Abstract
MicroRNAs are small non‐coding RNAs that play critical roles in the regulatory mechanisms involving cell differentiation, proliferation, apoptosis and tumorigenesis. Recent research efforts have been conducted to apply these discoveries into clinical functions, including the early diagnosis and therapeutic outcome of patients with cancer. Previous studies have shown that microRNA‐149 (miR‐149) is dysregulated in various human cancers and exerts its effects on tumorigenesis and tumour progression. In this review, we summarized the potential roles of miR‐149 dysregulation and its target genes during tumorigenesis and clinical treatment of human cancers.
Keywords: cancer, chemotherapy, diagnosis, dysregulation, miR‐149, prognosis
1. INTRODUCTION
Human cancer is a major public health issue worldwide because of its malignant characteristics and high mortalities.1 After decades of research, the mechanisms of cancer have accumulated an abundant and complex knowledge, such as the sustaining proliferative signalling, evading growth suppressors and activating invasion and metastasis during the stepwise development of cancer.2 Recently, it has been demonstrated that mircoRNAs (miRNAs) play important roles in the pathogenesis of cancers and interact with various signalling pathways in distinct cancers.3 Moreover, miRNAs are validated as biomarkers in cancer diagnosis and prognosis.4 Therefore, the mechanisms of cancers associated with miRNAs become necessary to be understood.
MicroRNAs are a class of small (20‐22 nucleotides) non‐coding RNAs that function as negative regulators of specific protein‐coding genes expression and, thus, play vital roles in the regulation of gene expression.5 In recent years, accumulating evidence has revealed that the dysregulation of miRNAs in human cancers are involved in tumour cell proliferation, apoptosis, differentiation, invasion and metastasis,3, 6 which has been linked with the aetiology, progression and prognosis of cancer. In addition, it was validated that miRNA genes could function as proto‐oncogenes or tumour suppressors due to the type of tumours.7 Further illustration of the association between miRNAs and carcinogenesis may contribute to understand cancers deeply and help to identify more potential diagnostic and prognostic parameters and, more importantly, to find potential therapeutic targets in clinical treatments for cancers.
MiR‐149 is a new‐found miRNA recently. In the human genome, the miRNA‐149 (miR‐149) gene is located on chromosome 2 at 2q37.3 (Gene ID: 406941), the first intron of human glypican 1 (GPC1).8 Mounting evidence indicates that miR‐149 may play a dual role in tumorigenesis. To be specific, miR‐149 is generally recognized as a tumour suppressor with reduction in distinct cancers,9, 10, 11, 12 while it is also reported that miR‐149 could function as an oncogene.8, 13, 14 In this review, we will summarize all the functions of miR‐149 reported and its involving signalling pathways including numerous targets associated with cancer hallmarks, such as proliferation, resistance to apoptosis, epithelial‐mesenchymal transition (EMT) and invasiveness in a variety of human cancers. Furthermore, the potential roles of miR‐149 as a biomarker for diagnosis and prognosis of cancers and potential use of cancer treatment will be also discussed.
2. BIOGENESIS AND STRUCTURE OF miR‐149
The biogenesis of miR‐149 is consistent with canonical miRNA biogenesis pathway (Figure 1A).15 First, miR‐149 is transcribed by RNA polymerase II as part of capped and polyadenylated primary transcripts (pri‐miR‐149) in nucleus. The primary transcript is cleaved to a stem‐loop precursor (pre‐miR‐149) containing 89‐nt by the Drosha RNase III enzyme. The predicted secondary structure based on RNA structure online software (RNAfold web server) is shown in Figure 1B. Then, pre‐miR‐149 is exported to the cytoplasm with the help of RanGTP‐dependent Exprotin5 and then further cleaved by the cytoplasmic Dicer RNase III to generate a miRNA/miRNA*duplex, which contains the mature miR‐149 (also known miR‐149‐5p) and antisense miR‐149* (also known miR‐149‐3p) products (Figure 1C). Based on numerous studies, miR‐149‐5p functions during various pathways through targeting various mRNAs in numerous tissues, while miR‐149‐3p may have specific functions in some specific cancer types and specific cases as well, such as melanoma under endoplasmic stress.8 Interestingly, it was reported that miR‐149‐5p and miR‐149‐3p were highly co‐expressed in brain and endothelial cell‐enriched tissues, such as lung and the well‐accepted angiogenic tissue and participate in the modulation of endothelial cell proliferation in response to fibroblast growth factor (FGF) via fine tuning levels of GPC1 and FGFR1.16 In fact, the majority of studies so far have been focused on miR‐149‐5p, and just few studies were focused on miR‐149‐3p. In this review, we will discuss both of the roles of miR‐149‐5p and miR‐149‐3p in human cancers.
Figure 1.
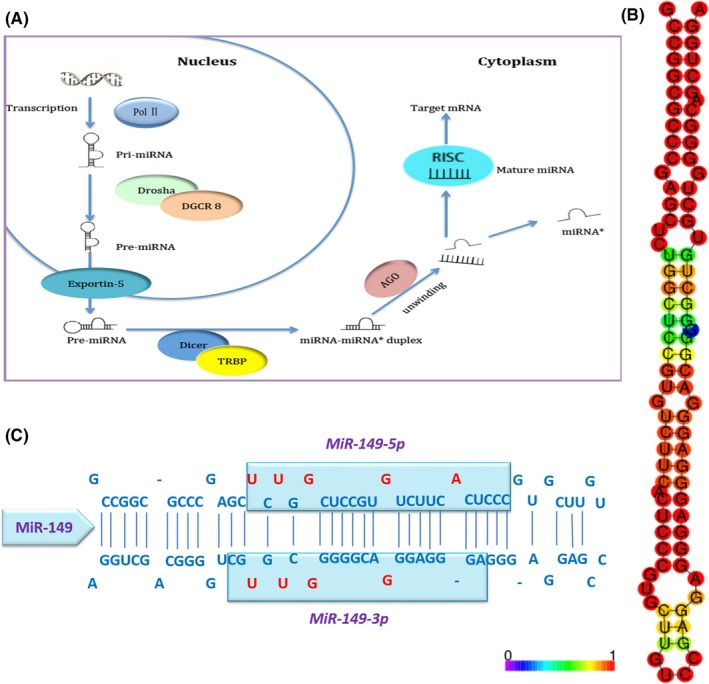
The sequences and biogenesis pathway of mature miR‐149. A, Model for miR‐149 processing: DROSHA cleavages pri‐miRNA into a stem‐loop structural pre‐miRNA, then be transferred to cytoplasm and removes the loop region by DICER, leaving the mature sequence. B, Predicted secondary structure of miR‐149. C, Mature miR‐149 and miR‐149* are 23 and 21 bp separately
3. DYSREGULATION AND DUAL ROLES OF miR‐149 IN HUMAN CANCERS
Accumulating evidence has suggested that miRNAs may play critical roles in tumorigenesis, including proliferation, apoptosis, cell cycle, metastasis, etc. Interestingly, miR‐149 has been shown to function as both a tumour suppressor and an onco‐miR during the development of various types of cancers (Table 1). For instance, compared with normal tissues, the level of miR‐149‐5p was found lower in non‐small‐cell lung cancer (NSCLC), hepatocellular carcinoma (HCC), gastric cancer (GC), colorectal cancer (CRC), breast cancer (BC), renal cell carcinoma (RCC), prostate cancer, glioma and upper tract urothelial carcinoma (UTUC) and have distinct functions, including proliferation inhibition, apoptosis induction, cell‐cycle regulation and metastasis promotion, in various cancers separately.9, 10, 11, 12, 17, 18, 19, 20, 21, 22, 23 On the contrary, it has also been reported that miR‐149‐5p may be upregulated and functions as an oncogene in some certain tumours, including melanoma, nasopharyngeal carcinoma (NPC), glioma, T‐cell acute lymphocytic leukaemia (T‐cell ALL) and castration‐resistant prostate cancer (CRPC).8, 13, 14, 24, 25 For instance, an oncogenic role for miR‐149‐5p was seen in NPC in which significantly increased.
Table 1.
Dysregulation of miR‐149‐5p and miR‐149‐3p and their targets and functions in various tumours
| Cancer type | MiR‐149 form | Expression | Function targets | Biological functions | References |
|---|---|---|---|---|---|
| Glioma | miR‐149‐5p | Up | Caspase‐2 | Inhibiting apoptosis | 24 |
| miR‐149‐3p | Down | Akt1 and E2F1 | Inducing apoptosis | 68 | |
| miR‐149‐5p | Down | p‐AKT1, PCNA, CyclinD1, MMP‐2 | Inhibiting proliferation and invasion | 21, 38 | |
| miR‐149‐5p | Down | RAP1B, CD47, CCNI, NXF1 | Inhibiting proliferation and migration | 22 | |
| CRC | miR‐149‐5p | Down | FOXM1 | Suppressing metastasis; mediating chemoresistance | 32, 74 |
| miR‐149‐5p | Down | SP1 | Inhibiting proliferation and invasion | 18 | |
| miR‐149‐5p | Down | – | Iron‐related genes, associated with invasion depth of CRC | 19 | |
| miR‐149‐5p | Down | SRPX2 | Inhibiting migration or invasion | 34 | |
| NSCLC | miR‐149‐5p | Down | FOXM1 | Suppressing EMT | 12 |
| SCC of NSCLC | miR‐149‐5p | Higher than adenocarcinoma | ABCC3, MUC1, CEACAM6 | – | 91 |
| BC | miR‐149‐5p | Down | Rap1a/1b | Inhibiting migration | 10 |
| miR‐149‐5p | Down | GIT1 | Inhibiting migration, invasion and metastasis | 35 | |
| miR‐149‐5p | Down | ErbB3 | Inhibiting proliferation | 36 | |
| miR‐149‐5p | Down | NDST1 | Associated with chemoresistance | 84 | |
| miR‐149‐3p | Down | GIT1 | Suppressing proliferation and metastasis | 77 | |
| Early‐relapsing BC | miR‐149‐5p | Down | – | Inhibiting metastasis | 93 |
| HCC | miR‐149‐5p | Down | Akt1 | Inhibiting proliferation | 9 |
| miR‐149‐5p | Down | PPM1F | Inhibiting metastasis | 76 | |
| miR‐149‐5p | Down | PARP2 | Tumour onco‐effect | 92 | |
| HCC with hepatitis C virus infected | miR‐149‐3p | Down | AAK1, PHLPPL | – | 105 |
| GC | miR‐149‐5p | Down | ZBTB2 | Inhibiting proliferation and cell cycle | 17 |
| miR‐149‐3p | Down | Wnt‐1 | Inhibiting tumour progression | 99 | |
| CAFs in GC | miR‐149‐5p | Down | IL‐6, EP2 | Inhibiting metastasis | 37 |
| NPC | miR‐149‐5p | Up | E‐cadherin | Promoting proliferation, mobility and invasion | 14 |
| miR‐149‐5p | Up | Smad2, TP63, CNTNAP2 | Promoting tumour progression | 78 | |
| miR‐149‐5p | ‐ | IL‐6 | Promoting tumour progression | 30 | |
| RCC | miR‐149‐5p | Down | – | Suppressing cell proliferation and migration, promoting apoptosis | 11 |
| ccRCC | miR‐149‐5p | Down | FOXM1, LOX, KCNAB1, KCNMA1 | Inhibiting proliferation, migration and invasion | 39, 73 |
| MiR‐149‐3p | Down | FOXM1 | Inhibiting proliferation, migration and invasion | 73 | |
| PC | miR‐149‐5p | Up | SOX2, NANOG, Oct4 | Promoting proliferation | 29 |
| miR‐149‐3p | Down | – | As one of diagnostic marker | 105 | |
| CRPC | miR‐149‐5p | Up | Ras, Rho, the SCF complex | Interfering cell cycle | 25 |
| T‐cell ALL | miR‐149‐3p | Up | JunB | Promoting proliferation and suppressing apoptosis | 13 |
| Melanoma | miR‐149‐3p | Up | GSK3α | Apoptosis resistance | 8 |
| miR‐149‐5p | Up | – | Correlated to metastasis | 70 | |
| Cervical carcinoma | miR‐149‐3p | Down | Akt1 and E2F1, b‐Myb | Inducing apoptosis | 68 |
| Uveal melanoma | miR‐149‐3p | – | – | Correlating with liver metastasis | 80 |
| Laryngeal squamous cell carcinoma | miR‐149‐5p | Down | – | Inhibiting proliferation, correlated to tumour stage and metastasis situation | 67 |
| Oesophagus adenocarcinoma | miR‐149‐5p | Down | – | – | 83 |
| UTUC | miR‐149‐5p | Down | – | Associated with tumour progression | 23 |
| Chordoma | miR‐149‐3p | Up | MKP, PKA, MEKK2β | Promoting tumour progression | 40 |
| OS | miR‐149‐5p | Up | BMP9 | Inhibiting apoptosis | 69 |
| BCa | miR‐149‐3p | Down | S100A4 | Inhibiting proliferation, migration and invasion | 79 |
BC, breast cancer; BCa, Bladder cancer; CAF, cancer‐associated fibroblasts; ccRCC, clear cell renal cell carcinoma; CRC, colorectal cancer; CRPC, castration‐resistant prostate cancer; GC, gastric cancer; HCC, hepatocellular carcinoma; NPC, nasopharyngeal carcinoma; NSCLC, non‐small‐cell lung cancer; OS, osteocarcinoma; PC, prostate cancer; RCC, renal cell carcinoma; SCC, squamous cell cancer; T‐cell ALL, T‐cell acute lymphoblastic leukaemia; UTUC, upper tract urothelial carcinoma.
3.1. The upstream and downstream pathways involving in miR‐149 dysregulation
There is an evolutionarily conserved cascade reaction of signal transduction concerning to the elementary cellular processes involving miR‐149 (Table 1). In this review, we made a brief summarization of the current knowledge associated with the regulatory network of miR‐149 (Figure 2). Accumulating evidence shows that miRNA dysregulation is contributed by both genetic and epigenetic alterations.26 It was demonstrated that in melanoma, p53 directly upregulates miR‐149‐3p and then in turn targets GSK‐3α, resulting in elevated expression of Mcl‐1 and resistance to apoptosis.8 XB130, also known as actin filament‐associated protein 1‐like 2 (AFAP1L2), is one of adaptor protein family.27 Takeshita et al found that XB130 regulates expression of miR‐149‐5p negatively in human cancer cells, and the latter inhibits cell proliferation by directly targeting FOSL1 (also called Fra‐1), which is a proto‐oncogene.28 In addition, it was verified that miR‐149‐5p along with miR‐126 contributes to syndecan‐1‐dependent cell growth and syndecan‐1 regulates cell proliferation by enhancing miR‐126‐ and miR‐149‐5p‐mediated expression of stem cell‐related factors, SOX2, NANOG and Oct4, in prostate cancer.29 A new study demonstrated that LncRNA‐LINC00460 contributed to the tumorigenesis of NPC through sponging miR‐149‐5p and then upregulating IL‐6.30 Considering that aberrant DNA methylation is one of the major causes of miRNA dysregulation in cancer among the epigenetic alterations and approximately half of human miRNAs are associated with CpG islands (CGI) including miR‐149‐5p,31, 32 the methylation variations about miR‐149 have raised more and more concern recent years. Gathering evidence has reported that miR‐149‐5p was methylated and dysregulated in several types of cancers, such as BC, GC, CRC, GBM (glioblastoma multiforme) and cervical carcinomas.18, 33, 34, 35, 36 Li et al validated that PGE2 induces the DNA methylation in cancer‐associated fibroblasts (CAFs) from human GC tissues, resulting in miR‐149‐5p downregulation and the subsequent functions of carcinogenesis in GC.37 Wang et al identified that miR‐149‐5p is silenced in human CRC cells, and its downregulation is associated with hypermethylation of the neighbouring CpG island (CGI).18 Moreover, it has been shown that miR‐149‐5p is downregulated in GBM, which is at least, in part, due to the hypermethylated promoter region of miR‐149‐5p.38
Figure 2.
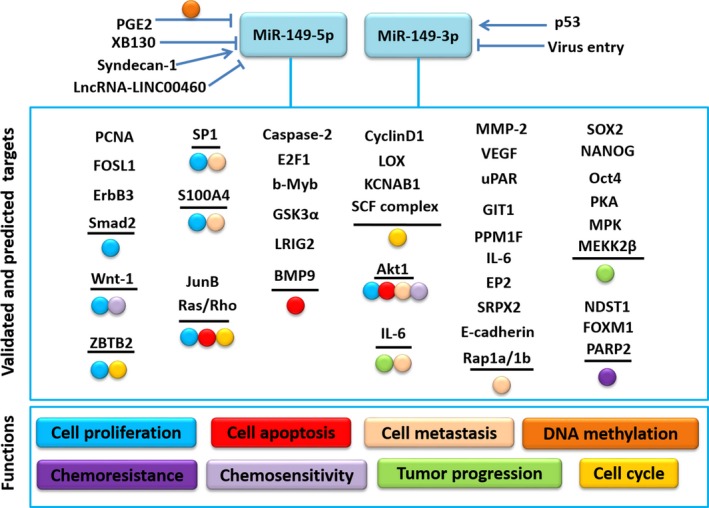
The upstream regulation and downstream targets of miR‐149. MiR‐149 regulates distinct cellular processes by participating multiple targeting, including cell proliferation, cell apoptosis, cell metastasis, chemoresistance and so on. The details could be acquired in the context
Growing evidence has shown that miR‐149 has numerous direct and indirect downstream factors. For instance, according to Fan's study,13 miRNA‐149‐3p was highly expressed in T‐cell ALL cell lines and T‐cell ALL patients' bone marrow samples and it promotes cell proliferation, interferes cell cycle and suppresses cell apoptosis by targeting JunB and modulating other specific effectors, such as p21, cyclinD1, 4EBP1 and p70s6k. Bischoff et al found that miR‐149‐5p is downregulated in basal breast cancer compared with other types of BC and functions as a tumour suppressor.10 On the basis of KEGG pathway analysis, miR‐149‐5p affects the ErbB pathway. Later in 2015, they studied further and provided evidence that miR‐149‐5p together with some other miRNAs directly targets ErbB3 to block HRG‐mediated viability of BC cells and hinders HRG‐induced MAPK and PI3K signalling and thus exerts tumour suppressive properties.36 There was a study that depicts a relatively convenient and efficient method to gene targets of dysregulated miRNAs in human cancers. It shows that LOX, KCNMA1 and KCNAB1, which are oncogenes, are 3 predicted representative, direct targets of miR‐149‐5p.39 Apart from of these, miR‐149‐3p along with 4 other miRNAs (miR‐663a, miR‐1908, miR‐2861 and miR‐3185) was upregulated in chordoma tissues compared with notochord tissues, and the 5 miRNAs were predicted to target genes encoding 7 elevated mRNAs (FGF2, JUND, DUSP4, MAP3K3, TGFB1, PRKACA and RAPGEF2), which are all involved in MAPK signalling pathway. Thereby, it was speculated that the 5 miRNAs play vital roles in the regulation of MAPK pathways and have important effects in tumorigenesis of chordoma as a result.40
3.2. Single‐nucleotide polymorphisms (SNPs) in pre‐miR‐149
To date, the literatures have suggested that the functional consequences of SNPs in pri‐miRNAs refer to mature miRNA processing and levels and subsequently contribute to cancer risk, prognosis and survival. The molecular mechanisms underlying the genetic associations of miR‐SNPs with various cancers are complicated and differ by cancer site.41 So far, there have been 2 SNPs in pre‐miR‐149 studied frequently, rs2292832 (G/T or C/T) and rs71428439 (A/G; located in the stem‐loop region of miR‐149), which are both located in other regions of pre‐miR‐149 except 5p or 3p mature miR‐149 regions, and could influence the maturation of miR‐149 and lead to a change in the structure of pre‐miR‐149.42 SNPs in pre‐miRNA genes are thought to affect function through 2 main mechanisms: first, through changing the sequence specificity of miRNA core regions and altering the combining affinity between miRNA and mRNA; second, through influencing the maturation process of miRNA and altering its expression level. For SNP of miR‐149, the latter mechanism is applicative. The association between miR‐149 SNPs and various cancer risks has been explored in numerous previous studies. However, controversial experimental data were shown.
For miR‐149 rs2292832 polymorphism, Li et al reported that rs2292832 is associated with a higher risk of digestive cancer risk, though the results were negative as regard to whole cancer risk.43 Interestingly, another study showed that rs2292832 marginally contributes to gastrointestinal cancer susceptibility based on the pooled studies, especially for Asians. However, no significant associations were found when the analysis was stratified by cancer type, including CRC and GC.44 It is a pity that in some studies no association was shown between rs2292832 with overall cancer risks.45, 46, 47, 48, 49, 50 As regard to every concrete cancer type, the results in GC, squamous cell carcinomas of the non‐oropharynx (SCCNOP), BC and papillary thyroid cancer (PTC) were consistent, while the results in other types of cancer are conflicting as well. Here, we mainly summarized the positive reports. It was shown that miR‐149 rs2292832 CC genotype was associated with a higher risk of GC in the Greek population, the heterozygote TC genotype was associated with a decreased risk of gastric cancer,51 and there were marginally significant associations for has‐miR‐149 rs2292832 with GC risk in male population. And interestingly, tea drinking was a protective factor in miR‐149 rs2292832 for GC and could modify the associations of miR‐149 polymorphisms with gastrointestinal cancer risk.52 A recent study examined the effect of rs2292832 SNP on biological behaviours of PTC and found that CC genotype was involved in increased susceptibility of PTC and more advanced local progression through reducing the expression level of mature miR‐149 and further targeting its predicted downstream genes, PDGFRA and CD47, to function as a tumour suppressor.53 In SCCNOP, patients having CC wild‐type genotype had significantly better overall, disease‐specific and disease‐free survival especially those who ever smoked and treated with chemoradiation.54 Researchers failed to find any significant association with the risk of BC in any genetic model, including that in Chinese women. In lung cancer, the patients with C allele of mir‐149 have a better overall survival,55 especially in women NSCLC.56, 57 Two research groups found that this SNP may be associated with increased risk of CRC in population‐based studies for recessive model, and miR‐149 rs2292832 polymorphism was associated with a significantly increased susceptibility of CRC in the TT homozygote, particularly in Asians.58, 59 Furthermore, miR‐149 CT/CC genotype carriers had increased susceptibilities to CRC among patients engaging in smoke inhalation, suggesting that smoking might have the potential to modify the relationship of miR‐149 with CRC.52 It was reported that rs2292832 was associated with significantly decreased risk of HCC, and the CC or CT genotype had a higher survival rate in patients at OKUDA stage II.60 Another study showed that the miR‐149 CC genotype has non‐significant increased risk of HCC, particularly in HBV‐infected patients.61 In head and neck squamous cell carcinoma (HNSCC), Tu et al reported that the expression of miR‐149 is decreased and that is correlated with an unfavourable survival of patients.62 Moreover, the T/T genotype from the rs2292832 polymorphism predicted the worse prognosis of buccal mucosa carcinoma subset of HNSCC, since the T variant in pre‐miR‐149 hinders the transform of pre‐miR‐149 to mature ones and thus changes the abundance of mature miR‐149, resulting in the poorer patient survival. To our interest, there was no association between separate SNP of miR‐149 with risk of HNSCC. However, the miR‐149 rs2292832 polymorphism along with other 3 common polymorphisms (miR‐146a, miR‐196a and miR‐499) may have a joint effect on the risk of HNSCC in a risk‐genotype dose‐response manner, especially in subjects with 4 risk genotypes.63 Moreover, it was found that the CC genotype of rs2292832 polymorphism was associated with risk of oral squamous cell carcinoma (OSCC) in South Indian population.64
For miR‐149 rs71428439 polymorphism, it was previously showed that rs71428439 resulted in a change in the structure of the miR‐149 precursor and the G‐allelic miR‐149 precursor displayed a lower production of mature one.42 Wu and colleagues found that patients with GG genotype express lower level of miR‐149 thus higher AKT1 (a pre‐validated 149 target in vitro) and have significantly higher risk of HCC in the Chinese Han population.65 Another study by Wang et al showed that rs7142843 was significantly associated with increased ccRCC risk and may be a new biomarker for ccRCC susceptibility.66
4. MiR‐149's ROLES AND FUNCTIONS IN DIFFERENT CANCERS
4.1. MiR‐149 and proliferation
Sustaining proliferative signalling is one of the most significant hallmarks of cancers. Recent studies have shown that miR‐149 plays important effect on cell proliferation of various cancers. Pan et al investigated the effects of lentivirus‐mediated overexpression of miR‐149‐5p upon the level of phosphated‐AKT1 (p‐AKT1), proliferating cell nuclear antigen (PCNA), matrix metallopeptidase‐2 (MMP‐2) and CyclinD1 in U251 cells.21 The results suggested that miR‐149‐5p targets p‐AKT1, PCNA to inhibit glioma cell proliferation via blockade of the AKT1 signalling. The same conclusion was reached in Ghasemi's study in GBM.38 Similarly, Zhang and colleagues validated that miR‐149‐5p was downregulated in HCC cells and tissues, and that miR‐149‐5p inhibited cell proliferation, invasion activities and migration activities of HepG2 cells by directly targeting AKT1. Furthermore, the expression level of miR‐149‐5p was relevant to tumour stage of HCC patients. The samples at advanced tumour stage have lower miR‐149‐5p expression compared with those at early tumour stage.9 These findings were similar to the conclusions of Wang et al on gastric cancer (GC) as well.17 Also in laryngeal squamous cell carcinoma, miR‐149‐5p inhibits the proliferation and its low level is associated with stages, differentiation and metastasis.67 The above studies hitherto show that miR‐149‐5p inhibits the proliferation of glioma, HCC and GC cells.
4.2. MiR‐149 and apoptosis
Apoptosis is known as programmed cell death process occurring in multicellular organisms, which is also an obstacle of cancer. As a consequence, hindering of apoptosis in cells which gets from oncogenic mutations can contribute to tumour progression, and inversely, apoptosis induction can repress tumour progression as well.
There have been several studies showing the anti‐ or pro‐apoptosis effect of miR‐149. Jin et al performed one study and validated a special mechanism, p53–miR‐149‐3p–GSK3α–Mcl‐1 pathway, involved in the survival of melanoma cells under endoplasmic reticulum (ER) stress.8 In their results, elevated miR‐149‐3p suppressed GSK3‐α expression and thus upregulated the expression level of Mcl‐1, leading to apoptotic resistance of melanoma cells. To our surprise, miR‐149‐3p induces apoptosis by repressing v‐akt murine thymoma viral oncogene homologue 1 (AKT1) and E2F transcription factor 1 (E2F1) expression in neuroblastoma and HeLa cells.68 A novel study showed that in osteocarcinoma (OS) patients, upregulation of miR‐149‐5p and the resultant downregulation of its target bone morphogenetic protein 9 (BMP9) inhibit apoptosis and then promote the progression of OS.69
Interestingly, just as above mentioned, Pan's study in GM cells in 2012 validated that miR‐149‐5p is lower in glioma and targets p‐AKT1, PCNA to inhibit glioma cell proliferation and thus hinders glioma development.21 Nevertheless this conclusion is contrary to Shen's,24 which was performed in 2016. They demonstrated that the expression level of miR‐149‐5p is higher in glioma and inhibits cell apoptosis and promotes cell viability via caspase‐2‐p53‐p21 pathway. More rigorous and convincing studies need to be performed and reach a convincing conclusion.
There is not only one apoptosis pathway correlated to miR‐149‐5p and miR‐149‐3p in distinct tumours, and the detailed mechanisms in specific tumour should be explored further.
4.3. MiR‐149 and cell cycle
It is generally recognized that cell cycle is a crucial process in which every component has distinct functions and the natural biochemical procedure impeded in some certain phase will affect the proceeding of cell cycle and lead to disorders involved in cancers. ZBTB2 was demonstrated as a TF in GC which can suppress the ARF‐HDM2‐p53‐p21 pathway to repress cell‐cycle progression by inhibiting the transcription of ARF, p53 and p21, and inducing HDM2 expression. It was shown that miR‐149‐5p is reduced in GC cell lines and clinical samples and ectopic miR‐149‐5p expression induces G0/G1 arrest in vitro, which indicates that miR‐149‐5p plays its role of repressing GC cell‐cycle progression by directly targeting ZBTB2.17 Pan's investigation about the effects of miR‐149‐5p overexpression upon the level of p‐AKT1, PCNA, MMP‐2 and CyclinD1 in U251 cells also showed that miR‐149‐5p targets CyclinD1 to induce glioma cell cycle evading via blocking the AKT1 signalling.21 Moreover, miR‐149‐5p was found upregulated in melanoma tumour tissue, and it may have important effects on G1/S checkpoint abnormalities by regulating cell response to TP53/RB1 activation and TGFβ/SMAD signalling pathways together with some other miRNAs.70 Also, Zhu and colleagues performed Gene Ontology (GO) analysis, pathway enrichment analysis and miRNA‐mRNA interaction network construction analysis and validated that miR‐149‐5p was upregulated with some other miRNAs in castration‐resistant prostate cancer (CRPC). Speculated that miR‐149‐5p along with some other miRNAs might contribute to the development of CRPC through Ras and Rho (proteins related to the G1/S transition), and the SCF complex (the Skp1‐cullin‐F‐box ubiquitin ligase (SCF) complex, related to cell cycle).25
4.4. MiR‐149 and metastasis (including EMT, migration and invasion)
Cancer is one of the most difficult issues and leads to death worldwide, mainly because of the development of metastasis at advanced stages of different cancers. Tumour metastasis contains a series of steps including separation, migration, invasion and so on. Furthermore, EMT has vital effects on the process of cancer metastasis since its invasive and metastatic properties on cancer cells.71 In view of advanced Forkhead box M1 (FOXM1) expression coincided with many cancers metastasis,72 one study found that dual strands of pre‐miR‐149 inhibit migration and invasion through targeting FOXM1 in RCC.73 Ke et al also demonstrated that miR‐149‐5p inhibited EMT and metastasis of NSCLC cells by directly targeting FOXM1.12 A similar mechanism in CRC was validated by Xu et al who demonstrated that miR‐149‐5p was obviously low in CRC tissues and that significantly repressed migration and invasion of CRC cells by targeting FOXM1.74 Another study in CRC suggested that the downregulation of miR‐149‐5p contributes to the regulation of the SRPX2 (Sushi repeat containing protein, X‐linked 2) transcript level, which may partly functions as a suppressor in migration or invasion through uPAR and FAK signalling.34 Pan et al also found that miR‐149‐5p inhibits glioma migration and invasion through downregulation of one of its targets, MMP‐2 expression via blockade of the AKT1 signalling.21 Since that SRPX2 is known to exert migration enhancing function in GC cells.75 Luo and colleagues found that miR‐149‐5p level is significantly lower, which was correlated with distant metastasis and TNM stage of HCC.76 Their further research suggested that miR‐149‐5p suppressed the invasion and migration of HCC through targeting PPM1F (protein phosphatase, Mg(2+)/Mn(2+)‐dependent, 1F), which is involved in the motility and adhesion of cancers by regulating cytoskeleton remodelling. It was reported that the expression of miR‐149‐5p was significantly downregulated in grade I–IV astrocytomas and was negatively correlated with tumour stage. Also, RAP1B, CD47, CCNI and NXF1 were targets of miR‐149‐5p. Of these, RAP1B was validated the inverse correlation with miR‐149 in grade I–IV astrocytomas, which suggested that miR‐149‐5p may inhibit the proliferation and migration of glioblastomas, at least partly, by targeting RAP1B.22 Recently, Chan et al and Dong et al demonstrated that miR‐149‐5p and miR‐149‐3p both can target GIT1, G‐protein‐coupled receptor kinase‐interacting protein 1, and negatively regulate GIT1 in the way of suppressing migration, invasion and metastasis in BC cells. Also, low level of miR‐149‐5p and high level of GIT1 is associated with advanced stages and lymph node metastasis of BC.35, 77 To our surprise, miR‐149‐5p was found downregulated in basal breast cancer compared with other types of BC and blocked the tumour cells to spread and migrate by suppressing aberrant Rac activation by targeting small GTPases Rap1a and Rap1b, which are downstream of integrin receptors in Bischoff's study.10
As above discussed, the majority studies showed that miR‐149‐5p inhibits EMT and metastasis in most cancers. However, in NPC, the studies showed opposite conclusion. These studies indicated that miR‐149‐5p was upregulated in NPC cell lines and promoted the proliferation, mobility and invasion of NPC cells. Apart from these, miR‐149‐5p may be involved in the regulation of EMT via downregulating the E‐cadherin level, while the inhibitor of miR‐149‐5p upregulated the E‐cadherin level highly.14, 78 These facts indicate that miR‐149 may have different functions about tumour metastasis based on specific cancer type.
For miR‐149‐3p, a novel study demonstrated that its expression significantly hinders cell proliferation, migration and invasion in BC cells through one of its target S100A4 in Bladder cancer (BCa) cells.79 Another study validated that the expression of miR‐149‐3p can be used to evaluate the metastasis‐free survival in uveral melanoma (UM) patients and shows a statistically significant correlation with liver metastasis.80 Knowing well of the mechanisms by which miR‐149 is associated with tumour metastasis will guide people to explore more effective directions of cancer treatment.
4.5. MiR‐149 and tumour microenvironment
Tumour development relies on the interactions between tumour and the surrounding microenvironment. Recent studies have demonstrated that cancer‐associated fibroblasts (CAFs) play important roles in the crosstalk between tumour cells and their microenvironment to promote tumour growth, although they are not malignant themselves.81 MiR‐149 has also been found to be correlated with tumour microenvironment. Li and colleagues found that miR‐149‐5p is significantly downregulated due to PGE2‐induced DNA methylation in CAFs from human GC tissues and is a critical factor for the transformation of NFs into CAFs in GC.37 MiR‐149‐5p targets IL‐6 and downregulates its expression to inhibit fibroblast activation and thus weakens EMT and stem‐like properties of GC cells. Also, EP2 is validated as another target of miR‐149‐5p. That is to say, PGE2 binds to EP2 and induces the hypermethylation and suppression of miR‐149‐5p, reversing the downregulation of IL‐6 and EP2 expression, which indicates that there exists a positive feedback loop in CAFs in which PGE2 triggers the hypermethylation of miR‐149‐5p. Based on the above results, the authors concluded that miR‐149‐5p mediates the crosstalk between tumour cells and CAFs in GC in a miR‐149‐5p‐IL‐6‐dependent manner and highlighted the potential of interfering miRNAs in stromal cells to improve cancer therapy. Exosomes derived from tumours are bioactive vesicles that have vital effects on crosstalk between tumour and normal cells through cell recruitment to the malignant process and they are supposed to be involved in regulating the tumour microenvironment.82 Pfeffer et al found that miR‐149‐5p is upregulated in melanoma tumour tissue and the exosomes derived from plasma of metastatic sporadic melanoma patients, indicating that exosomes may have partial effects on malignancy of melanoma.70 In addition, compared with adjacent normal tissue, miR‐149‐5p was downregulated in adenocarcinoma of the oesophagus and was merely or not detectable in exosomes isolating from serum of oesophageal cancer patients, while some other miRNAs, such as miR‐223‐5p and miR‐483‐5p, are upregulated, which may favour to identify an exosomal onco‐miRs profiles.83
4.6. MiR‐149 and tumour chemoresistance
Chemotherapy is one of the essential methods to resist cancers. However, the resistance to chemotherapeutic agents is a clinical obstacle to the successful treatment of cancer, mainly because chemoresistant cancer cells have specific aberrant apoptotic response to drugs and acquire enhanced cell survival signals. MiR‐149 has been shown to be associated with chemoresistance as well. He et al found that miR‐149‐5p is downregulated due to hypermethylation of its 5′‐UTR and is involved in chemoresistance in adriamycin‐resistant human BC cells.84 The underlying mechanism is that miR‐149 targets GlcNAc N‐deacetylase/N‐sulfotransfer‐ase‐1 (NDST1) and then promotes the transform of tumour cells to more malignant ones via the activation of HS (heparin sulphate)‐mediated pathways. Based on the previous study by Liu, it was demonstrated that miR‐149‐5p inhibits the migration and invasion of CRC cells by targeting FOXM1. The author made further study on the roles of miR‐149‐5p in the chemoresistance of CRC cells to 5‐Fluorouracil (5‐FU). They unravelled that re‐expression of miR‐149‐5p could increase the sensitivity of 5‐FU‐resistant CRC cells to 5‐FU, that is, reverse the 5‐FU resistance of CRC cells through attenuating 5‐FU‐induced apoptosis, partly by targeting FOXM1. Thus, there is a potential strategy focusing on miR‐149‐5p/FOXM1 signalling to improve the treatment for 5‐FU‐resistant CRC.32 Except for the above, there is a mechanism from Jaiswal's research showing that microparticles (MPs) are membrane vesicles released from normal and malignant cells and then bud and detach from donor cells and carry the essential enzymes, including Drosha, Dicer and Argonaute, for miRNA biogenesis.85 Apart from this, the miRNAs including miR‐149‐3p, which is transferred by MPs and increased relative to their donor cells, plays an important role in the regulation of biological processes involved in anti‐cancer drug resistance. Moreover, the malignancy‐related upregulated expression of miR‐149‐3p and other several miRNAs may serve as potential biomarkers in the treatment of cancer.
5. CLINICAL APPLICATIONS OF miR‐149 IN HUMAN CANCERS
5.1. MiR‐149 as a biomarker for diagnosis and prognosis
It was demonstrated that miRNAs are present in the serum and plasma of humans and their levels in serum are stable.86 Emerging evidence suggested that serum miRNAs can serve as potential biomarkers for the detection of various cancers87 and specific miRNA profiles were capable of distinguishing malignant and non‐malignant tissues, raising the feasibility of miRNAs in early detection of cancer, prognosis informing and treatment response monitoring.88, 89 MiR‐149 can be a biomarker as well. It was found that miR‐149 was downregulated in PC and it combined with other 5 miRNAs (miR‐96, miR‐181b, miR‐182, miR‐205, miR‐375) having an overall correct classification of 80% (AUV = 0.88), which can serve as one of powerful diagnostic markers for PC.20 Berghmans et al performed a prospective study on patients with advanced NSCLC and treated with cisplatin and vinorelbine. They found that miR‐149‐5p and miR‐375 could predict response and progression‐free survival in those patients.90 Molina‐Pinelo et al identified miRNA‐dependent mRNA distinct profiles, aiming to discriminate SCC from adenocarcinoma histological subtypes of NSCLC. Interestingly, it was found that miR‐149‐5p was over‐expressed in SCC compared with adenocarcinoma and miR‐149‐5p's predicted target ABCC3 is validated and downregulated in SCC.91 In HCC, miR‐149 was found downregulated significantly and was associated with poor clinicopathologic features, such as larger tumour volume, capsular and vascular invasion, lymph node metastasis, high histological grade, TNM and BCLC grade. Also, low miR‐149‐5p was correlated with a low survival rate of HCC, as a result of being predictive factors for poor prognosis of HCC.9, 76, 92 In BC, Perez‐Rivas and colleagues identified 5 miRNAs, including miR‐149‐5p, downregulated in tumours from patients with early relapse, and found that the 5‐miRNA signature represents high‐risk early recurrence and a shorter relapse‐free survival (RFS), as a result of having predictive value to distinct non‐relapsing from early‐relapsing patients and predict patients who have higher risk to develop metastasis early after primary surgery.93 Moreover, there was one study showing that miR‐149‐5p expression is downregulated in progressing upper tract urothelial carcinoma (UTUC). They identified the expression of miR‐149‐5p as independently associated with pathological tumour progression, tumour stage and cancer‐specific survival and thus can be independent prognostic factors of cancer‐specific survival.23 In addition, similar to several other studies about the function of miR‐149‐5p in glioma, Xue et al validated that miR‐149‐5p was obviously lower, which is associated with Akt/mTOR signalling, and the level of miR‐149‐5p was significantly correlated with patients' survival duration.94 Generally, miR‐149‐5p expression might be an eligible and independent indicator of diagnosis and prognosis of some cancers.
5.2. MiR‐149 as a therapeutic target for human cancers
Growing evidence has shown different effects of distinct drugs on different cancers. There have been several definite researches about association between miR‐149 and chemotherapy agents. One recent study95 showed that the expression of miR‐149 and miR‐128 was reduced in glioblastoma and the expression of miR‐149‐5p is negatively correlated with grade, which is consistent with studies by Li.22 The 2 miRNAs function as tumour suppressors via their common target Rap1B, resulting in changes in the actin cytoskeleton and inhibition of cell adhesion and invasion and cell proliferation. Temozolomide (TMZ), as the standard first‐line agent for GBM, inhibits proliferation. Interestingly, miR‐149‐5p and miR‐128 increase this effect and hinder invasion by targeting Rap1B‐mediated cytoskeletal alteration and related molecules, including Cdc42, RhoA and N‐cadherin. miR‐128 and miR‐149‐5p enhanced the chemosensitivity of human glioblastoma cells to TMZ. There is another study by Ru et al on the drugs treated glioma.96 Quinidine, which is a voltage‐gated K+ channel blocker, could inhibit cell proliferation and induce apoptosis in human glioma U87‐MG cells. Researchers found that after quinidine treatment, the expression of miR‐149‐3p and miR‐424‐5p was elevated and decreased, respectively, thus indicating that the anti‐proliferation and pro‐apoptosis effect in glioma of quinidine was mediated at least partly via regulating miRNA expression. In addition, it was demonstrated that bafilomycin A1 inhibits proliferation, invasion of the BEL‐7402 liver cancer and HO‐8910 ovarian cancer cell lines and induces upregulation of miR‐149‐5p in certain pathways. It is speculated that miR‐149‐5p with some other dysregulated miRNAs altered by bafilomycin A1 in the 2 cell lines may represent promising targets for anti‐cancer therapies.97 As mentioned above, low miR‐149‐5p and positive PARP‐2 (Poly(ADP‐ribose) polymerase‐1), which is also validated critical in tumour development,98 are involved in pathogenesis of HCC and both miR‐149‐5p overexpression and PARP‐2 inhibitor have anti‐tumour effect, although targeting PARP‐2 alone is a better strategy to treat with HCC in that PARP‐2 inhibitor is a chemo/radio sensor and can be used to strengthen chemotherapy and radiotherapy in HCC patients. Based on the study by Cao and colleagues, miR‐149‐3p was downregulated in GC but was upregulated by 18β‐glycyrrhetinic acid (GRA), which is an anti‐tumour agent by suppressing the tumorigenesis of GC.99 The possible mechanism is that GRA upregulates miR‐149‐3p, resulting in downregulation of Wnt‐1, and then together with amelioration of the inflammatory microenvironment through downregulation of COX‐2 to inhibit the initiation and progression of GC. Moreover, a latest study performed by Si et al described that after dioscin treatment, miR‐149‐3p was upregulated and induced apoptosis and suppressed tumour growth in pancreatic cancer through inhibiting the Akt1 signalling pathway.100
Based on the above clinical findings about the relationships between miR‐149 and several specific agents towards some specific cancers, these findings revealed that it is very promising for miR‐149 to be applied to the treatment of different tumours. Actually, miRNA‐based therapeutics have brought more and more concern recently,101 including miRNA mimics and anti‐miRs, aiming at tumour suppressors or onco‐miRs, respectively.102 However, it remains obstacles about the use of miRNA‐cased therapies, for example, the biological instability of the oligonucleotides and the poor cellular uptake. Fortunately, researchers have been making numerous attempts to solve the problems, mainly including different chemical modifications and delivery systems.103 As to tissue or cell‐specific targeting, several encapsulation methods, such as EnGeneIC Delivery Vehicle (EDV) nanocells (also called TargomiRs),104 have been developed. What a pity is that there is no study about agents directly targeting miR‐149 yet. Since miR‐149‐5p generally acts as a tumour suppressor in most cancers as above mentioned, the miR‐149‐5p mimics combining useful encapsulated and delivery methods may be a promising direction for treatment of those tumours.
6. CONCLUSION AND FUTURE VISION
Accumulating evidence hints that miRNAs are significant regulators of various cancers and could be utilized as novel biomarkers for cancer diagnosis, prognosis and treatment. In the past decades, miR‐149 was verified to be aberrantly expressed in most cancers and widely studied in tissue or blood and has emerged as essential and critical components involved in tumorigenesis. In this review, we collected and introduced the already published literatures presenting miR‐149 dysregulation in human malignancies and the mechanisms underlying miR‐149‐mediated carcinogenesis, such as proliferation, apoptosis, EMT and metastasis. To our best knowledge, this is the first review that systematically and mainly pays attention to the miR‐149 roles in tumour development and progression. Although there are still some clinical roles of miR‐149 in various cancers unravelled, there is no doubt that miR‐149 is referred to the regulation of different pathways that are involved in development and progression of many type of cancers, and may act as a useful biomarker in diagnosis, prognosis and therapy prediction of human cancers.
CONFLICTS OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (NSFC) (grant number: 81472266, 81772996) and the Excellent Youth Foundation of Jiangsu Province, China (grant number: BK20140032).
Zhi Y, Zhou H, Mubalake A, et al. Regulation and functions of MicroRNA‐149 in human cancers. Cell Prolif. 2018;51:e12465 10.1111/cpr.12465
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5‐29. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 3. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857‐866. [DOI] [PubMed] [Google Scholar]
- 4. Melo SA, Esteller M. Dysregulation of microRNAs in cancer: playing with fire. FEBS Lett. 2011;585:2087‐2099. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281‐297. [DOI] [PubMed] [Google Scholar]
- 6. Iorio MV, Croce CM. Causes and consequences of microRNA dysregulation. Cancer J. 2012;18:215‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11:252‐263. [DOI] [PubMed] [Google Scholar]
- 8. Jin L, Hu WL, Jiang CC, et al. MicroRNA‐149*, a p53‐responsive microRNA, functions as an oncogenic regulator in human melanoma. Proc Natl Acad Sci USA. 2011;108:15840‐15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Guo X, Xiong L, et al. Comprehensive analysis of microRNA‐regulated protein interaction network reveals the tumor suppressive role of microRNA‐149 in human hepatocellular carcinoma via targeting AKT‐mTOR pathway. Mol Cancer. 2014;13:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bischoff A, Huck B, Keller B, et al. miR149 functions as a tumor suppressor by controlling breast epithelial cell migration and invasion. Cancer Res. 2014;74:5256‐5265. [DOI] [PubMed] [Google Scholar]
- 11. Jin L, Li Y, Liu J, et al. Tumor suppressor miR‐149‐5p is associated with cellular migration, proliferation and apoptosis in renal cell carcinoma. Mol Med Rep. 2016;13:5386‐5392. [DOI] [PubMed] [Google Scholar]
- 12. Ke Y, Zhao W, Xiong J, Cao R. miR‐149 inhibits non‐small‐cell lung cancer cells EMT by targeting FOXM1. Biochem Res Int. 2013;2013:506731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. S‐j F, H‐b L, Cui G, et al. miRNA‐149* promotes cell proliferation and suppresses apoptosis by mediating JunB in T‐cell acute lymphoblastic leukemia. Leuk Res. 2016;41:62‐70. [DOI] [PubMed] [Google Scholar]
- 14. Luo Z, Zhang L, Li Z, et al. miR‐149 promotes epithelial‐mesenchymal transition and invasion in nasopharyngeal carcinoma cells. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University. Medical sciences. 2011;36:604‐609. [DOI] [PubMed] [Google Scholar]
- 15. Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43:892‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chamorro‐Jorganes A, Araldi E, Rotllan N, Cirera‐Salinas D, Suarez Y. Autoregulation of glypican‐1 by intronic microRNA‐149 fine tunes the angiogenic response to FGF2 in human endothelial cells. J Cell Sci. 2014;127:1169‐1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Y, Zheng X, Zhang Z, et al. MicroRNA‐149 inhibits proliferation and cell cycle progression through the targeting of ZBTB2 in human gastric cancer. PLoS ONE. 2012;7:e41693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang F, Ma YL, Zhang P, et al. SP1 mediates the link between methylation of the tumour suppressor miR‐149 and outcome in colorectal cancer. J Pathol. 2013;229:12‐24. [DOI] [PubMed] [Google Scholar]
- 19. Hamara K, Bielecka‐Kowalska A, Przybylowska‐Sygut K, Sygut A, Dziki A, Szemraj J. Alterations in expression profile of iron‐related genes in colorectal cancer. Mol Biol Rep. 2013;40:5573‐5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaefer A, Jung M, Mollenkopf H‐J, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126:1166‐1176. [DOI] [PubMed] [Google Scholar]
- 21. Pan SJ, Zhan SK, Pei BG, Sun QF, Bian LG, Sun BM. MicroRNA‐149 inhibits proliferation and invasion of glioma cells via blockade of AKT1 signaling. Int J Immunopathol Pharmacol. 2012;25:871‐881. [DOI] [PubMed] [Google Scholar]
- 22. Li D, Chen P, Li XY, et al. Grade‐specific expression profiles of miRNAs/mRNAs and docking study in human grade I‐III astrocytomas. OMICS. 2011;15:673‐682. [DOI] [PubMed] [Google Scholar]
- 23. Izquierdo L, Ingelmo‐Torres M, Mallofre C, et al. Prognostic value of microRNA expression pattern in upper tract urothelial carcinoma. BJU Int. 2014;113:813‐821. [DOI] [PubMed] [Google Scholar]
- 24. Shen X, Li J, Liao W, et al. microRNA‐149 targets caspase‐2 in glioma progression. Oncotarget. 2016;7:26388‐26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu J, Wang S, Zhang W, et al. Screening key microRNAs for castration‐resistant prostate cancer based on miRNA/mRNA functional synergistic network. Oncotarget. 2015;6:43819‐43830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Huang J, Yang N, et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proc Natl Acad Sci USA. 2006;103:9136‐9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamanaka D, Akama T, Fukushima T, et al. Phosphatidylinositol 3‐kinase‐binding protein, PI3KAP/XB130, is required for cAMP‐induced amplification of IGF mitogenic activity in FRTL‐5 thyroid cells. Mol Endocrinol. 2012;26:1043‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takeshita H, Shiozaki A, Bai XH, et al. XB130, a new adaptor protein, regulates expression of tumor suppressive microRNAs in cancer cells. PLoS ONE. 2013;8:e59057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujii T, Shimada K, Tatsumi Y, Fujimoto K, Konishi N. Syndecan‐1 responsive microRNA‐126 and 149 regulate cell proliferation in prostate cancer. Biochem Biophys Res Commun. 2015;456:183‐189. [DOI] [PubMed] [Google Scholar]
- 30. Kong Y‐G, Cui M, Chen S‐M, Xu Y, Xu Y, Tao Z‐Z. LncRNA‐LINC00460 facilitates nasopharyngeal carcinoma tumorigenesis through sponging miR‐149‐5p to up‐regulate IL6. Gene. 2018;639:77‐84. [DOI] [PubMed] [Google Scholar]
- 31. Weber B, Stresemann C, Brueckner B, Lyko F. Methylation of human microRNA genes in normal and neoplastic cells. Cell Cycle. 2007;6:1001‐1005. [DOI] [PubMed] [Google Scholar]
- 32. Liu X, Xie T, Mao X, Xue L, Chu X, Chen L. MicroRNA‐149 increases the sensitivity of colorectal cancer cells to 5‐fluorouracil by targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2016;39:617‐629. [DOI] [PubMed] [Google Scholar]
- 33. Wilting SM, Verlaat W, Jaspers A, et al. Methylation‐mediated transcriptional repression of microRNAs during cervical carcinogenesis. Epigenetics. 2013;8:220‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oster B, Linnet L, Christensen LL, et al. Non‐CpG island promoter hypomethylation and miR‐149 regulate the expression of SRPX2 in colorectal cancer. Int J Cancer. 2013;132:2303‐2315. [DOI] [PubMed] [Google Scholar]
- 35. Chan SH, Huang WC, Chang JW, et al. MicroRNA‐149 targets GIT1 to suppress integrin signaling and breast cancer metastasis. Oncogene. 2014;33:4496‐4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bischoff A, Bayerlova M, Strotbek M, Schmid S, Beissbarth T, Olayioye MA. A global microRNA screen identifies regulators of the ErbB receptor signaling network. Cell Commun Signal. 2015;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li P, Shan JX, Chen XH, et al. Epigenetic silencing of microRNA‐149 in cancer‐associated fibroblasts mediates prostaglandin E2/interleukin‐6 signaling in the tumor microenvironment. Cell Res. 2015;25:588‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ghasemi A, Fallah S, Ansari M. MicroRNA‐149 is epigenetically silenced tumor‐suppressive microRNA, involved in cell proliferation and downregulation of AKT1 and cyclin D1 in human glioblastoma multiforme. Biochem Cell Biol. 2016;94:569‐576. [DOI] [PubMed] [Google Scholar]
- 39. Liu H, Brannon AR, Reddy AR, et al. Identifying mRNA targets of microRNA dysregulated in cancer: with application to clear cell Renal Cell Carcinoma. BMC Syst Biol. 2010;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Long C, Jiang L, Wei F, et al. Integrated miRNA‐mRNA analysis revealing the potential roles of miRNAs in chordomas. PLoS ONE. 2013;8:e66676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding SL, Wang JX, Jiao JQ, et al. A pre‐microRNA‐149 (miR‐149) genetic variation affects miR‐149 maturation and its ability to regulate the Puma protein in apoptosis. J Biol Chem. 2013;288:26865‐26877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li L, Liu T, Li Z, Zhang L, Zhang Z. The miR‐149 rs2292832 T/C polymorphism may decrease digestive cancer susceptibility: an updated meta‐analysis. Int J Clin Exp Med. 2015;8:15351‐15361. [PMC free article] [PubMed] [Google Scholar]
- 44. Li L, Sheng Y, Lv L, Gao J. The association between two microRNA variants (miR‐499, miR‐149) and gastrointestinal cancer risk: a meta‐analysis. PLoS ONE. 2013;8:e81967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma XP, Zhang T, Peng B, Yu L, Jiang DK. Association between microRNA polymorphisms and cancer risk based on the findings of 66 case‐control studies. PLoS ONE. 2013;8:e79584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu L, Zhou X, Qiu MT, Yin R, Wu YQ, Xu L. Lack of association between hsa‐miR‐149 rs2292832 polymorphism and cancer risk: a meta‐analysis of 12 studies. PLoS ONE. 2013;8:e73762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu Y, Yu CY, Wang JL, Guan J, Chen HY, Fang JY. MicroRNA sequence polymorphisms and the risk of different types of cancer. Sci Rep. 2014;4:3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He B, Pan Y, Cho WC, et al. The association between four genetic variants in microRNAs (rs11614913, rs2910164, rs3746444, rs2292832) and cancer risk: evidence from published studies. PLoS ONE. 2012;7:e49032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Srivastava K, Srivastava A. Comprehensive review of genetic association studies and meta‐analyses on miRNA polymorphisms and cancer risk. PLoS ONE. 2012;7:e50966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y‐G, Shi J‐X, Song C‐H, et al. Association of mir‐499 and mir‐149 polymorphisms with cancer risk in the Chinese population: evidence from published studies. Asian Pac J Cancer Prev. 2013;14:2337‐2342. [DOI] [PubMed] [Google Scholar]
- 51. Dikeakos P, Theodoropoulos G, Rizos S, Tzanakis N, Zografos G, Gazouli M. Association of the miR‐146aC > G, miR‐149T > C, and miR‐196a2T > C polymorphisms with gastric cancer risk and survival in the Greek population. Mol Biol Rep. 2014;41:1075‐1080. [DOI] [PubMed] [Google Scholar]
- 52. Mw Z, Mj J, Yx Y, et al. Associations of lifestyle‐related factors, hsa‐miR‐149 and hsa‐miR‐605 gene polymorphisms with gastrointestinal cancer risk. Mol Carcinog. 2012;51:E21‐E31. [DOI] [PubMed] [Google Scholar]
- 53. Wei WJ, Lu ZW, Li DS, et al. Association of the miR‐149 Rs2292832 polymorphism with papillary thyroid cancer risk and clinicopathologic characteristics in a Chinese population. Int J Mol Sci. 2014;15:20968‐20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang C, Sturgis EM, Chen X, Zheng H, Wei Q, Li G. Pre‐miRNA variants as predictors of clinical outcome in patients with squamous cell carcinomas of the nonoropharynx. Oncotarget. 2016;7:26444‐26453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu Z, Chen J, Tian T, et al. Genetic variants of miRNA sequences and non–small cell lung cancer survival. J Clin Investig. 2008;118:2600‐2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xia L, Ren Y, Fang X, et al. Prognostic role of common microRNA polymorphisms in cancers: evidence from a meta‐analysis. PLoS ONE. 2014;9:e106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hong MJ, Choi YY, Jang JA, et al. Association between genetic variants in pre‐microRNAs and survival of early‐stage NSCLC. J Thorac Oncol. 2013;8:703‐710. [DOI] [PubMed] [Google Scholar]
- 58. Liu XX, Wang M, Xu D, et al. Quantitative assessment of the association between genetic variants in microRNAs and colorectal cancer risk. Biomed Res Int. 2015;2015:276410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Du W, Ma X‐L, Zhao C, et al. Associations of single nucleotide polymorphisms in miR‐146a, miR‐196a, miR‐149 and miR‐499 with colorectal cancer susceptibility. Asian Pac J Cancer Prev. 2014;15:1047‐1055. [DOI] [PubMed] [Google Scholar]
- 60. Kim WH, Min KT, Jeon YJ, et al. Association study of microRNA polymorphisms with hepatocellular carcinoma in Korean population. Gene. 2012;504:92‐97. [DOI] [PubMed] [Google Scholar]
- 61. Liu MF, Chen WQ, He YZ, Gu YL. Role of miR‐149C>T polymorphisms on the risk of hepatocellular carcinoma in a Chinese population. Genet Mol Res. 2014;13:7184‐7189. [DOI] [PubMed] [Google Scholar]
- 62. Tu H‐F, Liu C‐J, Chang C‐L, et al. The association between genetic polymorphism and the processing efficiency of miR‐149 affects the prognosis of patients with head and neck squamous cell carcinoma. PLoS ONE. 2012;7:e51606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Z, Li G, Wei S, et al. Genetic variants in selected pre‐microRNA genes and the risk of squamous cell carcinoma of the head and neck. Cancer. 2010;116:4753‐4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sushma PS, Jamil K, Kumar PU, Satyanarayana U, Ramakrishna M, Triveni B. Genetic variation in microRNAs and risk of oral squamous cell carcinoma in South Indian population. Asian Pac J Cancer Prev. 2015;16:7589‐7594. [DOI] [PubMed] [Google Scholar]
- 65. Wu J, Lv S, An J, Lu C. Pre‐miR‐149 rs71428439 polymorphism is associated with increased cancer risk and AKT1/cyclinD1 signaling in hepatocellular carcinoma. Int J Clin Exp Med. 2015;8:13628‐13633. [PMC free article] [PubMed] [Google Scholar]
- 66. Wang Z, Wei M, Ren Y, et al. miR149 rs71428439 polymorphism and risk of clear cell renal cell carcinoma: a case‐control study. Tumour Biol. 2014;35:12127‐12130. [DOI] [PubMed] [Google Scholar]
- 67. Xu Y, Lin Y‐P, Yang D, Zhang G, Zhou H‐F. Clinical significance of miR‐149 in the survival of patients with laryngeal squamous cell carcinoma. Biomed Res Int. 2016;2016:8561251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lin RJ, Lin YC, Yu AL. miR‐149* induces apoptosis by inhibiting Akt1 and E2F1 in human cancer cells. Mol Carcinog. 2010;49:719‐727. [DOI] [PubMed] [Google Scholar]
- 69. Xie Z, Xu J, Peng L, Gao Y, Zhao H, Qu Y. miR‐149 promotes human osteocarcinoma progression via targeting bone morphogenetic protein 9 (BMP9). Biotech Lett. 2017;40:47‐55. [DOI] [PubMed] [Google Scholar]
- 70. Pfeffer SR, Grossmann KF, Cassidy PB, et al. Detection of exosomal miRNAs in the plasma of melanoma patients. J Clin Med. 2015;4:2012‐2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial‐mesenchymal transitions in cancer. Cancer Res. 2012;72:4883‐4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wierstra I. FOXM1 (Forkhead box M1) in tumorigenesis: overexpression in human cancer, implication in tumorigenesis, oncogenic functions, tumor‐suppressive properties, and target of anticancer therapy Adv Cancer Res. 2013;119:191‐419. [DOI] [PubMed] [Google Scholar]
- 73. Okato A, Arai T, Yamada Y, et al. Dual strands of pre‐miR‐149 inhibit cancer cell migration and invasion through targeting FOXM1 in renal cell carcinoma. Int J Mol Sci. 2017;18:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu K, Liu X, Mao X, et al. MicroRNA‐149 suppresses colorectal cancer cell migration and invasion by directly targeting forkhead box transcription factor FOXM1. Cell Physiol Biochem. 2015;35:499‐515. [DOI] [PubMed] [Google Scholar]
- 75. Tanaka K, Arao T, Maegawa M, et al. SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int J Cancer. 2009;124:1072‐1080. [DOI] [PubMed] [Google Scholar]
- 76. Luo G, Chao Y‐L, Tang B, et al. miR‐149 represses metastasis of hepatocellular carcinoma by targeting actin‐regulatory proteins PPM1F. Oncotarget. 2015;6:37808‐37823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dong Y, Chang C, Liu J, Qiang J. Targeting of GIT1 by miR‐149* in breast cancer suppresses cell proliferation and metastasis in vitro and tumor growth in vivo. Onco Targets Ther. 2017;10:5873‐5882. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78. Luo Z, Zhang L, Li Z, et al. An in silico analysis of dynamic changes in microRNA expression profiles in stepwise development of nasopharyngeal carcinoma. BMC Med Genomics. 2012;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang D, Du G, Xu A, Xi X, Li D. Expression of miR‐149‐3p inhibits proliferation, migration, and invasion of bladder cancer by targeting S100A4. Am J Cancer Res. 2017;7:2209‐2219. [PMC free article] [PubMed] [Google Scholar]
- 80. Venkatesan N, Kanwar J, Deepa PR, et al. Clinico‐pathological association of delineated miRNAs in uveal melanoma with monosomy 3/disomy 3 chromosomal aberrations. PLoS ONE. 2016;11:e0146128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Warnecke‐Eberz U, Chon S‐H, Holscher AH, Drebber U, Bollschweiler E. Exosomal onco‐miRs from serum of patients with adenocarcinoma of the esophagus: comparison of miRNA profiles of exosomes and matching tumor. Tumour Biol. 2015;36:4643‐4653. [DOI] [PubMed] [Google Scholar]
- 84. He DX, Gu XT, Li YR, Jiang L, Jin J, Ma X. Methylation‐regulated miR‐149 modulates chemoresistance by targeting GlcNAc N‐deacetylase/N‐sulfotransferase‐1 in human breast cancer. FEBS J. 2014;281:4718‐4730. [DOI] [PubMed] [Google Scholar]
- 85. Jaiswal R, Luk F, Gong J, Mathys J‐M, Grau GER, Bebawy M. Microparticle conferred microRNA profiles ‐ implications in the transfer and dominance of cancer traits. Mol Cancer. 2012;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cortez MA, Bueso‐Ramos C, Ferdin J, Lopez‐Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997‐1006. [DOI] [PubMed] [Google Scholar]
- 88. Ke XS, Liu CM, Liu DP, Liang CC. MicroRNAs: key participants in gene regulatory networks ‐ commentary. Curr Opin Chem Biol. 2003;7:516‐523. [DOI] [PubMed] [Google Scholar]
- 89. Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Berghmans T, Ameye L, Willems L, et al. Identification of microRNA‐based signatures for response and survival for non‐small cell lung cancer treated with cisplatin‐vinorelbine A ELCWP prospective study. Lung Cancer. 2013;82:340‐345. [DOI] [PubMed] [Google Scholar]
- 91. Molina‐Pinelo S, Gutiérrez G, Pastor MD, et al. MicroRNA‐dependent regulation of transcription in non‐small cell lung cancer. PLoS ONE. 2014;9:e90524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin L, Zhang Y‐D, Chen Z‐Y, Chen Y, Ren C‐P. The clinicopathological significance of miR‐149 and PARP‐2 in hepatocellular carcinoma and their roles in chemo/radiotherapy. Tumour Biol. 2016;37:12339‐12346. [DOI] [PubMed] [Google Scholar]
- 93. Perez‐Rivas LG, Jerez JM, Carmona R, et al. A microRNA signature associated with early recurrence in breast cancer. PLoS ONE. 2014;9:e91884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Xue L, Wang Y, Yue S, Zhang J. Low MiR‐149 expression is associated with unfavorable prognosis and enhanced Akt/mTOR signaling in glioma. Int J Clin Exp Pathol. 2015;8:11178‐11184. [PMC free article] [PubMed] [Google Scholar]
- 95. She X, Yu Z, Cui Y, et al. miR‐128 and miR‐149 enhance the chemosensitivity of temozolomide by Rap1B‐mediated cytoskeletal remodeling in glioblastoma. Oncol Rep. 2014;32:957‐964. [DOI] [PubMed] [Google Scholar]
- 96. Ru Q, Tian X, Pi MS, et al. Voltagegated K+ channel blocker quinidine inhibits proliferation and induces apoptosis by regulating expression of microRNAs in human glioma U87MG cells. Int J Oncol. 2015;46:833‐840. [DOI] [PubMed] [Google Scholar]
- 97. Lu X, Chen L, Chen Y, Shao Q, Qin W. Bafilomycin A1 inhibits the growth and metastatic potential of the BEL‐7402 liver cancer and HO‐8910 ovarian cancer cell lines and induces alterations in their microRNA expression. Exp Ther Med. 2015;10:1829‐1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yelamos J, Farres J, Llacuna L, Ampurdanes C, Martin‐Caballero J. PARP‐1 and PARP‐2: new players in tumour development. Am J Cancer Res. 2011;1:328‐346. [PMC free article] [PubMed] [Google Scholar]
- 99. Cao D, Jia Z, You L, et al. 18β‐glycyrrhetinic acid suppresses gastric cancer by activation of miR‐149‐3p‐Wnt‐1 signaling. Oncotarget. 2016;7:71960‐71973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Si L, Xu L, Yin L, et al. Potent effects of dioscin against pancreatic cancer via miR‐149‐3P‐mediated inhibition of the Akt1 signalling pathway. Br J Pharmacol. 2017;174:553‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell‐free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145. [DOI] [PubMed] [Google Scholar]
- 102. Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discovery. 2010;9:775‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discovery. 2017;16:203. [DOI] [PubMed] [Google Scholar]
- 104. Taylor K, Howard CB, Jones ML, et al. Nanocell targeting using engineered bispecific antibodies. mAbs. 2015;7:53‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu X, Wang T, Wakita T, Yang W. Systematic identification of microRNA and messenger RNA profiles in hepatitis C virus‐infected human hepatoma cells. Virology. 2010;398:57‐67. [DOI] [PubMed] [Google Scholar]


