Abstract
Background:
Post-treatment morbidity among subjects with drug-resistant tuberculosis (DR-TB) is unclear.
Methods:
This was a cross-sectional study of patients from Tbilisi, Georgia with cavitary DR-TB and an outcome of cure. Participants had a chest X-ray (CXR), St. George Respiratory Quality (SGRQ) survey, and pulmonary function tests (PFTs) performed. Correlations between SGRQ and PFT results and factors associated with pulmonary impairment were examined.
Results:
Among 58 subjects (median age 31 years), 40% used tobacco, 59% had prior TB, and 47% underwent adjunctive surgical resection. The median follow-up time was 41 months. Follow-up CXR revealed fibrosis in 30 subjects (52%) and bronchiectasis in seven (12%). The median forced expiratory volume (FEV1)/forced vital capacity (FVC) ratio was 0.72, with 24 subjects (41%) having a ratio of ≤0.70. Significant correlations existed between PFT measures and overall and component SGRQ scores. In linear regression, age, prior TB, and CXR fibrosis or bronchiectasis were significantly associated with decreased pulmonary function. Adjunctive surgery was significantly associated with a higher percent predicted FEV1 and FVC.
Conclusions:
A high proportion of DR-TB subjects had residual pulmonary impairment, particularly with recurrent TB and severe radiological disease. The association of surgical resection with improved lung function deserves further study. PFTs and SGRQ may both be useful to evaluate lung health.
Keywords: St. George Respiratory Quality survey, Lung health, Tuberculosis, Impairment, Pulmonary function, Surgery
Introduction
While the mortality burden of tuberculosis (TB) is well documented, with an estimated 1.7 million deaths per year, the long-term morbidity of patients successfully completing treatment is not clear (WHO, 2016; Harries et al., 2016). The limited available data suggest that post-treatment TB patients are at higher risk of death as compared to the general population and have higher rates of chronic obstructive pulmonary disease (COPD) (Harries et al., 2016; Miller et al., 2015; Shuldiner et al., 2016; Allwood et al., 2013). Better understanding of the long-term morbidities of TB patients may help in the design and implementation of interventions aimed at optimizing quality of life and productivity.
The association of pulmonary TB and post-treatment lung impairment was first recognized a century ago (Garvin et al., 1918a; Garvin et al., 1918b); however, many unanswered questions regarding the relationship between TB and lung impairment persist. In a 2013 systemic review, 17 of 19 studies reported a positive association of pulmonary TB and chronic airflow obstruction, yet with marked heterogeneity between study designs and methods (Allwood et al., 2013). Additionally, no study included patients with drug-resistant TB. There is a need to better understand the scope of chronic lung impairment among TB patients post-treatment in order to identify predictors of and thus potential interventions for post-treatment lung impairment. A recent editorial highlighted this issue, urging closer attention to the coexistence of TB and chronic lung disease (Harries et al., 2016).
The main goals of this pilot study were to examine the prevalence and clinical predictors of lung impairment among subjects who had previously completed treatment for pulmonary multidrug-resistant and extensively drug-resistant (M/XDR)-TB and who received a favorable outcome, and to evaluate the relationship between adjunctive surgical resection and lung impairment. Focus was placed on M/XDR-TB given the lack of data within this group and because drug-resistant TB is a major problem in the country of Georgia, with rates of MDR-TB being 12% among new subjects and 33% among previously treated subjects (WHO, 2016). In regards to surgical resection, high rates of favorable outcomes among MDR-TB subjects undergoing adjunctive surgery have been shown previously, and it was aimed to further evaluate the consequences or benefits of surgery in regards to long-term lung health (Vashakidze et al., 2013).
Study population and methods
Setting
The study was conducted at the National Center for TB and Lung Disease (NCTLD) in Tbilisi, Georgia, which contains the National TB Thoracic Surgery Program. All patients with M/XDR-TB are treated with directly observed therapy and according to World Health Organization (WHO) guidelines. Subjects are chosen for surgery based on the recommendations of an institutional drug-resistant TB treatment committee and according to guidelines as described previously (Vashakidze et al., 2013; Kempker et al., 2012).
Study design
This was a cross-sectional study of subjects from a retrospective countrywide cohort of 408 individuals with cavitary M/XDR-TB disease who initiated treatment during 2009–2011. From this cohort, only subjects with a clinical outcome of completed or cured (n = 213), as defined by the WHO (World Health Organization, 2011), and who were from Tbilisi (n = 106) were contacted by phone for participation. A total of 242 phone calls were placed to 97 of 106 eligible subjects, and 58 study subjects were enrolled. All enrolled subjects came to the NCTLD for a one-time study visit. The study was approved by the institutional review boards of the NCTLD and Emory University.
Study visit
During the study visit, all study participants had their weight measured, a sputum sample collected for acid-fast bacillus (AFB) smear microscopy and culture (Bablishvili et al., 2015), and posterior–anterior chest radiography (CXR) and spirometry performed, and all completed a St. George Respiratory Quality (SGRQ) survey. Two chest radiologists at the NCTLD jointly reviewed all CXRs and used a consensus approach to record the presence or absence of cavitary disease, infiltrates, nodules, fibrosis, bronchiectasis, and pleurodiaphragmatic adhesions. Spirometry was performed by a trained study investigator using a MIR spirolab III (Medical International Research, Rome, Italy), with the following parameters obtained: forced vital capacity (FVC), percent predicted FVC, forced expiratory volume (FEV1), percent predicted FEV1, and peak expiratory flow (PEF) (Quanjer et al., 1993). FEV1 and FVC percent predicted were standardized for age, sex, and height (Quanjer et al., 1993). The severity of any spirometric abnormality was based on FEV1 and categorized by the Global Initiative for Chronic Obstructive Lung Disease(GOLD) criteria: GOLD 1 (>80), GOLD 2 (50–79), GOLD 3 (30–49), and GOLD 4 (<30) (Pellegrino et al., 2005). Only spirometry results with an American Thoracic Society (ATS) quality score of A–C were accepted.
The SGRQ is a 50-item survey designed to measure the impact of obstructive lung disease on overall health and perceived well-being (Jones et al., 1992; Jones et al., 1991). It contains three components: symptoms, activity level, and the impact of lung health on daily life. The SGRQ is scaled from 0 (optimal) to 100 (worst); weighted responses are used to produce a score for each component and an aggregate score (Jones et al., 1992; Jones et al., 1991).
Data analysis
All data analyses were performed with RStudio (version0.99.491; RStudio, Inc.). All figures were created in RStudio using the ggplot2 package (version 1.0.1). The two-sided Chi-square and t-test statistics were used to compare characteristics by adjunctive surgery status. A Pearson correlation coefficient was calculated to determine correlations between spirometric and SGRQ measures. Linear regression was performed to examine associations between patient characteristics and various spirometric measures and total SGRQ scores.
Results
Fifty-eight subjects were enrolled, including 27 (47%) who had adjunctive surgical resection. At the time of initial TB presentation, all subjects had cavitary lesions on CXR and the majority (59%) had a history of prior TB treatment. The median time from completion of drug-resistant TB treatment to study visit was 41.2 months. At follow-up, all subjects had a negative AFB sputum smear microscopy and culture. The median age at the time of follow-up was 31.2 years and the median body mass index (BMI) was 23.9 kg/m2, and there was a predominance of male patients (57%). Current tobacco use was 40%, the most common medical comorbidity was diabetes mellitus (9%), and no subject had either a history of chronic lung disease or HIV. In comparison to those who received antibiotic therapy alone, those who underwent adjunctive surgical resection were significantly more likely to be younger (26.2 vs. 35.0 years) and have pre XDR or XDR TB (63% vs. 26%). They were less likely to have had prior TB (41% vs. 74%) or to have bilateral cavitary disease at baseline (4% vs. 39%). Further patient characteristics and comparisons are shown in Table 1.
Table 1.
Characteristics of the overall study cohort and by adjunctive surgical resection status.
| Variables | Total n = 58 (%) |
Surgical resection n = 27 (%) |
No surgical resection n = 31 (%) |
p-Valuea |
|---|---|---|---|---|
| Age, yearsb | 31 (24–42) | 26 (23–39) | 35 (28–45) | 0.01 |
| Male sex | 33 (57) | 14 (52) | 19 (61) | 0.65 |
| BMI (kg/m2) at follow-upb | 24 (21–27) | 23 (21–26) | 24 (21.5–26.9) | 0.89 |
| Tobacco use | 23 (40) | 10 (37) | 13 (42) | 0.91 |
| Alcohol use | 11 (19) | 3 (11) | 8 (26) | 0.28 |
| Diabetes | 5 (9) | 1 (4) | 4 (13) | 0.44 |
| Hepatitis C | 4 (7) | 1 (4) | 3 (10) | 0.71 |
| TB case definition | 0.02 | |||
| New | 24 (41) | 16 (59) | 8 (26) | |
| Prior TB | 34 (59) | 11 (41) | 23 (74) | |
| Baseline CXR findings | ||||
| Multilobar | 36 (62) | 14 (52) | 22 (71) | 0.22 |
| Bilateral | 25 (43) | 7 (26) | 18 (58) | 0.03 |
| Bilateral cavities | 13 (22) | 1 (4) | 12 (39) | <0.01 |
| Follow-up CXR findings (n = 54) | ||||
| Multiple nodules | 22 (38) | 0 | 22 (71) | <0.01 |
| Cavity | 12 (21) | 0 | 12 (39) | <0.01 |
| Fibrosis | 30 (52) | 5 (19) | 25 (81) | <0.01 |
| Bronchiectasis | 7 (12) | 0 | 7 (23) | 0.02 |
| Pleural adhesions | 10 (17) | 4 (15) | 6 (19) | 0.73 |
| Drug resistance | ||||
| Pre XDR or XDR | 25 (43) | 17 (63) | 8 (26) | 0.01 |
| Surgery type | ||||
| Lobectomy | 10 (37) | |||
| Segmentectomy | 15 (56) | |||
| Thoracoplasty | 2 (7) | |||
| Treatment duration | ||||
| Monthsb | 23.0 (21–25) | 25 (22–27) | 22 (21–24) | 0.02 |
| Follow-up timec | ||||
| Monthsb | 41.2 (29–50) | 39 (33–46) | 43 (29–51) | 0.53 |
| Pulmonary function | ||||
| FVC (ml)b | 3520 (2760–4780) | 3985 (3078–4953) | 3300 (2575–4455) | 0.07 |
| FEV1 (ml)b | 2600 (1870–3837) | 2990 (2070–3900) | 2050 (1515–3520) | 0.10 |
| Peak expiratory flowb | 75 (52–91) |
BMI, body mass index; TB, tuberculosis; CXR, chest X-ray; XDR, extensively drug-resistant; FVC, forced vital capacity; FEV, forced expiratory volume.
Two-sided Chi-square and t-test statistics used.
Values are given as the median (interquartile range).
Time from end of treatment to follow-up study visit.
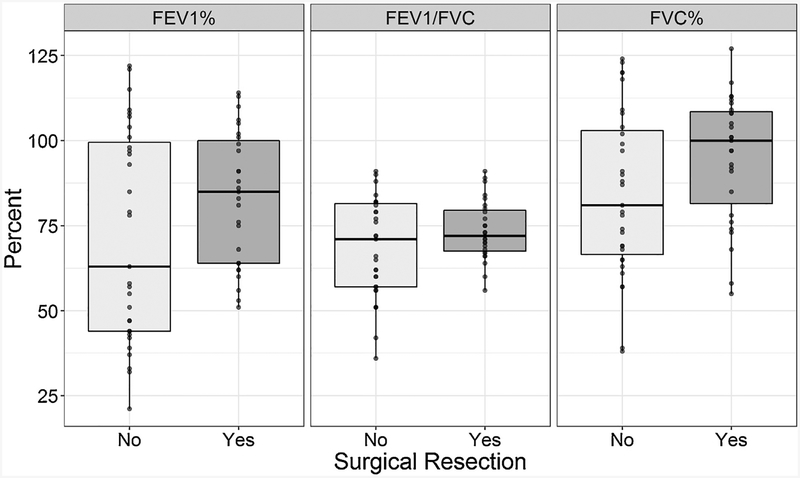
All 58 subjects completed spirometry and a SGRQ (Table 2, Figures 1 and 2). Among the entire group, 41% had a FEV1/FVC ratio <0.7. Comparing the groups by surgical status, there were similar distributions of FEV1/FVC <0.7 and FVC. Regarding the distribution of FEV1, there were significantly more non-surgical subjects with a lower percent predicted FEV1. Among surgical patients there were three (11%) with FEV1 <60% predicted, while in the non-surgical patients there were 48% in these GOLD 3 (45%) and GOLD 4 (3%) airflow limitation categories.
Table 2.
Pulmonary impairment and SGRQ scores after treatment among subjects with multidrug-resistant tuberculosis.
| Variables | Total n = 58 (%) |
Surgical resection n = 27 (%) |
No surgical resection n = 31 (%) |
p-Value |
|---|---|---|---|---|
| FVC, % predicted | 0.21 | |||
| 0–30 | 0 | 0 | 0 | |
| 30–59 | 6 (10) | 2 (7) | 4 (13) | |
| 60–79 | 16 (27) | 5 (19) | 11 (35) | |
| ≥80 | 36 (62) | 20 (74) | 16 (51) | |
| Median (IQR) | 92 (73–108) | 100 (82–109) | 81 (67–103) | |
| FEV1, % predicted | 0.01 | |||
| 0–30 | 1 (2) | 0 | 1 (3) | |
| 30–59 | 17 (29) | 3 (11) | 14 (45) | |
| 60–79 | 11 (19) | 8 (30) | 3 (10) | |
| ≥80 | 29 (50) | 16 (59) | 13 (42) | |
| Median (IQR) | 80 (55–101) | 85 (64–100) | 63 (44–100) | |
| FEV1/FVC ratio | 0.37 | |||
| <70 | 24 (41) | 9 (33) | 15 (48) | |
| ≥70 | 34 (59) | 18 (67) | 16 (52) | |
| Median (IQR) | 72 (63–81) | 72 (68–80) | 71 (57–82) | |
| SGRQ scoresa | ||||
| Symptoms | 27.5 (10.3–55.6) | 20.5 (7.5–34.0) | 39.9 (20.8–74.5) | <0.01 |
| Activity | 23.3 (5.3–32.6) | 17.1 (2.6–23.7) | 23.7 (8.6–73.4) | <0.01 |
| Impact | 3.6 (0–13.0) | 2.0 (0–9.6) | 5.1 (0.8–38.3) | 0.01 |
| Total (max 100) | 11.8 (6.3–23.9) | 9.5 (4.6–16.4) | 14.2 (9.4–53.6) | <0.01 |
SGRQ, St. George Respiratory Questionnaire; IQR, interquartile range; FVC, forced vital capacity; FEV, forced expiratory volume. FVC, forced vital capacity; FEV1, forced expiratory volume.
Median values with interquartile range.
Figure 1.
Distribution of spirometry results in post-treatment tuberculosis subjects by adjunctive surgical resection status.
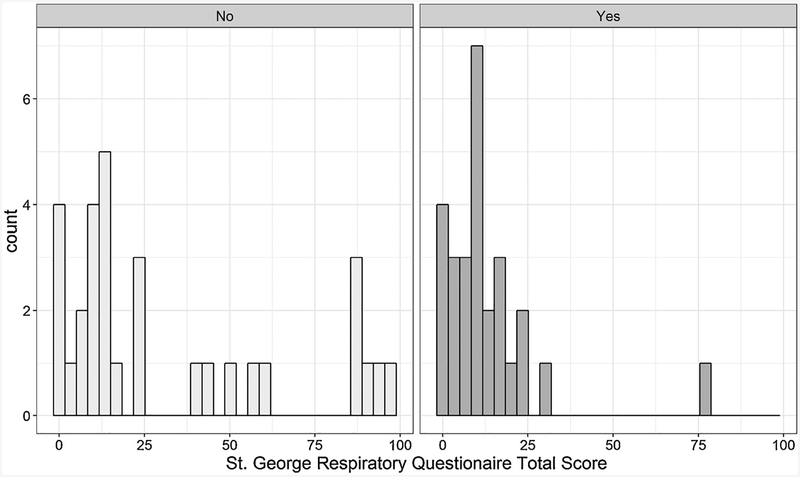
Figure 2.
Distribution of St. George Respiratory Questionnaire results in post-treatment tuberculosis subjects by adjunctive surgical resection status.
Among the entire group, the total median SGRQ score was 12/100 (lower scores being better). Notably, 44% of patients described their health between fair to poor, 36% of subjects had a cough at least a few days a week, 26% reported their chest condition interferes or prohibits them from work, and 53% reported that their chest condition prevents them doing things that they would like to do. Six subjects (10%) reported receiving medications for their chest condition, with three feeling like their medication was not helping much. In comparison to those who received antibiotic therapy alone, those who underwent adjunctive surgery had significantly lower total SGRQ scores (9.5 vs.14.2) and lower scores in the symptom, activity, and impact domains. Full SGRQ results are shown in the Supplementary Material (Appendix A).
In assessing the relationships between SGRQ scores and spirometry, SGRQ scores negatively correlated with all spirometry results (Table 3). The correlations were significant for the associations between each spirometry value and total SGRQ score (Pearson correlation coefficient range −0.31 to −0.50), as well as with each SGRQ component.
Table 3.
Correlations between pulmonary function test results and SGRQ scores (n = 58).a
| Variables | SGRQ scoresb | |||
|---|---|---|---|---|
| Symptom | Activity | Impact | Total | |
| FVC | −0.30* | −0.35** | −0.26* | −0.31* |
| FVC % | −0.41** | −0.41** | −0.33* | −0.39** |
| FEV1 | −0.33* | −0.41** | −0.30* | −0.36** |
| FEV1% | −0.42** | −0.46** | −0.36** | −0.42** |
| FEV1/FVC | −0.38** | −0.48** | −0.37** | −0.43** |
| PEF | −0.50** | −0.55** | −0.43** | −0.50** |
| FEV25–75 | −0.28* | −0.34** | −0.27* | −0.31* |
SGRQ, St. George Respiratory Questionnaire; FVC, forced vital capacity; FEV1, forced expiratory volume; PEF, peak expiratory flow.
Utilizing Pearson’s correlation coefficient.
Significant at
p ≤ 0.05,
p ≤ 0.01.
Table 4 reports the results of linear regression analyses examining associations between variables with measures of spirometry and total SGRQ scores. In the models for percent predicted FEV1 and FVC, the factors significantly associated with both lower percent predicted FEV1 and FVC included age, prior TB, fibrosis, and bronchiectasis. The only factor associated with higher percent predicted FEV1 and FVC was surgery. There were similar trends in models for FEV1/FVC and PEF. In an adjusted linear regression model for total SGRQ, the factors significantly associated with a higher (worse) score included age and the presence of fibrosis on CXR.
Table 4.
Results of linear regression analysis for spirometric measures of air flow among post-treatment tuberculosis subjects (n = 58).
| Variable | Pulmonary function testa | SQRG total scorea,b | |||
|---|---|---|---|---|---|
| FEV1% predictedc | FVC% predictedc | FEV1/FVC | PEF | ||
| Age, years | −0.87** | −0.88** | −0.31* | −1.00** | 1.19** |
| Male | −2.58 | −9.0 | 0.31 | 1.74 | 2.94 |
| Follow-up BMI | −0.09 | −0.39 | 0.17 | 0.57 | −0.24 |
| Tobacco use | −0.23 | −0.14 | −1.65 | −1.82 | 4.00 |
| Alcohol use | −17.22 | −13.89 | −8.81* | −7.75 | −8.76 |
| Hepatitis C | −24.49 | −10.03 | −16.14* | −26.27 | 7.17 |
| Prior TB | −16.83* | −12.28* | −7.70* | −18.31* | 12.04 |
| Baseline resistance | |||||
| Pre-XDR/XDR | −1.11 | 2.49 | −1.98 | 7.94 | −4.45 |
| Initial CXR | |||||
| Bilateral | −13.66* | −9.89 | −6.99* | −13.97 | 0.92 |
| Follow-up CXR | |||||
| Fibrosis | −18.7** | −18.5** | −5.47 | −19.44* | 13.99 |
| Cirrhosis | −13.8 | −9.70 | −7.78* | −12.08 | 26.66** |
| Bronchiectasis | −39.7** | −36.36** | −11.34* | −30.32** | 11.60 |
| Pleural adhesions | −9.19 | −5.61 | −4.66 | −12.86 | −0.50 |
| Surgery | |||||
| Yes | 14.74* | 14.78* | 5.23 | 13.32 | −11.78 |
| Segmentectomy | 14.0 | 5.53 | 6.83 | 15.00 | 6.88 |
SGRQ, St. George Respiratory Questionnaire; FVC, forced vital capacity; FEV1, forced expiratory volume; PEF, peak expiratory flow; BMI, body mass index; TB, tuberculosis; CXR, chest radiography; XDR, extensively drug-resistant.
Significant at
p ≤ 0.05,
p ≤ 0.01.
Controlled for age and sex.
Standardized for age, sex, and height.
Discussion
This study took its point of departure at the poorly studied time period of 2–4 years after the completion of TB treatment and explored the relationships between lung function, respiratory symptoms, adjunctive lung surgery, and other clinical factors among successfully treated drug-resistant TB subjects. The notable findings are that in this young group of subjects (median age 31 years), there was a high proportion with signs of significant impairment in lung function and that subjects undergoing adjunctive surgery had lower rates of impairment in lung function and less respiratory symptoms. Specifically, 41% had evidence of obstruction with an FEV1/FVC <0.7, with 36% of non-surgical subjects having a percent predicted FEV1 in the severe to very severe impairment range as compared to none in the surgery group. These results bring further needed attention to the issue of chronic lung disease post TB and highlight the need for further research including studies on the association between adjunctive surgery and long-term lung health.
The high rate of subjects with signs of obstructive pulmonary disease (41%) is striking given the young age of the cohort. To give some perspective, a population survey of persons >40 years old from 12 countries found a COPD prevalence of between 7% and 12%, including 7.2% among persons 40–49 years of age from Russia, the country most similar to Georgia (Landis et al., 2014). The rate of lung obstruction in the present study was slightly higher than the 36% rate of COPD found in a prospective population study among persons who had smoked continuously for 25 years (Lokke et al., 2006). While the finding of chronic airflow obstruction among patients with pulmonary TB has been shown before, the available data are still scarce and studies are difficult to compare given variations in study design and variables collected (Allwood et al., 2013). In two studies that similarly evaluated subjects with pulmonary TB after successful completion of treatment (between 20 and 156 weeks after treatment), 24% and 34% of subjects were found to have chronic airway obstruction by spirometry (Pasipanodya et al., 2007; de la Mora et al., 2015). The present study results along with available data suggest that in high burden TB countries, TB disease may be a significant cause of COPD, which is responsible for more than three million deaths worldwide annually, with 90% of these occurring in low to middle-income countries (Allwood et al., 2013; Rabe and Watz, 2017).
Likely reasons for the high rate of lung impairment found in this study include a cohort of patients with MDR and XDR cavitary TB, many of whom were retreatment cases. Cavitary disease is generally considered an indicator of severe and advanced TB disease, and therefore the external generalizability of the study findings may be limited to similar patients with worse symptoms and lung function. As found by others, this study identified a correlation between severity of disease based on CXR findings and worse lung impairment (de la Mora et al., 2015; Long et al., 1998; de Valliere and Barker, 2004). Radiological findings may thus be a useful clinical aid in determining which TB patients may be at higher risk of post-treatment lung impairment. Given the frequent delay in diagnosis and the long and less effective standard second-line treatment regimens for patients with MDR-TB, it is plausible that more lung damage and therefore lung impairment may occur during treatment (Dheda et al., 2017). A brief review of the literature identified two additional studies evaluating lung function among patients with pulmonary MDR-TB, both of which included patients with and without cavitary disease. Each involved 33 subjects with MDR-TB, and rates of airway obstruction by spirometry were 17% and 51% of subjects (de Valliere and Barker, 2004; Byrne et al., 2017). In the study by Byrne et al., patients with MDR-TB had slightly higher rates of chronic airway obstruction than subjects with drug-susceptible TB (17% vs. 14%) and were more likely than non-TB controls to have airway obstruction (adjusted odds ratio 4.9, 95% confidence interval 1.3–18.8) (Byrne et al., 2017).
It was found that subjects undergoing adjunctive surgery had less respiratory symptoms at follow-up and that surgery was associated with a higher percent predicted FEV1 and FVC. While surgery has been associated with improved clinical outcomes among subjects with MDR-TB, the present study appears to be the first evaluation of its impact on long-term lung health among drug-resistant TB subjects (Fox et al., 2016). While this association may be due in part to a healthier patient bias, given subjects undergoing surgery were younger and less likely to have prior TB and bilateral cavitary disease, there may be additional reasons. It has recently been shown that even among TB subjects with a successful treatment outcome, many have persistent pulmonary inflammation as indicated by positron emission tomography/computed tomography (PET/CT) scan (Malherbe et al., 2016). One intriguing hypothesis is that through surgical removal of the main focus of disease, you remove driving force inflammation and subsequent lung tissue fibrosis, retraction, and other parenchymal and airway changes (Dheda et al., 2005).
As in a prior study, significant correlations were found between the SGRQ and spirometry results among the TB subjects (Pasipanodya et al., 2007). The SGRQ thus provides another tool to implement for the screening of patients for respiratory symptoms associated with COPD and their impact on quality of life. To help with interpretation of the SGRQ scores, a change in value of 4 is considered clinically significant, and when compared to results from a European population sample among persons aged 39–49 years, the present study results for total (11.8 vs. 5.8), symptoms (27.5 vs. 8.0), impact (3.6 vs. 2.7), and activity (23.3 vs.9.3) are all higher (indicating worse values) (Ferrer et al., 2002). Another notable finding from the SGRQ is that despite many subjects with evidence of obstructive disease by spirometry, only six were receiving medications and half of these felt like it was helping them. This finding highlights the need to improve linkages between national TB programs and respiratory medicine services so that subjects can receive the care needed, including both medical care and pulmonary rehabilitation (Harries et al., 2016; Rabe and Watz, 2017).
Study limitations included the enrollment of a convenience sample of subjects who were contacted by phone and agreed to a follow-up research assessment. While the visit was not linked with any incentives, it did take place at the NCTLD and, therefore, there may be a selection bias towards participants who wanted to be assessed because they were experiencing post-treatment symptoms. This would tend to overestimate the lung impairment present in the target population. Conversely, it may be speculated that the participants were biased towards those who were healthy enough to come in for the study visit. It is also unclear how enrolling only patients from the capital city of Tbilisi may have affected the generalizability of the results. It is also important to note that the results do not include MDR-TB subjects with outcomes of treatment failure and loss to follow-up, groups likely to experience higher rates of lung impairment compared to successfully treated subjects. Additionally, it was not possible to assess pretreatment baseline measures of pulmonary function or change over time. To address these limitations, the authors are currently initiating a study on lung function among drug-susceptible and MDR-TB subjects after the successful completion of treatment, as well as healthy community controls. This study will include the use of chest CT, which has increased sensitivity and specificity in detecting abnormalities as compared to CXR.
In conclusion, this study highlights the need for further understanding of pulmonary impairment following the completion of TB treatment, particularly in areas where there are high rates of recurrence and drug resistance. The description of a high proportion of lung impairment at an early stage of life may be associated with significant consequences across the life course. If further studies confirm these findings, then there is an urgent need for preventive and mitigating therapies in this vulnerable group.
Supplementary Material
Funding
This work was supported in part by the National Institutes of Health Fogarty International Center (D43TW007124) and the National Institute of Allergy and Infectious Diseases (K23AI103044; R21AI122001). During time of work, JAK received support from the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and KL2 TR000455.
Ethical approval
Emory IRB approval number IRB00073233; NCTLD Georgia IRB approval number IORG0006411.
Footnotes
Conflict of interest
No conflicts exist for any of the authors.
Appendix A.: Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2019.02.039.
References
- Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 2013;86(1):76–85. [DOI] [PubMed] [Google Scholar]
- Bablishvili N, Tukvadze N, Avaliani Z, Blumberg HM, Kempker RR. A comparison of the Xpert((R)) MTB/RIF and GenoType((R)) MTBDRplus assays in Georgia. Int J Tuberc Lung Dis 2015;19(6):676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne AL, Marais BJ, Mitnick CD, Garden FL, Lecca L, Contreras C, et al. Chronic airflow obstruction after successful treatment of multidrug-resistant tuberculosis. ERJ Open Res 2017;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Valliere S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2004;8(6):767–71. [PubMed] [Google Scholar]
- de la Mora IL, Martinez-Oceguera D, Laniado-Laborin R. Chronic airway obstruction after successful treatment of tuberculosis and its impact on quality of life. Int J Tuberc Lung Dis 2015;19(7):808–10. [DOI] [PubMed] [Google Scholar]
- Dheda K, Booth H, Huggett JF, Johnson MA, Zumla A, Rook GA. Lung remodeling in pulmonary tuberculosis. J Infect Dis 2005;192(7):1201–9. [DOI] [PubMed] [Google Scholar]
- Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med 2017;5(4):291–360. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Villasante C, Alonso J, Sobradillo V, Gabriel R, Vilagut G, et al. Interpretation of quality of life scores from the St George’s Respiratory Questionnaire. Eur Respir J 2002;19(3):405–13. [DOI] [PubMed] [Google Scholar]
- Fox GJ, Mitnick CD, Benedetti A, Chan ED, Becerra M, Chiang CY, et al. Surgery as an adjunctive treatment for multidrug-resistant tuberculosis: an individual patient data metaanalysis. Clin Infect Dis 2016;62(7):887–95. [DOI] [PubMed] [Google Scholar]
- Garvin A, Lundsgaard C, Van Slyke DD. Studies of lung volume: II. Tuberculous men. JExp Med 1918a;27(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin A, Lundsgaard C, Van Slyke DD. Studies of lung volume: III. Tuberculous women. J Exp Med 1918b;27(1):129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries AD, Ade S, Burney P, Hoa NB, Schluger NW, Castro JL. Successfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatment. Int J Tuberc Lung Dis 2016;20(8):1010–4. [DOI] [PubMed] [Google Scholar]
- Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med 1991;85(Suppl B)25–31 (discussion 33–7). [DOI] [PubMed] [Google Scholar]
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992;145(6):1321–7. [DOI] [PubMed] [Google Scholar]
- Kempker RR, Vashakidze S, Solomonia N, Dzidzikashvili N, Blumberg HM. Surgical treatment of drug-resistant tuberculosis. Lancet Infect Dis 2012;12(2):157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SH, Muellerova H, Mannino DM, Menezes AM, Han MK, van der Molen T, et al. Confront COPD International Patient Survey: methods, COPD prevalence, and disease burden in 2012–2013. Int J Chron Obstruct Pulmon Dis 2014;9:597–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax 2006;61(11):935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest 1998;113(4):933–43. [DOI] [PubMed] [Google Scholar]
- Malherbe ST, Shenai S, Ronacher K, Loxton AG, Dolganov G, Kriel M, et al. Persisting positron emission tomography lesion activity and Mycobacterium tuberculosis mRNA after tuberculosis cure. Nat Med 2016;22(10):1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TL, Wilson FA, Pang JW, Beavers S, Hoger S, Sharnprapai S, et al. Mortality hazard and survival after tuberculosis treatment. Am J Public Health 2015;105(5):930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasipanodya JG, Miller TL, Vecino M, Munguia G, Bae S, Drewyer G, et al. Using the St. George respiratory questionnaire to ascertain health quality in persons with treated pulmonary tuberculosis. Chest 2007;132(5):1591–8. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26(5):948–68. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:5–40. [PubMed] [Google Scholar]
- Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017;389(10082):1931–40. [DOI] [PubMed] [Google Scholar]
- Shuldiner J, Leventhal A, Chemtob D, Mor Z. Mortality after anti-tuberculosis treatment completion: results of long-term follow-up. Int J Tuberc Lung Dis 2016;20(1):43–8. [DOI] [PubMed] [Google Scholar]
- Vashakidze S, Gogishvili S, Nikolaishvili K, Dzidzikashvili N, Tukvadze N, Blumberg HM, et al. Favorable outcomes for multidrug and extensively drug resistant tuberculosis patients undergoing surgery. Ann Thorac Surg 2013;95(6):1892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Global tuberculosis report. 2016. [Google Scholar]
- World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. WHO/HTM/TB20116. 2011. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.