences were detected (two-tailed Student’s t-test, t6 = 0.12, p = 0.91). (d) Quantitative RT-PCR analysis of daily expression of Pax6 in the retina of the adult rat housed under a 14 : 10 light/dark schedule. Values on graphs represent the mean ± SEM of three different pools of three retinae at each time point. For comparison, the daily pattern of Pax4 expression is shown (dashed line). Diurnal differences in Pax6 expression were not detected in the retina (one-way ANOVA, F5,12 = 0.66, p = 0.66).
Discussion
The results presented here represent the first demonstration of Pax4 expression in retinal photoreceptor cells. The finding that Pax4 expression is highest in the adult argues against a role in development, as is the case with Pax6. Rather, it would appear that Pax4 may play a role in regulating gene expression in the mature photoreceptor. The developmental appearance of Pax4 mRNA is coincident with that of genes required for core photoreceptor functions, e.g. photoreception and transduction (Ho et al. 1986; Treisman et al. 1988; Gonzales-Fernandes and Healy 1990; Babila et al. 1992; Cepko 1996; Morrow et al. 1998). A post-developmental role of Pax4 is further supported by the temporal correlation between the retinal appearance of Pax4 at the end of the first postnatal week and the dramatic increase in the proportion of postmitotic photoreceptor cells during the first postnatal week in the rodent retina (Alexiades and Cepko 1996; Cepko et al. 1996; Livesey and Cepko 2001). Interestingly, photoreceptors with adult morphology and functional synapses appear during the second and third postnatal week in the rat retina (Weidman and Kuwabara 1969), at which time the retinal Pax4 expression reaches a plateau at maximal levels.
The developmental pattern of Pax4 expression in the retina is in marked contrast to that in the pancreatic islet, in which the gene is strongly expressed only in early development (Smith et al. 1999; Wang et al. 2004). On the other hand, the developmental pattern of retinal expression of Pax4 is generally similar to that in the pineal gland (Rath et al. 2008). Therefore, it appears that even though Pax4 has a developmental role in the pancreas in cell fate determination (Sosa-Pineda et al. 1997), this may not be the case in the retina and pineal gland, because it is not expressed early in development. Rather, the late appearance is more in line with a primary role in the differentiated pinealocyte and photoreceptor cell. As indicated in the introduction, these tissues express the same homeobox genes, including Otx2 and Crx (Bovolenta et al. 1997; Chen et al. 1997; Rath et al. 2006, 2007), which are also expressed in the adult (Rath et al. 2006, 2007). In this light, the Pax4 findings support the view that homeobox genes in general may not be exclusively dedicated to developmental events, and adds to an emerging field of homeobox genes in the adult with high expression in tissues undergoing frequent renewal such as the gastrointestinal tract (reviewed by Morgan 2006); however, neuronal tissues, e.g. the retina and the pineal gland, are not characterized by continuous proliferation and maturation. In these tissues, homeobox genes may be required to maintain phenotype and normal function in the mature cell. It is of interest to note that a function for retinal Otx2 protein in the adult has recently been documented (Sugiyama et al. 2008); in this case, the homeoprotein has been shown to be transported trans-synaptically to the visual cortex, where it facilitates postnatal plasticity of a neural circuit.
The postnatal expression of Pax6 in amacrine cells and ganglion cells of the rat retina confirms previous studies in the mouse (Jones et al. 1998) and human (Stanescu et al. 2007). Even though, the Pax6 gene is not expressed in mature photoreceptors, as is the case with Pax4, these data also suggest a potential role of Pax6 in maintaining the phenotype of mature neurons of the inner retina. Notably, the differential expression of Pax4 and Pax6 in the retina partly mirrors the situation in the pancreatic islet, in which Pax6 is expressed in several endocrine cells, whereas in the mature pancreas, Pax4 is acting at low levels specifically in the β-cells (Brun and Gauthier 2008). As indicated above, Pax4 is known to suppress Pax6 transactivating functions in pancre atic gene expression (Campbell et al. 1999; Fujitani et al. 1999; Kalousová et al. 1999; Smith et al. 1999; Petersen et al. 2000). As Pax6 is involved in maintenance of multipotent photoreceptor progenitors (Marquardt et al. 2001), this leads us to speculate that Pax4 could actively suppress this Pax6-dependent state in the postnatal photoreceptor thereby promoting the fully differentiated phenotype.
In the retina, Pax4 expression exhibits a marked diurnal rhythm with high transcript levels during daytime, but it remains to be established whether rhythmic Pax4 expression is translated into rhythmic changes in Pax4 protein. If this is the case, it would seem reasonable to pursue the possibility that Pax4 plays a 24-hour suppressive role to the dynamic daily changes in retinal gene expression in view of the evidence that Pax4 is known to act as a transcriptional repressor (Smith et al. 1999); however, studies on Pax4 protein are currently hindered by the unavailability of useful anti-sera.
We recently found that pineal Pax4 expression exhibits a similar diurnal rhythm with low night-time transcript levels in the pineal gland; this nocturnal suppression is mediated by sympathetic stimulation of gland acting via a cAMP-dependent mechanism (Rath et al. 2008). In general, daily rhythms in the pineal gland are driven by the endogenous circadian clock of the suprachiasmatic nucleus from which a multisynaptic neural circuit controls nocturnal release of norepinephrine from sympathetic nerves in the gland (Klein et al. 1971; Møller and Baeres 2002). The finding here that diurnal Pax4 expression in the retina is not influenced by removal of the sympathetic input to the head is predictable, because the light-sensitive part of the retina, pars optica retinae, is not innervated by the sympathetic nervous system; rather, this rhythm is controlled by an intrinsic circadian clock driving retinal circadian oscillations (Tosini and Menaker 1996; Iuvone et al. 2005; Tosini et al. 2008). However, the presented data are not incompatible with the possibility that light directly influences daily changes in Pax4 expression. Pineal/retinal differences in molecular regulation of rhythmic gene expression have been reported for Aanat in the rat retina; rhythmic expression of this gene seems to reflect the action of the retinal circadian clock mediated via E-box elements in the Aanat promoter (Chen and Baler 2000); accordingly, it will be of interest to determine whether a similar mechanism is also involved in circadian expression of Pax4 in the retina.
Supplementary Material
Acknowledgements
This study was supported by the Lundbeck Foundation, the Danish Medical Research Council (grant numbers 271-07-0412 and 271-06-0754), the Novo Nordisk Foundation, the Carlsberg Foundation, Fonden til Lægevidenskabens Fremme, Simon Fougner Hartmanns Familiefond and the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. We wish to thank Mrs Ursula Rentzmann for expert histological assistance.
Abbreviations used:
- Aanat
arylalkylamine N-acetyltransferase
- E
embryonic day
- P
postnatal day
- PBS
phosphate-buffered saline
- qRT-PCR
quantitative real-time PCR
- ZT
zeitgeber time
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Specificity control of radiochemical in situ hybridization analysis of Pax4 and Pax6 expression by use of sense probes.
Figure S2 Reverse transcription-PCR analysis of Pax4 mRNA isoforms in the rat retina.
Table S1 Primers used for reverse-transcription PCR analysis of Pax4 mRNA isoforms.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexiades MR and Cepko C (1996) Quantitative analysis of proliferation and cell cycle length during development of the rat retina. Dev. Dyn 205, 293–307. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R and Gruss P (2001) Pax6 lights up the way for eye development. Curr. Opin. Cell Biol 13, 706–714. [DOI] [PubMed] [Google Scholar]
- Babila T, Schaad NC, Simonds WF, Shinohara T and Klein DC (1992) Development of MEKA (phosducin), Gβ, Gγ and S-antigen in the rat pineal gland and retina. Brain Res 585, 141–148. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Mallamaci A, Briata P, Corte G and Boncinelli E (1997) Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J. Neurosci 17, 4243–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun T and Gauthier BR (2008) A focus on the role of Pax4 in mature pancreatic islet beta-cell expansion and survival in health and disease. J. Mol. Endocrinol 40, 37–45. [DOI] [PubMed] [Google Scholar]
- Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle E, Wollheim CB and Gauthier BR (2004) The diabetes-linked transcription factor Pax4 promotes beta-cell proliferation and survival in rat and human islets. J. Cell Biol 167, 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SC, Cragg H, Elrick LJ, Macfarlane WM, Shennan KIJ and Docherty K (1999) Inhibitory effect of Pax4 on the human insulin and islet amyloid polypeptide (IAPP) promoters. FEBS Lett 463, 53–57. [DOI] [PubMed] [Google Scholar]
- Cepko CL (1996) The patterning and onset of opsin expression in vertebrate retinae. Curr. Opin. Neurobiol 6, 542–546. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M and Ezzeddine D (1996) Cell fate determination in the vertebrate retina. Proc. Natl Acad. Sci. USA 93, 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W and Baler R (2000) The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Mol. Brain Res 81, 43–50. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang QL, Nie Z, Sun H, Lennon G, Copeland NG, Gilbert DJ, Jenkins NA and Zack DJ (1997) Crx, a novel Otx-like paired-homeodomain protein, binds to and transactivates photoreceptor cell-specific genes. Neuron 19, 1017–1030. [DOI] [PubMed] [Google Scholar]
- Chi N and Epstein JA (2002) Getting your Pax straight: Pax proteins in development and disease. Trends Genet 18, 41–47. [DOI] [PubMed] [Google Scholar]
- Collin JP (1971) Differentiation and regression of the cells of the sensory line in the epiphysis cerebri, in The Pineal Gland (Wol-stenholme GEW and Knight J, eds.), pp. 79–125. Churchill Livingstone, Edinburgh. [Google Scholar]
- Estivill-Torrús G, Vitalis T, Fernández-Llebrez P and Price DJ (2001) The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mech. Dev 109, 215–224. [DOI] [PubMed] [Google Scholar]
- Fujitani Y, Kajimoto Y, Yasuda T et al. (1999) Identification of a portable repression domain and an E1A-responsive activation domain in Pax4: a possible role of Pax4 as a transcriptional repressor in the pancreas. Mol. Cell. Biol 19, 8281–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Li T, Davis FC and Cepko CL (1999) Retinopathy and attenuated circadian entrainment in Crx-deficient mice. Nat. Genet 23, 466–470. [DOI] [PubMed] [Google Scholar]
- Gehring WJ (1998) Master control genes in development and evolution Yale University Press, New Haven. [Google Scholar]
- Gehring WJ (2005) New perspectives on eye development and the evolution of eyes and photoreceptors. J. Hered 96, 171–184. [DOI] [PubMed] [Google Scholar]
- Gonzales-Fernandes F and Healy JI (1990) Early expression of the gene for interphotoreceptor retinol-binding protein during photoreceptor differentiation suggests a critical role for the interphotoreceptor matrix in retinal development. J. Cell Biol 111, 2775–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley JC, Davidson DR and Hill RE (1995) The role of Pax-6 in eye and nasal development. Development 121, 1433–1442. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND and van Heyningen V (1991) Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, 522–525. [DOI] [PubMed] [Google Scholar]
- Ho AK, Somers RL and Klein DC (1986) Development and regulation of rhodopsin kinase in rat pineal and retina. J. Neuro-chem 46, 1176–1179. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC and Chaurasia SS (2005) Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res 24, 433–456. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Thomas MR and Neal MJ (1998) Expression of Pax-6 mRNA in the retinal degeneration (rd) mouse. Biochem. Biophys. Res. Commun 252, 236–240. [DOI] [PubMed] [Google Scholar]
- Kalousová A, Benes V, Paces J and Kozmik Z (1999) DNA binding and transactivating properties of the paired and homeodomain protein Pax4. Biochem. Biophys. Res. Commun 259, 510–518. [DOI] [PubMed] [Google Scholar]
- Klein DC (2004) The 2004 Aschoff/Pittendrigh lecture: Theory of the origin of the pineal gland – a tale of conflict and resolution. J. Biol. Rhythms 19, 264–279. [DOI] [PubMed] [Google Scholar]
- Klein DC, Weller JL and Moore RY (1971) Melatonin metabolism: neural regulation of pineal serotonin:acetyl coenzyme A N-acetyl-transferase activity. Proc. Natl Acad. Sci. USA 68, 3107–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroma BM, Yang JM and Sundin OH (1997) The Pax6 homeobox gene is expressed throughout the corneal and conjuctival epithelia. Invest. Ophthalmol. Vis. Sci 38, 108–120. [PubMed] [Google Scholar]
- Livesey FJ and Cepko CL (2001) Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci 2, 109–118. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F and Gruss P (2001) Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43–55. [DOI] [PubMed] [Google Scholar]
- Møller M and Baeres FMM (2002) The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res 309, 139–150. [DOI] [PubMed] [Google Scholar]
- Møller M, Phansuwan-Pujito P, Morgan KC and Badiu C (1997) Localization and diurnal expression of mRNA encoding the beta1-adrenoceptor in the rat pineal gland: an in situ hybridization study. Cell Tissue Res 288, 279–284. [DOI] [PubMed] [Google Scholar]
- Morgan M (2006) Hox genes: a continuation of embryonic patterning? Trends Genet 22, 67–69. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Belliveau MJ and Cepko CL (1998) Two phases of rod photoreceptor differentiation during rat retinal development. J. Neurosci 18, 3738–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida A, Furukawa A, Koike C, Tano Y, Aizawa S, Matsuo I and Furukawa T (2003) Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci 6, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Petersen HV, Jørgensen MC, Andersen FG, Jensen J, Nielsen TF, Jørgensen R, Madsen OD and Serup P (2000) Pax4 represses pancreatic glucagon gene expression. Mol. Cell. Biol. Res. Com-mun 3, 249–254. [DOI] [PubMed] [Google Scholar]
- Philips GT, Stair CN, Lee HY, Wroblewski E, Berberoglu MA, Brown NL and Mastick GS (2005) Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev. Biol 279, 308–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath MF, Muñoz E, Ganguly S, Morin F, Shi Q, Klein DC and Møller M (2006) Expression of the Otx2 homeobox gene in the developing mammalian brain: embryonic and adult expression in the pineal gland. J. Neurochem 97, 556–566. [DOI] [PubMed] [Google Scholar]
- Rath MF, Morin F, Shi Q, Klein DC and Møller M (2007) Ontogenetic expression of the Otx2 and Crx homeobox genes in the retina of the rat. Exp. Eye Res 85, 65–73. [DOI] [PubMed] [Google Scholar]
- Rath MF, Bailey MJ, Kim JS, Ho AK, Gaildrat P, Coon SL, Møller M and Klein DC (2008) Developmental and diurnal dynamics of Pax4 expression in the mammalian pineal gland: Nocturnal down-regulation is mediated by adrenergic-cyclic AMP signaling. Endocrinology 10.1210/en.2008-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz-Laser B, Estreicher A, Gauthier BR, Mamin A, Edlund H and Philippe J (2002) The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia 45, 97–107. [DOI] [PubMed] [Google Scholar]
- Sakamoto K and Ishida N (1998) Circadian expression of serotonin N-acetyltransferase mRNA in the rat retina. Neurosci. Lett 245, 113–116. [DOI] [PubMed] [Google Scholar]
- Smith SB, Ee HC, Conners JR and German M (1999) Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol. Cell. Biol 19, 8272–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa-Pineda B, Chowdhury K, Torres M, Oliver G and Gruss P (1997) The Pax4 gene is essential for differentiation of insulin-producing β-cells in the mammalian pancreas. Nature 386, 399–402. [DOI] [PubMed] [Google Scholar]
- Stanescu D, Iseli HP, Schwerdtfeger K, Ittner L, Remé CE and Hafezi F (2007) Continuous expression of the homeobox gene Pax6 in the ageing human retina. Eye 21, 90–93. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A and Gruss P (1997) Pax6 is required for differentiation of glucagon-producing α-cells in mouse pancreas. Nature 387, 406–409. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A and Hensch TK (2008) Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134, 386–387. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Yagui K, Sakurai K, Hashimoto N, Saito Y and Kanatsuka A (1998) Molecular cloning of rat Pax4: identification of four isoforms in rat insulinoma cells. Biochem. Biophys. Res. Commun 248, 153–156. [DOI] [PubMed] [Google Scholar]
- Tosini G and Menaker M (1996) Circadian rhythms in cultured mam-malian retina. Science 272, 419–421. [DOI] [PubMed] [Google Scholar]
- Tosini G, Pozdeyev N, Sakamoto K and Iuvone PM (2008) The circadian clock system in the mammalian retina. Bioessays 30, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman JE, Morabito MA and Barnstable CJ (1988) Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Mol. Cell. Biol 8, 1570–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C and Gruss P (1991) Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113, 1435–1449. [DOI] [PubMed] [Google Scholar]
- Wang J, Elghazi L, Parker SE, Kizilocak H, Asano M, Sussel L and Sosa-Pineda B (2004) The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic β-cell differentiation. Dev. Biol 266, 178–189. [DOI] [PubMed] [Google Scholar]
- Weidman TA and Kuwabara T (1969) Development of the rat retina. Invest. Ophthalmol. Vis. Sci 8, 60–69. [PubMed] [Google Scholar]
- Xu S, Sunderland ME, Coles BLK, Kam A, Holowacz T, Ashery-Padan R, Marquardt T, McInnes RR and van der Kooy D (2007) The proliferation and expansion of retinal stem cells require functional Pax6. Dev. Biol 304, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.