Abstract
Multiple drugs classes have shown incremental benefits in heart failure with reduced ejection fraction. Most of these trials were designed to achieve specific doses of the investigational agent. Clinical practice guidelines recommend using the same target dosing of therapies, as tolerated. However, with the increasing number of available therapies, clinicians face the challenge of simultaneously using several drugs, achieving target doses, and managing side effects that are often overlapping. Blood pressure, renal function, hyperkalemia, and other factors may impede achieving target doses of all medications, leaving clinicians with dilemmas as to how to sequence and dose these various classes of drugs. The guideline directed eligibility for certain drugs and devices requires stability on maximally tolerated doses of background therapies. However, significant variability exists in dosing achieved in clinical practice. We discuss the existing background data regarding the doses of heart failure medications in clinical trials and in practice, and provide recommendations on how to navigate this complex therapeutic decision-making.
Multiple drugs, including angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), beta-blockers, mineralocorticoid-receptor antagonists (MRAs), and isosorbide dinitrate and hydralazine combination, are now available for the management of patients with heart failure with reduced ejection fraction (HFrEF). Recently, ivabradine and an angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril/valsartan, have also shown incremental benefits. Clinical trials with these therapies tested them in addition to existing standard of care at the time the trial was being conducted, or in the case of ARNI, as a replacement for ACEi therapy. With the exception of the Systolic Heart failure treatment with the lf inhibitor ivabradine (SHIFT) trial, which titrated ivabradine to a targeted heart rate, all other HFrEF trials were guided by protocols designed to achieve specific target doses of the investigational agent, or the highest tolerated dose.
Guidelines recommend and the most optimal outcomes are achieved (including lower mortality) with target dosing of HFrEF guideline-directed medical therapies. However, with the increasing number of available medical therapies for HFrEF, clinicians now face the challenge of simultaneously using several drugs, achieving target doses, and managing drug side effects, which are often overlapping. Worsening heart failure (HF) symptoms, low blood pressure, or comorbidities like chronic kidney disease, hyperkalemia, chronic lung disease, etc. may impede achieving target doses of all medications, leaving clinicians with dilemmas as to how to sequence and dose these various drugs. Furthermore, the guideline-directed eligibility for certain drugs (including ivabradine) and devices requires stability on maximally-tolerated doses of background therapies. Significant variability exists in dosing achieved in clinical practice and optimization of HF medication dosing has not received the attention it deserves. Herein, we discuss the existing data regarding the doses of HF medications in trials and in practice, and provide recommendations on how to navigate this complex therapeutic decision-making.
SELECTION OF TARGET DOSES IN PHASE II TRIALS
Although exceptions do exist, drug dosing in early phase trials is often not titrated to specific pharmacodynamics or until a particular physiological response is achieved. Rather, starting from very low doses, therapies are successively titrated to higher doses until a maximally tolerated dose is identified based on symptoms, hemodynamics (e.g. blood pressure), or toxicity. Non-specific markers of improvement such as natriuretic peptide levels or left ventricular ejection fraction (EF) are often measured at such doses to inform future development efforts. However, such biological measures are not yet considered acceptable surrogates for identifying the maximally effective dose for a therapy. This lack of targeting a specific biologic response in HF drug development contrasts with other diseases like hypertension or diabetes mellitus. While there is evidence that higher doses of some therapies may provide modest incremental benefit, lower doses may still provide significant value compared with no therapy. Importantly, beta blockers result in left ventricular reverse remodeling in a dose-dependent manner, [1, 2] a mechanism hypothesized to mediate the mortality benefit with these agents. [3, 4] Further, similar dose-dependent benefits have been observed with higher-dose beta-blocker therapies on more definitive endpoints, such as HF hospitalizations and all-cause mortality. However, despite suggestion of modest benefits on clinical outcomes with high dose over lower dose ACEi and ARB therapy, to our knowledge no such dose-related benefits on cardiac remodeling have been shown to date with non-beta-blocker therapies. [5, 6]
DOSES OF BASELINE MEDICATIONS IN PHASE III CLINICAL TRIALS
Due to application of strict inclusion/exclusion criteria, patients in phase III drug trials are more likely to be on background evidence-based therapies than community-based populations. This may be related to the enrolled patient cohort, who tend to be younger, with fewer comorbid conditions, and/or better access to care than the HF population at large. [7–9] However, even in this setting, some patients in trials are not on all evidence-based therapies at baseline, let alone guideline-directed target doses (Table 1). In many trials, doses of baseline medications are not reported, or when reported, it is frequently not documented whether higher doses had been attempted but not tolerated. As such, the incremental value of novel drugs over ideal background therapy in every patient is almost never known. Instead, trials inform the incremental efficacy and safety over a regimen of guideline-directed medical therapy (GDMT) over a range of background therapy dosing. While it is expected that not all patients will be able to tolerate maximum doses of standard therapies prior to enrollment, trials generally recommend “optimization of HF medications” per HF guidelines prior to enrollment at the discretion of the local investigator. However, the “optimization” of background therapy called for in clinical trials is not standardized and rarely protocolized.
Table 1:
Baseline therapy in heart failure clinical trials
| Trial | Beta blockers | ACEi/ARB | MRA | ISDN | Digoxin | Loop diuretic |
|---|---|---|---|---|---|---|
| V-Heft [64] | 35% | 93% | - | - | 67% | 85% |
| CONSENSUS [65] | 3% | - | 52% | 46% | 93% | 98% |
| SOLVD Treatment Trial [11] | 8% | - | - | 51% | 67% | - |
| MERIT-HF [13] | - | 96% | - | - | 64% | 90% |
| COPERNICUS [66] | - | 97% | 20% | - | 66% | 99% |
| COMET [67] | - | 92% | 11% | 33% | 59% | 99% |
| RALES [14] | 11% | 95% | - | - | 74% | 100% |
| CHARM-Alternative [68] | 55% | - | 24% | 43% | 45% | 85% |
| A-HeFT [16] | 74% | 87% | 39% | - | 60% | 90% |
| EMPHASIS [69] | 87% | 93% | - | - | 27% | 85% |
ACEi/ARB: angiotensin converting enzyme inhibitor/angiotensin receptor blocker; MRA: mineralocorticoid receptor antagonist; ISDN: Isosorbide dinitrate
DOSES OF INVESTIGATIONAL DRUGS IN PHASE III CLINICAL TRIALS
While target doses of the investigational agent are specified per protocol in phase III trials and generally achieved in the majority of patients, there are some patients who are unable to tolerate such doses (Table 2). The Studies of Left Ventricular Dysfunction (SOLVD) Prevention trial in asymptomatic patients with an EF ≤35% targeted 20 mg but achieved only 12.7 mg daily of enalapril. [10] Inability to reach target dose was driven by dizziness, cough, and hypotension. The SOLVD Treatment trial demonstrated a significant reduction in mortality with enalapril [11] after achieving 16.6 mg daily dose while targeting 20 mg daily, a dose that was achieved in less than half the participants. Side effects were reported in 87% of the patients with enalapril, but high rates of side effects were also reported with placebo (82%). Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart failure (MERIT-HF) [12] showed a mortality reduction of 31% when targeting 200 mg/day. This was achieved in 64% of patients and the mean dose was 159 mg/day. [13] In the Carvedilol Prospective Randomized Cumulative Survival Study (COPERNICUS) [7] trial, 4 in 5 patients were able to reach the target dose of 50mg daily. Further, side effects reported with carvedilol were actually significantly less than those reported with placebo.
Table 2.
Doses of various interventions in heart failure clinical trials
| Trial | Drug | Target Daily Dose |
Percent Reaching Target |
Mean Daily Dose |
|---|---|---|---|---|
| V-HeFT [64] | Hydralazine/Isosorbide dinitrate | 112.5/160mg | 55% | 270mg/136mg |
| CONSENSUS [65] | Enalapril | 40mg | - | 18.4mg |
| SOLVD Treatment [11] | Enalapril | 20 mg | - | 16.6mg |
| MERIT-HF [13] | Metoprolol CR/XL | 200mg | 64% | 159mg |
| COPERNICUS [66] | Carvedilol | 50mg | 80% | 45mg |
| COMET [67] | Metoprolol tartrate/ carvedilol | 100/50mg | 75%/78% | 85mg/42 mg |
| RALES [14] | Spironolactone | 25mg | - | 26mg |
| CHARM-ALTERNATIVE [68] | Candesartan | 32mg | - | 23mg |
| A-HeFT [16] | Hydralazine/Isosorbide dinitrate | 225/120 mg | 68% | 143mg/76mg |
| EMPHASIS [69] | Eplerenone | 50mg | 60.2% | 39.1 mg |
In the Randomized Aldactone Evaluation Study (RALES), the MRA, spironolactone, was compared to placebo starting at 25 mg with an option to titrate up to 50 mg at 8 weeks.[14] Mean daily dose in the trial was 26 mg, and at this dose a decrease in mortality of 30% was observed compared to placebo. Titration in RALES was limited by severe hyperkalemia in only 2% of patients; however, widespread use of spironolactone following the publication of RALES led to an increase in hospitalizations for hyperkalemia shortly after the trial was published.[15] This was largely attributed to sub-optimal prescribing, laboratory monitoring, and follow-up practices; as such, detailed guidelines were developed to guide appropriate patient selection for MRA therapy and on-treatment monitoring.
The African-American Heart Failure Trial (A-HeFT) trial demonstrated improved survival among patients self-identifying as African American with the use of fixed-dose isosorbide dinitrate (20 mg) and hydralazine (37.5 mg) three times daily vs. placebo.[16] In the trial, doses were titrated to target dose in only 68% of patients with a mean number of tablets per day of 3.8. Almost 30% of patients complained of dizziness with use of the study drug. It is notable that a substantial number of patients in HF trials receiving placebo have high rates of side effects reported or are not able to achieve target dosing of placebo.
The Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) compared sacubitril/valsartan (at a dose of 200 mg twice daily) to enalapril (at a dose of 10 mg twice daily) and demonstrated improved survival with ARNI as compared with ACEi.[8] Secondary analysis showed similar magnitude benefit with ARNI over ACE inhibitor for patients who achieved either low, or intermediate, or high doses.[17] [8] However, the dosing achieved in the PARADIGM-HF trial must be viewed in the context of a study design including a run-in period, in contrast to most prior landmark HFrEF trials. Specifically, 20% of patients enrolled in the trial failed the run-in period and did not receive randomized therapy, with the most common reason for run-in failure being adverse events.
The above examples highlight that oftentimes symptoms intrinsic to worsening HF and symptoms specific to a HF medication itself can be difficult to differentiate. Conceptualizing this difference is important in medication management in response to any given symptom. Greater achievement of target dosing of investigational therapies in clinical trials and routine practice may be facilitated by enhanced recognition of background adverse effects related to HF in placebo-treated patients. It is important to note that in most trials the eligibility criteria exclude patients at higher risk for side effects and intolerance, e.g. advanced age, severe comorbid conditions, hypotension, or poor renal function.
DOSE RANGING PHASE III CLINICAL TRIALS
Randomized dose-ranging clinical trials assessing outcomes are rare in HF. The Assessment of Treatment with Lisinopril and Survival (ATLAS) trial randomized 3164 patients with EF ≤30% [5] to low- (2.5–5 mg) or high-dose (32.5–35 mg) lisinopril. There was no significant difference between the two groups for all-cause mortality, but the combined endpoint of all-cause mortality and HF hospitalization was reduced by 15% with high dose (p<0.001). The Heart Failure Endpoint evaluation of Angiotensin II Antagonist Losartan (HEAAL) study enrolled 3846 patients with EF ≤40% to high- (150 mg) or low-dose (50 mg) losartan. [6] High dose was not associated with improved mortality but there was a 13% reduced risk for HF hospitalizations (p=0.03). Higher doses were associated with modest increase in reversible adverse events including hyperkalemia, hypotension, and renal impairment. Based on these results, there are some benefits between low and high dose of ACEi or ARBs, especially with respect to morbidity more than mortality, and the difference in efficacy between intermediate and high doses are likely to be more modest.
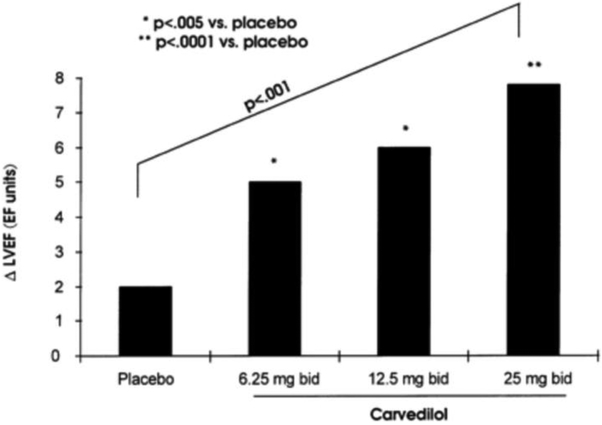
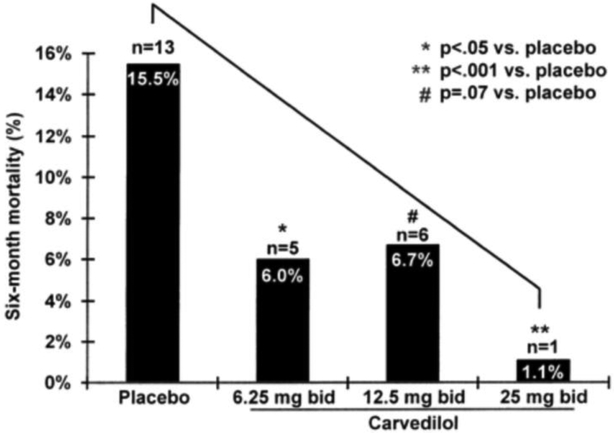
A smaller trial evaluated doses of carvedilol in HFrEF. [18] [2] Target and achieved doses were similar in the three dosing groups (achieved/target: 6.25±0/6.25 mg; 12.3±1.1/12.5 mg; and 23.7±4.0/25 mg). There were dose-related improvements in EF (Figure 1a), cardiovascular hospitalizations, and mortality (Figure 1b); however, there were relatively few events in this study. In addition, the Cardiac Insufficiency Bisoprolol Study (CIBIS) I trial utilized bisoprolol 5 mg daily as a target dose, yet failed to achieve a significant mortality reduction. With CIBIS II, utilizing bisoprolol 10 mg daily as a target dose, a statistically significant 34% mortality reduction was achieved; however there are certainly limitations with cross trial comparisons.
Figure 1: Dose ranging effect of carvedilol.
Ejection fraction (2a), and 6-month mortality (2b).
There are no dose ranging studies with MRAs for clinical efficacy. One small study randomized patients to 1 of 5 parallel treatment groups: placebo or spironolactone at a single daily dose of 12.5, 25, 50, or 75 mg for 12 weeks for safety and tolerability assessment. Definitive clinical outcomes were not evaluated in this small study but the incidence of hyperkalemia (serum potassium ≥ 5.5 mmol/L) was 5% among patients receiving placebo, vs. 5%, 13%, 20%, and 24% for the 12.5-, 25-, 50- and 75-mg spironolactone treatment groups, respectively. [19] Due to these dose-dependent risks of hyperkalemia, clinicians tend to prefer lower-dose MRA therapy and alternative dosing strategies than employed in pivotal clinical trials. More data are needed regarding the efficacy of such low MRA doses and greater attention is needed to achieving target doses in clinical practice. Alternatively, a lower risk of hyperkalemia with ARNI therapy as compared to ACEi may allow for safer uptitration of concurrent MRA therapy. [20] Similarly, there is no significant dose ranging data evaluating hydralazine or nitrates, including use of either agent alone. [21]
DOSE RESPONSE RELATIONSHIP IN SECONDARY ANALYSIS OF CLINICAL TRIALS
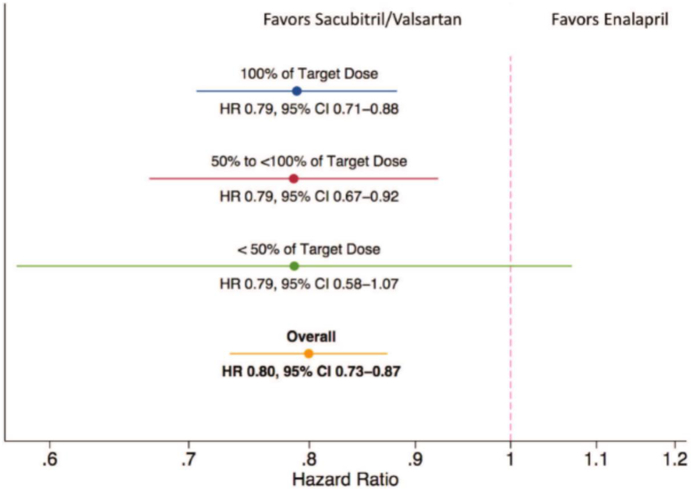
Secondary analysis of clinical trials has shown benefits with high vs. low dose therapy, [22] [23] e.g. in the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) trial, [24] higher beta blocker dose was associated with improved outcomes. However, despite multivariate modeling, such results are confounded by the clinical stability of patients who were able to tolerate higher doses. Even then, the data are inconsistent. In the PARADIGM-HF trial,[8] those needing dose reduction at some point during the trial were at higher risk of events. However, the magnitude of benefit with low, moderate, or high dose sacubitril/valsartan relative to corresponding enalapril doses were similar. [25] (Figure 2) These findings suggest that ARNI offers advantages to ACEi across the dosing range. However, these data do not specifically address differences in efficacy and safety between lower and higher ARNI doses.
Figure 2: Outcomes with sacubitril/valsartan relative to enalapril.
Participants taking lower than target sacubitril/valsartan doses had a lower risk of the primary event compared with those taking similar doses of enalapril.
DOSES USED IN CLINICAL PRACTICE
Practice guidelines recommend the use of evidence-based medications at trial recommended doses for all HF patients, as tolerated. [26, 27] However, in clinical practice, dosing typically falls short of that achieved in clinical trials. For instance, in the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) registry, among cardiology practices at baseline, only a third of the patients were treated at target doses of ACEi [9] and 20% at beta-blocker target doses. [28] This is in contrast with the rates of target dosing achieved in clinical trials, e.g. 59% in COPERNICUS trial, 64% in MERIT-HF, and 84% in the Valsartan Heart Failure Trial [Val-HeFT]. [29–31] In addition, data from the quality of adherence to guideline recommendations for life-saving treatment in heart failure: an international survey (QUALIFY) an international prospective observational longitudinal survey of 7,092 HF outpatients found that the proportion of patients at target dose of HF medication was low (28% for ACEi, 15% for beta-blockers, 7% for ARBs, and 7% for ivabradine). [32] Another study showed that only 7% of eligible African American outpatients with HF were actually receiving the recommended therapy with combination hydralazine/isosorbide dinitrate. [33] Furthermore, in a registry of hospitalized HF patients, only 22% of African Americans and 13% of all eligible patients were discharged on hydralazine/isosorbide dinitrate. [34] Although these findings in aggregate may be related to less effort and attention to achieving target dosing, it may also reflect the ability of “real-world” patients to tolerate GDMT achieved in clinical trials. Indeed, in practice, there are often challenges with up-titrating therapies, especially medications with potential for hypotension and renal dysfunction side effects, and in patients with co-morbid conditions such as chronic obstructive pulmonary disease (COPD). Oftentimes there is concern regarding beta-blockers in this population given the increased risk of hospitalization for COPD with beta-blocker use, especially non-selective beta-blockers such as carvedilol. [35] Studies show that patients with HF and COPD are prescribed all HF medications at a lower rate than those without COPD, however the discrepancy is most pronounced with beta-blockers. [36]
In dedicated HF disease management programs and studies utilizing standardized protocol for medication dosing, it has been suggested a majority of patients can achieve target dosing. [37] Without systematic approaches to care for these challenging patients it may be more difficult to reach target doses GDMT [9] for many reasons. Yet, such effort is worthwhile; in the Coreg (carvedilol) Heart Failure Registry (COHERE) study, despite enrolling older patients with substantial comorbidities, most patients were able to achieve target doses of beta-blockers with a focused effort. [37]
TRADEOFFS WITH TARGET DOSES OF DIFFERENT MEDICATION CLASSES
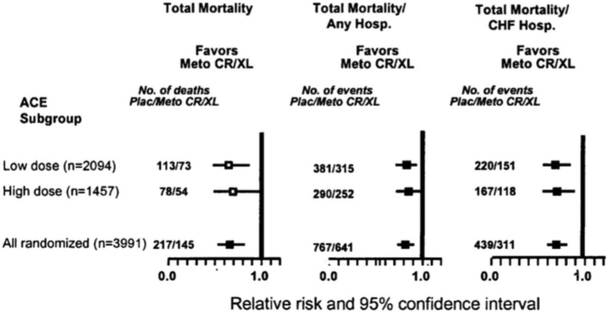
Though many patients can tolerate target doses of GDMT, there are many for which this may not be the case. Nonetheless, even in these circumstances, achievement of target dosing for at least 1 therapeutic class of medication may be possible. For example, patients on low doses of ACEi in the MERIT-HF trial were able to tolerate higher doses of beta-blockers in modestly higher proportion. [38] The mortality benefit of combined beta-blocker and ACEi therapy was apparent in both the low- and high-dose ACEi groups (Figure 3). [38] Similar analysis from COPERNICUS showed that the outcomes improved to a similar degree with carvedilol in patients receiving various doses of ACEi. [39]
Figure 3: Benefit with high vs. low dose ACE inhibitor on top of beta-blocker therapy.
Point estimates of relative risk and 95% confidence intervals in the two angiotensin-converting enzyme inhibitor dose groups for various outcomes.
BIOMARKER GUIDED MEDICATION TITRATION
The natriuretic peptides, B-type natriuretic peptide (BNP) and N-terminal pro–B-type natriuretic peptide (NT-proBNP), have demonstrated both diagnostic and prognostic value in patients with HF. [40, 41] A decrease in natriuretic peptide levels over a period of follow-up has been associated with improved outcomes, including morbidity and mortality. [42, 43] Importantly, HF therapy guided by natriuretic peptides has not been shown to improve outcomes in HF patients [44] [45] Beta-blockers have been shown to substantially decrease natriuretic peptide levels in the long run, [46] as have ACEi/ARBs [47] and MRAs. [48] One recent study demonstrated improved outcomes with patients who attained a significant reduction in NT-proBNP <1000; importantly, treatment with sacubitril/valsartan was nearly twice as likely as enalapril to achieve reductions in NT-proBNP to this level. [49] More data are needed regarding doses of medications and their interaction with natriuretic peptides, or other biomarkers, and clinical outcomes; and importantly if doses should be specifically titrated to achieve specific biomarker levels rather than the current recommendation for maximally tolerated dosing.
PRACTICAL OPTIONS TO MAXIMIZE TARGET DOSING
While there is little to no evidence on what specific strategies work best, given the benefits of target dosing highlighted above, we propose practical considerations for providers. Although these strategies are largely empiric and require prospective validation, they hold promise. Every effort to maximize target dosing of HF therapy should be made as evidence suggests that target doses of at least select components of GDMT may reduce mortality and morbidity. [26, 27] Furthermore, more structured implementation and employment of a dedicated nurse facilitator may improve guideline-directed dose titration. In a small, randomized clinical trial, target dosing of beta-blockers was achieved to a greater extent over a median of 12 months in the nursing facilitator group compared with routine clinical practice. [50] Although this study did not find utility of clinical reminders to patients and providers, new algorithms leveraging natural language processing in the electronic health records may allow for specific targeting of patients at suboptimal dosing regimens. [51] In many cases, nurse- or pharmacist-driven dosing protocols can result in faster up-titration with more frequent visits and greater number of medication changes. This may partly relate to developing better patient rapport, improving recognition of common adverse drug-related effects, and appropriately responding to patient symptom reporting.
Appropriate blood pressure and heart rate targets need to be clearly defined, targeting symptomatic blood pressure and heart rate reduction as indications for stopping uptitration as opposed to arbitrary asymptomatic thresholds. However, this does require close patient monitoring and careful history taking.[7, 12] If symptomatic hypotension prevents adequate uptitration of GDMT, providers should consider potential hypovolemia (with concomitant reduction in diuretics, if appropriate), or discontinuation of any medications that lower blood pressure without proven outcomes benefits in HFrEF patients (e.g. calcium channel blockers). Patients should be counseled on the importance of their medication, the concept of “target doses” (to mitigate resistance to frequent titration), and how to manage minor side effects with lifestyle changes, e.g. avoidance of sudden changes in posture to attenuate orthostatic symptoms. Switching agents within a drug class may also improve tolerance to GDMT. [52] Splitting of dosing regimens over a 24-hour period and avoiding intake of all vasoactive medications at once may limit blood pressure swings. Furthermore, referral to a cardiologist or HF program for assistance can often help if patients are unable to reach target dosing in the primary care setting. [53] (Table 3)
Table 3:
Practical considerations for maximal medical therapy in heart failure
| Concern | Solution |
|---|---|
| Clinical time constraints |
|
| Low blood pressure to add new medications |
|
| Orthostatic symptoms |
|
| Low enough blood pressure/heart rate to titrate medications |
|
| Fatigue |
|
PRACTICAL CONSIDERATIONS FOR MANAGEMENT OF OVERLAPPING MEDICATION SIDE EFFECTS
Challenges achieving target dosing are particularly germane in patients with borderline blood pressure or renal function. Many patients with HFrEF are elderly or have concomitant diabetes mellitus, which further exaggerates these risks. [54] For patients with borderline blood pressure, it is uncertain whether to use higher doses of ACEi/ARB/ARNI and lower doses of beta blockers, or vice versa. A similar conundrum exists for the use of MRAs and ACEi/ARB/ARNI for those at risk for hyperkalemia or renal dysfunction. [55] In some cases, using moderate to higher doses of one class of agents may completely preclude the use of other medication altogether.
For the broader HFrEF population, there are four biologic targets that have been shown to improve outcomes, including angiotensin II, norepinephrine, aldosterone, and vasoactive peptides. [56, 57] In general, it is the best practice to target all of these pathways and not leave one unattended. Ivabradine is a special case targeting elevated heart rate but only in patients who are in sinus rhythm on maximally-tolerated beta-blocker doses. [58] For African American patients who have persistently limiting class III and IV symptoms despite achieving optimal therapy otherwise, addition of hydralazine and isosorbide dinitrate is further recommended.
Data suggests that achieving high doses of one therapy and not focusing on other therapies is less beneficial. The CIBIS III trial [59–61] randomized patients to either an initial strategy of ACEi or beta-blockers. Whichever drug was started first, either the ACEi or the beta-blocker, ended up achieving higher relative doses than the medication started second. While there were no differences in outcomes overall with either strategy, a strong predictor of outcome was whether the patient was on monotherapy for 6 months before the second class of drug was initiated. Thus, these data suggest targeting all relevant pathways is more important than achieving higher doses of one and ignoring other drugs. [59–61] This is especially important considering the fact that other than beta-blockers, the dose response data with other agents is less robust.
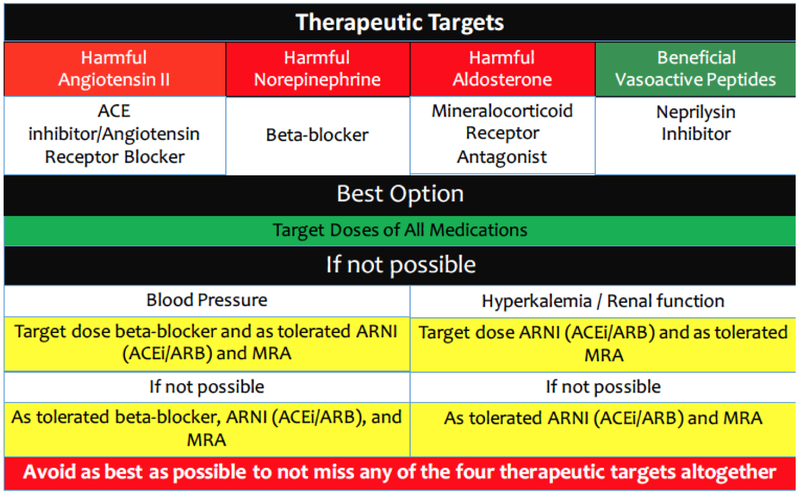
Recently, the American College of Cardiology Expert Consensus Pathway for HF Therapies addressed some of the issues of dosing of various medications (Figure 4)[62]:
Figure 4:
Suggested hierarchy of medication titration in heart failure.
In all patients, it is best to achieve maximum doses of all four biologic targets including angiotensin II modulation, beta blockade, aldosterone antagonism, and neprilysin inhibition.
If this is not possible, then the second-best option is to use lower doses of all drugs rather than higher doses of one and omitting another.
If the patient is able to tolerate higher doses of one but lower doses of the other therapy due to blood pressure, then preferences should to be given to beta blockers over angiotensin II modulation based on better dose response data with adrenergic blockade. [1] [18] [2]
If concerns are related to renal function or hyperkalemia, then higher doses of angiotensin II modulating drugs should be preferred, with lower doses of MRA used. [5] [6] Secondary analyses from PARADIGM-HF suggest that there may be less hyperkalemia with ARNI vs. ACEi, therefore sacubitril/valsartan may have an advantage in these settings. [8]
Table 4:
Target doses of heart failure medications from clinical trials [70]
| Starting dose (mg) | Target dose (mg) | |
|---|---|---|
| ACE Inhibitors | ||
| Captopril | 6.25 mg thrice a day | 50 mg thrice a day |
| Enalapril | 2.5 mg twice a day | 10–20 mg twice a day |
| Fosinopril | 5–10 mg daily | 40 mg daily |
| Lisinopril | 2.5–5.0 mg daily | 20–40 mg daily |
| Ramipril | 1.25–2.5 mg daily | 10 mg daily |
| Quinapril | 5 mg twice a day | 20 mg twice a day |
| Trandolapril | 1 mg daily | 4 mg daily |
| Angiotensin receptor blockers | ||
| Candesartan | 4–8 mg daily | 32 mg daily |
| Valsartan | 20–40 mg twice a day | 160 mg twice a day |
| Losartan | 25–50 mg daily | 50–150 mg daily |
| Angiotensin receptor neprilysin inhibitor | ||
| Sacubitril/valsartan | 49/51 mg twice a day | 97/103 mg twice a day |
| Beta-blockers | ||
| Bisoprolol | 1.25 mg daily | 10 mg daily |
| Carvedilol | 3.125 mg twice a day | 50 mg twice a day |
| Metoprolol succinate | 12.5–25 mg daily | 200 mg daily |
| Nebivolol | 1.25 mg daily | 10 mg daily |
| Mineralocorticoid-receptor antagonists | ||
| Eplerenone | 25 mg daily | 50 mg daily |
| Spironolactone | 12.5–25 mg daily | 25 mg daily/twice a day |
| If-channel blocker | ||
| Ivabradine | 5 mg twice a day | 7.5 mg twice a day |
| Isosorbide dinitrate and hydralazine | ||
| Hydralazine | 25–50 mg thrice a day | 100 mg thrice a day |
| Isosorbide dinitrate | 20–30 mg thrice a day | 40mg thrice a day |
FUTURE RESEARCH AND CLINICAL CONSIDERATIONS.
Given the general lack of biologically-guided therapeutic targets in HF, the priority should be to conduct phase II trials with more focus on dose identification based on pharmacodynamics profile rather than tolerability, through either molecular imaging or soluble biomarkers as targets. In phase III trials, increased focus should be placed on investigating the safety and efficacy of HF medications in a randomized, controlled manner. Post-approval quality improvement and education efforts should focus on achieving doses targeted in clinical trials (Table 4), recognizing that in real life, relatively fewer patients achieve target doses for a variety of reasons. Available evidence suggests existence of dose-response curves for many HF medications with improved outcomes at higher doses, with beta-blockers having the strongest such relationship. As such, strong emphasis is warranted on maximally targeting each pathway known to improve HF outcomes, including angiotensin II, beta-blockade, aldosterone, and vasoactive peptides. If a patient is unable to tolerate maximal doses of all medications, lower doses of all medications are preferred over a high dose therapy of one and no coverage of other pathways. Side effects and tolerability are emerging as major concerns in contemporary HF drug development. [63] While the magnitude of benefit may be debated, lower doses are nevertheless associated with benefit. It is critical for clinicians to recognize the important contribution of each targeted pathway in the HF armamentarium and to maximize each of these therapies to the highest tolerated dose. Combining practical approaches with sound clinical judgment to optimize this important aspect of HF patient care is the key to improving outcomes for HF patient
Acknowledgments
DISCLOSURES:
CY, and AA report no disclosures. CNM reports consulting for Abbott and St. Jude Medical. GCF reports research funding from NIH and consultant to Amgen, Janssen, Novartis, Medtronic, and St Jude Medical. SDA reports research support from DZHK Germany, European Union, Vifor International & Abbott Vascular, and consultant to Astra-Zeneca, Bayer, Boehringer Ingelheim, CVRx, Janssen, Novartis, Servier and Vifor International. MV is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). SJG is supported by the NHLBI T32 postdoctoral training grant (5T32HL069749–14) and a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis. JLJ report received grant support from Roche Diagnostics, Siemens, Singulex, Novartis, and Prevencio, and consulting for Roche Diagnostics, Critical Diagnostics, Abbott, Philips, and Novartis, and participates in clinical endpoint committees/data safety monitoring boards for Novartis, Amgen, Janssen, and Boehringer Ingelheim. MG reports being consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, CorThera, Cytokinetics, DebioPharm SA, Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical and Trevena Therapeutics. GF report consults to Bayer, Boehringer Ingelheim, Novartis, and Servier. JB report research support from the NIH, European Union, and Patient Centered Outcomes Research Institute, and consultant to Amgen, Astra-Zeneca, Bayer, Boehringer Ingelheim, BMS, CVRx, Janssen, Luitpold, Medtronic, Novartis, Relypsa, Vifor, ZS Pharma.
REFERENCES
- 1.Olsen SL, et al. , Carvedilol improves left ventricular function and symptoms in chronic heart failure: a double-blind randomized study. J Am Coll Cardiol, 1995. 25(6): p. 1225–31. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, et al. , Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation, 1996. 94(11): p. 2807–16. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa T, et al. , Cardiac adrenergic receptor effects of carvedilol. Eur Heart J, 1996. 17 Suppl B: p. 8–16. [DOI] [PubMed] [Google Scholar]
- 4.Bristow MR and Gilbert EM, Improvement in cardiac myocyte function by biological effects of medical therapy: a new concept in the treatment of heart failure. Eur Heart J, 1995. 16 Suppl F: p. 20–31. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, et al. , Comparative effects of low and high doses of the angiotensin-converting enzyme inhibitor, lisinopril, on morbidity and mortality in chronic heart failure. ATLAS Study Group. Circulation, 1999. 100(23): p. 2312–8. [DOI] [PubMed] [Google Scholar]
- 6.Konstam MA, et al. , Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet, 2009. 374(9704): p. 1840–8. [DOI] [PubMed] [Google Scholar]
- 7.Packer M, et al. , Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med, 2001. 344(22): p. 1651–8. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJ, et al. , Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med, 2014. 371(11): p. 993–1004. [DOI] [PubMed] [Google Scholar]
- 9.Heywood JT, et al. , Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy: report from IMPROVE HF. Circ Heart Fail, 2010. 3(5): p. 596–605. [DOI] [PubMed] [Google Scholar]
- 10.Investigators S, et al. , Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med, 1992. 327(10): p. 685–91. [DOI] [PubMed] [Google Scholar]
- 11.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med, 1991. 325(5): p. 293–302. [DOI] [PubMed] [Google Scholar]
- 12.Hjalmarson A, et al. , Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA, 2000. 283(10): p. 1295–302. [DOI] [PubMed] [Google Scholar]
- 13.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet, 1999. 353(9169): p. 2001–7. [PubMed] [Google Scholar]
- 14.Pitt B, et al. , The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med, 1999. 341(10): p. 709–17. [DOI] [PubMed] [Google Scholar]
- 15.Juurlink DN, et al. , Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med, 2004. 351(6): p. 543–51. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AL, et al. , Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med, 2004. 351(20): p. 2049–57. [DOI] [PubMed] [Google Scholar]
- 17.Vardeny O, et al. , Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail, 2016. 18(10): p. 1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osterziel KJ, Carvedilol in heart failure: the MOCHA trial. Circulation, 1997. 96(8): p. 2742; author reply 2743–4. [PubMed] [Google Scholar]
- 19.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol, 1996. 78(8): p. 902–7. [DOI] [PubMed] [Google Scholar]
- 20.Desai AS, et al. , Reduced Risk of Hyperkalemia During Treatment of Heart Failure With Mineralocorticoid Receptor Antagonists by Use of Sacubitril/Valsartan Compared With Enalapril: A Secondary Analysis of the PARADIGM-HF Trial. JAMA Cardiol, 2017. 2(1): p. 79–85. [DOI] [PubMed] [Google Scholar]
- 21.Cole RT, et al. , Hydralazine and isosorbide dinitrate in heart failure: historical perspective, mechanisms, and future directions. Circulation, 2011. 123(21): p. 2414–22. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar SR, et al. , Do evidence-based treatments provide incremental benefits to patients with congestive heart failure already receiving angiotensin-converting enzyme inhibitors? A secondary analysis of one-year outcomes from the Assessment of Treatment with Lisinopril and Survival (ATLAS) study. Clin Ther, 2004. 26(5): p. 694–703. [DOI] [PubMed] [Google Scholar]
- 23.Fiuzat M, et al. , Heart Rate or Beta-Blocker Dose? Association With Outcomes in Ambulatory Heart Failure Patients With Systolic Dysfunction: Results From the HF-ACTION Trial. JACC Heart Fail, 2016. 4(2): p. 109–15. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, et al. , Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA, 2009. 301(14): p. 1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vardeny O, et al. , Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yancy CW, et al. , 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation, 2017. [DOI] [PubMed] [Google Scholar]
- 27.Hunt SA, et al. , ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation, 2005. 112(12): p. e154–235. [DOI] [PubMed] [Google Scholar]
- 28.Gheorghiade M, et al. , Medication dosing in outpatients with heart failure after implementation of a practice-based performance improvement intervention: findings from IMPROVE HF. Congest Heart Fail, 2012. 18(1): p. 9–17. [DOI] [PubMed] [Google Scholar]
- 29.Cohn JN, Tognoni G, and Valsartan I Heart Failure Trial, A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med, 2001. 345(23): p. 1667–75. [DOI] [PubMed] [Google Scholar]
- 30.Krum H, et al. , Effects of initiating carvedilol in patients with severe chronic heart failure: results from the COPERNICUS Study. JAMA, 2003. 289(6): p. 712–8. [DOI] [PubMed] [Google Scholar]
- 31.Wikstrand J, et al. , How should subgroup analyses affect clinical practice? Insights from the Metoprolol Succinate Controlled-Release/Extended-Release Randomized Intervention Trial in Heart Failure (MERIT-HF). Card Electrophysiol Rev, 2003. 7(3): p. 264–75. [DOI] [PubMed] [Google Scholar]
- 32.Komajda M, et al. , Physicians’ adherence to guideline-recommended medications in heart failure with reduced ejection fraction: data from the QUALIFY global survey. Eur J Heart Fail, 2016. 18(5): p. 514–22. [DOI] [PubMed] [Google Scholar]
- 33.Yancy CW, et al. , Adherence to guideline-recommended adjunctive heart failure therapies among outpatient cardiology practices (findings from IMPROVE HF). Am J Cardiol, 2010. 105(2): p. 255–60. [DOI] [PubMed] [Google Scholar]
- 34.Golwala HB, et al. , Use of hydralazine-isosorbide dinitrate combination in African American and other race/ethnic group patients with heart failure and reduced left ventricular ejection fraction. J Am Heart Assoc, 2013. 2(4): p. e000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sessa M, et al. , Relationship between heart failure, concurrent chronic obstructive pulmonary disease and beta-blocker use: a Danish nationwide cohort study. Eur J Heart Fail, 2017. [DOI] [PubMed] [Google Scholar]
- 36.Canepa M, et al. , Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur J Heart Fail, 2017. [DOI] [PubMed] [Google Scholar]
- 37.Franciosa JA, et al. , Beta-blocker therapy for heart failure outside the clinical trial setting: findings of a community-based registry. Am Heart J, 2004. 148(4): p. 718–26. [DOI] [PubMed] [Google Scholar]
- 38.Ghali JK, et al. , Consistency of the beneficial effect of metoprolol succinate extended release across a wide range dose of angiotensin-converting enzyme inhibitors and digitalis. J Card Fail, 2004. 10(6): p. 452–9. [DOI] [PubMed] [Google Scholar]
- 39.Krum H, et al. , Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. Eur J Heart Fail, 2004. 6(7): p. 937–45. [DOI] [PubMed] [Google Scholar]
- 40.Januzzi JL Jr., et al. , Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med, 2006. 166(3): p. 315–20. [DOI] [PubMed] [Google Scholar]
- 41.Fonarow GC, et al. , Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol, 2007. 49(19): p. 1943–50. [DOI] [PubMed] [Google Scholar]
- 42.Hartmann F, et al. , Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation, 2004. 110(13): p. 1780–6. [DOI] [PubMed] [Google Scholar]
- 43.Bettencourt P, et al. , N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation, 2004. 110(15): p. 2168–74. [DOI] [PubMed] [Google Scholar]
- 44.Stienen S, et al. , NT-proBNP-Guided Therapy in Acute Decompensated Heart Failure: The PRIMA II Randomized Controlled Trial. Circulation, 2017. [DOI] [PubMed] [Google Scholar]
- 45.Felker GM, et al. , Effect of Natriuretic Peptide-Guided Therapy on Hospitalization or Cardiovascular Mortality in High-Risk Patients With Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA, 2017. 318(8): p. 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frantz RP, et al. , Carvedilol therapy is associated with a sustained decline in brain natriuretic peptide levels in patients with congestive heart failure. Am Heart J, 2005. 149(3): p. 541–7. [DOI] [PubMed] [Google Scholar]
- 47.Masson S, et al. , Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol, 2008. 52(12): p. 997–1003. [DOI] [PubMed] [Google Scholar]
- 48.Tsutamoto T, et al. , Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol, 2001. 37(5): p. 1228–33. [DOI] [PubMed] [Google Scholar]
- 49.Zile MR, et al. , Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J Am Coll Cardiol, 2016. 68(22): p. 2425–2436. [DOI] [PubMed] [Google Scholar]
- 50.Ansari M, et al. , Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation, 2003. 107(22): p. 2799–804. [DOI] [PubMed] [Google Scholar]
- 51.Vaduganathan M, et al. , Integrating electronic health records into the study of heart failure: promises and pitfalls. Eur J Heart Fail, 2017. [DOI] [PubMed] [Google Scholar]
- 52.Butler J, et al. , Tolerability to beta-blocker therapy among heart failure patients in clinical practice. J Card Fail, 2003. 9(3): p. 203–9. [DOI] [PubMed] [Google Scholar]
- 53.Bhatt AS, et al. , Achieving a Maximally Tolerated beta-Blocker Dose in Heart Failure Patients: Is There Room for Improvement? J Am Coll Cardiol, 2017. 69(20): p. 2542–2550. [DOI] [PubMed] [Google Scholar]
- 54.Maeder MT, et al. , Incidence, clinical predictors, and prognostic impact of worsening renal function in elderly patients with chronic heart failure on intensive medical therapy. Am Heart J, 2012. 163(3): p. 407–14, 414 e1. [DOI] [PubMed] [Google Scholar]
- 55.Vardeny O, et al. , Incidence, predictors, and outcomes related to hypo- and hyperkalemia in patients with severe heart failure treated with a mineralocorticoid receptor antagonist. Circ Heart Fail, 2014. 7(4): p. 573–9. [DOI] [PubMed] [Google Scholar]
- 56.Triposkiadis F, et al. , The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol, 2009. 54(19): p. 1747–62. [DOI] [PubMed] [Google Scholar]
- 57.Braunwald E, Heart failure: pathophysiology and treatment. Am Heart J, 1981. 102(3 Pt 2): p. 486–90. [DOI] [PubMed] [Google Scholar]
- 58.Teerlink JR, Ivabradine in heart failure--no paradigm SHIFT...yet. Lancet, 2010. 376(9744): p. 847–9. [DOI] [PubMed] [Google Scholar]
- 59.Willenheimer R, et al. , Effect on survival and hospitalization of initiating treatment for chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence: results of the randomized Cardiac Insufficiency Bisoprolol Study (CIBIS) III. Circulation, 2005. 112(16): p. 2426–35. [DOI] [PubMed] [Google Scholar]
- 60.Sliwa K, et al. , Impact of initiating carvedilol before angiotensin-converting enzyme inhibitor therapy on cardiac function in newly diagnosed heart failure. J Am Coll Cardiol, 2004. 44(9): p. 1825–30. [DOI] [PubMed] [Google Scholar]
- 61.Funck-Brentano C, et al. , Influence of order and type of drug (bisoprolol vs. enalapril) on outcome and adverse events in patients with chronic heart failure: a post hoc analysis of the CIBIS-III trial. Eur J Heart Fail, 2011. 13(7): p. 765–72. [DOI] [PubMed] [Google Scholar]
- 62.Yancy CW, et al. , 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol, 2018. 71(2): p. 201–230. [DOI] [PubMed] [Google Scholar]
- 63.Butler J, et al. , Exploring New Endpoints for Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail, 2016. 9(11). [DOI] [PubMed] [Google Scholar]
- 64.Cohn JN, et al. , Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med, 1986. 314(24): p. 1547–52. [DOI] [PubMed] [Google Scholar]
- 65.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med, 1987. 316(23): p. 1429–35. [DOI] [PubMed] [Google Scholar]
- 66.Packer M, et al. , Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med, 2001. 344(22): p. 1651–8. [DOI] [PubMed] [Google Scholar]
- 67.Poole-Wilson PA, et al. , Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet, 2003. 362(9377): p. 7–13. [DOI] [PubMed] [Google Scholar]
- 68.Granger CB, et al. , Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet, 2003. 362(9386): p. 772–6. [DOI] [PubMed] [Google Scholar]
- 69.Zannad F, et al. , Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med, 2011. 364(1): p. 11–21. [DOI] [PubMed] [Google Scholar]
- 70.Yancy CW, et al. , 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail, 2017. [Google Scholar]