Abstract
Long non‐coding RNAs (lncRNAs) participate in the complex network of cancer and play an important role in tumourigenesis and progression. BRAF activated non‐coding RNA (BANCR), a 4‐exon transcript of 693‐bp, was first discovered as an oncogenic long non‐coding RNA in BRAFV 600E melanomas cells in 2012 and was related to melanoma cell migration. Besides melanoma, increasing evidence has explored the potential role of BANCR in the development and progression of multiple other human malignancies, such as retinoblastoma, lung cancer, gastric cancer etc. since its discovery. The expression pattern of BANCR varies in different types of cancers, either as a tumour suppressor or as an accelerator. Functional BANCR may serve as a promising biomarker for cancer diagnosis as well as prognosis evaluation. BANCR‐targeted intervention may also become a valuable novel therapeutic tool against human malignancies. This review summarized the advanced research progresses concerning the expression and role of BANCR in different human malignancies.
Keywords: BANCR, long noncoding RNAs (lncRNAs), tumour accelerator, tumour suppressor
1. INTRODUCTION
Cancer is a group of malignancy diseases with uncontrolled cell growth, migration and invasion. With hundreds of types, cancer is the leading cause of cancer related death worldwide. In 2015, an estimated 90.5 million people were diagnosed with cancer which has incidence of 14.1 million new cases per year, 15.7% of all human death are caused by cancer.1 Present therapeutic methods for advanced stage cancers still have some limitations and early stage cancers tend to have a poor diagnosis due to the lack of efficient biomarkers. Therefore, identification of these potential efficient biomarkers may help in the early stage diagnosis of cancers.
Long non‐coding RNAs (long ncRNAs, lncRNA), transfer RNA (tRNA), ribosomal RNA (rRNA), small nucleolar RNAs (snoRNA), small nuclear RNA (snRNA) and microRNAs (miRNAs) all belong to the non‐coding RNAs family. Due to the fact that non‐coding RNAs do not encode any proteins, they were considered to possess no biological function and once were neglected for a long time. Recently, more and more researches (studies) reveal that non‐coding RNAs can be transcripted and exert essentially biological roles at RNA levels. The difference between lncRNAs and other members of non‐coding RNAs family is that lncRNAs are transcripts >200 nucleotides, while the others are not. Though emerging and not well‐characterized as microRNAs, lncRNAs play a vital role in the regulation of diverse biological processes, such as proliferation, apoptosis, invasion, differentiation, and etc. Increasing evidence demonstrates that lncRNAs are also abnormally expressed in varieties of cancers, such as melanoma, has roles in tumourigenesis and progression. lncRNAs are emerging as new biomarkers in many human malignancies.2 Some studies have focused on analysing molecular mechanisms and networks, aiming to expose their role in the development of cancer. However, the exact underlying biological roles of lncRNAs in cancers remain largely unknown.
Potential medical applications of lncRNAs have gained extensive attention in recent years. Among lncRNAs, BRAF‐activated non‐coding RNA (BANCR) is a 4‐exon transcript of 693‐bp in a 60 kb gene desert region on chromosome 9. It was first identified in 2012 year by Flockhart RJ et al. as a tumour accelerator via RNA‐seq screening in BRAFV600E melanocyte cells.3 However, its expression and clinical significance in other human malignancies remains unknown. This review will elucidate the advanced research progresses on the different expression pattern of BANCR, as well as its specific biological role it exhibited in different human cancers (Table 1). BANCR may serve as a novel biomarker for cancer diagnosis and disease prognosis evaluation. BANCR‐targeting inventions may be explored as a promising therapeutic option in cancer treatments.
Table 1.
Functional characterization of BANCR in various tumours
| Tumour type | Expression | Role | Related molecules | Phenotypes affected | Ref. |
|---|---|---|---|---|---|
| Melanoma | Up‐regulation | Tumour accelerator | Ras‐1, ERK, JNK, miR‐204, Notch2 | Proliferation, migration, invasion, apoptosis | 3, 6, 7 |
| Retinoblastoma | Proliferation, migration, invasion | 8 | |||
| Osteosarcoma | Proliferation, migration, invasion | 19 | |||
| Endometrial cancer | MEK/ERK1/2,MMP1,MMP2 | Proliferation, migration, invasion | 20 | ||
| Oesophageal cancer | Proliferation, migration, invasion | 24 | |||
| Gastric cancer | microRNA‐9,NF‐κB1 | Proliferation, apoptosis, metastasis | 28, 29, 30, 31 | ||
| Hepatocellular carcinoma | Vimentin, E‐cadherin protein, ERK, JNK | Proliferation, apoptosis, migration, invasion | 36, 37 | ||
| Colorectal cancer | Up‐regulation | Tumour accelerator | ERK, Vimentin, E‐cadherin protein | Proliferation, migration, and EMT | 41, 42, 43 |
| Down‐regulation | Tumour suppresser | p21 protein | Proliferation | 40 | |
| Papillary thyroid carcinoma | Up‐regulation | Tumour accelerator | cyclin D1, TSHR | Proliferation, apoptosis and autophagy | 45, 46 |
| Down‐regulation | Tumour suppresser | ERK1/2, p38 MAPK | Proliferation apoptosis, migration and invasion | 47 | |
| Bladder cancer | Down‐regulation | Tumour suppresser | Proliferation, apoptosis and migration | 48 | |
| Lung caner | HDAC3, p38 MAPK, JNK, E‐cadherin, N‐cadherin,Vimentin | Proliferation, apoptosis, migration, invasion and EMT | 51, 52, 54 |
Note: BANCR, BRAF‐activated non‐coding RNA; ERK, extracellular signal‐regulated kinase; JNK, c ‐ Jun N ‐ terminal Kinase; MAPK, mitogen‐activated protein kinase; MMP1, matrix metalloproteinase 1; MMP2, matrix metalloproteinase 2; F‐κB1, Nuclear factor kappa B1; EMT, epithelial to mesenchymal transition; TSHR, thyroid stimulating hormone receptor; HDAC3, Histone deacetylase.
2. BANCR IN HUMAN MALIGNANCIES
2.1. Melanoma
Melanoma is a type of malignant cancer that originates from the melanocytes, which may occur in the skin, eye, mouth, intestines etc. Being the most aggressive skin cancer with rapid progression,4 melanoma is the primary cause of skin cancer mortality in the United States.5 The prognosis of melanoma is good if the disease is detected early and intervened timely. However, melanomas are sometimes inclined to be neglected when they appear as a small dot in the early stage, though all the melanomas are malignant. In‐depth understanding of the complex process of melanomagenesis, both epigenetic and genetic, may facilitate prevention, diagnosis and early intervention for melanomas.
LncRNAs can alter gene regulation and lead to epigenetic changes. BANCR, as one of the important lncRNAs, was found to be a potential melanoma biomarker. BANCR was overexpressed in BRAFV600E melanocyte cells and crucial for melanoma cell migration.3 BANCR was also highly expressed in human melanoma tissues and cells when compared to naïve controls (melanocytic nevus or human melanocytes),6, 7 and the expression level of BANCR increased with clinical tumour stages.6 Higher expression of BANCR was also found to be associated with a lower survival rate in melanoma patients. BANCR promoted melanoma cells proliferation, migration, invasion and inhibited apoptosis. These effects can be augmented by BANCR overexpression and inhibited by BANCR knockdown.6, 7 Tumourigenicity assay in nude mice further demonstrated that BANCR knockdown inhibited tumour growth in vivo.6, 7 Raf‐ERK1/2,6 JNK signalling pathways,6 and BANCR/miR‐204/Notch2 signalling were demonstrated to regulate the tumour progression of melanoma.
These results indicate that BANCR participates in the cell proliferation of melanoma both in vitro and in vivo. Raf‐ERK1/2, JNK, miR‐204, Notch2‐targeted interpretations may provide novel options in malignant melanoma treatments.
2.2. Retinoblastoma (Rb)
Long non‐coding RNA BANCR regulates growth as well as metastasis and is associated with poor prognosis in retinoblastoma.8 Rb is an uncommon childhood malignant tumour of the retina. Nearly 300 new cases of Rb are diagnosed in America and 5000‐8000 cases are newly diagnosed worldwide, every year.9 Untreated Rb is fatal within 2 years mainly because of intracranial spread as well as disease dissemination.10 Nowadays, therapy rate for Rb is high in the developed world due to early diagnosis, along with aggressive multimodal therapy. The available effective treatment includes photocoagulation,11, 12 systemic chemotherapy,13 external beam radiotherapy,14, 15 and enucleation, etc.3, 16 Further discovery of the biomarkers of Rb progression may contribute to the establishment of both novel molecular prognostic factors and therapeutic strategies.
Su et al reported that BANCR is overexpressed in 60 retinoblastoma tissues and cell lines by using RT‐PCR, which is related to tumour size, optic nerve invasion, as well as choroidal invasion.8 Besides, multivariate analysis revealed that increased expression of BANCR was a poor independent prognostic factor for Rb cases. Knocking down the expression of BANCR notably inhibited the proliferation and migration of Rb cell in vitro.8 To sum up, BANCR plays an important role in the aggressiveness and prognosis of Rb and may act as a novel target for Rb treatment as well as prognostic prediction.
2.3. Osteosarcoma (OS)
Osteosarcoma is the most usual primary malignant bone tumour that occurs mainly in young adults.17 In spite of recent progressions in multimodal treatments such as tumour excision and adjuvant chemotherapy, survival expectancy is still limited.18 The accurate mechanisms of the formation and progression of OS are ambiguous, and further identifying the biomarker of OS progression is essential for improving the diagnosis as well as treatment of OS. Peng et al. reported that the overexpression of BANCR was observed in OS clinical specimens and cell lines, which was linked with tumour size, metastasis, and advanced TNM staging.19 Elevated BANCR expression in OS is a poor independent prognostic factor. Downregulated BANCR inhibited OS cell proliferation, invasion and induced cell apoptosis in vitro.19 These findings illustrates that BANCR may perform as a tumour promoter in OS and could be beneficial as a therapeutic target for OS.
2.4. Endometrial cancer (EC)
Endometrial cancer is a cancer of the uterine endometrium of the women uterus or womb, and is prevalent in females. EC falls into two categories (Type 1 and Type 2) based on clinical and endocrine features, with Type 1 being the most common one that occurs before and around the time of menopause. Awareness of the EC pathogenesis is not fully clear. The expression level of BANCR, MMP2 and MMP1 were detected to be significantly higher in the tissues from type 1 EC patients than from normal endometrium tissues by qRT‐PCR. And highly expressed BANCR was revealed to be correlated with pathological grade, Federation International of Gynecology and Obstetrics (FIGO) stage, myometrial invasion, lymph node metastasis and enhanced expression of matrix metalloproteinases 1 (MMP1) and matrix metalloproteinases 2 (MMP2).20 Knockdown of BANCR with si‐BANCR dramatically suppressed cell proliferation, migration, and invasion of EC cell lines (HEC‐1A cells and Ishikawa cells). It was further elucidated that BANCR induced cell proliferation, migration and invasion via activating ERK/MAPK/MMPs signalling pathway in EC cell lines, suggesting that BANCR plays roles in the EC progression, and BANCR may be explored as a promising biomarker for prognostic evaluation, as well as a potential therapeutic target for type 1 EC. In addition, the expression of BANCR in type 2 EC is still not clear, so more in vitro and in vivo studies based on human EC as well as animal EC model are needed.20
2.5. Oesophageal cancer
Oesophageal cancer is the ninth and sixth most common cause of cancer‐related death among females and male, respectively, worldwide.21 Even though, pathogenesis of oesophageal cancer is not fully illustrated, various factors such as tobacco, alcohol, reflux esophagitis, dietary habits, nutrition, as well as environmental factors may contribute to the initiation of oesophageal cancer.22 Presently, there are still no ideal biomarkers that can be used as a screening tool for oesophageal cancer strategies.23
Liu et al revealed that BANCR levels in plasma were considerably higher in oesophageal squamous cell carcinoma (ESCC) patients compared with normal controls.24 Moreover, increased expression of BANCR was correlated with advanced TNM stage, more lymph node metastasis, as well as the shorter survival of ESCC patients. The expression level of BANCR improved with the disease progression, which reverts to normal after the resection of the tumour. Increased BANCR expression was confirmed to be an independent prognostic factor in ESCC patients.24 In conclusion, BANCR may be a promising tumour biomarker for early detection, and a potential therapeutic target for ESCC patients. Further studies on the biological function of BANCR in plasma and tissue are needed.
2.6. Gastric cancer (GC)
Among the malignant cancers, GC is ranked as the most common cancer worldwide. Being a disease of high mortality, it is the second commonest cause of cancer‐related death worldwide. Although, the treatment option for GC patients is still challenging, surgery and systemic chemotherapy are the mainstay treatments.25
Moreover, the exact molecular mechanism of GC is still obscured. Molecular biomarker plays a critical role in the diagnosis, prognosis and treatment of most cancers.26, 27 Hence, it is urgent to find new molecular markers in early diagnosis of GC and investigate therapeutic targets for improving patient outcomes.
Up‐regulation of BANCR expression was detected in human GC tissues in comparison with matched normal tissues or cell lines.28 A close relationship between BANCR up‐regulation and tumour size, invasion depth, advanced TNM stage, regional lymph nodes metastasis, as well as distant metastasis was noted in GC patients.29, 30, 31 In survival analysis, high expression of BANCR in GC patients was an independent unfavourable prognostic factor.29, 30, 31 These findings show that BANCR might play an important role in GC development and tumourigenesis, which may be linked with poor prognosis. Zhang et al. revealed down‐regulation of BANCR hindered GC cell growth and induced cell apoptosis. Furthermore, it also leads to a sharp decrease in NF‐κB1 (P50/105) expression, as well as 3′UTR of NF‐κB1 activity. NF‐κB1 overexpression overturned the effect of BANCR on GC cell growth and apoptosis. MiroRNA‐9 (miR‐9) targeted NF‐κB1, and miR‐9 inhibitor could also reverse the roles of BANCR on GC cell growth and apoptosis. Therefore, both NF‐κB1 and miR‐9 were involved in the effects of BANCR in GC cell growth and apoptosis.28 In conclusion, BANCR has been found to be linked with tumour suppressor or oncogenic pathways of GC, and altered expression of BANCR was associated with the occurrence and development of GC. This finding provides evidence that BANCR may be considered as an early detection biomarker as well as a novel therapeutic target in GC. However, there is still lacking the clear analysis of specific downstream proteins in details, as well as its role in multidrug resistance, which is one of the major reasons for therapy failure in GC. Thus, the role of BANCR in the development and progression of GC still has many limitations, and further researches are needed.
2.7. Hepatocellular carcinoma (HCC)
Hepatocellular carcinoma is the most common primary malignancy of the liver, and is responsible for approximately 600 000 HCC‐related deaths every year, worldwide.32 Even though various diagnostic and treatment methods are available for HCC, still the prognosis is poor.33, 34 It is urgent to discover effective biomarkers for diagnosis as well as targets for therapy, which will play an important role for the diagnosis and treatment of HCC.35
Zhou et al found that the expression of BANCR was significantly increased in HCC tissues in comparison with adjacent noncancerous tissues.36 The expression of BANCR was also remarkably upregulated in HCC cell lines.36, 37 Further analysis showed that high expression of BANCR is associated with high tumour grade, venous infiltration, large tumour size and metastasis stage, as well as shorter overall survival. In HCC patients, it is identified that BANCR overexpression is an independent unfavourable prognostic factor.36 Furthermore, downregulation of BANCR in HCC cells has inhibited cell proliferation, induced cell apoptosis, weakened invasion, and migration of HCC cells, resulted in downregulated vimentin, as well as upregulated the levels of E‐cadherin protein.36 It was also demonstrated that ERK and JNK signalling pathways were involved in the BANCR‐associated malignancy of HCC cells whereas, p38 MAPK signalling pathway was not involved.
These findings implied that BANCR might donate to HCC initiation and progression and its beneficial effects as a novel prognostic marker and a therapeutic target for HCC.
2.8. Colorectal cancer (CRC)
Colorectal cancer is the third most common tumour and has the third highest mortality rate, worldwide.38 Surgery, radiation therapy, chemotherapy and targeted therapy are the main treatments options for CRC. In spite of the death rate has decreased for many years mainly due to the advancement of standard treatment methods,39 the occurrence of relapses and the unfavourable prognosis still considerably influences the consequences of treating CRC. Consequently, it is vital to detect novel CRC‐specific biomarkers in CRC.
Shi et al reported that the expression of BANCR in CRC tissues was down‐regulated in comparison with normal tissues, and BANCR overexpression has inhibited CRC cell growth both in vitro and in vivo.40 They also found that pcDNA‐BANCR‐mediated CRC cell proliferation was linked with G0/G1 cell‐cycle arrest and induction of apoptosis via the regulation of p21 protein.40 On the contrary, some researches reported that BANCR was overexpressed in CRC tissues, which was considerably related with lymph node metastasis, tumour stage, as well as poor prognosis.41, 42, 43 EMT was induced by BANCR via a MEK/extracellular signal‐regulated kinase‐dependent mechanism.42 Li et al found that fentanyl indicated anti‐tumour like effects on CRC cells, including hindered cell migration and invasion. Fentanyl induced the upregulation of BANCR and the downregulation of Ets‐1 in CRC cells. Further studies revealed that Ets‐1 negatively regulated the expression of BANCR through the deacetylation of histones H3 within the promoter of BANCR.44
As a conclusion, these results showed the significance of BANCR in the molecular etiology of CRC and suggested the potential application of BANCR as an essential diagnostic marker and therapeutic target of CRC. The variation between the expression profile and function among different researches is probably due to the difference in the study size as well as diverse ethnic groups. Larger studies and extensive functional researches are required to confirm these findings.
2.9. Papillary thyroid carcinoma (PTC)
Papillary thyroid carcinoma is the most common type of thyroid cancers. The prognosis of majority PTC is excellent if treated timely. An ideal biomarker for PTC may help in early detection and intervention, but it has not been identified yet. The role of BANCR in the pathogenesis of PTC remains largely unknown. Based on different studies, the role of BANCR varies either as an oncogene or tumour suppressor.45, 46, 47 Wang et al. in 2014 and Zheng et al. in 2016 reported that BANCR was upregulated in the PTC tissues and PTC cells (IHH‐4 cells) when compared with paired normal controls. BANCR overexpression leads to increased cell proliferation via enhancing autophagy and inhibiting apoptosis of PTC cells, which was reversed by BANCR knockdown.45 It was further demonstrated by BANCR knockdown technique that BANCR may contribute to PTC through cyclin D1 and thyroid stimulating hormone receptor (TSHR) regulation.46 These two studies indicate that BANCR is an oncogenic lncRNA in PTC.45 Whereas, Liao et al. in 2017 found that the expression of BANCR was lower in PTC tissues when compared to naïve controls, and decreased expressed BANCR was positively correlated with poor prognosis (advanced PTC stage, tumour size and multifocal lesions). Overexpression of BANCR inhibited cell proliferation and promoted apoptosis of PTC cells (TPC‐1 and K1 cells), resulting in the inhibition of metastasis of PTC evidenced by the xenograft mouse models. It was further confirmed that BANCR inhibited PTC tumourigenesis partially via the inhibition of the ERK/MAPK pathways. This study suggests that BANCR may be functioned as a tumour suppressor in PTC.47 Different sample numbers, different PTC cell lines, different PTC differentiation status may account for the inconsistent results of BANCR expression patterns in PTC tumourigenesis. Further studies with larger samples are needed to solve the above puzzle.
2.10. Bladder cancer (BC)
Bladder cancer is a common malignant cancer with frustrated survival rate worldwide. Lack of thorough understanding of underlying molecular mechanism partially accounts for the high fatality rate of BC. The expression level of BANCR RNA was found to be dramatically downregulated in human BC tissues, and decreased BANCR was revealed to be positively correlated with the TNM stage by clinicopathologic analysis.48 The expression level of BANCR RNA was also downregulated in BC cell lines (SW780 and T24). Once BANCR was overexpressed with pcDNA‐BANCR vector in BC cells, cell proliferation was inhibited, apoptosis was induced and migration was suppressed significantly.48 These results indicate that BANCR participates in BC as a tumour suppressor, and it may serve as a potential biomarker and candidate therapeutic target for BC.
2.11. Lung cancer (LC)
Lung cancer, including small cell lung cancers (SCLCs) and non‐small cell lung cancers (NSCLCs), are one of the most aggressive malignant tumours, threating to human health. NSCLCs are the predominant forms of LC and account for the majority of cancer‐related deaths, worldwide,49 with an estimated 5‐years survival rate of 11%.50 Metastasis is a crucial problem in NSCLC induced death, and understanding of the molecular mechanisms in metastasis may facilitate in identifying new therapeutic targets for anti‐cancer therapy. Epithelial‐mesenchymal transition (EMT), a biological process with epithelial cells lose their polarity and transit into a mesenchymal phenotype, is a vital step in cancer metastasis. BANCR expression was significantly downregulated in LC tissues (both SCLCs and NSCLCs) and LC cell lines.51, 52 Reduced BANCR was correlated with larger tumour size, advanced pathological stage, metastasis distance and shorter overall survival, revealing that decreased BANCR may serve as an independent biomarker for poor prognosis of NSCLC.51 While overexpression of BANCR with pcDNA‐BANCR transfection led to the significant inhibition of cell viability, migration, invasion and promotion of apoptosis.51, 52 Activation of p38 MAPK and JNK can promote LC tumour progression.53 Overexpression of BANCR also led to the downregulation of p‐p38 and p‐JNK,52 indicating that p38 MAPK and JNK were involved in the BANCR‐related cell proliferation and migration of LC. To the opposite, BANCR knockdown with RNA interference significantly promoted cell proliferation, migration and invasion of LC.51, 52
Enhanced BANCR was also revealed to inhibit EMT in NSCLC through the upregulation of E‐cadherin, and downregulation of N‐cadherin along with Vimentin expression.51 Histone deacetylase (HDAC) inhibitor (trichostatin A) treatment or HDAC3 knockdown increased BANCR expression in NSCLC cell lines,51 indicating that histone deacetylation is also involved in the negative regulation of BANCR.54 BANCR was found to be elevated in mice receiving radiation therapy, and HDAC3 overexpression significantly reversed the effect of radiation therapy on BANCR expression. These findings indicate that BANCR may serve as a potential biomarker for LC prognosis and a target for LC chemotherapy.
Further molecular investigations of LC are still needed, for example, the BACNR orientated EMT regulation in NSCLC, which may facilitate the understanding of LC oncogenesis and the discovery of lncRNA‐targeted therapeutics.
3. CONCLUSIONS
Emerging evidence reveals that lncRNAs plays an important role in the complex network of cancer and is also linked with tumourigenesis and progression. BANCR was first identified as an oncogenic lncRNAs in BRAFV600E melanomas cells. The clinical significance of BANCR, and its' molecular mechanisms in controlling cancer are unclear. Overview of BANCR in human malignancies may provide new insights into the mechanisms of cancer development. This review elucidates the advanced researches and progresses with the possible role of BANCR in different human malignancies (Table 1), as well as the underlying mechanism of BANCR involved (Figure 1). Studies show that the expression pattern and role of BANCR varies in different types of cancers, either as a tumour suppressor or tumour accelerator. The expression tendency of BANCR was inconsistent and the role of BANCR was even opposite in the same type of cancer (e.g. PTC, CRC) in different researches sometimes. Functional BANCR may serve as a promising biomarker for cancer diagnosing and prognosis evaluation, and BANCR‐target interventions may also become a valuable novel therapeutic tool against human malignancies, though more‐in‐depth studies are needed which may explain the expression differences in BANCR in some human malignancies.
Figure 1.
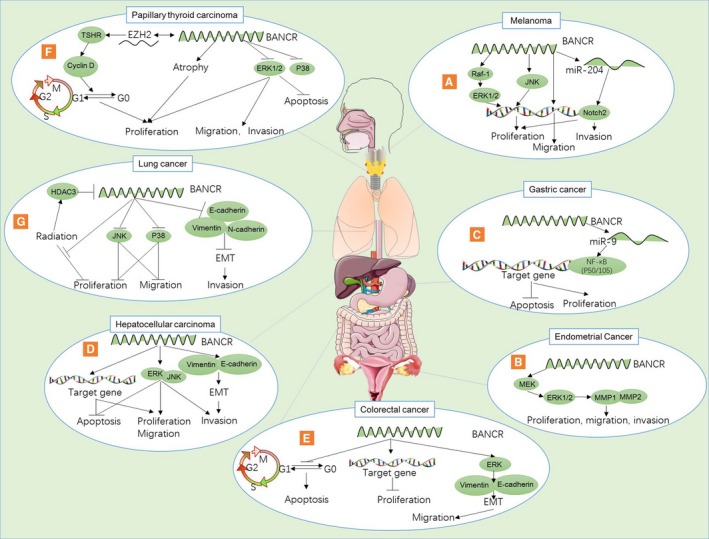
The different regulatory mechanisms of BANCR in different human malignancy. (A). BANCR induced proliferation via the activation of the Ras‐1/ERK and JNK pathway. BANCR also promoted migration and invasion in melanoma. BANCR promotes the proliferation and invasion of melanoma by functioning as a competing endogenous RNA to upregulate Notch2 expression by sponging miR‐204. (B). BANCR promote cell proliferation, migration, and invasion by activating MEK/ERK1/2 signalling pathway that regulates MMP1 and MMP2 in endometrial cancer. (C). BANCR induced cell proliferation and inhibited apoptosis via the upregulation of MiroRNA‐9 targeted NF‐kB1 expression in gastric cancer. (D). BANCR inhibited cell apoptosis and induced cell proliferation, migration and invasion via activating the ERK and JNK pathways. BANCR also induced invasion by EMT (upregulated vimentin, and downregulated E‐cadherin) in hepatocellular carcinoma. (E). BANCR inhibited cell proliferation by increasing p21 protein, and induced apoptosis by induction of G0/G1 arrest in colorectal cancer. BANCR promoted cell migration by EMT in colorectal cancer. (F). BANCR inhibited proliferation, migration, metastasis by inhibition of ERK1/2 and p38 pathways in papillary thyroid carcinoma. It also induced apoptosis in papillary thyroid carcinoma. BANCR increased papillary thyroid carcinoma cell proliferation by activating autophagy, as well as activating cell cycle via EZH2/TSHR/Cyclin D pathway. (G). BANCR inhibited proliferation, migration by inhibition of JNK and P38 pathways in lung cancer. BANCR inhibited invasion of lung cancer by inhibition of EMT. Radiation upregulated BANCR's expression via the upregulation of HDAC3 and HDAC3 reversed the BANCR's protective effect on radiation‐induced cell death reversed radiation induced cell in lung cancer
ACKNOWLEDGMENTS
Not Applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
CONSENT FOR PUBLICATION
Not applicable.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
AUTHORS' CONTRIBUTIONS
The topic was conceptualized by C.W.L. and D.D.Z. X.F.L., J.L.H., and T.X. contributed to the literature database search, and writing of the manuscript. C.W.L. and D.D.Z. contributed to vital revising. O.P.P. contributed to English Polishing. All authors read and approved the final manuscript.
Liu X‐F, Hao J‐L, Xie T, et al. The BRAF activated non‐coding RNA: A pivotal long non‐coding RNA in human malignancies. Cell Prolif. 2018;51:e12449 10.1111/cpr.12449
Xiu‐Fen Liu and Ji‐Long Hao have contributed equally to this work.
Contributor Information
Cheng‐Wei Lu, Email: lcwchina800@sina.com.
Dan‐Dan Zhou, Email: zhoudan0928@sohu.com.
REFERENCES
- 1. Mortality GBD. Causes of Death C. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weidle UH, Birzele F, Kollmorgen G, Ruger R. Long non‐coding RNAs and their role in metastasis. Cancer Genomics Proteomics. 2017;14:143‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flockhart RJ, Webster DE, Qu K, et al. BRAFV600E remodels the melanocyte transcriptome and induces BANCR to regulate melanoma cell migration. Genome Res. Jun 2012;22:1006‐1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893‐2917. [DOI] [PubMed] [Google Scholar]
- 5. Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008;84:528‐536. [DOI] [PubMed] [Google Scholar]
- 6. Li R, Zhang L, Jia L, et al. Long non‐coding RNA BANCR promotes proliferation in malignant melanoma by regulating MAPK pathway activation. PLoS ONE. 2014;9:e100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai B, Zheng Y, Ma S, et al. BANCR contributes to the growth and invasion of melanoma by functioning as a competing endogenous RNA to upregulate Notch2 expression by sponging miR204. Int J Oncol. Dec 2017;51:1941‐1951. [DOI] [PubMed] [Google Scholar]
- 8. Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non‐coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. Sep 2015;36:7205‐7211. [DOI] [PubMed] [Google Scholar]
- 9. Villegas VM, Hess DJ, Wildner A, Gold AS, Murray TG. Retinoblastoma. Curr Opin Ophthalmol. 2013;24:581‐588. [DOI] [PubMed] [Google Scholar]
- 10. Abramson DH. Retinoblastoma in the 20th century: past success and future challenges the Weisenfeld lecture. Invest Ophthalmol Vis Sci. Aug 2005;46:2683‐2691. [DOI] [PubMed] [Google Scholar]
- 11. Shields CL, Shields JA. Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra‐arterial chemotherapy. Curr Opin Ophthalmol. May 2010;21:203‐212. [DOI] [PubMed] [Google Scholar]
- 12. Melamud A, Palekar R, Singh A. Retinoblastoma. Am Fam Physician. 2006;73:1039‐1044. [PubMed] [Google Scholar]
- 13. Gatta G, Capocaccia R, Stiller C, et al. Childhood cancer survival trends in Europe: a EUROCARE Working Group study. J Clin Oncol. 2005;23:3742‐3751. [DOI] [PubMed] [Google Scholar]
- 14. Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. Jun 2011;21:354‐361. [DOI] [PubMed] [Google Scholar]
- 15. Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Can Res. 2011;71:3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non‐coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31:4577‐4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. Oct 2010;8:705‐718. [PubMed] [Google Scholar]
- 18. Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011‐2018. [DOI] [PubMed] [Google Scholar]
- 19. Peng ZQ, Lu RB, Xiao DM, Xiao ZM. Increased expression of the lncRNA BANCR and its prognostic significance in human osteosarcoma. Genet Mol Res. 2016. Mar 28;15(1). 10.4238/gmr.15017480. [DOI] [PubMed] [Google Scholar]
- 20. Wang D, Wang D, Wang N, Long Z, Ren X. Long non‐coding RNA BANCR promotes endometrial cancer cell proliferation and invasion by regulating MMP2 and MMP1 via ERK/MAPK signaling pathway. Cell Physiol Biochem. 2016;40:644‐656. [DOI] [PubMed] [Google Scholar]
- 21. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 22. Palladino‐Davis AG, Mendez BM, Fisichella PM, Davis CS. Dietary habits and esophageal cancer. Dis Esophagus. Jan 2015;28:59‐67. [DOI] [PubMed] [Google Scholar]
- 23. Gao QY, Fang JY. Early esophageal cancer screening in China. Best Pract Res Clin Gastroenterol. Dec 2015;29:885‐893. [DOI] [PubMed] [Google Scholar]
- 24. Liu Z, Yang T, Xu Z, Cao X. Upregulation of the long non‐coding RNA BANCR correlates with tumor progression and poor prognosis in esophageal squamous cell carcinoma. Biomed Pharmacother. 2016;82:406‐412. [DOI] [PubMed] [Google Scholar]
- 25. Kanat O, O'Neil BH. Metastatic gastric cancer treatment: a little slow but worthy progress. Med Oncol. Mar 2013;30:464. [DOI] [PubMed] [Google Scholar]
- 26. Fan YH, Ye MH, Wu L, et al. Overexpression of miR‐98 inhibits cell invasion in glioma cell lines via downregulation of IKKepsilon. Eur Rev Med Pharmacol Sci. Oct 2015;19:3593‐3604. [PubMed] [Google Scholar]
- 27. Mirzaei H, Gholamin S, Shahidsales S, et al. MicroRNAs as potential diagnostic and prognostic biomarkers in melanoma. Eur J Cancer. Jan 2016;53:25‐32. [DOI] [PubMed] [Google Scholar]
- 28. Zhang ZX, Liu ZQ, Jiang B, et al. BRAF activated non‐coding RNA (BANCR) promoting gastric cancer cells proliferation via regulation of NF‐kappaB1. Biochem Biophys Res Comm. 2015;465:225‐231. [DOI] [PubMed] [Google Scholar]
- 29. Zhang K, Shi H, Xi H, et al. Genome‐wide lncRNA microarray profiling identifies novel circulating lncRNAs for detection of gastric cancer. Theranostics. 2017;7:213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fan YH, Ye MH, Wu L, Wu MJ, Lu SG, Zhu XG. BRAF‐activated lncRNA predicts gastrointestinal cancer patient prognosis: a meta‐analysis. Oncotarget. 2017;8:6295‐6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li L, Zhang L, Zhang Y, Zhou F. Increased expression of LncRNA BANCR is associated with clinical progression and poor prognosis in gastric cancer. Biomedicine Pharmacother. 2015;72:109‐112. [DOI] [PubMed] [Google Scholar]
- 32. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 33. Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. Aug 2006;10:99‐111. [DOI] [PubMed] [Google Scholar]
- 34. Attwa MH, El‐Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1632‐1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu J, Han J, Zhang J, et al. The long noncoding RNAs PVT1 and uc002mbe.2 in sera provide a new supplementary method for hepatocellular carcinoma diagnosis. Medicine. 2016;95:e4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou T, Gao Y. Increased expression of LncRNA BANCR and its prognostic significance in human hepatocellular carcinoma. World J Surg Oncol. 2016;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li J, Wang J, Zhou W, Zhang S, Le Y, He R. Downregulation of BRAF‐activated non‐coding RNA suppresses the proliferation, migration and invasion, and induces apoptosis of hepatocellular carcinoma cells. Oncol Lett. Oct 2017;14:4751‐4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sostres C, Gargallo CJ, Lanas A. Aspirin, cyclooxygenase inhibition and colorectal cancer. World J Gastrointest Pharmacol Ther. 2014;5:40‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Altobelli E, Lattanzi A, Paduano R, Varassi G, di Orio F. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med. May 2014;62:132‐141. [DOI] [PubMed] [Google Scholar]
- 40. Shi Y, Liu Y, Wang J, et al. Downregulated long noncoding RNA BANCR promotes the proliferation of colorectal cancer cells via downregualtion of p21 expression. PLoS ONE. 2015;10:e0122679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang R, Du L, Yang X, et al. Identification of long noncoding RNAs as potential novel diagnosis and prognosis biomarkers in colorectal cancer. J Cancer Res Clin Oncol. Nov 2016;142:2291‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guo Q, Zhao Y, Chen J, et al. BRAF‐activated long non‐coding RNA contributes to colorectal cancer migration by inducing epithelial‐mesenchymal transition. Oncol Lett. Aug 2014;8:869‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shen X, Bai Y, Luo B, Zhou X. Upregulation of lncRNA BANCR associated with the lymph node metastasis and poor prognosis in colorectal cancer. Biol Res. 2017;50:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li AX, Xin WQ, Ma CG. Fentanyl inhibits the invasion and migration of colorectal cancer cells via inhibiting the negative regulation of Ets‐1 on BANCR. Biochem Biophys Res Comm. 2015;465:594‐600. [DOI] [PubMed] [Google Scholar]
- 45. Wang Y, Guo Q, Zhao Y, et al. BRAF‐activated long non‐coding RNA contributes to cell proliferation and activates autophagy in papillary thyroid carcinoma. Oncol Lett. Nov 2014;8:1947‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zheng H, Wang M, Jiang L, et al. BRAF‐activated long noncoding RNA modulates papillary thyroid carcinoma cell proliferation through regulating thyroid stimulating hormone receptor. Cancer Res Treat. Apr 2016;48:698‐707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liao T, Qu N, Shi RL, et al. BRAF‐activated LncRNA functions as a tumor suppressor in papillary thyroid cancer. Oncotarget. 2017;8:238‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He A, Liu Y, Chen Z, et al. Over‐expression of long noncoding RNA BANCR inhibits malignant phenotypes of human bladder cancer. J Exp Clin Cancer Res. 2016;35:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10‐29. [DOI] [PubMed] [Google Scholar]
- 50. Thomson CS, Forman D. Cancer survival in England and the influence of early diagnosis: what can we learn from recent EUROCARE results? Br J Cancer. 2009;101(Suppl 2):S102‐S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun M, Liu XH, Wang KM, et al. Downregulation of BRAF activated non‐coding RNA is associated with poor prognosis for non‐small cell lung cancer and promotes metastasis by affecting epithelial‐mesenchymal transition. Mol Cancer. 2014;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jiang W, Zhang D, Xu B, et al. Long non‐coding RNA BANCR promotes proliferation and migration of lung carcinoma via MAPK pathways. Biomed Pharmacother. 2015;69:90‐95. [DOI] [PubMed] [Google Scholar]
- 53. Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nature Rev Cancer. 2009;9:537‐549. [DOI] [PubMed] [Google Scholar]
- 54. Chen JX, Chen M, Zheng YD, Wang SY, Shen ZP. Up‐regulation of BRAF activated non‐coding RNA is associated with radiation therapy for lung cancer. Biomed Pharmacother. 2015;71:79‐83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


