To the editor:
We read with interest the article by Tian et al,1 describing the role of MicroRNA‐9 (miR‐9) in the regulation of myeloid‐derived suppressor cell (MDSC) differentiation and function by targeting the runt‐related transcription factor 1 (Runx1). This finding has revealed post‐transcriptional regulation of Runx1 by miR‐9. RUNX1, the master regulator of hematopoiesis,2 is known to regulate megakaryocyte (MK) polyploidization and cytoskeleton rearrangement in the process of MK maturation and pro‐platelet formation.3, 4 Specifically, RUNX1 sequence is conserved for miR‐9 among vertebrates and miR‐9 was also identified with decreasing intensity of expression during human MK ontogeny,5 which shows the functional significance of miR‐9/RUNX1 axis during evolution. Thus, miR‐9 could be a potential therapeutic target in neonatal thrombocytopenia and other platelet disorders.
Thrombocytopenia, the deficiency of platelets in the blood, is a major clinical problem encountered among several conditions and is common in all sick and preterm neonates admitted to NICU; the primary outcome of thrombocytopenia in infants is the incidence and severity of intra‐ventricular haemorrhages (IVH), which is a leading cause of poor neurological outcome and mortality in sick neonates.6, 7 It is believed that developmental differences between neonatal and adult MKs contribute to this vulnerability.8 Specifically, neonatal MK progenitors possess a high proliferative potential and give rise to MKs smaller and of low ploidy that generate fewer platelets compared with adult MKs. The regulatory mechanisms underlying these developmental differences are unknown, but we have revealed a critical role of small non‐coding RNAs (miRNAs) in the regulation of MK development.9 Further, RUNX1 emerged as a putative target of miR‐9 by several bioinformatic databases such as TargetScan, miRbase and RNAhybrid. In our miRNA array study of neonatal vs adult MKs, four other RUNX1 targeting miRNAs were identified with higher levels of expression in neonatal MKs, although miR‐9 was the highest up‐regulated miRNA (Table 1, P < .05). Hsa‐miR‐9 and RUNX1 mRNA duplex showed minimum free energy of −17.6 kcal/mole using bioinformatic tool RNAhybrid (Figure 1A).
Table 1.
RUNX1 targeting miRNAs expression in CB‐ and PB‐derived MKs. miR‐9 exhibited the largest developmental difference, being expressed at levels 20‐fold higher in neonatal compared with adult MKs
| miRNA ID | P‐value | CB/PB expression | Putative target |
|---|---|---|---|
| hsa‐miR‐370 | .05 | 2.03 | RUNX1 |
| hsa‐miR‐192 | .04 | 2.91 | RUNX1 |
| hsa‐miR‐129‐5p | .05 | 3.89 | RUNX1 |
| hsa‐miR‐215 | .05 | 5.91 | RUNX1 |
| hsa‐miR‐9 | .03 | 21.4 | RUNX1 |
Figure 1.
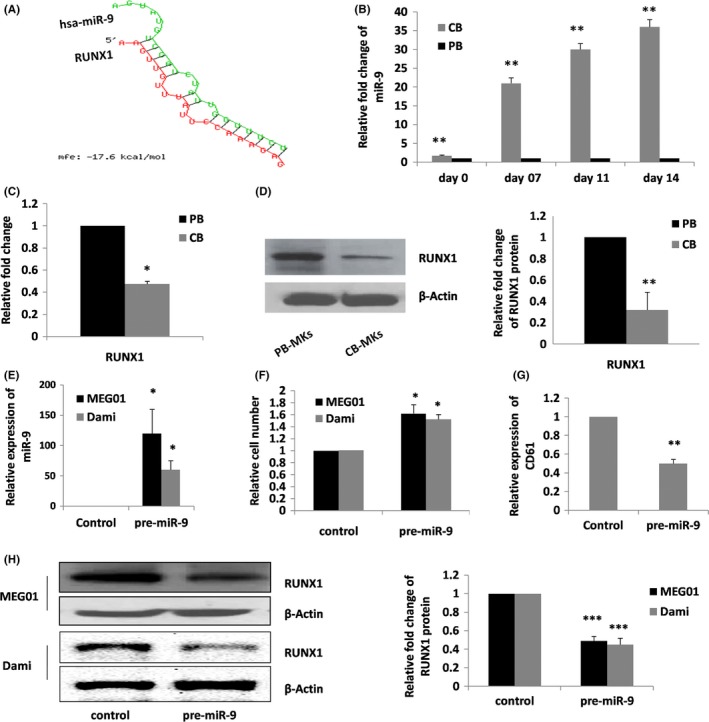
hsa‐miR‐9 and RUNX1 expression is inversely related in neonatal vs adult MKs. (A) Minimum free energy (mfe) duplex of the hsa‐miR‐9 and RUNX1 analysed by RNAhybrid. (B) hsa‐miR‐9 levels were measured in human cord blood (CB)‐ and adult peripheral blood (PB)‐CD34+ progenitor‐derived MKs. miR‐9 values were normalized against U6 (internal control), and miR‐9 levels in CB at different stages (0 day, 7 days, 11 days, 14 days) of MK differentiation were expressed as relative to miR‐9 levels in PB cells at the same stage of MK differentiation. hsa‐miR‐9 levels were significantly higher in CB‐ compared with PB‐derived mature MKs (n = 3, **P = .02). (C) RUNX1 mRNA levels were measured in human CB‐ and adult PB‐CD34+ progenitor‐derived MKs. RUNX1 mRNA levels in MKs derived from CB expressed as relative to RUNX1 levels in PB‐derived MKs at day 14 (n = 3, *P < .05). (D) RUNX1 protein levels expressed as a ratio to β‐actin were significantly lower in CB‐ compared with adult PB‐derived MKs (n = 3, **P = .02). (E) hsa‐miR‐9 expression was >60‐fold higher in pre‐mir‐9 transfected cells than cells transfected with the control (n = 3, P < .05). (F) The rate of cell proliferation was also affected with ~1.6‐fold increase in pre‐miR‐9 transfected cells compared with control (n = 3, *P = .05). (G) The megakaryocyte marker, CD61 expression was also reduced after 72‐hour post‐transfection (n = 3, **P = .02). (H) After 72 hours of transfection, RUNX1 protein level was significantly reduced in pre‐miR‐9 transfected cells compared with non‐targeting sequence transfected cells (n = 3, ***P < .001). Bars represent mean ± SD of three independent experiments
To test the role of miR‐9 in RUNX1 regulation in megakaryocytes, we cultured human CB‐ and adult PB‐CD34+ cells (n = 3 for each group) in the presence of serum free medium with TPO (50 ng/mL), as previously described10; after 14 days of culture, >90% of cells were MKs (CD41+). The expression levels of hsa‐miR‐9 and RUNX1 mRNA were measured by qRT‐PCR (n = 3) and were normalized against internal control U6 and β‐actin, respectively.10, 11 Initially, we confirmed the hsa‐miR‐9 expression levels and found that CB levels were significantly higher compared with PB‐derived MKs, and these differences were consistent through all the stages (0, 7, 11, 14 days) of megakaryocytopoiesis (n = 3, P = .02; Figure 1B), whereas its target RUNX1 mRNA was observed with significant lower expression in CB‐derived MKs compared with PB‐derived MKs (n = 3, P < .05; Figure 1C). RUNX1 protein levels were determined by western blot using anti‐RUNX1 (Santa Cruz, CA, USA) and anti‐β‐actin (Santa Cruz) antibodies. RUNX1 protein levels were quantified by densitometry using the ImageJ system and normalized with β‐actin. We further confirmed the developmental differences between CB‐derived MKs and PB‐derived MKs in RUNX1 protein expression (n = 3, P = .02; Figure 1D). To prove the functional significance of hsa‐miR‐9 in RUNX1 regulation, we transfected MEG01 (human megakaryoblastic leukaemia cell line) and Dami (human megakaryocytic leukaemia cell line) cells with either pre‐miR‐9 or scrambled control (Cy3 labelled) using lipofectamine Hi‐perfect (Qiagen, Hilden, Germany). MEG01 and Dami cells, respectively, were maintained in DMEM and RPMI with 10% FBS. After 72 hours of transfection, we observed a significant increase in the miR‐9 levels (n = 3, P < .05; Figure 1E), along with high cell proliferation rate (n = 3, P < .05; Figure 1F) and reduced MK marker, CD61 expression (n = 3, P = .02; Figure 1G). Further, lower RUNX1 protein levels (n = 3, P < .001; Figure 1H) were noticed using western blot analysis.
Over the past many years, multiple reports have identified the essential role of miRNAs in the regulation of lineage‐selective TFs.10 In vertebrates, recent studies evidenced the development stage and cellular context dependent role of miR‐9 on cell proliferation, migration and differentiation.12 In this study, we observed developmental differences in the expression levels of hsa‐miR‐9 in neonatal vs adult MKs (Figure 1B), and similar developmental differences were also observed in embryonic stem cell MKs vs adult MKs.5 Hypothetically, down‐regulated miRNAs during MK development and differentiation unblock mRNAs involved in developmental maturation and differentiation. Interestingly in our study, hsa‐miR‐9 and its putative target RUNX1 were inversely related in neonatal vs adult MKs (Figure 1B‐D).
RUNX1 is a critical regulator of embryonic and adult definitive hematopoiesis.13 In human, loss of function mutations in RUNX1 causes familial platelet disorder with predisposition to thrombocytopenia.14 In adult mice, conditional Runx1 deletion generates a phenotype characterized by abundant small low ploidy MKs in the bone marrow, which closely resembles normal neonatal megakaryocytopoiesis.15 In this study, we found a significant lower expression of RUNX1 in CB‐derived MKs compared with PB‐derived MKs (Figure 1C,D), and these developmental differences in the expression levels of RUNX1 could be under the post‐transcriptional control of hsa‐miR‐9. Our miR‐9 transfection study in MEG01 and Dami cells showed a significantly reduced expression of RUNX1 (Figure 1H). Interestingly, we found increased rate of cell proliferation and reduced level of MK‐specific marker, CD61 in miR‐9 mimic transfected cells as compared with control (Figure 1F,G). Our findings are consistent with those of Ben‐Ami et al16 observed in HEK293 cells. Notably, Tian et al1 also described the role of miR‐9 for translational attenuation of Runx1 in MDSC differentiation and function.
In conclusion, our current findings are in line with previous observation indicating molecular differences in neonatal vs adult MKs. This study clearly reveals the developmental differences in the expression of hsa‐miR‐9 and its target RUNX1. Further our observations associate the reduced level of RUNX1 with higher expression of miR‐9 in neonatal MKs. Our data provide the first evidence of an important role of hsa‐miR‐9 in human megakaryocyte development, and for the first time identify a miRNA as a molecular regulator of the differences between neonatal and adult MKs. A known target of miR‐9 is the key MK transcription factor RUNX1, which is crucial for the regulation of MK specification, maturation and thrombopoiesis.17 Taken together, this study shows the functional significance of hsa‐miR‐9 in the regulation of cell proliferation by targeting RUNX1 in human MK development. The higher expression of miR‐9 may contribute to the developmentally different and disease susceptible phenotype of neonatal MKs via regulating the RUNX1 expression; thus, it could be a potential target in neonatal thrombocytopenia and other platelet disorders.
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the Department of Biotechnology (DBT) grants for Rapid Grant for Young Investigators (RGYI) and Human Developmental and Disease Biology grants of Government of India. We appreciate the funding in form of Council of Scientific and Industrial Research (CSIR) and UGC Fellowships from Government of India.
REFERENCES
- 1. Tian J, Rui K, Tang X, et al. MicroRNA‐9 regulates the differentiation and function of myeloid‐derived suppressor cells via targeting Runx1. J Immunol. 2015;195:1301‐1311. [DOI] [PubMed] [Google Scholar]
- 2. Kuvardina ON, Herglotz J, Kolodziej S, et al. RUNX1 represses the erythroid gene expression program during megakaryocytic differentiation. Blood. 2015;125:3570‐3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bluteau D, Glembotsky AC, Raimbault A, et al. Dysmegakaryopoiesis of FPD/AML pedigrees with constitutional RUNX1 mutations is linked to myosin II deregulated expression. Blood. 2012;120:2708‐2718. [DOI] [PubMed] [Google Scholar]
- 4. Lordier L, Bluteau D, Jalil A, et al. RUNX1‐ induced silencing of non‐muscle myosin heavy chain IIB contributes to megakaryocyte polyploidization. Nat Commun. 2012;3:717. [DOI] [PubMed] [Google Scholar]
- 5. Bluteau O, Langlois T, Rivera‐Munoz P, et al. Developmental changes in human megakaryopoiesis. J Thromb Haemost. 2013;11:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 6. Al‐Abdi SY, Al‐Aamri MA. A systematic review and meta‐analysis of the timing of early intraventricular hemorrhage in preterm neonates: clinical and research implications. J Clin Neonatol. 2014;3:76‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129:2829‐2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sola‐Visner M, Sallmon H, Brown R. New insights into the mechanisms of non‐immune thrombocytopenia in neonates. Semin Perinatol. 2009;33:43‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raghuwanshi S, Karnati HK, Sarvothaman S, et al. microRNAs: key players in hematopoiesis. Adv Exp Med Biol. 2015;887:171‐211. [DOI] [PubMed] [Google Scholar]
- 10. Liu ZJ, Italiano J Jr., Ferrer‐Marin F, et al. Developmental differences in megakaryocytopoiesis are associated with up‐ regulated TPO signaling through mTOR and elevated GATA‐1 levels in neonatal megakaryocytes. Blood. 2011;117:4106‐4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Undi RB, Gutti U, Gutti RK. Role of let‐7b/Fzd4 axis in mitochondrial biogenesis through wnt signaling: In neonatal and adult megakaryocytes. Int J Biochem Cell Biol. 2016;79:61‐68. [DOI] [PubMed] [Google Scholar]
- 12. Yuva‐Aydemir Y, Simkin A, Gascon E, et al. MicroRNA‐9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8:557‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swiers G, de Bruijn M, Speck NA. Hematopoietic stem cell emergence in the conceptus and the role of Runx1. Int J Dev Biol. 2010;54:1151‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michaud J, Wu F, Osato M, et al. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 2002;99:1364‐1372. [DOI] [PubMed] [Google Scholar]
- 15. Growney JD, Shigematsu H, Li Z, et al. Loss of Runx1 perturbs adult hematopoiesis and is associated with a myeloproliferative phenotype. Blood. 2005;106:494‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ben‐Ami O, Pencovich N, Lotem J, et al. A regulatory interplay between miR‐27a and Runx1 during megakaryopoiesis. Proc Natl Acad Sci USA. 2009;106:238‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Draper JE, Sroczynska P, Leong HS, et al. Mouse RUNX1C regulates premegakaryocytic/erythroid output and maintains survival of megakaryocyte progenitors. Blood. 2017;130:271‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]


