Abstract
Tumour necrosis factor alpha (TNFα) and interferon gamma (IFNγ) were originally found to be produced by inflammatory cells and play important roles in the immune system and surveillance of tumour growth. By activating distinct signalling pathways of nuclear factor‐κB (NF‐κB), mitogen‐activated protein kinase (MAPK), and JAK/STAT, TNFα and IFNγ were reported to effectively trigger cell death and perform powerful anti‐cancer effects. In this review, we will discuss the new advancements of TNFα and IFNγ in anti‐cancer therapy.
1. INTRODUCTION
Tumour necrosis factor alpha (TNFα), a member of the TNF superfamily, was reported to be mainly produced by macrophages and capable of replicating the ability of endotoxin in inducing haemorrhagic tumour necrosis.1 A number of studies demonstrated it as a potent inflammatory cytokine inducing complex immune responses2 and also performing anti‐cancer effects. TNFα was the first cytokine to be employed for cancer treatment. It exerts anti‐tumour activity through complex mechanisms of induction of inflammatory and immune responses, tumour cell apoptosis/necrosis and extensive thrombosis and destruction of tumour vasculature.3 By now, many studies have been conducted to evaluate the anti‐cancer efficacy of TNFα in various tumour types and some are even put into clinical trials.
The other modulator interferon gamma (IFNγ), which is a cytokine, belongs to a type II interferon group and plays critical roles in both host defence and immune regulation. Mature forms of natural human or murine IFNγ comprise of glycosylated polypeptides of 143 and 134 amino acids, respectively and homodimerize to form a non‐covalently linked 50 kDa protein. The understanding of cell biology and physiology of IFNγ started from the initial description of its anti‐viral activities produced by phytohaemagglutinin‐activated human leucocytes.4 The later discovery that patients with deficiency in IFNγ production or signalling are highly susceptible to rare mycobacterial infections highlighted the importance of IFNγ in preventing infectious diseases.5 IFNγ is now clearly depicted to exert its effects by binding to distinct high affinity receptors of IFNGR1 and IFNGR2 and subsequently activate a specific signal transduction pathway termed JAK‐STAT pathway to regulate transcription of IFNγ‐inducible genes mediating specific IFNγ‐dependent cellular responses6 of apoptosis etc. Studies then focused on a critical role of endogenously produced IFNγ in promoting host responses to tumours, which then evoked many interests on its anti‐cancer function and clinical application. Despite the overwhelming evidence indicating the anti‐tumour activity of IFNγ, there are still some studies revealing its pro‐tumourigenic activities based on the cellular, microenvironment and/or molecular context.7 Therefore, the anti‐cancer therapeutic application of IFNγ should be carefully evaluated.
Despite the promising anti‐cancer potential of the two cytokines, their clinical application is still hindered by severe toxicity after systemic administration. Many strategies have been investigated to reduce their systemic toxicity.8 Fusion proteins consisting of a cytokine and a recombinant peptide are regarded as a novel class of “armed” antibodies acting as delivery vehicles and increasing the therapeutic index of pro‐inflammatory cytokines.9 So far, various ligands targeting tumour‐associated antigens have been employed to combine with cytokines as fusion proteins, which help for the specific accumulation of cytokines at tumour sites. But the tumour‐targeting and therapeutic effects have variable outcomes and should be evaluated from case to case. For example, some pro‐inflammatory cytokines as IL‐2, IL‐12 and TNFα that fused to L19 (specific to spliced EDB domains) or F8 (specific to spliced EDA domains) exhibited impressive anti‐cancer activity with selective uptake at the tumour site, while IL‐7, IL‐17, IL‐15 and IL‐18 showed limitations either in tumour‐targeting or therapy.10, 11, 12 Our review here will mainly discuss the anti‐cancer mechanisms of TNFα and IFNγ and their selective delivery systems and potential clinical application in cancer therapy.
2. THE ANTI‐CANCER ACTIVITY OF TNFα
Tumour necrosis factor alpha consists of 3 non‐covalently linked TNFα monomers, ~17.5 kDa each, which forms a compact bell‐shaped homotrimer.13, 14 The soluble homotrimeric TNFα can be released via proteolytic cleavage by a metalloprotease, the TNFα converting enzyme. TNFα was reported to bind to 2 receptors, TNFR1 and TNFR2, where TNFR1 is constitutively expressed in most tissues and considered as a death receptor, and TNFR2 is mainly expressed in cells of the immune system.15 Upon TNFα binding, TNFRs form homotrimers which cause conformational changes to the receptors with a series of intracellular events leading to the activation of 3 major signalling cascades, namely the nuclear factor kappa B (NF‐κB) pathway, the mitogen‐activated protein kinase (MAPK) pathway and the induction of death signalling.8
Tumour necrosis factor alpha plays a paradoxical role in cancer biology in which its induction of cancer cell death or survival depends on the cellular context. TNFα was initially isolated from the sera of mice treated with bacterial endotoxin and it was found to be able to replicate the ability of endotoxin in inducing haemorrhagic tumour necrosis. After that, numerous studies were conducted to investigate its clinical applications especially in cancer therapy. It has been discovered that TNFα can lead to massive haemorrhagic necrosis of transplanted tumours.2 Although TNFα shows potent anti‐tumour activity in various animal cancer models, this cytokine unselectively binds not only to tumour cells and endothelial cells, but also to normal cells and blood vessels, to produce non‐specific damage to various cell types. This could cause severe toxicity after systemic administration, even at doses far below the therapeutic window. Various phase I and phase II clinical trials were conducted in the 1980s and 1990s for systemic treatment of recombinant human TNFα (rhTNFα), using TNFα either as a single agent or in combination with other cytokines, chemotherapy or radiotherapy. However, the results were disappointing due to significant toxicities and very limited beneficial outcome.16 The main clinical trials and toxicities associated with systemic administration with TNFα have been reviewed previously.16 The common dose limiting side effects include hypotension, rigors, phlebitis, thrombocytopenia, leucopenia and hepatotoxicity. Other general symptoms include fever, fatigue, nausea/vomiting, malaise and weakness, headache, chest tightness, low back pain, diarrhoea and shortness of breath.16, 17, 18, 19, 20 For the above reason, the clinical use of TNFα is now confined to isolated limb perfusion (ILP) in combination with melphalan for soft tissue sarcoma and melanoma. Many efforts have been paid to augment the anti‐tumour effect of TNFα while to reduce its systematic toxicity, including passive targeting by PEGylation, cell‐based therapy, gene therapy with inducible or tissue‐specific promoters, shielding or encapsulation of TNFα, antibody‐TNFα conjugate, vascular targeting TNFα coupled to tumour‐homing peptides and TNFα mutants.8, 21, 22 Lately, it is reported that systemic administration of TNF‐expressing tumour cells can reduce the growth of both primary tumours and metastatic colonies in immunocompetent mice by homing to tumours, locally releasing TNFα, damaging neovascular endothelia and inducing massive cancer call apoptosis.23 At the same time, it can minimize the common side effects. However, more pre‐clinical and clinical studies are needed to fully assess the safety and efficacy of this approach.
3. TNFα AND TUMOUR ANGIOGENESIS
As early as the 1990s, TNFα has been reported to exert synergic anti‐tumour effects when combined with other chemotherapeutic drugs. Such synergism is mainly based on the alteration of endothelial barrier function, reduction of tumour interstitial pressure and finally improvement of drug delivery to the tumours.24, 25 It has also been proposed that the anti‐tumour activity of TNFα depends on indirect mechanisms of selective obstruction and damage of tumour‐associated blood vessels and activation of immune responses rather than having toxic effects directly on tumour cells.26, 27, 28, 29 Studies then found out that isolated limb or hepatic perfusion with high dose of TNFα in combination with melphalan (a chemotherapeutic drug) produced high complete response rates in patients with melanoma or sarcoma of the extremities,30, 31 as well as regression of bulky hepatic cancer confined to the liver.32 The micro‐ and macro‐vasculature in tumours was observed to be extensively damaged after isolated perfusion to limbs with TNFα in combination with IFNγ and melphalan.33
However, as we mentioned above, TNFα showed non‐specificity in cancer therapy, which hampered its systemic administration. Therefore, specially homing TNFα to tumour vessels could be a powerful anti‐tumour strategy. By in vivo phage ligand capable of homing to tumour vessels and is first explored to fuse with TNFα to effectively homing TNFα to tumours. By coupling TNFα to CNGRC as a compound of NGR‐TNF, it can deliver pictogram doses of TNFα into tumours, which indeed successfully hyper‐concentrated TNFα at tumours and enhanced the immunotherapeutic properties of TNFα.14 Studies investigating the structure‐activity and receptor‐binding of NGR‐TNF fusion proteins showed that NGR peptide did not influence and prevent folding, oligomerization, and the interaction between TNFα with TNFα receptors.14 Studies were then conducted in the in vivo murine tumour models showing that compared to TNFα, low doses of NGR‐TNF could greatly inhibit tumour growth and enhance chemotherapeutic efficacy of doxorubicin and melphalan,34 indicating that the conjugation with NGR did not influence the biological effect of TNFα in vivo. Besides the direct inhibition of tumour growth by NGR‐TNF, many efforts were paid to explore its capacity to improve response to chemotherapy by altering tumour vasculature and tumour microenvironment. Since TNFα itself could alter endothelial barrier function and synergistically improve drug concentration in tumours, one study in 2006 aimed at evaluating the biological effects of NGR‐TNF on tumour vasculature at low doses in lymphoma‐bearing mice.35 This study demonstrated an increase in vascular permeability after NGR‐TNF treatment. However, two hours after NGR‐TNF treatment, there was a decrease in tumour hypoxia and an increase in labelling index of the S‐phase marker bromodeoxyruridine, which could lead to increased tumour growth. However, after 1 day of treatment, the in vivo tumour growth decreased, implying that other potentially long‐lasting effects of NGR‐TNF did occur. This study underlines the importance of timing for the combined treatment of NGR‐TNF with other therapeutic agents.
By targeting tumour vessels, NGR‐TNF was proven to exert synergistic anti‐tumour effects with melphalan, doxorubicin, cisplatin, gemcitabine and paclitaxel in RMA lymphoma‐bearing mice.36 Similar to TNFα, a primary mechanism for NGR‐TNF to produce synergic effects with chemotherapeutic drugs was related to disassembly of endothelial VE‐cadherin‐dependent adherence junctions and alteration of endothelial barrier function in tumours, increase of tumour perfusion and reduction of interstitial pressure. Currently, NGR‐TNF, either alone or in combination with chemotherapy, has been tested in various clinical studies in cancer patients.37, 38
Tumours can develop new strategies to impair effector T lymphocyte function39 and cause hypoxic microenvironment to form new vessels that are disorganized, tortuous and more leaky than the normal ones. NGR‐TNF, on other hand, even at low doses, was identified to upregulate endothelial cell adhesion molecules in tumour vessels and enhance the local production of immunomodulating cytokines in tumour‐bearing mice, thereby favouring the extravasation of immune cells and improving therapeutic activity of immunotherapy.40
In addition to NGR‐TNF, other tumour vessel homing derivatives of TNFα, such as fusion protein with ACDCRGDCFCG or CisoDGRC peptides (both ligands of αv integrins)41 or with the single chain Fv Ab L19,42 can be exploited to produce synergic effects with chemotherapeutic drugs and enhance immune response in tumours. One example is the RGD peptide which can recognize various αβ integrins heterodimers.43 Interestingly, the αvβ3 heterodimer is overexpressed in blood vessels in tumours. Therefore, this receptor could be exploited as a pharmacological target to deliver cytokines to tumour blood vessels.44, 45 Subnanogram doses of RGD‐TNFα prepared by recombinant DNA technology were sufficient to enhance anti‐tumour effects in combination with melphalan in subcutaneous murine B16F1 melanomas and RMA‐T lymphomas. However, the trimetric RGD‐TNFα fusion protein hardly folded in a homogeneous manner due to 4 Cys residues involved in the structure of RGD peptide.46 In this regard, NGR‐TNFα was preferentially chosen for clinical study. Another peptide named RGR selected by phage display in pancreatic tumours showed special affinity to angiogenic vessels in insulinomas.47 It has been used as a carrier to deliver therapeutic proteins, such as TNFα and IFNγ to the targeted site for cancer therapy. Johansson et al48 demonstrated that intratumoural low‐dose of RGR‐TNFα (2 μg over 2 weeks) caused initial vessel activation and stabilization, enhanced vascular functionality, decreased vascular leakiness and T‐cell infiltration mediated by CD8+effector cells. Recently, our group has found a tumour vascular‐homing peptide TCP‐1 (a 9‐amino acid cyclic peptide) based on an in vivo phage library screening against an orthotopic colorectal cancer developed in mice.49 This peptide can specifically recognize the neovasculature of the colorectal tumour but not normal tissues in different organs. Our study showed that TCP‐1/TNFα could synergize with 5‐FU to inhibit orthotopic colorectal cancer growth. TCP‐1/TNFα normalized tumour blood vessels, increased the absorption of 5‐FU into the tumour and also facilitated the infiltration of immune cells into the neoplasm.50 Thus, TCP‐1/TNFα could be a novel agent targeting colorectal cancer tumour vessels and improve drug delivery and immune response in tumours.
4. THE ANTI‐CANCER ACTIVITY OF IFNγ
Angiogenesis is a basic process in promoting tumour growth. Numerous studies so far have focused on the angiogenic process, in an attempt to explore new strategies against tumour growth. Angiogenesis has been revealed to be a highly regulated process involving the balance between pro‐ and anti‐angiogenic factors and the interaction between the immune and endothelial cells. Vascular endothelial growth factor (VEGF) is an important pro‐angiogenic molecule in the tumour microenvironment, whose upregulation has been shown to contribute to tumour‐associated angiogenesis, and tumour‐associated macrophages (TAMs) are one of the main sources of VEGF.51 It has been found that IFNγ can reduce the expression of mouse‐VEGF, inhibit tumour angiogensis52 and induce blood vessel destruction and necrosis.53 Study also revealed that IFNγ could promote monocytes/macrophages infiltrating into tumour tissues and inhibit them to differentiate into TAMs.52 Therefore, IFNγ reduced angiogenesis by inhibiting TAM differentiation and VEGF expression in the tumour microenvironment.
As early as 1992, it was reported that the administration of recombinant IFNγ and a synthetic lipid A subunit analogue (GLA‐60) could inhibit tumour‐associated angiogenesis synergistically in C57BL/6 mice, perhaps partially depending on the induction of endogenous TNFα.54 Other mechanisms have also been proposed for IFNγ to inhibit tumour angiogenesis. For example, IFNγ can induce non‐haematopoietic cells to secrete interferon‐inducible protein 10(IP‐10), leading to blockade of tumour angiogenesis and inhibition of tumour growth.55 Specially targeting cancer‐associated fibroblasts by IFNγ to inhibit fibroblasts‐induced tube formation of H5V endothelial cells was reported to inhibit tumour vascularization.56
Besides anti‐angiogenesis effect, IFNγ could exert its anti‐cancer effect by inducing chemokine and cytokine secretion in the tumour microenvironment, as well as upregulating MHC class I and II to stimulate anti‐tumour immunity. Studies in recurring superficial transitional bladder carcinoma57 and ovarian cancers58 demonstrated significant increases of T cells infiltrating into the neoplasm after administration of IFNγ, which favoured a good prognosis in cancer patients. Moreover, IFNγ itself has direct anti‐proliferative activity on ovarian cancer cells by inducing tumour cell growth arrest and apoptosis59 and could achieve an increased complete/partial response. Several clinical trials have been conducted for IFNγ. It is proven that IFNγ when used as an adjuvant therapy, could prolong the survival in ovarian cancer patients.60 Also intraperitoneally given, IFNγ has been shown to achieve surgically documented responses by intraperitoneal treatment in the second‐line therapy of ovarian cancer.61 Moreover, when administered intravesically, IFNγ was found to be effective against bladder tumour recurrence.57 In spite of the encouraging result in the above clinical trials, a lack of beneficial effect was seen in metastatic renal‐cell carcinoma,62 advanced colon cancer63 or small‐cell lung cancer,64 advanced measurable pancreatic adenocarcinoma and also advanced breast cancer.65 Thus, the anti‐cancer effect is only on certain kinds of cancer if not for all cancers.
Similar to TNFα, systematic administration of IFNγ also faces the same problem of systemic toxicity and low anti‐cancer efficacy. The clinical anti‐cancer effects of IFNγ are summarized in Table 1. The most common adverse effects are “flu‐like,” such as fever, headache, chills or fatigue. Other common side effects include diarrhoea, nausea, vomiting and anorexia. Reversible and transient increases in hepatic transaminase and decrease in granulocyte and leucocyte counts were also seen.65, 67, 69, 82, 83, 84 Fusion proteins of IFNγ with NGR to form IFNγ‐NGR could successfully target IFNγ to tumour vessels. However, excessive stimulation of IFNγ receptors by frequent administration of low doses of IFNγ‐NGR could activate counter‐regulatory mechanisms and inhibit ongoing anti‐tumour response.41 It was found that repeated treatment of IFNγ‐NGR increases dindoleamine 2,3‐dioxygenase (IDO) and caused excessive stimulation of tryptophan catabolism and inhibited anti‐tumour immunity.85 Combination of IFNγ‐NGR with IDO inhibitors was then reported to overcome resistance of IFNγ‐NGR in nu/nu mice bearing RMA lymphoma.85 F8 antibody was another ligand specially targeting EDA domain of fibronectin, a tumour‐associated antigen expressed in the vasculature and stroma of almost all tumour types. Fusion conjugate of F8 to IFNγ retained the biological activity of both the antibody and the cytokine moiety in vitro,9 and showed dose‐dependent activity with a clear superiority over untargeted recombinant IFNγ.9 Platelet‐derived growth factor‐beta receptor (PDGFbR)‐binding carrier (pPB‐HSA) has been used as fusing peptide to specially target IFNγ to stromal fibroblasts and pericytes (2 components of tumour stroma).The pPB‐HSA‐IFNγ conjugate successfully activated IFNγ‐signalling (pSTAT1α), inhibited the activation and migration of NIH3T3 fibroblasts and hampered fibroblasts‐induced tube formation of H5V endothelial cells.56 This provides new types of drugs to target tumour stromal cells in cancer therapy.
Table 1.
Clinical studies of single agent IFNγ in different types of cancer
| Study | Total no. of patients | Phase | Tumour type | Route | Dose of IFN gamma | Schedule | MTD | ORRa | Major reported toxicities |
|---|---|---|---|---|---|---|---|---|---|
| Foon66 | 11 | N/A |
Melanoma Adenocarcinoma lung Multiple myeloma Renal cell carcinoma Giant cell sarcoma Hairy cell leukaemia |
IM(6) or IV(5) | 0.05‐10 mg/m2 | Twice weekly and the IV dose was infused over 5 min | NR | 0% | Fever, chills, fatigue, anorexia and granulocytopenia |
| Kurzrock67 | 10 |
Renal cell carcinoma; Sarcomas Colon adenocarcinoma Nodular poorly Differentiated lymphocytic lymphoma, Carcinoid Multiple myeloma Adenocarcinoma of the lung |
IM and IV | 0.01‐2.5 mg/m2 | A twice weekly schedule with IM injections alternating with IV bolus injections. A minimum period of 72 h between injections | NR | 0% | Fever, chills and fatigue after both routes of administration and granulocytopenia after IM | |
| Muss65 | 15 | II | Advanced carcinoma of the breast | IV | 2 mg/m2 | Five consecutive days every other week | NR | 0% | Flu‐like symptoms and nausea, vomiting and anorexia, hepatic toxicity |
| Boue68 | 29 | I | Advanced malignancy | IV | 0.01‐5 mg/m2 | Every 72 h for 15 days | NR | 3.4% | Fever, chills, nausea, vomiting and hypocholesterolaemia |
| Vadhan‐Raj69 | 16 | I | Advanced malignancy | IV | 0.1, 0.5, or 1.0 mg/m2/d | Six‐hour IV infusions daily, 5 days a week for 2 weeks. After a 2‐week rest period, the IV treatment cycle was repeated | NR | 12.5% | Fever, chills, fatigue and myalgias |
| D'Acquisto70 | 27 | I | Refractory ovarian carcinoma | IP | 0.5‐8 IU/m2 | Weekly | NR | 0% | Fever, myalgias and flu‐like symptoms, transaminase elevation |
| Lane71 | 16 | I | Acquired immunodeficiency syndrome (AIDS) patients with Kaposi's sarcoma | IM and IV | 0.001, 0.01, 0.1, or 1.0 mg/m2 | Single dose followed 4 days later by a 10‐day course of daily therapy. Following a 1‐week washout period, repeated administration by the alternate route | 0.1‐1.0 mg/m2 | NR | Fever, headache, fatigue, nausea and hepatitis |
| Yoshida72 | 15 | II | Advanced hepatocellular carcinoma | IV | 1.6‐2.4 × 107units | Five consecutive days every 2 weeks. | NR | 0% | Flu‐like symptoms, pyrexia, anorexia, nausea and vomiting, headache, sore throat and hepatotoxicity |
| Jett64 | 100 | III | Small‐cell lung cancer | SC | 4 × 106 U/d | Daily for 6 months | NR | 0% | Chills, myalgia, lethargy, and alteration of mood‐personality |
| Pujade‐Lauraine61 | 108 | / | Ovarian cancer with residual disease after first line cisplatin‐based chemotherapy | IP | 20 × 106 IU/m2 | Twice a week for 3‐4 months | NR | 31.6% | Fever, flu‐like syndrome, neutropenia and liver enzyme disturbances |
| Giannopoulos57 | 123 | Superficial transitional cell carcinoma of the bladder | Intravesical Instillations | 1.5 × 107 IU/instillation | Eight weekly instillations followed by four biweekly and then by eight monthly instillations | NR | NR | Cystitis‐like symptoms | |
| Rinehart73 | 13 | I/II | Metastatic renal cell carcinoma | IV | Twice weekly | NR | 0% | Anorexia, fever and malaise | |
| Quesada74 | 33 | II | Metastatic renal cell carcinoma | IM (15) and IV (18) |
IM: 0.25‐1.0 mg/m2
IV: 0.01‐0.05 mg/m2 |
Daily | NR | 7% for IM and 6% for IV | Fatigue, anorexia, weight loss, leucopenia, abnormalities in liver function tests and hypertriglyceridaemia |
| Garnick75 | 42 | I/II | Advanced renal cell carcinoma | IV | 10‐3000 mcg/m2 | Either a daily 2‐h infusion or 24‐h infusion for 7 days every 3 weeks for at least 2 cycles. Maintenance program of 5 days of recombinant interferon gamma administered every 3‐4 weeks | 3000 mcg/m2 | 9.8% | Leucopenia, chills, fevers, rigors and hepatotoxicity |
| Aulitzky76 | 22 | II | Metastatic renal cell carcinoma | SC | 100 μg | Once weekly | NR | 30% | Fever, fatigue, chills, febrile reactions, fatigue and malaise |
| Ellerhorst77 | 35 | II | Metastatic renal cell carcinoma | SC | 100 μg | Once weekly | NR | 15% | Low grade fever, chills and myalgias |
| Gleave62 | 181 | / | Metastatic renal cell carcinoma | SC | 60 mcg/m2 | Once weekly | NR | 4.4% | Chills, fever, asthenia and headaches |
| Small78 | 207 | / | Metastatic renal cell carcinoma | SC | 60 mcg/m2 | Once weekly | NR | 3% | Chills, fever, asthenia, nausea and headache |
| Creagan79 | 28 | II | Disseminated malignant melanoma | IM | 0.25 mg/m2 on days 1‐7 followed by a daily dose of 0.5 mg/m2 if tolerated | Daily | NR | 11.1% | Moderate to severe fever greater than 37°C (100%), fatigue (59%), chills (37%) and mild to moderate myalgias (64%) |
| Ernstoff80 | 30 | I/II | Metastatic melanoma | IV | 3‐3000 mcg/m2 over either 2 or 24 h | Daily | 1000 mcg/m2 | 6.7% | Fever, chills, myalgias, headache, fatigue, neutropenia, elevations in liver enzymes, tachyarrhythmias and change in mental status |
| Schiller81 | 89 | II/III | Metastatic melanoma | IV | 0.01‐0.90 mg/m2 | Three times per week for at least 8 weeks or until progressive disease | NR | 5% | Fever and chills and hepatic toxicity |
Objective response rate calculated using number of patients evaluable for response where available.
ORR: objective response rate; NR: not reported in study; IM: intramuscular; IV: intravenous.
5. CO‐ADMINISTRATION OF TNFα AND IFNγ IN ANTI‐CANCER THERAPY
Both TNFα and IFNγ demonstrated inspiring anti‐cancer effects in in vitro and in vivo studies. However, both of them when administrated alone presented limited therapeutic responses in clinics. Numerous studies were then conducted to focus on the synergic anti‐cancer effects of both TNFα and IFNγ, especially in the induction of cellular apoptosis. As early as 1988, recombinant TNFα and IFNγ have already been reported to induce synergic anti‐proliferative effects on human pancreatic tumour cell lines.86 TNFα combined with IFNγ could accelerate NF‐κB‐mediated apoptosis through enhancement of fas expression in colon cancer cells.87 Such effect was also depicted in ewing tumour cells.88 Nitric Oxide expression and activation of PI3‐kinase‐dependent signalling cascade were also involved in mediating the synergistic pro‐apoptotic effects of TNFα and IFNγ.89 Kim et al90 found out that IFNγ sensitizes MIN6N8 insulinoma cells to TNFα‐induced apoptosis by inhibiting NF‐κB‐mediated XIAP upregulation. Kulkarni et al91 then reported that IFNγ can sensitize the human salivary gland cell line, HSG, to TNFα‐induced activation of dual apoptotic pathways. Hairy cell leukaemia was reported to extremely sensitive to IFNγ, and further studies decoded that exposure of hairy cells (HCs) to IFNγ resulted in a marked increase of TNFα secretion, which was then solidly identified to be attributable to suppression of IAP (inhibitors of apoptosis), a protein known to be regulated by the cytoprotective NF‐κB‐dependent arm of TNFα signalling.92 Synergistic activation of JNK/MAPK induced by TNFα and IFNγ to activate apoptosis was observed in pancreatic β‐cells via the p53 and ROS pathway.93
There are many other mechanisms underlying the synergism between TNFα and IFNγ besides the synergic apoptosis‐inducing effects. Studies hypothesized that although TNFα and IFNγ were not required by cytolytic effect on CD8+ T cells (CTLs) for perforin‐mediated killing of antigen‐expressing tumour cells, tumour antigen‐specific CTLs must secrete TNFα and IFNγ for the destruction of tumour stroma.94 Moreover, TNFα and IFNγ produced by NK cells could induce target cell cytolysis through upregulation of ICAM‐1.95 Lately, TNFα and IFNγ were reported to co‐operate together to induce senescence in numerous murine and human cancers by induction of permanent growth arrest in G1/G0, activation of p16INK4a, and downstream Rb hypophosphorylation at serine 795.96
Malignant tumours evolve along multistage programs of establishing a tumour stroma, neoangiogenesis and reprogramming of cell metabolism, finally leading to the expression of tumour‐associated antigens (TAA).97 This evolving process mainly depends on innate immune cells to induce aberrant vessel growth and adaptive immune response against TAA, which play important roles in the transition of premalignant dysplasia into carcinoma and further cancer progression.98, 99 Many focuses have been put on CTL to develop tumour immune therapy, and later a more efficient IFNγ‐producing CD4+ cell (Th1) was recognized to prevent transplant tumours growth and development by regulating multistage carcinogenesis through cytokine signals. Both tumour necrosis factor p55 receptor (TNFR1) signalling and IFNγ signalling were found to be essential for dominant anti‐tumour effects of Tag‐specific Th1 cells. Absence of either TNFR1 signalling or IFNγ signalling determined Tag‐specific Th1 cells to induce tumour dormancy or promote multistage carcinogenesis,97 which was another solid evidence supporting the co‐operative effects of TNFα and IFNγ in anti‐tumour treatment. Our recent study using tumour vasculature homing peptide TCP‐1 showed that targeted combination therapy with TCP‐1/TNFα and TCP‐1/IFNγ could remarkably inhibit orthotopic colorectal tumour growth by inducing tumour necrosis without causing significant systematic toxicity.100 The anti‐tumour effects of TNFα and IFNγ either using alone or in combination are summarized in Figure 1. Our result emphasizes the therapeutic potential of co‐administration of targeted TNFα and IFNγ for cancer treatment and the utility of TCP‐1 peptide as a tumour‐targeting agent in colorectal cancer. Comprehensive toxicity study is still needed before further application of this combination of treatment for type of cancer.
Figure 1.
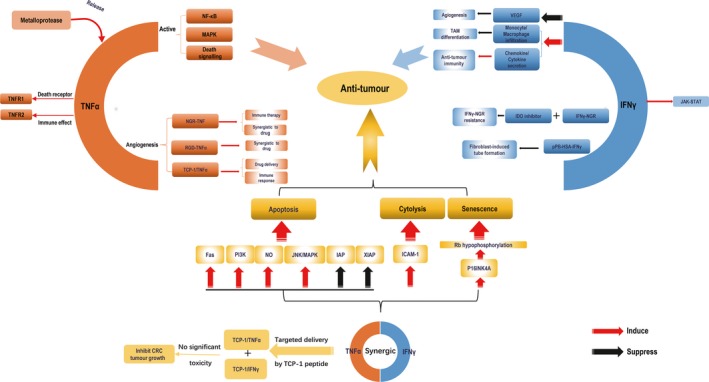
The anti‐tumour effect of TNFα and IFNγ alone and in combination. The soluble homotrimeric TNFα released by metalloprotease bind to death receptor TNFR1 and immune system receptor TNFR2,which can activate 3 signalling cascades including NF‐κB, MAPK and death signalling. The tumour‐homing TNFα has significant anti‐tumour effect. NGR‐TNF can enhance TNFα delivery and immunotherapeutic effect without influencing the biological effect of TNFα in vivo. At the same time, it can also synergize with chemotherapeutic drug. Another peptide RGD can also recognize the tumour blood vessel and RGD‐TNFα has synergistic anti‐tumour effect with chemotherapeutic drug. Besides, TCP‐1/TNFα increased the absorption of drug and immune response in tumours. The 2 receptors IFNGR1/IFNGR2 of IFNγ can activate JAK‐STAT pathway to regulate cell apoptosis. Study indicated that IFNγ could reduce the mouse‐VEGF and promote monocytes/macrophages infiltrating and chemokine/cytokine secretion to inhibit tumour growth. IFNγ‐NGR could target IFNγ to tumour vessels. Combination of IFNγ‐NGR with IDO inhibitors could overcome resistance of IFNγ‐NGR caused by excessive stimulation of tryptophan catabolism. The pPB‐HSA‐IFNγ also successfully activated IFNγ‐signalling, inhibited the activation and migration of fibroblasts and hampered fibroblasts‐induced tube formation of endothelial cells. TNFα combined with IFNγ has been shown to have synergic anti‐tumour effect via various pathways. Lately, targeted delivery of TCP‐1/TNFα and TCP‐1/IFNγ to tumour blood vessel has been demonstrated to significantly inhibit orthotopic colorectal tumour growth without significant systematic toxicity
6. CONCLUSION
Tumour necrosis factor alpha and IFNγ are now affirmed as pro‐inflammatory cytokines and also produce effective anti‐tumour effects. Their clinical application was limited due to the toxicity and counter‐regulatory mechanisms. Such limitations could partially be overcome by fusion of TNFα and IFNγ to peptides or antibodies targeting tumour epithelial, endothelial or stromal cells.13, 101 An alternative strategy of targeted delivery of TNFα by TNF‐expressing cancer cells has lately been demonstrated. The safety issues in clinical context await further assessment.23 The multifunctional properties of TNFα and IFNγ and the newly discovered targeted delivery strategies may well result in a more optimistic clinical applications of these 2 cytokines in cancer treatment in a foreseeable future.
CONFLICT OF INTEREST
The authors declare that the fundings mentioned in the Acknowledgments section do not lead to any conflict of interest. Additionally, the authors declare that there is no conflict of interest regarding the publication of this manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (Grant nos. 81503093, 81602166, and 81672444), the Joint Funds of the Southwest Medical University &Luzhou (2016LZXNYD‐T01 and 2017LZXNYD‐Z05).
Shen J, Xiao Z, Zhao Q, et al. Anti‐cancer therapy with TNFα and IFNγ: A comprehensive review. Cell Prolif. 2018;51:e12441 10.1111/cpr.12441
Correction added on 24 January 2019 after first online publication: The corresponding author details was previously incorrect and is now updated in this version.
Contributor Information
Zhangang Xiao, Email: xzg555898@hotmail.com.
Chi H. Cho, Email: chcho@cuhk.edu.hk
REFERENCES
- 1. Carswell EA, Old LJ, Kassel RL, et al. An endotoxin‐induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666‐3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lejeune FJ, Lienard D, Matter M, et al. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- 3. Mortara L, Balza E, Sassi F, et al. Therapy‐induced antitumor vaccination by targeting tumor necrosis factor alpha to tumor vessels in combination with melphalan. Eur J Immunol. 2007;37:3381‐3392. [DOI] [PubMed] [Google Scholar]
- 4. Wheelock EF. Interferon‐like virus‐inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965;149:310‐311. [PubMed] [Google Scholar]
- 5. Jouanguy E, Lamhamedi‐Cherradi S, Lammas D, et al. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nat Genet. 1999;21:370‐378. [DOI] [PubMed] [Google Scholar]
- 6. Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95‐109. [DOI] [PubMed] [Google Scholar]
- 7. Zaidi MR, Merlino G. The two faces of interferon‐gamma in cancer. Clin Cancer Res. 2011;17:6118‐6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai W, Kerner ZJ, Hong H, et al. Targeted cancer therapy with tumor necrosis factor‐alpha. Biochem Insights. 2008;2008:15‐21. [PMC free article] [PubMed] [Google Scholar]
- 9. Hemmerle T, Neri D. The dose‐dependent tumor targeting of antibody‐IFNgamma fusion proteins reveals an unexpected receptor‐trapping mechanism in vivo. Cancer Immunol Res. 2014;2:559‐567. [DOI] [PubMed] [Google Scholar]
- 10. Kaspar M, Trachsel E, Neri D. The antibody‐mediated targeted delivery of interleukin‐15 and GM‐CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res. 2007;67:4940‐4948. [DOI] [PubMed] [Google Scholar]
- 11. Pasche N, Frey K, Neri D. The targeted delivery of IL17 to the mouse tumor neo‐vasculature enhances angiogenesis but does not reduce tumor growth rate. Angiogenesis. 2012;15:165‐169. [DOI] [PubMed] [Google Scholar]
- 12. Pasche N, Woytschak J, Wulhfard S, et al. Cloning and characterization of novel tumor‐targeting immunocytokines based on murine IL7. J Biotechnol. 2011;154:84‐92. [DOI] [PubMed] [Google Scholar]
- 13. Tandle A, Hanna E, Lorang D, et al. Tumor vasculature‐targeted delivery of tumor necrosis factor‐alpha. Cancer. 2009;115:128‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Curnis F, Sacchi A, Borgna L, et al. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13). Nat Biotechnol. 2000;18:1185‐1190. [DOI] [PubMed] [Google Scholar]
- 15. Zhang G. Tumor necrosis factor family ligand‐receptor binding. Curr Opin Struct Biol. 2004;14:154‐160. [DOI] [PubMed] [Google Scholar]
- 16. Roberts NJ, Zhou S, Diaz LA Jr, et al. Systemic use of tumor necrosis factor alpha as an anticancer agent. Oncotarget. 2011;2:739‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furman WL, Strother D, McClain K, et al. Phase I clinical trial of recombinant human tumor necrosis factor in children with refractory solid tumors: a Pediatric Oncology Group study. J Clin Oncol. 1993;11:2205‐2210. [DOI] [PubMed] [Google Scholar]
- 18. Creaven PJ, Plager JE, Dupere S, et al. Phase I clinical trial of recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 1987;20:137‐144. [DOI] [PubMed] [Google Scholar]
- 19. Creagan ET, Kovach JS, Moertel CG, et al. A phase I clinical trial of recombinant human tumor necrosis factor. Cancer. 1988;62:2467‐2471. [DOI] [PubMed] [Google Scholar]
- 20. Kimura K, Taguchi T, Urushizaki I, et al. Phase I study of recombinant human tumor necrosis factor. Cancer Chemother Pharmacol. 1987;20:223‐229. [DOI] [PubMed] [Google Scholar]
- 21. Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Horssen R, Ten Hagen TL, Eggermont AM. TNF‐alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397‐408. [DOI] [PubMed] [Google Scholar]
- 23. Dondossola E, Dobroff AS, Marchio S, et al. Self‐targeting of TNF‐releasing cancer cells in preclinical models of primary and metastatic tumors. Proc Natl Acad Sci USA. 2016;113:2223‐2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kristensen CA, Nozue M, Boucher Y, et al. Reduction of interstitial fluid pressure after TNF‐alpha treatment of three human melanoma xenografts. Br J Cancer. 1996;74:533‐536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suzuki S, Ohta S, Takashio K, et al. Augmentation for intratumoral accumulation and anti‐tumor activity of liposome‐encapsulated adriamycin by tumor necrosis factor‐alpha in mice. Int J Cancer. 1990;46:1095‐1100. [DOI] [PubMed] [Google Scholar]
- 26. Gasparri A, Moro M, Curnis F, et al. Tumor pretargeting with avidin improves the therapeutic index of biotinylated tumor necrosis factor alpha in mouse models. Cancer Res. 1999;59:2917‐2923. [PubMed] [Google Scholar]
- 27. Nawroth P, Handley D, Matsueda G, et al. Tumor necrosis factor/cachectin‐induced intravascular fibrin formation in meth A fibrosarcomas. J Exp Med. 1988;168:637‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nawroth PP, Stern DM. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986;163:740‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palladino MA Jr, Shalaby MR, Kramer SM, et al. Characterization of the antitumor activities of human tumor necrosis factor‐alpha and the comparison with other cytokines: induction of tumor‐specific immunity. J Immunol. 1987;138:4023‐4032. [PubMed] [Google Scholar]
- 30. Lienard D, Ewalenko P, Delmotte JJ, et al. High‐dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52‐60. [DOI] [PubMed] [Google Scholar]
- 31. Fraker DL, Alexander HR, Andrich M, et al. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: results of a tumor necrosis factor dose‐escalation study. J Clin Oncol. 1996;14:479‐489. [DOI] [PubMed] [Google Scholar]
- 32. Alexander HR Jr, Bartlett DL, Libutti SK, et al. Isolated hepatic perfusion with tumor necrosis factor and melphalan for unresectable cancers confined to the liver. J Clin Oncol. 1998;16:1479‐1489. [DOI] [PubMed] [Google Scholar]
- 33. Eggermont AM, Schraffordt Koops H, Lienard D, et al. Isolated limb perfusion with high‐dose tumor necrosis factor‐alpha in combination with interferon‐gamma and melphalan for nonresectable extremity soft tissue sarcomas: a multicenter trial. J Clin Oncol. 1996;14:2653‐2665. [DOI] [PubMed] [Google Scholar]
- 34. Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J Clin Invest. 2002;110:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Laarhoven HW, Gambarota G, Heerschap A, et al. Effects of the tumor vasculature targeting agent NGR‐TNF on the tumor microenvironment in murine lymphomas. Invest New Drugs. 2006;24:27‐36. [DOI] [PubMed] [Google Scholar]
- 36. Sacchi A, Gasparri A, Gallo‐Stampino C, et al. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature‐targeted tumor necrosis factor‐alpha. Clin Cancer Res. 2006;12:175‐182. [DOI] [PubMed] [Google Scholar]
- 37. Gregorc V, Santoro A, Bennicelli E, et al. Phase Ib study of NGR‐hTNF, a selective vascular targeting agent, administered at low doses in combination with doxorubicin to patients with advanced solid tumours. Br J Cancer. 2009;101:219‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gregorc V, Zucali PA, Santoro A, et al. Phase II study of asparagine‐glycine‐arginine‐human tumor necrosis factor alpha, a selective vascular targeting agent, in previously treated patients with malignant pleural mesothelioma. J Clin Oncol. 2010;28:2604‐2611. [DOI] [PubMed] [Google Scholar]
- 39. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Calcinotto A, Grioni M, Jachetti E, et al. Targeting TNF‐alpha to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. 2012;188:2687‐2694. [DOI] [PubMed] [Google Scholar]
- 41. Curnis F, Gasparri A, Sacchi A, et al. Targeted delivery of IFNgamma to tumor vessels uncouples antitumor from counterregulatory mechanisms. Cancer Res. 2005;65:2906‐2913. [DOI] [PubMed] [Google Scholar]
- 42. Borsi L, Balza E, Carnemolla B, et al. Selective targeted delivery of TNFalpha to tumor blood vessels. Blood. 2003;102:4384‐4392. [DOI] [PubMed] [Google Scholar]
- 43. Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861‐3863. [DOI] [PubMed] [Google Scholar]
- 44. Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Avraamides CJ, Garmy‐Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zarovni N, Monaco L, Corti A. Inhibition of tumor growth by intramuscular injection of cDNA encoding tumor necrosis factor alpha coupled to NGR and RGD tumor‐homing peptides. Hum Gene Ther. 2004;15:373‐382. [DOI] [PubMed] [Google Scholar]
- 47. Joyce JA, Laakkonen P, Bernasconi M, et al. Stage‐specific vascular markers revealed by phage display in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2003;4:393‐403. [DOI] [PubMed] [Google Scholar]
- 48. Johansson A, Hamzah J, Payne CJ, et al. Tumor‐targeted TNFalpha stabilizes tumor vessels and enhances active immunotherapy. Proc Natl Acad Sci USA. 2012;109:7841‐7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li ZJ, Wu WK, Ng SS, et al. A novel peptide specifically targeting the vasculature of orthotopic colorectal cancer for imaging detection and drug delivery. J Control Release. 2010;148:292‐302. [DOI] [PubMed] [Google Scholar]
- 50. Lu L, Li ZJ, Li LF, et al. Vascular‐targeted TNFalpha improves tumor blood vessel function and enhances antitumor immunity and chemotherapy in colorectal cancer. J Control Release. 2015;210:134‐146. [DOI] [PubMed] [Google Scholar]
- 51. Allavena P, Sica A, Solinas G, et al. The inflammatory micro‐environment in tumor progression: the role of tumor‐associated macrophages. Crit Rev Oncol Hematol. 2008;66:1‐9. [DOI] [PubMed] [Google Scholar]
- 52. Sun T, Yang Y, Luo X, et al. Inhibition of tumor angiogenesis by interferon‐gamma by suppression of tumor‐associated macrophage differentiation. Oncol Res. 2014;21:227‐235. [DOI] [PubMed] [Google Scholar]
- 53. Briesemeister D, Sommermeyer D, Loddenkemper C, et al. Tumor rejection by local interferon gamma induction in established tumors is associated with blood vessel destruction and necrosis. Int J Cancer. 2011;128:371‐378. [DOI] [PubMed] [Google Scholar]
- 54. Saiki I, Sato K, Yoo YC, et al. Inhibition of tumor‐induced angiogenesis by the administration of recombinant interferon‐gamma followed by a synthetic lipid‐A subunit analogue (GLA‐60). Int J Cancer. 1992;51:641‐645. [DOI] [PubMed] [Google Scholar]
- 55. Qin Z, Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677‐686. [DOI] [PubMed] [Google Scholar]
- 56. Bansal R, Tomar T, Ostman A, et al. Selective targeting of interferon gamma to stromal fibroblasts and pericytes as a novel therapeutic approach to inhibit angiogenesis and tumor growth. Mol Cancer Ther. 2012;11:2419‐2428. [DOI] [PubMed] [Google Scholar]
- 57. Giannopoulos A, Constantinides C, Fokaeas E, et al. The immunomodulating effect of interferon‐gamma intravesical instillations in preventing bladder cancer recurrence. Clin Cancer Res. 2003;9:5550‐5558. [PubMed] [Google Scholar]
- 58. Marth C, Fiegl H, Zeimet AG, et al. Interferon‐gamma expression is an independent prognostic factor in ovarian cancer. Am J Obstet Gynecol. 2004;191:1598‐1605. [DOI] [PubMed] [Google Scholar]
- 59. Wall L, Burke F, Barton C, et al. IFN‐gamma induces apoptosis in ovarian cancer cells in vivo and in vitro. Clin Cancer Res. 2003;9:2487‐2496. [PubMed] [Google Scholar]
- 60. Windbichler GH, Hausmaninger H, Stummvoll W, et al. Interferon‐gamma in the first‐line therapy of ovarian cancer: a randomized phase III trial. Br J Cancer. 2000;82:1138‐1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pujade‐Lauraine E, Guastalla JP, Colombo N, et al. Intraperitoneal recombinant interferon gamma in ovarian cancer patients with residual disease at second‐look laparotomy. J Clin Oncol. 1996;14:343‐350. [DOI] [PubMed] [Google Scholar]
- 62. Gleave ME, Elhilali M, Fradet Y, et al. Interferon gamma‐1b compared with placebo in metastatic renal‐cell carcinoma. Canadian Urologic Oncology Group. N Engl J Med. 1998;338:1265‐1271. [DOI] [PubMed] [Google Scholar]
- 63. Wiesenfeld M, O'Connell MJ, Wieand HS, et al. Controlled clinical trial of interferon‐gamma as postoperative surgical adjuvant therapy for colon cancer. J Clin Oncol. 1995;13:2324‐2329. [DOI] [PubMed] [Google Scholar]
- 64. Jett JR, Maksymiuk AW, Su JQ, et al. Phase III trial of recombinant interferon gamma in complete responders with small‐cell lung cancer. J Clin Oncol. 1994;12:2321‐2326. [DOI] [PubMed] [Google Scholar]
- 65. Muss HB, Caponera M, Zekan PJ, et al. Recombinant gamma interferon in advanced breast cancer: a phase II trial. Invest New Drugs. 1986;4:377‐381. [DOI] [PubMed] [Google Scholar]
- 66. Foon KA, Sherwin SA, Abrams PG, et al. A phase I trial of recombinant gamma interferon in patients with cancer. Cancer Immunol Immunother. 1985;20:193‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kurzrock R, Rosenblum MG, Sherwin SA, et al. Pharmacokinetics, single‐dose tolerance, and biological activity of recombinant gamma‐interferon in cancer patients. Cancer Res. 1985;45:2866‐2872. [PubMed] [Google Scholar]
- 68. Boue F, Pastran Z, Spielmann M, et al. A phase I trial with recombinant interferon gamma (Roussel UCLAF) in advanced cancer patients. Cancer Immunol Immunother. 1990;32:67‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vadhan‐Raj S, Al‐Katib A, Bhalla R, et al. Phase I trial of recombinant interferon gamma in cancer patients. J Clin Oncol. 1986;4:137‐146. [DOI] [PubMed] [Google Scholar]
- 70. D'Acquisto R, Markman M, Hakes T, et al. A phase I trial of intraperitoneal recombinant gamma‐interferon in advanced ovarian carcinoma. J Clin Oncol. 1988;6:689‐695. [DOI] [PubMed] [Google Scholar]
- 71. Lane HC, Davey RT Jr, Sherwin SA, et al. A phase I trial of recombinant human interferon‐gamma in patients with Kaposi's sarcoma and the acquired immunodeficiency syndrome (AIDS). J Clin Immunol. 1989;9:351‐361. [DOI] [PubMed] [Google Scholar]
- 72. Yoshida T, Okazaki N, Yoshino M, et al. Phase II trial of high dose recombinant gamma‐interferon in advanced hepatocellular carcinoma. Eur J Cancer. 1990;26:545‐546. [DOI] [PubMed] [Google Scholar]
- 73. Rinehart JJ, Malspeis L, Young D, et al. Phase I/II trial of human recombinant interferon gamma in renal cell carcinoma. J Biol Response Mod. 1986;5:300‐308. [PubMed] [Google Scholar]
- 74. Quesada JR, Kurzrock R, Sherwin SA, et al. Phase II studies of recombinant human interferon gamma in metastatic renal cell carcinoma. J Biol Response Mod. 1987;6:20‐27. [PubMed] [Google Scholar]
- 75. Garnick MB, Reich SD, Maxwell B, et al. Phase I/II study of recombinant interferon gamma in advanced renal cell carcinoma. J Urol. 1988;139:251‐255. [DOI] [PubMed] [Google Scholar]
- 76. Aulitzky W, Gastl G, Aulitzky WE, et al. Successful treatment of metastatic renal cell carcinoma with a biologically active dose of recombinant interferon‐gamma. J Clin Oncol. 1989;7:1875‐1884. [DOI] [PubMed] [Google Scholar]
- 77. Ellerhorst JA, Kilbourn RG, Amato RJ, et al. Phase II trial of low dose gamma‐interferon in metastatic renal cell carcinoma. J Urol. 1994;152:841‐845. [DOI] [PubMed] [Google Scholar]
- 78. Small EJ, Weiss GR, Malik UK, et al. The treatment of metastatic renal cell carcinoma patients with recombinant human gamma interferon. Cancer J Sci Am. 1998;4:162‐167. [PubMed] [Google Scholar]
- 79. Creagan ET, Ahmann DL, Long HJ, et al. Phase II study of recombinant interferon‐gamma in patients with disseminated malignant melanoma. Cancer Treat Rep. 1987;71:843‐844. [PubMed] [Google Scholar]
- 80. Ernstoff MS, Trautman T, Davis CA, et al. A randomized phase I/II study of continuous versus intermittent intravenous interferon gamma in patients with metastatic melanoma. J Clin Oncol. 1987;5:1804‐1810. [DOI] [PubMed] [Google Scholar]
- 81. Schiller JH, Pugh M, Kirkwood JM, et al. Eastern cooperative group trial of interferon gamma in metastatic melanoma: an innovative study design. Clin Cancer Res. 1996;2:29‐36. [PubMed] [Google Scholar]
- 82. Miller CH, Maher SG, Young HA. Clinical use of interferon‐gamma. Ann NY Acad Sci. 2009;1182:69‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Balachandran S, Adams GP. Interferon‐gamma‐induced necrosis: an antitumor biotherapeutic perspective. J Interferon Cytokine Res. 2013;33:171‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bennett CL, Vogelzang NJ, Ratain MJ, et al. Hyponatremia and other toxic effects during a phase I trial of recombinant human gamma interferon and vinblastine. Cancer Treat Rep. 1986;70:1081‐1084. [PubMed] [Google Scholar]
- 85. Gasparri AM, Jachetti E, Colombo B, et al. Critical role of indoleamine 2,3‐dioxygenase in tumor resistance to repeated treatments with targeted IFNgamma. Mol Cancer Ther. 2008;7:3859‐3866. [DOI] [PubMed] [Google Scholar]
- 86. Schmiegel WH, Caesar J, Kalthoff H, et al. Antiproliferative effects exerted by recombinant human tumor necrosis factor‐alpha (TNF‐alpha) and interferon‐gamma (IFN‐gamma) on human pancreatic tumor cell lines. Pancreas. 1988;3:180‐188. [DOI] [PubMed] [Google Scholar]
- 87. Kimura M, Haisa M, Uetsuka H, et al. TNF combined with IFN‐alpha accelerates NF‐kappaB‐mediated apoptosis through enhancement of Fas expression in colon cancer cells. Cell Death Differ. 2003;10:718‐728. [DOI] [PubMed] [Google Scholar]
- 88. Abadie A, Wietzerbin J. Involvement of TNF‐related apoptosis‐inducing ligand (TRAIL) induction in interferon gamma‐mediated apoptosis in Ewing tumor cells. Ann NY Acad Sci. 2003;1010:117‐120. [DOI] [PubMed] [Google Scholar]
- 89. Wright K, Kolios G, Westwick J, et al. Cytokine‐induced apoptosis in epithelial HT‐29 cells is independent of nitric oxide formation. Evidence for an interleukin‐13‐driven phosphatidylinositol 3‐kinase‐dependent survival mechanism. J Biol Chem. 1999;274:17193‐17201. [DOI] [PubMed] [Google Scholar]
- 90. Kim HS, Kim S, Lee MS. IFN‐gamma sensitizes MIN6N8 insulinoma cells to TNF‐alpha‐induced apoptosis by inhibiting NF‐kappaB‐mediated XIAP upregulation. Biochem Biophys Res Commun. 2005;336:847‐853. [DOI] [PubMed] [Google Scholar]
- 91. Kulkarni K, Selesniemi K, Brown TL. Interferon‐gamma sensitizes the human salivary gland cell line, HSG, to tumor necrosis factor‐alpha induced activation of dual apoptotic pathways. Apoptosis. 2006;11:2205‐2215. [DOI] [PubMed] [Google Scholar]
- 92. Baker PK, Pettitt AR, Slupsky JR, et al. Response of hairy cells to IFN‐alpha involves induction of apoptosis through autocrine TNF‐alpha and protection by adhesion. Blood. 2002;100:647‐653. [DOI] [PubMed] [Google Scholar]
- 93. Kim WH, Lee JW, Gao B, et al. Synergistic activation of JNK/SAPK induced by TNF‐alpha and IFN‐gamma: apoptosis of pancreatic beta‐cells via the p53 and ROS pathway. Cell Signal. 2005;17:1516‐1532. [DOI] [PubMed] [Google Scholar]
- 94. Zhang B, Karrison T, Rowley DA, et al. IFN‐gamma‐ and TNF‐dependent bystander eradication of antigen‐loss variants in established mouse cancers. J Clin Invest. 2008;118:1398‐1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wang R, Jaw JJ, Stutzman NC, et al. Natural killer cell‐produced IFN‐gamma and TNF‐alpha induce target cell cytolysis through up‐regulation of ICAM‐1. J Leukoc Biol. 2012;91:299‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Braumuller H, Wieder T, Brenner E, et al. T‐helper‐1‐cell cytokines drive cancer into senescence. Nature. 2013;494:361‐365. [DOI] [PubMed] [Google Scholar]
- 97. Muller‐Hermelink N, Braumuller H, Pichler B, et al. TNFR1 signaling and IFN‐gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507‐518. [DOI] [PubMed] [Google Scholar]
- 98. Boon T, Coulie PG, Van den Eynde BJ, et al. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175‐208. [DOI] [PubMed] [Google Scholar]
- 99. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shen J, Li ZJ, Li LF, et al. Vascular‐targeted TNFalpha and IFNgamma inhibits orthotopic colorectal tumor growth. J Transl Med. 2016;14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Curnis F, Sacchi A, Gasparri A, et al. Isoaspartate‐glycine‐arginine: a new tumor vasculature‐targeting motif. Cancer Res. 2008;68:7073‐7082. [DOI] [PubMed] [Google Scholar]


