Abstract
Endometriosis is an estrogen-dependent and progesterone-resistant gynecological inflammatory disease of reproductive-age women. Current hormonal therapies targeting estrogen can be prescribed only for a short time. It indicates a need for non-hormonal therapy. ERK1/2 and AKT pathways control several intracellular signaling molecules that control growth and survival of cells. Objectives of the present study are to (i) determine the dual inhibitory effects of ERK1/2 and AKT pathways on proliferation, survival, and apoptosis of human endometrioitc epithelial cells and stromal cells in vitro; (ii) on growth and survival of endometrioitc lesions in vivo in xenograft mouse model of endometriosis of human origin; and (iii) establish the associated ERK1/2 and AKT downstream intracellular signaling modules in the pathogenesis of endometriosis. Our results indicated that combined inhibition of ERK1/2 and AKT highly decreased the growth and survival of human endometriotic epithelial cells and stromal cells in vitro and suppressed the growth of endometriotic lesions in vivo compared to inhibition of either ERK1/2 or AKT pathway individually. This cause-effect is associated with dysregulated intracellular signaling modules associated with cell cycle survival, and apoptosis pathways. Collectively, our results indicate that dual inhibition of ERK1/2 and AKT pathways could emerge as potential non-hormonal therapy for the treatment of endometriosis.
INTRODUCTION
Endometriosis is an estrogen-dependent and progesterone-resistant gynecological inflammatory disease of reproductive-age women. The prevalence of endometriosis is ~5-10% in reproductive-age women, and it increases to 20-30% in women with subfertility, and further it increases to 40-60% in women with pain and infertility [1,2]. Endometriosis is clinically and pathologically characterized by the presence of functional endometrium as heterogeneous lesions or phenotypes outside the uterine cavity. At the time of clinical presentation, most women have established active endometriosis for a long period of time 8-10 years [1,2], and majority of these women experience pelvic pain, infertility, and recurrence of disease. The current anti-estrogen therapies can be prescribed only for a short time because of the undesirable side effects on menstruation, pregnancy, and bone health, and failure to prevent recurrence.
The pathogenesis of endometriosis is an enigma in reproductive medicine. The most widely accepted hypothesis first advanced by Sampson in 1921 is that viable endometrial tissue fragments move in a retrograde fashion through the fallopian tubes into the pelvic cavity during menstruation [3]. One of the important behaviors of the endometriotic cells is resistant to apoptosis [4-9]. We and others have proposed that therapeutic strategies to intervene survival or apoptosis pathways in endometriotic lesions may lead to the identification of effective treatment modalities for endometriosis [4-10].
Extracellular signal-regulated kinase (ERK1/2) and phosphatidylinositide 3-kinase (PI3K) and AKT/protein kinase B (PI3K-AKT) are the well-studied pathways which regulate proliferation, survival, and apoptosis of the cells by integrating multiple intracellular signaling modules [11-14]. Upstream, ERK1/2 is activated by a small G protein Ras-Raf family members followed by MEK1/2. Upstream, AKT is activated by PI3K followed by PDK1. Downstream, ERK1/2 or AKT regulates several signaling molecules that include protein kinases, protein phosphatases, receptors, transcriptional factors, and several other proteins. Recent studies have identified a role for multiple redundant and complementary intracellular cell signaling modules such as Ras-Raf-ERK1/2-p90RSK [15-18], PI3K-AKT-p70S6K-mTOR [17-19], ERK1/2 or AKT-IκBα-NFκB [20], and ERK1/2 or AKT-Wnt-βcatenin pathways [21-23] in proliferation, survival, and apoptosis of several mammalian cell types.
To date, much information is available on the role of ERK1/2 or AKT signaling in proliferation, growth and survival of a variety of cells [11-13,24,25]. Relatively, a small number of studies have demonstrated molecular link between ERK1/2 or AKT pathways and endometriosis [25-32]. No studies have reported combined inhibition of ERK1/2 and AKT pathways in endometriosis. In early 2009, we have reported that Bcl2, Bcl-XL, pBad112, pBad136, pERK1/2, pAKT, active-βcatenin, and NFκB proteins are highly expressed in the epithelial cells and stromal cells of the peritoneal endometriotic lesions in women compared to endometrium from the healthy women [10]. Later studies by other groups, using human tissues, cell cultures, and animal models, confirmed that ERK1/2 and AKT pathways are involved in the growth and survival of peritoneal endometriotic lesions. AKT and ERK1/2 pathways are temporally activated during establishment of endometriosis [27,29]. Inhibition of AKT with inhibitor MK2206 or ERK1/2 with inhibitor U0126 did not increase the expression of cl-caspase-3 in primary cultured stromal cells derived from deep endometriotic lesions from women [28]. By contrast, either inhibition of AKT or ERK1/2 with the same inhibitors increased expression of cl-caspase-3 in primary cultured stromal cells derived from endometrioma [29]. The difference in activation of caspase-3 by AKT or ERK1/2 pathways in these two studies may be due to the sensitivity of endometriotic stromal cells derived from different lesional phenotypes or existence of compensatory mechanisms between AKT and ERK1/2 pathways. Interestingly, inhibition of AKT pathway resulted in activation of ERK1/2 pathway; similarly, inhibition of ERK1/2 pathway resulted in activation of AKT pathway in primary cultured endometriotic cells derived from deep endometriotic lesions from women [28] and in other cancer or tumor cells [14,33-36]. Inhibition of ERK1/2 or AKT pathway partially decreased proliferation and viability of human endometriotic stromal cells in vitro, and growth of endometriotic lesions in mouse model of endometriosis in vivo [27-29]. This partial growth inhibitory or apoptotic effect appears to be due to compensatory mechanisms between the ERK1/2 and AKT pathways.
The remarkable redundancy of ERK1/2 and AKT signaling pathways that control interactions among proliferation, survival, and apoptosis underscores the importance of combined inhibition of the ERK1/2 and AKT pathways to suppress the growth and survival of endometriotic lesions. The primary objectives of the present study are to determine the dual inhibitory effects of ERK1/2 and AKT pathways (i) on proliferation, survival, and apoptosis of the human endometrioitc epithelial cells and stromal cells in vitro and (ii) on growth and survival of the endometrioitc lesions in vivo in xenograft mouse model of endometriosis of human origin. (iii) An additional objective is to establish the associated ERK1/2 and AKT downstream intracellular signaling modules in the pathogenesis of endometriosis.
MATERIALS AND METHODS
Materials:
General chemicals and reagents used in the study were molecular and cell biological grade from Sigma-Aldrich (St. Louis, MO), Fisher Scientific (Pittsburgh, PA), VWR (Radnor, PA) or Invitrogen Life Technologies Inc (Carlsbad, CA). Details of the antibodies and concentrations used are given in Table-1.
Table 1:
Details of the antibody used.
| Details of Antibodies Used | Manufacturer | Cat # | Concentration Used in WB (ICC) |
|---|---|---|---|
| Anti-human rabbit monoclonal pAKT | Cell Signaling | 4060 | 1:1000 (1:100) |
| Anti-human rabbit monoclonal pERK1/2 | Cell Signaling | 4370 | 1:1000 (1:100) |
| Anti-human rabbit polyclonal β-catenin | Cell Signaling | 9562 | 1:1000 |
| Anti-human rabbit polyclonal p-p90RSK | Cell Signaling | 9344 | 1:500 |
| Anti-human rabbit monoclonal t-p90RSK | Cell Signaling | 9355 | 1:1000 |
| Anti-human rabbit polyclonal p-p70S6K | Cell Signaling | 9204 | 1:500 |
| Anti-human rabbit polyclonal t-p70s6K | Cell Signaling | 9202 | 1:1000 |
| Anti-human rabbit polyclonal p-mTOR1 r | Cell Signaling | 2971 | 1:1000 |
| Anti-human rabbit polyclonal t-mTOR-1 | Cell Signaling | 2972 | 1:1000 |
| Anti-human rabbit polyclonal b-Catenin | Cell Signaling | 9562 | 1:1000 |
| Anti-human rabbit polyclonal NFkB-p65 | Cell Signaling | 3034 | 1:1000 |
| Anti-human rabbit polyclonal c-Jun | Cell Signaling | 9162 | 1:500 |
| Anti-human rabbit polyclonal c-Fos | Cell Signaling | 4384 | 1:500 |
| Anti-human rabbit polyclonal Sp1 | Santa Cruz | Sc-59 | 1:1000 |
| Anti-human rabbit polyclonal p-CREB | Cell Signaling | 9191 | 1:500 |
| Anti-human rabbit polyclonal ETS-1 | Santa Cruz | SC-112 | 1:1000 |
| Anti-human rabbit polyclonal EGR-1 | Cell Signaling | 4152 | 1:1000 |
| Anti-human mouse monoclonal CDK1 | Cell Signaling | 9116 | 1:1000 |
| Anti-human rabbit monoclonal CDK2 | Abcam | ab32147 | 1:1000 |
| Anti-human mouse monoclonal CDK4 | Cell Signaling | 2906 | 1:1000 |
| Anti-human mouse monoclonal CDK6 | Cell Signaling | 3136 | 1:1000 |
| Anti-human mouse monoclonal Cyclin A | Cell Signaling | 4656 | 1:2000 |
| Anti-human rabbit polyclonal Cyclin B1 | Abcam | ab2949 | 1:2000 |
| Anti-human mouse monoclonal Cyclin D1 | Cell Signaling | 2926 | 1:1000 |
| Anti-human rabbit polyclonal Cyclin D2 | Cell Signaling | 2924 | 1:1000 |
| Anti-human mouse monoclonal Cyclin D3 | Cell Signaling | 2936 | 1:1000 |
| Anti-human rabbit polyclonal Cyclin E2 | Cell Signaling | 4132 | 1:1000 |
| Anti-human rabbit polyclonal Bcl-2 | Santa Cruz | SC-783 | 1:1000 |
| Anti-human rabbit polyclonal Bcl-XL | Cell Signaling | 2762 | 1:1000 |
| Anti-human rabbit polyclonal XIAP | Cell Signaling | 2042 | 1:1000 |
| Anti-human mouse polyclonal p-Bad112 | Cell Signaling | 9296 | 1:500 |
| Anti-human rabbit polyclonal p-Bad136 | Cell Signaling | 9295 | 1:500 |
| Anti-human rabbit polyclonal t-Bad | Cell Signaling | 9292 | 1:500 |
| Anti-human rabbit polyclonal t-Bax | Cell Signaling | 2774 | 1:1000 |
| Anti-human rabbit polyclonal cl-Caspase3 | Cell Signaling | 9661 | 1:1000 (1:100) |
| Anti-human mouse monoclonal cl-PARP | Abcam | ab110315 | 1:1000 (1:100) |
| Anti-human mouse monoclonal β-actin | Sigma-Aldrich | A2228 | 1:10000 |
| Anti-Mouse goat polyclonal IgG1 Secondary Antibody, Alexa Fluor 488 conjugate | Invitrogen | A21121 | (1:250) |
| Anti-Rabbit goat polyclonal IgG (H+L) Secondary Antibody, Alexa Fluor 488 conjugate | Invitrogen | A11008 | (1:250) |
| Anti-Mouse goat polyclonal IgG (H+L) Secondary Antibody, Alexa Fluor 594 conjugate | Invitrogen | A11032 | (1:500) |
| Anti-Rabbit goat polyclonal IgG (H+L) Secondary Antibody, Alexa Fluor 594 conjugate | Invitrogen | A11037 | (1:500) |
Human Endometriotic Cell Lines:
Immortalized endometriotic epithelial cell line 12Z and stromal cell line 22B used in this study were derived from active red peritoneal endometriosis lesions during the proliferative phase of the menstrual cycle from woman suffering from endometriosis for more than 8 years [37]. These 12Z and 22B cells share several phenotypic and molecular characteristics of primary cultured endometriotic cells [37]. Accumulating information from our and other laboratories indicate that 12Z and 22B cells are a potential model system to study the progressive phase of endometriosis [10,37-39]. Importantly, xenograft of a mixed population of these 12Z and 22B cells into the peritoneal cavity of immunocompromised mice is able to proliferate, attach, invade, reorganize and establish peritoneal endometriosis-like lesions and that histomorphology is similar to that of spontaneous peritoneal endometriosis in women [10,40]. We have shown that 12Z and 22B cells express p-ERK1/2 and p-AKT proteins at the basal level [10]. Therefore, inhibition of ERK1/2 and AKT is the best approach to investigate the role of ERK1/2 and AKT interactive signaling in the pathogenesis of endometriosis.
In Vitro Experiment-Pharmacologic Approach:
These well-characterized 12Z and 22B cells were cultured in DMEM/F12 without special steroid treatment containing 10% fetal bovine serum (FBS) and penicillin (100 U/ml), streptomycin (100 μg/ml) and amphotericin-B 2.5 μg/ml in a humidified 5% CO2 and 95% air at 37°C as we described previously [10,38,39,41]. At 70-80% confluency the cells were cultured in DMEM/F12 with 1% dextran-charcoal-treated fetal bovine serum (DC-FBS) and treated with MEK1/2 inhibitor (U0126) to suppress ERK1/2 pathway and/or PI3K inhibitor (LY294002) to suppress AKT pathway in vehicle (1% DMSO) in plain media for 24h.
In Vitro Experiment- siRNA Approach:
SiRNA experiments were performed as we reported [10]. Briefly, 12Z and 22B cells (3.0 ×105/well) were cultured as described above in six-well tissue culture plates. At 70-80% confluency, cells were used for ERK1 or AKT knock-down experiments using SMARTpool-ON-TARGETplus siRNA (ERK1 siRNA, L-003555-00-0005 and AKT1 siRNA, L-003000-00-0005) delivered by DharmaFect-1 as we described previously [10] and per manufacturer’s instructions (Dharmacon Inc, Lafayette, CO). As an internal control, scrambled siRNA was used. Fluorescence labeled siGLO RISC-free siRNA was transfected separately and transfection efficiency was estimated using a fluorescence microscope. Transfection efficiency more than 80% was considered as optimal conditions for further experiments. Efficiency of siRNA on silencing of ERK-1 and AKT genes was assessed by qRT-PCR 48 h post-transfection. Knock-down efficiency was 70-80% in both 12Z and 22B cells.
Cell Proliferation Assay:
12Z and 22B cells (1×105/well) were cultured in DMEM/F12 with 10% FBS in six-well plates. At 70-80% confluency the cells were cultured in DMEM/F12 with 1% dextran charcoal treated fetal bovine serum (DC-FBS) for 24h. In dose-response experiment, the cells were treated with different doses (0, 1, 10, 20, 50, 75, and 100 μM in vehicle 1% DMSO) for MEK1/2 inhibitor U01260 to suppress ERK1/2 pathway or PI3K inhibitor LY294002 to suppress AKT pathway for 24h in plain media. Based on this dose-response experiments, the optimal dose for each inhibitor was selected and the cells were treated with MEK1/2 inhibitor U0126 (20μm), PI3K inhibitor (LY294002, 50μm) or combination of both for 24h. These inhibitors competitively bind and inhibit their functions [42-44]. For siRNA study, after 24 h post-transfection of siRNA the medium was replaced and the cells were cultured in plain media which was considered as time 0 h, and cell proliferation was estimated at 24 h as described above. Number of cells were counted using a Coulter counter [45,46]. The total number of cells in control considered as 100%. Data were expressed as mean ± SEM of three independent experiments conducted in duplicate.
Cell Cycle Analysis:
12Z and 22B cells were cultured in T-75 flasks and treated as described above. The cells were first fixed in 1% buffered paraformaldehyde saline for 15 min on ice, and then fixed in ice cold 70% ethanol and kept at −20°C for 30 min. The cells were rehydrated in PBS for 15 min, treated with DNase-free RNAse (100 μg/ml), and stained with propidium iodide (25 μg/ml) in staining buffer (100 mM Tris, PH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.1% Nonidet P-40) for 30 min at room temperature. The number of cells distributed in G1, S, G2-M phases of cell cycle was determined by fluorescence-activated cell sorting (FACS) analysis of propidium-stained cells distribution using a flowcytometer (FACSCaliber, Becton Dickinson, San Jose, CA) and ModFit LT program (Verity Software House) and as we reported [46]. Data were expressed as mean ± SEM of three independent experiments.
Cell Apoptosis, Terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) assay and Flowcytometry:
The cells were harvested, mixed together, and resuspended at the concentrations of 1×106 cells/ml. Nicks in the DNA were determined by terminal deoxynucleotidyl transferase (TdT) and 5-bromo-2'-deoxyuridine 5'-triphosphate (BrdUTP) labeling using APO-BrdU TUNEL Assay Kit. Detection of BrdU incorporation at DNA break sites was achieved through Alexa Fluor 488 dye–labeled anti-BrdU antibody. Numbers of apoptotic cells were analyzed by a flowcytometer (FACSCaliber, Becton Dickinson, San Jose, CA) using Cell Quest software as we reported [10].
Protein Extraction and Western Blot:
Total protein was isolated from endometriotic cells and immunoblotting/western blotting was performed as we described previously [10,45,47]. Briefly, the cells were harvested using 1% Trypsin-EDTA and pelleted. The cell lysates were sonicated in sonication buffer which consisted of 20mM Tris-Hcl, 0.5mM EDTA, 100 μM DEDTC, 1% Tween, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail tablets: complete EDTA-free (1 tablet/50 ml) and PhosStop (1 tablet /10 ml). Sonication was performed using a Microson ultrasonic cell disruptor (Microsonix Incorporated, Farmingdale, NY). Protein concentration was determined using the Bradford method[48] and a Bio-Rad Protein Assay kit. Protein samples (75 μg) were resolved using 7.5%, 10% or 12.5% SDS-PAGE. Chemiluminescent substrate was applied according to the manufacturer's instructions (Pierce Biotechnology). The blots were exposed to Blue X-Ray film and densitometry of autoradiograms was performed using an Alpha Imager (Alpha Innotech Corporation, San Leandro, CA).
Immunoprecipitation:
12Z and 22B cells were cultured, treated, harvested, and then total cell lysates were prepared as described above. Total cell lysate (1 mg) was precleared by incubating with appropriate preclearing matrix (Santa Cruz Biotechnology) for 30 min at 4°C. The precleared cell lysate was incubated with primary antibody overnight at 4°C at the recommended concentrations given by manufacturers (Cell Signaling Technology and/or Santa Cruz Biotechnology), and then further incubated with ImmunoCruz immunoprecipitation optima (Santa Cruz Biotechnology) overnight at 4°C and as we reported [10]. Protein-antibody complexes were precipitated using protocols provided by Santa Cruz Biotechnology and/or Cell Signaling Technology.
Xenograft Rag2γ(c) Mouse Model of Endometriosis of Human Origin:
The human endometriotic epithelial cells 12Z were transduced with lentivirus containing NEF-Green plasmid and endometriotic stromal 22B cells was transduced with lentivirus NEF-Red and stable 12Z-GFP and 22B-RFP cell lines were established as we reported [40]. The 12Z-GFP and 22B-RFP cells were cultured as described above. At 70% confluency, the 12Z-GFP and 22B-RFP cells were processed for xenograft as we described previously [40].
All procedures were approved by the Institutional Animal Care and Use Committee at Texas A&M University. Rag2γ(c) mice were purchased (Taconic Biosciences, Inc) and then breeding colony was established, housed, and maintained at Laboratory Animal Resources and Research (LARR), Texas A&M University as described above. Rag2γ(c) mice (~22-25 gm, ovary intact, cyclic, not treated with estradiol) were included in the study. At 8 weeks of age, peritoneal endometriosis was induced by xenograft of 12Z-GFP (3×106) and stromal cells 22B-RFP(0.5×106) were mixed with 250μl of DMEM/F12 and 50μl of matrigel as we reported [40]. Day of xenograft was considered as day 1. The experimental mice were treated with MEK1/2 inhibitor U0126 to suppress ERK1/2 pathway, PI3K inhibitor LY294002 to suppress AKT pathway or combination of both treatments to suppress ERK1/2 and AKT pathways in vehicle (5% DMSO in 300μl sterile PBS, i.p) from days 15-28 of xenograft. Mice were necropsied on days 29-30 on E2-phase, based on vaginal cytology.
In Study-1, Group1 control mice (n=3) were treated with vehicle (5% DMSO in 300μl sterile PBS, i.p); Group-2 mice (n=3) were treated with MEK1/2 inhibitor U0126 (25mg/kg) in vehicle. Group-3 mice (n=3) were treated with MEK1/2 inhibitor U0126 (50mg/kg) in vehicle. Group-4 mice (n=3) were treated PI3K inhibitor LY294002 (25mg/kg) in vehicle. Group-5 mice (n=3) were treated with PI3K inhibitor LY294002 (50mg/kg) in vehicle.
In Study-2, Group-1 control mice (n=6) were treated with vehicle. Group-2 mice (n=6) were treated with MEK1/2 inhibitor U0126 (25mg/kg) and PI3K inhibitor LY294002 (25mg/kg) in vehicle. Group-3 mice (n=6) were treated with U0126 (50mg/kg) and LY294002 (50mg/kg) in vehicle.
Fluorescence Stereo Microscopy Imaging and Evaluation of Endometriotic Lesions:
Rag2g(c) experimental endometriosis mice were euthanized and blood collected as we described previously [40]. Then, the entire abdominal cavity was examined under fluorescence zoomstereo dissection microscope to determine the dissemination of 12Z-GFP and 22B-RFP clusters of endometriotic lesions. The fluorescent endometriotic lesions were recorded, tracked, and images captured under GFP and RFP filters at 1X magnification. Intensity of GFP and RFP in each image (clusters of lesions) was quantified using Image-Pro Plus as described below and expressed in numerical data as we reported [40]. The Nikon AZ100 Fluorescence stereomicroscope is equipped with AZ100 Plan Fluor Objectives 1x, 2x and 5x, fluorescent light source-excite series 120 PC, Nikon DS QiMc digital camera, and Nikon NIS Elements BR 3.22 software. All the lesions were dissected under the fluorescence zoomstereo dissection microscope and care was taken not to include the underlying peritoneal tissues. Grossly, the experimental endometriotic lesions were measured in two dimensions, the larger denoted ‘a’ and the smaller denoted ‘b’, and total volume, calculated using the formula V= axb2x0.5 [40]. Portions of endometriotic lesions were embedded in Optimal Cutting Temperature (OCT) compound and cryopreserved.
Immunocytochemistry (ICC):
Immunocytochemistry was performed according the protocol provided by Cell Signaling Technology (Danvers, MA) and as we reported [40]. The endometriotic lesion cryosections (10μm) were fixed in 2% PFA for 15 min at room temperature and followed by fixed in methanol for 10 min at 4°C. The tissue sections were incubated with primary antibodies for overnight at 4°C. The sections were further incubated with Alexa Fluor 488 and Alexa Fluor 594 conjugated secondary antibodies for 60 min at room temperature. Nuclei were stained with DAPI (ProLong Gold antifade, Molecular Probes). For the negative control, serum or IgG from respective species with reference to the primary antibody at the respective dilution was used.
Digital images were captured using a Zeiss Axioplan 2 Research Microscope (Carl Zeiss, Thornwood, NY) with an Axiocam HR digital color camera. The intensity of staining for each protein was quantified using Image-Pro Plus 6.3 image processing and analysis software according to the manufacturer’s instructions (Media Cybernetics, Inc; Bethesda, MD). The detailed methods for quantification are given in the instruction guide: “The Image-Pro Plus: The proven solution for image analysis.” In brief: a minimum 3 images of at 400X magnification were captured randomly without hot-spot bias in each tissue section per animal. The integrated optical intensity (IOD) of immunostaining was quantified under RGB mode. Numerical data were expressed as least square mean ± SEM. This technique is more quantitative than conventional blind scoring systems and the validity of quantification was reported previously by our group [40].
Statistical Analyses:
Statistical analyses were performed using general linear models of Statistical Analysis System (SAS, Cary, NC). Effects of inhibition of ERK1/2 and AKT pathways on expression levels of different proteins in 12Z and 22B cells in vitro, growth of endometriotic lesions, and relative expression of proteins in glandular epithelial cells and stromal cells of endometriotic lesions were analyzed by one-way analysis of variance (ANOVA) followed by Tukey-Kramer HSD test. The numerical data are expressed as mean ± SEM. Statistical significance was considered at P<0.05.
RESULTS
ERK1/2 and AKT Interactive Cell Signaling Pathways:
In order to understand the dual role of ERK1/2 and AKT pathways in the pathogenesis of endometriosis, we first determined their interactive cell signaling pathways (Fig-1).
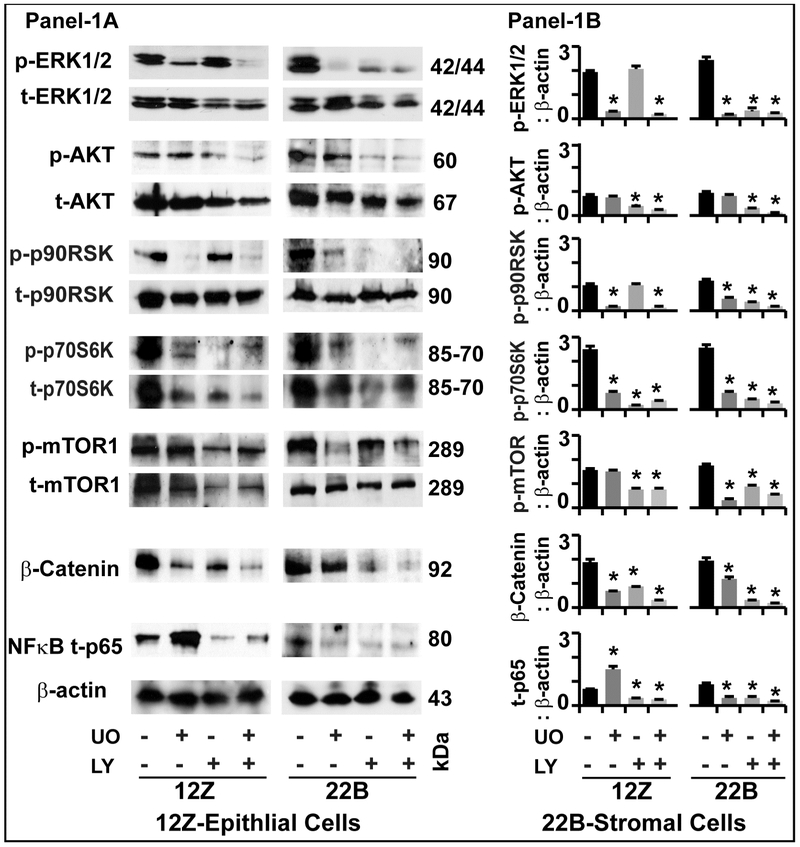
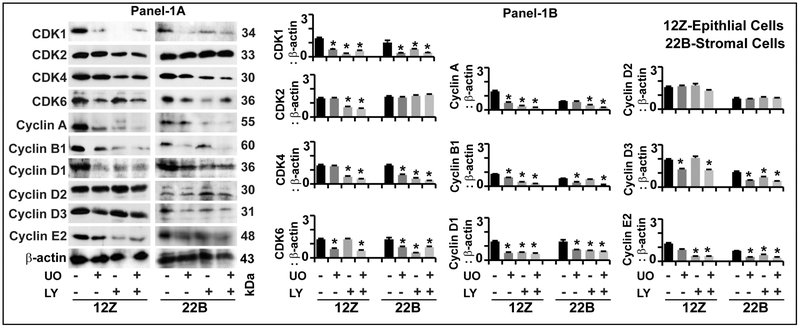
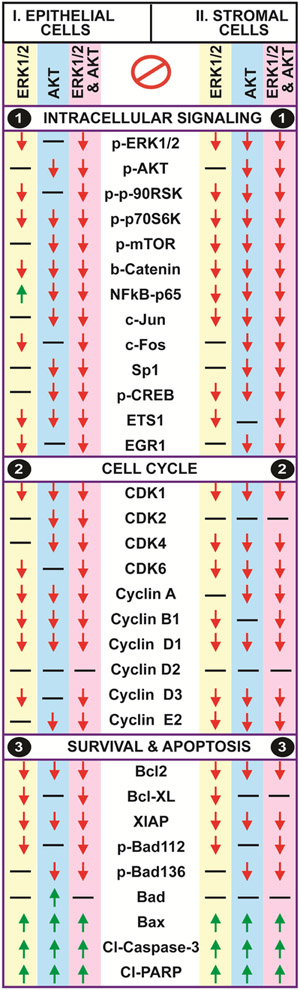
Fig-1: Effects of ERK1/2 and AKT pathways on intracellular signaling proteins in human endometriotic cells:
Panel-1A: Representative Immunoblot. Panel 1B: Histogram. The human endometriotic epithelial cells 12Z and stromal cells 22B were treated with MEK1/2 inhibitor (U0126, 20μm) to suppress ERK1/2 pathway or PI3K inhibitor (LY294002, 50μm) to suppress AKT pathway for 24h. Expression of important downstream signaling proteins were analyzed by western blot. β-actin protein was measured as an internal control. The densitometry of autoradiograms was performed using an Alpha Imager. Data expressed in integrated density value (IDV). *- control vs. treatment, p<0.05, n=3. See Materials and Method section for additional experimental details.
p-ERK1/2:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of p-ERK1/2 protein in epithelial cells and stromal cells. Inhibition of AKT pathway did not decrease the expression of p-ERK1/2 protein in epithelial cells but decreased (p<0.05) its expression in stromal cells. Combined inhibition of both pathways highly decreased (p<0.05) the expression of p-ERK1/2 protein in both epithelial cells and stromal cells.
p-AKT:
Inhibition of ERK1/2 pathway did not decrease the expression of p-AKT protein in epithelial cells and stromal cells. Inhibition of AKT pathway decreased the expression of p-AKT protein in epithelial cells and stromal cells. Combined inhibition of both pathways highly decreased (p<0.05) the expression of p-AKT protein in epithelial cells but not in stromal cells.
p-p90RSK:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of p-p90RSK protein in epithelial cells and stromal cells. Inhibition of AKT did not decrease the expression of p-p90RSK protein in epithelial cells; in contrast, decreased (p<0.05) its expression in stromal cells. Combined inhibition of ERK1/2 and AKT pathways highly decreased (p<0.05) the expression of p-p90RSK protein in stromal cells but not in epithelial cells.
p-p70S6K:
Inhibition of ERK1/2, AKT or combined inhibition of both pathways decreased (p<0.05) the expression of p-p70S6K protein in epithelial cells as well as in stromal cells.
p-mTOR1:
Inhibition of ERK1/2 pathway did not decrease the expression of p-mTOR1 protein in epithelial cells; in contrast, decreased (p<0.05) its expression in stromal cells. Inhibition of AKT decreased (p<0.05) the expression of p-mTOR1 protein in both epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of p-mTOR1 protein in both epithelial cells and stromal cells.
β-Catenin:
Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) the expression of β-Catenin protein in epithelial cells as well as in stromal cells.
NFkB-p65:
Inhibition of ERK1/2 pathway increased (p<0.05) the expression of NFkB-p65 protein in epithelial cells, in contrast; decreased (p<0.05) its expression in stromal cells. Inhibition of AKT pathways decreased (p<0.05) the expression of NFkB-p65 protein in both epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of NFkB-p65 protein in both epithelial cells and stromal cells.
Analyses of these multiple downstream signaling proteins indicate the existence compensatory interactions between ERK1/2 and AKT pathways in an epithelial-stromal cell specific manner in human endometriotic stromal cells.
ERK1/2 and AKT Interactive Transcriptional Factors:
In order to further understand downstream signaling mechanisms we determined the dual inhibitory effects of ERK1/2 and AKT pathways on regulation of transcriptional factors (Fig-2).
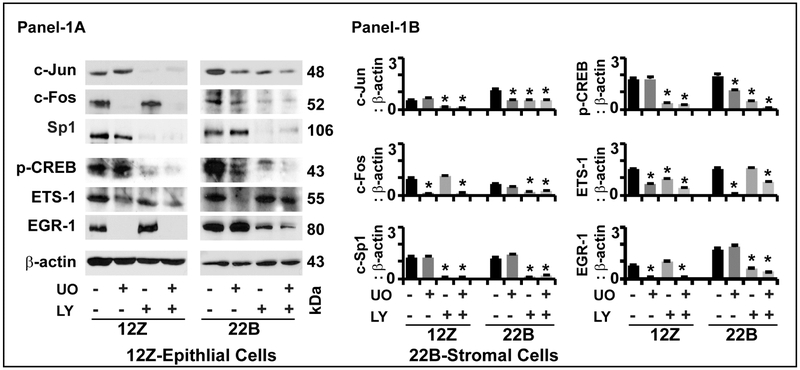
Fig-2: Effects of ERK1/2 and AKT pathways on regulation of transcriptional factors in human endometriotic cells:
Panel-1A: Representative Immunoblot. Panel 1B: Histogram. The human endometriotic epithelial cells 12Z and stromal cells 22B were treated with MEK1/2 inhibitor (U0126, 20μm) to suppress ERK1/2 pathway or PI3K inhibitor (LY294002, 50μm) to suppress AKT pathway for 24h. Expression of important downstream transcriptional factor proteins were analyzed by western blot. β-actin protein was measured as an internal control. The densitometry of autoradiograms was performed using an Alpha Imager. Data expressed in integrated density value (IDV). *- control vs. treatment, p<0.05, n=3. See Materials and Method section for additional experimental details.
c-Jun:
Inhibition of ERK1/2 pathway did not decrease the expression of c-Jun protein in epithelial cells, in contrast; decreased (p<0.05) its expression in stromal cells. Inhibition of AKT pathway decreased (p<0.05) the expression of c-Jun protein in epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of c-Jun protein in epithelial cells and stromal cells.
c-FOS:
Inhibition of ERK1/2 decreased (p<0.05) the expression of c-FOS protein in epithelial cells and stromal cells. Inhibition of AKT pathway did not decrease the expression of c-FOS protein in epithelial cells but decreased (p<0.05) its expression in stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of c-FOS protein in both epithelial cells and stromal cells.
Sp1:
Inhibition of ERK1/2 pathway did not decrease the expression of Sp1 protein in epithelial cells and stromal cells. Inhibition of AKT pathway decreased (p<0.05) the expression of Sp1 protein in both epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways highly decreased (p<0.05) the expression of Sp1 protein in both epithelial cells and stromal cells.
p-CREB:
Inhibition of ERK1/2 pathway did not decrease the expression of p-CREB protein in epithelial cells but decreased (p<0.05) its expression in stromal cells. Inhibition of AKT pathway decreased (p<0.05) the expression of p-CREB protein in both epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways highly decreased (p<0.05) the expression of p-CREB protein in both epithelial cells and stromal cells.
ETS1:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of ETS1 protein, inhibition of AKT pathway did not decrease its expression epithelial cells and stromal cells. Combined inhibition ERK1/2 and AKT pathways decreased (p<0.05) the expression of ETS1 protein epithelial cells and stromal cells.
EGR-1:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of EGR-1 protein in epithelial cells but did not decrease its expression in stromal cells. Inhibition of AKT did not decrease the expression of EGR-1 protein in epithelial cells but decreased (p<0.05) its expression in stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of EGR-1 protein in both epithelial cells and stromal cells.
These results together indicate that dual inhibition of ERK1/2 and AKT pathways regulates multiple transcriptional factors in an epithelial-stromal cell specific and pathway-dependent pathway in human endometrioitc cells.
Cell Proliferation and Cell Cycle Regulation
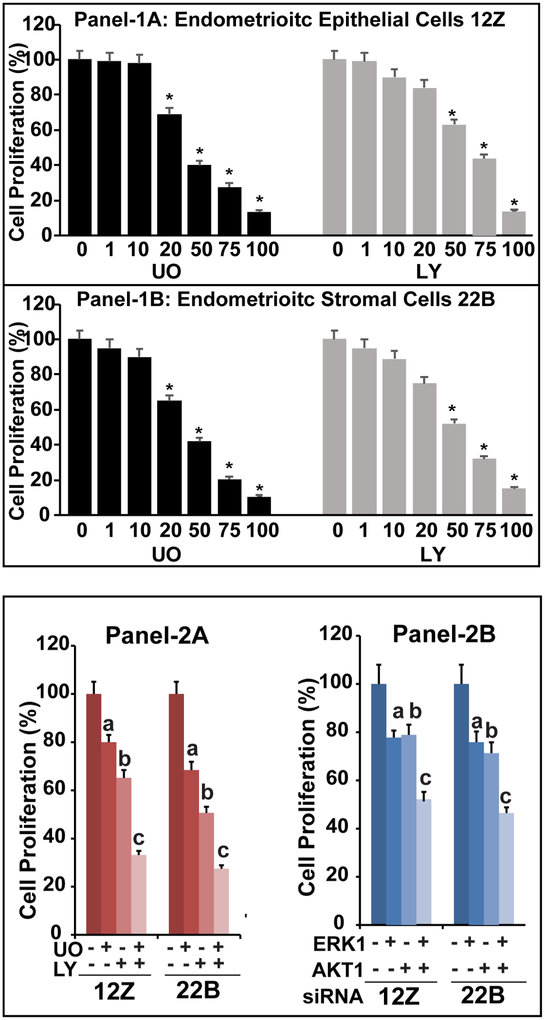
We determined the dual inhibitory effects of ERK1/2 and AKT interactive pathways on proliferation of human endometriotic epithelial cells and stromal cells (Fig-3). Pharmacological inhibition of ERK1/2 or AKT pathways dose-dependently (p<0.05) decreased the proliferation both endometriotic epithelial cells 12Z (Panel-1A) and stromal cells 22B (Panel-1B). Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) proliferation of endometriotic epithelial cells up to 20%, 34% or 68% respectively compared to control (Panel-2A). Equally, pharmacological inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) proliferation of endometriotic stromal cells up to 32%, 49%, and 74% respectively compared to control (Panel-2A). Similarly, silencing of ERK1, AKT or both genes using siRNA decreased (p<0.05) proliferation of endometriotic epithelial cells up to 22%, 23% or 48% and proliferation of endometriotic stromal cells up to 22%, 29%, and 53% respectively compared to control (Panel-2B). In both epithelial cells and stromal cells, combined inhibition of ERK1/2 and AKT pathways caused higher (p<0.05) inhibitory effects on cell proliferation compared to inhibition of either ERK1/2 or AKT pathway alone.
Fig-3: Effects of ERK1/2 and AKT pathways on proliferation of human endometriotic cells.
Panel-1: The human endometriotic epithelial cells 12Z (Panel-1A) and stromal cells 22B (Panel-1B) were treated with MEK1/2 inhibitor (U0126, 0, 1, 10, 20, 50, 75, and 100 μM) to suppress ERK1/2 pathway and/or PI3K inhibitor (LY294002, 0, 1, 10, 20, 50, 75, and 100 μM) to suppress AKT pathway in vehicle (1% DMSO) in plain media for 24h. *- control vs. treatment, p<0.05, n=3. Panel-2A: The optimal concentration for was selected based on its effects on proliferation of 12Z and 22B cells. The 12Z cells and stromal cells 22B were treated with MEK1/2 inhibitor (U0126, 20μm) and/or PI3K inhibitor (LY294002, 50μm) for 24h. Panel-2B: ERK1 and AKT genes were silenced using siRNA approach. The number of live cells were counted at 48h post-transfection. In all experiments, the number of cells were counted using a Coulter counter and considered as 100% present in control. Data were expressed as mean ± SEM of three independent experiments conducted in duplicate. a- control vs inhibition of ERK1/2 pathway, b- control vs inhibition of AKT pathway, c- control vs. combined inhibition of ERK1/2 and AKT pathways, p<0.05, n=3. See Materials and Method section for additional experimental details.
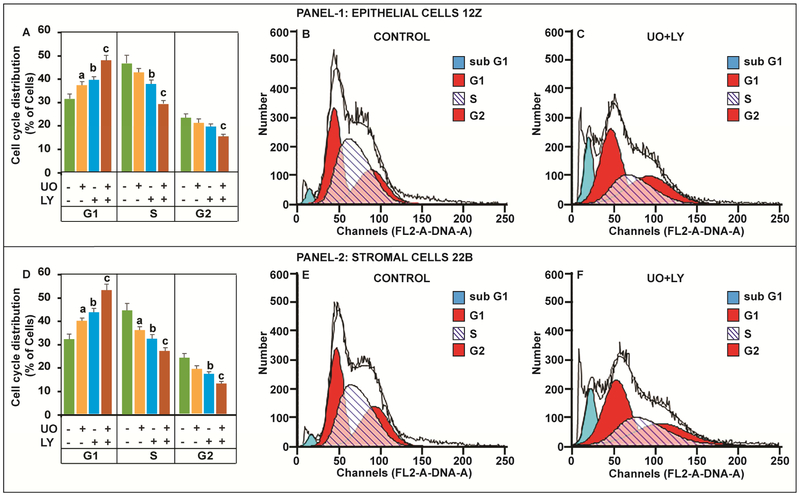
Next, we analyzed progression of human endometriotic cells through cell cycle. Results (Fig-4) indicated that combined inhibition of ERK1/2 and AKT pathways arrested (p<0.05) the progression of endometriotic epithelial cells and stromal cells in the G1 phase and concomitantly decreased (p<0.05) progression of these cells through S phase and G2 phase of the cell cycle compared to inhibition of either ERK1/2 or AKT pathway individually. These results indicate that dual inhibition of ERK1/2 and AKT pathways highly (p<0.05) affects the progression of human endometriotic cells through G1-S and G2-M phases of the cell cycle compared to inhibition of a single pathway.
Fig-4: Effects of ERK1/2 and AKT pathways on cell cycle regulation in human endometriotic cells.
The human endometriotic epithelial cells 12Z (Panel-1) and stromal cells 22B (Panel-2) were treated with MEK1/2 inhibitor (U0126, 20μm) to suppress ERK1/2 pathway and/or PI3K inhibitor (LY294002, 50μm) to suppress AKT pathway for 24h. Distribution of cells in different phases of cell cycle was measured by fluorescence activated cell sorting. Histograms (A,D) show the effects of inhibition of ERK1/2, AKT or combination of both pathways on distribution of cells in G1, S, and G2 phases of the cell cycle. Representative FL2A plot shows the gated Sub G1/ G1/ S/G2 cells in (B, E) control and (C, F) combined inhibition of ERK1/2 and AKT pathways. a- control vs inhibition of ERK1/2 pathway, b- control vs inhibition of AKT pathway, c- control vs. combined inhibition of ERK1/2 and AKT pathways, p<0.05, n=3. See Materials and Method section for additional experimental details.
In order to understand the cell cycle dysregulation, we further determined the regulation of cell cycle regulatory proteins in endometrioitc epithelial cells and stromal cells (Fig-5).
Fig-5: Effects of ERK1/2 and AKT pathways on cell cycle regulatory proteins in human endometriotic cells.
Panel-1A: Representative Immunoblot. Panel 1B: Histogram. The human endometriotic epithelial cells 12Z and stromal cells 22B were treated with MEK1/2 inhibitor (U0126, 20μm) to suppress ERK1/2 pathway or PI3K inhibitor (LY294002, 50μm) to suppress AKT pathway for 24h. Expression of important cell cycle regulatory proteins were analyzed by western blot. β-actin protein was measured as an internal control. The densitometry of autoradiograms was performed using an Alpha Imager. Data expressed in integrated density value (IDV). *- control vs. treatment, p<0.05, n=3. See Materials and Method section for additional experimental details.
CDK1:
Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) the expression of CDK1 protein in epithelial cells and stromal cells.
CDK2:
Inhibition of ERK1/2 pathway did not decrease the expression of CDK2 protein in epithelial cells or stromal cells. Inhibition of AKT pathway decreased (p<0.05) the expression of CDK2 protein in epithelial cells but not in stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of CDK2 protein in epithelial cells but not in stromal cells.
CDK4:
Inhibition of ERK1/2 pathway did not decrease the expression of CDK4 protein in epithelial cells but decreased (p<0.05) its expression in stromal cells. Inhibition of AKT pathway decreased (p<0.05) the expression of CDK4 protein in epithelial cells as well as in stromal cells. Combined inhibition of ERK1/2 and AKT pathways highly decreased (p<0.05) the expression of CDK2 protein in stromal cells but not in epithelial cells.
CDK6:
Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) the expression of CDK6 protein in epithelial cells and stromal cells.
Cyclins A, B1, D1, E2:
Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) the expression of cyclin A, cyclin B1, cyclin D1, and cyclin E2 proteins in epithelial cells and stromal cells; whereas, it did not decrease the expression of cyclin D2 protein in both epithelial cells and stromal cells.
Cyclin D3:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of cyclin D3 protein in epithelial cells and stromal cells. Inhibition of AKT did not decrease the expression of cyclin D3 protein in epithelial cells but decreased (p<0.05) its expression in stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of cyclin D3 protein in both epithelial cells as well as stromal cells.
These results indicate that inhibition of ERK1/2 and AKT pathways dysregulate cell cycle regulatory proteins involved in G1-S and G2-M transition in an epithelial and stromal cell-specific and pathway-dependent pattern.
Cell Apoptosis and Intrinsic Apoptotic Pathways
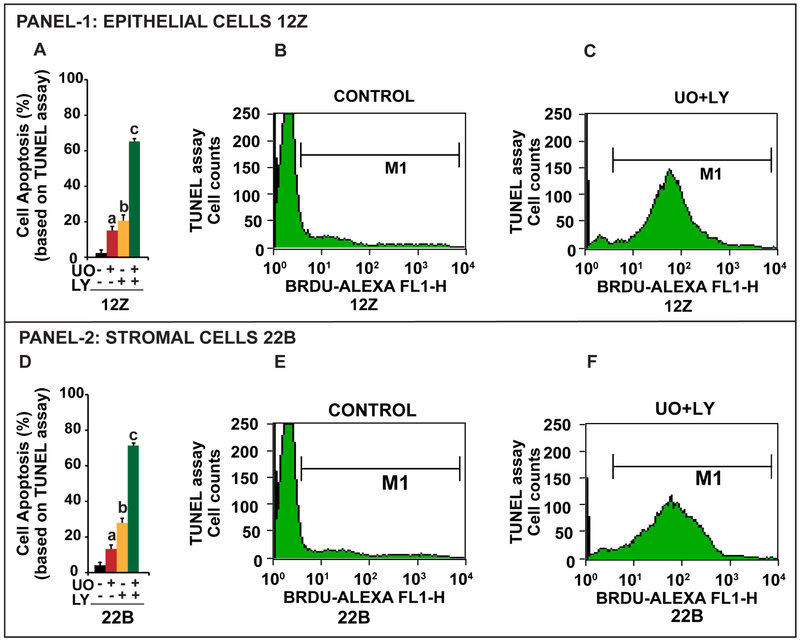
It is evident from cell cycle analyses (Fig-4) that inhibition ERK1/2 and AKT pathways increased (p<0.05) the accumulation of cells in sub G0/G1 phase of the cell cycle, suggesting transition of cells to apoptotic phase. Therefore, we determined the dual inhibitory effects of ERK1/2 and AKT pathways on the cells that undergo extensive DNA degradation during the late stages of apoptosis by TUNEL assay. Results (Fig-6) indicated that inhibition of ERK1/2, AKT, or combination of ERK1/2 and AKT pathways induced (p<0.05) apoptosis of endometriotic epithelial cells 12Z (17%, 20%, 65% respectively) and stromal cells 22B (16%, 29%, and 72%respectively). Combined inhibition of ERK1/2 and AKT pathways induced higher (p<0.05) apoptosis compared to inhibition of either ERK1/2 or AKT pathway alone in both epithelial cells and stromal cells.
Fig-6: Effects of ERK1/2 and AKT pathways on apoptosis of human endometriotic cells.
The human endometriotic epithelial cells 12Z (Panel-1) and stromal cells 22B (Panel-2) were treated with MEK1/2 inhibitor (U0126, 20μm) to suppress ERK1/2 pathway or PI3K inhibitor (LY294002, 50μm) to suppress AKT pathway for 24h. Nicks in the DNA were determined by TUNEL assay and numbers of apoptotic cells were analyzed by a flowcytometer. Histograms (A,D) show the effects of inhibition of ERK1/2, AKT or combination of both pathways on apoptosis of cells. Representative FL1H plot shows the gated apoptotic cells in (B, E) control and (C, F) combined inhibition of ERK1/2 and AKT pathways. a- control vs inhibition of ERK1/2 pathway, b- control vs inhibition of AKT pathway, c- control vs. combined inhibition of ERK1/2 and AKT pathways, p<0.05, n=3. See Materials and Method section for additional experimental details.
In order to understand the molecular and cellular mechanisms, we determined the underlying apoptotic signaling pathways in human endometrioitc cells. (Fig-7).
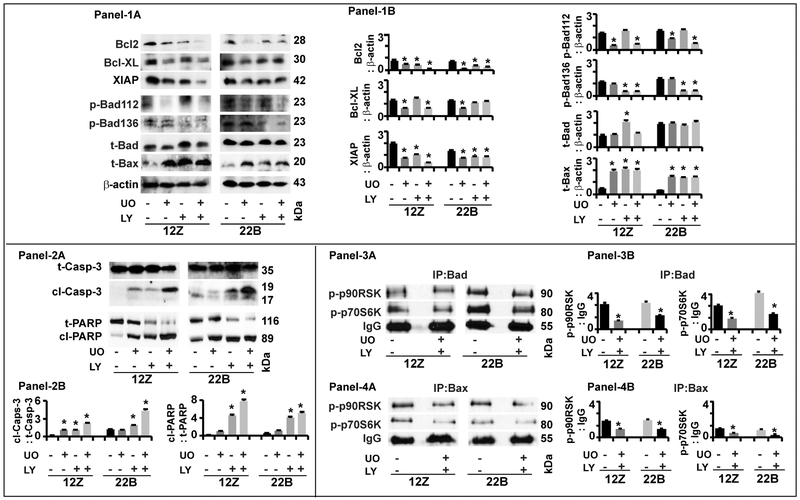
Fig-7: Effects of ERK1/2 and AKT pathways on intrinsic apoptosis pathway proteins in human endometriotic cells.
Panel-1: Antiapoptotic and proapoptotic proteins. (1A) Representative Immunoblot and (1B) Histogram. Panel-2: Caspase-3 and PARP proteins. (2A) Representative Immunoblot and (2B) Histogram. Panel-3: Interactions between Bad and p-p90RSK and p-p70S6K. (3A) Representative immunoprecipitation/immunoblot and (3B) Histogram. Panel-4: Interactions between Bax and p-p90RSK and p-p70S6K. (4A) Representative immunoprecipitation/immunoblot and (4B) Histogram. The human endometriotic epithelial cells 12Z and stromal cells 22B were treated with MEK1/2 inhibitor (U0126, 20μm) to suppress ERK1/2 pathway or PI3K inhibitor (LY294002, 50μm) to suppress AKT pathway for 24h. Expression of intrinsic apoptosis pathway proteins were analyzed by western blot. β-actin protein was measured as an internal control. Protein-protein interaction was determined by immunoprecipitation. IgG was measured as internal control. The densitometry of autoradiograms was performed using an Alpha Imager. Data expressed in integrated density value (IDV). *- control vs. treatment, p<0.05, n=3. See Materials and Method section for additional experimental details.
Bcl2:
Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) the expression of Bcl2 protein in an epithelial cells and stromal cells-specific and pathway-dependent pattern.
Bcl-XL:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of Bcl-XL protein in epithelial cells and stromal cells. Inhibition of AKT did not decrease the expression of Bcl-XL protein in epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) the expression of Bcl-XL protein in epithelial cells but not in stromal cells.
XIAP:
Inhibition of ERK1/2, AKT or combination of both pathways decreased (p<0.05) the expression of XIAP protein in an epithelial cells and stromal cells-specific and pathway-dependent pattern.
pBad112:
Inhibition of ERK1/2 pathway decreased (p<0.05) the expression of p-Bad112 protein in epithelial cells and stromal cells. Inhibition of AKT pathway (p<0.05) did not affect expression of p-Bad112 protein in both epithelial cells and stromal cell types. Combined inhibition of ERK1/2 and AKT pathways highly decreased (p<0.05) the expression of p-Bad112 protein in stromal cells but not in epithelial cells.
pBad136:
Inhibition of ERK1/2 pathway did not affect expression of p-Bad136 protein in epithelial and stromal cells. Inhibition of AKT pathway decreased (p<0.05) the expression of p-Bad136 protein in both epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways highly decreased (p<0.05) the expression of p-Bad136 protein in epithelial cells and stromal cells.
Bad:
Inhibition of ERK1/2 did not modulate the expression of total-Bad protein in both epithelial cells and stromal cells. In contrast, inhibition of AKT pathway increased (p<0.05) the expression of total-Bad protein in epithelial cells but not in stromal cells. Combined inhibition of ERK1/2 and AKT pathways did not show any additional inhibitory effects on expression of total-Bad protein in both epithelial cells and stromal cell types.
Bax:
Inhibition of ERK1/2, AKT or combination of both pathways increased (p<0.05) the expression of total-Bax protein in epithelial cells and stromal cells.
Cl-Capase-3:
Inhibition of ERK1/2 pathway cleaved (p<0.05) caspase-3 protein in epithelial cells but not in stromal cells. Inhibition of AKT pathway cleaved (p<0.05) caspase-3 protein in both epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways showed higher (p<0.05) effects on cleavage of caspase-3 protein in both epithelial cells and stromal cells.
Cl-PARP:
Inhibition of ERK1/2 or AKT pathway cleaved (p<0.05) PARP protein in epithelial cells and stromal cells. Combined inhibition of ERK1/2 and AKT pathways highly (p<0.05) cleaved PARP protein in both epithelial cells and stromal cells.
ERK1/2 or AKT and Bax or Bad interactions:
We further determined interactions between ERK1/2-p90RSK and AKT-p70S6K and proapoptotic proteins Bax and Bad. Results indicated that combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) interactions between Bad and p-p90RSK and Bad and p-p70S6K proteins in both epithelial cells and stromal cells. Similarly, combined inhibition of ERK1/2 and AKT pathways decreased (p<0.05) interactions between Bax and p-p90RSK and Bax and p-p70S6K proteins in both epithelial cells and stromal cells.
These results together indicate that dual inhibition of ERK1/2 and AKT pathways activates intrinsic apoptosis mechanisms in an epithelial cells and stromal cell-specific and pathway-dependent pathway in human endometrioitc cells.
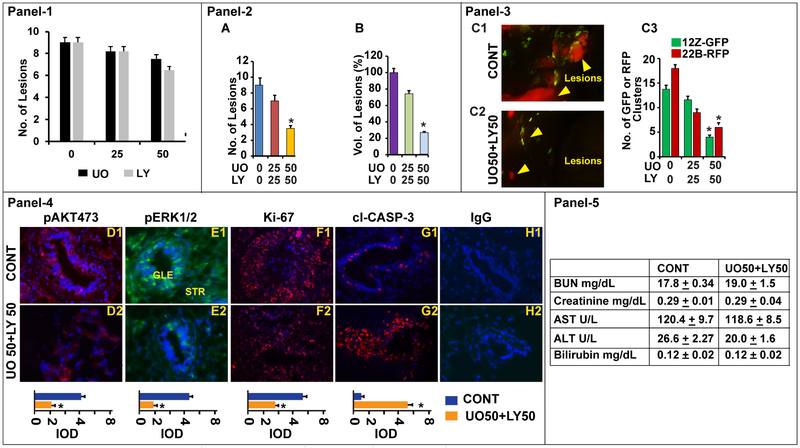
Experimental Endometriosis In vivo:
We determined the effects of inhibition of ERK1/2, AKT or ERK1/2 and AKT pathways on growth and survival of endometriotic lesions in xenograft mice of model of experimental endometriosis in vivo (Fig-8). We first determined the effects of inhibition of ERK1/2 or AKT pathways. Results (Panel-1) indicated that inhibition of either pathway did not decrease the growth of endometrioitc lesions. By contrast, dual inhibition of ERK1/2 and AKT pathways decreased (p<0.05) total number (Panel-2A) and total volume (Panel-2B) of endometriotic lesions in a dose dependent manner. It decreased ~20% of endometriotic lesions at 25mg/kg, whereas, it decreased ~70% of endometriotic lesions at 50mg/kg. In addition, fluorescent microscopy cell-specific analyses (Panel-3, C1-C3) indicated that dual inhibition of ERK1/2 and AKT pathways decreased the quantity of epithelial cells (12-GFP) and stromal cells (22B-RFP) in the endometriotic lesions in vivo. Immunocytochemistry analyses (Panel-4, D-H) indicated that dual inhibition of ERK1/2 and AKT pathways decreased the expression of pERK1/2 and pAKT proteins. In addition, it increased the expression of apoptosis marker protein cl-Caspase-3 protein and concomitantly decreased the expression of cell proliferation marker protein ki67 in both epithelial cells (12-GFP) and stromal cells (22B-RFP) of the endometriotic lesions in vivo. Biochemical analyses (Panel-5) indicated that experimental mice treated with ER1/2 and AKT inhibitors for 2 weeks did not develop toxicity on kidney, heart, and liver functions. These results together indicate that dual inhibition of ERK1/2 and AKT pathways decreased proliferation and induced apoptosis of both epithelial cells and stromal cells of the endometriotic lesions.
Fig-8: Effects of ERK1/2 and AKT pathways on growth and survival of endometriotic lesions.
A mixture of human endometriotic epithelial cells 12Z-GFP and stromal cells 22B-RFP suspension was injected into the peritoneal cavity of Rag2g(c) mice and peritoneal endometriosis was induced (day 1). The endometriosis mice were treated with MEK1/2 inhibitor U0126 (UO@ 0, 25, 50 mg/kg) to suppress ERK1/2 pathway and/or PI3K inhibitor LY294002 (LY@ 0, 25, 50 mg/kg) to suppress AKT pathway from days 15-28. The mice were necropsied on day 29-30 on E2-phase of the estrus cycle. Panel-1: Histogram shows the dose-dependent inhibitory effects of ERK1/2 (n=3) or AKT (n=3) pathway on growth of endometriotic lesions. Panel-2: Histogram shows the dose-dependent effects of combined inhibition of ERK1/2 or AKT pathways (n=6) on growth of endometriotic lesions, (A) number of lesions and (B) volume of lesions. Panel-3: Dose-dependent effects @ UO50/LY50 is shown. (C1-C2) Fluorescence zoomstereo microscopy examination of dissemination of 12Z-GFP and 22B-RFP cells of endometriotic lesions in the peritoneal cavity, yellow arrows show the lesions. (C3) Histogram shows number of 12Z-GFP and 22B-RFP cells in these endometriotic lesions. Panel-4: Expression of (D1-D2) pAKT, (E1-E2) pERK1/2, (F1-F2) ki-67, and (G1-G2) cl-Caspase-3 proteins in the endometriotic lesions. (H1-H2): Negative control IgG. GLE: Glandular epithelial cells. STR: Stromal cells. Relative expression was quantified using Image Pro-Plus. Panel-5: Biochemical profile. *- control vs. treatment, p<0.05, n=6 mice.
DISCUSSION
Interactions among survival, antiapoptotic, and proapoptotic pathways determine survival or apoptosis of the cells. The well-studied signaling pathways that govern survival of cells are Ras-Raf-ERK1/2-p90RSK [16,17,49-51], PI3K-AKT-p70S6K [17,19,50,51], IκBα-NFκB [20], and Wnt-β-catenin pathways [21-23]. In the present study, we determined downstream signaling modules which are coordinately regulated by ERK1/2 and AKT pathways in human endometriotic cells. Results indicate that inhibition of ERK1/2 pathway decreases the expression of p-ERK1/2 protein in endometriotic epithelial cells and decreases the expression of p-ERK1/2 and p-AKT proteins in endometriotic stromal cells. Inhibition of AKT pathway decreases the expression of p-AKT protein but not p-ERK1/2 protein in endometriotic epithelial cells and stromal cells. Notably, inhibition of ERK1/2 pathway alone represses the ERK1/2-p90RSK, ERK1/2-p70RSK, and ERK1/2-β-Catenin but not the ERK1/2-mTOR1 or ERK1/2-NFkBp65 signaling modules in endometriotic epithelial cells; in contrast, it represses all these signaling modules in endometriotic stromal cells. Inhibition of AKT pathway alone represses the AKT-p70RSK, AKT-mTOR1, AKT-β-Catenin, AKT-NFkBp65 but not the AKT-p90RSK signaling modules in endometriotic epithelial cells; in contrast, it represses all these signaling modules in endometriotic stromal cells. Importantly, combined inhibition of ERK1/2 and AKT pathways represses the ERK1/2+AKT-p90RSK, ERK1/2+AKT-p70RSK, ERK1/2+AKT-β–Catenin, ERK1/2+AKT-mTOR1, and ERK1/2+AKT-NFkBp65 signaling modules in both endometriotic epithelial cells and stromal cells. Inhibition of ERK1/2 pathway decreases the expression of c-Fos, ETS-1, and EGR-1 proteins in endometriotic epithelial cells and decreases the expression of c-Jun, p-CREB, and ETS-1 proteins in endometriotic stromal cells. Inhibition of AKT decreases the expression of c-Jun, SP1, p-CREB, ETS-1 proteins in endometriotic epithelial cells and decreases the expression of c-Jun, C-Fos, Sp1, p-CREB, and EGR-1 proteins in endometriotic stromal cells. Importantly, combined inhibition of both ERK1/2 and AKT pathways decreases the expression of all these transcriptional factors in endometriotic epithelial cells and stromal cells. These results clearly indicate that ERK1/2 and AKT pathways are interacting and coordinately regulate multiple downstream signaling modules in an epithelial cells and stromal cell-specific and pathway-dependent ways in human endometriotic cells. Our new findings together indicate the existence of compensatory mechanisms between ERK1/2 and AKT pathways on regulation of down-stream signaling pathways, and strongly point out a need for dual inhibition of these two pathways in endometriosis.
We determined the effects of inhibition of ERK1/2 and AKT pathways on proliferation of human endometrioitc cells and the underlying molecular mechanisms. Results indicate that the combined inhibition of both ERK1/2 and AKT pathways causes higher inhibitory effects on proliferation of epithelial cells and stromal cells compared to inhibition of either ERK1/2 or AKT pathway. In support of this, the cell cycle analyses indicate that combined inhibition of ERK1/2 and AKT pathways decreases the progression of epithelial cells and stromal cells through G1-S and G2-M check-points. Next, we examined whether the cell cycle arrest is associated with regulation of respective CDKs and cyclins. Selective CDK/cyclin complexes are activated at different phases/check-points of the cell cycle [52-55]. Cyclin D1/D2/D3 and CDK4/6 complexes are activated in early to mid G1-phase; cyclin E/CDK2 complexes are required for the G1/S transition; cyclin A/CDK2 complex is essential for the progression of S-phase/DNA synthesis; and cyclin A-B/CDK1 is necessary for G2-M transition [52-55]. Results of the present study indicate that downregulation of cyclins and CDK complexes is responsible for deregulated progression of endometriotic epithelial cells and stromal cells through G1-S and G2-M check-points. In cyclin D1/D2/D3 and CDK4/6 complexes, expression of cyclin D2 protein is not decreased in contrast expression of D2, D3, and CDK4 and CDK6 proteins are decreased in both epithelial cells and stromal cells. It suggests suppression of D1 and D3 along with CDK4/6 is sufficient to decrease the progression of human endometriotic cells through G1-phase of the cell cycle. In cyclin E/CDK2 complexes, expression of CDK2 is not decreased but expression of cyclin E2 is decreased in stromal cells, suggesting suppression of cyclin E2 is sufficient to regulate the progression of human endometriotic cells through G1-S transition. These results together indicate that inhibition of ERK1/2 and AKT pathways suppresses the proliferation of human endometriotic epithelial cells and stromal cells through dysregulated cell cycle mechanisms. Evidently, these results support the existence of compensatory mechanisms between ERK1/2 and AKT pathways and confirm the need for dual inhibition of both ERK1/2 and AKT pathways to suppress proliferation of human endometriotic epithelial cells and stromal cells.
We determined the effects of inhibition of ERK1/2 and AKT pathways on apoptosis or survival of human endometrioitc cells and underlying molecular mechanisms. Members of the Bcl-2 family play pivotal roles in cell survival or apoptosis [56-58]. The Bcl-2 family includes anti-apoptotic (Bcl-2 and Bcl-XL) and pro-apoptotic (Bad and Bax) members [56-58]. Bcl-2 and Bcl-XL proteins are localized exclusively in the mitochondria and control its potential to prevent the release of cytochrome C into the cytosol [56-58]. In addition, activation of NFκB and β-catenin signaling pathways increases expression of Bcl2 and Bcl-XL proteins in the mitochondria [20,22]. Results of the present study indicate that inhibition of ERK1/2 pathway decreases the expression of Bcl2, Bcl-XL, and XIAP proteins in endometriotic epithelial cells and stromal cells. Inhibition of AKT pathway decreases the expression of Bcl2 and XIAP proteins but not Bcl-XL protein in endometriotic epithelial cells and stromal cells. Combined inhibition of both pathways decease the expression of Bcl2, Bcl-XL, and XIAP proteins in endometriotic epithelial cells and decrease the expression of Bcl2 and XIAP but not Bcl-XL proteins in endometriotic stromal cells. These results indicate that Bcl2, Bcl-XL, XIAP proteins are the downstream targets for ERK1/2 pathway; whereas, Bcl2 and XIAP but not Bcl-XL proteins are down-stream targets for AKT pathway in human endometriotic epithelial cells and stromal cells.
Activation of Ras-Raf-ERK1/2 and PI3K-AKT signaling modules phosphorylates/inactivates Bad at serine 112 or 136 [17,19,50,51] and activation of PI3K-AKT phosphorylates/inactivates Bax at serine 184 [59-62]. Phosphorylation of Bad and Bax at these specific sites sequestrates them in the cytosol with 14-3-3 proteins, prevents translocation of Bad and Bax proteins from the cytosol into the mitochondria and interactions with antiapoptotic proteins Bcl-2 and Bcl-XL, and thus inhibits apoptosis [23,63,64]. Apoptotic stimuli dephosphorylate Bad and Bax, dissociate them from 14-3-3 proteins, translocate them from the cytosol into the mitochondria, mediate interactions between Bad/Bax and Bcl-2/Bcl-xL, and facilitate release of cytochrome C from the mitochondria into the cytosol [23,63-65]. Results of the present study indicate that inhibition of ERK1/2 pathway decreases the expression of p-Bad112 protein but not pBad136 protein; in contrast, inhibition of AKT pathway decreases the expression of p-Bad136 protein but not p-Bad112 protein in endometriotic epithelial cells and stromal cells. These results indicate that p-Bad112 is a downstream target for ERK1/2 pathway and p-Bad136 is a down-stream target for AKT pathways in human endometriotic epithelial cells and stromal cells. Inhibition of ERK1/2 pathway does not increase t-Bad protein in both epithelial cells and stromal cells; whereas, inhibition of AKT pathway does increase expression of t-Bad protein in epithelial cells but not in stromal cells. These results together indicate that inhibition ERK1/2 pathways increases phosphorylation of Bad protein at serine 112, and inhibition of AKT increases phosphorylation of Bad protein at serine 136 in both epithelial cells and stromal cells. Importantly, inhibition of ERK1/2, AKT, or combination both pathways increases the expression of t-Bax protein endometrioitc epithelial cells and stromal cells. These results together indicate that ERK1/2 and AKT pathways targets Bad and Bax proteins in human endometriotic epithelial cells and stromal cells.
Release of cytochrome C from the mitochondria into the cytosol activates caspase-3 which in turn activates nuclear PARP and other proteins that are required to complete programmed cell death [23,63-65]. Results of the present study indicate that inhibition of ERK1/2 or AKT pathways cleaves or activates caspase-3 and PARP proteins, and combined inhibition of both pathways highly cleaves caspase-3 and PARP proteins to a greater degree in endometriotic epithelial cells and stromal cells. These result together indicate that ERK1/2 and AKT pathways target caspase-3 and PARP proteins and thereby activates intrinsic apoptotic pathways in human endometriotic epithelial cells and stromal cells.
Activation of ERK1/2-p90RSK [16,17,49-51] and AKT-p70S6K [17,19,50,51] signaling modules phosphorylates Bad protein at serine 112 or 136 and activation of PI3K-AKT phosphorylates Bax protein at serine 184[59-62] in tumor cells. Results of the present study indicate that combined inhibition of ERK1/2 or AKT pathways decreases the interactions between p-p90RSK and Bad and Bax proteins, and decreases interaction between p-p70S6K and Bad and Bax proteins in endometrioitc epithelial cells and stromal cells. These results evidently demonstrate that Bad and Bax proteins are down-stream targets for p90RSK and p70S6K in human endometriotic epithelial cells and stromal cells.
Finally, we examined the role of ERK1/2 and AKT pathways in growth and survival of endometrioitc lesions in vivo. Results indicate that combined inhibition of ERK1/2 and AKT pathways decreases the growth and survival of endometriotic lesions dose-dependently up to 70% compared to inhibition of either ERK1/2 or AKT pathway. Combined inhibition of ERK1/2 and AKT decreases the expression of cell proliferation marker protein ki67 and increases the expression of apoptosis marker protein caspase-3 in the epithelial cells and stromal cells of the endometriotic lesions. Together, these results indicate that dual inhibition of ERK1/2 and AKT pathways decrease the growth and survival of endometriotic lesions by decreasing proliferation and inducing apoptosis of epithelial cells and stromal cells of the endometriotic lesions. In this study, we treated the experimental mice for 2 weeks and no toxicity was observed on kidney, heart, and liver function at biochemical level. However, future studies with different doses for longer duration is required.
We and others have shown that relative expressions of proteins involved in ERK1/2 and AKT signaling including p-Bad112, p-Bad136, Bcl2, Bcl-XL, p-ERK1/2, p-AKT, p-IκB and β-catenin are significantly higher in ectopic endometriotic tissues compared to eutopic endometrial tissues in women [10] and animal models of endometriosis [27-29]. These results unequivocally indicate that ERK1/2, AKT, NFκB or β-catenin pathways are highly activated in endometriosis. Results of the present study clearly indicate that ERK1/2 and AKT pathways interactively regulate these signaling proteins in human endometrioitc cells in an epithelial cells and stromal cell specific-pattern in vitro and in vivo. Thus, it supports the role of hyperactivated ERK1/2 and AKT interactive pathways in the pathogenesis of endometriosis.
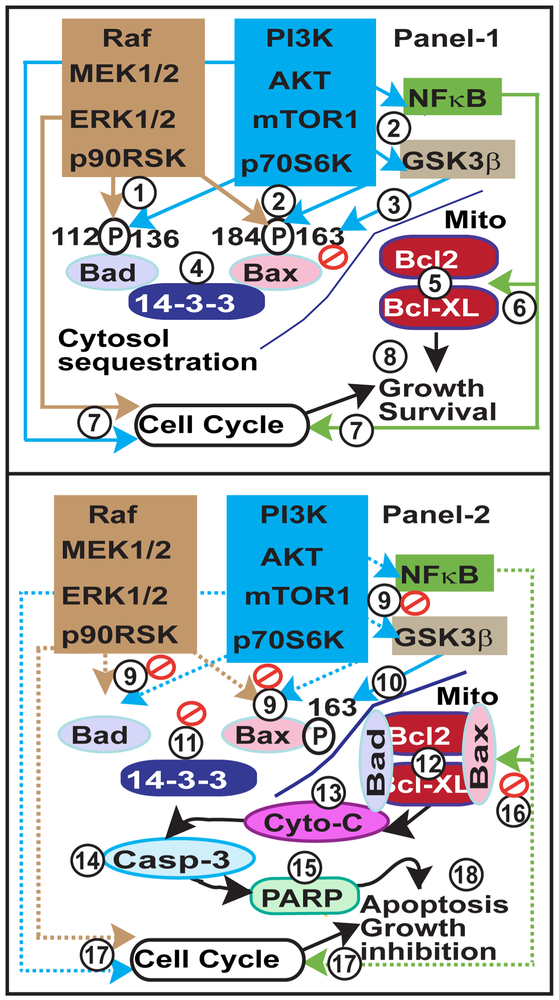
Analysis of ERK1/2 and AKT pathways on growth and survival of human endometriotic cells has revealed a complex organization of signaling modules which are regulated in an epithelial cells and stromal cell-specific pattern, as shown in Fig-9. Our new results strongly indicate that ability of human endometriotic cells to circumvent apoptosis signals is associated with increased ERK1/2 and AKT interactive cell signaling pathways. Based on the results of the present study, we propose molecular mechanisms by which dual inhibition of ERK1/2 and AKT pathways suppresses growth and survival of human endometriotic cells, as illustrated in Fig-10. The remarkable redundancy of signaling pathways that control interactions among proteins involved in cell proliferation, cell cycle, cell survival, and cell apoptosis confirm the need for dual inhibition of ERK1/2 and AKT pathways for the treatment of endometriosis mainly progressive stage of the disease with red lesions. One of the limitations of the current study is that we used human immortalized human endometiotic cell lines from peritoneal red lesions and immunocompromised Rag2g(c) mice. Under clinical condition in women, the endometriotic lesions are heterogeneous such as red, white, blue, and black phenotypes with different biochemical properties. Therefore, more preclinical studies using heterogeneous lesional phenotypes, different formats of ERK1/2 and AKT inhibitors, and additional mice and primate models are required to move this research forward.
Fig-9: ERK1/2 and AKT interactive and compensatory pathways in intracellular signaling modules, cell cycle regulation, and intrinsic apoptosis in human endometriotic cells.
Panel-1:ERK1/2 and AKT interactive pathways in human endometriotic epithelial cells. Panel-2: ERK1/2 and AKT interactive pathways in human endometriotic stromal cells.
Fig-10: Working model on ERK1/2 and AKT interactive pathways in growth and survival of endometriotic lesions.
Panel-1: (1) Activation of ERK1/2-p90RSK pathway phosphorylates Bad at serine 112 and Bax at serine 184. (2) Activation of AKT-mTOR1-p70S6K pathways phosphorylates Bad at serine 136, and Bax at serine 184. In addition, AKT phosphorylates (2) GSK3β at serine 9 and inhibits its ability to (3) phosphorylate Bax at serine 163, which is necessary for conformation and translocation of Bax into mitochondria. These signaling interactions (4) sequestrate p-Bad and p-Bax proteins in the cytosol with 14-3-3 proteins, and (5) prevent translocation of p-Bad/p-Bax into the mitochondria, and their interactions with Bcl-2/Bcl-XL proteins. (6) Further, activation of multiple ERK1/2 and AKT signaling modules (6) increases expression of Bcl2 and Bcl-XL and cell cycle regulatory proteins. Together, the hyperactivated ERK1/2 and AKT pathways (7) regulate cell cycle and (8) promote growth and survival of endometriosis. Panel 2: (9) Inhibition of ERK1/2- p90RSK, AKT-mTOR1-p70S6K and AKT-GSK3β modules in turn dephosphorylates (9) Bad112/136 and Bax184 and (10) phosphorylates Bax163. These signaling interactions (11) dissociate Bad and Bax from 14-3-3 protein, (12) translocate them into the mitochondria and (13-15) activate intrinsic apoptotic pathways. In addition, suppression of multiple ERK1/2 and AKT signaling modules regulate (16) expression of Bcl2 and Bcl-XL proteins and (17) cell cycle regulatory proteins. Together, inhibition of ERK1/2 and AKT interactive pathways (18) decrease growth and apoptosis of endometriosis.
In conclusion, results of the present study collectively indicate that inhibition of the ERK1/2 and AKT pathways decreases the growth and survival of endometriotic cells and endometriotic lesions through multiple mechanisms. Our new results: (i) establish interactive-compensatory mechanisms between the ERK1/2 and AKT pathways in the pathogenesis of endometriosis; and (ii) indicate a need for dual inhibition of these two pathways for the treatment of endometriosis. (iii) Dual inhibition of the ERK1/2 and AKT pathways could emerge as a potential non-steroidal therapy for the treatment of endometriosis in women.
Acknowledgement:
This work is partially supported by National Institute of Child Health and Human Development (NICHD) Grants HD065138, HD066248 and HD079625. We thank previous and current lab members of Dr. Arosh’s laboratory for the technical assistance and animal husbandry during the course of the study.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Bulun SE (2009) Endometriosis. N Engl J Med 360, 268–79. [DOI] [PubMed] [Google Scholar]
- [2].Giudice LC and Kao LC (2004) Endometriosis. Lancet 364, 1789–99. [DOI] [PubMed] [Google Scholar]
- [3].Sampson J (1927) Peritoneal endometritis due to menstrual dissemination of endometrial tissu into the peritoneal cavity. Am J Obstet Gynecol 14, 442–469. [Google Scholar]
- [4].Harada T, Kaponis A, Iwabe T, Taniguchi F, Makrydimas G, Sofikitis N, Paschopoulos M, Paraskevaidis E and Terakawa N (2004) Apoptosis in human endometrium and endometriosis. Hum Reprod Update 10, 29–38. [DOI] [PubMed] [Google Scholar]
- [5].Harada T, Taniguchi F, Izawa M, Ohama Y, Takenaka Y, Tagashira Y, Ikeda A, Watanabe A, Iwabe T and Terakawa N (2007) Apoptosis and endometriosis. Front Biosci 12, 3140–51. [DOI] [PubMed] [Google Scholar]
- [6].Izawa M, Harada T, Deura I, Taniguchi F, Iwabe T and Terakawa N (2006) Drug-induced apoptosis was markedly attenuated in endometriotic stromal cells. Hum Reprod 21, 600–4. [DOI] [PubMed] [Google Scholar]
- [7].Agic A, Djalali S, Diedrich K and Hornung D (2009) Apoptosis in Endometriosis. Gynecol Obstet Invest 68, 217–223. [DOI] [PubMed] [Google Scholar]
- [8].Nasu K, Nishida M, Ueda T, Takai N, Bing S, Narahara H and Miyakawa I (2005) Bufalin induces apoptosis and the G0/G1 cell cycle arrest of endometriotic stromal cells: a promising agent for the treatment of endometriosis. Mol Hum Reprod 11, 817–23. [DOI] [PubMed] [Google Scholar]
- [9].Nasu K, Yuge A, Tsuno A, Nishida M and Narahara H (2009) Involvement of resistance to apoptosis in the pathogenesis of endometriosis. Histol Histopathol 24, 1181–92. [DOI] [PubMed] [Google Scholar]
- [10].Banu SK, Lee J, Speights VO Jr, Starzinski-Powitz A and Arosh JA (2009) Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkB and b-catenin pathways and activation of intrinsic apoptotic mechanisms. Molecular Endocrinology 23, 1291–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Asati V, Mahapatra DK and Bharti SK (2016) PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. Eur J Med Chem 109, 314–41. [DOI] [PubMed] [Google Scholar]
- [12].Caunt CJ, Sale MJ, Smith PD and Cook SJ (2015) MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat Rev Cancer 15, 577–92. [DOI] [PubMed] [Google Scholar]
- [13].Fouque A, Jean M, Weghe P and Legembre P (2016) Review of PI3K/mTOR Inhibitors Entering Clinical Trials to Treat Triple Negative Breast Cancers. Recent Pat Anticancer Drug Discov 11, 283–96. [DOI] [PubMed] [Google Scholar]
- [14].Saini KS, Loi S, de Azambuja E, Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and Piccart-Gebhart MJ (2013) Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev 39, 935–46. [DOI] [PubMed] [Google Scholar]
- [15].Apkarian AV, Bushnell MC, Treede RD and Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9, 463–84. [DOI] [PubMed] [Google Scholar]
- [16].Zandi R, Larsen AB, Andersen P, Stockhausen MT and Poulsen HS (2007) Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal 19, 2013–23. [DOI] [PubMed] [Google Scholar]
- [17].Sastry KS, Karpova Y and Kulik G (2006) Epidermal growth factor protects prostate cancer cells from apoptosis by inducing BAD phosphorylation via redundant signaling pathways. J Biol Chem 281, 27367–77. [DOI] [PubMed] [Google Scholar]
- [18].Berkley KJ, Rapkin AJ and Papka RE (2005) The pains of endometriosis. Science 308, 1587–9. [DOI] [PubMed] [Google Scholar]
- [19].Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y and Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91, 231–41. [DOI] [PubMed] [Google Scholar]
- [20].Kumar A, Takada Y, Boriek AM and Aggarwal BB (2004) Nuclear factor-kappaB: its role in health and disease. J Mol Med 82, 434–48. [DOI] [PubMed] [Google Scholar]
- [21].Castellone MD, Teramoto H, Williams BO, Druey KM and Gutkind JS (2005) Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science 310, 1504–10. [DOI] [PubMed] [Google Scholar]
- [22].Grigoryan T, Wend P, Klaus A and Birchmeier W (2008) Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev 22, 2308–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB and Greenberg ME (2000) 14–3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6, 41–51. [PubMed] [Google Scholar]
- [24].Mundi PS, Sachdev J, McCourt C and Kalinsky K (2016) AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol 82, 943–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi J, Jo M, Lee E, Lee DY and Choi D (2015) Dienogest enhances autophagy induction in endometriotic cells by impairing activation of AKT, ERK1/2, and mTOR. Fertil Steril 104, 655–64 e1. [DOI] [PubMed] [Google Scholar]
- [26].Cinar O, Seval Y, Uz YH, Cakmak H, Ulukus M, Kayisli UA and Arici A (2009) Differential regulation of Akt phosphorylation in endometriosis. Reprod Biomed Online 19, 864–71. [DOI] [PubMed] [Google Scholar]
- [27].Kim TH, Yu Y, Luo L, Lydon JP, Jeong JW and Kim JJ (2014) Activated AKT pathway promotes establishment of endometriosis. Endocrinology 155, 1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Matsuzaki S and Darcha C (2015) Co-operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro. Hum Reprod 30, 1606–16. [DOI] [PubMed] [Google Scholar]
- [29].Eaton JL, Unno K, Caraveo M, Lu Z and Kim JJ (2013) Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. J Clin Endocrinol Metab 98, E1871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McKinnon BD, Kocbek V, Nirgianakis K, Bersinger NA and Mueller MD (2016) Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum Reprod Update 22. [DOI] [PubMed] [Google Scholar]
- [31].Murk W, Atabekoglu CS, Cakmak H, Heper A, Ensari A, Kayisli UA and Arici A (2008) Extracellularly signal-regulated kinase activity in the human endometrium: possible roles in the pathogenesis of endometriosis. J Clin Endocrinol Metab 93, 3532–40. [DOI] [PubMed] [Google Scholar]
- [32].Velarde MC, Aghajanova L, Nezhat CR and Giudice LC (2009) Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3',5'-cyclic adenosine 5'-monophosphate inhibition of cyclin D1. Endocrinology 150, 4701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Luca A, Maiello MR, D'Alessio A, Pergameno M and Normanno N (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16 Suppl 2, S17–27. [DOI] [PubMed] [Google Scholar]
- [34].Hoeflich KP, O'Brien C, Boyd Z, Cavet G, Guerrero S, Jung K, Januario T, Savage H, Punnoose E, Truong T, Zhou W, Berry L, Murray L, Amler L, Belvin M, Friedman LS and Lackner MR (2009) In vivo antitumor activity of MEK and phosphatidylinositol 3-kinase inhibitors in basal-like breast cancer models. Clin Cancer Res 15, 4649–64. [DOI] [PubMed] [Google Scholar]
- [35].Mendoza MC, Er EE and Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 36, 320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, Gili M, Russillo M, Parra JL, Singh S, Arribas J, Rosen N and Baselga J (2011) PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 30, 2547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zeitvogel A, Baumann R and Starzinski-Powitz A (2001) Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol 159, 1839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kochunov P, Glahn DC, Fox PT, Lancaster JL, Saleem K, Shelledy W, Zilles K, Thompson PM, Coulon O, Mangin JF, Blangero J and Rogers J (2010) Genetics of primary cerebral gyrification: Heritability of length, depth and area of primary sulci in an extended pedigree of Papio baboons. Neuroimage 53, 1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lee J, Banu SK, Subbarao T, Starzinski-Powitz A and Arosh JA (2011) Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol 332, 306–13. [DOI] [PubMed] [Google Scholar]
- [40].Arosh JA, Lee J, Balasubbramanian D, Stanley JA, Long CR, Meagher MW, Osteen KG, Bruner-Tran KL, Burghardt RC, Starzinski-Powitz A and Banu SK (2015) Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc Natl Acad Sci U S A 112, 9716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lee J, Banu SK, Burghardt RC, Starzinski-Powitz A and Arosh JA (2013) Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits adhesion of human endometriotic epithelial and stromal cells through suppression of integrin-mediated mechanisms. Biol Reprod 88, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Coleman RA, Smith WL and Narumiya S (1994) International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46, 205–29. [PubMed] [Google Scholar]
- [43].Woodward DF, Pepperl DJ, Burkey TH and Regan JW (1995) 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2 receptor antagonist. Biochem Pharmacol 50, 1731–3. [DOI] [PubMed] [Google Scholar]
- [44].Crider JY, Griffin BW and Sharif NA (2000) Endogenous EP4 prostaglandin receptors coupled positively to adenylyl cyclase in Chinese hamster ovary cells: pharmacological characterization. Prostaglandins Leukot Essent Fatty Acids 62, 21–6. [DOI] [PubMed] [Google Scholar]
- [45].Banu SK, Lee J, Satterfield MC, Spencer TE, Bazer FW and Arosh JA (2008) Molecular cloning and characterization of prostaglandin transporter in ovine endometrium: Role of mitogen activated protein kinase pathways in release of prostaglandin F2 alpha. Endocrinology 149, 219–231. [DOI] [PubMed] [Google Scholar]
- [46].Lee J, Banu SK, Rodriguez R, Starzinski-Powitz A and Arosh JA (2010) Selective blockade of prostaglandin E2 receptors EP2 and EP4 signaling inhibits proliferation of human endometriotic epithelial cells and stromal cells through distinct cell cycle arrest. Fertil Steril 93, 2498–506. [DOI] [PubMed] [Google Scholar]
- [47].Arosh JA, Banu SK, Chapdelaine P, Emond V, Kim JJ, MacLaren LA and Fortier MA (2003) Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology 144, 3076–91. [DOI] [PubMed] [Google Scholar]
- [48].Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–54. [DOI] [PubMed] [Google Scholar]
- [49].Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH and Hung MC (2005) Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol Cell 19, 159–70. [DOI] [PubMed] [Google Scholar]
- [50].Sastry KS, Smith AJ, Karpova Y, Datta SR and Kulik G (2006) Diverse antiapoptotic signaling pathways activated by vasoactive intestinal polypeptide, epidermal growth factor, and phosphatidylinositol 3-kinase in prostate cancer cells converge on BAD. J Biol Chem 281, 20891–901. [DOI] [PubMed] [Google Scholar]
- [51].She QB, Solit DB, Ye Q, O'Reilly KE, Lobo J and Rosen N (2005) The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 8, 287–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Johnson DG and Walker CL (1999) Cyclins and cell cycle checkpoints. Annu Rev Pharmacol Toxicol 39, 295–312. [DOI] [PubMed] [Google Scholar]
- [53].Malumbres M and Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30, 630–41. [DOI] [PubMed] [Google Scholar]
- [54].Sanchez I and Dynlacht BD (2005) New insights into cyclins, CDKs, and cell cycle control. Semin Cell Dev Biol 16, 311–21. [DOI] [PubMed] [Google Scholar]
- [55].Schwartz GK and Shah MA (2005) Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol 23, 9408–21. [DOI] [PubMed] [Google Scholar]
- [56].Adams JM and Cory S (2007) The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 26, 1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kroemer G, Galluzzi L and Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87, 99–163. [DOI] [PubMed] [Google Scholar]
- [58].Papadopoulos K (2006) Targeting the Bcl-2 family in cancer therapy. Semin Oncol 33, 449–56. [DOI] [PubMed] [Google Scholar]
- [59].Arokium H, Ouerfelli H, Velours G, Camougrand N, Vallette FM and Manon S (2007) Substitutions of potentially phosphorylatable serine residues of Bax reveal how they may regulate its interaction with mitochondria. J Biol Chem 282, 35104–12. [DOI] [PubMed] [Google Scholar]
- [60].Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL and Henson PM (2004) Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279, 21085–95. [DOI] [PubMed] [Google Scholar]
- [61].Nechushtan A, Smith CL, Hsu YT and Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18, 2330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Yamaguchi H and Wang HG (2001) The protein kinase PKB/Akt regulates cell survival and apoptosis by inhibiting Bax conformational change. Oncogene 20, 7779–86. [DOI] [PubMed] [Google Scholar]
- [63].Zha J, Harada H, Yang E, Jockel J and Korsmeyer SJ (1996) Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14–3-3 not BCL-X(L). Cell 87, 619–28. [DOI] [PubMed] [Google Scholar]
- [64].Saito A, Hayashi T, Okuno S, Ferrand-Drake M and Chan PH (2003) Overexpression of copper/zinc superoxide dismutase in transgenic mice protects against neuronal cell death after transient focal ischemia by blocking activation of the Bad cell death signaling pathway. J Neurosci 23, 1710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang X and Wang X (2004) Cytochrome C-mediated apoptosis. Annu Rev Biochem 73, 87–106. [DOI] [PubMed] [Google Scholar]