Abstract
Objectives
To investigate the mechanism of mechanical stimulation in bone formation and regeneration during distraction osteogenesis.
Materials and methods
In this study, microarray technology was used to investigate the time course of bone‐related molecular changes in distraction osteogenesis in rats. Real‐time PCR and Western‐blot analyses were used to confirm the expression of genes identified in microarrays. Meanwhile, we used a lentivirus vector to inhibit Fak expression, in order to identify the osteogenic effect of Fak and Fak‐Mapk pathway during distraction osteogenesis.
Results
Several components of the Wnt and Hippo pathways were found to be up‐ or down‐regulated during distraction osteogenesis by microarray. Meanwhile, it was found that Fak, Src, Raf‐1, Erk1, Jnk and p38‐Mapk were up‐regulated during gradual distraction, compared with consolidation. To further determine whether Fak‐Mapk pathway played an important role in distraction osteogenesis, Fak was disrupted with a lentivirus vector. The expressions levels of p‐Fak, p‐Erk1/2, p‐JNK and p‐p38Mapk were decreased. Meanwhile, a poor early and late osteogenesis effect was found in the shRNA‐Fak group.
Conclusion
It was inferred that the mechanical stimulus induces increased expression of Fak and activates Fak‐Mapk pathway, by activation of Erk, Jnk and p38‐Mapk pathway, and that Fak at least, in part, plays an important role in maintaining osteogenic effect by activating Fak‐Mapk pathway during distraction osteogenesis.
1. INTRODUCTION
Distraction osteogenesis (DO), a well‐established surgical procedure, initiates a biomechanical process that stimulates an endogenous biological reaction to create new bone. Since studied by Ilizarov,1 distraction osteogenesis has evolved into a surgical technique for correction of craniofacial deformities, deficiencies and increasing limb length2, 3
One of the main problems is the long‐treatment phase during which patients must wear distractor devices and manage a psychological and economic burden. Consequently, surgeons are seeking any improvement to shorten treatment phase and accelerate osteogenesis. Previous studies have reported that distraction osteogenesis resulted in the activation of signalling pathways. Mechanical tension‐stress up‐regulated the entire Bmp pathway during DO.4 The spatial and temporal localization of Wnt pathway is increased, due to distraction strain, during DO.5, 6 Meanwhile, some studies found the tension‐stress induced some genes and transcription factors up‐regulated or down‐regulated during DO.7, 8, 9, 10, 11 However, the molecular mechanisms of DO regulating osteogenic effect remain elusive.
Therefore, we explore the molecular basis and local microenvironment regulations in DO by microarray technology. Microarray technology was used to survey bone‐related mRNA expression in the distracted callus during different phases of DO. We identified the significantly differential expressed genes and found 3 functional groups of signalling pathways (Wnt, Hippo and Fak‐Mapk pathways). Wnt and Fak‐Mapk pathways were more activated during gradual distraction, while Hippo pathway was more activated during consolidation. The expressions of Fak, Src, Raf‐1, Erk1, Jnk and p38‐Mapk were up‐regulated during gradual distraction, compared with consolidation.
Mechanical stimulation plays a critical role in bone remodelling and the maintenance of homeostasis.12, 13, 14 Focal adhesion kinase (Fak), an important mechanotransduction involved gene, is shown to play an important role in fluid shear stress‐induced bone formation in osteoblast.15 Studies of mechanotransduction‐induced osteogenesis in vivo are still lacking. In this study, we used shRNA technology to inhibit Fak expression and investigated whether the shRNA‐Fak‐induced osteogenic effect decreased during DO. The shRNA knockdown of Fak inhibited the Fak‐Mapk pathway, which was verified by immunohistochemistry, real‐time PCR and Western‐blot, and delayed new bone formation by verification of histological and micro‐CT analyses. These data showed Fak and Fak‐Mapk pathway at least, in part, plays an important role in osteogenic effect during DO.
2. MATERIALS AND METHODS
2.1. Animals
Sprague‐Dawley rats (10‐week‐old male, weight 320‐350 g) were chosen as the research subjects. Animals were housed in a light/dark and temperature‐controlled room. The distraction osteogenesis model in rats was approved by the Ethics Committee of the University of Sichuan. All procedures that involved animals followed the Care and Use Committee guidelines of the University of Sichuan. The number of qualitative qualification is SCXK‐2009‐0009.
2.2. Surgery and distraction protocol
The rats were anaesthetized with 10% chloral hydrate (3.0 mL/kg). Rats underwent osteodistraction on the right tibia. Briefly, a longitudinal incision was made along the mid‐diaphysis of the right tibia to expose the diaphysis. The periosteum was not stripped and remained attached to the tibia. A 0.2 mm drill bit was used to drill through the lateral and medial cortices of the tibia and to form 4 small holes. Then, 4 titanium mini‐screws were placed across the holes (Figure 1A). The external distractor (orthodontic expansion) was fixed to 4 screws with acrylic resin (Methyl methacrylate) (Figure 1B). A transverse osteotomy was performed in the middle of the diaphysis between the 2nd and 3rd screws (Figure 1C, D). The ipsilateral fibula was fractured. After these procedures, the muscles and the skins were sutured (Figure 1E).
Figure 1.
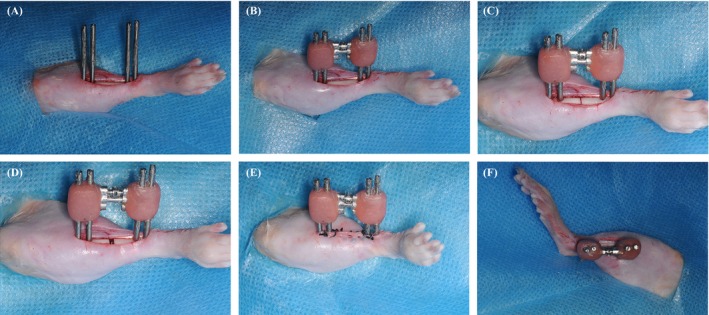
The surgical procedure for the right tibia model of distraction osteogenesis. (A) 4 titanium mini‐screws were placed across the right tibia. (B) The titanium external distractor was fixed to 4 screws with acrylic resin. (C) A transverse osteotomy was carried out at the middle of the tibia. (D) A distance was distracted with the orthodontic expansion. (E) The muscles and the skins were returned to their normal positions and were sutured. (F) The external distractor was parallel to the diaphysis of the right tibia
Distraction osteogenesis was divided into 3 phases: the latency period of 5 days after surgery; the active distraction period of 14 days, during which gradual distraction was initiated at a rate of 0.2 mm/12 h (total 5.6 mm); and the consolidation period of 8 weeks, during which the external distractor was held in situ as a fixator.6
2.3. Agilent 4*44k rat microarray gene analysis
The rats were randomly divided into 3 experimental groups: the middle of distraction (12 days post‐operation), the end of distraction (19 days post‐operation) and 1 week of consolidation (26 days post‐operation).
Before microarray analysis, the specimens were dissected from the tibia. The muscle and tendon were removed. The periosteum and distraction callus were dissected and stored in liquid nitrogen. Total RNA was extracted with Trizol reagent and further purified with an RNeasy mini kit and RNase‐Free. The RIN number was checked. And qualified total RNA was amplified and labelled with a Low Input Quick Amp Labelling Kit, One‐Coloe (Cat#5190‐2305; Agilent Technologies, Santa Clara, CA, USA), and labelled cRNA was purified with an RNeasy mini kit (QIAGEN, GmBH, Germany). Each slide was hybridized with 1.65 μg of Cy3‐labelled cRNA with a Gene Expression Hybridization Kit (Santa Clara, CA, USA).
Slides were scanned by the Agilent Microarray Scanner (Santa Clara, CA, USA), and date were extracted and normalized. The online sas analysis system was used for microarray gene analysis. The samples from 3 groups were compared with a single‐factor analysis of variance. Pairwise comparisons were performed with t test between the 3 groups (12 days post‐operation, 19 days post‐operation and 26 days post‐operation).
The criteria for filtering changed genes on the basis of microarray data were as follows: (i) FC (fold change): in pairwise comparisons, the genes were identified as up‐regulated when FC was ≥ 2, and as down‐regulated when FC was ≤ 0.5, (ii) P value: the change in P value was quantified in pairwise comparisons and was set at < 0.05.
2.4. Construction of a recombinant lentivirus vector expressing shRNA for Fak
The lentivirus expression vector was constructed with the lenti‐X shRNA expression system (Clontech, Mountain View, CA, USA). The design of shRNA for Fak (CTATGAAGTGTTAGAGAAA) strictly complied with the previously described rules. The cDNA olives were annealed and ligated into a GV118 (BamHI/EcoRI‐digested pLVX‐shRNA1) vector. The vectors were purified and cotransfected with a Lenti‐X HT Packaging Mix into HEK293T packaging cells with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). Then, the virus‐containing 293T cell culture supernatants were collected 48 hours after transfection, centrifuged at 4000 g for 10 minutes, filtered and stored at −80°C. The virus titre of LV‐Fak‐RNAi was 3E+8.
2.5. Real‐time quantitative RT‐PCR
Total RNA was extracted from the distraction tissues with Trizol. Total RNA was reverse transcribed to produce cDNA using the First Strand cDNA Synthesis Kit (Thermo, Pittsburgh, PA, USA), according to manufacturer's protocol. Fluorescence‐based real‐time PCR was carried out with an ABI Power SYBR Green PCR Master Mix (ABI, USA). The primer sequences were derived from gene sequences available through GenBank (Table 1). Relative expression was calculated for each gene by the 2−∆∆Ct method, normalized with GAPDH expression and presented as fold changes relative to the control. The samples that were used for RT‐PCR were not the same as the ones for microarray.
Table 1.
Rat primer for RT‐PCR assays
| Target gene | Primer sequence(5′‐3′) | ||
|---|---|---|---|
| Name | Accession | Direction | |
| Panx3 | NM_199398 | Forward | CCAGTGGGTTAGACCTGATTTCA |
| Reverse | GGCTAGCCTTGGAGAATCAGTTC | ||
| Wnt4 | NM_053402 | Forward | GCCATTGAGGAGTGCCAATA |
| Reverse | AACATATGCGGTAGAGAAGTCG | ||
| Lrp5 | NM_001106321 | Forward | CCATACAGGCCCTACATCATTC |
| Reverse | GATGGACCTGAACTTAAGCCTG | ||
| Ccl3 | NM_013025 | Forward | GGCTTGAGCCCCAGAACA |
| Reverse | TGGCAGCAAACAGCTTATAGGA | ||
| Fak | NM_013081 | Forward | TGAGCCACTGGCCATTGA |
| Reverse | GCTGCTGGTGGAATGCTAGAG | ||
| Raf‐1 | NM_012639 | Forward | ATGTGCGGAATGGGATGAG |
| Reverse | TCGGTGTTCCAATCTAAGCG | ||
| Src | NM_031977 | Forward | CAAGATCACTAGACGGGAATCAG |
| Reverse | GTTTCACATTTAGGCCCTTGG | ||
| Erk1 | NM_017347 | Forward | CTGGACCGGATGTTAACCTTTA |
| Reverse | GATACTAGGCTCTCTACTTGGTC | ||
| Jnk | NM_053829 | Forward | CAGAGGTCATTCTCGGCATG |
| Reverse | TTTCTCCCATAATGCACCCC | ||
| P38‐Mapk | NM_031020 | Forward | TGATACAAAGACGGGACATCG |
| Reverse | CACATCCAACAGACCAATCAC | ||
| GAPDH | NM_017008 | Forward | TCTGTGTGGATTGGTGGCTCTA |
| Reverse | CTGCTTGCTGATCCACATCTG | ||
2.6. Western blot
Distraction callus protein was extracted with the Protein Extraction Kit (KeyGen, Nanjing, China). Protein concentration was analysed with the BCA Protein Assay Reagent Kit. Each sample protein was analysed by electrophoresis in 10% acrylamide gels (with SDS) and transferred to transfer buffer at RT with shaking. To block the non‐specific binding site (without SDS), the membrane was immersed into 5% non‐fat milk in Tris‐buffered saline solution for 2 hours. The membrane was incubated for 2 hours with primary antibodies against Fak, p‐Fak, Erk1, Erk2, p‐Erk1/2, JNK, p‐JNK, p38Mapk, p‐p38Mapk, Raf‐1, Src, c‐fos and OPN (Abcam, Santa, Gene Tex and R&D, US) in 10 mL of 5% non‐fat milk in TBST solution. Each membrane was probed with GAPDH and anti‐tubulin as a loading control.
2.7. Injection of shRNA in bony callus
The rats were randomly divided into 3 groups. The same volume (100 uL) of saline solution (control), gutted shRNA vectors (shRNA‐neg) and Fak‐specific shRNA (shRNA‐Fak) were injected into the bony callus, 6 days after the operation, and injections were repeated every 4 days for a total of 3 injections per animals.
2.8. Immunohistochemistry
Distracted tibiae were embedded in 3% paraformaldehyde for 36 hours, decalcified in 20% ethylenediamine tetraacetic acid for 1 month, embedded in paraffin and were sectioned at a thickness of 5 μm. The immunolocalization of Fak, a critical gene involved in mechanotransduction pathway, was examined at the end of distraction (19 days). Blocked sections were incubated with a rabbit polyclonal antibody against Fak (Abcam, US, 1:200) and developed with a DAB colour development kit (ZSJQ‐BIO, china).
2.9. Radiographic, micro‐CT and histological analysis
To verify the osteogenic effect of our new distraction model, radiography was performed at the middle and end of the gradual distraction (12 and 19 days after operation) and at 2, 4 and 8 weeks of consolidation (33, 47 and 75 days after operation). The right tibias were scanned with a high‐resolution micro‐CT scanner (Y. Cheetah, Germany) and reconstructed with an isotropic voxel size of 10 μm. For histological analysis, the distraction tissues were decalcified and paraffin‐embedded, sectioned at 5 μm and stained with haematoxylin and eosin. The micro‐CT analysis and histological stain were performed at end of distraction and at 2, 6 and 8 weeks of consolidation (19, 33, 61 and 75 days after operation).
Moreover, to verify the important role of Fak in the osteogenesis effect during DO, micro‐CT analysis (Control, siena‐neg and shRNA‐Fak) was performed at 33 and 61 days, and the histological stain was performed at 19 days after operation.
3. RESULTS
3.1. Radiographic, micro‐CT and histological analysis of distraction osteogenesis models
Because we used a new model of DO, we initially evaluated the osteogenic effect. The model of DO shows good contraposition on X‐ray images (Figure 2E). During DO, almost no calcified areas were observed within the distracted gap at the middle of distraction (Figure 2F). At the end of distraction, the radiolucent areas remained at the centre of the distracted gap; however, tiny calcified areas were observed near the bone fragments (Figure 2G). At 33, 47 and 61 days, progressive bone formation occurred within the distracted gap (Figure 2H, I, K, L). Histological images revealed that the remodelling of new bone was formed by endochondral (mainly early phase of distraction) and intramembranous (initially late phase of distraction and mainly consolidation) ossification during DO. When distraction began, the callus was elongated and filled with longitudinally orientated fibrous tissue and newly formed blood vessels around the centre of distracted gap. Around the distal end of fibrous tissue, the new bone was formed by endochondral ossification, in which chondroblast‐like cells, hypertrophic chondrocytes and cartilage were observed. Following distraction, cartilaginous callus was initially resorbed and replaced by bone, which was formed by intramembranous ossification. We observed osteoblasts and osteocytes in the distracted callus at the end of distraction. (Figure 2A). During consolidation, the cartilaginous callus was progressively resorbed and was replaced by new bone, which was formed more by intramembranous ossification instead of endochondral ossification. The new bone progressively bridged the distracted gap, in which bone marrow tissue and a small amount of cartilage were found (Figure 2B, C). At 75 days, the complete bone was remodelled into mature cortical bone, and the medullary canal and cortical bone were indistinguishable (Figure 2D, J, M).
Figure 2.
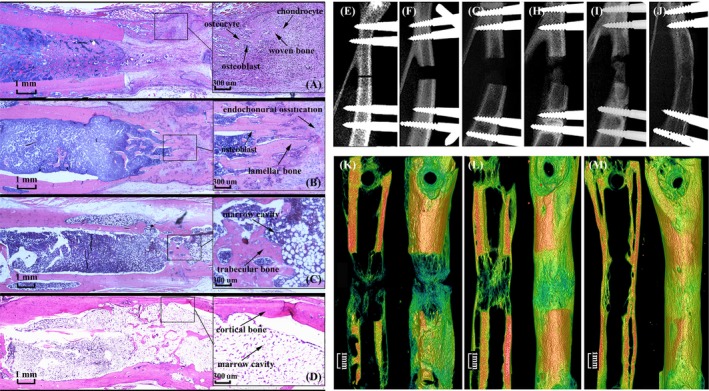
(A‐D) The histological stains were performed at the end of distraction (19 days after operation), and at 2, 6 and 8 weeks of consolidations (33, 61 and 75 days after operation). (E‐J) The X‐ray was performed at the healing phase of bone fracture (5 days after operation), the middle of distraction (12 days after operation), the end of distraction (19 days after operation), and at 2, 4 and 8 weeks of consolidations (33, 47 and 75 days after operation). (K‐M), micro‐CT examinations were performed at 2, 6 and 8 weeks of consolidations (33, 61 and 75 days after operation). (n = 8 rats/group)
3.2. Microarray analyses of distraction osteogenesis
To identify the bone‐related molecular mechanisms of biological processes that occur during DO, we evaluated Agilent 4*44k Rat microarray slides to compare gene expression profiles at 12, 19 and 26 days. The significantly altered genes were described by scatter and volcano plots, which are presented in Figure 3.
Figure 3.
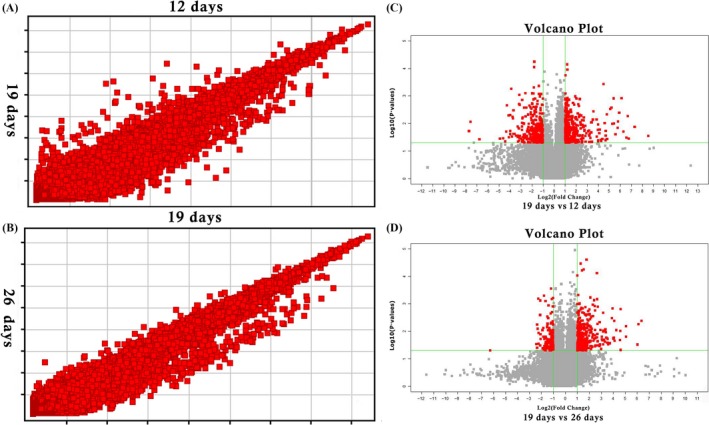
Scatter and volcano plots analysis diagram. (A) The scatter at 19 days vs at 12 days after operation. (B) The scatter at 19 days vs at 26 days after operation. (C) Volcano plots at 19 days vs at 12 days after operation. (D) Volcano plots at 19 days vs at 26 days after operation
3.2.1. Identification of ossification‐related genes and molecular signalling pathways
Some altered genes were chosen (FC ≤ 0.2 or ≥ 5). In the comparison of 19 and 12 days, the expression levels of Dlx5, Wnt5b, Itga10, Col2a1, Col11a2 and Col11a1 were noticeably up‐regulated at 19 days vs 12 days, especially Col2a1. In addition, the results showed increased expression of Panx3, Ifitm5 and Smpd3, which play important roles in early bone formation.16, 17, 18 sFRP4, Twsg1 and Ccl3 were noticeably down‐regulated at 19 days vs 12 days. In the comparison of 19 and 26 days, the expression levels of Sox9, Lcn2 and Itga2 were noticeably down‐regulated at 26 days. Vallet found that Ccl3 played an important role in the inhibition of osteoblast function and differentiation.19 Up‐regulated Lcn2 and down‐regulated Lepr were previously reported to be essential positive and negative regulators of osteoblast differentiation and function in response to mechanical force 20, 21
To identify whether the ossification‐related genes and molecular pathways are activated during DO, we examined 3 functional groupings of differential gene expression, including those with roles in the Wnt, Hippo and Fak‐Mapk pathway. The results for pathways and genes were presented in Table 2 (P < .05). At 19 days, the expression of genes was greater or lower than that at 12 and 26 days by a factor of 2. All results show that the Wnt pathway was more activated at 19 days, while the Hippo pathway was more activated during 26 days.
Table 2.
Differentially expressed genes involved in the Wnt, Hippo and Fak‐Mapk signalling
| Gene description | GenBank | Fold change | |
|---|---|---|---|
| 19 days vs 12 days | 19 days vs 26 days | ||
| Wnt signalling pathway | |||
| Wingless‐type MMTV integration site family, member 4 (Wnt4) | NM_053402 | 3.857 142 | 2.777 022 |
| Wingless‐type MMTV integration site family, member 10a (Wnt10a) | NM_001108227 | 2.829 97 | 3.435 399 |
| Wingless‐type MMTV integration site family, member 10b (Wnt10b) | NM_001108111 | 2.502 069 | 2.540 512 |
| Frizzled family receptor 1 (Fzd‐1) | NM_021266 | 3.985 11 | NS |
| Frizzled family receptor 2 (Fzd‐2) | NM_172035 | 2.401 284 | 2.705 707 |
| Frizzled family receptor 9 (Fzd‐9) | NM_153305 | NS | 0.223 223 |
| Low‐density lipoprotein receptor‐related protein 5 (Lrp5) | NM_001106321 | 2.265 443 | 4.379 979 |
| Secreted frizzled‐related protein 1 (sFRP‐1) | XM_001072532 | 4.414 605 | 2.725 474 |
| Secreted frizzled‐related protein 2 (sFRP‐2) | NM_001100700 | 4.324 393 | 3.200 82 |
| Glycogen synthase kinase 3 beta (Gsk‐3beta) | NM_032080 | 0.375 044 | 0.432 978 |
| Dickkopf 1 (Dkk‐1) | NM_001106350 | 0.248 414 | 0.432 848 |
| Transcription factor 7, Tell specific (Tcf‐7) | XM_001073458 | 3.831 727 | 2.728 356 |
| Lymphoid enhancer binding factor 1 (Lef‐1) | NM_130429 | 4.583 25 | 2.476 436 |
| Catenin (cadherin‐associated protein), beta 1 (β‐catenin) | NM_053357 | 4.015 773 | 4.265 686 |
| Hippo signalling pathway | |||
| Mammalian sterile 20‐like kinase 1 (MST1) | NM_001107800 | NS | 0.397 01 |
| Large tumour suppressor kinase 1 (LATS1) | NM_01134543 | 3.258 143 | 0.340 747 |
| Yes‐associated protein 1 (YAP1) | NM_001034002 | NS | 3.878 762 |
| Mitogen‐activated protein kinase kinase kinase kinase 1(MAP4K1) | NM_001106243 | NS | 0.459 191 |
| Mitogen‐activated protein kinase kinase kinase kinase 4(MAP4K4) | NM_001106904 | 2.121 188 | 0.262 66 |
| Salvador family WW domain containing protein 1 (Sav1) | NM_001097581 | NS | 0.445 885 |
| MOB kinase activator 1A (MOB1A) | NM_001033891 | 0.329 036 | 0.404 453 |
| Fak‐Mapk signalling pathway | |||
| Focal adhesion kinase(FAK) | NM_013081 | NS | 4.229 253 |
| Extracellular signal‐regulated kinase‐1(ERK1) | NM_017347 | 2.956 338 | 4.425 792 |
| Raf‐1 proto‐oncogene, serine/threonine kinase (Raf‐1) | NM_012639 | NS | 2.729 553 |
| SRC proto‐oncogene, non‐receptor tyrosine kinase (Src) | NM_031977 | 2.545 700 | 3.437 297 |
| c‐Jun N‐terminal kinase (Jnk) | NM_053829 | NS | 4.034 904 |
| P38 mitogen‐activated protein kinase (p38‐Mapk) | NM_031020 | 2.519 176 | 3.411 471 |
Mechanotransduction plays a critical role in the new bone formation during DO by converting the physical stimulus into a cellular response and accelerating bone formation. The expression levels of Fak, Raf‐1 and Jnk remained unchanged at 12 and 19 days and were down‐regulated at 26 days. The protein expression of Erk1, p38‐Mapk and Src increased gradually from 12 to 19 days and declined at 26 days (P < .05). These results show that the mechanical stimulus induces increased expression of Fak, and activates Fak‐Mapk pathway, by activation of Erk, Jnk and p38‐Mapk pathway. The results for pathways and genes were presented in Table 2 (P < .05).
3.2.2. Real‐time PCR and Western‐blot analysis
To verify the microarray results, real‐time PCR was performed on the distracted samples obtained on 12, 19 and 26 days. The results showed that the mRNA expression levels of Erk1, Src, p38‐Mapk, Wnt4, Lrp5 and Panx3 were increased (P < .05) at 19 days vs at 12 days; the expression levels of Ptch1 and Ccl3 were decreased (P < .05); and the expression of Fak, Raf‐1 and Jnk did not significantly change. At 19 days vs at 26 days, the expression levels of Fak, Raf‐1, Erk1, Src, p38‐Mapk, Wnt4, Lrp5 and Jnk were up‐regulated. All results of real‐time PCR were similar to the expression changes observed by microarray analysis and are shown in Figure 4
Figure 4.
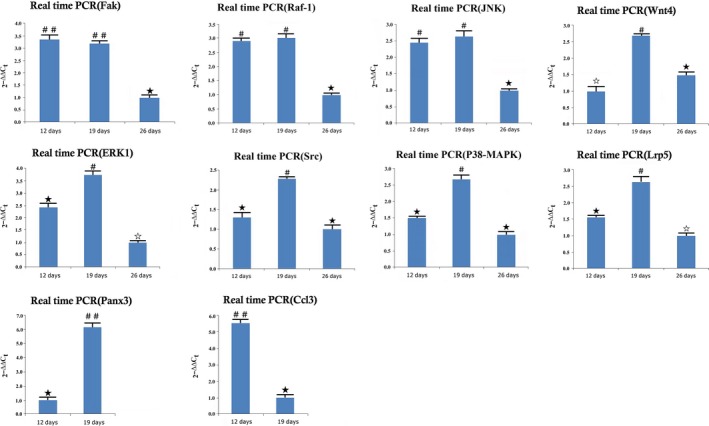
The relative mRNA expression of Fak, Raf‐1, Src, Erk1, Jnk, p38‐Mapk, Wnt4, Lrp5, Panx3 and Ccl3 in 12, 19 and 26 days after operation (n = 8 rats/each time point) (# vs ★: P < .05; ★ vs ☆: P < .05; ## vs ★: P < .01)
To confirm the protein expression changes of the related genes, Western blot was performed on the same time course (Figure 5). Compared with the protein expression levels at 12 days, the Fak, Raf‐1 and Jnk expressions remained unchanged at 19 days and were down‐regulated at 26 days. The protein expression of Erk1, p38‐Mapk and Src increased gradually from 12 to 19 days and declined at 26 days. All Western blot results were consistent with the mRNA expression results of RT‐PCR and microarray analysis.
Figure 5.
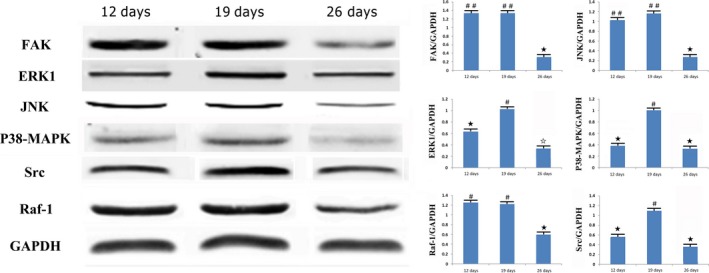
Comparison of patterns and quantification of the expression of Fak, Raf‐1, Src, Erk1, Jnk and p38‐Mapk in 12, 19 and 26 days after operation (n = 8 rats/each time point) (# vs ★: P < .05; ★ vs ☆: P < .05: ## vs ★: P < .01)
3.3. ShRNA‐Fak knockdown of Fak reduced the osteogenic effect of distraction osteogenesis
3.3.1. shRNA knockdown of Fak during distraction osteogenesis
The recombinant lentivirus vectors express green fluorescent protein (GFP) in the shRNA constructs; thus, we monitored GFP expression to examine the delivery efficiency of the lentivirus vectors. The results showed that the vectors effectively infected the cells of the distracted callus in the shRNA‐Fak and shRNA‐neg group, whereas no GFP‐positive cells were present in the control group (Figure 6 G‐I).
Figure 6.
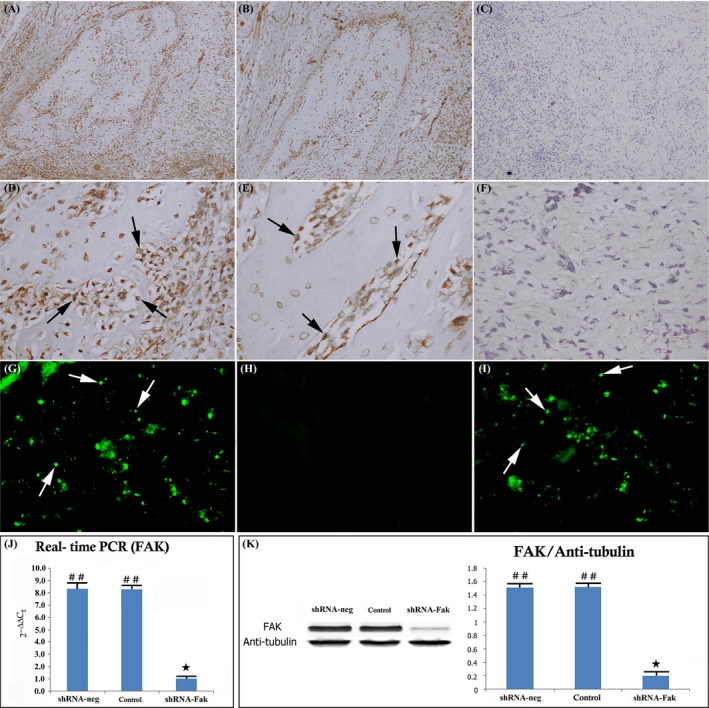
shRNA knockdown of Fak expression in the distracted callus. Immunohistochemical image showing FAK expression in the shRNA‐neg (A, D), control group (B, E) and shRNA‐Fak (C, F) (original magnification ×100 and ×400). No FAK protein expression was present with FAK antibody staining in the shRNA‐Fak group. (C, F) The infected callus region showing GFP expression in the shRNA‐neg (G), control (H) and shRNA‐Fak group (I). Mesenchymal cells in callus region were efficiently infected by the lentivirus constructs and express GFP in both shRNA‐neg (G) and shRNA‐Fak group (I). RT‐PCR and Western blot analysis showed that the level of FAK was significantly reduced in shRNA‐Fak group (J, K). (n = 8 rats/group) (## vs ★: P < .01) (n = 8 rats/group)
To verify that the expression of Fak mRNA and protein was down‐regulated by shRNA during DO, we examined the mRNA and protein levels in the distracted callus in the control, shRNA‐neg and shRNA‐Fak group by RT‐PCR and Western blot. The result showed that the Fak mRNA and protein levels in the shRNA‐Fak group had significantly decreased compared with those in the control and shRNA‐neg group (Figure 6 A‐F, J, K).
3.3.2. Fak knockdown caused a decrease in the expression of p‐Fak, p‐Erk1/2, p‐JNK and p‐p38‐Mapk in the distracted callus
To investigate whether shRNA‐Fak decreased the expression of Fak‐Mapk pathway components, we examined the protein levels of pathway‐related proteins (p‐Fak, p‐Erk1/2, p‐JNK and p‐p38Mapk). The results showed that the protein levels of p‐Fak, p‐Erk1/2, p‐JNK and p‐p38Mapk were significantly decreased in the shRNA‐Fak group, compared with the control and shRNA‐neg group; however, the expression of Erk1/2, JNK and p38‐Mapk remained unchanged (Figure 7).
Figure 7.
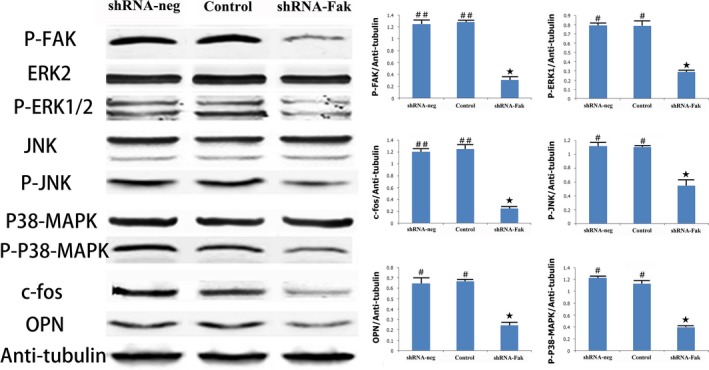
Comparison of patterns and quantification of the expressions of Fak‐Mapk signalling components: p‐Fak, Erk2, p‐Erk1/2, JNK, p‐JNK, p38‐Mapk and p‐p38Mapk in the shRNA‐neg, shRNA‐Fak and control group. (n = 8 rats/each time point) (# vs ★: P < .05; ## vs ★: P < .01)
3.3.3. Fak knockdown reduced the early and late osteogenic effect of distraction osteogenesis
To determine whether shRNA‐Fak decreased the osteogenic effect in distracted areas, histology and micro‐CT were used to analyse the early and late osteogenic effect during DO. At 19 days after operation, the distracted areas showed 3 osteogenic zones in the control and shRNA‐neg groups, whereas almost no calcified areas were observed in the shRNA‐Fak group (Figure 8). Micro‐CT analysis of 33 days showed that less bone trabecula was formed in the shRNA‐Fak group, compared with the control and shRNA‐neg group. Moreover, at 61 days after operation, the complete bone was remodelled into mature cortical bone in the control and shRNA‐neg group, whereas the bone fragments were not fused in the shRNA‐Fak group (Figure 8). Compared with the control and shRNA‐neg groups, Western blot analysis showed that the protein expression level of osteocalcin (OCN), an osteoblast differentiation maker and c‐fos were decreased in the shRNA‐Fak group (Figure 9).
Figure 8.
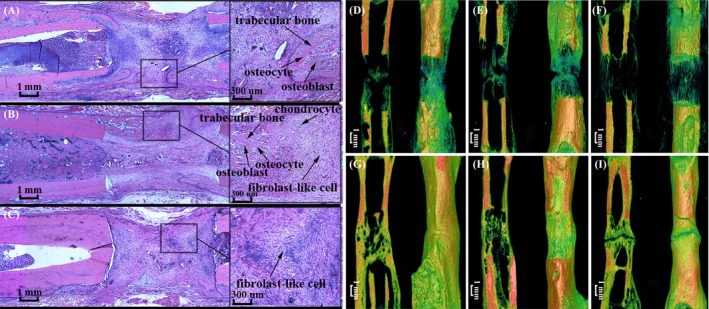
Representative histological image of the distracted callus from the shRNA‐neg (A), control (B) and shRNA‐Fak group (C). Representative micro‐CT images from the shRNA‐neg (33 days, D and 61 days, G), control (33 days, E and 61 days, H) and shRNA‐Fak group (33 days, F and 61 days, I). The results of the histology and micro‐CT showed a poor early and late osteogenic effect in the ShRNA‐Fak group (C, F and I). (n = 8 rats/group)
Figure 9.
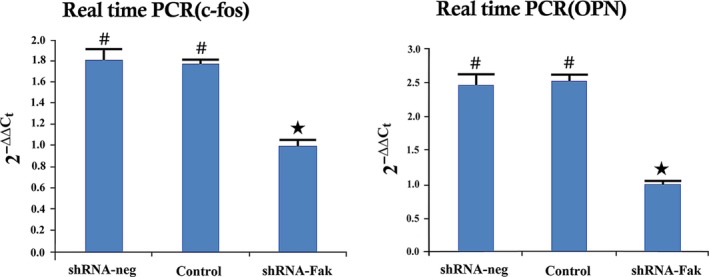
RT‐PCR and Western blot analysis showed that the levels of OPN and c‐fos were significantly reduced in the shRNA‐Fak group (n = 8 rats/each time point) (# vs ★: P < .05)
4. DISCUSSION
Distraction osteogenesis is an endogenous bone tissue engineering technique and imposes a specific size and frequency of mechanical force to induce endogenous bone formation and remodelling in the distraction gap.1 In spite of the application of this instrument for the regeneration and reconstruction of bone, the molecular mechanism of DO remains unknown. Mechanical stimulation is essential for the promotion of osteoblast and chondrocyte differentiation, bone formation, the prevention of bone resorption and the maintenance of skeletal homeostasis.12 Previous studies had been reported that mechanical stimulation influences the osteogenic differentiation of MSCs through inducing the expression of osteogenic genes and the activity of ALP, OCN and Runx2 in vitro.22, 23, 24 However, mechanisms of mechanical stimulus‐induced osteogenesis in vivo were still not clearly elucidated. Distraction osteogenesis is a suitable tool for researching the mechanism of mechanical microenvironments of bone formation and regeneration in vivo.
4.1. Altered expression of bone formation‐related molecular pathway components during distraction osteogenesis
In this study, several components of the Wnt, Hippo and Fak‐Mapk pathways were found to be up‐ or down‐regulated during different phases of DO by microarray. The results revealed that the Wnt pathway was more activated at the end of distraction, while the Hippo pathway was more activated during consolidation. These results were consistent with the previous studies, in which Wnt pathways was reported to play important roles in bone formation during DO.5, 6 However, The studies of whether Fak‐Mapk pathway was activated or not during DO remain unclear.
Previous studies demonstrated that mechanical stimulation could induce changes in molecular function, demonstrated by the up‐ or down‐regulation of transcription factors,25, 26 increased mRNA synthesis,27 and cause changes in gene expression.28, 29 Focal adhesion kinase (FAK) is a non‐receptor tyrosine kinase that associates with integrins at focal adhesion in the Integrin‐Fak pathway.30 Subsequently, Fak was activated via a phosphorylation event, which provides a binding site for Src and other molecules.31 The C‐terminal domain of Fak can associate with other factors and connect the focal adhesion with the actin cytoskeleton.32 Fak can, in turn, activate downstream signalling mediators, including Raf and Ras.15, 33 Raf is an upstream kinase of the mitogen‐activated protein kinase.15 The Fak‐Mapk pathway has previously been found to play important roles in signal transduction during cell migration, cellular proliferation and differentiation.24 However, it is unclear whether Fak‐Mapk pathway was activated during DO. In this study, we used gene microarray to analyse the expressions of altered genes and signalling pathways. We found Erk1, p38‐Mapk and Src increased gradually from 12 to 19 days and declined at 26 days after operation. Fak, Raf‐1 and Jnk remained unchanged at 12 and 19 days, and were down‐regulated at 26 days after operation. These data suggest that the up‐regulated expression of Fak and activation of Fak‐Mapk pathway were induced by mechanical stimulus in DO.
4.2. Identifying the osteogenic effect of Fak and Fak‐Mapk signalling during the distraction osteogenesis
Previous studies had demonstrated that Fak was a regulator of focal adhesion dynamics and cell movent,33, 34 which aggregated with integrins at focal adhesion complexes, and was activated through phosphorylation in response to cell adhesion, mechanical stimulus and fluid shear stress.35, 36, 37 Fak was also thought to have a vital role in signalling transduction during cell differentiation, migration, growth, proliferation and survival.38, 39, 40 Young has demonstrated the role of Fak in osteoblast differentiation, with a conditional FAK−/− osteoblast in Fluid shear stress.15 We suspected that Fak may act as mechanoreceptors to sense the stimulation and influenced the osteogenic effect by Fak‐Mapk pathway during DO. To explore this possibility, a lentivirus vector was used to inhibit Fak expression, in order to identify the osteogenic effect of Fak and Fak‐Mapk pathway during DO. The phosphorylation level of Fak was noticeably declined in the shRNA‐Fak group, compared with the shRNA‐neg and control group. The downstream factors p‐Erk1/2, p‐JNK and p‐p38Mapk were also found to be declined in the shRNA‐Fak group, as detected via Western blot. Compared with the control and shRNA‐neg groups, disturbed early and late osteogenesis was found in the shRNA‐Fak group. This result indicated that Fak knockdown lead to an inhibited effect on Fak‐Mapk pathway and an obstructive effect on the osteogenesis in the distracted callus during DO. However, in addition to Integrin‐Fak pathway, Wnt, Bmp and TGF also induced the activation of Mapk pathway. Therefore, these results suggested that Fak at least, in part, plays an important role in osteogenic effect by activating Fak‐Mapk pathway during DO.
In summary, a comparative temporal analysis of altered genes in different phases of DO provides more precise insights about the mechanisms of bone formation. We demonstrate Fak, Raf‐1, Src, Erk1, Jnk and p38‐Mapk were increased during gradual distraction, compared to consolidation. In addition, to further examine the role of the Fak and Fak‐Mapk pathway in the osteogenic effect, a lentivirus vector was used to inhibit Fak expression. These data show that the inhibition of Fak expression decreased the expression of components of the Fak‐Mapk pathway and resulted in a poor early and late osteogenic effect of DO. All results indicate that the mechanical stimulus induces increased expression of Fak and activates Fak‐Mapk pathway, by activation of Erk, Jnk and p38‐Mapk pathway, and that the low osteogenic effect of DO induced by Fak knockdown is mediated at least, in part, through suppression of Mapk pathway. Fak plays an important role in maintaining osteogenic effect by activating Fak‐Mapk pathway during DO.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
HUMAN AND ANIMAL RIGHTS
This study was conducted in accordance with the National Institutes of Health guide for the care and use of Laboratory animals.
ACKNOWLEDGEMENTS
This study was supported by grants from the NSFC (81621062, 81271106 and 81100779), and the Sichuan Province Science and Technology Innovation Team Program(2017TD0016)
Song J, Ye B, Liu H, et al. Fak‐Mapk, Hippo and Wnt signalling pathway expression and regulation in distraction osteogenesis. Cell Prolif. 2018;51:e12453 10.1111/cpr.12453
Jian Song and Bin Ye are the authors who contributed equally to this work.
Contributor Information
Jing Hu, Email: drhu-vip@sohu.com.
En Luo, Email: luoen521125@sina.com.
REFERENCES
- 1. Ilizarov GA. The tension‐stress effect on the genesis and growth of tissues. Part I. The influence of stability of fixation and soft‐tissue preservation. Clin Orthop Relat Res. 1989;238:249‐281. [PubMed] [Google Scholar]
- 2. Kessler P, Schultze‐Mosgau S, Neukam FW, et al. Lengthening of the reconstructed mandible using extraoral distraction devices: report of five case. Plast Reconstr Surg. 2003;111:1400‐1404. [DOI] [PubMed] [Google Scholar]
- 3. Aldegheri R. Distraction osteogenesis for lengthening of the tibia in patients who have limb‐length discrepancy or short stature. J Bone Joint Surg Am. 1999;81:624‐634. [DOI] [PubMed] [Google Scholar]
- 4. Khanal A, Yoshioka I, Tominaga K, et al. The BMP signaling and its Smads in mandibular distraction Osteogenesis. Oral Dis. 2008;14:347‐355. [DOI] [PubMed] [Google Scholar]
- 5. Kasaai B, Moffatt P, Al‐Salmi L, et al. Spatial and Temporal Localization of WNT Signaling Proteins in a Mouse Model of Distraction osteogenesis. J Histochem Cytochem. 2012;60:219‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Luo E, Bi R, et al. Wnt/beta‐catenin signaling is required for distraction osteogenesis in rats. Connect Tissue Res. 2017;1:1‐10. [DOI] [PubMed] [Google Scholar]
- 7. Xue J, Peng J, Yuan M, et al. NELL1 promote shigh‐quality bone regeneration in rat femoral distraction osteogenesis model. Bone. 2011;48:485‐495. [DOI] [PubMed] [Google Scholar]
- 8. Stewart KJ, Weyand B, van't Hof RJ, et al. A quantitative analysis of the effect of insulin‐like growth factor‐1 infusion during mandibular distraction osteogenesis in rabbits. Br J Plast Surg. 1999;52:343‐350. [DOI] [PubMed] [Google Scholar]
- 9. Eckardt H, Bundgaard KG, Christensen KS, et al. Effects of locally applied vascular endothelial growth factor (VEGF) and VEGF‐inhibitor to the rabbit tibia during distraction osteogenesi. J Orthop Res. 2005;21:335‐340. [DOI] [PubMed] [Google Scholar]
- 10. Raschke MJ, Bail H, Windhagen HJ, et al. Recombinant growth hormone accelerates bone regenerate consolidation in distraction osteogenesis. Bone. 1999;24:81‐88. [DOI] [PubMed] [Google Scholar]
- 11. Yeung HY, Lee SK, Fung KP, et al. Expression of basic fibroblast growth factor during distraction osteogenesis. Clin Orthop Relat Res. 2011;69:2860‐2871. [DOI] [PubMed] [Google Scholar]
- 12. Hillam RA, Skerry TM. Inhibition of bone resorption and stimulation of formation by mechanical loading of the modeling rat ulna in vivo. J Bone Miner Res. 1995;10:683‐689. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Yang X, Yue Y, et al. Cyclic mechanical stress modulates neurotrophic and myelinating gene expression of Schwann ceell. Cell Prolif. 2015;48:59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li G, Fu N, Yang X, et al. Mechanical compressive force inhibits adipogenesis of adipose stem cells. Cell Prolif. 2013;46:586‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yong SR, Gerard‐O'Riley R, Kim JB, et al. Focal adhesion kinase is important for fluid shear stress‐induced mechanotransduction in osteoblast. J Bone Miner Res. 2009;24:411‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bond SR, Lau A, Penuela S, Sampaio AV, et al. Pannexin 3 is a novel target for Runx2, expressed by osteoblasts and mature growth plate chondrocytes. J Bone Miner Res. 2011;26:2911‐2922. [DOI] [PubMed] [Google Scholar]
- 17. Aubin I, Adams CP, Opsahl S, et al. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet. 2005;37:803‐805. [DOI] [PubMed] [Google Scholar]
- 18. Cho TJ, Lee KE, Lee SK, et al. A single recurrent mutation in the 5′‐UTR of IFITM5 causes osteogenesis imperfecta type V. Am J Hum Genet. 2012;91:343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vallet S, Pozzi S, Patel K, et al. A novel role for CCL3 (MIP‐1α) in myeloma‐induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25:1174‐1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veeriah V, Zanniti A, Paone R, Chatterjee S, et al. Interleukin‐1β, lipocalin 2 and nitric oxide synthase 2 are mechano‐responsive mediators of mouse and human endothelial cell‐osteoblast crosstalk. Sci Rep. 2016;6:29880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kapur S, Amoui M, Kesavan C, et al. Leptin receptor (Lepr) is a negative modulator of bone mechanosensitivity and genetic variations in Lepr may contribute to the differential osteogenic response to mechanical stimulation in the C57BL/6J and C3H/HeJ pair of mouse strains. J Biol Chem. 2010;285:37607‐37618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grellier M, Bareille R, Bourget C, et al. Responsiveness of human bone marrow stromal cells to shear stress. J Tissue Eng Regen Med. 2009;3:302‐309. [DOI] [PubMed] [Google Scholar]
- 23. Stiehler M, Bunger C, Baatrup A, et al. Effect of dynamic 3D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 2009;89:96‐107. [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Zong C, Li B, et al. The interaction between β1 integrins and ERK1/2 in osteogenic differentiation of human mesenchymal stem cells under fluid shear stress modelled by a perfusion system. J Tissue Eng Regen Med. 2014;8:85‐96. [DOI] [PubMed] [Google Scholar]
- 25. Jurek B, Slattery DA, Hiraoka Y, et al. Oxytocin regulates stress‐induced Crf gene transcription through CREB‐regulated transcription coactivator 3. J Neurosci. 2015;35:12248‐12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nozaki JI, Kubota H, Yoshida H, et al. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2 +/Akita, pancreatic β cells. Genes Cells. 2004;9:261‐270. [DOI] [PubMed] [Google Scholar]
- 27. Maigaard K, Hageman I, Jørgensen A, et al. Electroconvulsive stimulations prevent chronic stress‐induced increases in L‐type calcium channel mRNAs in the hippocampus and basolateral amygdala. Neurosci Lett. 2012;516:24‐28. [DOI] [PubMed] [Google Scholar]
- 28. Rolli M, Kotlyarov A, Sakamoto KM, et al. Stress‐induced stimulation of early growth response gene‐1 by p38/stress‐activated protein kinase 2 is mediated by a cAMP‐responsive promoter element in a MAPKAP kinase 2‐independent manner. J Biol Chem. 1999;274:19559‐19564. [DOI] [PubMed] [Google Scholar]
- 29. Autelitano DJ. Stress‐induced stimulation of pituitary POMC gene expression is associated with activation of transcription factor AP‐1 in hypothalamus and pituitary. Brain Res Bull. 1998;45:75‐82. [DOI] [PubMed] [Google Scholar]
- 30. Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15:954‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;18:516‐523. [DOI] [PubMed] [Google Scholar]
- 32. Schaller M, Hidebrand J, Shannon J, et al. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2‐dependent binding of pp60src. Mol Cell Biol. 1994;14:1680‐1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Laser M, Willey CD, Jiang W, et al. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem In vitro. 2000;45:24‐30. [DOI] [PubMed] [Google Scholar]
- 34. Parsions JT, Martin KH, Slack JK, et al. Focal adhesion kinase: a regulator of adhesion dynamics and cell movement. Oncogene. 2000;19:5606‐5613. [DOI] [PubMed] [Google Scholar]
- 35. Burridge K, Turner C, Romer L, et al. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yano Y, Geibel J, Sumpio BE. Tyrosine phosphorylation of pp125FAK and paxillin in aortic endothelial cells induced by mechanical strain. Am J Physiol. 1996;271:635‐649. [DOI] [PubMed] [Google Scholar]
- 37. Ishida T, Peterson TE, Kovach NL, et al. MAP kinase activation by flow in endothelial cells: role of beta1 integrins and tyrosine kinase. Circ Res. 1996;79:310‐316. [DOI] [PubMed] [Google Scholar]
- 38. Clark EA, Brugge JS. Integrins and signal transduction pathway: the road taken. Science. 1995;268:233‐239. [DOI] [PubMed] [Google Scholar]
- 39. Ilić D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110:401‐407. [DOI] [PubMed] [Google Scholar]
- 40. Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883‐885. [DOI] [PubMed] [Google Scholar]


