Abstract
The mesocorticolimbic pathway is canonically known as the “reward pathway”. Embedded within the center of this circuit is the striatum, a massive and complex network hub that synthesizes motivation, affect, learning, cognition, stress, and sensorimotor information. While striatal subregions collectively share many anatomical and functional similarities, it has become increasingly clear that it is an extraordinarily heterogeneous region. In particular, the nucleus accumbens (NAc) medial shell has repeatedly demonstrated that the rules dictated by more dorsal aspects of the striatum do not apply, or are even reversed in functional logic. These discrepancies are perhaps most easily captured when isolating the functions of various neuromodulatory peptide systems within the striatum. Endogenous peptides are thought to play a critical role in modulating striatal signals to either amplify or dampen evoked behaviors. Here we describe the anatomical-functional backdrop upon which several neuropeptides act within the NAc to modulate behavior, with a specific emphasis on nucleus accumbens medial shell and stress responsivity. Additionally, we propose that as the field continues to dissect fast neurotransmitter systems within NAc, we must also provide considerable contextual weight to the roles that local peptides play in modulating these circuits to more comprehensively understand how this important subregion gates motivated behaviors.
Introduction
The striatum is a major site of convergence in the forebrain, synthesizing motivation, affect, cognition, and sensorimotor information. Upon the integration of these various signals, the striatum transforms this composite signal into an observable behavioral action/output. However, while the entire striatum appears to follow the same basic influx/outflux of information (described below), multiple lines of evidence suggest that there are many anatomically and functionally distinct areas that merit classifying the striatum into discrete subregions. In this review, we describe the underlying architectural makeup of the striatum, as well as the overarching general circuit pathways. We use this foundational information as a contrast point to discuss recent advances in our understanding of the ventromedial-most subregion, nucleus accumbens (NAc) medial shell, describing the unusual patterns specifically isolated to this area. Next, we introduce the prominent role of the endogenous opioid system in this region, highlighting their common features and generally accepted roles in the striatum. These features are then contrasted to the more heterogenous NAc medial shell to highlight how chemically similar peptidergic entities can diverge in their neuromodulatory output functions. Finally, we outline critical, lingering questions related to studying these neuropeptide systems, and how technological advances may reveal surprising nuance to neuropeptide function in the NAc, as well as the brain at large.
Canonical architecture of the striatum
The striatum is traditionally considered to have five discernable cell types: two projection populations, and three interneuron populations (Figure 1). The two projection populations are described as medium spiny neurons (MSNs or SPNs), owing to their medium-sized soma (~14um in diameter), and the high density of dendritic spines on their dendritic arbors (CAJAL, 1911; Meredith et al., 2008; Wilson et al., 1983). They also account for ~90–95% of all neurons in the striatum. Interestingly, despite the high density of spines, electrophysiological recordings of putative MSNs indicate an extremely low baseline firing rate (~1Hz), suggesting a strong, tonic GABAergic tone. Additionally, MSNs are known to express high levels of leaky potassium channels, which further hyperpolarize the cells (Karschin et al., 1996; Perez et al., 2006; Talley et al., 2001). Nevertheless, this quality permits high fidelity signaling of evoked spiking activity.
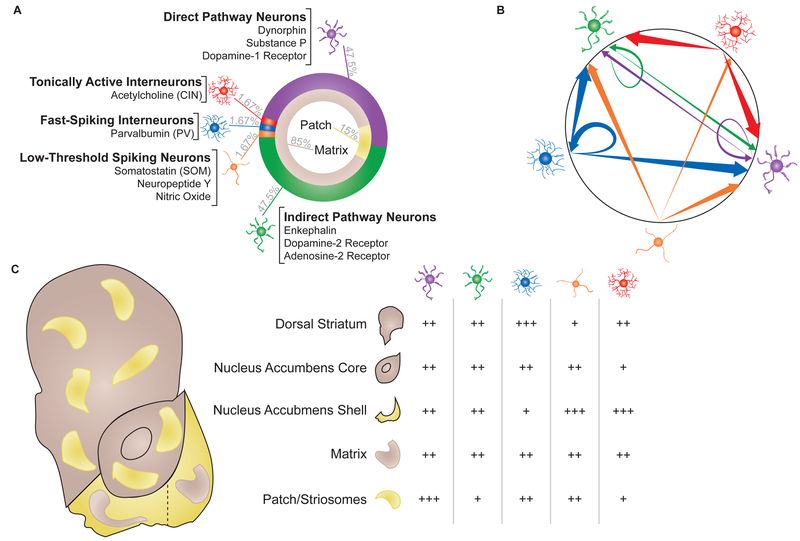
Figure 1. Basic structural anatomy of the striatum.
(A, left) Ring charts showing relative expression (%, gray text) of cell types (outer ring) or patch/matrix (inner ring). Cell types labeled with known and exclusive markers for each population. (B, right) Intra-striatal connectivity schematic showing preferential connections between cell types. Arrows signify projection target, circled arrows signify synapses onto other neurons of the same type. Cell populations: purple, direct pathway; green, indirect pathway; blue, fast-spiking interneuron; orange, low-threshold spiking interneurons; red, tonically active interneurons. Arrow thickness: thin, low connectivity; medium, moderate connectivity; thick, strong connectivity. (C, bottom, left) Schematic of rodent striatum, dividing dorsal, core, and shell into separable subregions(lateral and medial divided by dashed line). Matrix (brown) and patch (yellow) shaped and distributed to show relative expression in each subregion. (right) Table showing relative distribution of different cell types within each region of the striatum. Direct pathway neurons, purple; indirect pathway neurons, green; tonically active interneurons, red; fast-spiking interneurons, blue; low-threshold spiking interneurons, orange. +, low relative expression; ++, average relative expression; +++ high relative expression.
Though seemingly homogenous, several neurochemical and receptor markers reveal a highly organized system that divides MSNs into what are known as “direct” and “indirect” pathways. Markers for the direct pathway include the expression of dopamine 1 receptors (D1), dynorphin, and substance P. In contrast, the indirect pathway expresses dopamine 2 receptors (D2), adenosine 2A receptors (A2A) and enkephalin. These designations are extraordinarily well conserved evolutionarily, with dynorphin and substance P highly colocalizing in cats, pigeons, turtles, and rats, and neither colocalizing with enkephalin (Anderson and Reiner, 1990; Besson et al., 1990; Schiffmann and Vanderhaeghen, 1993). The “direct” and “indirect” labels describe the downstream efferent destinations. The direct pathway directly innervates the midbrain in the substantia nigra (SN) and ventral tegmental area (VTA). By contrast, the indirect pathway targets pallidal and hypothalamic structures, which then go on to innervate the midbrain. The efferents of the striatum are topographically organized. Dorsolateral direct pathway neurons innervate the dorsolateral-most subregions of the SN pars reticulata, and more ventromedial projections innervate more ventromedial sites (Haber et al., 2000). Though not well studied, several studies have begun to investigate how local MSNs can affect other MSN activity (Figure 1) (Tecuapetla et al., 2009; Tejeda et al., 2017). About 15% of MSNs synapse onto nearby MSNs, with preferential innervation on matching pathways (i.e., direct MSNs contact direct MSNs). Interestingly, indirect neurons also synapse on direct MSNs, though to a lesser degree than to their same pathway constituents. On the other hand, direct pathway MSNs almost never synapse onto indirect pathway neurons (Planert et al., 2010; Tejeda et al., 2017). Such a relationship strongly suggests an imbalanced local inhibition favoriting indirect inhibition of direct pathway neurons. Because MSNs are typically studied in either isolation or as a single mass, there is very little analysis of concomitant and causal lateral modulation of MSN activity. Along with the cytoarchitectonic and projection specialization of the MSNs, the two populations also demonstrate unique electrophysiological characteristics (Gertler et al., 2008). Though both populations have low baseline firing rates, direct pathway MSNs are typically more hyperpolarized and are resistant to depolarization via injected current (i.e., they have a higher rheobase). These differences appear to be related to the overall dendritic surface area, as direct pathway MSNs typically have more primary dendrites than indirect pathway MSNs (Gertler et al., 2008). In other words, the constraints of having more overall surface area from the greater spines density on direct neurons supersedes the net excitatory effect of having more glutamatergic contact points.
Making up the other 5% of neurons in striatum are the local interneuron populations (Kawaguchi, 1993; Tepper et al., 2010). Though initially characterized by the unique firing patterns, further work has characterized these populations as (1) fast spiking/parvalbumin-releasing (PV), (2) persistent low threshold/somatostatin-releasing (SOM), and (3) tonically active/acetylcholine-releasing (CIN) interneurons. More recently, interest has begun to refocus on several other potential interneurons, including a calretinin expressing population (which partially overlaps with PV interneurons) and a tyrosine hydroxylase expressing population that is only found in the dorsomedial most portions of dorsal striatum (Tepper et al., 2010). While not much is known about these populations, continued advances in genetic screening (e.g. rna-seq) will likely facilitate their study.
PV/FSI neurons have similar soma sizes as MSNs, but are noticeably aspiny (Figure 1) (Gerfen et al., 1985; Kita et al., 1990). Morphologically, they emit about 4–8 dendrites that arborize into second and third order branches. These arborizations result in PV neurons extending into a sphere reaching ~300μm in diameter. Emerging from the cell bodies, PV axons sprout incredibly dense collaterals, more or less matching the arborization displayed by the dendrites. Extrapolating from Koós and Tepper, about 16 PV/FSIs can converge onto a single MSN, and upwards of 300 MSNs can be contacted by a single PV/FSI (Figure 1) (Koós and Tepper, 1999). Indeed, the likelihood of a PV neuron synapsing onto an MSN within its arborized space has been reported as higher than 50%, though PV neurons appear to avoid other non-PV interneurons (Gittis et al., 2010; Planert et al., 2010). These multilateral connections allow PV neurons to dramatically hyperpolarize nearby MSNs (Kubota et al., 1993). Considering that PV/FSI’s may further expand their coordinated sphere of influence via gap junctions, it is likely that PV/FSI networks can organize large portions of striatum (Figure 1) (Hjorth et al., 2009; Kita et al., 1990). Recent work by Owen et al. (Owen et al., 2018) experimentally demonstrated this by showing that selective inhibition or ablation of PV/FSI neurons resulted in increased, though desynchronous firing of MSNs. However it should be noted that the precise role of PV/FSIs in coordinating MSN activity remains a complex and ongoing area of study (Berke, 2008; Marche and Apicella, 2017).
PV neurons were first characterized by their highly unique electrophysiological signature (Kawaguchi, 1993). Specifically, they exhibit little to no baseline activity (similarly to MSNs), but when stimulated, emit trains of action potentials over 400Hz. Summarizing several decades of work, PV neurons appear to exert their effects via brief, intense hyperpolarizations, and do so in large part through the recruitment of Kv 3.1 channels (Kotaleski et al., 2006; Lenz et al., 1994). As mentioned above, PV neurons also exhibit gap junctions, allowing for nearly synchronous firing between cells (Hjorth et al., 2009; Tepper et al., 2010). However, whether such synchronous firing occurs in vivo is still debated, as coordinated activity among PV neurons does not appear to manifest itself during salient behavioral tasks (Berke, 2008; Yamada et al., 2016).
Spatially, PV neurons appear to be well distributed across the striatum, present in both patch and, to a lesser degree, matrix subcompartments (patch and matrix are described in greater details below), where they appear to synapse equally onto direct and indirect pathway MSNs (Banghart et al., 2015; Cowan et al., 1990; Kubota et al., 1993). However, emerging evidence suggests that there may be subpopulations of PV neurons depending on the precise striatal anatomical site. For example, Monteiro et al. (Monteiro et al., 2018) recently showed that dorsomedial PV neurons, compared to their dorsolateral counterparts, have greater intrinsic excitability (at least in males) and are especially sensitive to cingulate inputs, consistent with recent tracing studies showing cingulate-PV connectivity (Mailly et al., 2013). To date, it remains unknown whether there are further dissociable populations of PV neurons related to patch versus matrix inputs.
The second interneuron population is the persistent and low threshold spiking neurons (Figure 1) (Kawaguchi, 1993). This population is highly enriched in the neuropeptide somatostatin (SOM), as well as neuropeptide Y (NPY) and nitric oxide synthase (NOS) (Kubota et al., 1993; Vincent and Johansson, 1983). Like MSNs and PV neurons, they are medium sized, though typically a little larger than both. As implied by their name, SOM neurons have a low threshold Ca2+ spike, which is compounded by their relatively depolarized resting membrane potential. Unlike PV neurons, SOM action potentials are few in number but last an unusually long time, often taking longer than 30ms for the action potential to decay (Kawaguchi, 1993; Straub et al., 2016). Their dendritic arborizations are few in number and aspiny, but can extend to more than a quarter of a millimeter from the soma (DiFiglia and Aronin, 1982; Vincent and Johansson, 1983). The axonal branches that stem from SOM neurons largely synapse on MSNs, and in particular the more distal portions of the MSN dendrites (Figure 1) (Aoki and Pickel, 1990; Straub et al., 2016). Additionally, recent experiments by Straub et al. have demonstrated that SOM neurons (but not PV neurons) also synapse onto CINs, verifying anatomical observations first made decades ago (Vuillet et al., 1992). In opposition to PV neurons, SOM neurons do not appear to form unified networks with other SOM neurons. Instead, their long axons target distant MSNs, outside of the local fields of other SOM or PV neurons. It has been suggested that this organizational structure may explain why paired recordings of SOM neurons and their post-synaptic targets have been difficult to replicate, since most paired studies of interneurons are done closer to the stimulated cell body or in preparations in which the long range post-synaptic target may have been disconnected (Gittis et al., 2010; Straub et al., 2016). Alternatively, others have suggested that SOM neurons may act principally through their released peptides (Tepper et al., 2010), providing a second means for how SOM neurons regulate striatal activity (Lopez-Huerta et al., 2008). Though the functional contribution of the spatial organization of SOM neurons is not well characterized, it is worth noting that SOM neurons appear to mirror PV neurons in overall expression patterns across striatum. Specifically, SOM neurons are expressed most densely in ventromedial striatum and least densely in dorsolateral zones (Beal et al., 1983; Wasilewska et al., 2011), though appear to be equally expressed in patch and matrix subzones (Figure 1) (Rymar et al., 2004). Recent work (Ribeiro et al., 2018) provides some insight into the role and mechanism of SOM neurons in ventromedial striatum/nucleus accumbens. Using multiple approaches, Ribeiro and colleagues showed that optogenetic stimulation of SOMs increased a cocaine place preference, whereas inhibition suppressed preference for cocaine. These effects appear to be related to specific changes in transcription factor expression in SOM neurons, such as JundD, though there are certainly more mechanisms to uncover. Whether similar mechanisms of action apply to other reward-seeking behaviors remain unknown; nevertheless, these results provide a strong foundation for future interrogation of SOM function.
The final group of striatal neurons are the tonically active cholinergic interneurons (CINs) (Figure 1). Though few in number, the ~20–30um diameter soma and 1 millimeter extension of its arbors allow these neurons to coordinate activity across vast swathes of striatal tissue (Chuhma et al., 2014; Ma et al., 2014). This population was first identified in the late 1800’s, though it would take nearly 100 years before they were identified as cholinergic (Bolam et al., 1984; Kolliker, 1986). CINs have a high baseline firing rate of 5Hz (Zhou et al., 2002), encoding salient information through transient pauses in activity (Aosaki et al., 1994; Atallah et al., 2014; Shimo and Hikosaka, 2001). Because of the density of local innervation from CINs, it has been posited that CINs may engage in both synaptic and volume transmission, potentially even maintaining some degree of tonic acetylcholine (ACh) (Descarries et al., 1997). Interestingly, reports of direct actions of CINs on MSNs have been comparatively rare compared to the other interneurons. Instead, CINs appear to activate surrounding tissue via disinhibition of incoming dopamine terminals or coordination with other interneuron groups (Cachope et al., 2012; Nelson et al., 2014; Threlfell et al., 2012). However, more recent evidence suggests that CINs may act directly on MSNs via muscarinic receptors, providing yet another mechanism through which CINs can cohesively modulate striatal activity (Mamaligas and Ford, 2016). Morphologically, CINs appear to be either “spidery” or “nonspidery”, referencing the density of their dendritic arborization (Gonzales and Smith, 2015). In either case, CINs typically have a few, very large dendritic stems emerging from an otherwise smooth soma and can extend up to 400μm from the soma (Phelps and Vaughn, 1986; Phelps et al., 1985). In general, CINs appear to be robustly expressed in ventromedial striatum (Figure 1) (Matamales et al., 2016). However, emerging cellular and electrophysiological quantification suggest that the density of CIN expression may not necessarily be predictive of their overall functional impact on the neural activity across specific subregions of striatum. For example, stimulation of D2 receptors on CINs results in burst-pause firing in NAc shell, but only pauses in dorsal striatum (Chuhma et al., 2014). Continued investigation into how the function of these neurons varies across striatal sites, intersected with how their neuronal profiles may change, will yield vital information for modeling how the striatum as a heterogenous structure gates behavioral output.
In addition to the discrete classification of the neurons in striatum, there is another key feature that further compartmentalizes this region: the patch and the matrix (Figure 1) (Brimblecombe and Cragg, 2017). This calbindin-rich matrix accounts for a majority of tissue in the striatum, filling anywhere from 85–90% of the striatum. Interspersed within the matrix are small patches, also called striosomes, identifiable by the high expression of mu opioid receptors (MOPRs) and low expression of calbindin, and low levels of acetylcholinesterase (Gerfen et al., 1985; Pert et al., 1976). Each patch contains direct and indirect MSNs, though direct pathway neurons are expressed at slightly higher levels than indirect MSNs (Gerfen and Young, 1988). Direct and indirect pathway efferent patterns are also conserved, regardless of patch/matrix compartmentalization, though communication between direct and indirect MSNs is segregated by patch/matrix localization (e.g., patch indirect MSNs only synapse onto patch direct MSNs, not matrix direct MSNs). Interestingly, recent evidence suggests that general direct and indirect MSNs may not be homogenously distributed across the striatum. For example, Gangarossa et al. (Gangarossa et al., 2013) show that the caudal most portion of dorsal striatum completely lacks indirect MSNs, despite containing direct MSNs and interneurons. This caudal zone of dorsal striatum also appears to be completely composed of matrix, being both rich in calbindin and poor in MOPR expression.
Traditionally, striatal patches are thought to receive more “limbic” afferents, and the matrix receiving more sensorimotor afferents (Friedman et al., 2015; Gerfen, 1984). This informational segregation was further supported by the exclusivity of the two compartments from each other, since MSNs do not typically communicate across patch/matrix divisions (Bolam et al., 1988; Lopez-Huerta et al., 2016). However, increasing evidence suggests that both the anatomical and functional divisions between these two compartments are not as isolated as was once believed. For example, work by Smith et al. (Smith et al., 2016) now show that in addition to identifiable patch and matrix compartments, there also appears to be a third “exo-patch” compartment. Exo-patches appear to resemble patches based on neurochemical and electrophysiological properties, but are not located in dense MOPR expressing locations. Strikingly, all three compartments receive both limbic and sensorimotor information, making it difficult to relate the traditional functional explanations into our current anatomical understanding of the canonical patch and matrix system. However, by capitalizing on the known cortico-striatal connectivity, Friedman et al. (Friedman et al., 2015) showed that preferential modulation of striosome or matrix activity appeared to affect cost-benefit behaviors differentially. Specifically, inhibition of striosome-biased prelimbic cortical inputs resulted in rats choosing a high reward/high cost stimulus over a low reward/low cost stimulus more often under conditions in which the high cost normally deterred high reward selection. Normal cost-avoidance behaviors were maintained in the other behavioral tasks. By contrast, inhibition of matrix-biased inputs from anterior cingulate cortex resulted in rats preferring larger rewards more often, regardless of whether they were comparing the relative value of two rewards (no associated costs), or the rewards within cost/benefit conflicts. These results provide some of the clearest associations between patch/matrix anatomical divisions and function to date. Future studies linking patch/matrix divisions to function will be essential for teasing apart how this unusual anatomical phenotype contributes to motivated behavioral output, though this will likely be a significant challenge, as patch/matrix ratios are not uniform across striatum.
Introduction to Ventral Striatum: Nucleus Accumbens Core
The ventral striatum is composed of three major subnuclei, the core, and lateral and medial shell. These subregions were first noted by Záborszky and colleagues (Záborszky et al., 1985), who reported strong differences in acetylcholinesterase expression within NAc that coincided with differences in the compactness of cellular distribution. The core wraps around the anterior commissure, extending ventrolaterally from the ventral point of the lateral ventricle, similar in shape to a slanted olive or grape (Figure 1). The core is then encapsulated by NAc shell (with ventral shell once being referred to as the ventrolateral striatal pocket (Nauta et al., 1978)). Generally speaking, NAc core shares several anatomical characteristics with dorsal striatum. Similar cellular distributions exist throughout the structure, and the subsequent MSN outputs follow a direct/indirect division (though the pathways may not be divisible by D1/D2 expression patterns, see (Creed et al., 2016; Kupchik et al., 2015)). However, NAc core projections follow a slightly different trajectory than its dorsal counterparts, preferentially innervating the ventral tegmental area over substantia nigra, though altogether the pattern is consistent with the “striatal-loops” described by Haber and others (Haber et al., 2000).
Though NAc core generally shares similar architectural features with dorsal striatum, there are some notable differences (differences that are further exaggerated in NAc shell, described below). First, NAc core MSNs are slightly smaller in size than their counterpart dorsal cells, averaging around 12μm in diameter (Meredith et al., 1992). However, these MSNs do appear to be homogenously distributed throughout the core, with little to no notable phenotypic alterations throughout the structure. The core also represents a shift in the preferred inputs, with specialized afferents from mid-rostral insula, rostrolateral portions of VTA, and reciprocal connections with dorsal ventral pallidum (Ikemoto, 2007; Saunders et al., 2018; Wright and Groenewegen, 1996). Though not well characterized, there does appear to be some localization of function within the NAc core. For example, deep brain stimulation (130Hz) of dorsal NAc core facilitates fear extinction, whereas stimulation in ventral NAc core (below the anterior commissure) enhances fear learning (Rodriguez-Romaguera et al., 2012). However, these results are complicated to interpret because of later reports showing that the same stimulation parameters in dorsal NAc core could enhance drug seeking during extinction trials (Martínez-Rivera et al., 2016). Altogether, these results point to potentially clinically relevant subregional specificity of function. However, as discussed below, despite some of these anatomical nuances, the NAc core appears to be more similar to dorsal striatum than its ventral striatal neighbor, NAc shell.
Unusual Architectural Phenotype of Nucleus Accumbens Medial Shell
The striatum as a whole is a well-organized network hub that possesses a large degree of architectural standardization. However, cumulative evidence along anatomical and functional dimensions from the last 30 years now indicates that the ventromedial-most segment of the striatum does not, in fact, follow the outlined “striatal rules” described above. While “ventral striatum” is often used synonymously with “nucleus accumbens”, ventral striatum proper includes both core and shell of nucleus accumbens, as well as olfactory tubercle. Here, we will discuss one specific region, NAc medial shell, and describe the striatal features that are maintained in this subnucleus, as well as which features are either ambiguous or are missing altogether.
As with the NAc core and dorsal striatum, the NAc medial shell possess two major classes of MSN projection neurons and three classes of interneurons. While technically the same class of neuron, NAc medial shell MSNs can be more accurately described as “medium-small, sort of spiny” neurons. Indeed, their spine density is roughly 20% less compared to NAc core (Meredith et al., 1992). The cell bodies are also smaller, closer to 10μm in diameter (~30% smaller than dorsal striatal MSNs). Despite the reductions in spine density, NAc MSNs appear to be the most active MSNs in the striatum, having baseline firings rates at around 1.5–3Hz, with the indirect pathway MSN neurons being especially excitable (Ma et al., 2012; Roitman et al., 2005; Taha and Fields, 2005; Tejeda et al., 2017).
When it comes to classifying “direct” and “indirect” pathways, NAc medial shell again shows a confusing phenotype. Historically, direct and indirect pathways are divided into D1-expressing and D2-expressing populations, respectively. However, multiple reports indicate that anywhere from 5% to more than 30% of NAc MSNs can actually express both receptor subtypes, making it difficult to know how or if such co-expression affects down-stream targets (Al-Hasani et al., 2015; Bertran-Gonzalez et al., 2008; Kupchik et al., 2015). Further complicating the role of dopamine in NAc MSNs is the additional expression of D3 receptors, which are not known to be expressed in any other region of the striatum (Landwehrmeyer et al., 1993; Sokoloff et al., 1992a). Like D2s, D3 receptors are Gi coupled, and can be found on either direct or indirect pathway neurons (Le Moine and Bloch, 1996; Sokoloff et al., 1992b), though their affinity for dopamine is much higher. Our understanding for how D3 receptors, and dopamine in general, may affect MSN activity and function in NAc shell remains a complex and rich field of study (Berke, 2018; de Jong et al., 2018; Pich and Collo, 2015). Another potentially unique subsystem in NAc shell includes the peptide cocaine-and-amphetamine-regulated transcript (CART). Though difficult to distinguish between core and shell, there does appear to be an unusually high prevalence of this peptide, particularly within D1/dynorphin MSNs at rostral sites of NAc. How this specific CART population contributes functionally to direct versus indirect pathway output (e.g., specialized collaterals to indirect pathway targets) remains underexplored and relatively unknown (Hubert et al., 2010).
Unlike the MSNs, whose presence is undeniable in NAc medial shell, the PV interneuron population common to the dorsal striatum is comparatively absent from this region (Kubota et al., 1993). Multiple groups have demonstrated a strong dorsolateral to ventromedial reduction in PV cells, though few groups have examined what this difference means at a functional level in terms of information processing and behavioral output. Indeed, the increase in available molecular-genetic tools to isolate discrete cell types has demonstrated a robust role for PV neurons in modulating local dorsal striatum MSN activity, which has yielded several thoughtful and intriguing models for striatal regulation of behavior (Monteiro et al., 2018; Tepper et al., 2008). But a key question remains: do these same models apply to medial shell, in which PV neurons are found in substantially less numbers than in dorsal striatum? Certainly, in vitro electrophysiological studies have demonstrated that PV neurons have the ability to modulate local MSNs, though visualizing real-time activity of these neurons in awake behaving animals, or selectively disrupting PV neuronal activity remain open areas of exploration within this subnucleus (Fino et al., 2018). Notably, as PV neurons decrease in number in NAc medial shell, there is a coincident increase in SOM and CIN neurons (Figure 1). As alluded to above, greater SOM expression likely suggests that this population plays a larger role in modulating local MSN and CIN activity in the region. However, it is difficult to speculate how the greater expression of SOM neurons affects local NAc medial shell function as there are almost no reported direct recordings of medial shell SOM neurons, nor are there studies elucidating how they integrate signals with other cells in the region to alter specific behaviors. Even CINs display a unique medial shell phenotype. Quantification of CINs in the late 1980’s showed that they are most robustly expressed in caudomedial shell, and appear to favor the ventral zones as well (Meredith et al., 1989). With the advent of in vivo cell-type targeting tools, cumulative studies appear to suggest that CINs play a modulatory or faciliatory role, rather than a directive role in behavior. For instance, stimulation or inhibition of CINs does not drive appetitive or aversive motivation or learning, but loss of CIN firing during cocaine exposure blocks the formation and extinction of a cocaine CPP (Lee et al., 2016; Witten et al., 2010). Interestingly, CIN activation also decreases hunger-enhanced food intake (but does not block it) and facilitates cocaine CPP extinction (Aitta-Aho et al., 2017; Lee et al., 2016). This could be related to how CIN modulates local plasticity of MSNs (Lee et al., 2016), but further investigations are needed to dissect the biological mechanisms underlying their complex mediation of behavior.
Perhaps the greatest difference between medial shell and the rest of the striatum concerns the patch/matrix dichotomy. For reasons that remain unclear, the ratio of patch and matrix is flipped in medial shell, such that the calbindin-poor zones dominate the region, and are interspersed by smaller pockets of calbindin-rich matrix. The difference is so robust that comparative studies between core and shell in the early 1990’s consistently used calbindin binding as a boundary marker between the two subregions (Jongen-Rêlo et al., 1994; Jongen-Rëlo et al., 1993). Interestingly, despite the greater patch representation, acetylcholinesterase and acetylcholine-positive neurons are expressed more robustly in the shell compartment (though still predominately limited to the matrix), and there is evidence that Substance P is also more highly expressed in shell as well (Miyamoto et al., 2018). The inversion of the patch/matrix ratio is further reinforced by the high and well distributed expression of MOPRs throughout the shell (Mansour et al., 1994). The functional impact of an inversed patch/matrix distribution is a difficult topic on which to speculate. The NAc shell certainly has unique efferent/afferent patterns, but little has been done to resolve the functional intersection of afferents and patch/matrix targets (Berendse et al., 1988; Wright et al., 1996).
Finally, while the medial NAc shell does follow the topographic input/output schema suggested by dorsal striatum, there are notable surprises (Fig. 2). For example, medial shell receives direct inputs from lateral hypothalamus, and in particular from LH orexin and melanin-concentrating hormone populations (Baldo et al., 2003; Diniz and Bittencourt, 2017). This reciprocal connection provides the medial shell with unique access to metabolic and motivational information. Similarly, the caudal nucleus of the solitary tract (NTS) sends long-range, catecholamine and peptide rich projections directly to medial shell (Table 1) (Delfs et al., 1998; Wang et al., 2015). This comparatively understudied connection could act to directly relay important visceral information to medial shell to modulate motivated and stress-related behavior. Even connections that should be similar (i.e., strong dopaminergic inputs) appear to be qualitatively different. For example, Zahm et al., using electron microscopy, found that tyrosine hydroxylase positive axons in NAc medial shell were highly vascularized and preferentially synapsed onto dendritic shafts (Zahm, 1992). In contrast, dorsal striatal inputs preferred to synapse onto dendritic spines, with NAc core dopamine inputs somewhere in the middle (though still somewhat spine preferring). Altogether, NAc medial shell, while sharing surface level similarities with the rest of the striatum, possesses a number of unusual and singular features. As molecular tools for targeting distinct features of the neuronal milieu continue to develop, it will be important to update our models for how medial shell synthesizes and encodes information along anatomical, functional, and cell-type specific axes.
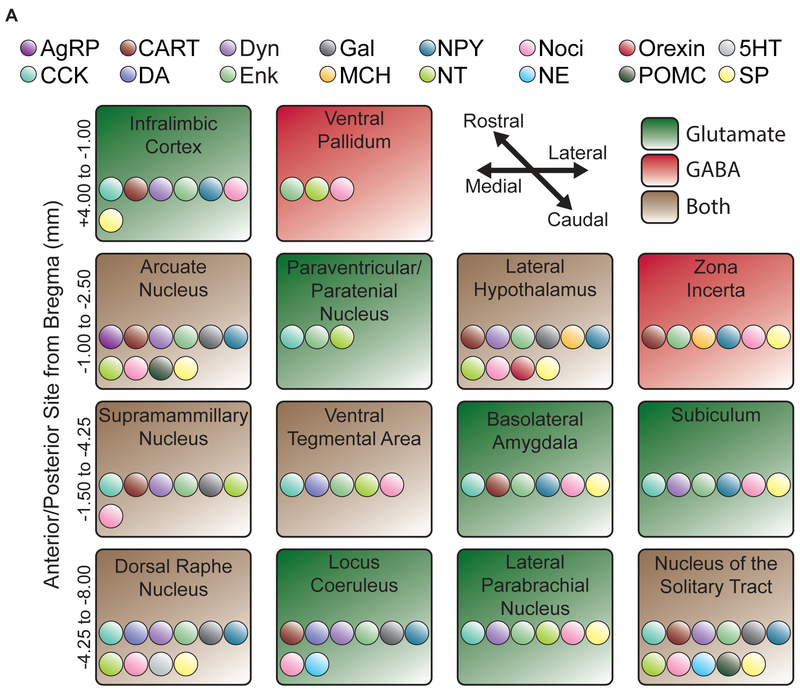
Figure 2. Known projections and potential neuromodulatory afferents to NAc medial shell.
Schematic listing the known afferents to NAc medial shell. Afferents are roughly divided by rostrocaudal (top, rostral) and mediolateral coordinates (left, medial). Afferents are labeled by whether they send glutamatergic or GABAergic efferents: glutamate (green); GABA (red); or both (brown). Potential coreleased neuromodulators are denoted by individually colored dots within each afferent. Whether or not the listed peptide is actually coreleased in NAc is unknown, but the existence of the modulator in the afferent seed allows for the possibility. Abbreviations: AgRP, agouti-related peptide; CCK, cholecystokinin; CART, cocaine and amphetamine related transcript; DA, dopamine; Dyn, dynorphin; Enk, enkephalin, Gal, galanin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; NT, neurotensin; Noci, nociceptin; NE, norepinephrine; Orexin, orexin/hypocretin; POMC, proopiomelanocortin; 5HT, Serotonin; SP, Substance P.
Table 1. Cellular localization of peptide receptors in NAc medial shell.
Receptor systems and specific receptors with common abbreviations (first and second columns). Known cell-type in NAc shell on which the receptor has been shown to express. Most receptors appear to be expressed on the direct and indirect pathway neurons, but this is largely due to interneuron colocalization being untested, rather than ruled out. Cases in which expression is inferred, but not actually shown, on direct/indirect medium spiny neurons are marked with “?”. Studies cited limited to nucleus accumbens neurons (shell and core).
| Peptide Receptor System | Receptors | Cell-type Localization | References |
|---|---|---|---|
| Cholecystokinin Receptor | CCKB | Medium Spiny Neurons? | (Kombian et al., 2004) |
| Corticotropin Releasing Factor Receptor | CRFR1 CRFR2 |
- | (Lemos et al., 2012) |
| Melanin-Concentrating Hormone Receptor | MCH1R | Direct Pathway Indirect Pathway |
(Georgescu et al., 2005) |
| Melanocortin Receptor | MC4 | Primarily Direct Pathway Indirect Pathway |
(Hsu et al., 2005; Pandit et al.,2015) |
| Neurokinin Receptor | NK1 | Acetylcholine Interneuron | (Kombian et al., 2003) |
| Neuropeptide Y Receptor | Y1 | Direct Pathway Indirect Pathway |
(van den Heuvel et al., 2015) |
| Neurotensin Receptor | NT1 | - | (Cáceda et al., 2005) |
| Opioid Receptor | μ | Direct Pathway Indirect Pathway |
(Guttenberg et al., 1996; Lindskog et al., 1999; Mansour et al., 1994) |
| Orexin Receptor | OX2R | - | (Marcus et al., 2001) |
| Oxytocin Receptor | OTR | - | (Dölen et al., 2013; Ross et al., 2009) |
| Somatostatin Receptor | SSTR1? | Medium Spiny Neurons? | (Lopez-Huerta et al., 2008; Raynor et al., 1993) |
From Structure to Function: Opioids in NAc
The endogenous opioid system is made up of four interacting receptor/ligand pairs: mu opioid receptors and Beta-Endorphin (BEND), delta opioid receptors (DOPR) and enkephalins (ENK), kappa opioid receptors (KOPR) and dynorphins (DYN), and nociceptin receptors (NOPR) and nociceptin (PNOC). All four opioid receptors are Gi-coupled seven-transmembrane receptors that, when stimulated, activate GIRKs (Kir 3.1), inhibit calcium channels, and suppress cAMP. Upon phosphorylation of the C-terminus, arrestin signaling cascades typically result in the internalization of the receptor and activation of MAPK (among other kinases). Though distinct receptors, the four receptors share some components of their amino acid compositions, which can contribute to their ability to have modest affinity for multiple endogenous opioid peptide ligands (more below) (Al-Hasani and Bruchas, 2011; Corder et al., 2018; Toll et al., 2016).
As is typical of many neuropeptides, the precursor peptides are thought to be constructed in the nucleus and later packaged and sent to axon terminals located within dense core vesicles. During their journey to the bouton, the precursor peptides are spliced into smaller functional opioid ligands via trypsin-like enzymes and prohormone convertases (carboxypeptidase-like enzymes) (Costa et al., 1987; Fricker and Snyder, 1982). Unlike small molecule transmitters, opioids may communicate either via synaptic transmission or volumetric release (Banghart and Sabatini, 2012; Duggan, 2000), though it remains unclear if or when either approach is used, and if so, under what contexts. This remains a critically understudied area in the field, but one that requires high resolution sensors or tools to resolve (discussed below). Regardless, opioids do appear to capitalize on “non-synaptic” signaling, which is reflected by the typically high expression of extra-synaptic opioid receptors (Svingos et al., 1996, 1998, 1999). Some have even reported active opioid peptides traveling as far as 100μm from the initial release site (Chavkin, 2013; Drake et al., 1994). While the reason for the extra-synaptic localization of the receptors remains an active area of study, it is important to consider how this distribution pattern may impact “discrete signaling” events.
Despite their complexity, the opioid system has shown itself to be a useful lens through which to examine how the architectural differences across the striatum manifest as differences in function. Many groups have discussed at length some of these functions, especially concerning the role of D1 versus D2 expressing neurons and dopamine in mesocorticolimbic systems (Becker, 2016; Francis and Lobo, 2017; Humphries and Prescott, 2010; Ostroumov and Dani, 2018). Here, we refocus these discussions on endogenous opioids and related peptides, exploring several potential frameworks for how peptidergic systems can act as critical players to powerfully augment striatal activity, especially within NAc medial shell. In particular, we will consider how opioids and peptides are recruited in a state dependent manner, particularly in stress-like states to augment cellular and behavioral activity.
Kappa/Dynorphin and Stress
Stressors and stress responses are naturally occurring phenomenon that are critical for guiding both approach and avoidance behaviors in the natural environment. As a central hub in mesocorticolimbic circuitry, NAc medial shell is well positioned to respond to stress signals and transform them into motivated coping behaviors (Beck and Fibiger, 1995; Land et al., 2009; Robinson and Berridge, 2013; Vialou et al., 2015). But are opioids in NAc medial shell involved in this process? Evidence suggests they are, and there is some indication that specific opioids may have specialized roles within discrete cell types as well as distinct anatomical locations.
The KOPR/dynorphin system is primarily known for its role in aversive signaling, especially within the context of stress. Several studies systemically modulating the KOPR/dynorphin system have demonstrated that its general activation is important for conditioned place avoidances, shock avoidance, odor avoidances, social defeat, and learned helplessness in the forced swim model (Barr et al., 1994; Bruchas et al., 2011; Donahue et al., 2015; Land et al., 2008, 2009; McLaughlin et al., 2006; Tang and Collins, 1985). Subsequent brain site selective studies then indicated that KOPR/dynorphin may in fact have several sites in which it can act to augment aversive processing. One early study by Bals-Kubik et al. showed that KOPR agonist microinjections into NAc, VTA, medial PFC, or LH were sufficient to drive a conditioned place avoidance (Bals-Kubik et al., 1993). Others have since shown that KOPR/dynorphin in specific brain sites are involved in modulating many stress-related behavior, such as parts of the amygdala for fear conditioning or anxiogenesis (Bruchas et al., 2009; Crowley et al., 2016; Knoll et al., 2011), central amygdala for pain processing (Nation et al., 2018), NAc shell for selective aggression in pair-bonded prairie voles (Resendez et al., 2012), and dorsal raphe and VTA for intrinsic KOPR aversion (Chefer et al., 2013; Ehrich et al., 2015; Land et al., 2009).
KOPR/Dynorphin and NAc
Though fewer studies have examined how KOPR/dynorphin mediates stress-related aversion in NAc, cumulative evidence suggests multiple sites of action (Bruchas et al., 2007; Land et al., 2009; Shirayama et al., 2004). Currently, KOPRs appear to exert most of their actions on presynaptic afferents, having been shown to modulate glutamatergic, dopaminergic, and serotonergic inputs. Glutamate signaling in NAc shell is likely important for tuning specific behavioral responses by supplying important cognitive and saliency information (Britt et al., 2012; Reed et al., 2018; Tejeda et al., 2017). It is interesting to note that despite the numerous specific glutamatergic afferents, non-projection specific glutamatergic modulation of NAc shell activity still produces cohesive, intense behavioral responses. For example, glutamate disruption via pharmacological blockade of AMPA receptors can induce intense food intake or fearful/defensive behaviors in rats (depending on the particular subregion) (Reynolds and Berridge, 2001, 2008; Richard and Berridge, 2013). Additionally, inhibition of individual glutamatergic inputs in rostral NAc shell is also sufficient to enhance food intake (Reed et al., 2018). In contrast, optogenetic stimulation of prefrontal, hippocampal, or amygdalar inputs are all sufficient to drive self-stimulation, as does direct stimulation of NAc MSNs (Britt et al., 2012). While the precise mechanisms explaining these dichotomous glutamatergic effects are likely complex (Francis and Lobo, 2017), strong evidence suggests KOPRs may be involved in modulating NAc shell glutamate signaling. In vitro recordings in NAc have shown that KOPR activation swiftly decreases neuronal activity in NAc by reducing postsynaptic mEPSPs, suggesting that it may act to suppress glutamate (Hjelmstad and Fields, 2001). More recent work by Tejeda et al. have further pinpointed the presynaptic glutamatergic site to be BLA terminals (Tejeda et al., 2017). Using optogenetics, conditional KOPR knockouts, and pharmacology with whole-cell patch clamp recordings, they were able to definitively show that KOPRs are limited in their modulation of hippocampal inputs, but yield large reductions in BLA glutamatergic terminal activity. Additionally, they demonstrated that KOPRs interact with postsynaptic NAc D1 and D2 MSNs differently to ultimately bias NAc signaling toward D1 or D2 MSN activity. How these particular KOPR-mediated synaptic regulatory properties map onto behavioral outcomes requires further in vivo circuit mapping approaches, yet at least some of the variation observed in BLA glutamate afferent activity may be related to differences in the rostrocaudal effects of KOPRs (more below) (Castro and Berridge, 2014a; Reed et al., 2018).
A second population that KOPRs act on to modulate behavior are the dopaminergic terminals arriving into the NAc shell from VTA. VTA dopamine (DA) to NAc is perhaps best known for its role in incentive motivation and reward-prediction error encoding (Hamid et al., 2016; Humphries and Prescott, 2010; de Jong et al., 2018; Mirenowicz and Schultz, 1996; Steinberg et al., 2013), both of which are greatly affected by stress states. In what is now recognized as a classic demonstration of DA/KOPR interactions, Carlezon et al. (Carlezon et al., 1998) showed that the rewarding effects of cocaine could be regulated by changes in CREB in NAc, and that these CREB regulated effects were further controlled by KOPR signaling. Since then, multiple groups have shown interactions between KOPR and DA systems, especially within NAc (Chefer et al., 2000, 2013, Ehrich et al., 2014, 2015; Heidbreder et al., 1998). Collectively, these studies suggest that acute KOPR stimulation results in decreased DA activity, whereas chronic or extended pretreatment increases DA activity. Such an interaction is likely relevant for KOPR mediated decreases and increases in drug-seeking responses that occur during or after stress (Wee and Koob, 2010), and also suggests that KOPRs may play a pivotal role in driving both active and passive responses to stressors.
KOPR/Dynorphin and Appetitive Motivation
While the studies discussed so far have focused on the role of KOPR/dynorphin in mediating aversive stress behaviors, there is a burgeoning literature suggesting that it may also be involved in appetitive behaviors. Though not often considered in models of NAc processing, some of the earliest studies on the KOPR/dynorphin system reliably demonstrated that systemic stimulation resulted in increased ingestive behaviors (Cooper et al., 1985; Morley and Levine, 1983). Relatedly, Singh and Desiraju showed that LH injections of dynorphin facilitated VTA/nigral electrical self-stimulation (i.e., less stimulation was needed to achieve the same behavioral response), again showing facilitated appetitive motivated behavior in response to KOPR/dynorphin activation (Singh and Desiraju, 1988). A third example for KOPR/dynorphin involvement in appetitive motivation includes a series of experiments by Bodnar and colleagues. They showed that while KOPR is not sufficient to drive intake behavior in NAc medial shell, loss of KOPR function prevents MOPR, DOPR, GABA receptor, and food deprived stimulated intake (Bodnar et al., 1995; Khaimova et al., 2004; Ragnauth et al., 2000). Though generally understudied, part of what may underlie an appetitive KOPR/dynorphin system is that its appetitive effects may be highly anatomically localized, particularly in the NAc medial shell. So far, two studies in the last several years have begun to highlight how anatomical differences may manifest in drastically different behavioral phenotypes after KOPR/dynorphin activation. The first study was a behavioral pharmacology mapping experiment in which the selective KOPR agonist U50488H was microinjected throughout NAc medial shell, and affective orofacial ‘liking’ reactions and motivated food ‘wanting’ were monitored and compared to vehicle test days (Castro and Berridge, 2014a). As summarized by Castro and Berridge (2014b), “‘Liking’ and ‘disgust’ are placed in quotation marks to acknowledge that these are objective positive or negative hedonic reactions that are not necessarily accompanied by subjective feelings of pleasure or disgust (even if they often are), and to distinguish them from the everyday use of the English term, liking. Similarly, ‘wanting’ in quotes refers specifically to the motivation process of incentive salience, which also can occur in brain and behavioral responses either with or without accompanying subjective feelings of ordinary wanting” (Castro and Berridge, 2014b). In the study by Castro and Berridge, it was found that KOPR stimulation within the rostral half of NAc medial shell increased ‘liking’ reactions to sweet sucrose (in what we now refer to as a “hedonic hotspot”), whereas the same KOPR stimulation in caudal sites reduced ‘liking’ reactions (i.e., a hedonic coldspot”). These results provided the first evidence that KOPR may actually be directly involved in modulating positively valenced behaviors. Concurrent work by Al-Hasani et al. (Al-Hasani et al., 2015) showed that direct optogenetic stimulation of dynorphinergic neurons in medial shell generated either a real-time place preference (RTPP) or avoidance (RTPA) depending on whether dorsal or ventral sites were stimulated. As mentioned above, KOPR, in part, acts by modulating presynaptic afferents. Considering that reward responses from glutamate afferents vary according to rostrocaudal site, KOPR activation can bias signaling toward direct or indirect pathway excitation, and that discrete changes in D1 or D2 activity can further gate glutamate-modulated behaviors, it seems likely that NAc KOPR acts at microcircuit levels to titrate and scale eventual MSN activity (Reed et al., 2018; Richard and Berridge, 2011; Tejeda et al., 2017). These microcircuits may help explain why KOPRs appear to have multiple anatomically segregated mechanism of action, such as rostral/caudal versus dorsal/ventral sites in NAc shell. At the very least, there is now evidence that points toward an appetitive role for KOPRs, at least within NAc medial shell. The growing field of an appetitive KOPR/dynorphin system, coupled with the large literature on its role in aversion suggest that it is involved in modulating all sorts of stress-related behaviors. Future work examining whether well-known aversion circuits can be refashioned under various conditions (i.e. stress, drug abuse, pain, etc) into appetitive circuits would be an exciting step forward in understanding its role in behavior.
MOPRs: Facilitators of Motivation
Though all four receptors are implicated in motivated behaviors, MOPRs are perhaps the most well described, and have long been associated with appetitive motivation and affect. Indeed, the use of psychoactive compounds that act on the MOPR system have been used for millennia, though even early studies on morphine use/abuse noted it had many complex effects on the central and peripheral nervous system (Mattison, 1891). These early observations are once again pertinent, as synthetic and prescription opioid use and abuse has skyrocketed within the last decade (Centers for Disease Control and Prevention (CDC), 2012; Volkow et al., 2018). As most of these abused drugs are highly potent MOPR agonists, it is worth revisiting 20th century scientific history, as reappraisals of these studies may yield new insights into our modern epidemic.
With the discovery and development of MOPR selective ligands and experimental tools, studies in the 1960’s and early 1970’s were able to systematically demonstrate MOPR agonists (e.g., morphine) were rewarding and primarily acted to enhance motivated behaviors (Frenk and Rogers, 1979; Katz and Steinberg, 1972; Khavari et al., 1975; Kumar et al., 1968). The later generation of the MOPR knockout and conditional knockout mouse lines solidified MOPRs role in motivated behaviors (Matthes et al., 1996; Weibel et al., 2013). Notably, many behaviors that can be positively augmented by MOPR recruitment (e.g. analgesia, food intake, social investigation) do not require MOPR to generate normal/baseline behaviors (pain avoidance, ad libitum food intake, sniffing and play behavior) (Contet et al., 2006; Gavériaux-Ruff et al., 2008; Papaleo et al., 2007; Weibel et al., 2013). This likely indicates that MOPRs function primarily as an evoked “state-dependent” modulatory system, recruited to enhance behavioral responses to stress (e.g., analgesia, hunger-enhanced intake, pair-bond formation), rather than a necessary initiator of behaviors, per se.
During the 1980’s several studies demonstrated that at least part of the rewarding effects of MOPR activation were driven through central mechanisms via intraventricular microinjections. Based on early autoradiographic and in situ hybridization studies, regions like NAc/striatum, VTA, and PAG immediately garnered interest as potentially important sites for understanding the actions of MOPR within the CNS (Herkenham and Pert, 1980; Mansour et al., 1994). Indeed, electrical stimulation of PAG to induce opioid release had already been suggested as a key site for opioid analgesia, and dopamine lesions in NAc via 6-hydroxydopamine showed attenuated heroin reward (Spyraki et al., 1983; Yeung et al., 1977). Since then, numerous targeted pharmacological studies have revealed that the role of MOPR in modulating motivation is far more complex than initially hypothesized (Castro and Berridge, 2014, 2017; Charbogne et al., 2017; Corder et al., 2017; Mahler and Berridge, 2009; Mena et al., 2011; Parker et al., 2014; Peciña and Berridge, 2005; Ragnauth et al., 2000; Smith et al., 2018; Wager et al., 2007; Wassum et al., 2009, 2011).
MOPRs in NAc
One factor that appears to contribute to at least some of the variance across studies is the subregional specificity of MOPR recruitment, especially within NAc medial shell. This work largely begins with a landmark study by Bals-Kubik et al. (Bals-Kubik et al., 1993), in which the MOPR agonist DAMGO was injected into multiple sites throughout the brain to ascertain whether its activation was sufficient to engage reward systems as measured by a conditioned place preference (CPP). Surprisingly, the only area that did so was the VTA; NAc MOPR stimulation had no effect on place preference. Later that same year Bakshi and Kelley published data showing that MOPR stimulation in NAc was more than sufficient to drive intense food intake, implicating MOPR in NAc in reward regulation (Bakshi and Kelley, 1993). Since then, several investigators have corroborated Kelley’s initial findings, and further work has demonstrated that MOPR stimulation throughout the striatum is sufficient to drive not only food intake, but many types of motivated behaviors (Peciña and Berridge, 2000; Ragnauth et al., 2000; Zhang and Kelley, 1997). For example, MOPR activation in NAc medial shell could modulate drug seeking behaviors, pair-bond formation in prairie voles, sexual behaviors in rats, and operant responding for food rewards (Cui et al., 2014; Hanlon et al., 2004; Resendez et al., 2013; Wiskerke et al., 2011). While it is tempting to assume that many of these behaviors are the result of MOPR altering the hedonic qualities of the reward, work by Peciña and Berridge suggest that MOPRs may have multiple, distinct mechanisms for separately modulating affect and motivation (Peciña and Berridge, 2005). Specifically, using a microinjection mapping approach, in which the behavioral effects of a drug infusion was mapped onto the histologically verified injection site, they identified the rostrodorsal zone of NAc medial shell as a MOPR “hedonic hotspot”. In contrast, MOPR stimulation in the caudal half of NAc medial shell actually suppressed ‘liking’ in a “hedonic coldspot” (note: the MOPR and KOPR hot and coldspots appear to overlap considerably). However, despite the anatomically localized effects on hedonic reactions, MOPR stimulation at all sites in NAc increased food intake. In a follow up study, Castro and Berridge mapped the same hedonic hotspot and coldspot, and demonstrated that MOPR stimulation could generate a CPP, but only if the activated sites were restricted to the hedonic hotspot; MOPR stimulation in caudal NAc shell did not result in a CPP, similar to the absence of an effect observed by Bals-Kubik et al. in NAc core (Bals-Kubik et al., 1993; Castro and Berridge, 2014a). Finally, Smith and Berridge showed that the mechanisms underlying MOPR control of motivation and affect are likely independent, as MOPR blockade in ventral pallidum (which likewise houses a localized hotspot) prevented concurrent NAc MOPR stimulated enhancement of affective ‘liking’ reactions, but left MOPR stimulated eating intact (Smith and Berridge, 2007). Cumulatively, investigations into the role of MOPRs over the last 50–60 years have gone from gross systematic demonstrations of its various roles in behavior, to central versus peripheral mechanisms, to nucleus-focused studies, to a subregional specificity of function. Alongside these anatomical refinements have been increasingly sophisticated molecular studies that have begun isolating the contributions of G-protein versus arrestin signaling, transcription factor analysis modifications and the effects of protein-protein interactions, like the proposed dimerization of GPCRs (Anderson et al., 2017; Fox et al., 2018; Heshmati et al., 2018; Taniguchi et al., 2017). Moving forward, studies will need to incorporate these molecular mechanisms into subregion specific locations in order to better understand how a single peptide GPCR can selectively yet broadly modulate behaviors in a neural circuit-specific manner.
Retuning NAc through DOPRs
The DOPR/enkephalin system has been well characterized by several investigators. Here, we describe the known roles of DOPRs in stress and motivation, and examine how these roles apply to their function in NAc medial shell. Like other opioid systems, DOPR stimulation consistently generates analgesic responses for both spinal and supraspinal pain (Corder et al., 2018; Filliol et al., 2000; François and Scherrer, 2017; Pradhan et al., 2014). It also causes anxiolytic states, increasing time spent in open arms of elevated mazes and time spent in the center zone of open field assays. Importantly, DOPR knockout mice show increased anxiogenic responses, suggesting that, unlike the MOPR system, basal DOPR activity is necessary for normal function. It also implies that DOPRs may be involved in modulating stress-reactivity, with low levels allowing for increased stress responses, and high levels subduing them (Filliol et al., 2000). Indeed, recent studies have shown that DOPR activation is sufficient to prevent vulnerable phenotypes from emerging after repeated social defeat (Henry et al., 2018). Similarly, DOPR stimulation can alleviate hyperalgesia and pain-associated avoidance, again buffering against the effects of stressful experiences (Pradhan et al., 2014).
While direct experimentation on the role of DOPRs in NAc medial shell and stress are scant, potential inferences can still be made based on the available literature. During the 1990’s, Ann Kelley and Rich Bodnar published numerous studies examining the role of DOPRs on food intake (Bakshi and Kelley, 1993; Bodnar et al., 1995; Ragnauth et al., 2000). Collectively, they showed that DOPR stimulation was able to enhance intake similarly to MOPR stimulation, and likely interacts with other opioids to do so. Additionally, several studies have noted that DOPR stimulation increases locomotor behavior (Bakshi and Kelley, 1993; Katsuura and Taha, 2010; Zhang and Kelley, 1997). Considering that DOPRs likely play a role in reducing anxiety, it is likely that DOPR activation rapidly enhances the incentive value of positive stimuli, which could manifest behaviorally as increased appetitive food intake or increased locomotor/exploratory behavior. But even beyond enhanced appetitive motivation, DOPR also appears to actively suppress aversive incentives, such as the protective nature of DOPR stimulation on repeated stress (social defeat) or alcohol withdrawal (Alongkronrusmee et al., 2016; Henry et al., 2018). A bimodal capacity for DOPR function is consistent with recent observations that NAc medial shell contains a DOPR hedonic hotspot and hedonic coldspot that appears to completely map onto the MOPR and KOPR hot and coldspots described above (Castro and Berridge, 2014a). While that study only evaluated DOPR’s role in modulating responses to an appetitive stimulus (1% sucrose solution), it still demonstrates a multiplicative role for DOPR signaling within medial shell. It would be worthwhile for future studies to examine how DOPR affects aversive stimuli (e.g., bitter quinine) to ascertain whether there is a similar suppressive effect on affective reactions.
Given the array and diversity of behaviors that the DOPR/enkephalin system can modulate, parsing the specific mechanisms of action becomes a necessary endeavor. To date, evidence suggests that DOPRs preferentially express on CINs (Bertran-Gonzalez et al., 2013), though they are also expressed on indirect pathway/enkephalin neurons too, at least in dorsal striatum (Banghart et al., 2015; Lindskog et al., 1999; Noble and Cox, 1995). Furthermore, Banghart et al. provide evidence for a special role for DOPRs in patch compartments of dorsal striatum. Here, DOPR activation appears to reduce lateral GABAergic inhibition from indirect pathway neurons onto direct pathway neurons (Banghart et al., 2015). Whether such a mechanism is also present in the patch-rich NAc medial shell remains untested, but regardless of whether DOPRs act through inhibition of lateral silencing or via CINs still suggests that the primary role of DOPRs in NAc is to indirectly modulate MSN activity via retuning local activity. This would be consistent with behavioral evidence showing that the DOPR/enkephalin system is already active, even at basal states, carefully shifting the balance of striatal activity to allow for adaptive responding to various stimuli.
Nociceptin: A Motivational Suppressor?
Since its initial discovery, nociceptin has been thought to possess anxiolytic properties, possibly by retuning responses to facilitate stress reduction (Griebel et al., 1999; Jenck et al., 1997; Köster et al., 1999; Reinscheid and Civelli, 2002). This can manifest as an acute event (Devine et al., 2003; Nazzaro et al., 2010), or as a response to repeated stress (Köster et al., 1999). Due to their consistent anxiolytic effects, a growing literature has targeted sites like central amygdala and bed nucleus of the stria terminalis (Ciccocioppo et al., 2003, 2014; Cruz et al., 2012; Delaney et al., 2012). In each of these sites, NOPR/nociceptin appears to consistently reduce stress responses. In contrast to the clear role of NOPR/nociceptin in many limbic sites, understanding its role in NAc medial shell has been less well investigated. Early exploration into the role of NOPR/nociceptin, while informative, often neglected NAc medial shell, instead favoring the more microdialysis accessible core (Lutfy et al., 2001; Murphy et al.,1996). Though not directly translatable, it is possible that nociceptin could have similar physiological effects in core as it does in shell, suggesting that NOPR stimulation in shell would likewise result in reduced dopamine signaling (Vazquez-DeRose et al., 2013). In this sense, the NOPR/nociceptin system may be more similar to the KOPR/dynorphin system, with whom it also shares more genetic similarities. However, cumulative evidence suggests that the NOPR/nociceptin system is more complex. Since the mid-1990’s, intracerebroventricular injections of nociceptin have failed to induce a conditioned place preference or avoidance (making it neither like a MOPR nor a KOPR agonist) (Devine et al., 1996). Yet, infusions of nociceptin will block the formation of a morphine CPP without altering other learning behaviors or morphine sensitized locomotion (though NOPR stimulation does prevent cocaine enhanced locomotion) (Ciccocioppo et al., 2000; Murphy et al., 1999; Vazquez-DeRose et al., 2013). One explanation for the failure of NOPR/nociceptin to actively generate preferences or avoidances could be that it acts as a general dampening signal, reducing the incentive value of all stimuli regardless of affective valence. This could account for why it appears to reduce stress related behaviors (dampening aversive experiences) as well as reward behaviors like morphine CPP. However, when nociceptin is locally infused in NAc medial shell of ad libitum fed rats, food intake is enhanced to a similar degree as MOPR or DOPR stimulated eating (Stratford et al.,1997). While other corroborations of an active role for NOPR/nociceptin are quite rare, it is of great interest to determine whether NOPR may also be involved in modulating motivated or stress behaviors similarly to the other opioid receptors, in addition to its saliency suppressive role elsewhere in the brain.
Additional Neuropeptides in NAc Shell: An Emerging Field
We have discussed the role of opioids in NAc, with a particular emphasis on known and potential anatomical localization of function, primarily because they have been the most widely studied in this region. However, it is important to consider that beyond opioids, many other neuropeptides are enriched in NAc and have been shown to powerfully modulate behaviors (For a summary see Table 2). As one might expect, many neuropeptides that enter NAc arisec from hypothalamic populations, such as orexin/hypocretin (O/H), melanin-concentrating hormone (MCH), or agouti-related peptide (AgRP) (Fig. 2). Others are more likely derived from local sources, such as Substance P (SP) or somatostatin (SOM), and others still may come from cortical or brainstem sites. Regardless, the potential for peptidergic modulation of neural activity in NAc is clear, which makes the dearth of studies available for discussion surprising, especially in light of the wealth of information that has been accumulated for opioids in this subnucleus. On the one hand, there are quite a few studies that have looked at the descriptive qualities of peptides in NAc (e.g., oxytocin), such as changes in RNA expression or overlap with immediate early gene expression. But on the other hand, most of these studies do not evaluate these peptides through experimental means, leaving their exact contribution to behavior fairly ambiguous. However, some consideration for how various peptides contribute to behavior can be extrapolated from systemic or non-site specific studies, such as with SP. For example, neurokinin 1 knockout mice fail to learn a morphine CPP but show a robust cocaine CPP (Murtra et al., 2000), suggesting an interaction between MOPR, SP, and dopamine systems that collectively contribute to reward processing. Furthermore, electrophysiological recordings in NAc (specific site not defined) show that while SP stimulation reduces EPSPs, it also enhances dopamine release, which may be relevant for how it affects reward signaling (Kombian et al., 2003). However, as expected, the role of SP in NAc shell specifically is somewhat more difficult to ascertain, as direct stimulation of SP does not generate a CPP (despite presumably enhancing dopamine release), yet loss of SP function prevents ethanol reinstatement, suggesting that it is necessary for some motivated behaviors (Schank et al., 2015; Schildein et al., 1998). Generally, though, if we focus in on NAc shell, it appears that the trend of peptides facilitating behaviors holds up, with several peptides being shown to enhance dopamine transmission (Marshall et al., 1991; Sørensen et al., 2009), overtly enhancing appetitive motivated behaviors (Brown et al., 2000; Georgescu et al., 2005; Peciña et al., 2006; Thorpe and Kotz, 2005; Yu et al., 2016), and even enhancing aversive motivated behaviors (Bosch et al., 2016; Chen et al., 2012) (Table 2, Figure 3A and B). Indeed, only a few examples show suppressive effects of peptide stimulation, such as α-melanocyte-stimulating hormone suppressing food intake and ethanol intake (Carvajal et al., 2017; Pandit et al., 2015) and CART stimulation reducing cocaine and food reward (Jaworski et al., 2008; Yang et al., 2005). While specialized study of peptides in NAc shell is currently underpowered, multiple reports of peptide function in NAc core may provide some insight into when/how these systems may be engaged. For example, changes in MSN excitability (which has been linked to depressive-like phenotypes) after chronic stress is MCR4-dependent (Lim et al., 2012), indicating that the melanocortin system is likely an active suppressor of motivated behaviors across ventral striatal circuitry. By contrast, other neuropeptides appear to maintain their facilitative role in behavior, increasing social interaction, locomotion, and drug reward (Dölen et al., 2013; Hayes et al., 2012; Kohli et al., 2019; Schwartz et al., 2014; Vadnie et al., 2014). Such broad function of the peptidergic systems is consistent with the role of many neurochemical systems within ventral striatum (Table 2). Moving forward in this field, it will be of interest to determine whether these other peptide systems are using similar mechanisms of action (e.g., same cell types) across striatal subregions, and whether they have potential localization of function as opioids do with affective ‘liking’ systems.
Table 2. Functional role of neuropeptides in NAc medial shell.
Non-opioidergic peptides with accompanying common abbreviation (first column). Specific anatomical site in NAc medial shell tested, based on reported placement maps or photomicrograph images (second column). Major function of the peptide system from cited publication (third column). “Stimulation” refers to either peptide or selective ligand infusions, not to genetically labeled populations. Specific references for each finding are listed in the fourth column. Studies in which placements were only textually described were excluded from the table due to unreliability of inferring actual placements from reported placements (dilemma described in text).
| Peptide System | Anatomical Site in NAc Shell | Finding | Reference |
|---|---|---|---|
|
α-Melanocyte-Stimulating Hormone (MSH) |
Rostral, Dorsal | Stimulation reduces food reward | (Pandit et al., 2015) |
| Rostral, Dorsal | Stimulation reduces ethanol intake | (Carvajal et al., 2017) | |
|
Cholecystokinin (CCK) |
Rostral | Modulates dopamine release | (Marshall et al., 1991) |
| Caudal | Stimulation increases DA release | (Ladurelle et al., 1994) | |
|
Cocaine and Amphetamine Related Transcript (CART) |
Ventral | Stimulation reduces cocaine reward, but not food intake | (Jaworski et al., 2008) |
| Rostral | Stimulation reduces food intake | (Yang et al., 2005) | |
|
Cortico tropin-Releasing Factor (CRF) |
Caudal, Ventral | Stimulation increases avolition, anxiety, and ACh release | (Chen et al., 2012) |
| Rostral | Blockade prevents nicotine withdrawal reward deficits | (Marcinkiewcz et al., 2009) | |
| Nonspecific | Stimulation increases locomotion | (Holahan et al., 1997) | |
| Caudal, Dorsal | Stimulation increases PIT | (Peciña et al., 2006)(Peciña et al., 2006) | |
|
Melanin-Concentrating Hormone (MCH) |
Nonspecific | Stimulation increases food intake, blockade suppresses intake | (Georgescu et al., 2005) |
| Dorsal | Blockade reduces cocaine reward | (Chung et al., 2009) | |
|
Neuropeptide Y (NPY) |
Ventral | Stimulation causes CPP | (Brown et al., 2000) |
| Nonspecific | Stimulation increases food reward | (Pandit et al., 2014) | |
| Rostral | Stimulation increases DA release | (Sørensen et al., 2009)(Sørensen et al., 2009) | |
|
Neurotensin (NT) |
Nonspecific | Stimulation blunts presynaptic D2 activity on DA terminals | (Fawaz et al., 2009) |
| Rostral | Blockade increases cocaine self-administration | (Ramos-Ortolaza et al., 2009) | |
|
Orexin/Hypocretin (O/H) |
Nonspecific | Stimulation increases food intake and locomotion | (Thorpe and Kotz, 2005) |
| Rostral | Stimulation increases ‘liking’ | (Castro et al., 2016) | |
| Nonspecific | Blockade prevents stress primed CPP reinstatement | (Qi et al.,2013) | |
| Rostral | Blockade reduces ethanol reward | (Lei et al., 2016) | |
| Rostral, Ventral | Stimulation enhances DA-induced turning | (Kotani et al., 2008) | |
|
Oxytocin (OT) |
Nonspecific | Reduced tone increases passive coping | (Bosch et al., 2016) |
| Rostral | Stimulation increases social reward | (Yu et al., 2016) | |
| Nonspecific | Increasing receptor expression increases maternal behaviors | (Keebaugh and Young, 2011) | |
| Rostral | Blockade prevents pairbond formation | (Liu and Wang, 2003) | |
|
Somatostatin (SOM) |
Rostral, Ventral | Stimulation enhances DA-induced turning | (Ikeda et al., 2009) |
|
Substance P (SP) |
Nonspecific | Stimulation does not generate CPP | (Schildein et al., 1998) |
| Rostral | Blockade reduces stress-reinstatement of ethanol | (Schank et al., 2015) |
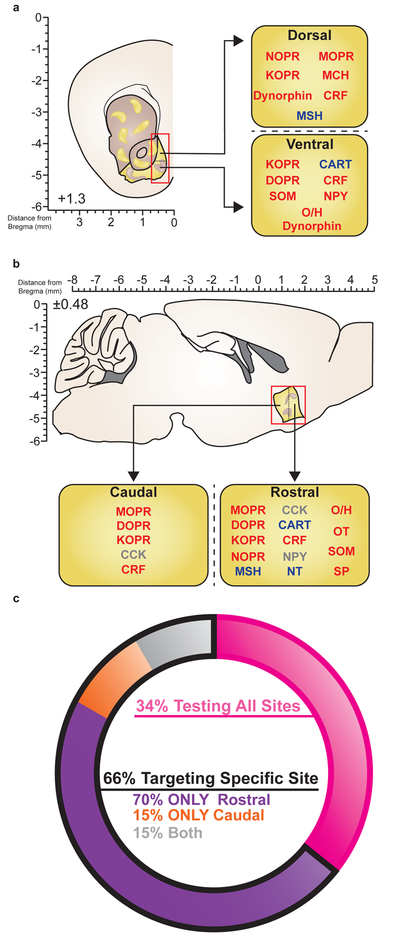
Figure 3. Peptidergic localization of function within NAc medial shell.
(A) Schematic showing site specific manipulations of various peptides in NAc medial shell, divided by dorsal versus ventral targets. Red text, enhancement of behavior; blue text, suppression of behavior; gray text, non-behavioral study. (b) Schematic showing site specific manipulations of various peptides in NAc medial shell, divided by rostral versus caudal targets. (c) Ring charts show proportion of peptide studies targeting all sites in NAc (pink) versus specific cites (black outline). Of the studies targeting specific sites, proportion of peptide studies in NAc medial shell targeting rostral (purple), caudal (orange), or both (gray) sites. Studies cited can be found in Table 2.
In considering whether non-opioid peptides show specialized localization of function across NAc shell, it may come as a surprise that of the studies that both targeted NAc shell and visualized placements, only ~34% tested all or most sites in NAc (Figure 3C, Table 2). This suggests that the majority of experiments across these different neuropeptide systems only evaluated their role in particular subregions. A further analysis of the “shown placements” studies even further narrowed their scope, as ~70% of them exclusively targeted rostral NAc shell, and only ~15% exclusively targeted caudal NAc shell. What might account for this unusual preference across studies and peptides for rostral NAc? As discussed in a review by Richard et al. (Richard et al., 2013) (refer to Figure 1), the scaling system in the commonly used brain atlas by Paxinos and Watson shifted in the late 2000’s, changing the rostral border of NAc from ~1.6 to ~2.2mm anterior to Bregma in the coronal plane; the sagittal plane has remained consistent and appears to accurately reflect the current coronal borders of NAc across atlas editions (Paxinos and Watson, 1998, 2007). The discrepancy between stereotaxic coordinates and accompanying images creates an illusory interpretation of placements such that textually reported coordinates (+1.7mm anterior to Bregma, the “middle”) differed from the actual placements, which clearly favored rostral NAc as shown by either summary placement figures or actual photomicrographs. We anticipate that research into the role of non-opioid peptides in NAc will begin to fill in the missing gaps in understanding their function over previous decades, which may have important implications for how the differential milieu of neurotransmitters in the NAc can elicit varied behavioral responses depending on their site of action.
Peptidergic Transmission in the Age of Optogenetics
This review examines the special role of neuropeptides in the structurally and functionally unique NAc medial shell. We have focused here mostly on the opioids, and other recent reviews have examined other neuropeptides like orexin/hypocretin, corticotropin-releasing factor, and ghrelin (Burdakov, 2018; Ferrario et al., 2016; James et al., 2017; Mantsch et al., 2016; Nevárez and de Lecea, 2018; Quadros et al., 2016; Revitsky and Klein, 2013). Together they all demonstrate the impact that peptides can have on influencing various types of motivated behaviors. However, as modern cell-type selective neuroscience tools have continued to develop, the incorporation of peptides into experimental designs has, for the most part, not been a major priority. Most studies using vGluT or vGAT-cre lines almost always ignore potential coreleased peptides after optogenetic or chemogenetic manipulations, and the control experiments using localized pharmacology to account for possible peptidergic effects are almost never published. While it is appreciated that these experiments are challenging, it has left a big gap in our understanding for how peptides function in the context of modern neural circuit analysis. Ironically, while the generation of novel cell type specific cre-driver lines frequently relies on unique peptide expression, the vast majority of studies that use these peptidergic lines choose to study the binary effects of the coreleased fast neurotransmitters (GABA or glutamate) and not the peptide. While examining glutamatergic and GABAergic transmission remains a crucial component for understanding neural circuits, we argue that continuing to draw causal conclusions of neural systems without considering the endogenous role of co-packaged neuropeptides is misrepresenting of how these systems actually function to shape behavior. Of course, the major reasons for the failure to integrate peptides into circuit studies is not due to disinterest in peptides, but rather because of technological limitations, such as the very real difficulty of measuring neuropeptide release in vivo (e.g, microdialysis) with high spatial and temporal resolution. It can certainly be accomplished, but only at timescales that are magnitudes greater than what is available for faster transmitter systems (e.g., catecholamines) (Al-Hasani et al., 2018). Thus, time-locking neuropeptide release to receptor activation to behavior is a substantial challenge. However, while this is a difficult task, it will be of critical importance to examine how peptides influence and interact with dopamine, serotonin, glutamate and GABA microcircuits to ultimately influence behavior. Finally, we caution that we must include neuropeptides into our neural models to avoid overly simplistic, binary explanations for how brain systems function. While it may be simpler to model net gains or losses, it is likely that neuropeptides provide the mechanisms for how these systems achieve and shape net differences in activity within circuits. Indeed, every major projection to NAc shell possess multiple potentially coreleased peptides, which could dramatically modify how those inputs regulate behaviors (Figure 2). Therefore, by incorporating peptides into fast-transmitter models, and specifically within network hubs, such as the NAc shell, it will become necessary to integrate peptidergic processes into our descriptions of endogenous neural mechanisms.
Looking forward, there are several emerging technologies that will facilitate investigations into the complex signaling and functional properties of peptides, both observationally and experimentally. As a step toward understanding the role of a neurochemical in awake, behaving animals, monitoring and quantifying endogenous activity has proven to be a prolific enterprise. One of the first widely used techniques to measure in vivo neurochemical activity was microdialysis, which grew in popularity during the 1980’s. Since then, microdialysis has continued to excel as a method for collecting and distinguishing multiple neurochemicals from a single sample (DiFeliceantonio et al., 2012; Pontieri et al., 1996; Thompson et al., 2000). While opioids and related peptides had long been proven to be difficult to collect, recent modifications now allow for comparatively faster sample collection times (12–15 minutes) (DiFeliceantonio et al., 2012; Zhou et al., 2015). While 15 minute collection periods are still more than 10x longer than other neuromodulators (dopamine ~1 minute), this first step in opioid detection preempted the more recent development of an opto-dialysis probe (Al-Hasani et al., 2018). Using this device, Al-Hasani et al. showed that optogenetic stimulation of the GABAergic D1/dynorphin neurons in NAc could reliably induce opioid release, as well as concurrently detect changes in GABA and glutamate. In the future, it would be well worth investigating whether stimulation of other well studied amino-acid pathways (e.g., cortical inputs to striatum, habenular inputs to VTA) likewise result in amino-acid and peptide corelease, and whether various stimulation parameters elicit unique combinations.
As an alternative to comprehensive peptide collection, receptor-based fluorescent imaging may provide another route for studying peptidergic systems (Jing et al., 2018; Patriarchi et al., 2018; Sun et al., 2018). One exciting development on this front includes a construct recently generated by Patriarchi et al. called dLight1 (Patriarchi et al., 2018). Here, they inserted a cpGFP molecule into the third intracellular loop of a dopamine receptor that produces photons following a conformational change triggered by ligand binding. Importantly, while these genetically encoded fluorescent sensors are nearly identical to native receptors, their activation does not recruit G-protein or beta-arrestin signaling pathways. Thus, these receptors can theoretically track ligand binding without interfering with endogenous signaling at native receptors. The process for generating dLight was successfully replicated for several other GPCR families, including the opioidergic MOPRs and KOPRs. Future studies capitalizing on these dLight variants could dramatically reshape how we understand and model neuromodulatory circuits. Another exciting development in receptor-based technology includes ligand-dependent, receptor-specific, light-gated labeling constructs that serve to link receptor activity with functional manipulations (i.e., Tango). Initially introduced in 2008 (Barnea et al., 2008), the Tango approach functions by using endogenous signaling pathways to induce the expression of novel proteins. More specifically, an artificial transcription factor is first tethered to the intracellular domain of a GPCR, with a modified cleavage site linking the two. Within the same cell, a protease is tethered to endogenous signaling effectors (i.e., beta-arrestin), which is brought to the receptor after ligand binding. The close proximity of the proteins allows the protease to cleave the transcription factor from the receptor, which can then access the nucleus and promote the generation of novel proteins. Ten years later, the Tango approach has been updated to include a light-gated feature, further refining the ability to link receptor action with temporal resolution (iTango and SPARK) (Kim et al., 2017; Lee et al., 2017). Unlike the original Tango, iTango has split the protease molecule such that part of the molecule is carried by the effector (similar to the original model), and the other half is linked to a light-sensitive protein. Thus, without both light delivery and ligand binding, the link between the receptor and transcription factor cannot be denatured to release the transcription factor. Alternatively, SPARK has made the binding site of the protease light dependent, thereby making cleavage only possible during close protein-protein interactions and light exposure. Because eventual protein transcription is now temporally gated, it is possible to determine if a specific receptor was activated during a specific behavioral event or experience. The customizability of this technique is also striking, as the transcription factor could be as simple as a fluorescent protein to identify labeled subpopulations, or as dramatic as caspase to induce subpopulation lesions. Being able to either correlate or experimentally manipulate GPCR-expressing systems with a single technique will be essential for parsing apart the specific roles of GPCRs in awake, behaving animals (for a review on these approaches, see (Spangler and Bruchas, 2017)).
While often the first step towards understanding the potential role of a neurochemical system is to monitor its endogenous activity, a major limiting factor for fully understanding neuropeptides is the inability to acutely/experimentally induce neuronal activity patterns that generate peptidergic signals. Historically, the only available tool involved chronically implanting metal cannulas into specific brain sites and injecting selective pharmacological agents, followed by behavioral testing. In vivo microinjections (as discussed in length above) have been, and continue to be, an invaluable tool for relating specific neurochemical systems to discrete behaviors. However, this approach is unable to 1) identify which cells the drug is acting on to mediate its effect; 2) to determine when, during the course of a behavioral test session, the receptors are specifically engaged (as infused drugs often take substantial time before degradation), and 3) be repeated over extended time courses as each microinjection damages the brain slightly during the infusion process. To begin resolving some of these deficits, multiple groups have begun to develop optogenetically activated chimeric G-protein coupled receptors (optoXRs) (Li et al., 2015; Siuda et al., 2015a, 2015b, 2016; Spangler and Bruchas, 2017; van Wyk et al., 2015). OptoXRs function by swapping the extracellular binding domains of a GPCR with a photosensitive GPCR. Importantly, the intracellular loops of the specific GPCR being modified is conserved; thus the receptor is now light-instead of ligand-gated, and, when activated, recruits endogenous receptor effector pathways. Thus, by using conventional optogenetic implants, it is possible to selectively stimulate specific peptide receptors under discrete, time locked conditions. So far, opto-XRs have been developed for MOPRs, β-adrenergic receptors 1 and 2, adenosine 2A receptors, and metabotropic glutamate 6 receptors. While a powerful technique, a present limiting factor is that each optoXR has to be modeled and constructed separately, so creating a widely available library from which to work will take time to develop. Relatedly, optoXRs with mutations relevant to specific intracellular signaling cascades (e.g., beta-arrestin phosphorylation sites) or allosteric modulator binding sites are few in number, and will take substantial characterization and development before they can be used. As opto-XRs and related constructs continue to improve, a more proximally useable technological advance include wireless optofluidic devices (Jeong et al., 2015; Noh et al., 2018). These are all-in-one devices that are able to optically stimulate neurons via LED, and/or infuse various pharmacological agents. These devices are advantageous relative to traditional cannulations in that the they minimize the bulk of neural damage that typically occurs during the implantation process; once implanted, the devices show similar, if not lower, immunoreactivity and glial responses compared to their metal cannula counterparts. Additionally, subsequent infusions are less damaging, as there is no “re-insertion” of a microinjector. This could potentially allow for either many microinjections or long-term microinjections that are less harmful to the overall health of the tissue. Notably, because these devices are wirelessly powered and controlled, both optogenetic stimulation and drug infusions are substantially less stressful for the animal being tested. Future iterations of these devices will continue to refine their functionality by making them more lightweight and increasing the accessibility of the user interface to facilitate implementation. Lastly, steady progress in the field of optopharmacology shows great promise for their use in behavioral neuroscience (Banala et al., 2018; Berry et al., 2017; Hagen et al., 2008; Kienzler and Isacoff, 2017; Matsuzaki et al., 2001). Optopharmacology (sometimes also called “photo-pharmacology”) specializes in the use of caged ligands that, when infused, are biologically inactive due to an interfering chemical (the cage). When exposed to light, the cage is either separated from the ligand or conformationally triggered to allow higher affinity binding with local receptors. Recently, this approach has been applied to opioid peptides in slice, where targeted UV stimulation was shown to successfully uncage enkephalin and dynorphin ligands within the area illuminated (Banghart and Sabatini, 2012; Banghart et al., 2015). Used in vivo, this tool could be used to activate specific neurochemical systems at time-locked behavioral events, transforming the information acquired during observational studies (e.g., by using dLight) into experimental practice. In their current state, most caged ligands function unidirectionally; they can only be irreversibly uncaged or photoswitched. In the future, it will be of great interest to design ligands that can be uncaged and switched in dynamically reversible manner to specifically induce neurochemical signals that are closer to endogenous signaling kinetics.
Conclusions
Here we have consolidated the intersection between the unique anatomical features of NAc medial shell and its functional implications. Briefly, NAc medial shell possesses smaller, less spiny MSNs, greater expression of acetylcholinesterase, lower expression of parvalbumin interneurons, higher expression of somatostatin interneurons, and possesses unusual afferent and efferent pathways that are not consistent with its core or dorsal striatal counterparts. These anatomical idiosyncrasies manifest as likely biological mechanisms for functionally unique subregions, evidenced here through the actions of opioids in NAc medial shell. Cumulative evidence indicates some overlap in function across opioid and neuropeptide subsystems, but also definitive parcellations for each. Additionally, a survey of studies examining the role of nonopioid peptides in NAc shows that they, too, currently lack resolution in terms of the anatomical heterogeneity in NAc shell, leaving an open question as to whether their actions might also be anatomically segregated. Finally, as the experimental tools continue to advance, it will be vital for the field to consider how the underlying anatomical features (many of which have been detailed since the 1980’s, but inaccessible for experimental manipulation) play roles in the precise physiological function of various neurotransmitter systems and circuits. In particular, we note that our understanding for how opioid-fast transmitter systems interact to modulate behavior endogenously lags far behind our examination of the fast-transmitters alone. In conclusion, we propose that future work (extending beyond NAc medial shell) explore the precise anatomical profiles of systems of interest in order to yield a deeper understanding for how neural circuits control cognition, emotional behavior, and action selection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aitta-Aho T, Phillips BU, Pappa E, Hay YA, Harnischfeger F, Heath CJ, Saksida LM, Bussey TJ, and Apergis-Schoute J (2017). Accumbal Cholinergic Interneurons Differentially Influence Motivation Related to Satiety Signaling. eNeuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, and Bruchas MR (2011). Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115, 1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo C-O, Park SI, Marcinkiewcz CM, Crowley NA, et al. (2015). Distinct Subpopulations of Nucleus Accumbens Dynorphin Neurons Drive Aversion and Reward. Neuron 87, 1063–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, Wong J-MT, Mabrouk OS, McCall JG, Schmitz GP, Porter-Stransky KA, Aragona BJ, Kennedy RT, and Bruchas MR (2018). In vivo detection of optically-evoked opioid peptide release. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alongkronrusmee D, Chiang T, and van Rijn RM (2016). Involvement of delta opioid receptors in alcohol withdrawal-induced mechanical allodynia in male C57BL/6 mice. Drug Alcohol Depend. 167, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KD, and Reiner A (1990). Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: an evolutionarily conserved feature of basal ganglia organization.J. Comp. Neurol 295, 339–369. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Wissman AM, Chemplanikal J, Buzin N, Guzman D, Larson EB, Neve RL, Nestler EJ, Cowan CW, and Self DW (2017). BDNF-TrkB controls cocaine-induced dendritic spines in rodent nucleus accumbens dissociated from increases in addictive behaviors. Proc. Natl. Acad. Sci. U. S. A 114, 9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C, and Pickel VM (1990). Neuropeptide Y in cortex and striatum. Ultrastructural distribution and coexistence with classical neurotransmitters and neuropeptides. Ann. N. Y. Acad. Sci 611, 186–205. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, and Kimura M (1994). Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. J. Neurosci. Off. J. Soc. Neurosci 14, 3969–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah HE, McCool AD, Howe MW, and Graybiel AM (2014). Neurons in the ventral striatum exhibit cell-type-specific representations of outcome during learning. Neuron 82, 11451156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi VP, and Kelley AE (1993). Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J. Pharmacol. Exp. Ther 265, 1253–1260. [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, and Kelley AE (2003). Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J. Comp. Neurol 464, 220–237. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, and Shippenberg TS (1993). Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J. Pharmacol. Exp. Ther 264, 489–495. [PubMed] [Google Scholar]
- Banghart MR, and Sabatini BL (2012). Photoactivatable neuropeptides for spatiotemporally precise delivery of opioids in neural tissue. Neuron 73, 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banghart MR, Neufeld SQ, Wong NC, and Sabatini BL (2015). Enkephalin Disinhibits Mu Opioid Receptor-Rich Striatal Patches via Delta Opioid Receptors. Neuron 88, 1227–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Wang S, and Carden S (1994). Aversive properties of the kappa opioid agonist U50,488 in the week-old rat pup. Psychopharmacology (Berl.) 113, 422–428. [DOI] [PubMed] [Google Scholar]
- Beal MF, Domesick VB, and Martin JB (1983). Regional somatostatin distribution in the rat striatum. Brain Res. 278, 103–108. [DOI] [PubMed] [Google Scholar]
- Beck CH, and Fibiger HC (1995). Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. Off. J. Soc. Neurosci 15, 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB (2016). Sex differences in addiction. Dialogues Clin. Neurosci 18, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Voorn P, te Kortschot A, and Groenewegen HJ (1988). Nuclear origin of thalamic afferents of the ventral striatum determines their relation to patch/matrix configurations in enkephalin-immunoreactivity in the rat. J. Chem. Neuroanat 1, 3–10. [PubMed] [Google Scholar]
- Berke JD (2008). Uncoordinated firing rate changes of striatal fast-spiking interneurons during behavioral task performance. J. Neurosci. Off. J. Soc. Neurosci 28, 10075–10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2018). What does dopamine mean? Nat. Neurosci 21, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, and Girault J-A (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. Off. J. Soc. Neurosci 28, 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Laurent V, Chieng BC, Christie MJ, and Balleine BW (2013). Learning-related translocation of δ-opioid receptors on ventral striatal cholinergic interneurons mediates choice between goal-directed actions. J. Neurosci. Off. J. Soc. Neurosci 33, 1606016071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson MJ, Graybiel AM, and Quinn B (1990). Co-expression of neuropeptides in the cat’s striatum: an immunohistochemical study of substance P, dynorphin B and enkephalin. Neuroscience 39, 33–58. [DOI] [PubMed] [Google Scholar]
- Bloem B, Huda R, Sur M, and Graybiel AM (2017). Two-photon imaging in mice shows striosomes and matrix have overlapping but differential reinforcement-related responses. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, and Cooper ML (1995). General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 700, 205–212. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Wainer BH, and Smith AD (1984). Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience 12, 711–718. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Izzo PN, and Graybiel AM (1988). Cellular substrate of the histochemically defined striosome/matrix system of the caudate nucleus: a combined Golgi and immunocytochemical study in cat and ferret. Neuroscience 24, 853–875. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Dabrowska J, Modi ME, Johnson ZV, Keebaugh AC, Barrett CE, Ahern TH, Guo J, Grinevich V, Rainnie DG, et al. (2016). Oxytocin in the nucleus accumbens shell reverses CRFR2-evoked passive stress-coping after partner loss in monogamous male prairie voles. Psychoneuroendocrinology 64, 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimblecombe KR, and Cragg SJ (2017). The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem. Neurosci 8, 235–242. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, and Bonci A (2012). Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Coscina DV, and Fletcher PJ (2000). The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides 21, 1279–1287. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, and Chavkin C (2007). Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J. Neurosci. Off. J. Soc. Neurosci 27, 11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, and Chavkin C (2009). CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PloS One 4, e8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, et al. (2011). Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71, 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D (2018). How orexin signals bias action: Hypothalamic and accumbal circuits. Brain Res. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM, and Cheer JF (2012). Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAJAL S (1911). Histologie du syste me nerveux de I’Homme et des verte be s. Maloine Paris 2, 891–942. [Google Scholar]
- Carlezon WA, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, and Nestler EJ (1998). Regulation of cocaine reward by CREB. Science 282, 2272–2275. [DOI] [PubMed] [Google Scholar]
- Carvajal F, Lerma-Cabrera JM, Alcaraz-Iborra M, Navarro M, Thiele TE, and Cubero I (2017). Nucleus Accumbens MC4-R Stimulation Reduces Food and Ethanol Intake in Adult Rats Regardless of Binge-Like Ethanol Exposure during Adolescence. Front. Behav. Neurosci 11, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, and Berridge KC (2014). Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting.” J. Neurosci 34, 4239–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, and Berridge KC (2017). Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc. Natl. Acad. Sci. U. S. A 114, E9125–E9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro DC, Terry RA, and Berridge KC (2016). Orexin in Rostral Hotspot of Nucleus Accumbens Enhances Sucrose “Liking” and Intake but Scopolamine in Caudal Shell Shifts “Liking” Toward “Disgust” and “Fear.” Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2012). CDC grand rounds: prescription drug overdoses - a U.S. epidemic. MMWR Morb. Mortal. Wkly. Rep 61, 10–13. [PubMed] [Google Scholar]
- Charbogne P, Gardon O, Martín-García E, Keyworth HL, Matsui A, Mechling AE, Bienert T, Nasseef T, Robé A, Moquin L, et al. (2017). Mu Opioid Receptors in Gamma-Aminobutyric Acidergic Forebrain Neurons Moderate Motivation for Heroin and Palatable Food. Biol. Psychiatry 81, 778–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C (2013). Dynorphin--still an extraordinarily potent opioid peptide. Mol. Pharmacol 83, 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Morón JA, Hope B, Rea W, and Shippenberg TS (2000). Kappa-opioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience 101, 619–627. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Bäckman CM, Gigante ED, and Shippenberg TS (2013). Kappa opioid receptors on dopaminergic neurons are necessary for kappa-mediated place aversion. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 38, 2623–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-W, Rada PV, Bützler BP, Leibowitz SF, and Hoebel BG (2012). Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience 206, 155–166. [DOI] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li C-Y, Belluzzi JD, Bonci A, and Civelli O (2009). The melanin-concentrating hormone system modulates cocaine reward. Proc. Natl. Acad. Sci. U. S. A 106, 6772–6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, and Massi M (2000). Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol 404, 153—159. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, and Massi M (2003). The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J. Neurosci. Off. J. Soc. Neurosci 23, 9445–9451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, de Guglielmo G, Hansson AC, Ubaldi M, Kallupi M, Cruz MT, Oleata CS, Heilig M, and Roberto M (2014). Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: significance for anxiety-like behaviors. J. Neurosci. Off. J. Soc. Neurosci 34, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Gavériaux-Ruff C, Matifas A, Caradec C, Champy M-F, and Kieffer BL (2006). Dissociation of analgesic and hormonal responses to forced swim stress using opioid receptor knockout mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 31, 1733–1744. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Jackson A, and Kirkham TC (1985). Endorphins and food intake: kappa opioid receptor agonists and hyperphagia. Pharmacol. Biochem. Behav 23, 889–901. [DOI] [PubMed] [Google Scholar]
- Corder G, Tawfik VL, Wang D, Sypek EI, Low SA, Dickinson JR, Sotoudeh C, Clark JD, Barres BA, Bohlen CJ, et al. (2017). Loss of μ opioid receptor signaling in nociceptors, but not microglia, abrogates morphine tolerance without disrupting analgesia. Nat. Med 23, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Castro DC, Bruchas MR, and Scherrer G (2018). Endogenous and Exogenous Opioids in Pain. Annu. Rev. Neurosci 41, 453–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ, Emson PC, and Heizmann CW (1990). Parvalbumin-containing GABAergic interneurons in the rat neostriatum. J. Comp. Neurol 302, 197–205. [DOI] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, and Lüscher C (2016). Convergence of Reinforcing and Anhedonic Cocaine Effects in the Ventral Pallidum. Neuron 92, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, Yu W, Schools ZL, Krashes MJ, et al. (2016). Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Rep. 14, 2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, and Roberto M (2012). Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol. Psychiatry 71, 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Ostlund SB, James AS, Park CS, Ge W, Roberts KW, Mittal N, Murphy NP, Cepeda C, Kieffer BL, et al. (2014). Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat. Neurosci 17, 254–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney G, Dawe KL, Hogan R, Hunjan T, Roper J, Hazell G, Lolait SJ, and Fulford AJ (2012). Role of nociceptin/orphanin FQ and NOP receptors in the response to acute and repeated restraint stress in rats. J. Neuroendocrinol 24, 1527–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, and Aston-Jones GS (1998). Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 806, 127–140. [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, and Steriade M (1997). Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol 53, 603–625. [DOI] [PubMed] [Google Scholar]
- Devine DP, Reinscheid RK, Monsma FJ, Civelli O, and Akil H (1996). The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res. 727, 225–229. [DOI] [PubMed] [Google Scholar]
- Devine DP, Hoversten MT, Ueda Y, and Akil H (2003). Nociceptin/orphanin FQ content is decreased in forebrain neurones during acute stress. J. Neuroendocrinol 15, 69–74. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, and Aronin N (1982). Ultrastructural features of immunoreactive somatostatin neurons in the rat caudate nucleus. J. Neurosci. Off. J. Soc. Neurosci 2, 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz GB, and Bittencourt JC (2017). The Melanin-Concentrating Hormone as an Integrative Peptide Driving Motivated Behaviors. Front. Syst. Neurosci 11, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue RJ, Landino SM, Golden SA, Carroll FI, Russo SJ, and Carlezon WA (2015). Effects of acute and chronic social defeat stress are differentially mediated by the dynorphin/kappa-opioid receptor system. Behav. Pharmacol 26, 654–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, and Chavkin C (1994). Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J. Neurosci. Off. J. Soc. Neurosci 14, 3736–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan AW (2000). Neuropeptide spread in the brain and spinal cord. Prog. Brain Res 125, 369–380. [DOI] [PubMed] [Google Scholar]
- Ehrich JM, Phillips PEM, and Chavkin C (2014). Kappa opioid receptor activation potentiates the cocaine-induced increase in evoked dopamine release recorded in vivo in the mouse nucleus accumbens. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 39, 3036–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich JM, Messinger DI, Knakal CR, Kuhar JR, Schattauer SS, Bruchas MR, Zweifel LS, Kieffer BL, Phillips PEM, and Chavkin C (2015). Kappa Opioid Receptor-Induced Aversion Requires p38 MAPK Activation in VTA Dopamine Neurons. J. Neurosci. Off. J. Soc. Neurosci 35, 12917–12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawaz CS, Martel P, Leo D, and Trudeau L-E (2009). Presynaptic action of neurotensin on dopamine release through inhibition of D(2) receptor function. BMC Neurosci. 10, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Labouèbe G, Liu S, Nieh EH, Routh VH, Xu S, and O’Connor EC (2016). Homeostasis Meets Motivation in the Battle to Control Food Intake. J. Neurosci. Off. J. Soc. Neurosci 36, 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gavériaux-Ruff C, Dierich A, LeMeur M, et al. (2000). Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet 25, 195–200. [DOI] [PubMed] [Google Scholar]
- Fino E, Vandecasteele M, Perez S, Saudou F, and Venance L (2018). Region-specific and state-dependent action of striatal GABAergic interneurons. Nat. Commun 9, 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ME, Chandra R, Menken MS, Larkin EJ, Nam H, Engeln M, Francis TC, and Lobo MK (2018). Dendritic remodeling of D1 neurons by RhoA/Rho-kinase mediates depression-like behavior. Mol. Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, and Lobo MK (2017). Emerging Role for Nucleus Accumbens Medium Spiny Neuron Subtypes in Depression. Biol. Psychiatry 81, 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk H, and Rogers GH (1979). The suppressant effects of naloxone on food and water intake in the rat. Behav. Neural Biol 26, 23–40. [DOI] [PubMed] [Google Scholar]
- Friedman A, Homma D, Gibb LG, Amemori K-I, Rubin SJ, Hood AS, Riad MH, and Graybiel AM (2015). A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict. Cell 161, 1320–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, Mailly P, De Bundel D, de Kerchove d’Exaerde A, Hervé D, Girault J-A, Valjent E, and Krieger P (2013). Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front. Neural Circuits 7, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavériaux-Ruff C, Karchewski LA, Hever X, Matifas A, and Kieffer BL (2008). Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur. J. Neurosci 27, 2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolaños CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, et al. (2005). The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J. Neurosci. Off. J. Soc. Neurosci 25, 2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR (1984). The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature 311, 461–464. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, and Young WS (1988). Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res. 460, 161–167. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Baimbridge KG, and Miller JJ (1985). The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc. Natl. Acad. Sci. U. S. A 82, 8780–8784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler TS, Chan CS, and Surmeier DJ (2008). Dichotomous anatomical properties of adult striatal medium spiny neurons. J. Neurosci. Off. J. Soc. Neurosci 28, 10814–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Nelson AB, Thwin MT, Palop JJ, and Kreitzer AC (2010). Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J. Neurosci. Off. J. Soc. Neurosci 30, 2223–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KK, and Smith Y (2015). Cholinergic interneurons in the dorsal and ventral striatum: anatomical and functional considerations in normal and diseased conditions. Ann. N. Y. Acad. Sci 1349, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Perrault G, and Sanger DJ (1999). Orphanin FQ, a novel neuropeptide with antistress-like activity. Brain Res. 836, 221–224. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, and Uylings HB (1997). The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J. Psychopharmacol. Oxf. Engl 11, 99–106. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, and McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. Off. J. Soc. Neurosci 20, 2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, and Berke JD (2016). Mesolimbic dopamine signals the value of work. Nat. Neurosci 19, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon EC, Baldo BA, Sadeghian K, and Kelley AE (2004). Increases in food intake or food-seeking behavior induced by GABAergic, opioid, or dopaminergic stimulation of the nucleus accumbens: is it hunger? Psychopharmacology (Berl.) 172, 241–247. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Schenk S, Partridge B, and Shippenberg TS (1998). Increased responsiveness of mesolimbic and mesostriatal dopamine neurons to cocaine following repeated administration of a selective kappa-opioid receptor agonist. Synap. N. Y. N 30, 255–262. [DOI] [PubMed] [Google Scholar]
- Henry MS, Bisht K, Vernoux N, Gendron L, Torres-Berrio A, Drolet G, and Tremblay M-È (2018). Delta Opioid Receptor Signaling Promotes Resilience to Stress Under the Repeated Social Defeat Paradigm in Mice. Front. Mol. Neurosci 11, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, and Pert CB (1980). In vitro autoradiography of opiate receptors in rat brain suggests loci of “opiatergic” pathways. Proc. Natl. Acad. Sci. U. S. A 77, 5532–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati M, Aleyasin H, Menard C, Christoffel DJ, Flanigan ME, Pfau ML, Hodes GE, Lepack AE, Bicks LK, Takahashi A, et al. (2018). Cell-type-specific role for nucleus accumbens neuroligin-2 in depression and stress susceptibility. Proc. Natl. Acad. Sci. U. S. A 115, 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmstad GO, and Fields HL (2001). Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J. Neurophysiol 85, 1153–1158. [DOI] [PubMed] [Google Scholar]
- Hjorth J, Blackwell KT, and Kotaleski JH (2009). Gap junctions between striatal fast-spiking interneurons regulate spiking activity and synchronization as a function of cortical activity. J. Neurosci. Off. J. Soc. Neurosci 29, 5276–5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Kalin NH, and Kelley AE (1997). Microinfusion of corticotropin-releasing factor into the nucleus accumbens shell results in increased behavioral arousal and oral motor activity. Psychopharmacology (Berl.) 130, 189–196. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Manvich DF, and Kuhar MJ (2010). Cocaine and amphetamine-regulated transcript-containing neurons in the nucleus accumbens project to the ventral pallidum in the rat and may inhibit cocaine-induced locomotion. Neuroscience 165, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, and Prescott TJ (2010). The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol 90, 385–417. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Kotani A, Koshikawa N, and Cools AR (2009). Somatostatin receptors in the nucleus accumbens modulate dopamine-dependent but not acetylcholine-dependent turning behaviour of rats. Neuroscience 159, 974–981. [DOI] [PubMed] [Google Scholar]
- Ikemoto S (2007). Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev 56, 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, and Aston-Jones G (2017). A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr. Top. Behav. Neurosci 33, 247281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, and Mark GP (2008). Injection of CART (cocaine-and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine selfadministration in rats. Behav. Brain Res 191, 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenck F, Moreau JL, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Nothacker HP, and Civelli O (1997). Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. U. S. A 94, 14854–14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuura Y, and Taha SA (2010). Modulation of feeding and locomotion through mu and delta opioid receptor signaling in the nucleus accumbens. Neuropeptides 44, 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DM, and Steinberg H (1972). Proceedings: Previous environment and responses to morphine. Br. J. Pharmacol 44, 350P. [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y (1993). Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. Off. J. Soc. Neurosci 13, 4908–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keebaugh AC, and Young LJ (2011). Increasing oxytocin receptor expression in the nucleus accumbens of pre-pubertal female prairie voles enhances alloparental responsiveness and partner preference formation as adults. Horm. Behav 60, 498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaimova E, Kandov Y, Israel Y, Cataldo G, Hadjimarkou MM, and Bodnar RJ (2004). Opioid receptor subtype antagonists differentially alter GABA agonist-induced feeding elicited from either the nucleus accumbens shell or ventral tegmental area regions in rats. Brain Res. 1026, 284–294. [DOI] [PubMed] [Google Scholar]
- Khavari KA, Peters TC, Baity PL, and Wilson AS (1975). Voluntary morphine ingestion, morphine dependence, and recovery from withdrawal signs. Pharmacol. Biochem. Behav 3, 1093–1096. [DOI] [PubMed] [Google Scholar]
- Kita H, Kosaka T, and Heizmann CW (1990). Parvalbumin-immunoreactive neurons in the rat neostriatum: a light and electron microscopic study. Brain Res. 536, 1–15. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, and Carlezon WA (2011). Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol. Psychiatry 70, 425433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolliker A (1986). Handbuch der Gewebelechre des Menchen (Kengleman).
- Kombian SB, Ananthalakshmi KVV, Parvathy SS, and Matowe WC (2003). Substance P depresses excitatory synaptic transmission in the nucleus accumbens through dopaminergic and purinergic mechanisms. J. Neurophysiol 89, 728–737. [DOI] [PubMed] [Google Scholar]
- Köster A, Montkowski A, Schulz S, Stübe EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, and Reinscheid RK (1999). Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc. Natl. Acad. Sci. U. S. A 96, 10444–10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaleski JH, Plenz D, and Blackwell KT (2006). Using potassium currents to solve signal-to-noise problems in inhibitory feedforward networks of the striatum. J. Neurophysiol 95, 331341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani A, Ikeda H, Koshikawa N, and Cools AR (2008). Role of orexin receptors in the nucleus accumbens in dopamine-dependent turning behaviour of rats. Neuropharmacology 54, 613–619. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Mikawa S, and Kawaguchi Y (1993). Neostriatal GABAergic interneurones contain NOS, calretinin or parvalbumin. Neuroreport 5, 205–208. [DOI] [PubMed] [Google Scholar]
- Kumar R, Steinberg H, and Stolerman IP (1968). Inducing a preference for morphine in rats without premedication. Nature 218, 564–565. [DOI] [PubMed] [Google Scholar]
- Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, and Kalivas PW (2015). Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat. Neurosci 18, 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladurelle N, Durieux C, Roques BP, and Daugé V (1994). Different modifications of the dopamine metabolism in the core and shell parts of the nucleus accumbens following CCK-A receptor stimulation in the shell region. Neurosci. Lett 178, 5–10. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, and Chavkin C (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. Off. J. Soc. Neurosci 28, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, and Chavkin C (2009). Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc. Natl. Acad. Sci. U. S. A 106, 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landwehrmeyer B, Mengod G, and Palacios JM (1993). Differential visualization of dopamine D2 and D3 receptor sites in rat brain. A comparative study using in situ hybridization histochemistry and ligand binding autoradiography. Eur. J. Neurosci 5, 145–153. [DOI] [PubMed] [Google Scholar]
- Le Moine C, and Bloch B (1996). Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with the D1 and D2 dopamine receptors. Neuroscience 73, 131–143. [DOI] [PubMed] [Google Scholar]
- Lee J, Finkelstein J, Choi JY, and Witten IB (2016). Linking Cholinergic Interneurons, Synaptic Plasticity, and Behavior during the Extinction of a Cocaine-Context Association. Neuron 90, 1071–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Wegner SA, Yu JH, Mototake A, Hu B, and Hopf FW (2016). Nucleus Accumbens Shell and mPFC but Not Insula Orexin-1 Receptors Promote Excessive Alcohol Drinking. Front. Neurosci 10, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz S, Perney TM, Qin Y, Robbins E, and Chesselet MF (1994). GABA-ergic interneurons of the striatum express the Shaw-like potassium channel Kv3.1. Synap. N. Y. N 18, 55–66. [DOI] [PubMed] [Google Scholar]
- Liu Y, and Wang ZX (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. [DOI] [PubMed] [Google Scholar]
- Lopez-Huerta VG, Tecuapetla F, Guzman JN, Bargas J, and Galarraga E (2008). Presynaptic modulation by somatostatin in the neostriatum. Neurochem. Res 33, 1452–1458. [DOI] [PubMed] [Google Scholar]
- Lopez-Huerta VG, Nakano Y, Bausenwein J, Jaidar O, Lazarus M, Cherassse Y, Garcia-Munoz M, and Arbuthnott G (2016). The neostriatum: two entities, one structure? Brain Struct. Funct 221, 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Do T, and Maidment NT (2001). Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl.) 154, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Feng Q, Ouyang L, Mu S, Liu B, Li Y, Chen S, and Lei W (2014). Morphological diversity of GABAergic and cholinergic interneurons in the striatal dorsolateral and ventromedial regions of rats. Cell. Mol. Neurobiol 34, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y-Y, Cepeda C, Chatta P, Franklin L, Evans CJ, and Levine MS (2012). Regional and cell-type-specific effects of DAMGO on striatal D1 and D2 dopamine receptor-expressing medium-sized spiny neurons. ASN Neuro 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, and Berridge KC (2009). Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J. Neurosci. Off. J. Soc. Neurosci 29, 6500–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailly P, Aliane V, Groenewegen HJ, Haber SN, and Deniau J-M (2013). The rat prefrontostriatal system analyzed in 3D: evidence for multiple interacting functional units. J. Neurosci. Off. J. Soc. Neurosci 33, 5718–5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaligas AA, and Ford CP (2016). Spontaneous Synaptic Activation of Muscarinic Receptors by Striatal Cholinergic Neuron Firing. Neuron 91, 574–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, and Watson SJ (1994). Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol 350, 412–438. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, and Shaham Y (2016). Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 335–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marche K, and Apicella P (2017). Changes in activity of fast-spiking interneurons of the monkey striatum during reaching at a visual target. J. Neurophysiol 117, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, and Bruijnzeel AW (2009). Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 34, 1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall FH, Barnes S, Hughes J, Woodruff GN, and Hunter JC (1991). Cholecystokinin modulates the release of dopamine from the anterior and posterior nucleus accumbens by two different mechanisms. J. Neurochem 56, 917–922. [DOI] [PubMed] [Google Scholar]
- Martínez-Rivera FJ, Rodriguez-Romaguera J, Lloret-Torres ME, Do Monte FH, Quirk GJ, and Barreto-Estrada JL (2016). Bidirectional Modulation of Extinction of Drug Seeking by Deep Brain Stimulation of the Ventral Striatum. Biol. Psychiatry 80, 682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matamales M, Götz J, and Bertran-Gonzalez J (2016). Quantitative Imaging of Cholinergic Interneurons Reveals a Distinctive Spatial Organization and a Functional Gradient across the Mouse Striatum. PloS One 11, e0157682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, et al. (1996). Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823. [DOI] [PubMed] [Google Scholar]
- Mattison J (1891). The Treatment of the Morphine-Disease. Indian Med. Gaz 26, 65–68. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, and Chavkin C (2006). Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 31, 1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, and Baldo BA (2011). Induction of hyperphagia and carbohydrate intake by μ-opioid receptor stimulation in circumscribed regions of frontal cortex. J. Neurosci. Off. J. Soc. Neurosci 31, 3249–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Blank B, and Groenewegen HJ (1989). The distribution and compartmental organization of the cholinergic neurons in nucleus accumbens of the rat. Neuroscience 31, 327345. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Agolia R, Arts MP, Groenewegen HJ, and Zahm DS (1992). Morphological differences between projection neurons of the core and shell in the nucleus accumbens of the rat. Neuroscience 50, 149–162. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, and Kelley AE (2008). The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct 213, 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro P, Barak B, Zhou Y, McRae R, Rodrigues D, Wickersham IR, and Feng G (2018). Dichotomous parvalbumin interneuron populations in dorsolateral and dorsomedial striatum. J. Physiol 596, 3695–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, and Levine AS (1983). Involvement of dynorphin and the kappa opioid receptor in feeding. Peptides 4, 797–800. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Ly HT, and Maidment NT (1996). Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience 75, 1–4. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, and Maidment NT (1999). Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 832, 168–170. [DOI] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, and De Felipe C (2000). Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature 405, 180–183. [DOI] [PubMed] [Google Scholar]
- Nation KM, De Felice M, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, and Porreca F (2018). Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain 159, 919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, and Domesick VB (1978). Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience 3, 385–401. [DOI] [PubMed] [Google Scholar]
- Nazzaro C, Barbieri M, Varani K, Beani L, Valentino RJ, and Siniscalchi A (2010). Swim stress enhances nociceptin/orphanin FQ-induced inhibition of rat dorsal raphe nucleus activity in vivo and in vitro: role of corticotropin releasing factor. Neuropharmacology 58, 457464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hammack N, Yang CF, Shah NM, Seal RP, and Kreitzer AC (2014). Striatal cholinergic interneurons Drive GABA release from dopamine terminals. Neuron 82, 6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevárez N, and de Lecea L (2018). Recent advances in understanding the roles of hypocretin/orexin in arousal, affect, and motivation. F1000Research 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, and Dani JA (2018). Inhibitory Plasticity of Mesocorticolimbic Circuits in Addiction and Mental Illness. Trends Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Berke JD, and Kreitzer AC (2018). Fast-Spiking Interneurons Supply Feedforward Control of Bursting, Calcium, and Plasticity for Efficient Learning. Cell 172, 683–695.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, Luijendijk MCM, Vanderschuren LJMJ, la Fleur SE, and Adan RAH (2014). Limbic substrates of the effects of neuropeptide Y on intake of and motivation for palatable food. Obes. Silver Spring Md 22, 1216–1219. [DOI] [PubMed] [Google Scholar]
- Pandit R, van der Zwaal EM, Luijendijk MCM, Brans MAD, van Rozen AJ, Oude Ophuis RJA, Vanderschuren LJMJ, Adan RAH, and la Fleur SE (2015). Central melanocortins regulate the motivation for sucrose reward. PloS One 10, e0121768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F, Kieffer BL, Tabarin A, and Contarino A (2007). Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur. J. Neurosci 25, 3398–3405. [DOI] [PubMed] [Google Scholar]
- Parker JG, Marshall JD, Ahanonu B, Wu Y-W, Kim TH, Grewe BF, Zhang Y, Li JZ, Ding JB, Ehlers MD, et al. (2018). Diametric neural ensemble dynamics in parkinsonian and dyskinetic states. Nature 557, 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker KE, Johns HW, Floros TG, and Will MJ (2014). Central amygdala opioid transmission is necessary for increased high-fat intake following 24-h food deprivation, but not following intra-accumbens opioid administration. Behav. Brain Res. 260, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, and Watson C (1998). The rat brain atlas in stereotaxic coordinates (San Dieogo Acad; ). [Google Scholar]
- Paxinos G, and Watson C (2007). The rat brain in stereotaxic coordinates (Amst; ). [DOI] [PubMed] [Google Scholar]
- Peciña S, and Berridge KC (2000). Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res. 863, 71–86. [DOI] [PubMed] [Google Scholar]
- Peciña S, and Berridge KC (2005). Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci. Off. J. Soc. Neurosci 25, 11777–11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peciña S, Schulkin J, and Berridge KC (2006). Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 4, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, and Snyder SH (1976). Opiate receptor: autoradiographic localization in rat brain. Proc. Natl. Acad. Sci. U. S. A 73, 3729–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps PE, and Vaughn JE (1986). Immunocytochemical localization of choline acetyltransferase in rat ventral striatum: a light and electron microscopic study. J. Neurocytol 15, 595–617. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, and Vaughn JE (1985). Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J. Comp. Neurol 238, 286–307. [DOI] [PubMed] [Google Scholar]
- Planert H, Szydlowski SN, Hjorth JJJ, Grillner S, and Silberberg G (2010). Dynamics of synaptic transmission between fast-spiking interneurons and striatal projection neurons of the direct and indirect pathways. J. Neurosci. Off. J. Soc. Neurosci 30, 3499–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Zyuzin J, and Charles A (2014). δ-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br. J. Pharmacol 171, 2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi K, Wei C, Li Y, and Sui N (2013). Orexin receptors within the nucleus accumbens shell mediate the stress but not drug priming-induced reinstatement of morphine conditioned place preference. Front. Behav. Neurosci 7, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros IMH, Macedo GC, Domingues LP, and Favoretto CA (2016). An Update on CRF Mechanisms Underlying Alcohol Use Disorders and Dependence. Front. Endocrinol 7, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnauth A, Moroz M, and Bodnar RJ (2000). Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 876, 76–87. [DOI] [PubMed] [Google Scholar]
- Ramos-Ortolaza DL, Negrón A, Cruz D, Falcón E, Iturbe MC, Cajigas MH, and Maldonado-Vlaar CS (2009). Intra-accumbens shell injections of SR48692 enhanced cocaine self-administration intake in rats exposed to an environmentally-elicited reinstatement paradigm. Brain Res. 1280, 124–136. [DOI] [PubMed] [Google Scholar]
- Reed SJ, Lafferty CK, Mendoza JA, Yang AK, Davidson TJ, Grosenick L, Deisseroth K, and Britt JP (2018). Coordinated Reductions in Excitatory Input to the Nucleus Accumbens Underlie Food Consumption. Neuron. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, and Civelli O (2002). The orphanin FQ/nociceptin knockout mouse: a behavioral model for stress responses. Neuropeptides 36, 72–76. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, and Aragona BJ (2012). κ-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J. Neurosci. Off. J. Soc. Neurosci 32, 6771–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Dome M, Gormley G, Franco D, Nevárez N, Hamid AA, and Aragona BJ (2013). μ-Opioid receptors within subregions of the striatum mediate pair bond formation through parallel yet distinct reward mechanisms. J. Neurosci. Off. J. Soc. Neurosci 33, 91409149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revitsky AR, and Klein LC (2013). Role of ghrelin in drug abuse and reward-relevant behaviors: a burgeoning field and gaps in the literature. Curr. Drug Abuse Rev 6, 231–244. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, and Berridge KC (2001). Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J. Neurosci. Off. J. Soc. Neurosci 21, 3261–3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, and Berridge KC (2008). Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat. Neurosci 11, 423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro EA, Salery M, Scarpa JR, Calipari ES, Hamilton PJ, Ku SM, Kronman H, Purushothaman I, Juarez B, Heshmati M, et al. (2018). Transcriptional and physiological adaptations in nucleus accumbens somatostatin interneurons that regulate behavioral responses to cocaine. Nat. Commun 9, 3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, and Berridge KC (2011). Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J. Neurosci. Off. J. Soc. Neurosci 31, 12866–12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, and Berridge KC (2013). Prefrontal cortex modulates desire and dread generated by nucleus accumbens glutamate disruption. Biol. Psychiatry 73, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Castro DC, Difeliceantonio AG, Robinson MJF, and Berridge KC (2013). Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neurosci. Biobehav. Rev 37, 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, and Berridge KC (2013). Instant transformation of learned repulsion into motivational “wanting.” Curr. Biol. CB 23, 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FHM, and Quirk GJ (2012). Deep brain stimulation of the ventral striatum enhances extinction of conditioned fear. Proc. Natl. Acad. Sci. U. S. A 109, 8764–8769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, and Carelli RM (2005). Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45, 587–597. [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, and Sadikot AF (2004). Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J. Comp. Neurol 469, 325–339. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Margolis EB, and Janak PH (2018). Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci 21, 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Nelson BS, Damadzic R, Tapocik JD, Yao M, King CE, Rowe KE, Cheng K, Rice KC, and Heilig M (2015). Neurokinin-1 receptor antagonism attenuates neuronal activity triggered by stress-induced reinstatement of alcohol seeking. Neuropharmacology 99, 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildein S, Agmo A, Huston JP, and Schwarting RK (1998). Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 790, 185–194. [DOI] [PubMed] [Google Scholar]
- Shimo Y, and Hikosaka O (2001). Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement. J. Neurosci. Off. J. Soc. Neurosci 21, 7804–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama G-I, Kawahara R, and Duman RS (2004). Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J. Neurochem 90, 1258–1268. [DOI] [PubMed] [Google Scholar]
- Singh J, and Desiraju T (1988). Differential effects of opioid peptides administered intracerebrally in loci of self-stimulation reward of lateral hypothalamus and ventral tegmental area--substantia nigra. NIDA Res. Monogr 87, 180–191. [PubMed] [Google Scholar]
- Smith KS, and Berridge KC (2007). Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J. Neurosci. Off. J. Soc. Neurosci 27, 1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJW, Ratnaseelan AM, and Veenema AH (2018). Robust age, but limited sex, differences in mu-opioid receptors in the rat brain: relevance for reward and drug-seeking behaviors in juveniles. Brain Struct. Funct 223, 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JB, Klug JR, Ross DL, Howard CD, Hollon NG, Ko VI, Hoffman H, Callaway EM, Gerfen CR, and Jin X (2016). Genetic-Based Dissection Unveils the Inputs and Outputs of Striatal Patch and Matrix Compartments. Neuron 91, 1069–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Andrieux M, Besancon R, Pilon C, Bouthenet ML, Souil E, and Schwartz JC (1992a). Localization and function of the D3 dopamine receptor. Arzneimittelforschung. 42, 224–230. [PubMed] [Google Scholar]
- Sokoloff P, Andrieux M, Besançon R, Pilon C, Martres MP, Giros B, and Schwartz JC (1992b). Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor. Eur. J. Pharmacol 225, 331–337. [DOI] [PubMed] [Google Scholar]
- Sørensen AT, Nikitidou L, Ledri M, Lin E-JD, During MJ, Kanter-Schlifke I, and Kokaia M (2009). Hippocampal NPY gene transfer attenuates seizures without affecting epilepsy-induced impairment of LTP. Exp. Neurol 215, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, and Phillips AG (1983). Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology (Berl.) 79, 278–283. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Holahan MR, and Kelley AE (1997). Injections of nociceptin into nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. Neuroreport 8, 423–426. [DOI] [PubMed] [Google Scholar]
- Straub C, Saulnier JL, Bègue A, Feng DD, Huang KW, and Sabatini BL (2016). Principles of Synaptic Organization of GABAergic Interneurons in the Striatum. Neuron 92, 8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Uhl GR, and Pickel VM (1996). Ultrastructural immunocytochemical localization of mu-opioid receptors in rat nucleus accumbens: extrasynaptic plasmalemmal distribution and association with Leu5-enkephalin. J. Neurosci. Off. J. Soc. Neurosci 16, 4162–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, and Pickel VM (1998). Cellular sites for activation of delta-opioid receptors in the rat nucleus accumbens shell: relationship with Met5-enkephalin. J. Neurosci. Off. J. Soc. Neurosci 18, 1923–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Colago EE, and Pickel VM (1999). Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J. Neurosci. Off. J. Soc. Neurosci 19, 1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha SA, and Fields HL (2005). Encoding of palatability and appetitive behaviors by distinct neuronal populations in the nucleus accumbens. J. Neurosci. Off. J. Soc. Neurosci 25, 11931202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AH, and Collins RJ (1985). Behavioral effects of a novel kappa opioid analgesic, U-50488, in rats and rhesus monkeys. Psychopharmacology (Berl.) 85, 309–314. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Carreira MB, Cooper YA, Bobadilla A-C, Heinsbroek JA, Koike N, Larson EB, Balmuth EA, Hughes BW, Penrod RD, et al. (2017). HDAC5 and Its Target Gene, Npas4, Function in the Nucleus Accumbens to Regulate Cocaine-Conditioned Behaviors. Neuron 96, 130–144.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Koós T, Tepper JM, Kabbani N, and Yeckel MF (2009). Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J. Neurosci. Off. J. Soc. Neurosci 29, 8977–8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Wu J, Kornspun AR, Pignatelli M, Kashtelyan V, Krashes MJ, Lowell BB, Carlezon WA, and Bonci A (2017). Pathway- and Cell-Specific Kappa-Opioid Receptor Modulation of Excitation-Inhibition Balance Differentially Gates D1 and D2 Accumbens Neuron Activity. Neuron 93, 147–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Wilson CJ, and Koós T (2008). Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res. Rev 58, 272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koós T, and Ibáñez-Sandoval O (2010). Heterogeneity and diversity of striatal GABAergic interneurons. Front. Neuroanat 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe AJ, and Kotz CM (2005). Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res. 1050, 156–162. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, and Cragg SJ (2012). Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Toll L, Bruchas MR, Calo’ G, Cox BM, and Zaveri NT (2016). Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev 68, 419–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-DeRose J, Stauber G, Khroyan TV, Xie XS, Zaveri NT, and Toll L (2013). Retrodialysis of N/OFQ into the nucleus accumbens shell blocks cocaine-induced increases in extracellular dopamine and locomotor activity. Eur. J. Pharmacol 699, 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Thibault M, Kaska S, Cooper S, Gajewski P, Eagle A, Mazei-Robison M, Nestler EJ, and Robison AJ (2015). Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 99, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, and Johansson O (1983). Striatal neurons containing both somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivities and NADPH-diaphorase activity: a light and electron microscopic study. J. Comp. Neurol 217, 264–270. [DOI] [PubMed] [Google Scholar]
- Volkow N, Benveniste H, and McLellan AT (2018). Use and Misuse of Opioids in Chronic Pain. Annu. Rev. Med 69, 451–465. [DOI] [PubMed] [Google Scholar]
- Vuillet J, Dimova R, Nieoullon A, and Kerkerian-Le Goff L (1992). Ultrastructural relationships between choline acetyltransferase- and neuropeptide y-containing neurons in the rat striatum. Neuroscience 46, 351–360. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, and Zubieta J-K (2007). Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U. S. A 104, 11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, and Zhan C (2015). Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat 9, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasilewska B, Robak A, Równiak M, Bogus-Nowakowska K, Najdzion J, Zakowski W, and Majewski M (2011). Distribution and chemical coding pattern of somatostatin immunoreactivity in the dorsal striatum of the guinea pig. Folia Histochem. Cytobiol 49, 690699. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT, and Balleine BW (2009). Distinct opioid circuits determine the palatability and the desirability of rewarding events. Proc. Natl. Acad. Sci. U. S. A 106, 12512–12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Balleine BW, and Maidment NT (2011). Micro-opioid receptor activation in the basolateral amygdala mediates the learning of increases but not decreases in the incentive value of a food reward. J. Neurosci. Off. J. Soc. Neurosci 31, 1591–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, and Koob GF (2010). The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl.) 210, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel R, Reiss D, Karchewski L, Gardon O, Matifas A, Filliol D, Becker JAJ, Wood JN, Kieffer BL, and Gaveriaux-Ruff C (2013). Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice. PloS One 8, e74706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Kitai ST, and Linder JC (1983). Three-dimensional structure of dendritic spines in the rat neostriatum. J. Neurosci. Off. J. Soc. Neurosci 3, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerke J, Schetters D, van Es IE, van Mourik Y, den Hollander BRO, Schoffelmeer ANM, and Pattij T (2011). μ-Opioid receptors in the nucleus accumbens shell region mediate the effects of amphetamine on inhibitory control but not impulsive choice. J. Neurosci. Off. J. Soc. Neurosci 31, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin S-C, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, and Deisseroth K (2010). Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, and Groenewegen HJ (1996). Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience 73, 359–373. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, and Groenewegen HJ (1996). Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J. Neurosci. Off. J. Soc. Neurosci 16, 1877–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Inokawa H, Hori Y, Pan X, Matsuzaki R, Nakamura K, Samejima K, Shidara M, Kimura M, Sakagami M, et al. (2016). Characteristics of fast-spiking neurons in the striatum of behaving monkeys. Neurosci. Res 105, 2–18. [DOI] [PubMed] [Google Scholar]
- Yang S-C, Shieh K-R, and Li H-Y (2005). Cocaine- and amphetamine-regulated transcript in the nucleus accumbens participates in the regulation of feeding behavior in rats. Neuroscience 133, 841–851. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Yaksh TL, and Rudy TA (1977). Concurrent mapping of brain sites for sensitivity to the direct application of morphine and focal electrical stimulation in the production of antinociception in the rat. Pain 4, 23–40. [DOI] [PubMed] [Google Scholar]
- Yu CJ, Zhang SW, and Tai FD (2016). Effects of nucleus accumbens oxytocin and its antagonist on social approach behavior. Behav. Pharmacol 27, 672–680. [DOI] [PubMed] [Google Scholar]
- Záborszky L, Alheid GF, Beinfeld MC, Eiden LE, Heimer L, and Palkovits M (1985). Cholecystokinin innervation of the ventral striatum: a morphological and radioimmunological study. Neuroscience 14, 427–453. [DOI] [PubMed] [Google Scholar]
- Zahm DS (1992). An electron microscopic morphometric comparison of tyrosine hydroxylase immunoreactive innervation in the neostriatum and the nucleus accumbens core and shell. Brain Res. 575, 341–346. [DOI] [PubMed] [Google Scholar]
- Zhang M, and Kelley AE (1997). Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl.) 132, 350–360. [DOI] [PubMed] [Google Scholar]
- Zhou F-M, Wilson CJ, and Dani JA (2002). Cholinergic interneuron characteristics and nicotinic properties in the striatum. J. Neurobiol 53, 590–605. [DOI] [PubMed] [Google Scholar]