Abstract
Purpose
There is no prevention or treatment for diabetic retinal neurodegeneration (DRN), which is a complication of diabetes that can occur independently of diabetic retinopathy (DR). We hypothesized that an intravitreal fluocinolone acetonide (FAc) implant may affect the rate of DRN when used in patients with diabetic macular edema (DME).
Methods
In this retrospective analysis, optical coherence tomography with neuroretinal analysis was obtained at 3-month intervals from 130 patients in the USER study both before (mean duration 903 days, range 35–4005 days) and after administration of FAc (mean 408 days, range 7 to 756 days). The rate of DRN was defined as the change over time on inner neuroretinal thickness using logistic regression. A DRN rate was calculated independently for two areas: region 1 located within 1.5 mm of the fovea, and region 2 from 1.5 mm to 3.0 mm from the fovea.
Results
In regions of the macula more than 1.5 mm from the central fovea, there was a statistically significant decrease in the rate of DRN in the post-FAc period. The pre-FAc neuroretinal loss in this area occurred at 4.0 μm/y, compared with a post-FAc loss rate of 1.1 μm/y (P = 0.001).
Conclusions
This retrospective study suggests that FAc may decelerate the rate of inner retinal thinning in patients with persistent DME. Further prospective studies are necessary to determine the effects of FAc on the rate of DRN in patients with DME.
Keywords: diabetic neuroretinal degeneration, nerve fiber layer, ganglion cell layer, inner plexiform layer, fluocinolone acetonide (FAc) implant
Increasing evidence suggests that diabetic retinal neurodegeneration (DRN) precedes the vascular changes that clinically define nonproliferative diabetic retinopathy (NPDR). Neuroretinal thinning has been documented in eyes with no or minimal NPDR in patients with type 1 and type 2 diabetes mellitus (DM)1–6 and correlates with visual function deficits.7 In a study of 45 patients with no to minimal NPDR, high-resolution optical coherence tomography (OCT) demonstrated thinning of the retinal nerve fiber layer (NFL) and ganglion cell/inner plexiform layer (GCL/IPL) occurring at rates of 0.25 μm/y and 0.29 μm/y, respectively.8 The rate of NFL thinning from aging alone has been estimated at 0.13 to 0.24 μm/y in healthy eyes.9,10 In donor eyes from humans with DM presenting an in vitro capillary density identical to control donor eyes, the NFL is statistically significantly thinner (Garmager A, et al. IOVS 2015;56:ARVO E-Abstract 4707). These findings have been supported by in vivo studies of type 1 and type 2 DM mouse models, which showed NFL and GCL thinning in the absence of pericyte loss (Jiao C, et al. IOVS 2015;56:ARVO E-Abstract 4708). In humans with type 1 DM and no to minimal NPDR, the defects on Humphrey 24-2 visual fields and diminished multifocal electroretinogram implicit times correlated with future development of microvasculopathy at corresponding retinal loci.11,12 Additionally, diabetic patients without retinopathy have decreased visual function within the central 4 degrees of the macula compared with nondiabetic patients.13
It is unclear whether neurodegeneration and microvasculopathy are causally linked or are independent manifestations of DM. Regardless, the ideal treatment for diabetic retinal disease may be intervention at the earliest detectable stage of neurovascular compromise. In rats with streptozotocin-induced DM, topical administration of somatostatin for 3 weeks resulted in relative preservation of retinal structure and function.14 In a large placebo-controlled trial of humans with type 2 DM, both topical brimonidine and somatostatin demonstrated benefit in a subset of patients affected by DRN. Unfortunately, no current therapies approved by the Food and Drug Administration are available to prevent DRN in human eyes.
Intravitreal steroid delivery has been shown to decrease or eliminate diabetic macular edema.15,16 Steroids are thought to decrease inflammatory cytokines, which have been implicated in diabetic macular edema (DME). These cytokines include interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1, interferon gamma-induced protein-10, and VEGF.17,18 Additionally, natural and synthetic steroids may have neuroprotective effects. In certain neurodegenerative disorders, such as Alzheimer's disease and Parkinson's disease, cell death occurs due to a multifactorial process involving oxidative stress, mitochondrial dysfunction, excitotoxicity, and neuroinflammation. In rats, levels of advanced glycation end-products (diabetic byproducts associated with neuropathy) correlated with elevated systemic TNF-α and IL-6.19 In studies of spinal cord contusion, rats treated with estradiol, dihydrotestosterone, or a combination of both, have shown a reduction of motor neuron dendritic damage compared with placebo.20 They have also demonstrated prevention of membrane cell damage and apoptosis when injured spinal cord tissue is exposed to progesterone or allopregnanolone.21
In two separate rat models of retinal neurodegeneration, the corticosteroid fluocinolone acetonide demonstrated neuroprotective effects as measured by histologic analysis and electroretinograms.22,23 The mechanisms by which steroids interact with neuronal cell lines are complex and remain to be elucidated in full. Nevertheless, given the potential of steroids for modulating neurodegeneration, we hypothesized that steroid treatment with fluocinolone acetonide (FAc) for DME might also alter the rate of progression of DRN, if, like DME, neurodegeneration also has an inflammatory component. Among the steroids approved to treat DME, FAc is unique in that it is difluorinated and penetrates the retina extremely well. The FAc implant (ILUVIEN; Alimera Sciences, Inc., Alpharetta, GA, USA) contains 0.19 mg of embedded active drug, and its delivery technology provides a consistent, low-dose therapy over a 3-year span. In the U.S. Retrospective Chart Review in Patients Receiving ILUVIEN (USER) study, patients with type 1 or 2 diabetes received an FAc implant for treatment of DME.24 At 36 months postinjection, the steady-state aqueous concentration ranged from 0.5 to 1.0 ng/mL, or approximately one-quarter to one-half of the mean concentration at 1 month after injection.25
In the USER study, the subgroup of patients with the greatest reduction in treatment frequency was the group with the best baseline visual acuity (VA) (≥20/40). VA was maintained post-0.2 μg/d FAc administration with a significantly reduced treatment frequency from one treatment every 2.9 months to one treatment every 22.0 months (P < 0.001). This trend continued in the intermediate subgroups: in the <20/40 to 20/100 VA subgroup, treatment frequency was reduced from once every 3.2 months to every 15.2 months (P < 0.001), and in the <20/100 to 20/200 VA subgroup, from once every 2.3 months to every 7.0 months (P < 0.001). In the subgroup of patients with the worst baseline VA (<20/200), frequency declined from once every 2.9 months to every 6.7 months (P = 0.026).24 Data from this study suggest that FAc offers a long-term adjunctive treatment to reduce injection burden for patients with DME.
The purpose of this retrospective analysis was to determine if there was a difference in the rate of DRN before and after treatment with FAc among 120 eyes (N = 130 patients) enrolled in the USER study. DRN was defined as thinning of the neuroretinal layers as determined by automated OCT with neuroretinal analysis, that is, automated segmentation to measure the combined thickness of the NFL and GCL/IPL. Furthermore, the aim was to investigate the rate of DRN before and after treatment with FAc at different distances from the foveal center.
Methods
Patients
The USER study was a multicenter, retrospective chart review conducted in the United States at four clinical sites: Cincinnati Eye Institute in Ohio, Georgia Retina in Georgia, Retina Health Institute in Florida, and Southern Eye in Mississippi. The study adhered to the guidelines of the Declaration of Helsinki, and the protocol and consent form were approved by an institutional review board. Each subject provided written informed consent. Patients must have received FAc before January 1, 2016, for the treatment of DME in at least one eye. USER collected all available data up to 3 years before and after FAc treatment. These data included macular images obtained with spectral-domain OCT (SD-OCT), performed with either Cirrus (Carl Zeiss Meditec, Inc., Jena, Germany) or Heidelberg (Heidelberg Engineering, Heidelberg, Germany) machines. At each visit, we collected all available data regarding measurement of VA, IOP, adverse events, and any administered treatments for DME, whether FAc or another treatment (i.e., focal laser, anti-VEGF injection, and all forms of sub-Tenon or intravitreal steroid administration). Eyes with better baseline VA scores were treated less frequently post-0.2 μg/d FAc administration than eyes with poorer baseline VA, and this reduction of treatment burden post-FAc occurred in a “dose-response type” fashion with regard to baseline VA.24
OCT Imaging and Segmentation
The Iowa Reference Algorithm (Retinal Image Analysis Lab, Iowa Institute for Biomedical Imaging, Iowa City, IA, USA) is a validated method for calculating GCL/IPL and NFL thickness in patients with type 1 diabetes and minimal diabetic retinopathy (DR).26 For all available SD-OCT images, the central subfield thickness (CST) was recorded. OCT images were segmented using the Iowa Reference Algorithm. Inner neuroretinal thickness was defined as the combined thickness of the NFL, GCL, and IPL, and was determined for all eyes from approximately 3 years before FAc up to 16 months afterward. The macula was divided into two regions of interest depending on distance from the foveal center (Fig. 1): region 1 being within 1.5 mm of the fovea, and region 2 from 1.5 mm to 3.0 mm from the fovea. At each visit, OCT data were acquired using either a Zeiss or Heidelberg OCT imaging platform. We corrected for differences in thickness measurements between platforms. For images obtained using the Zeiss Cirrus machine, we multiplied the raw thickness values by 99.5%; for images obtained using the Heidelberg Spectralis machine, we multiplied by 97.2%.27
Figure 1.
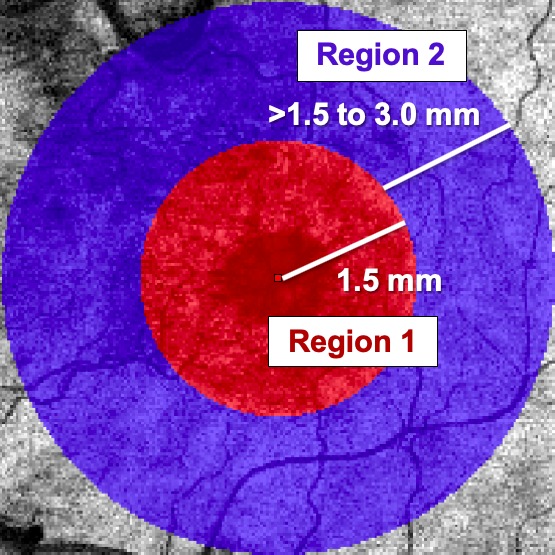
Regions of interest within the macula. Red circle indicates region of interest 1; blue ring indicates region of interest 2. The peripheral radius of each region is indicated by the distance in millimeters from the foveola. The red square indicates the foveola.
Iowa Reference Algorithm: Validation of Inner Neuroretinal Segmentation in DME
We modified the standard Iowa Reference Algorithm to follow the correct boundaries in the case of disorganization of the inner retinal layers and macular edema.28 The standard Iowa Reference Algorithm analyzes macular OCT scans at low resolution. Although this permits a fast, memory-efficient approach, it limits the ability to segment disrupted neuroretinal layers. Although the new method requires more processing time and memory, given the higher resolution at which the OCT scans are analyzed, more accurate layer segmentation is possible. Additionally, the modified algorithm uses wider smoothness constraints, allowing the boundary transitions to be detected even when the neuroretinal layers having large variation between two adjacent A-scans. In a pilot study, the original as well as modified Iowa Reference Algorithm was compared with manual OCT segmentation of 100 scans with macular edema due to retinal vascular disease by certified graders of the Vienna Reading Center. Compared with manual tracings, the mean unsigned and signed errors of automated retinal NFL thickness decreased from 6.88 ± 8.94 μm and 6.82 ± 8.99 μm to 3.12 ± 4.21 μm and 1.54 ± 5.02 μm. Those of GCL/IPL thickness decreased from 2.94 ± 4.14 μm and −2.27 ± 4.54 μm to 2.45 ± 3.47 μm and −0.59 ± 4.21 μm.29,30
Statistical Analysis
For each macular region of interest, absolute change in inner neuroretinal thickness over time was modeled as a mixed effect logistic regression. The patient was treated as a grouping factor to correct the clustering effect of repeated measurements per patient, and age and sex were additional fixed effects. The model equation was as follows:
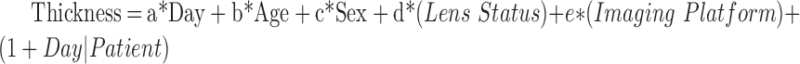 |
Multivariate regression was used to plot thickness in microns as a function of days after FAc injection at each of the two macular regions. Using ANOVA, we tested the difference between the pre- and posttreatment slope (i.e., the rate of neuroretinal thinning) in the two regions. The null hypothesis was that the slopes, or rates, were constant over the pretreatment and posttreatment periods and at each macular region. To address the potential confounder of lens status, which could affect OCT segmentation by change in magnification, we adjusted for subjects' lens status on study day 0 according to methods described by Darma et al.29
Results
Of the 130 patients (Table) from which data were collected, 160 eyes had received treatment with FAc. The mean age of the population was 69.6 years; 46% were females; 89% were Caucasian; 12.3% had type 1 DM; 68.8% were pseudophakic; and for the entire group, the mean hemoglobin A1C (HbA1C) was 7.07%. The mean follow-up time after FAc administration was 13.2 months, with 102 eyes (63.8%) followed for at least 6 months, 92 eyes (57.5%) for at least 12 months, and 50 eyes (31.2%) for at least 18 months. The mean pretreatment period for which data were available was 903 days, or approximately 30 months. Study subjects had received a mean of 1 DME treatment every 2.9 months (or 0.3 treatments per month) before receiving FAc. After treatment with FAc, the frequency of ancillary treatments decreased to a mean of one treatment every 14.3 months (P < 0.001).
Table.
Study Population Baseline Characteristics and Demographics
|
Intravitreal Fluocinolone Acetonide,
n
= 130 Patients, 160 Eyes |
|
| Patient age, mean y | 69.6 |
| Male, % | 53.8 |
| Race, % | |
| White | 89.2 |
| Black/African American | 6.2 |
| Asian | 0.8 |
| Other | 3.8 |
| Diabetes type, % | |
| Type 1 | 10.0 |
| Type 2 | 87.7 |
| Not Recorded | 2.3 |
| HbA1C, mean % | 7.07 |
| Time since diagnosis, mean y (range) | |
| Diabetes | 24.1 (2–56) |
| DME | 4.4 (0–32) |
| Lens status, % | |
| Phakic | 22.5 |
| Pseudophakic | 68.8 |
| Not recorded | 8.7 |
| Prior treatment, no. of eyes (%) | 146 (91.3) |
| Anti-VEGF | 123 (76.9) |
| Steroid | 90 (56.3) |
| Laser | 80 (50.0) |
| Duration of follow-up, mean d (range) | |
| Pre-FAc | 903.3 (35–4005) |
| Post-FAc | 407.8 (7–756) |
The total number of OCT scans analyzed was 1813 in pretreatment eyes and 903 in posttreatment eyes. Significant longitudinal change in macular CST was detected among the 120 eyes treated with FAc for which OCT data were available. The proportion of eyes with CST < 300 μm (i.e., with a normal range) increased over the course of follow-up, which ranged from 3 to 24 months. OCT segmentation demonstrated that the age- and sex-adjusted mean central neuroretinal (NFL + GCL/IPL) thickness was 115.6 ± 35.5 μm at the study visit before treatment with FAc. As expected, the FAc treatment caused a decrease in central mean thickness to 106.4 ± 38.9 μm (P = 0.003, Wilcoxon signed rank test) when considering the data from the last follow-up visit after the date of implantation for each treated eye.
The difference in the “pre” and “post” rate of inner neuroretinal loss was statistically significant in region 2, the ring of macula >1.5 mm and <3.0 mm from the fovea (P = 0.001) (Fig. 2). The rate of thinning before FAc was 0.011 μm/d, or 4.0 μm/y. After treatment with FAc, the rate of thinning declined to 0.003 μm/d, or 1.1 μm/y.
Figure 2.
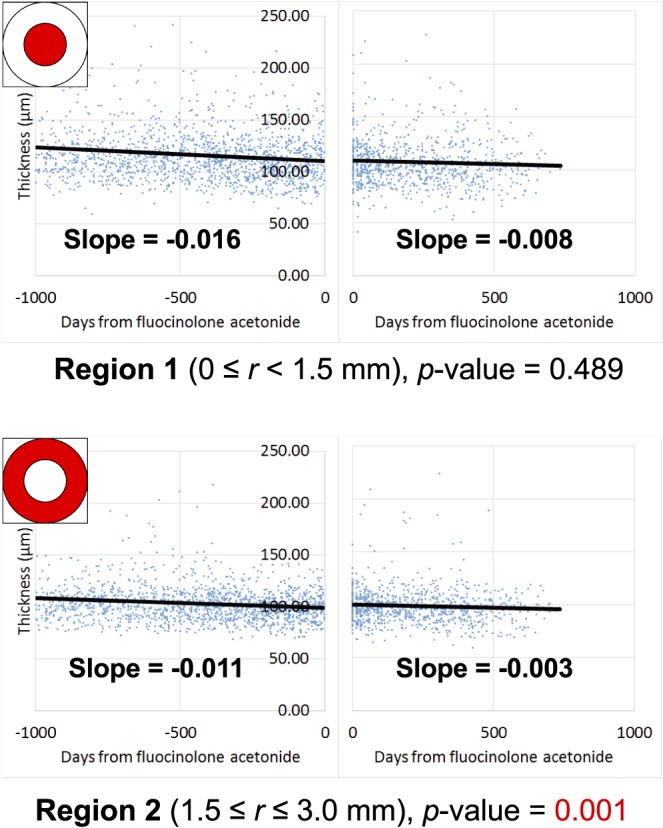
For all subjects at all time points, the inner neuroretinal thickness (defined as the sum of the NFL, GCL, and IPL thicknesses) is shown on the y-axis and time is shown on the x-axis, where t = 0 days at the time of FAc treatment. Results for region 1 are shown in the top half of the figure and for region 2 in the bottom half of the figure. Calculation of slope is corrected for age, sex, imaging platform (i.e., Zeiss versus Heidelberg), and lens status. The value of the slope represents the average adjusted inner neuroretinal loss per day.
Discussion
In this retrospective pilot study, the rate of DRN decreased in eyes with DME following treatment with FAc. Notably, the effect was seen only in macular region 2, greater than 1.5 mm from the foveal center. DRN may represent the first sign of retinal damage from diabetes; however, there are currently no neuroprotective treatments for patients with diabetes. This study showed that neuroretinal thinning slowed after treatment with FAc and suggests a potential role for FAc in patients who have DM and progressive thinning of the DRN. The population prevalence of DM has increased dramatically over the past several decades, and along with it, the incidence of new cases of DME and DR. Although it is currently unknown if there is a causative link between DRN and subsequent DME/DR, treatment at the earliest stages of DRN may reduce the development and progression of DR.
Although the magnitude of the change appears small, it must be interpreted in the context of neurodegenerative diseases. Normal senescence, on average, leads to NFL thinning determined by 0.13 to 0.24 μm/y.9,10 In a large study of patients with glaucoma, the decline in neuroretinal thickness between early (<6 dB perimetric loss) and severe glaucoma (>12 dB loss) ranged from 6 to 16 μm.30 In this study, inner neuroretinal thinning in region 2 declined from a rate of −0.011 μm/d (4.0 μm/y) to −0.003 μm/d (1.1 μm/y). Assuming the best-fit line could be extrapolated over the 36-month duration of FAc therapy, FAc could hypothetically prevent approximately 8.7 μm in absolute inner neuroretinal loss. This degree of neuropreservation is similar in magnitude to the loss in severe glaucoma and therefore may be visually significant. It should be noted that our study included GCL and IPL layers in inner neuroretinal thickness measurements; it is not clear what proportion of the change in neuroretinal thinning is attributable to the NFL versus the GCL/IPL.
The Iowa Reference Algorithm shows greater variability in segmentation accuracy within several hundred microns of the foveal center compared with regions farther away. This is because of the severe distortion from macular edema, which can also cause disruption of the inner retinal layers (DRIL), making the retinal layer boundaries hard to distinguish.31,32 It is currently unknown whether FAc could cause therapeutic repair of DRIL. The presence of DRIL in eyes with DME corresponds to VA. Although our analysis cannot exclude that rates of neuroretinal loss suggested by OCT analysis are confounded by changes in DRIL, it is possible that DRIL is a less significant variable in region 2, given that it represents an area greater than 1.5 mm from the foveal center; there is little to no data about DRIL in the parafoveal and perifoveal macula.
Barring potential adverse effects from steroid-responsive ocular hypertension, FAc offers significant benefits to patients because of its depot design. If, in fact, prospective studies confirm that FAc slows inner retinal thinning, studies for a neuroprotective indication may be warranted. These investigations would need to assess whether the maintenance of structural neural thickness correlates with maintenance of visual function. Contraindications to the FAc implant include active or suspected ocular/periocular infections, glaucoma with a cup-to-disc ratio of >0.8, and/or known hypersensitivity to any of the implant's components. Additionally, to mitigate the risk of IOP spikes, the FAc implant is contraindicated in patients who have had a clinically significant IOP elevation with exposure to prior ocular steroid. Serious adverse events include endophthalmitis, retinal detachment, and migration of the implant into the anterior chamber. In the USER study, 4 of the 21 phakic subjects required cataract extraction within 1 year of receiving the FAc implant due to steroid-associated cataract progression.15
A major limitation of this study is that data were analyzed retrospectively and were not available for all eyes at all points. The collected data were obtained from nonstandardized reports, mimicking “real-world” data tracking in clinical practice. Thus, undertreatment of DME may be a confounder, such that the preservation of neuroretinal thickness in reality is due to residual subclinical edema rather than to neuroprotection. Such subclinical DME, if persistent, may have long-term negative effects on visual function.
Additionally, patients may have received treatment for DME (including anti-VEGF, focal laser, or other steroids) during the pre-FAc period and/or post-FAc period. The number of adjunctive treatments varied based on baseline VA; patients with better baseline VA received fewer adjunctive post-FAc treatments. Therefore, it is conceivable that other treatments may have contributed to the trend in the rate of DRN; their direct effects on inner neuroretinal thickness have not been studied in a prospective, controlled fashion. Additionally, the study population was predominantly Caucasian with a relatively low mean HbA1C (7.07%). This limits generalizability of the study in ethnically diverse populations or those with poorly controlled diabetes. Additionally, because the patients in the study received FAc due to the presence of DME, it could be argued that patients without DME, who have fewer circulating cytokines, may not show a significant change in the rate of DRN after FAc injection.
This study suggests that neuroretinal thinning slowed after treatment with FAc. There may be a potential role for FAc in patients who have DM and progressive thinning of the DRN. However, our analysis is based on retrospective data, and therefore, further prospective studies will be needed to understand whether FAc or other intravitreal treatments affect the rate of DRN independently of variation in the natural disease process. For example, the preservation of neuroretinal thickness could reflect addition of tissue via gliosis, during the process of neurodegeneration,8,33 as opposed to a decreased rate of neural apoptosis. Further studies are needed to confirm the trend of slowed retinal thinning in patients with DM who have received FAc or another corticosteroid treatment. The ideal study design would involve a multicenter, prospective, randomized, sham-controlled trial in a population of people with diabetes who have not been diagnosed with DME or DR.
Acknowledgments
Supported by the Arnold and Mabel Beckman Initiative for Macular Research; the following National Eye Institute grants: R01 EY019112, R01 EY018853, and R01 EY17066; the Veterans Administration I01 CX000119; and Alimera Sciences, Inc. (Atlanta, GA, USA).
Disclosure: S.K. Lynch, None; K. Lee, None; Z. Chen, None; J.C. Folk, IDx (I, S); U. Schmidt-Erfurth, IDx (C); B.S. Gerendas, IDx (F, C); A. Wahle, None; C.C. Wykoff, IDx (F, C, S); M.D. Abramoff, IDx (F, C, I, E, S), P
References
- 1.DeBuc DC, Tatrai E, Laurik L, et al. Identifying local structural and optical derangement in the neural retina of individuals with type 1 diabetes. J Clin Exp Ophthalmol. 2013;4:289. [Google Scholar]
- 2.Vujosevic S, Midena E. Retinal layers changes in human preclinical and early clinical diabetic retinopathy support early retinal neuronal and Muller cells alterations. J Diabetes Res. 2013;2013:905058. doi: 10.1155/2013/905058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scarinci F, Picconi F, Virgili G, et al. Single retinal layer evaluation in patients with type 1 diabetes with no or early signs of diabetic retinopathy: the first hint of neurovascular crosstalk damage between neurons and capillaries? Ophthalmologica. 2017;237:223–231. doi: 10.1159/000453551. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk HW, Kok PH, Garvin M, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–3409. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk HW, Verbraak FD, Kok PH, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–3665. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Dijk HW, Verbraak FD, Kok PH, et al. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012;53:2715–2719. doi: 10.1167/iovs.11-8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk HW, Verbraak FD, Stehouwer M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011;51:224–228. doi: 10.1016/j.visres.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113:E2655–E2664. doi: 10.1073/pnas.1522014113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demirkaya N, van Dijk HW, van Schuppen SM, et al. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:4934–4940. doi: 10.1167/iovs.13-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieves-Moreno M, Martinez-de-la-Casa JM, Morales-Fernandez L, Sanchez-Jean R, Saenz-Frances F, Garcia-Feijoo J. Impacts of age and sex on retinal layer thicknesses measured by spectral domain optical coherence tomography with Spectralis. PLoS One. 2018;13:e0194169. doi: 10.1371/journal.pone.0194169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verrotti A, Lobefalo L, Altobelli E, Morgese G, Chiarelli F, Gallenga PE. Static perimetry and diabetic retinopathy: a long-term follow-up. Acta Diabetol. 2001;38:99–105. doi: 10.1007/s005920170021. [DOI] [PubMed] [Google Scholar]
- 12.Han Y, Bearse MA, Jr,, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004;45:948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson M, von Wendt G, Wanger P, Martin L. Early detection of macular changes in patients with diabetes using Rarebit Fovea Test and optical coherence tomography. Br J Ophthalmol. 2007;91:1596–1598. doi: 10.1136/bjo.2007.124461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez C, Garcia-Ramirez M, Corraliza L, et al. Topical administration of somatostatin prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2013;62:2569–2578. doi: 10.2337/db12-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campochiaro PA, Hafiz G, Shah SM, et al. Sustained ocular delivery of fluocinolone acetonide by an intravitreal insert. Ophthalmology. 2010;117:1393–1399.e3. doi: 10.1016/j.ophtha.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52:80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 17.Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–1746. [PMC free article] [PubMed] [Google Scholar]
- 18.Sohn HJ, Han DH, Kim IT, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152:686–694. doi: 10.1016/j.ajo.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Xiong DD, Zhang M, Li N, Gai JF, Mao L, Li M. Mediation of inflammation, obesity and fatty liver disease by advanced glycation endoproducts. Eur Rev Med Pharmacol Sci. 2017;21:5172–5178. doi: 10.26355/eurrev_201711_13835. [DOI] [PubMed] [Google Scholar]
- 20.Sengelaub DR, Han Q, Liu NK, et al. Protective effects of estradiol and dihydrotestosterone following spinal cord injury. J Neurotrauma. 2018;35:825–841. doi: 10.1089/neu.2017.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labombarda F, Ghoumari AM, Liere P, De Nicola AF, Schumacher M, Guennoun R. Neuroprotection by steroids after neurotrauma in organotypic spinal cord cultures: a key role for progesterone receptors and steroidal modulators of GABA(A) receptors. Neuropharmacology. 2013;71:46–55. doi: 10.1016/j.neuropharm.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Glybina IV, Kennedy A, Ashton P, Abrams GW, Iezzi R. Intravitreous delivery of the corticosteroid fluocinolone acetonide attenuates retinal degeneration in S334ter-4 rats. Invest Ophthalmol Vis Sci. 2010;51:4243–4252. doi: 10.1167/iovs.09-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glybina IV, Kennedy A, Ashton P, Abrams GW, Iezzi R. Photoreceptor neuroprotection in RCS rats via low-dose intravitreal sustained-delivery of fluocinolone acetonide. Invest Ophthalmol Vis Sci. 2009;50:4847–4857. doi: 10.1167/iovs.08-2831. [DOI] [PubMed] [Google Scholar]
- 24.Eaton A, Koh SS, Jimenez J, Riemann CD. The USER study: a chart review of patients receiving a 0.2 μg/day fluocinolone acetonide implant for diabetic macular edema. Ophthalmol Ther. 2019;8:51–62. doi: 10.1007/s40123-018-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campochiaro PA, Nguyen QD, Hafiz G, et al. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 2013;120:583–587. doi: 10.1016/j.ophtha.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Garvin MK, Abramoff MD, Wu X, Russell SR, Burns TL, Sonka M. Automated 3-D intraretinal layer segmentation of macular spectral-domain optical coherence tomography images. IEEE Trans Med Imaging. 2009;28:1436–1447. doi: 10.1109/TMI.2009.2016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashid A, Waldstein SM, Gerendas BS, et al. Reproducibility of retinal thickness measurements across spectral-domain optical coherence tomography devices using Iowa Reference Algorithm. arXiv:1612.06442 [physics.med-ph]. Available at: https://arxiv.org/abs/1612.06442. Accessed April 29, 2019.
- 28.Lee K, Zhang H, Wahle A, Abràmoff MD, Sonka M. Tavares J, Natal Jorge R, editors. Multi-layer 3D simultaneous retinal OCT layer segmentation: just-enough interaction for routine clinical use. Lecture Notes in Computational Vision and Biomechanics. In: eds. VipIMAGE 2017. ECCOMAS 2017. Cham, Switzerland: Springer; 2017:862–871.
- 29.Darma S, Kok PH, van den Berg TJ, et al. Optical density filters modeling media opacities cause decreased SD-OCT retinal layer thickness measurements with inter- and intra-individual variation. Acta Ophthalmol. 2015;93:355–361. doi: 10.1111/aos.12596. [DOI] [PubMed] [Google Scholar]
- 30.Bogunovic H, Kwon YH, Rashid A, et al. Relationships of retinal structure and humphrey 24–2 visual field thresholds in patients with glaucoma. Invest Ophthalmol Vis Sci. 2014;56:259–271. doi: 10.1167/iovs.14-15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun JK, Lin MM, Lammer J, et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 2014;132:1309–1316. doi: 10.1001/jamaophthalmol.2014.2350. [DOI] [PubMed] [Google Scholar]
- 32.Sun JK, Radwan SH, Soliman AZ, et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes. 2015;64:2560–2570. doi: 10.2337/db14-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch SK, Abramoff MD. Diabetic retinopathy is a neurodegenerative disorder. Vision Res. 2017;139:101–107. doi: 10.1016/j.visres.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


