Abstract
Objectives
Urinary tract infection, urinary frequency, urgency, urodynia and haemorrhage are common post‐operative complications of thulium laser resection of the prostate (TmLRP). Our study mainly focuses on the role of finasteride in prostate wound healing through AR signalling.
Materials and methods
TmLRP beagles were randomly distributed into different treatment groups. Serum and intra‐prostatic testosterone and DHT level were determined. Histological analysis was conducted to study the re‐epithelialization and inflammatory response of the prostatic urethra in each group. We investigated the role of androgen in proliferation and inflammatory response in prostate. In addition, the effects of TNF‐α on prostate epithelium and stromal cells were also investigated.
Results
Testosterone and DHT level increased in testosterone group and DHT decreased in finasteride group. Accelerated wound healing of prostatic urethra was observed in the finasteride group. DHT suppressed proliferation of prostate epithelium and enhanced inflammatory response in prostate. We confirmed that DHT enhanced macrophages TNF‐α secretion through AR signalling. TNF‐α suppressed proliferation of prostate epithelial cells and retarded cell migration. TNF‐α also played a pivotal role in suppressing fibroblasts activation and contraction.
Conclusion
Testosterone treatment repressed re‐epithelialization and wound healing of prostatic urethra. Finasteride treatment may be an effective way to promote prostate re‐epithelialization.
Keywords: DHT, finasteride, TmLRP, TNF‐α, wound healing
1. INTRODUCTION
Benign prostatic hyperplasia (BPH) is characterized as a non‐malignant enlargement of the prostate, which is one of the most common diseases in ageing men.1 The most frequent problem of BPH is lower urinary tract symptoms (LUTS).2, 3 Surgical intervention is an ideal way for treatment of patients with moderate‐to‐severe LUTS.4 Multiple surgical techniques have emerged since decades ago, among which transurethral resection of prostate (TURP) has been recognized as the gold standard. However, urologists have raised concern about the disadvantages of TURP including transurethral resection (TUR) syndrome, blood loss, incontinence, balance disturbances and retrograde ejaculation.5, 6 Meanwhile, laser therapy is arisen as advanced minimally invasive surgical treatment for BPH. Thulium laser resection of the prostate (TmLRP) has been proved to be a safer and more efficient therapy. Nowadays, TmLRP goes further beyond as a replacement for the gold standard TURP. Inevitably, post‐operative complications such as urinary tract infection, urinary frequency, urgency, urodynia and haemorrhage occurred due to the thermal injury of the laser causing tissue necrosis and secondary sloughing.7 Forementioned complications would reduce after the prostatic urethra wound healing, which is a complicated and dynamic tissue repair process including re‐epithelialization and angiogenesis. Epithelialization involves the proliferation and migration of epithelial cells across the wounded bed. Specific immune responses, including inflammatory cells, cytokines and extracellular matrix, are relevant to the healing of wound. Thus, understanding of the mechanism and process of prostatic urethra wound healing is critical to guide the clinical intervention to reduce LUTS post‐operative complications. Unfortunately, few studies have focused on this area.
In this study, we established TmLRP beagle models with different treatments. The process of re‐epithelialization and changes of inflammatory response are assessed by haematoxylin and eosin (H&E) staining, immunohistochemical analysis and immunofluorescent staining. Besides the in vivo experiment, we also identified the effect of DHT and AR signalling on prostate epithelial cells proliferation in vitro. In addition, the role of TNF‐α was also investigated to explore the effect of inflammatory response activated by testosterone treatment on wound healing of prostate.
2. MATERIALS AND METHODS
2.1. Canines and treatments
A total of 24 healthy adult male beagles aged 2 years were obtained from Shanghai General Hospital. All procedures were done in accordance with guidelines of the Chinese Council on Animal Care. Protocols were approved by the Medical Science Ethics Committee of Shanghai General Hospital. The animals were randomly divided into 3 groups: testosterone group, finasteride group and control group. Animals in testosterone group and finasteride group were fed with 80 mg testosterone undecanoate (Catalent, France, Beinheim S. A.) for 1 week or finasteride (Merck & Co., Inc., Kenilworth, NJ, USA) at a dose of 0.5 mg kg−1 body weight per day for 3 months, respectively, before the TmLRP until they were killed.
2.1.1. Androgen and estradiol determination
Blood was collected from the forelimb vein and centrifuged at 3000 r/min for 20 minutes. The upper serum was collected and stored at −80°C for testosterone and DHT test. The resected prostate tissue samples subjected to TmLRP were cut into pieces on an icy table and 9 times the tissue weight of phosphate buffered solution (PBS) was added to each sample. The samples were homogenized in a Homogenate Machine Pro Scientific 200 (Pro Scientific Co., Inc., Oxford, CT, USA) and centrifuged at 3000 r min−1 for 20 minutes. The supernatant was used for protein quantification and to analyse the testosterone and DHT levels. The canine testosterone, canine dihydrotestosterone and canine estradiol ELISA Kit (Cusabio Biotech Co., Ltd., Wuhan, China) were used for testosterone, DHT and estradiol analysis in serum and prostate tissues.
2.1.2. Cell lines and treatment
The WPMY‐1 human prostate stromal cell line was commercially obtained from the American Type Culture Collection (ATCC) (Manassas, VA) and was maintained in Dulbecco's Minimal Essential Medium (DMEM). BPH‐1 human prostate epithelial cell line was kindly provided by Dr. Ju Zhang (Nankai University, China) and was cultured in RPMI‐1640 media. Media were supplemented with 10% fetal bovine serum (FBS) (Life Technologies, USA), 100 units per mL penicillin and 100 μg mL−1 streptomycin (Life Technologies, Camarillo, CA, USA).
Human normal prostate stromal cells (PrSC) from older donors undergoing radical prostatectomy were obtained from previous established cells in our laboratory and immortalized by simian virus 40 large T antigens. Cells were cultured in RPMI‐1640 media supplemented with 10% FBS.
Human normal prostate epithelial cells (PrECs) from young donors were obtained commercially from Pricell (Wuhan, China) and immortalized by simian virus 40 large T antigen. PrEC and all its derivatives were grown in Keratinocyte Serum Free defined media supplemented with growth factors (GFs) [standard K‐SFM] as supplied by manufacture (Life Technologies, NY, USA).
THP‐1 was cultured in RPMI‐1640 Phenol red free medium supplemented with 10% charcoal‐stripped FBS. THP‐1 monocytes were seeded in 6‐well cell culture plates at 5 × 105 cells per well and differentiated into macrophages in RPMI media containing 20 ng mL−1 phorbol 12‐myristate 13‐acetate (PMA) for 48 hours according to previously established protocols.8 After differentiation, THP‐1 macrophages were activated with low‐dose lipopolysaccharide (LPS) at 10 ng mL−1 for another 24 hours followed by either incubation with different concentration of DHT (1 nM or 10 nM) or left untreated. Both DHT and PMA were all obtained from Sigma (St. Louis, MO). LPS was obtained from Beyotime (Shanghai, China). Culture supernatants were collected after 12‐hour incubation, filter sterilized, and stored at −80°C until ready for ELISA analysis.
2.1.3. Lentiviral infection of cells
The AR and TNFR1 shRNA lentivirus particles were obtained from GenePharma. Ltd (Shanghai, China). The sequences of the AR and TNFR1 shRNAs are listed in Table S1. Lentiviral transduction of THP‐1 cells with particles for shRNAs targeting AR (Target sequence: NM_000044) and prostate epithelial cells (PrEC and BPH‐1) with particles for shRNAs targeting TNFR1 (Target sequence: NM_001065) or Scrambled (Scr) non‐target negative control was performed according to the previous established assays.9, 10 Briefly, 24 hours prior to transduction, THP‐1 cells or prostate epithelial cells were plated in 6‐well plates at 40‐50% confluency in culture medium. The following day, the viral stocks were added to the cells in the presence of 8 μg mL−1 of polybrene (Santa Cruz, USA). After 24 hours of transduction, medium containing viral particles was removed and replaced with fresh medium to proliferate until a sufficient cell number. Then, clones stably expressing shRNA were selected with medium containing appropriate concentration of puromycin. After 3‐4 weeks, the cells were collected and examined for GFP and AR or TNFR1 expression.
To construct of stable AR overexpression prostate stromal cells (WPMY‐1 and PrSC) and prostate epithelial cells (PrEC and BPH‐1), lentivirus particles obtained from Obio Ltd (Shanghai, China) were added to the cell culture medium in the presence of polybrene (μg mL−1) to incubate for 24 hours. Cells were refreshed with culture medium and cultured for another 3 days to allow target protein expression. Fluorescence microscopy was used to monitor the infection efficiency via checking the green fluorescence signal. AR expression was tested by western blot.
2.1.4. RNA extraction and quantitative real‐time PCR analysis
The animals were killed, and total RNAs were isolated from fresh prostate tissue using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. One milligram of total RNA was subjected to reverse transcription using PrimeScript™ RT Master Mix (Takara, Japan). Quantitative real‐time PCR (qRT‐PCR) was conducted using a QuantStudio™ 6 Flex Real‐Time PCR System with SYBR green to determine the genes mRNA expression levels. Primers used were concluded in Table S1. Expression levels were normalized to the expression of GAPDH RNA.
2.1.5. Cytokine detection in urine and cell supernatants by ELISA
Conditioned medium was collected from THP‐1 culture after the incubations described above. The cytokine (TNF‐α) in the supernatants was determined using a commercial ELISA kit (R&D Diagnostics, MN, USA). The assay was performed according to manufacturers’ instructions.
For detection of urine TNF‐α production, morning urine samples were collected from each canine through the bladder fistula on days 3, 5, 7, 10, 14, 21, 28, and before the thulium laser surgery. The urine samples were centrifuged at 1000 × g for 5 minutes and the supernatant was used for analysis. Urinary TNF‐α quantification was assessed by ELISA commercial kit, which is specific for canine TNF‐α evaluation (RayBiotech, Inc., Norcross GA, USA).
2.1.6. Tissue and cell lysis and Western blot analysis
Primary antibodies were p21, p27, SAPK/JNK, Phospho‐SAPK/JNK (Thr183/Tyr185), GAPDH (Cell Signaling); c‐ myc, cyclin D1, TNF‐α, TNFR1, β‐catenin, Histone H3 (Abcam).
Cells were lysed in RIPA (1% sodium deoxycholate, 0.1% SDS, 1% Triton X‐100, 10 mM Tris‐HCl [pH 8.0], 0.14 M NaCl) with protease and phosphatase inhibitors. Nuclear extracts were prepared as described previously.11 Lysates were centrifuged at 12 000 g for 15 minutes at 4°C. Supernatants were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS‐PAGE). After transfer to nitrocellulose or polyvinylidene fluoride (PVDF), membranes were blocked in milk or bovine serum albumin (BSA) and incubated at 4°C overnight with primary antibody. Detection was performed by enhanced chemiluminescence after 2‐hour incubation with horseradish‐peroxidase (HRP)‐conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA) and visualized by enhanced chemiluminescence reagent (Thermo Scientific, USA).
2.1.7. In vitro growth assays
For the MTT assay, cells were plated in 96‐well plates and treated with DHT or TNF‐α or corresponding doses of dimethylsulfoxide (DMSO). Cell viability was determined at 24, 48, 72, 96 and 120 hours after transfection. All cells were incubated with 10 μl of 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT, Sigma‐Aldrich, St. Louis, MO) solution (5 mg mL−1) in PBS for 4 hours. Fifty microlitre DMSO was added for 10 minutes after aspiration of the MTT solution. Plates were reading using xx at 560 nm. Clonogenic assays were conducted according to the previous established assays.12
2.1.8. Cell cycle and apoptosis assays
Fluorescence activated cell sorting (FACS) analysis was done after treatment with DHT or TNF‐α. The cells were harvested, washed with cold PBS, and re‐suspended in the nuclear stain propidium iodide (PI) solution for cell cycle analysis or stained with PI and Annexin‐V‐FITC using ANNEXIN‐V‐FITC/PI KIT (Beyotime, China) for apoptosis analysis according to the manufacturer's protocol. Stained cells were immediately analysed by FACS (BD Accuri C6; BD Biosciences).
2.1.9. Immunohistochemistry and immunofluorescence assays
Prostate tissues were collected from canines after being killed and fixed in paraformaldehyde for 12 hours, embedded in paraffin and sectioned. Then, the tissue sections were stained with H&E and subjected to immunohistochemistry (IHC) or immunofluorescence (IF). As for immunohistochemistry, tissue sections were incubated with primary antibodies against TNF‐α (1:100, Abcam). As a negative control, sections were treated with PBS in place of the primary IgG, which in all cases yielded no staining. Primary antibody was detected using the Vectastain ABC peroxidase kits (Vector Labs, Peterborough, UK). Immunoreactivity was identified with the peroxidase‐anti‐peroxidase technique using diaminobenzidine as a chromogenic substrate. For immunofluorescence assays, tissue sections were incubated with a blocking solution (1% BSA in PBS) for 1 hour at room temperature and then with primary antibody against CD86 (1:100, Abcam) overnight at 4°C, followed by incubating with Alexa Fluor® 594 goat anti‐mouse IgG (1:500, Life Technologies) at 37°C for 30 minutes. Next, the sections were washed in PBS, incubated with 4′,6‐diamindino‐2‐phenylindole dihydrochloride (1:100, Sigma‐Aldrich) for nuclear staining, sealed with aqueous mounting medium (R&D Systems) and visualized using fluorescence microscopy. Quantification of cell numbers per unit area was performed using ImagePro Plus software.
Immunofluorescence for cells was similar to the description above. Briefly, cells were plated on fibronectin‐coated glass coverslips in 24‐well tissue‐culture plates. Cells were treated and incubated followed by fixed with warmed 4% formaldehyde and permeabilized with 0.1% Triton X‐100. After incubated with a blocking solution and then with primary antibody against α‐SMA (1:200, Abcam) or Ki67 (1:200, Abcam) followed by incubated with Alexa Fluor® 594 secondary antibodies (1:250), glass coverslips sealed with antifade mounting medium and visualized with fluorescence microscopy.
2.1.10. Wound‐healing assays
Cells were seeded in 6‐well plates in RPMI 1640 with 5% FBS. After overnight attachment and growth, cells were starved in serum‐free RPMI medium for 24 hours before incubation with or without 10 nM DHT or TNF‐α. The cell sheets were wounded with a plastic pipette tip when they attained a confluent monolayer. This generated a gap in the monolayer, and the ability of cells to migrate into the cleared section was observed and recorded photographically at different times at fixed points in the wound. All the experiments were done in triplicate.
2.1.11. Assay of collagen gel contraction
The contractile activity of fibroblasts cultured on type I rat tail collagen was assessed according to previous established assay.13 Briefly, to form the gel disc, WPMY‐1‐AR or human primary prostate stromal cell was re‐suspended in 400 μl DMEM (with 5% charcoal‐stripped FBS) at the concentration of 3 × 106 cells per mL and mixed with 200 μL collagen suspension (3 mg mL−1). The mixture (500 μL) containing stromal cells was transferred into each well of 24‐well plate and incubated at 37°C for 1 hour in order to facilitate polymerization. After solidification, dissociate the gel gently from the well and add 600 μL serum‐free DMEM containing TGF‐β1 (3 ng mL−1) with presence of DHT (10 nM) or TNF‐α (50 ng mL−1) or vehicle (0.1% BSA). After stimulation, the diameter of the gels was determined using Image‐Pro Plus at 24, 48 and 72 hours.
2.1.12. Patient treatment and urine cytokine detection
Urine cytokine was determined in patients undergoing TmLRP for treatment of BPH at Shanghai General Hospital from May 2015 to January 2017. In total, 50 patients were enrolled in our study. Twenty‐five patients who had taken finasteride (5mg, po, Qd) regularly for more than 3 months were set as the finasteride group. Another 25 patients who had never taken finasteride were set as the control group.
Morning urine samples were collected from each patient before the surgery and in 6 days after the surgery. The urine specimens were centrifuged at 1000 × g for 5 min, and the supernatant was used for analysis. Urinary inflammatory cytokine quantification was assessed by ELISA using commercial kits for human IL‐6, IL‐12 and TNF‐α (RayBiotech, Inc., Norcross GA, USA). Written informed consent was obtained from the each participant prior to enrollment, and approval for the study was obtained from the Ethics Committee of the Shanghai General Hospital.
2.1.13. Statistics
The data are presented as mean ± SEM unless specified. For parametric analyses, 2‐tailed Student's t test or one‐way ANOVA was used. For nonparametric analyses, Mann‐Whitney U test was used. All statistical calculations were performed with the Statistical Package for the Social Sciences for Windows version 17 (SPSS Inc., Chicago, IL, USA). A 2‐sided P value of <.05 was considered statistically significant.
3. RESULTS
3.1. Finasteride reduced intra‐prostatic DHT and accelerated re‐epithelialization of the prostatic urethra
Observation of cell re‐epithelialization by H&E straining started 1 week after TmLRP. In the first week, we observed focal re‐epithelialization in the process of wound regeneration at the cavity surface by 1‐2 layers of epithelium cells with distinct squamous metaplasia in residual acinar and in ductal prostatic epithelium in finasteride group. Meanwhile, thin germinating epithelium layer extended to some area of lumen of the cavity in residual gland duct in control group (Figure 1A, yellow arrow). No obvious proliferating epithelial cells originated from the underlying prostatic epithelium, with more areas of cavitation, coagulation necrosis, acute inflammatory exudation on the wound surface and fewer proliferations of the glandular elements were observed in testosterone group. There is one sensible difference manifested between groups in 2 weeks later after operation. Remarkable epithelium regeneration comprising of 3‐5 layers of cells was observed on the cavity surface in finasteride group. Regenerative layer in control group, with 2‐4 layers thick, is thinner than finasteride group. In contrast, inflammation has not completely subsided in testosterone group, with 1‐2 layers or discontinuous regenerative epithelium. All the regenerative epithelial cells have not gain polarity yet. Significant difference appeared in third week. Re‐epithelialization was essentially complete in finasteride group. Cells gained polarity and were morphological similar with normal urothelium. In control group, the re‐epithelialized layer was almost with the same thickness as finasteride group, but cells were still squamous metaplasia like. Epithelium in both groups was much thicker than that in testosterone group. Faster than testosterone group, re‐epithelialization was completed in finasteride group and control group 4 weeks after surgery (Figure 1A). In addition, cell number of regenerative epithelium was also counted every week. Regenerated cells increased significantly every week in all the groups. Except for the last week, there are more cell cells in finasteride group compared with control group. Testosterone group has the lowest cell number during 4 weeks (Figure 1B).
Figure 1.
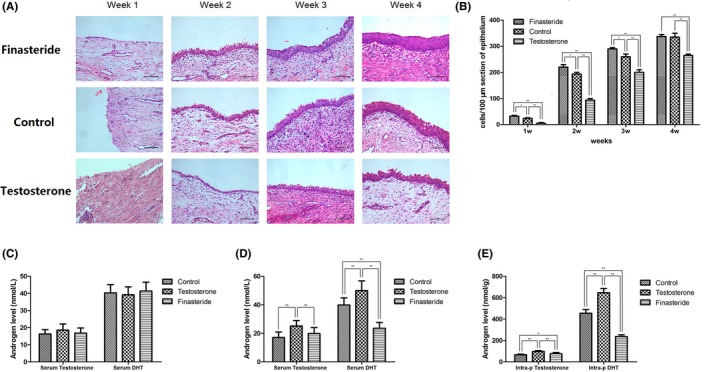
(A) H&E straining of the prostate sample at each time point after operation. (B) Quantification of regenerative epithelium. (C) Serum testosterone and DHT level before treatment. (D) Serum testosterone and DHT level after treatment. (E) Intra‐prostatic testosterone and DHT level after treatment
Serum testosterone and DHT level were measured before and after finasteride or testosterone treatment (Table 1). No differences were observed in serum testosterone and DHT before canines received testosterone or finasteride (Figure 1C). After drug treatment, both serum and intra‐prostatic testosterone and DHT significantly increased in testosterone group, with serum DHT decreased in finasteride group (Figure 1D). Intra‐prostatic testosterone and DHT were also determined after treatment. When compared with control group, testosterone treatment increased testosterone and DHT, while finasteride treatment decreased DHT level. Significant testosterone increase was observed in finasteride group. We assumed that this is the result of reciprocal increase by blocking the conversion of testosterone to DHT (Figure 1E). Previous studies showed that finasteride treatment could affect serum estradiol, which plays an important role in the aetiology of BPH through intra‐prostatic oestrogen receptors.14 We observed higher level of estradiol in finasteride treated beagles compared with other groups. However, there is no significant intra‐prostatic estradiol difference among 3 groups. We speculate that estradiol is not a predominant factor involved in prostate re‐epithelialization (Figure S1).
Table 1.
Testosterone and DHT level of canines before and after treatment
| Groups | Before treatment | After treatment | ||||
|---|---|---|---|---|---|---|
| Serum Testosteronenmol L−1 | Serum DHTnmol L−1 | Serum Testosteronenmol L−1 | Serum DHTnmol L−1 | Intra‐prostatic Testosteronenmol g−1 tissue | Intra‐prostatic DHTnmol g−1 tissue | |
| Control group | 16.32 ± 2.50 | 4.02 ± 0.486 | 17.06 ± 3.87 | 3.98 ± 0.50 | 70.26 ± 6.87 | 45.42 ± 3.48 |
| Testosterone group | 18.52 ± 3.62 | 3.91 ± 0.463 | 25.16 ± 3.80 | 4.99 ± 0.67 | 96.79 ± 7.72 | 64.58 ± 4.00 |
| Finasteride group | 16.75 ± 3.02 | 4.13 ± 0.517 | 19.87 ± 4.13 | 2.35 ± 0.41 | 83.09 ± 10.95 | 23.61 ± 1.68 |
3.2. DHT inhibited the growth of prostate epithelium through down‐regulation of c‐myc
Prostate epithelial cells overexpressing wild‐type androgen receptor were established by lentivirus transfection and the following puromycin selection, which termed as BPH‐1‐AR and PrEC‐AR. Previous studies demonstrated that androgen induced AR‐mediated growth arrest of prostate epithelial cells. In this study, we confirmed DHT significantly reduced number of colonies and cell viabilities through a concentration‐dependent way (Figure 2A,B). However, this effect was diminished in cells absent of AR (data not shown). Interestingly, we found that the growth of BPH‐1‐AR and PrEC‐AR accelerated when supplemented with conditional media of WPMY‐1‐AR compared with control group. The reasonable explanation is that proliferation of epithelial cells requires the presence of continuous growth factors secreted by stromal cells. However, this growth promotion effect was suspended when DHT was added (Figure 2B). Ki67 staining also indicated that the proliferation of BPH‐1‐AR and PrEC‐AR was remarkably reduced by DHT (Figure 2C). In addition, we tested the expression of nuclear c‐myc, which is reported to express in proliferating prostate epithelial cells and can be transcriptional repressed by DHT. Western blot analysis indicated that after more than 4 hours treatment with DHT, BPH‐1‐AR and PrEC‐AR lost nuclear c‐myc expression (Figure 2D). Androgen‐dependent AR‐mediated growth suppression was associated with increased expression of the CDK inhibitors p21, instead of p27, and subsequently reduced expression of cyclin D1 (Figure 2E). Flow cytometric analyses of DNA content documented that DHT exposure of prostate cells induced cell growth arrest in phase G0/G1 (Figure 2F). To conclude, these results suggested that DHT may suppress the re‐epithelialization of prostate by reducing the proliferation of epithelial cells through AR signalling.
Figure 2.
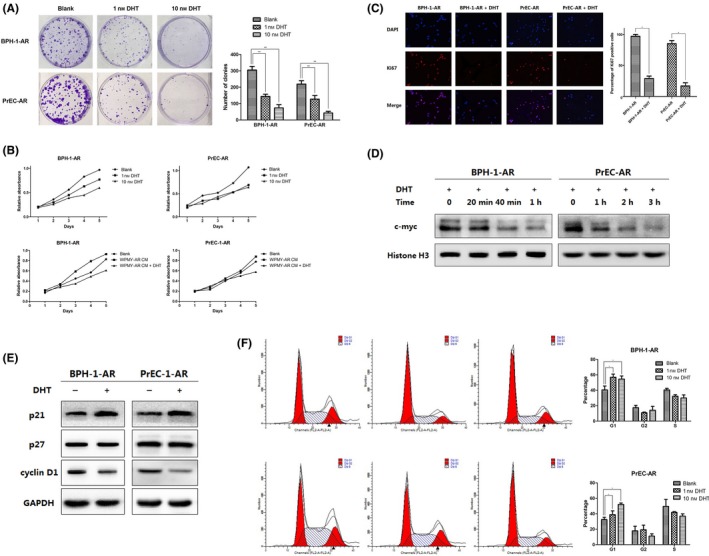
(A & B) In vitro proliferation assays of BPH‐1‐AR and PrEC‐AR with or without DHT treatment. (C) Ki67 staining of BPH‐1‐AR and PrEC‐AR. (D) Western blot analysis of nuclear c‐myc expression. (E) Western blot analysis of p21, p27 and cyclin D1 expression. (F) Flow cytometric analyses of cell cycle
3.3. Testosterone treatment increased inflammation and enhanced TNF‐α creation in wounds
Inflammation also plays an important role in wound healing. M1 macrophages, which may contribute to the impairment of wound healing, are characterized as pro‐inflammatory macrophages. We stained M1 macrophages with CD 86+ primary antibodies and found that M1 macrophages were significantly reduced in finasteride and control group, especially in first and second week. The amount of M1 macrophages was even lower in finasteride group than control group (Figure 3A). As M1 macrophages are main source of TNF‐α, we measured TNF‐α production in wound. Immunohistochemistry analysis manifested that TNF‐α protein level was increased in testosterone group when compared with finasteride and control group in 1‐2 weeks (Figure 3B). Similarly, real‐time PCR and western blot analysis also revealed that the expression of TNF‐α increased in the wounds of the testosterone group in 1‐3 weeks. At the same time, TNF‐α expression was reduced in finasteride group when compared with control group, especially in first and second week (Figure 3C). In addition, we found that TNF‐α was remarkably reduced in urine, especially in the early stage (day 3 to day 5) in finasteride group, whereas testosterone treatment immensely enhanced and prolonged TNF‐α creation (Figure 3D). We also found that urine TNF‐α, IL‐6 and IL‐12, mainly released by M1 were significantly lower post‐operatively in patients taking finasteride (Figure 3E). This effect may be partially due to the effect that DHT could activate secretion of macrophages through AR. To test this, AR was successfully knockdown in THP‐1 cells, termed as THP‐1‐shAR (data not shown). Then, TNF‐α production in culture media of LPS‐stimulated THP‐1 and THP‐1‐shAR treated with or without DHT was determined by ELISA. As Figure 3F shows, TNF‐α production of THP‐1 was increased after continuously treated with DHT, but knockdown of AR impaired TNF‐α secretion of macrophages. These data implicated that TNF‐α was an inducible factor stimulated by DHT through AR signalling in prostate wound‐healing response.
Figure 3.
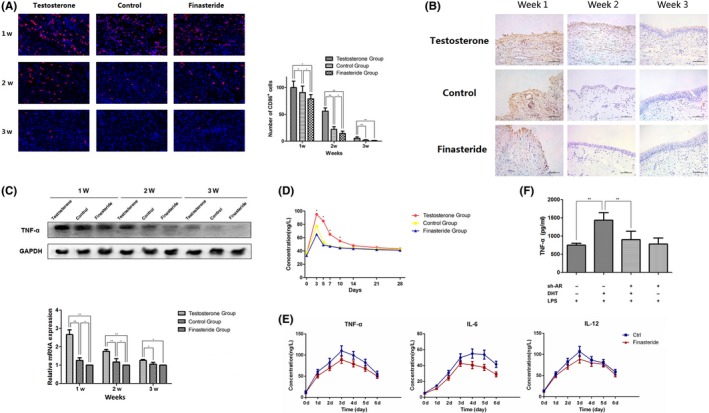
(A) Immunofluorescence of prostate tissues stained with CD 86+ primary antibodies at different time points. (B) Immunohistochemistry detecting TNF‐α production in wound. (C) Western blot analysis and real‐time PCR of the expression of TNF‐α in the prostate tissues. (D) TNF‐α in canine urine. (E) TNF‐α, IL‐6 and IL‐12 in patients’ urine post‐operatively. (F) TNF‐α production in culture media of LPS‐stimulated THP‐1 and THP‐1‐shAR treated with or without DHT
3.4. TNF‐α suppressed proliferation and retarded migration of prostate epithelial cells through TNFR1
Previous study revealed that pathological level (100 ng mL−1) of TNF‐α exerts inhibition role of proliferation and migration through TNFR1, and physiological TNF level (1 ng mL−1) enhances migration of epithelial cells.15, 16 β‐catenin is a bifunctional protein, translocating from cytosol into the nucleus to activate downstream gene transcription like c‐myc and cyclin D1 and promote cell proliferation. In growing PrEC, β‐catenin/TCF‐4 signalling is activated with presence of growth factors (EGF, IGF‐1, and FGFs). Such β‐catenin activation is dependent on wnt‐independent pathway.12 This study confirmed that deprivation of growth factors inhibited proliferation of PrEC, and reduced expression of β‐catenin (Figure 4A,B). We also conducted 2 level of TNF‐α treatment to investigate the effect of TNF‐α on prostate epithelial cells. The results revealed that lower level of TNF‐α (1 ng mL−1) had no effect on proliferation of prostate epithelial cells, whereas TNF‐α at higher level (50 ng mL−1) inhibited cell growth through reducing the expression of β‐catenin and cyclin D1 (Figure 4A,B). This effect can be interrupted by knockdown of TNFR1 (Figure 4A,B). As TNFR1 is known to promote cell death, we also explored the role of TNF‐α on apoptosis. No notable apoptosis of PrEC was induced by 50 ng mL−1 TNF‐α, which is in line with the previous study (Figure 4C).17 Lastly, we asked whether TNF‐α effected migration of epithelial cells. Wound closure model in the presence or absence of 20 ng mL−1 TNF‐α showed that TNF‐α prevented wounds closure, but TNF‐α showed loss of the inhibitory effect on wound closure of cells lack TNFR1 (Figure 4D).
Figure 4.
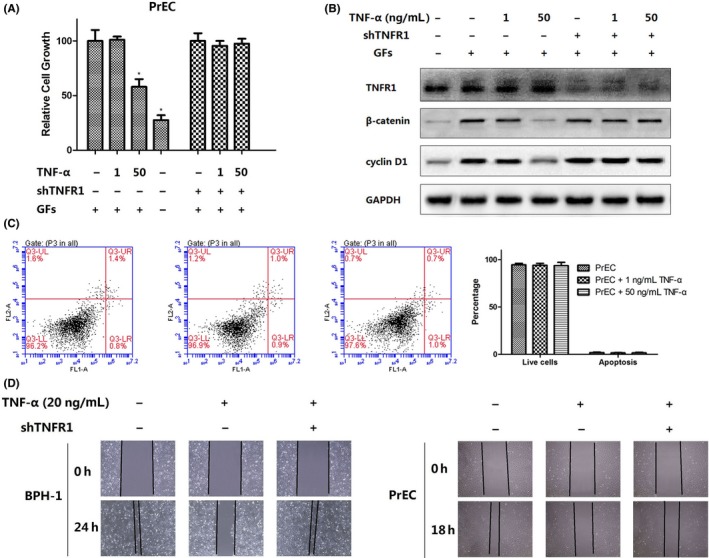
(A) MTT assays of prostate epithelial cells treated with TNF‐α. (B) Western blot analysis of TNFR1, β‐catenin and cyclin D1. (C) Fluorescence activated cell sorting (FACS) analysis of the role of TNF‐α on apoptosis. (D) Wound‐healing assays of migration of epithelial cells treated with TNF‐α
3.5. Fibroblast activation and wound contraction are suppressed by TNF‐α
As an active phenotype of fibroblasts, myofibroblast is characterized as the expression of a‐smooth muscle actin (α‐SMA). The increased level of α‐SMA found in myofibroblast is potentially important for the contractile features of these cells under the condition of normal tissue repairment. Desmouliere et al18 reported that TGF‐β induces α‐SMA expression in human dermal fibroblasts. We found TGF‐β could also increase the α‐SMA expression in prostate fibroblast cells (WPMY‐AR and PrSC‐AR) (Figure 5A). However, treated with both TGF‐β (1 ng mL−1) and TNF‐α (5 ng mL−1) could reverse the ability of TGF‐β to up‐regulate α‐SMA, indicating that TNF‐α has antagonistic activity against TGF‐β in prostate fibroblasts (Figure 5A,B). It is reported that recruitment of tumour necrosis factor receptor‐associated factor family members activates multiple signal transduction pathways like JNK, and suppresses TGF‐β‐induced α‐SMA expression.19 As shown in Figure 5C, TNF‐α treatment leads to phosphorylation of JNK, and blockade of JNK pathway by SP600125 (30 mM) reversed suppression effect of TNF‐α on α‐SMA (Figure 5D). Collagen gel contraction mediated by WPMY‐AR and PrSC‐AR was inhibited by 68.4% and 48.1% when treated with TNF‐α (Figure 5E). Wound‐healing assays also revealed the inhibition role of TNF‐α on prostate fibroblasts (Figure 5F). Intriguingly, no difference was observed in fibroblasts added with DHT (Figure 5A,B and D,F). In conclusion, we showed that TNF‐α, instead of DHT, impairs wound‐healing responses of prostate fibroblasts.
Figure 5.
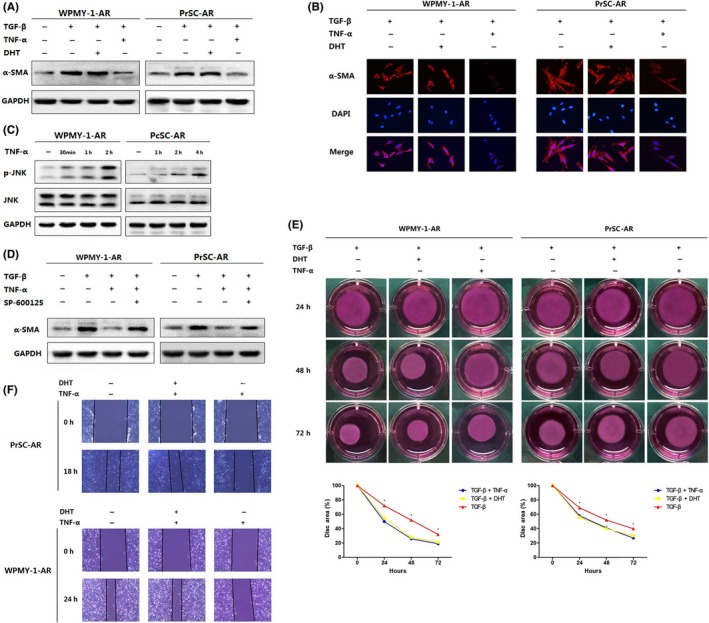
(A & B) TGF‐β induced α‐SMA expression. (C & D) JNK activation assessment with TNF‐α and/or SP600125 (30 mM) treatment. (E) Collagen gel contraction of WPMY‐AR and PrSC‐AR. (F) Wound‐healing assays of prostate fibroblasts
4. DISCUSSION
Although TURP or TmLRP significantly reduces complications of BPH, post‐operative complications such as urinary tract infection, urinary frequency, urgency, urodynia and haemorrhage sometimes happen before complete re‐epithelialization of the prostatic epithelium. Therefore, understanding of wound healing of prostatic urethra and developing the administration and intervention strategies to accelerate wound‐healing process after TURP or TmLRP is of great value.
Previous studies revealed that wound healing of elderly males were slower than females, with reduced matrix deposition and an enhanced inflammatory response,20 indicating that androgen has a negative impact on the cutaneous wound healing. In line with these results, the general AR knockout (ARKO) mice showed faster healing of the cutaneous wounds and collagen deposition and reduced inflammation than wild‐type mice, suggesting a pivotal role of AR signalling in suppressing wound healing.21 Prostate is a sex steroid hormone reactive organ. Circulating testosterone primarily derived from both testes and adrenal cortex is necessary for the gland development, maintenance, and function.22 Dihydrotestosterone (DHT), formed by conversion of testosterone by the enzyme 5‐α‐reductase (5AR) in prostate tissue,23 maintains much higher intra‐prostatic concentration than serumal. It is a principal androgen metabolite and exhibits higher bioactivity in prostate. However, no study was performed to investigate the influence of androgen on post‐operative prostate wound healing. In this study, we successfully established beagle TmLRP models with testosterone or anti‐androgen treatment. Serum and intra‐prostatic testosterone and DHT were tested and we found a significant reduction of DHT in finasteride‐treated canine prostate. Based on the results of H&E staining, acceleration of prostatic urethra re‐epithelialization was observed in finasteride group compared with testosterone group.
Similar with cutaneous wound healing, 3 overlapping cell functions, proliferation, migration and differentiation, are thought to be responsible for the process of re‐epithelialization.24 Till now, a large number of independent groups have clarified AR and AR signalling as suppressor of proliferation of prostate epithelial cells.25, 26, 27, 28 The secretory‐luminal cells are the most majority cell type within an adult prostate epithelial stem cell unit.12 However, these terminal differentiated expressing AR are usually proliferative quiescent, based on the observation that only <1% of prostate epithelial cells are proliferative.29 Furthermore, the studies of selective AR knock out by transgenic technique in mouse secretory‐luminal cells revealed that AR deficiency contributed to the hyperplasia of prostate epithelium.30, 31 All these results indicate that AR and testosterone treatment might attenuate the process of prostate re‐epithelialization. The observation based on our in vivo study by H&E staining showed that the number of epithelial layers decreased in testosterone treatment group at each time points, and finasteride treatment “thickening” the regenerative prostatic epithelium. Animal ethology observation revealed that finasteride treatment attributed to less and shorter phase of post‐operative complications, indicating the suppressing role of androgen, or DHT to be more specific, in the re‐epithelialization process of TmLRP.
The prostate epithelial stem cells are located in the basal epithelium layer in niches.12 These prostate basal cells account for the majority of epithelium proliferation in normal condition due to the self‐renew and transient amplifying ability and form the hierarchically expanding nature of prostate epithelium. The proliferation of these prostate basal cells needs paracrine‐secreted growth factors diffusing from the stroma into the epithelium collectively termed “andromedins.” The secretion of andromedins needs AR signalling activated by androgen in prostate stromal cells.32, 33, 34 In the present in vitro study, we confirmed that AR suppressed growth of prostate epithelial cells even in the presence of supporting stromal cells, which is in line with the fact that androgen‐dependent AR signalling helps to prevent prostatic epithelium hyperplasia even in the presence of continuous andromedins production.35, 36
Lizamma et al12 found that the growth arrest of prostate epithelial cells was due to the activation of AR by down‐regulating the expression and transcription activity of c‐myc. Treatment of AR‐positive epithelial cells with androgen resulted in growth repression accompanied with up‐regulation of p21 and p27, which are cyclin‐dependent kinase inhibitors (CKI) repressing c‐myc.25 In this study, we also observed down‐regulation of c‐myc in AR‐positive prostate epithelial cells treated with DHT. Cells growth was arrested in G0, with up‐regulation of p21 not p27 and down‐regulation of cyclin D1. Androgen responsive element has been identified on the promoter of the p21 gene,37 and increased expression of p21 may be partly due to the transcriptional mechanisms regulated by active AR.
In addition, macrophages and inflammatory response during wounding healing were studied in each group. As a key player that drives wound inflammation, wound‐site macrophages are important in clearance of dead cells and debris within the wound. Macrophages may polarize along 2 lines that have functional differences, pro‐inflammatory macrophages (M1), and anti‐inflammatory macrophages (M2).38 M1 macrophages exhibit pro‐inflammatory phenotype and mainly secret pro‐inflammatory cytokines that exert the function of removing pathogens and damaged tissues.39, 40 M1‐like macrophages are predominate in early stage of wound healing and replaced by M2 macrophages in later stage of repair. The appropriate hierarchical presence of M1 and M2 macrophages is crucial for favourable wound healing. An excessive or aberrant inflammatory response is recognized as a major contributing factor to delayed healing in both animal and human models. It is reported that greater and prolonged infiltration by inflammatory M1 contributed to the impairment of wound healing.41 We found extended duration of inflammatory phase in tissues of testosterone supplement group, related with retarded re‐epithelialization in prostate.
TNF‐α is a regulator of angiogenesis, inflammation and fibroplasia during repair, and M1 macrophages are main source of TNF‐α. Ashcroft et al20 observed dampened inflammatory response and reduced TNF‐α tissue expression in castrated animals, which was the first report of the association between TNF‐α and androgen. Apart from that, AR blockade by flutamide accelerated the healing response in a similar fashion to castration, indicating that AR activation suppressed wound healing by enhancing local TNF‐α expression.20 Similar results were also observed in our study that testosterone treatment enhanced TNF‐α expression and prolonged inflammatory response (M1 macrophages) in prostate tissue. In vitro studies have demonstrated that testosterone treatment could directly activate macrophages and improve the production of inflammatory factors like TNF‐α and IL‐6.20, 42, 43 As natural metabolite of testosterone, DHT can exert more potent biological effects on processes of physiology and pathology, including tissue repair. Through inhibiting the conversion of testosterone to DHT by feeding finasteride, DHT level was significantly reduced in canine prostate tissues, indicating that DHT mainly exerts the suppression effect in prostatic wound healing. All these results are in line with the studies conducted on cutaneous wound healing.42, 44 Similar to the previous study,21 we found DHT increased TNF‐α secretion of human macrophages activated by LPS, and this effect can be weakened by AR knockdown.
As a potent inflammatory cytokine expressed during the inflammatory phase of wound healing, TNF‐α inhibited production of α‐smooth muscle actin (α‐SMA) and cell migration and contraction. Activation of JNK pathway and subsequently smad3 phosphorylation reduction contributed to this effect in other type of fibroblasts.19, 45 We observed similar effect in prostate fibroblasts, and this effect was inhibited by JNK inhibitor SP600125. In addition, TNF‐α inhibited proliferation and migration of prostate epithelial cells though TNFR1. These effects were also true in other types of epithelial cells.15, 46 Taken together, TNF‐α secreted by macrophages may be also a key factor in attenuating the process of prostatic wound healing.
Different from castration, finasteride does not block androgen biological synthesis. There is still significant level of circulating and intra‐prostate testosterone and DHT (Figure 1C‐E). Finasteride significantly reduced DHT level, with increased level of testosterone. In combination of our in vitro study, these results indicate that it is DHT, not testosterone, worsens prostate re‐epithelialization and promotes inflammation. Two 5alpha‐reductase isozymes exist in human body, as type I 5alpha‐reductase predominantly expresses in liver and skin and type II 5alpha‐reductase primarily expresses in prostate. In situ hybridization and immunohistochemical localization studies showed that stromal cells and basal epithelium of the prostate maintained high level of type II 55alpha‐reductase.47 It is reasonable that testosterone to DHT conversion is significantly suppressed in the basal cell layer and stromal cells, contributing to the accelerated wound healing. Also, finasteride treatment would reduce the number of blood vessels in the prostate, resulting in less inflammatory cell infiltration and acute inflammatory exudation. The improved wound condition may promote re‐epithelialization of prostatic urethra after surgery.
Some issues exist and need to be further investigated. We observed increased level of serum estradiol after finasteride treatment (Figure S1). Although intra‐prostatic estradiol did not change, the effect of circulating estradiol on prostate wound healing is still unknown. As substantial studies showed that estradiol played a pivotal role in wound healing, it is interesting for further research focus on this field.
To conclude, we provide evidence that DHT performs key functions during prostate repair. Supplemented with type II 5α‐reductase inhibitor finasteride may promote re‐epithelialization of prostatic urethra after TmLRP by reducing the suppression effects of DHT and TNF‐α on wound healing, mirroring previous findings in cutaneous wound healing.42, 44 Recently, our team have demonstrated that castration induces basal cell proliferation and differentiation into urothelial cells,48 thus promoting re‐epithelialization and accelerating wound healing in the prostatic urethra after TmLRP. We confirmed that testosterone treatment regulates the conversion of M1 macrophage, resulting in enhanced inflammatory response and prolonged inflammatory phase. Conduct complete androgen deprivation on the BPH patients is impracticable. By repressing converting testosterone to DHT through inhibition of 5α‐reductase in prostate, finasteride should be a promising drug to reduce post‐operative complications. Further work is needed to validate the effectiveness of finasteride treatment on men. We hope our study could benefit BPH patients tormented by complications of TURP and TmLRP.
ACKNOWLEDGEMENTS
This study was supported financially by grants from the National Natural Science Foundation of China (no. 81570682, no. 81300625 and no. 81400755) and grants from Shanghai Municipal Health Bureau (2013ZYJB0102).
AUTHORS’ CONTRIBUTIONS
R.Z., X.W. and S.X. conceived the study. R.Z., X.W., C.J., F.S. and G.L. performed the experiments. B.H. and J.Z. aided beagles experiments. B.Y., Y.J. and Y.Z. analysed the data. B.H. and S.X. supervised the entire project. R.Z., X.W. and S.X. wrote and revised the manuscript. All authors reviewed the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Supporting information
Zhao R, Wang X, Jiang C, et al. Finasteride accelerates prostate wound healing after thulium laser resection through DHT and AR signalling. Cell Prolif. 2018;51:e12415 10.1111/cpr.12415
Ruizhe Zhao, XingJie Wang, Chenyi Jiang are co‐first authors because of equal contribution.
Contributor Information
Shujie Xia, Email: xsjurologist@163.com.
Bangmin Han, Email: hanbm@163.com.
REFERENCES
- 1. Issa MM, Regan TS. Medical therapy for benign prostatic hyperplasia – Present and future impact. Am J Manag Care. 2007;13(Suppl 1):S4‐S9. [PubMed] [Google Scholar]
- 2. Roehrborn CG. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am. 2011;95:87‐100. [DOI] [PubMed] [Google Scholar]
- 3. McNicholas T, Kirby R. Benign prostatic hyperplasia and male lower urinary tract symptoms (LUTS). BMJ Clin Evid. 2011;2011:1‐2. [PMC free article] [PubMed] [Google Scholar]
- 4. AUA guideline on management of benign prostatic hyperplasia . Chapter 1: diagnosis and treatment recommendations. J Urol. 2003;170:530‐547. [DOI] [PubMed] [Google Scholar]
- 5. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP) – Incidence, management, and prevention. Eur Urol. 2006;50:969‐979. [DOI] [PubMed] [Google Scholar]
- 6. Muntener M, Aellig S, Kuttel R, Gehrlach C, Hauri D, Strebel RT. Peri‐operative morbidity and changes in symptom scores after transurethral prostatectomy in Switzerland: results of an independent assessment of outcome. BJU Int. 2006;98:381‐383. [DOI] [PubMed] [Google Scholar]
- 7. Cao Y, Luo GH, Luo L, et al. Re‐epithelialization resulted from prostate basal cells in canine prostatic urethra may represent the ideal healing method after two‐micron laser resection of the prostate. Asian J Androl. 2015;17:831‐838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park EK, Jung HS, Yang HI, Yoo MC, Kim C, Kim KS. Optimized THP‐1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 2007;56:45‐50. [DOI] [PubMed] [Google Scholar]
- 9. Peng H, Wang H, Xue P, et al. Suppression of NRF2‐ARE activity sensitizes chemotherapeutic agent‐induced cytotoxicity in human acute monocytic leukemia cells. Toxicol Appl Pharmacol. 2016;292:1‐7. [DOI] [PubMed] [Google Scholar]
- 10. Yu S, Wang MW, Yao X, Chan FL. Establishment of a novel immortalized human prostatic epithelial cell line stably expressing androgen receptor and its application for the functional screening of androgen receptor modulators. Biochem Biophys Res Comm. 2009;382:756‐761. [DOI] [PubMed] [Google Scholar]
- 11. Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini‐extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antony L, van der Schoor F, Dalrymple SL, Isaacs JT. Androgen receptor (AR) suppresses normal human prostate epithelial cell proliferation via AR/beta‐catenin/TCF‐4 complex inhibition of c‐MYC transcription. Prostate. 2014;74:1118‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ngo P, Ramalingam P, Phillips JA, Furuta GT. Collagen gel contraction assay. Methods Mol Biol. 2006;341:103‐109. [DOI] [PubMed] [Google Scholar]
- 14. Usoro AJ, Obot AS, Ekaidem IS, Akaiso OE, Udoh AE, Akinloye O. Serum Testosterone, 17beta‐Estradiol and PSA levels in subjects with prostate disorders. Indian J Clin Biochem. 2015;30:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corredor J, Yan F, Shen CC, et al. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor‐dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953‐C961. [DOI] [PubMed] [Google Scholar]
- 16. Chang F, Lacey MR, Bouljihad M, Honer Zu, Bentrup K, Fortgang IS. Tumor necrosis factor receptor 1 functions as a tumor suppressor. Am J Physiol Gastrointest Liver Physiol. 2012, 302:G195‐G206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chopra DP, Menard RE, Januszewski J, Mattingly RR. TNF‐alpha‐mediated apoptosis in normal human prostate epithelial cells and tumor cell lines. Cancer Lett. 2004;203:145‐154. [DOI] [PubMed] [Google Scholar]
- 18. Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor‐beta 1 induces alpha‐smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldberg MT, Han YP, Yan C, Shaw MC, Garner WL. TNF‐alpha suppresses alpha‐smooth muscle actin expression in human dermal fibroblasts: an implication for abnormal wound healing. J Invest Dermatol. 2007;127:2645‐2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ashcroft GS, Mills SJ. Androgen receptor‐mediated inhibition of cutaneous wound healing. J Clin Investig. 2002;110:615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lai JJ, Lai KP, Chuang KH, et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF‐alpha expression. J Clin Investig. 2009;119:3739‐3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruchovsky N, Wilson JD. The intranuclear binding of testosterone and 5‐alpha‐androstan‐17‐beta‐ol‐3‐one by rat prostate. J Biol Chem. 1968;243:5953‐5960. [PubMed] [Google Scholar]
- 24. Sivamani K, Garcia MS, Isseroff RR. Wound re‐epithelialization: Modulating keratinocyte migration in wound healing. Front Biosci. 2007, 12:2849‐2868. [DOI] [PubMed] [Google Scholar]
- 25. Ling MT, Chan KW, Choo CK. Androgen induces differentiation of a human papillomavirus 16 E6/E7 immortalized prostate epithelial cell line. J Endocrinol. 2001;170:287‐296. [DOI] [PubMed] [Google Scholar]
- 26. Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1‐11. [PubMed] [Google Scholar]
- 27. Berger R, Febbo PG, Majumder PK, et al. Androgen‐induced differentiation and tumorigenicity of human prostate epithelial cells. Can Res. 2004;64:8867‐8875. [DOI] [PubMed] [Google Scholar]
- 28. Vander Griend DJ, Litvinov IV, Isaacs JT. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c‐Myc regulation. Int J Biol Sci. 2014, 10:627‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berges RR, Vukanovic J, Epstein JI, et al. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473‐480. [PMC free article] [PubMed] [Google Scholar]
- 30. Wu CT, Altuwaijri S, Ricke WA, et al. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc Natl Acad Sci USA. 2007;104:12679‐12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Simanainen U, McNamara K, Gao YR, Handelsman DJ. Androgen sensitivity of prostate epithelium is enhanced by postnatal androgen receptor inactivation. Am J Physiol Endocrinol Metab. 2009;296:E1335‐E1343. [DOI] [PubMed] [Google Scholar]
- 32. Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor‐10. A second candidate stromal to epithelial cell andromedin in prostate. J Biol Chemis. 1999;274:12827‐12834. [DOI] [PubMed] [Google Scholar]
- 33. Le H, Arnold JT, McFann KK, Blackman MR. DHT and testosterone, but not DHEA or E2, differentially modulate IGF‐I, IGFBP‐2, and IGFBP‐3 in human prostatic stromal cells. Am J Physiol Endocrinol Metab. 2006;290:E952‐E960. [DOI] [PubMed] [Google Scholar]
- 34. Ohlson N, Bergh A, Persson ML, Wikstrom P. Castration rapidly decreases local insulin‐like growth factor‐1 levels and inhibits its effects in the ventral prostate in mice. Prostate. 2006;66:1687‐1697. [DOI] [PubMed] [Google Scholar]
- 35. Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114‐118. [DOI] [PubMed] [Google Scholar]
- 36. De Marzo AM, Meeker AK, Epstein JI, Coffey DS. Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells. Am J Pathol. 1998;153:911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ. Androgen regulation of the cyclin‐dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol. 1999;13:376‐384. [DOI] [PubMed] [Google Scholar]
- 38. Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453‐461. [DOI] [PubMed] [Google Scholar]
- 39. Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733‐3739. [DOI] [PubMed] [Google Scholar]
- 40. Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanter JE, Kramer F, Barnhart S, et al. Diabetes promotes an inflammatory macrophage phenotype and atherosclerosis through acyl‐CoA synthetase 1. Proc Natl Acad Sci USA. 2012;109:E715‐E724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS. Androgens modulate the inflammatory response during acute wound healing. J Cell Sci. 2006;119:722‐732. [DOI] [PubMed] [Google Scholar]
- 43. Ashcroft GS, Mills SJ, Flanders KC, et al. Role of Smad3 in the hormonal modulation of in vivo wound healing responses. Wound Repair Regen. 2003;11:468‐473. [DOI] [PubMed] [Google Scholar]
- 44. Gilliver SC, Ruckshanthi JP, Hardman MJ, Zeef LA, Ashcroft GS. 5alpha‐dihydrotestosterone (DHT) retards wound closure by inhibiting re‐epithelialization. J Pathol. 2009;217:73‐82. [DOI] [PubMed] [Google Scholar]
- 45. Arancibia R, Oyarzun A, Silva D, Tobar N, Martinez J, Smith PC. Tumor necrosis factor‐alpha inhibits transforming growth factor‐beta‐stimulated myofibroblastic differentiation and extracellular matrix production in human gingival fibroblasts. J Periodontol. 2013;84:683‐693. [DOI] [PubMed] [Google Scholar]
- 46. Capaldo CT, Beeman N, Hilgarth RS, et al. IFN‐gamma and TNF‐alpha‐induced GBP‐1 inhibits epithelial cell proliferation through suppression of beta‐catenin/TCF signaling. Mucosal Immunol. 2012;5:681‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Steers WD. 5alpha‐reductase activity in the prostate. Urology. 2001;58(6 Suppl 1):17‐24. [DOI] [PubMed] [Google Scholar]
- 48. Wang XJ, Zhuo J, Luo GH, et al. Androgen deprivation accelerates the prostatic urethra wound healing after thulium laser resection of the prostate by promoting re‐epithelialization and regulating the macrophage polarization. Prostate. 2017;77:708‐717. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


